Abstract
A hallmark of heart-valve development is the swelling and deposition of extracellular matrix in the heart-valve region. Only myocardium overlying this region can signal to underlying endothelium and cause it to lose cell–cell contacts, delaminate, and invade the extracellular space abutting myocardium and endocardium to form endocardial cushions (EC) in a process known as epithelial to mesenchymal transformation (EMT). The heart-valve myocardium expresses bone morphogenetic protein-2 (Bmp2) coincident with development of valve mesenchyme. BMPs belong to the transforming growth factor beta superfamily (TGF-β) and play a wide variety of roles during development. We show that conditional ablation of Bmp2 in cardiac progenitors results in cell fate changes in which the heart-valve region adopts the identity of differentiated chamber myocardium. Moreover, Bmp2-deficient hearts fail to induce production and deposition of matrix at the heart-valve-forming region, resulting in the inability of the endothelium to swell and impairing the development of ECs. Furthermore, in collagen invasion assays, Bmp2 mutant endothelium is incapable of undergoing EMT, and addition of BMP2 protein to mutant heart explants rescues this phenotype. Our results demonstrate that Bmp2 is both necessary and sufficient to specify a field of cardiac progenitor cells as the heart-valve-inducing region amid developing atria and ventricles.
Keywords: Heart development, Endocardial cushion, epithelial-mesenchymal transition, EMT, Bmp2, Atrioventricular canal, AVC, Cardiac morphogenesis, Cardiac development, Bone morphogenetic protein
Introduction
Proper heart chamber and valve development is essential for life, and problems in the ontogeny of the heart valves and septa account for a preponderance of cases of congenital heart disease (Fishman and Chien, 1997; McFadden and Olson, 2002; Pierpont et al., 2000). The heart-valve progenitors originate adjoining the developing atria and ventricles in a region known as the atrioventricular canal (AVC), in an arrangement that prefigures the architecture of the adult cardiac chambers.
Several genes show restricted spatial expression along the antero-posterior axis of the developing heart (Mjaatvedt, 1999). They divide the heart into five regions: outflow tract (OT), right ventricle, left ventricle, atria, and inflow tract (IT). Concurrent with looping and growth of the ventricles, the ECs form and remodel to become the OT and AV valves (Bolender and Markwald, 1979; Fishman and Chien, 1997; McFadden and Olson, 2002). Inductive signals from the myocardium stimulate endothelial cells in these regions to undergo EMT (Mjaatvedt et al., 1987). The potential to respond to these signals is unique within the ECs, as atrial or ventricular endothelia cannot undergo transformation in vivo or in vitro even when exposed to suitable stimuli (Runyan and Markwald, 1983). Hence, mesenchyme formation within the heart depends on both a localized myocardial-inducing activity and an endothelial cell competence.
BMPs are part of the transforming growth factor beta superfamily (TGF-β) and have a broad variety of roles during development (Hogan, 1996). Bmp2, Bmp4 and Bmp5 are localized temporally and spatially to the myocardium overlying the AVC (Cai et al., 2005; Jiao et al., 2003; Lyons et al., 1990). Conventional generation of Bmp2 null embryos results in death by embryonic day 8.5 (Zhang and Bradley, 1996), prior to cushion formation. This has precluded the examination of Bmp2 loss-of-function during specification of AVC and EC morphogenesis. Conditional ablation of Bmp4 in the myocardium reveals that it regulates AV septation after cushions are formed (Jiao et al., 2003). In Bmp5-deficient embryos, heart development is normal (Kingsley et al., 1992). BMPs bind two serine/threonine kinase receptors, type I and type II. Conditional deletion of the type I receptor ALK3 in myocardium results in hypoplasia of AV cushions, thin myocardium and down-regulation of TGFβ2 (Gaussin et al., 2002). In these mutants, the AVC, albeit malformed, is patterned appropriately along the anterior–posterior axis. Recently, BMP2 protein was shown to be sufficient to induce EMT in monolayer cultures of mouse endothelium (Sugi et al., 2004). However, no molecule from the BMP subfamily, their receptors or intermediates have been implicated in the necessary upstream step of the initial establishment of the heart-valve-forming region along the anterior–posterior axis of the heart.
