Abstract
Quantification of insulin release from pancreatic islets of Langerhans is of interest for diabetes research. Typical insulin secretion experiments are performed using offline techniques that are expensive, slow, have low-throughput, and require multiple islets. We have developed a microfluidic device for high-throughput, automated, and online monitoring of insulin secretion from individual islets in parallel. This chip consists of 15 channel networks each capable of superfusing a single islet and mixing superfusate from each islet online with fluorescein isothiocyanate-labeled insulin and anti-insulin antibody for a competitive immunoassay. The resulting continuous reaction streams are periodically injected onto parallel electrophoresis channels where the mixtures are separated. The resulting traces are used to quantify relative insulin released from islets. Serial immunoassays were performed at 10 s intervals on all 15 channels, corresponding to 5400 immunoassays per hour, to create temporally resolved insulin release profiles that captured single islet secretion dynamics. The chip was used to demonstrate that free fatty acid induced lipotoxicity in islets eliminates pulsatile insulin secretion.
INTRODUCTION
Secretion of insulin from pancreatic islets of Langerhans, necessary for modulation of blood glucose levels, displays complex kinetics including biphasic1,2 and pulsatile3–5 responses to elevated glucose. Although the underlying mechanisms of such dynamics have yet to be fully elucidated, it is thought that these secretion patterns are important for insulin action on target tissues.6,7 Interest in oscillatory secretion has been heightened by the observation that individuals with type 2 diabetes and their near relatives have disrupted pulsatile secretion.8,9 Furthermore, oscillatory insulin secretion is considered an indicator of robust islet function and therefore might serve as a useful measure of quality of islets used for transplant, an experimental therapy for type 1 diabetes.10 For these reasons, measurements of insulin secretion dynamics have research and clinical significance.
Insulin secretion measurements are typically performed using enzyme-linked immunosorbent assay (ELISA) or radioimmunoassay (RIA).11,12 Nearly all such measurements are performed at low temporal resolution (minutes) on batches of islets because it is difficult and expensive to use immunoassays that have sufficient sensitivity to measure insulin release at high temporal resolution necessary for monitoring dynamics, especially from single islets. Single islet experiments are necessary for detecting oscillations to prevent destructive interference of out of phase oscillations from multiple islets in a large batch.13 Minimizing the number of islets required for experiments is also important because islets are difficult and expensive to harvest from subjects. The long analysis time of ELISA and RIA also hampers their use for evaluating islet quality prior to transplant where a short period between islet isolation and transplant is available. Thus, the ability to rapidly quantify temporally-resolved insulin secretion at the single islet level would have significant biomedical and clinical application.
To fill this need, we have previously developed a microfluidic chip that allows insulin secretion from individual islets to be continuously monitored.14 On this device, an islet is housed in a cell chamber on the chip and superfused with media while secreted insulin is continuously sampled from the chamber by electroosmotic flow (EOF). The sample stream is mixed on-line with fluorescein isothiocyanate-labeled insulin (FITC-ins) and anti-insulin antibody (Ab). Portions of this reaction mixture stream are periodically sampled and separated by capillary electrophoresis (CE) to quantify immunoassay products. This chip enables insulin secretion to be monitored with ~10 s temporal resolution from single islets with complete automation; however, throughput for islet experiments was low because only a single islet could be monitored at one time. To improve throughout, we developed a parallel channel system in which four independent cell monitoring networks were fabricated in one chip.15 Detection in the parallel system was accomplished using a rapid scanning confocal microscope. While improving throughput, this system had limited practicality because the LOD was barely sufficient to detect basal insulin secretion and the geometry of the chips combined with the limited field of view of the microscope prevented scale up beyond four channels. Both the single and four channel chips also suffered in that the approach to sampling superfusate from the cells had potential for inaccuracies associated with positioning cells within the chip and use of electroosmotic flow for sampling.
In this work, we address these limitations and further increase throughput resulting in a practical chip for quantitative monitoring of insulin secretion from 15 single islets in parallel. Multiplexed fluorescence detection of the 15 parallel CE networks is facilitated by a radial microchannel design16 in which all separation channels converge at a common point allowing fluorescence detection to be performed by imaging with a single objective lens on a microscope equipped with a sensitive CCD. This detector yielded greater than10-fold better fluorescence LOD even though it was used on more channels than the previous scanning system. Sampling from the islets is changed to allow improved quantification. As a demonstration of the utility of the chip, we show that islets isolated from mice have characteristic fast and slow oscillation patterns of insulin secretion, complementing previous studies.17,18 An additional set of experiments revealed that chronic exposure to free fatty acids in culture, a model of lipotoxicity,19 blunts pulsatile release from islets. The ability to perform these studies relies on the capacity to collect high throughput secretion data on the single islet level, a technique greatly facilitated with the presented microdevice and not possible with competing technologies. Furthermore, the general approach to detection, sampling, and assay should be of benefit in other cell-on-chip applications.
EXPERIMENTAL SECTION
Chemicals and Reagents
Unless otherwise noted, all reagents were from Fisher. Tricine was from MP Biomedicals (Aurora, OH). Collagenase type XI, insulin, ethylenediaminetetraacetic acid (EDTA), fluorescein, and Tween 20 were from Sigma (St. Louis, MO). Cell culture reagents were purchased from Invitrogen (Carlsbad, CA). Fluorescein isothiocyanate-labeled insulin (FITC-ins) was obtained from Molecular Probes (Eugene, OR), and monoclonal antibody (Ab) to human insulin was from Biodesign International (Saco, ME). Poly(dimethylsiloxane) (PDMS) was purchased from G.E. Silicones (Waterford, NY). All solutions were made with 18-MΩ deionized water from a Millipore (Bedford, MA) Milli-Q filtration system and filtered with 0.2 μm nylon syringe filters.
