Abstract
Each year more than 250,000 infants in the United States are exposed to artificial lighting in hospital nurseries with little consideration given to environmental lighting cycles. Essential in determining whether environmental lighting cycles need to be considered in hospital nurseries is identifying when the infant’s endogenous circadian clock becomes responsive to light. Using a non-human primate model of the developing human, we examined when the circadian clock, located in the hypothalamic suprachiasmatic nuclei (SCN), becomes responsive to light. Preterm infant baboons of different ages were exposed to light (5,000 lux) at night, and then changes in SCN metabolic activity and gene expression were assessed. After exposure to bright light at night, robust increases in SCN metabolic activity and gene expression were seen at ages that were equivalent to human infants at 24 weeks after conception. These data provide direct evidence that the biological clock of very premature primate infants is responsive to light.
Circadian rhythms are endogenously generated rhythms that have a period length of about 24 hrs and profoundly affect human physiology and behavior (1–3). Notable examples of circadian rhythms include the sleep–wake cycle and daily rhythms in hormone production (2–4).
Circadian rhythms are generated by a biological clock located in the hypothalamic suprachiasmatic nuclei (SCN) (1, 4–6). To ensure that the oscillations of the circadian clock are synchronized with the 24-hr light–dark cycle, circadian rhythms are entrained by light via the retinohypothalamic tract that projects from the retina to the SCN (7).
We now know that the SCN begin oscillating in utero (8, 9), and the circadian system can influence physiology at very early stages of development (10, 11). Depending on the species examined, photic regulation of the SCN develops either prenatally or postnatally (12–14). In humans, the retinohypothalamic tract has been identified at 36 weeks of gestation (15), and functional entrainment of circadian phase has been observed in term baboon infants (16). However, we do not know whether the primate clock is functionally responsive to light at earlier stages. Considering that more than 250,000 infants per year are exposed to artificial lighting in hospital nurseries in the United States (17), identifying when the primate SCN become responsive to light is of considerable clinical importance in determining whether environmental lighting cycles can indeed influence the developing infant.
Because of the limitations of studying humans, it is not possible to determine when the circadian clock of human infants becomes functionally responsive to light. Thus, to provide insights into the developing human clock, we studied baboons, because they are favored models for human preterm infants (11, 18–20). Here, we provide direct evidence that the biological clock of very premature primate infants is responsive to light.
MATERIALS AND METHODS
Animals.
Baboon infants (Papio spp.) were studied at the Southwest Foundation for Biomedical Research (San Antonio, TX) or at the University of Illinois (Chicago, IL). All studies were approved by the subcommittees for animal care at Yale University, University of Illinois, and the Southwest Foundation for Biomedical Research. After mating, pregnant dams were maintained in diurnal light–dark cycles consisting of 12 hrs of each per day (lights on 0700 to 1900 hrs; 1,000 lux or greater). Infants were delivered by Cesarean section at 1000 hrs on the appropriate postconceptual (PC) day and immediately blindfolded. Infants born at PC day 160 were kept in individual newborn incubators (Ohio, Madison, WI). Infants born earlier than 160 PC days were incubated, mechanically ventilated, and kept on warming tables (32°C). All infants were infused i.v. with 5% dextrose in 0.45% saline at a rate of 150 ml/kg per day. For ventilated infants, blood gases were obtained every 2–4 hrs, and oxygenation and ventilation were adjusted to maintain normal levels of pH, 02 pressure, and CO2 pressure. Constant illumination (1,000 lux) of the nursery was provided by Phillips Cool White fluorescent lights (Sommerset, NJ). For light-at-night studies, illumination was provided by 500-W halogen lamps. Light intensity was measured with a SPER Scientific light meter (Itaska, IL) that was placed near the animals’ eyes.
Deoxyglucose (DG) Studies.
DG (2-deoxy-d-[14C]glucose) studies were performed as described (21, 22). In brief, individual animals were exposed to light at specified times and, 5 min later, injected with 100 μCi/kg of DG (Amersham Pharmacia; specific activity = 60 Ci/mmol; 1 Ci = 37 GBq) via umbilical venous lines. Illumination was continued until the animals were euthanized 45 min later with an overdose of pentobarbital (100 mg/kg; i.v.). The hypothalamus was dissected, frozen in chilled 2-methylbutane (−20°C), and stored at −80°C. Slide-mounted frozen tissue sections (20 μm thick) were then air-dried and exposed to Kodak Biomax MR radiography film. SCN metabolic activity was assessed by determining the relative OD of the SCN (OD of SCN/OD of adjacent hypothalamus). For each specimen, the relative OD values for three consecutive sections in the midportion of the SCN were determined. To capture images, a Bio-Rad Gel Doc 1000 system was used, and OD values were computed with molecular analyst Software (Bio-Rad) with Kodak autoradiographic standards.