To circumvent Bmp2-deficient lethality and determine the role of Bmp2 in chamber specification, antero-posterior heart patterning and EC formation, we undertook a recombinase-based approach to eliminate Bmp2 specifically in the developing heart. Conditional ablation of Bmp2 in cardiac progenitors results in cell fate changes, in which the heart-valve forming region adopts the identity of differentiated chamber myocardium. Bmp2-deficient hearts fail to induce production and deposition of matrix at the heart-valve-forming region, resulting in the inability of the endothelium to swell and impairing the development of ECs. In collagen gel invasion assays, Bmp2 mutant endothelium is unable to undergo EMT, and addition of BMP2 protein to mutant heart explants rescues this process. Taken together, these results indicate that Bmp2 is both necessary and sufficient for the specification of the heart-valve-inducing region between the developing atria and ventricles.
Methods
Animals
Bmp2 floxed mice were generated in Vicki Rosen’s laboratory (manuscript in preparation). Briefly, the third exon of Bmp2 and part of its 3′UTR were flanked by loxP sites, whose excision creates a non-functional protein. DNAwas extracted from either tail biopsies or yolk sacs as follows: 1–2 mm of tail or one yolk sac was placed in a screw cap 1.5-ml tube, followed by addition of 200 μl of 50 mM NaOH, incubated at 95°C for 20 min and heavily vortexed until the material disintegrates. The preparation was quick-spun to prevent cross-contamination, and then 50 μl of 1 M Tris pH 8.0 was added to the mixture, mixed and spun for 5 min at maximum speed. We used 1 μl of the cleared supernatant per PCR reaction with the addition of 1.5 μl 25 mM MgCl2 in a 25 μl reaction. Bmp2 genotyping: 5′ GTGTGGTCCACCGCATCAC and 5′ GGCAGACATTGTATCTCTAGG amplify a 474-bp product from the wild type allele and a 545-bp product from the targeted unexcised allele. Reaction conditions were 30 cycles of 30 s at 95°C, 30 s at 65°C and 60 s at 72°C, and Taq (Roche) was used as the thermo-cycling polymerase. Cre genotyping: 5′ GCAGAACCTGAAGATGTTCGC and 5′ ACACCAGAGACGGAAATCCATC amplify a 494-bp product. Reaction conditions and DNA extractions were as above. All mice were maintained in a mixed C57BL6/129 genetic background. Bmp2wt/flox; Nkx2.5::Cre+ mice were crossed with Bmp2flox/flox mice to generate Bmp2flox/flox; Nkx2.5::Cre+(Bmp2Nkx2.5del) mice. Matings were timed by checking for plugs daily, with noon of the day of the plug defined as 0.5 dpc. All experiments involving mice were approved by the HMA Standing Committee on Animals.
Collagen gel assay for cushion mesenchyme formation
Embryos were collected in ice cold Hank’s buffered saline. AV canal explants were dissected away from isolated hearts using a gastromaster microdissection tool (Xenotek Engineering). Explants were placed on top of freshly prepared 1 mg/ml type I rat-tail collagen gels (BD Biosciences) in OptiMem media (Gibco), and covered with additional collagen. The collagen sandwich was then covered with OptiMem containing 1% fetal bovine serum and penicillin/streptomycin, supplemented with insulin/transferin/selenium (all reagents from Gibco) (Camenisch et al., 2002b) and incubated in a tissue culture incubator at 37°C in 5% CO2. Where indicated, 200 ng/ml of BMP2 (a gift from Vicki Rosen) was added to the media at the start of the explant culture.
Immunohistochemistry
Immuno-detections were performed as described (Sugi et al., 2004), with the following modifications: the primary antibodies, monoclonal anti-α-SMA-Cy3 conjugated (Clone 1A4, Sigma) and biotin-conjugated rat anti-mouse CD31 (PECAM1) monoclonal antibody (Clone MEC 13.3, BD Pharmingen), were incubated overnight, in the dark at 4°C, followed by extensive washes in PBS. To inactivate endogenous peroxidases, we treated explants with 0.3% H2O2 for 15 min followed by a 5 min wash in TNT (0.1 M Tris–HCl, pH 7.5, 0.15 M NaCl, 0.05% Tween-20). The explants were blocked for at least 1 h in TNB (0.1 M Tris–HCl, pH 7.5, 0.15 M NaCl, 0.5% Blocking reagent–Roche, and 10% heat inactivated sheep serum), followed by Strepavidin-POD antibody (Roche, 1:500 in TNB) incubation for 30 min, and washed three times in TNT, 5 min each, at room temperature. Tyramide amplification proceeded as per manufacturer’s protocol (Molecular Probes). Fluorophore tyramide stock solution was diluted 1:100 in amplification buffer with the addition of 0.0015% H2O2. Explants were incubated in tyramide working solution for 7 min and then washed 3 times in TNT, 5 min each, at room temperature. Nuclei were counterstained with DAPI. Images were obtained using a Leica-SP2 confocal microscope.