Buffer for immunoassay reagents was 50 mM NaCl, 1 mM EDTA, 20 mM tricine, 0.1% (w/v) Tween 20, and 0.7 mg mL−1 BSA, adjusted to pH 7.4. Balanced salt solution (BSS) used as a physiological buffer for islets experiments consisted of 125 mM NaCl, 5.9 mM KCl, 1.2 mM MgCl2, 2.4 mM CaCl2, 25 mM tricine, and 0.7 mg mL−1 bovine serum albumin (BSA), adjusted to pH 7.4 with NaOH. Separation buffer consisted of 20 mM NaCl and 150 mM tricine, adjusted to pH 7.4.
Microfluidic Chip Fabrication
The microchip design illustrated in Figure 1A was fabricated using previously described wet-chemical etching techniques.15 Channels were etched to 15 μm deep in borofloat glass with hydrofluoric acid solution. Access holes to channels were drilled with 360 μm diameter (Tartan Tool Co., Troy, MI) and 1 mm diameter drill bits (Euro Tool Inc., Grandview, MO). The four perfusion inlet connectors were from Upchurch Scientific (Oak Harbor, WA) and the remaining fluidic reservoirs were made in-house from polytetrafluoroethylene (PTFE) tubing cut to length. PTFE reservoirs were attached to the chip with epoxy (E-6000, Eclectic Products, Inc., Pineville, LA) and allowed to cure for 24 hours.
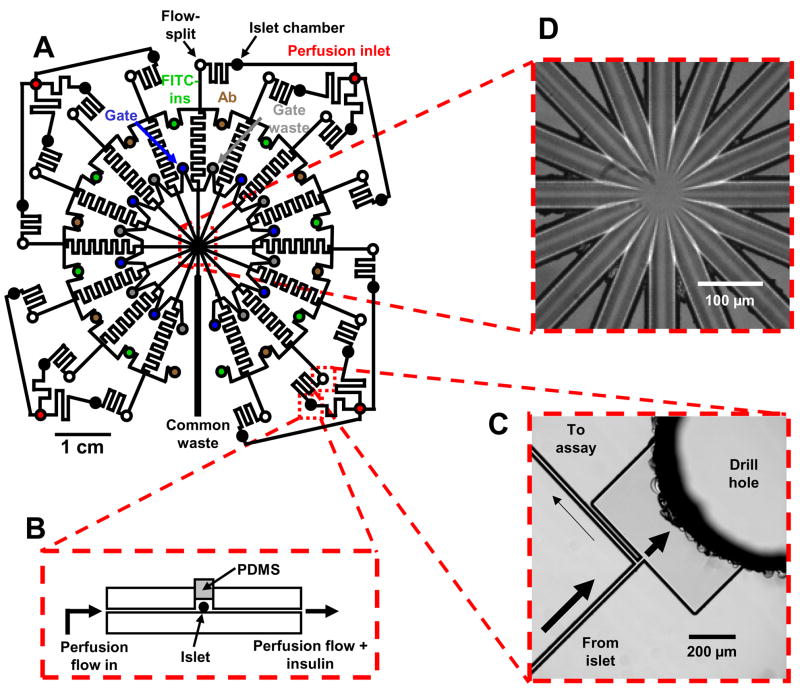
Figure 1.
Channel layout and images of detailed portions of a microfluidic chip for monitoring insulin secretion from 15 independent islets. (A) The channel network of the entire device. Microfluidic channels are indicated by solid black lines, and circles represent fluidic reservoirs. Each type of fluidic reservoir (holding a different solution) is color-coded for clarity. (B) Side-view representation (not to scale) of an islet perfusion chamber. Islets are loaded into the islet chamber with physiological buffer and then sealed with a PDMS plug under a stereomicroscope. Perfusion buffer flows over the entire islet and pushes all secreted insulin into the sampling channel. (C) CCD image of an on-chip flow-split that allows the fast flowing insulin sampling stream to be compatible with the slower flow of the EOF-driven immunoassay reagents. Arrows indicate direction and estimated magnitude of flow. (D) Brightfield image of the detection area taken with the CCD camera. Flow from 15 separation channels enter the center portion of the chip before flowing out through a single waste channel (bottom center in photograph).
Microfluidic Chip Operation
The microchip consists of 15 channel manifolds capable of CE-based immunoassays for continuous monitoring of insulin release from pancreatic islets. Flow through the radially designed separation channels converges at a common point to facilitate parallel fluorescence imaging detection (described later). Electrical connections to the chip were made with a chip-electrode interface built in-house. Two separate high voltage power supplies (CZE1000R, Spellman High Voltage Electronics, Hauppauge, NY) were used to apply potential to the waste reservoirs (-HV1 and -HV2) and a single high-voltage relay (Kilovac, Santa Barbara, CA), controlled by a LabVIEW program, connected the gate reservoirs to ground.
Pancreatic Islet Loading and Sampling of Insulin
In contrast with previous immunoassay chips,14,15 the device presented here performs islet perfusion, sampling, and introduction of secreted insulin to the on-chip assay entirely with hydrodynamic flow. Flow from a pressurized vial of perfusion buffer was split four ways with a five-port manifold before entering the chip where each stream was split again before reaching perfusion chambers. Chambers on the chip were heated to 37 °C by a heating strip (Minco, Minneapolis, MN) positioned beneath the chip.
For analysis, islets were housed in perfusion chambers on the chip that were sealed with PDMS plugs (Figure 1B). PDMS plugs could be removed so that islets could be taken out after experimentation and the chip could be reused. Care was taken to not damage the islets when sealing the chamber, and islets were checked for visible damage after every experiment. Plugs were fabricated by puncturing a thin slab of PDMS with small bore blunted-tip stainless steel tubing (20 gauge). The resulting plugs were cut to length with a scalpel under a stereomicroscope.
Islets were perfused at 500 nL min−1 with buffer that could be switched to apply different glucose concentrations or test compounds as desired. The pressure at the islet chamber associated with this low flow rate was not sufficient to break the reversible PDMS-glass seal. A low pressure drop in the islet chamber is also advantageous for maintaining islet heath. A perfusion flow rate of 500 nL min−1 is too high for downstream operations such as mixing with immunoassay reagents; therefore, a flow-split was added to the design (Figure 1C) so that only ~3.5 nL min−1 was passed to the assay region on the chip.