In Situ Hybridization.
Tissue sections adjacent to those used for DG studies were used. In situ hybridization for c-fos,per1 mRNA were performed by using methods similar to those described (16). The Riboprobe system (Promega) was used to generate [35S]α-thio-UTP-labeled antisense and sense cRNA probes. A 400-bp fragment of the mouse c-fos cDNA was used as the template for fos cRNA probe generation as described (11). A 400-bp fragment of the mouse per1 gene was used as the template for per1 cRNA probe generation. Slides were apposed to Kodak Biomax MR film to generate autoradiographic images. In situ hybridization signal was quantified by measuring the relative OD of the SCN as described above.
RESULTS
Photic Responsiveness of the SCN in Preterm Infants.
Previous studies have shown that the SCN of term baboon infants (gestation length 180–185 days) are responsive to light at birth and that photic responsiveness is retina-mediated (16). To address when the developing primate circadian system first becomes responsive to light, we studied progressively younger preterm baboon infants. To test for photic responsiveness, changes in SCN metabolic activity and gene expression were monitored after light exposure at night (16). If the SCN are innervated functionally by the retina, light exposure at night induces a large increase in SCN metabolic activity and gene expression, whereas changes are not seen if the SCN are not innervated by the retina (16).
To assess changes in SCN metabolic activity, we first studied SCN DG uptake in two preterm baboon infants at 160 PC days of age. After Cesarean-section delivery at 1000 hrs, animals were blindfolded immediately. Then, animals were exposed to light at night at 2230 hrs. One animal remained blindfolded during light exposure, and another animal was exposed to light directly (5,000 lux) without a blindfold.
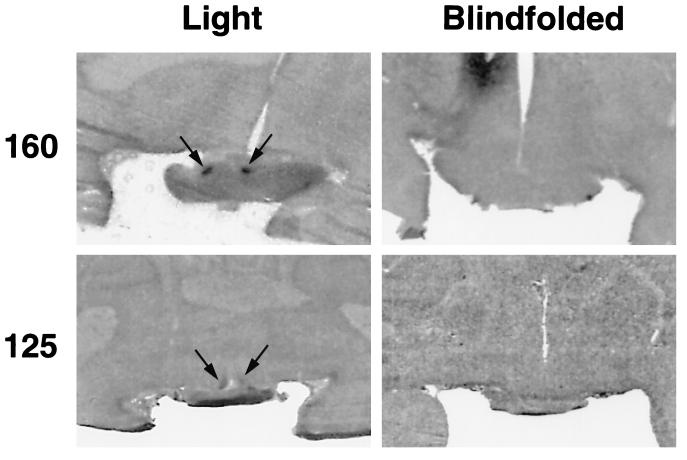
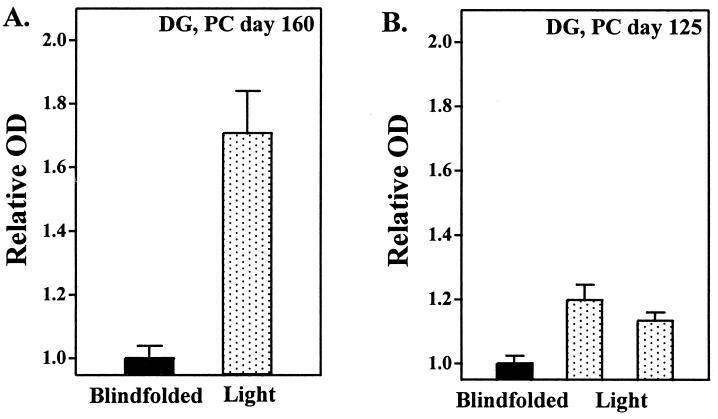
After light exposure, a robust increase in relative DG uptake was apparent in the autoradiographic images of the SCN of the animal that was not blindfolded (Figs. 1 and 2A). In contrast, no discernible SCN images were visible in autoradiographs generated from the animal that remained blindfolded during light exposure (Figs. 1 and 2A).
Figure 1.