Histology
Embryos were harvested in ice-cold PBS. Yolk sacs were kept for genotyping, while embryos were fixed in 4% PFA overnight at 4°C, followed by extensive PBS washes. Embryos were dehydrated using ethanol and embedded in paraffin, and 10-μm sections were collected. Hematoxylin and eosin staining was performed using standard procedures. For Alcian Blue staining, embryos were harvested as described above. After fixation, sections were briefly washed in distilled water and then washed for 3 min in 3% acetic acid. Specimens were counterstained in a 5% acetic acid solution with Alcian Blue at 0.15 mg/ml for 2 h, after which they were washed in water, mounted in aqueous media and photographed.
In situ hybridization
Whole-mount or section in situ hybridization was performed as described (Brent et al., 2003). DIG-labeled probes were detected with NBT/BCIP (Sigma). For section in situ hybridization, mouse embryos were collected and sectioned as above. Gata4, Mlc2v (Zeisberg et al., 2005), Id1 (Benezra et al., 1990), ANF and Tbx2 (Bruneau et al., 2001) probes were synthesized as described. Notch1 was transcribed from an EST (AW047868). The Msx2 probe was a gift from Richard Mass, Harvard Medical School. An 848-bp fragment of Bmp2 was generated by RT-PCR with the primers 5′ AACTAGAAGCCGTGGAGGAACTTCCA and 5′ TTGTGGAGGGCTGCGGGTGTCGTTAG. This fragment was cloned into pGem-T-easy vector (Promega). To synthesize the antisense probe, the vector was linearized with Nde1, and transcribed using T7 polymerase.
Results and discussion
Bmp2 is required for endocardial swelling and heart-valve mesenchyme formation
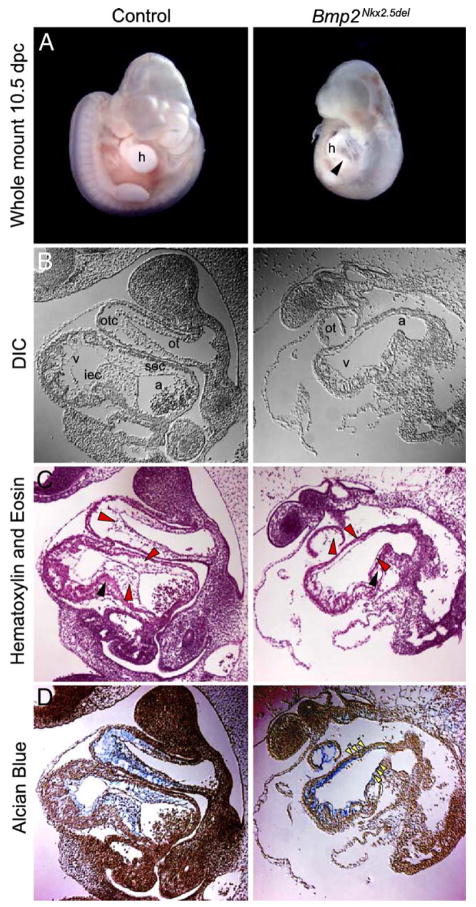
A cre-loxP strategy was employed in which a floxed Bmp2 allele (Bmp2flox; Rosen et al., manuscript in preparation) was deleted in cardiac progenitors by a 3′UTR-ires-Cre allele of the homeobox gene Nkx2.5 (Nkx2.5::Cre) (Stanley et al., 2002). We generated Bmp2flox/flox; Nkx2.5::Cre+ (Bmp2Nkx2.5del) embryos and discovered that Bmp2 ablation in cardiac progenitors causes death at 11.5 days post coitum (dpc) due to cardiac failure. Although we never recovered embryos past 11.5 dpc, all embryos at 10.5 dpc were alive as assessed by cardiac contractile activity at the time of harvesting. At 10.5 dpc, the AVC is barely visible on the leftside of the control embryos because of the expanding size of the developing chambers (Fig. 1A, left panel). At the same stage, Bmp2Nkx2.5del embryos have an abnormally patterned heart, and the region amid the atria and ventricles is readily visible with an uncharacteristic, straight morphology (Fig. 1A, right panel black arrowhead). Some of the Bmp2Nkx2.5del embryos have cardiac edema and pericardial effusion (data not shown). In addition, the Bmp2Nkx2.5del embryos are smaller, presumably as a secondary consequence of their cardiovascular defects. To analyze the morphological difference between control and Bmp2Nkx2.5del hearts, we examined sagittal sections by differential interference contrast microscopy and hematoxylin and eosin staining (Figs. 1B and C, respectively). In control embryos, the forming ECs separate the atria and ventricles, and as the chambers enlarge, the AV constriction appears at the inner curvature of the heart (Fig. 1B and black arrowhead in Fig. 1C). The Bmp2Nkx2.5del hearts lack ECs, EC mesenchyme (red arrowheads in Fig. 1C) and the AV constriction (compare black arrowheads in both panels of Fig. 1C). In contrast, while the