Insulin Secretion Assay and Calibration
Insulin that was secreted from the islet was introduced into the assay portion of the chip and mixed with FITC-ins and Ab streams controlled by EOF. FITC-ins and Ab reservoirs were grounded and −4kV was applied to the common waste (-HV2) reservoir allowing the sampled insulin to mix and react with the immunoassay reagents while flowing through the reaction channels. A potential of ~−1 kV was applied to the gating waste (-HV1) reservoirs, diverting flow from the reaction channels towards the gating waste (-HV1) reservoirs while the gate reservoirs were at ground. A single high-voltage relay was used to switch all the gate reservoirs from ground to float (open circuit) which allowed small plugs of the reaction mixture to enter the 15 separation channels. Injections of 0.5 s were performed at 9.5 s intervals. After the gate reservoirs were returned to ground, flow from the reaction channels was again diverted by separation buffer and the sample plugs in the separation channels were separated by CE. Fluorescence detection was performed at the point of separation channel convergence, yielding an effective separation distance of 1 cm.
Separation of the two fluorescent products (bound FITC-ins:Ab and free FITC-ins) allowed comparison of peak heights to be used to quantify the amount of insulin introduced to each channel network. Calibration of the microchip was performed by operating the device while perfusing insulin standards into the chip without the presence of islets. This allowed specific bound FITC-ins:Ab and free FITC-ins peak height ratios to be assigned to insulin concentrations.
Fluorescence Detection and Data Analysis
Simultaneous fluorescence detection of all separation channels was accomplished by collecting time-lapse intervals of fluorescence images using an inverted epi-fluorescence microscope (IX71, Olympus America, Inc., Melville, NY). Fluorescence excitation light was from a 300 W Xe arc lamp (LB-LS/30, Sutter Instrument Company, Novato, CA) and passed through a FITC filter cube (Semrock, Rochester, NY) before being focused on the chip detection region with an objective lens (Olympus America Inc., Melville, NY). Emitted fluorescence was collected with the same objective and detected using an electron-multiplying CCD camera (C9100-13, Hamamatsu Photonic Systems, Bridgewater, NJ). In order to image the entire detection region of the chip with the highest light gathering efficiency, a 20x objective lens (0.75 numerical aperture) that allowed for the collecting of 400 × 400 μm2 images was selected for fluorescence detection. A sample brightfield image taken using this objective (Figure 1D) shows all 15 separation channels and the common waste channel within the detection region.
Images were collected at ~28 Hz (to allow for adequate sampling of electrophoresis separations), stored, and analyzed with SlideBook software (Intelligent Imaging Innovations, Inc., Denver, CO). Fluorescence intensities from 35 μm diameter regions of interest that corresponded to each separation channel were extracted to produce parallel electropherograms that were analyzed using software written in-house.20
Calcium Flux Measurements
Intracellular calcium levels in islets were measured with Fura-2 using methods based on previously described techniques.21,22 Briefly, islets were loaded with 2 μM Fura-2 dye for 45 min at 37 °C prior to experiments. Islets were washed in Krebs Ringer Buffer, loaded into a microfluidic perfusion chamber and superfused with glucose and various drugs of interest. Once superfused, the dye was excited by 340 nM (Ca2+ complex) and 380 nM (free dye) light and the emission at 510 nM was collected, ratioed, and converted to Ca2+ concentration.
Isolation of Murine Islets
Pancreatic islets were isolated from 20 to 30 g male CD-1 mice as previously described.23 After isolation, islets were incubated at 37 °C and 5% CO2 in RPMI cell culture media supplemented with 10% fetal bovine serum, 100 units mL−1 penicillin, and 100 μg mL−1 streptomycin. Islets were used 1 – 6 days after isolation. Islets chosen for experiments were of average size (100 – 200 μm diameter) and with an intact membrane.
RESULTS AND DISCUSSION
Microfluidic System for Parallel Islet Analysis
The microfluidic chip developed for the analysis of insulin release from 15 independent pancreatic islets is illustrated in Figure 1A. The chip consists of 15 channel networks, each capable of automated single islet analysis with on-chip solution-phase immunoassays and CE separations with fluorescence detection.24 In each network, an islet is placed into a chamber on the chip that is reversibly sealed using a PDMS plug (Figure 1B). Islets are then superfused with buffer that could be switched to apply different glucose concentrations or other drugs as desired.
Flow coming from the islet chamber carrying secreted insulin is mixed on-chip with immunoassay reagents (FITC-ins and Ab). Streams of FITC-ins and Ab flowing from reservoirs to the serpentine reaction channel were controlled by EOF, and had significantly lower flow rates than the pressure-driven perfusion flow exiting the islet chamber. To allow for appropriate mixing of the three streams, a flow-split (Figure 1C) was incorporated downstream of the islet superfusion so that only ~3.5 nL min−1 of the islet superfusate passed to the assay region.
The sample and reagent streams mix and react while flowing down the reaction channel before coming to a microfluidic flow-gate injection cross25 and radially-situated channels for CE separations. Injectors were used to load portions of continuously-flowing reaction mixture onto all separation channels simultaneously every 10 s. Immunoreaction products are separated by CE as they flow towards the center of the chip and out through the common waste channel. Multiplexed detection of the parallel separations, performed by collecting fluorescence images of the chip area in Figure 1D, was used to monitor relative amounts of immunoassay products to determine rates of insulin release.