Autoradiographic images of preterm baboon-brain sections showing SCN DG uptake after exposure to 5,000 lux of light at night. The infants shown are either 160 (Upper) or 125 (Lower) PC days of age and were either blindfolded (Right) or directly exposed (Left) during light exposure. The images are at mid-SCN levels. Arrows identify the SCN.
Figure 2.
Relative OD values of individual animals generated from DG-uptake studies at PC days 160 (A) and 125 (B). Animals were exposed directly (dotted bars) or blindfolded (black bars) during exposure to 5,000 lux of light. Relative OD values are means ± SEM of three determinations at mid-SCN level per animal.
Next, to test for SCN photic responsiveness at younger ages, preterm infants at 125 PC days of age were studied. After Cesarean-section delivery, infants were ventilated mechanically because of their immature respiratory development. One infant was blindfolded and exposed to 5,000 lux of light at night, and two infants were exposed directly to 5,000 lux of light at night.
Similar to the results obtained at PC day 160, exposure to light resulted in a robust increase of relative SCN DG uptake, whereas no apparent changes in SCN DG uptake were seen in the autoradiographs generated from the blindfolded animal (Figs. 1 and 2B).
Next, one preterm infant at 120 PC days of age was exposed to 5,000 lux of light at night. In contrast to the experiments with the older animals, light exposure did not induce increases in DG uptake, as no discernible images of the SCN were seen in the autoradiographs.
Light-Induced Changes in SCN c-fos and per1 Gene Expression.
To complement DG studies, we also tested for light-induced changes in SCN c-fos and per1 mRNA expression. Both c-fos and per1 gene expression in the SCN is induced by light exposure at night if the SCN are innervated functionally by the retina (23–26). Therefore, in situ hybridization was performed on tissue sections adjacent to those used for DG studies.
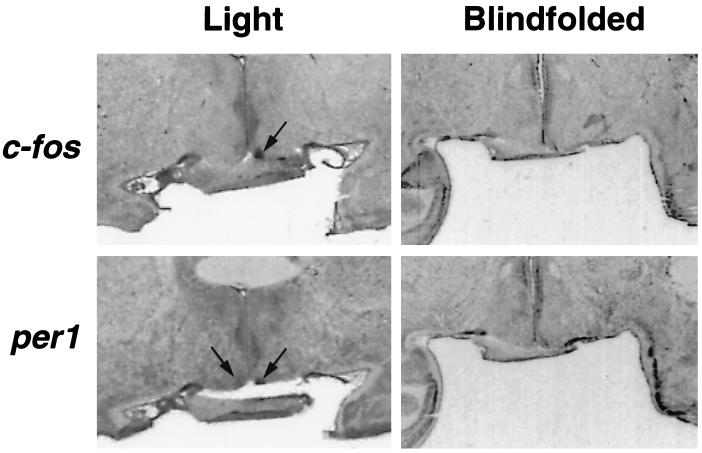
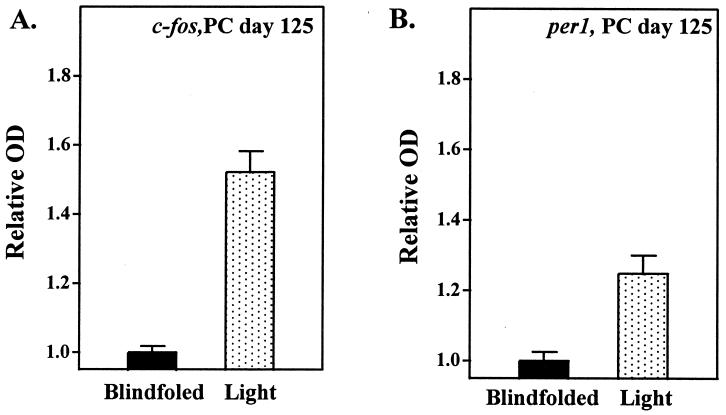
Strong hybridization signals in the SCN region of the animals exposed to light were seen for both c-fos and per1 mRNA at 160 and 125 PC days of age (Fig. 3 and 4). In contrast, no hybridization signals were seen in the infants born on PC day 160 or 125 that had been blindfolded during light exposure or in the infant born on PC day 120 exposed to light.
Figure 3.
In situ hybridization images generated with 35S-labeled cRNA probes showing c-fos (Upper) and per1 (Lower) mRNA expression at PC day 125 after exposure to light at night with (Right) or without (Left) blindfolds. Arrows identify the SCN.
Figure 4.
Relative OD values of the SCN autoradiographic images generated from c-fos (A) and per1 (B) mRNA expression studies. Animals were studied either with (black bars) or without (dotted bars) blindfolds during 5,000 lux of light exposure. Relative OD values are means ± SEM of three determinations at mid-SCN level.