receptor tyrosine kinases Erbb2 and Erbb3 knockout mice lack cushion mesenchyme (Camenisch et al., 2002b), they retain endocardial swells and normal AV constriction. The first morphological indication of commitment of AV canal myocardium to instruct the formation of the heart valves is the deposition and swelling of the extracellular matrix. Compared to the Erbb2 and Erbb3 defect, our Bmp2Nkx2.5del mutants arrest at a stage prior to matrix deposition, hence before specification of the AVC myocardium occurs, indicating that Bmp2 is required for endocardial swelling in addition to heart-valve mesenchyme formation. In contrast, the outflow tract of the heart is grossly normal in Bmp2Nkx2.5del embryos. The high contribution of neural crest mesenchyme to the development of this structure may contribute to the absence of a phenotype in this region.
Fig. 1.
Bmp2 is required for the formation of the endocardial cushions. (A) Control and Bmp2Nkx2.5del embryos during midgestation. Note that the atrioventricular constriction is absent in the mutant hearts, black arrowhead. h, Heart. (B) Differential interference contrast (DIC) microscopy of control and Bmp2Nkx2.5del heart sagittal sections. Photographs are from the left side of the embryos: atrial foramen (a) to the right, ventricle foramen (v) to the left, outflow tract foramen (ot) on top. otc, outflow tract cushion; sec, superior endocardial cushion; iec, inferior endocardial cushion. Magnification 200×. (C) Hematoxylin and eosin staining of sections through the heart of control and Bmp2Nkx2.5del hearts. The red arrowheads indicate endocardial cushion mesenchyme in the control and at the predicted location of endocardial cushions in a Bmp2Nkx2.5del heart. Note the presence of endocardial cushion cells in the control embryos and the absence of mesenchyme in Bmp2Nkx2.5del hearts. In addition, the atrioventricular constriction is absent in Bmp2Nkx2.5del hearts (black arrowhead). (D) Alcian Blue staining of acidic glycosaminoglycans. Bmp2Nkx2.5del hearts fail to deposit extracellular matrix at the heart-valve-forming region and fail to undergo endocardial swelling (compare blue staining, yellow arrowheads).
Bmp2 is essential for extracellular matrix deposition
We investigated the possibility that Bmp2 could regulate the increase of extracellular matrix deposition at the cushion forming regions, which is necessary for cushion mesenchyme formation (Camenisch et al., 2000). We examined the EC matrix of Bmp2Nkx2.5del hearts and found no increase in matrix deposition (yellow arrowheads, Fig. 1D) as assessed by binding of Alcian Blue to acidic glycosaminoglycans. The lack of matrix deposition in Bmp2Nkx2.5del ECs phenocopies hyaluronan synthase-2 (Has2)-deficient embryos (Camenisch et al., 2000). However, unlike our conditional Bmp2 knockouts, Has2 mutants also display vascular abnormalities and cardiomegaly. Therefore, it is unclear whether the cardiac defects in the Has2 mutant are due to loss of hyaluronan synthase-2 in the ECs or are secondary to the vascular phenotype. In contrast, our conditional ablation strategy takes advantage of a heart-specific promoter, which is not expressed in the extracardiac vasculature. And indeed, the yolk sacs of the Bmp2Nkx2.5del embryos show normal vasculature and blood formation (data not shown). We have demonstrated that Bmp2 is indispensable for extracellular matrix deposition, endocardial swelling and heart-valve mesenchyme formation.