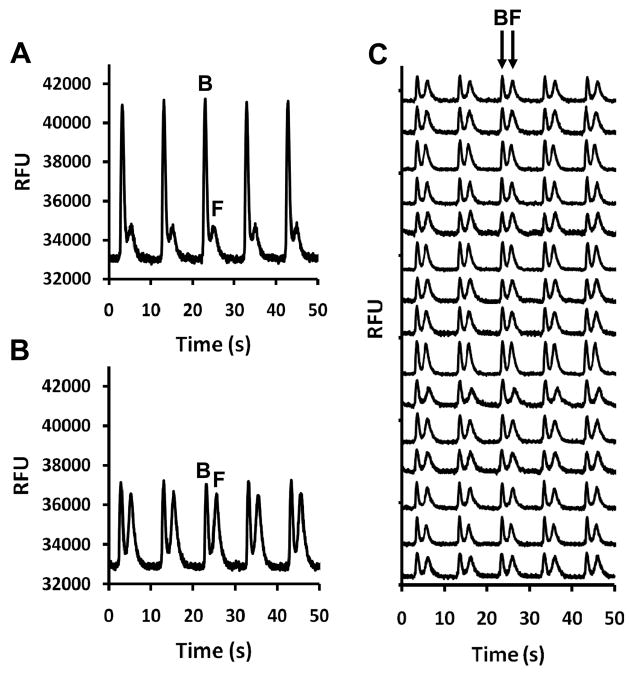
Examples of separations on single channels are shown in Figure 2A & B. Each panel shows five separations consisting of a peak for the FITC-ins:Ab complex (bound) and the slower migrating FITC-ins (free). The change in peak heights observed in panels A and B was caused by the addition of insulin which shifted the ratio of bound and free FITC-ins (B/F) through a competitive binding reaction. The performance of the device was found to be stable with RSDs of 5% in the B/F for 120 assays collected in 20 min. Fig. 2C illustrates parallel separations using all 15 networks on the chip. Migration time RSD across all 15 channels was < 4% (more than adequate for parallel separations controlled by a single injection trigger). Bound-to-free ratio RSD across the entire chip (9.6%) was slightly larger than calculated for single channels. The system generates 5,400 assays per hour at a reagent cost of $0.01 per assay illustrating the throughput and cost advantages of a microfluidic system.
Figure 2.
Typical serial and parallel electropherograms obtained with online mixing of immunoassay reagents. Injections were made every 10 s. FITC-ins bound to antibody [B] and free FITC-ins [F] are labeled accordingly. Data are shown in relative fluorescence units (RFUs). (A) Serial electropherograms collected when mixing 50 nM FITC-ins, 40 nM Ab, and 5 nM insulin standard. (B) Electropherograms collected after the introduction of 100 nM insulin standard into the same channel used in (A) illustrating the change in B/F. RFU values are shown in (A) and (B) to allow peak height comparisons. (C) Serial electropherograms collected in parallel after introduction of 100 nM insulin standard using all 15 channel networks. Traces are offset for clarity.
Improvements over Previous Islet Sampling Chips
The chip offers several advantages over previous chip designs developed to superfuse islets and monitor secretion by electrophoresis.14,15 Most importantly, the radial arrangement of channels allows scale up to 15 networks (although more are possible) thus substantially improving throughput of cellular experiments. The radial geometry has previously been used for high-throughput DNA analysis,26,27 off-line immunoassays,28 and enzyme assays;29 but this is the first report of such a geometry used for continuous cellular monitoring and on-line immunoassays.
The assay limit of detection (LOD) was lowered 10-fold in comparison to the four-sample chip that used a commercial scanning confocal detector.15 This improvement in LOD can be attributed to the increased sensitivity of the CCD-based detection scheme which in turn allowed for a decrease in immunoassay reagent concentrations (currently 50 nM FITC-ins and 40 nM Ab, previously 250 nM FITC-ins and 250 nM Ab). This improved LOD is significant as it allows for quantification of lower amounts of insulin that are secreted from an islet in low glucose conditions. The previous four-sample chip had an LOD of 10 nM insulin which corresponds to 35 pg min−1 at 600 nL min−1 islet perfusion. Because observed average secretion rates from islets perfused at 3 mM glucose are close to this value, this detection limit was barely sufficient to detect this lower level of release. The 15-sample chip has an LOD of ~3.5 pg min−1 using the same perfusion conditions meaning that basal secretion can be routinely detected and quantified with this device.
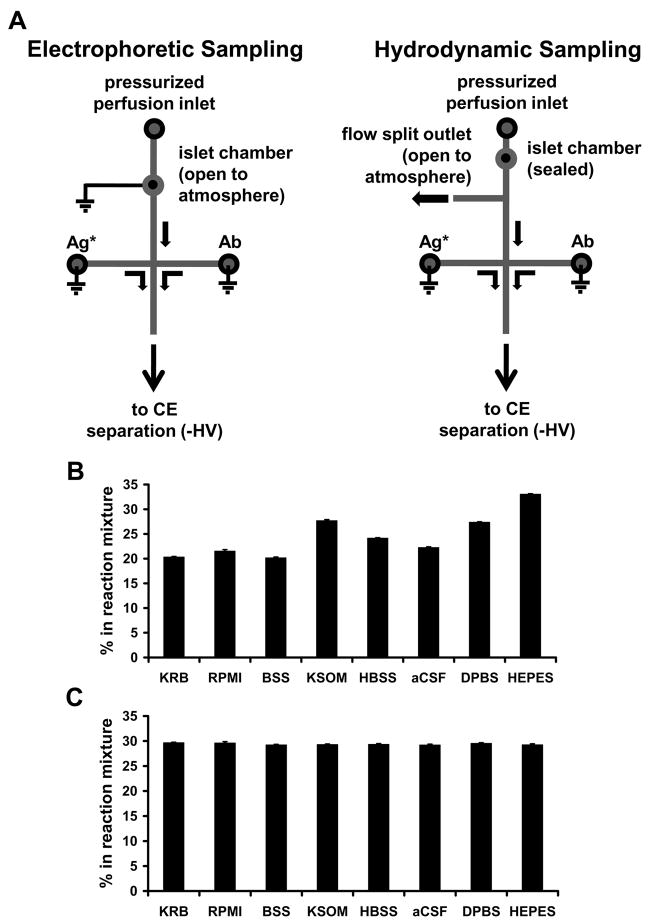
A final significant improvement was the approach to sampling from cells superfused on the chip. Sampling from cell chambers for coupling to electrophoretic monitoring has previously relied on EOF.14 In this previous system, superfusate flowed into an open cell chamber and exited into a reservoir above the cells (Figure 3A). A small fraction of the fluid in the cell chamber is continuously sampled by EOF. Although this approach gives a rapid response and allows cells to be easily loaded onto the chip, it has the significant disadvantage that the concentration of analyte sampled could depend upon the cell position within the chamber and location of release sites (heterogeneous secretion from the periphery of islets has been observed for example30), i.e. if the cells are placed close to the sampling channel higher concentrations would be sampled than if the cells were placed far from the sampling channel. Furthermore, the flow rate out of the sampling chamber depends on the type of buffer being used to superfuse the cells because of the sensitivity of EOF to ionic content and buffer additives (Figure 3B). These effects can reduce the accuracy and precision of measurements.