DISCUSSION
Because noninvasive methods for examining SCN metabolic activity in mammals are not available currently, baboons were used to provide insights into the development of SCN photic responsiveness. Baboons are favorable models for human preterm infants and have been used to develop important therapeutic interventions for preterm infants (11, 18–20, 27). The development of the prenatal visual system is also very similar among monkeys and humans, making non-human primates excellent models for the development of the visual system in humans (28).
To determine the developmental stage at which the primate SCN become responsive to light, light-at-night studies on preterm baboons were carried out with progressively younger infants. At PC days 160 and 125, which are equivalent 32 and 24 weeks of gestation for human fetuses, respectively (27), acute light exposure induced robust increases in SCN metabolic activity and induced c-fos and per1 gene expression. In contrast, at PC day 120, which is equivalent to 22 weeks of human gestation (27), light-induced changes in SCN metabolic rates and c-fos and per1 gene expression were not seen. Thus, there seems to be a developmental window in very premature primate infants when SCN photic responsiveness develops.
Because there are no humoral or behavior outputs of the circadian system until several weeks after term birth in human and baboons (11, 16), it is not possible for us to assess whether light can entrain circadian phase at such early ages. However, photic entrainment of circadian phase by low-intensity lighting (200 lux) can be seen in term newborn baboons (16). Thus, it is likely that circadian phase will be entrained at the age when the SCN are responsive to light.
Intriguing evidence suggests that cycled lighting influences human infant development and behavior. Hospitalized term infants cared for by a single provider in a regular light–dark cycle were found to sleep more at night and less during the day than infants with unstructured patterns of care (29). In one report involving a small number of subjects, preterm infants cared for in a nursery with strict light–dark cycles were reported to gain weight faster and develop sleep–wake cycles sooner than infants cared for in constant lighting (30). It has also been suggested that preterm infants cared for in nurseries with cycled lighting are less ill and grow faster than infants cared for in constant illumination (31); however, study subjects were randomized poorly in that report. The discovery of SCN photic responsiveness at developmental stages close to when human infant survival becomes possible (32, 33) thus provides an important foundation for assessing whether environmental lighting influences the developing newborn.
Overall, our results provide direct evidence that primate SCN are responsive to light at very premature stages, and we also show that light responsiveness is retina-mediated. Further consideration of the lighting cycles to which preterm infants are exposed is warranted.
Acknowledgments
We are indebted to Dr. Dee Carey (Southwest Foundation for Biomedical Research) and to Drs. Judy Lee and Jeffrey Fortman (University of Illinois) for their assistance. This work was supported by National Institutes of Health Grant R01NS32624.
ABBREVIATIONS
- DG
deoxyglucose
- PC
postconceptual
- SCN
suprachiasmatic nuclei
References
- 1.Moore R Y. J Biol Rhythms. 1993;8:S3–S9. [PubMed] [Google Scholar]
- 2.Moore-Ede M C, Czeisler C A, Richardson G S. N Engl J Med. 1983;309:469–476. doi: 10.1056/NEJM198308253090806. [DOI] [PubMed] [Google Scholar]
- 3.Moore-Ede M C, Czeisler C A, Richardson G S. N Engl J Med. 1983;309:530–536. doi: 10.1056/NEJM198309013090905. [DOI] [PubMed] [Google Scholar]
- 4.Klein D C, Moore R Y, Reppert S M. Suprachiasmatic Nucleus: The Mind’s Clock. New York: Oxford Univ. Press; 1991. [Google Scholar]