Bmp2Nkx2.5del atrioventricular endothelium fails to initiate EMT
Since Bmp2Nkx2.5del mutants live past stages of EC specification, we can examine the proximal tissue interactions between myocardium and endocardium during cushion morphogenesis at the AVC. When cultured in a collagen lattice, AVC explants reiterate EMT (Runyan and Markwald, 1983). Using this assay we determined the stage at which valve specification arrested in Bmp2Nkx2.5del hearts. After 48 h in culture, control explants produced a halo of mesenchymal cells (Fig. 2A, bright field), while Bmp2Nkx2.5del explants did not (Fig. 2B, bright field), indicating that Bmp2 is required to produce invasive mesenchyme. The absence of EMT in mutant explants at 48 h in culture verifies the fact that the AV canal is incapable of undergoing normal morphogenesis in these animals.
Fig. 2.
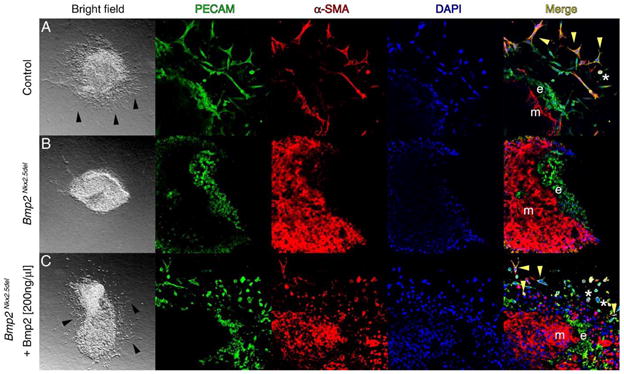
Bmp2 is necessary and sufficient for epithelial to mesenchymal transformation of the atrioventricular cushions. (A) Bright field and confocal micrographs of a representative control explant. Immunohistochemical detection of platelet cell adhesion molecule (PECAM, green) and anti-smooth muscle actin (a-SMA, red). Nuclei are stained with DAPI in blue. The merged images are on the right. Black arrowheads indicate cells with mesenchymal morphology. Yellow arrowheads show transformed mesenchymal cells, and asterisks show activated untransformed endothelial cells. e, Endothelium; m, myocardium. Magnification for bright field 6.3×; magnification for confocal micrographs 400×. (B) Bmp2Nkx2.5del representative explant, note the absence of cells with mesenchymal morphology (bright field) and the lack of activated and transformed endothelium (Merge). (C) BMP2 protein can rescue invasive mesenchyme formation (bright field), activation and transformation of the endothelium (Merge) in Bmp2Nkx2.5del explants.
BMP2 rescues EMT from AV explants deficient for Bmp2 by inducing cell–cell separation and invasive mesenchymal cell formation
As cardiac conditioned media can replace the requirement for myocardium to achieve EMT in culture (Mjaatvedt and Markwald, 1989), it has been proposed that myocardium secretes a molecule(s) that elicits endocardial cell differentiation. Bmp2 is thought to be this factor as it is only expressed in myocardium adjoining cushion-forming segments and induces EMT in monolayer cultures of AV endothelium (Sugi et al., 2004). To address physiological sufficiency, a biochemical replacement experiment was performed in Bmp2-deficient explants. We found that addition of 200 ng/ml BMP2 to Bmp2Nkx2.5del explants rescued cell–cell separation and invasive mesenchymal cell formation (Fig. 2C, bright field), indicating BMP2 is sufficient