Figure 3.
Comparison of electrophoretic and hydrodynamic on-chip sampling designs and performance. (A) Diagrams illustrating channel layout and islet position for electrophoretic and hydrodynamic insulin sampling. Lines indicate microfluidic channels, circles indicate fluidic reservoirs or islet chambers, and arrows indicate direction of fluid flow. For electrophoretic sampling, excess perfusion fluid flows out of the plane of the diagram towards the reader. (B & C) Comparison of online reagent mixing ratios on (B) electrophoretic- and (C) pressure-sampling chips with varying perfusion buffer compositions. Mixing ratios were determined using fluorescence microscopy. Solution compositions are noted in the Supporting Material. Data are averages of the measured mixing ratio at three time points, measured 2 minutes apart from one another. Error bars are ± 1 standard deviation.
The sampling system described here avoids these limitations. By capping the cell chamber with a PDMS plug it is possible use pressure to flow all of the secreted chemicals out of the chamber before splitting a small fraction to the sampling channel. The distance between the cell chamber and the flow split was sufficient to ensure that all released insulin mixed laterally within the channel to give a representative concentration in the split flow (Supporting Information, Figure S1). The use of pressure-driven flow also made the sampling rate much less dependent on perfusion buffer as illustrated in Figure 3C. This is important because it indicates that wide variety of buffers can be used without concern for the amount of sample that enters the sampling chamber. The use of a resealable PDMS plug with the chamber ensures that the sampling system retains the advantage of facile cell loading and removal. A possible concern with this approach is that the temporal resolution would be reduced by the need to wash fluid past the cell chamber which could broaden concentration changes by flow and diffusional broadening; however, the chamber could be completely washed out in 3.5 s and the temporal resolution was still about 20 s (Supporting Information, Figure S2).
Parallel Monitoring of Insulin Release from Islets
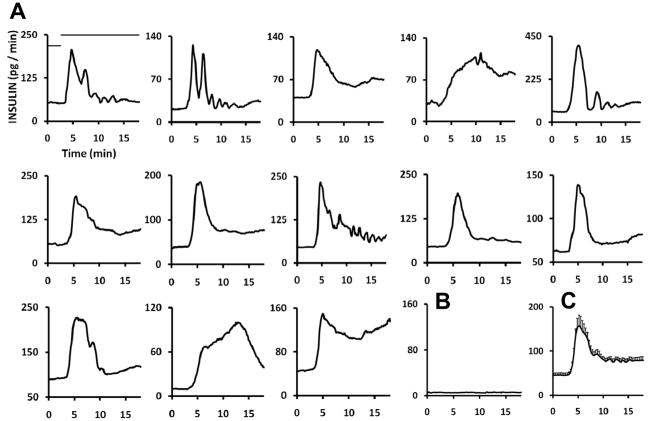
Functionality of the parallel microchip was demonstrated by simultaneously monitoring insulin secretion from 15 individual islets stimulated with glucose. Insulin secretion plots shown in Figure 4 were from islets treated with a step change in superfused glucose concentration (3 mM to 11 mM) at t = 3 min. In the experiment, 13 of the 15 islets showed an increased rate of release after stimulation. The majority of islets show pronounced 1st phase release of insulin (initial peak that occurs at approximately5 min) followed by a 2nd phase of sustained release; however, several islets show oscillatory secretion or a continual increase in release rate. Two islets had no response to glucose (sample shown in Figure 4B). It is unlikely that the variations shown here are due to disparities between parallel networks on the chip because of the reproducibility found among the networks and the use of calibration for each channel; rather, the differences are due to intrinsic disparities between islets. Indeed, similar variations in overall secretion rates and dynamics have been observed between repeated experiments performed on a single-sample device.14 An average of the plots obtained from the individuals (Figure 4C) shows a biphasic insulin release profile that is characteristic of pancreatic endocrine tissue stimulated with elevated glucose.2,11 The secretion rate after glucose stimulation (160 pg min−1 at the peak) is similar to that reported in studies using conventional islet perfusion systems.31,32
Figure 4.
Monitoring of single islet biphasic insulin release. (A) 13 single islet plots from a total of 15 islets stimulated with a step change in glucose concentration. Two islets showed no increase in secretion rate after stimulation with high levels of glucose. Bars, shown only on the upper left plot, indicate glucose concentration (low bar = 3 mM glucose, high bar = 11 mM glucose). (B) Secretion plot from an islet that showed no release of insulin during glucose perfusion. (C) Averaged plot from 13 single islet traces collected simultaneously with the 15-sample device. Error bars, placed on every other data point, are ±1 SEM (standard error of the mean).