- 5.Edgar D M, Dement W C, Fuller C A. J Neurosci. 1993;13:1065–1079. doi: 10.1523/JNEUROSCI.13-03-01065.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weaver D R. J Biol Rhythms. 1998;13:100–102. doi: 10.1177/074873098128999952. [DOI] [PubMed] [Google Scholar]
- 7.Morin L P. Brain Res Rev. 1994;19:102–107. doi: 10.1016/0165-0173(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 8.Reppert S M. Prog Brain Res. 1992;93:119–131. doi: 10.1016/s0079-6123(08)64568-9. [DOI] [PubMed] [Google Scholar]
- 9.Rivkees S A, Reppert S M. Horm Res. 1992;37:99–104. doi: 10.1159/000182409. [DOI] [PubMed] [Google Scholar]
- 10.Serron-Ferre M, Ducsay C A, Vlenzuela G J. Endocr Rev. 1993;14:594–609. doi: 10.1210/edrv-14-5-594. [DOI] [PubMed] [Google Scholar]
- 11.Rivkees S A. Pediatr Clin North Am. 1997;44:467–487. doi: 10.1016/s0031-3955(05)70486-7. [DOI] [PubMed] [Google Scholar]
- 12.Weaver D R, Reppert S M. Dev Brain Res. 1989;47:151–155. doi: 10.1016/0165-3806(89)90119-3. [DOI] [PubMed] [Google Scholar]
- 13.Duncan M J, Banister M J, Reppert S M. Brain Res. 1986;369:326–330. doi: 10.1016/0006-8993(86)90544-5. [DOI] [PubMed] [Google Scholar]
- 14.Reppert S M, Weaver D R, Rivkees S A. In: Development of Circadian Rhythmicity and Photoperiodism in Mammals. Reppert S M, editor. Ithaca, New York: Perinatology; 1989. pp. 25–44. [Google Scholar]
- 15.Glotzbach S T, Sollars P, Paragano R L, Pickard G H. Soc Neurosci Abstr. 1992;18:587. [Google Scholar]
- 16.Rivkees S A, Hofman P L, Fortman J. Proc Natl Acad Sci USA. 1997;94:292–297. doi: 10.1073/pnas.94.1.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Graven S N, Bowen F W J, Brooten D, Eaton A, Graven M N, Hack M, Hall L A, Hansen N, Hurt H, Kavalhuna R. J Perinatol. 1992;12:164–172. [PubMed] [Google Scholar]
- 18.Coalson J J. Biol Neonate. 1997;71:35–38. doi: 10.1159/000244452. [DOI] [PubMed] [Google Scholar]
- 19.Maeta H, Vidyasagar D, Raju T N, Bhat R, Matsuda H. Pediatrics. 1988;81:277–283. [PubMed] [Google Scholar]
- 20.Minoo P, Segura L, Coalson J J, King R J, DeLemos R A. Am J Physiol. 1991;261:L386–L392. doi: 10.1152/ajplung.1991.261.6.L386. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz W J, Gainer H. Science. 1977;197:1089–1091. doi: 10.1126/science.887940. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz W J, Reppert S M, Eagan S M, Moore-Ede M C. Brain Res. 1983;274:184–187. doi: 10.1016/0006-8993(83)90538-3. [DOI] [PubMed] [Google Scholar]
- 23.Zylka M J, Shearman L P, Weaver D R, Reppert S M. Neuron. 1998;20:1103–1110. doi: 10.1016/s0896-6273(00)80492-4. [DOI] [PubMed] [Google Scholar]
- 24.Shearman L P, Zylka M J, Weaver D R, Kolakowski L F J, Reppert S M. Neuron. 1997;19:1261–1269. doi: 10.1016/s0896-6273(00)80417-1. [DOI] [PubMed] [Google Scholar]
- 25.Albrecht U, Sun Z S, Eichele G, Lee C C. Cell. 1997;91:1055–1064. doi: 10.1016/s0092-8674(00)80495-x. [DOI] [PubMed] [Google Scholar]
- 26.Kornhauser J M, Mayo K E, Takahashi J S. Behav Genet. 1996;26:221–240. doi: 10.1007/BF02359382. [DOI] [PubMed] [Google Scholar]
- 27.Hendrickx A G. Embryology of the Baboon. London: Univ. Chicago Press; 1971. [Google Scholar]
- 28.Kostovic I, Rakic P. J Neurosci. 1984;4:25–42. doi: 10.1523/JNEUROSCI.04-01-00025.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sander L W, Julia H L, Stechler G, Burns P. Psychosom Med. 1972;34:270–282. doi: 10.1097/00006842-197205000-00009. [DOI] [PubMed] [Google Scholar]
- 30.Mann N P, Haddow R, Stokes L, Goodley S, Rutter N. Br Med J. 1986;293:1265–1267. doi: 10.1136/bmj.293.6557.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller C L, White R, Whitman T L. Infant Behav Dev. 1995;18:87–95. [Google Scholar]
- 32.Derth D P. Am J Obstet Gynecol. 1994;170:960–961. doi: 10.1016/s0002-9378(94)70325-6. [DOI] [PubMed] [Google Scholar]
- 33.McCormick M C, Wise P H. Curr Opin Pediatr. 1993;5:552–557. doi: 10.1097/00008480-199310000-00006. [DOI] [PubMed] [Google Scholar]