for EMT. To confirm that invasive mesenchymal cells form, we analyzed PECAM1 and smooth muscle alpha actin (SMA) expression to discern endothelial from mesenchymal cells (Sugi et al., 2004). The cell adhesion molecule PECAM1 is expressed in endothelial cells and downregulated during EMT in the AVC (Sugi et al., 2004). PECAM1 was maintained in endocardial cells of control, Bmp2Nkx2.5del, and Bmp2Nkx2.5del + 200 ng/ml BMP2 explants (Figs. 2A–C, PECAM). In control explants, SMA is expressed in invasive mesenchymal cells after EMT but not in endocardial cells (Sugi et al., 2004) (Fig. 2A, α-SMA). No SMA-expressing mesenchymal cells were detected in Bmp2Nkx2.5del explants (Fig. 2B, α-SMA), and upon addition of BMP2 to the media, SMA-positive invasive mesenchymal cells reappeared. Co-expression of SMA and PECAM1 only occurs in activated endothelial cells (asterisks in Figs. 2A and C, merged) and transformed mesenchymal cells, and it is indicative of EMT (Camenisch et al., 2002b) (yellow arrowheads in Fig. 2A, merged). Bmp2Nkx2.5del explants never exhibit co-expression of these markers, indicating that BMP2 is required for AVC endothelium to undergo EMT (e in Fig. 2B, merge). Addition of BMP2 to Bmp2Nkx2.5del explants rescues this activation and transformation (yellow arrowheads, Fig. 2C, merge). In order to better characterize the extent of rescue, we counted the number of invasive cells with mesenchyme cell morphology in all three conditions. While Bmp2Nkx2.5del explants never undergo EMT, we find that addition of 200 ng/ml of BMP2 to Bmp2Nkx2.5del explants results in a 30% rescue of invasive mesenchyme formation relative to controls, with a P value of 4 × 10−6 (Fig. 3). Although we cannot rule out the possibility that BMP2 may have a role in controlling proliferation of the AVC endothelium, we speculate that a mere increase in endothelial cell numbers will not necessarily result in a proportional increase in the formation of invasive mesenchyme. Local proliferation in the absence of EMT may result in formation of a multilayered non-invasive endothelium (Dor et al., 2001). Nevertheless, our experiments support our initial hypothesis that BMP2 is sufficient and necessary for inducing AVC EMT within the forming endocardial endothelium.
Fig. 3.
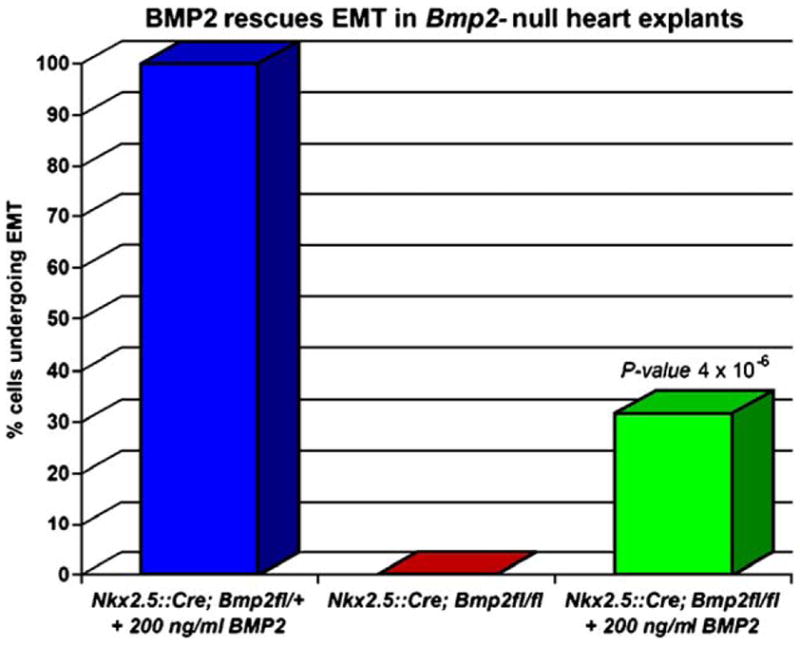
Addition of BMP2 protein can rescue invasive mesenchyme formation. Quantitative analysis of invasive mesenchyme formation at 48 h. One representative experiment in shown for control (blue, n = 5), Bmp2Nkx2.5del (red, n = 2), and rescued (green, n = 9) heart explants. The 30% rescue in mesenchyme formation in Bmp2Nkx2.5del explants after the addition of 200 ng/ml BMP2 is statistically significant, with a P value of 4 × 10−6.