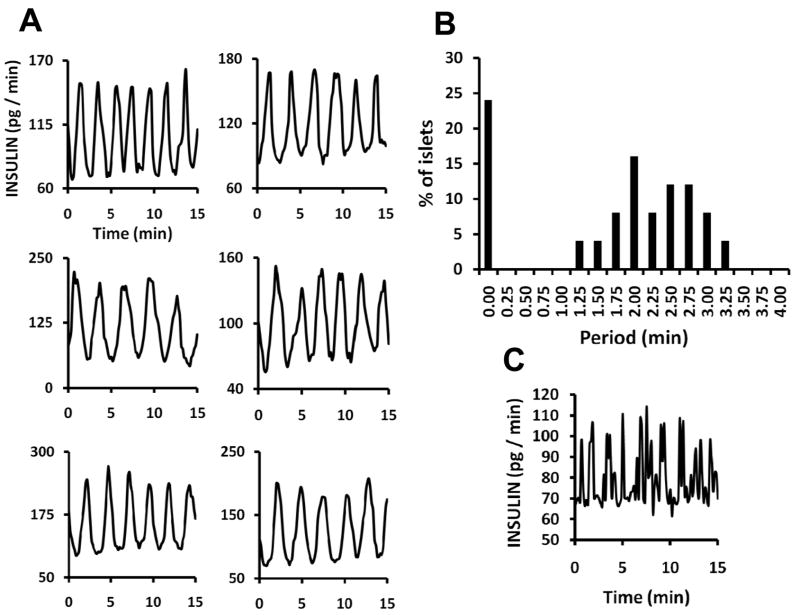
Transfer of islets directly to 10 mM glucose superfusion, without intervening low glucose concentrations, evoked oscillatory release in 90% of the islets tested (n = 28), which was more reliable than experiments in which the glucose was changed from 3 to 10 mM glucose (Figure 5A). Oscillation periods from a total of 25 islets treated with 10 mM glucose ranged from 1.25 to 3.25 minutes (Figure 5B). Additionally, 24% of the islets showed rapidly fluctuating secretion but not regular oscillations (sample shown in Figure 5C). Metabolic and intracellular Ca2+ oscillations (driving factors in insulin exocytosis) typically have a bimodal distribution with some islets having “slow” oscillations, similar to those in 5A, and others having “fast” oscillations with periods of ~10 s. 17,18,33 The irregular pulses in insulin secretion represented in Figure 5C are likely a correlate of fast Ca2+ fluxes. It is possible regular secretory oscillations are occurring, but the temporal resolution of the chip is insufficient to detect them. A second possibility is that the rapid Ca2+ oscillations do not support rapid oscillations in insulin secretion due to inefficiencies in the Ca2+-secretion coupling.
Figure 5.
Recordings of fast and slow oscillatory insulin release patterns. (A) Plots from six of 15 islets run simultaneously on the 15-islet chip showing oscillatory insulin release. Islets were stimulated with continuous flow of 10 mM glucose. (B) Plot comparing oscillation periods from a group of 25 islets from 2 mice tested with the 15-sample device over two experimental periods. These data show a bimodal distribution of periods including islets that showed no oscillations and islets that oscillated with periods of 1.25 – 3.25 min. (C) Example of release from an islet with irregular secretion patterns.
Effects of Chronic Fatty Acid Exposure on Pulsatile Secretion
We next used the chip to evaluate how long-term exposure to fatty acid in culture influences insulin secretory dynamics. Although fatty acids can potentiate insulin secretion over short exposures, incubation with fatty acid for 48 h impairs islet function resulting in disturbed metabolism34 and inhibited glucose-stimulated insulin secretion.19 This effect of fatty acids is considered a model of lipotoxicity, a potential mechanism of islet functional degradation in type 2 diabetes.35 Although chronic exposure to fatty acids has been shown to blunt first phase insulin release from islets,36 its effects on islet oscillations are not known.
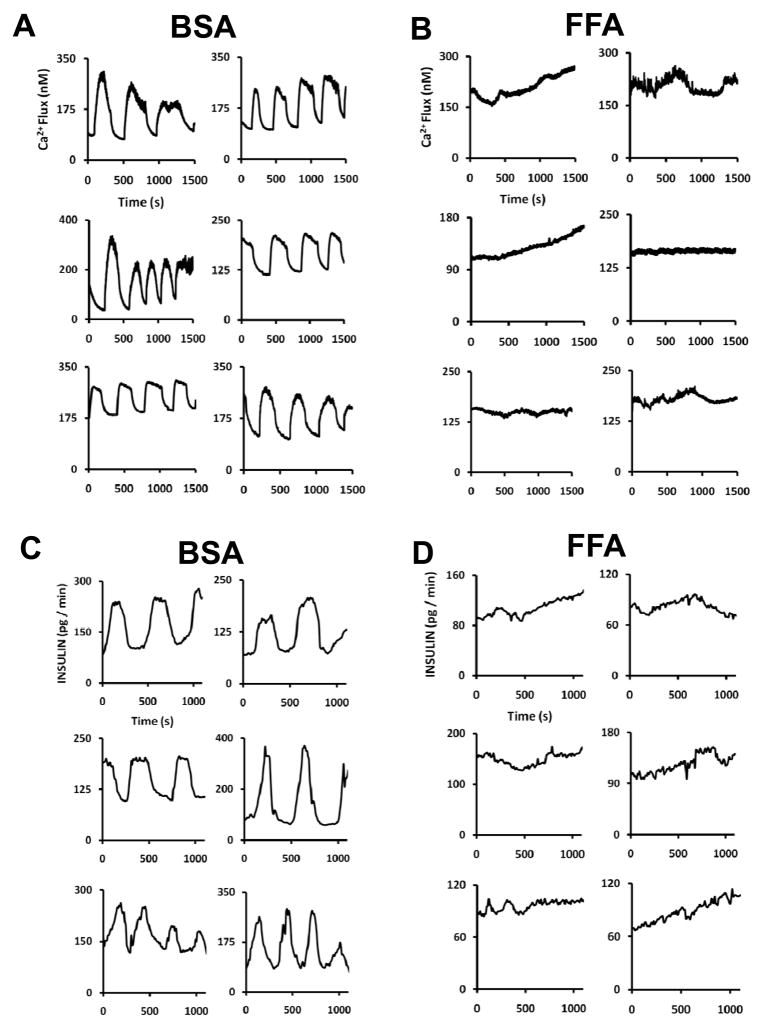
Islets were incubated for 48 h with 1 mM palmitic acid added to the media and then subjected to continuous superfusion with 10 mM glucose in the microfluidic device to induce oscillatory behavior while either intracellular Ca2+ levels or insulin release was monitored. Results presented in Figure 6 illustrate that overall Ca2+ flux and insulin release rates were lower from islets treated with palmitic acid incubation, in agreement with previous studies. The dynamic recordings shown here also reveal that the impaired insulin secretion extends to disrupted pulsatility. The islets affected by free fatty acid (FFA) incubation (panels B & D) show decreased oscillation amplitude as well as perturbed periods (either lacking oscillations completely or having potentially much slower oscillations than observed in panels A & C). These data suggest that FFA-induced lipotoxicity in islets can potentially affect mechanisms that control pulsatile secretion that possibly include oscillatory glycolysis,37 membrane potential, and ATP/ADP ratio.38 Furthermore, these results demonstrate the utility of the 15-islet chip for comparing single islet pulsatile release and highlight the potential importance of this type of data in diabetes research.