In the absence of Bmp2, the AV canal myocardium loses its identity as primary myocardium
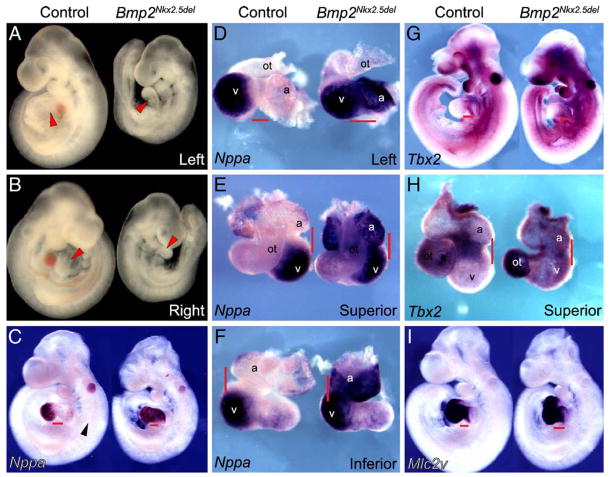
We have determined that the morphology of the region between atrial and ventricular foramens in Bmp2Nkx2.5del embryos is rather straight. The scarce matrix and close proximity to the myocardium suggests that the endothelium in this malformed area morphologically resembles that of the atria and ventricles. We also have shown that the defect in heart morphogenesis occurs prior to the increase in extracellular matrix deposition at the cushion-forming region, as the cardiac jelly never swells in Bmp2Nkx2.5del hearts. Additionally, we have demonstrated that Bmp2 is both essential and sufficient to transform AV canal endocardial cells to mesenchyme. Taken together, the defects we observed in morphogenesis and AVC patterning in Bmp2Nkx2.5del embryos suggest that Bmp2 may also be required for specification of segmental heart patterning. To investigate this, embryos were examined at 9.5 dpc when cushion endothelium is undergoing EMT (Camenisch et al., 2002a). Compared to controls, Bmp2Nkx2.5del hearts have a straight morphology on the left (compare red arrowheads Fig. 4A) and have defects with atrial rotation. However, the OT is present and looped. To determine if loss of Bmp2 alters gene expression in the putative AVC region, expression of Nppa, a gene exclusive to chamber myocardium (Christoffels et al., 2000), was examined. In 9.5 dpc control embryos, Nppa is expressed in myocardium of the outer curvature of the atrial and ventricular compartments as they differentiate and balloon out (Christoffels et al., 2000). The inner curvature forms a continuity of myocardium with IT, AVC, and OT, but it does not express Nppa and remains undifferentiated (Christoffels et al., 2000) (Fig. 4F, control, red bars). In Bmp2-deficient hearts, Nppa expression is expanded (Figs. 4C–F; Bmp2Nkx2.5del) into the atria, IT and inner curvature of the heart (red bars in Fig. 4C, red bars in D–F), suggesting that loss of Bmp2 causes primitive inner curvature myocardium to acquire the identity of differentiated outer curvature myocardium. Additionally, the expression of Nppa in the putative AVC region suggests that this heart-valve forming region has also adopted chamber myocardium identity.
Fig. 4.
Bmp2 instructs the development of the atrioventricular canal. (A, B) Control and Bmp2Nkx2.5del embryos at 9.5 days post-fertilization. (A) Left side view, the atrioventricular constriction is absent in mutant hearts (red arrowhead). (B) Right side view, the outflow tract is patterned normally in Bmp2Nkx2.5del hearts (red arrowhead). (C–F) Whole-mount in situ hybridization for Nppa, which marks differentiated chamber myocardium in control embryos. In Bmp2Nkx2.5del hearts, the atrioventricular canal adopts differentiated chamber myocardium fates as judged by the expansion of Nppa into the region where the atrioventricular canal should develop (C; compare red bars). Notice the gap of expression at the atrioventricular canal in the control embryo. (D–F) Nppa expression in control and Bmp2Nkx2.5del hearts at 9.5 days post-fertilization. Left (D), superior (E) and inferior (F) views. The position of the atrioventricular canal is indicated by the red bar. (G, H) Tbx2 expression in control and Bmp2Nkx2.5del hearts at 9.5 days post-fertilization. The atrioventricular canal adopts differentiated chamber myocardium fates as judged by the absence of Tbx2 in the region where the atrioventricular canal should form (red bars in panels G and H). (I) Mlc2v expression, which specifically marks the ventricles and AV canal, is expanded into the atrial rudiment of Bmp2Nkx2.5del hearts.