Figure 6.
Effects of long-term FFA islet exposure on pulsatile Ca2+ flux and insulin release. Single islet insulin secretion and Ca2+ flux were monitored during continuous perfusion of glucose after 48-hour incubation in RPMI media supplemented with either FFA-free BSA (A & C) or palmitic acid (B & D). Islets incubated in FFA showed little to no oscillatory behavior, while those treated with BSA showed typical response.
Reliability of Chip Fabrication and Operation
Overall the chips prepared for this study showed good reliability and reproducibility. Of 10 chips prepared, all had at least 13 channels functioning and could be reused 10 more times without failure of more channels. In all experiments performed using this device on control islets (over 200 islets in various experiments) the basal secretion rate was between 30 and 50 pg/min, the first phase peaked within 2 to 4 min, and the peak secretion was between 150 and 200 pg/min for a 3 to 10 mM glucose transition. Basal secretion rate, time to peak secretion, and peak level of secretion, was not significantly different from chip to chip or from different uses of the same chip. While achieving this level of performance is likely somewhat dependent on the operator, the results suggest the potential for reliable operation.
CONCLUSIONS
The studies presented here demonstrate the usefulness of a high-throughput single islet monitoring system by showing the capability of obtaining dynamic information on the single entity level at good throughput. While the data averaged from individual islets is comparable to that obtained by other methods, single islet data offers information on individual islet variation and dynamics of insulin secretion, which can be just as critical as the amount of insulin released as illustrated by the effect of fatty acids on oscillations.39 Underlying mechanisms behind specific patterns of secretion have not been firmly identified and there is much interest in the development of tools for investigating the fundamental causes of these dynamics and their potential involvement in diabetes.
A possible clinical application for high-throughput single islet secretion monitoring is evaluation of islets for transplant. Islets harvested for transplant undergo numerous stresses that could potentially damage islet health and function. Lack of methods for rapid testing of viability of harvested islets before implantation into a patient is one of several obstacles in developing successful islet transplant therapies. This microfluidic device may prove useful for batch testing of islets prior to transplant by providing rapid, quantitative assessment both amount and dynamics of insulin release. Such a tool may prove useful in improving islet transplant success. These potential applications are made possible by the improved throughput, sensitivity, and sampling made possible in this current design. While the system was designed for islet experiments, it could be applied to other tissue samples.
Supplementary Material
Acknowledgments
This work was supported by NIH R37 DK0469690 (R.T.K.). J.F.D. was partially supported by Pfizer, Inc.
LITERATURE CITED
- 1.Straub SG, Sharp SW. Diabetes Metab Res Rev. 2002;18:451–63. doi: 10.1002/dmrr.329. [DOI] [PubMed] [Google Scholar]
- 2.Nesher R, Cerasi E. Diabetes. 2002;51:S53–S59. doi: 10.2337/diabetes.51.2007.s53. [DOI] [PubMed] [Google Scholar]
- 3.Nunemaker CS, Zhang M, Wasserman DH, McGinness OP, Powers AC, Bertram R, Sherman A, Satin LS. Diabetes. 2005;54:3517–3522. doi: 10.2337/diabetes.54.12.3517. [DOI] [PubMed] [Google Scholar]
- 4.Luciani DS, Misler S, Polonsky KS. J Physiol. 2006;572:397–92. doi: 10.1113/jphysiol.2005.101766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Porksen N, Hollingdal M, Juhl C, Butler P, Veldhuis JD, Schmitz O. Diabetes. 2002;51:S245–254. doi: 10.2337/diabetes.51.2007.s245. [DOI] [PubMed] [Google Scholar]
- 6.Matthews DR, Naylor BA, Jones RG, Ward GM, Turner RC. Diabetes. 1983;32:617–621. doi: 10.2337/diab.32.7.617. [DOI] [PubMed] [Google Scholar]
- 7.Komjati M, Bratuschmarrain P, Waldhausl W. Endocrinology. 1986;118:312–319. doi: 10.1210/endo-118-1-312. [DOI] [PubMed] [Google Scholar]
- 8.Polonsky KS, Given BD, Hirsch LJ, Tillil H, Shapiro ET, Beebe C, Frank BH, Galloway JA, Vancauter E. New Engl J Med. 1988;318:1231–1239. doi: 10.1056/NEJM198805123181903. [DOI] [PubMed] [Google Scholar]
- 9.Ristow M, Carlqvist H, Hebinck J, Vorgerd M, Krone W, Pfeiffer A, Muller-Wieland D, Ostenson CG. Diabetes. 1999;48:1557–1561. doi: 10.2337/diabetes.48.8.1557. [DOI] [PubMed] [Google Scholar]
- 10.Meier JJ, Hong-McAtee I, Galasso R, Veldhuis JD, Moran A, Hering BJ, Butler PC. Diabetes. 2006;55:2324–2332. doi: 10.2337/db06-0069. [DOI] [PubMed] [Google Scholar]
- 11.Henquin JC, Nenquin M, Stiernet P, Ahren B. Diabetes. 2006;55:441–451. doi: 10.2337/diabetes.55.02.06.db05-1051. [DOI] [PubMed] [Google Scholar]
- 12.Nabe K, Fujimoto S, Shimodahira M, Kominato R, Nishi Y, Funakoshi S, Mukai E, Yamada Y, Seino Y, Inagaki N. Endocrinology. 2006;147:2717–2727. doi: 10.1210/en.2005-1260. [DOI] [PubMed] [Google Scholar]
- 13.Bergsten P. Am J Physiol Endocrinol Metab. 1998;274:E796–E800. doi: 10.1152/ajpendo.1998.274.5.E796. [DOI] [PubMed] [Google Scholar]
- 14.Shackman JG, Dahlgren GM, Peters JL, Kennedy RT. Lab Chip. 2005;5:56–63. doi: 10.1039/b404974h. [DOI] [PubMed] [Google Scholar]
- 15.Dishinger JF, Kennedy RT. Anal Chem. 2007;79:947–954. doi: 10.1021/ac061425s. [DOI] [PubMed] [Google Scholar]
- 16.Paegel BM, Emrich CA, Wedemayer GJ, Scherer J, Mathies RA. Proc Natl Acad Sci. 2002;99:574–579. doi: 10.1073/pnas.012608699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu YJ, Tengholm A, Grapengiesser E, Hellman B, Gylfe E. J Physiol-London. 1998;508:471–481. doi: 10.1111/j.1469-7793.1998.471bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bergsten P. Am J Physiol-Endoc Metab. 1995;268:E282–E287. doi: 10.1152/ajpendo.1995.268.2.E282. [DOI] [PubMed] [Google Scholar]
- 19.Biden TJ, Robinson D, Cordery D, Hughes WE, Busch AK. Diabetes. 2004;53:S159–S165. doi: 10.2337/diabetes.53.2007.s159. [DOI] [PubMed] [Google Scholar]
- 20.Shackman JG, Watson CJ, Kennedy RT. J Chromatogr A. 2004;1040:273–282. doi: 10.1016/j.chroma.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 21.Grynkiewicz G, Poenie M, Tsein RY. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- 22.Kulkarni RN, Roper MG, Dahlgren GM, Shih DQ, Kauri LM, Peters JL, Stoffel M, Kennedy RT. Diabetes. 2004;53:1517–1525. doi: 10.2337/diabetes.53.6.1517. [DOI] [PubMed] [Google Scholar]
- 23.Pralong WF, Bartley C, Wollheim CB. EMBO J. 1990;9:53–60. doi: 10.1002/j.1460-2075.1990.tb08079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schultz NM, Kennedy RT. Anal Chem. 1993;65:3161–3165. [Google Scholar]
- 25.Jacobson SC, Ermakov SV, Ramsey JM. Anal Chem. 1999;71:3273–3276. doi: 10.1021/ac990059s. [DOI] [PubMed] [Google Scholar]
- 26.Shi Y, Simpson PC, Scherer JR, Wexler D, Skibola C, Smith MT, Mathies RA. Anal Chem. 1999;71:5354–5361. doi: 10.1021/ac990518p. [DOI] [PubMed] [Google Scholar]
- 27.Emrich CA, Tian H, Medintz IL, Mathies RA. Anal Chem. 2002;74:5076–5083. doi: 10.1021/ac020236g. [DOI] [PubMed] [Google Scholar]
- 28.Bromberg A, Mathies RA. Electrophoresis. 2004;25:1895–1900. doi: 10.1002/elps.200305818. [DOI] [PubMed] [Google Scholar]
- 29.Pei J, Dishinger JF, Roman DL, Rungwanitcha C, Neubig RR, Kennedy RT. Anal Chem. 2008;80:5225–31. doi: 10.1021/ac800553g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qian WJ, Peters JL, Dahlgren GM, Gee KR, Kennedy RT. Biotechniques. 2004;37:922–933. doi: 10.2144/04376BI01. [DOI] [PubMed] [Google Scholar]
- 31.Henquin JC, Nenquin M, Szollosi A, Kubosaki A, Notkins AL. J Endocrinol. 2008;196:573–581. doi: 10.1677/JOE-07-0496. [DOI] [PubMed] [Google Scholar]
- 32.Cooksey RC, Pusuluri S, Hazel M, McClain DA. Am J Physiol-Endoc Metab. 2006;290:E334–E340. doi: 10.1152/ajpendo.00265.2005. [DOI] [PubMed] [Google Scholar]
- 33.Dahlgren GM, Kauri LM, Kennedy RT. Biochim Biophys Acta. 2005;1724:23–36. doi: 10.1016/j.bbagen.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 34.Kohnke R, Mei J, Park M, York DA, Erlanson-Albertsson C. Nutr Neurosci. 2007;10:273–278. doi: 10.1080/10284150701745910. [DOI] [PubMed] [Google Scholar]
- 35.Cnop M. Biochem Soc T. 2008;36:348–352. doi: 10.1042/BST0360348. [DOI] [PubMed] [Google Scholar]
- 36.Ayvaz G, Toruner FB, Karakoc A, Yetkin I, Cakir N, Arslan M. Diabetes Metab. 2002;28:S7–S12. [PubMed] [Google Scholar]
- 37.Tornheim K. Diabetes. 1997;46:1375–1380. doi: 10.2337/diab.46.9.1375. [DOI] [PubMed] [Google Scholar]
- 38.Detimary P, Gilon P, Henquin JC. Biochem J. 1998;333:269–274. doi: 10.1042/bj3330269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nunemaker CS, Wasserman DH, McGuinness OP, Sweet IR, Teague JC, Satin LS. Am J Physiol Endocrinol Metab. 2006;209:E523–E529. doi: 10.1152/ajpendo.00392.2005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.