A mechanism has been proposed wherein site-specific AVC formation results from localized repression of chamber differentiation in the heart-valve region (Habets et al., 2002). In this model, Tbx2, a transcriptional repressor (Jacobs et al., 2000), forms a complex with Nkx2.5 on T-box and NK-class elements within the Nppa promoter and suppresses Nppa activation in the AVC and OT (Habets et al., 2002). Therefore, we examined if misexpression of Nppa in atria, IT, and inner curvature of Bmp2-deficient hearts could result from the loss of Tbx2 expression. Tbx2 was expressed normally in AVC and OT of control hearts (Christoffels et al., 2004; Habets et al., 2002) (Figs. 4G–H, control). In Bmp2Nkx2.5del hearts, Tbx2 was absent from the AVC and inner curvature (Fig. 4H). These data suggest that Bmp2 instructs AVC development by regulating Tbx2 expression and is consistent with the model in which Tbx2 represses chamber myocardium fate and permits AVC differentiation. Interestingly, at earlier stages of heart development, Tbx2 expression actually correlates with decreased Bmp2 signaling, rather than Tbx2 being induced by BMP activity. These data come from several studies in which Tbx20 was shown to be a repressor of Bmp2 (Cai et al., 2005; Singh et al., 2005; Stennard et al., 2005). In two of these reports, the loss of Tbx20 resulted in the downregulation of Bmp2 and yet the upregulation of Tbx2 (Cai et al., 2005; Stennard et al., 2005). The third group, in contrast, found concomitant upregulation of Bmp2 and Tbx2 along the linear heart tube (Singh et al., 2005). In all three studies, embryos deficient for Tbx20 arrest at stages prior to cushion development, precluding the analysis of the relation between these genes in the later setting of cushion morphogenesis.
Bmp2 is required for specification of posterior heart structures
Prior to AV canal specification, during linear heart tube stages, Bmp2 is strongly expressed in the posterior pole of the heart where the atria develop; hence, it is possible that Bmp2 is required for atrial specification. In order to test this hypothesis, we looked at the expression of ventricular myosin light chain 2 (Mlc2v), a ventricular and AV canal-specific marker (Franco et al., 1999). In Bmp2Nkx2.5del hearts, Mlc2v expression is expanded into the atrial region (Fig. 4I). As noted in Figs. 4C–F, Nppa expression is dramatically upregulated in the atrial appendage of Bmp2Nkx2.5del hearts relative to controls. This high level of expression is characteristic of ventricular myocardium. Together, our data suggest that the atrial rudiment in Bmp2Nkx2.5del hearts is adopting the molecular identity of ventricular myocardium. Our mutant partially phenocopies the LIM homeodomain transcription factor Isl1 mutant (Cai et al., 2003). Isl1-deficient embryos lack outflow tract, right ventricle and have mispatterned atria. Similar to our Bmp2Nkx2.5del embryos, in Isl1-deficient embryos, Mlc2v expression is expanded into the atrial appendage. This accompanies a reduction in Bmp2 expression. The authors report that this phenotype most likely arises from the failure of a particular population of cardiac progenitors that express Isl1 to migrate into these developing heart structures. Hence, our results support a model in which Bmp2 is epistatic to Isl1 and necessary for the recruitment of cardiac progenitors to the posterior heart segments. The fact that Bmp2Nkx2.5del embryos still have a properly patterned outflow tract and a normal right ventricle suggests that Isl1 regulates other targets in these anterior structures.
To further examine the AVC myocardium of Bmp2Nkx2.5del hearts, we analyzed the expression of myocardial restricted genes by section in situ hybridization. We verified that Bmp2 was inactivated by Nkx2.5::Cre in the heart, as Bmp2 transcripts were not detected in AVC myocardium of Bmp2Nkx2.5del hearts but were present in controls (Fig. 5A, Bmp2Nkx2.5del, control). Section in situ hybridization for the chamber myocardium markers Nppa and the small muscle-specific protein Smpx (Palmer et al., 2001) confirmed the expansion of transcripts into the heart-valve field (compare black arrowhead pairs in Figs. 5B and C) and atria (Figs. 5B and C insets). Similar analysis for Tbx2 verified its downregulation in the AVC (Fig. 5D). Similar to Bmp2 and Tbx2, Msx2 (muscle segment homeobox 2) expression is restricted to the AVC myocardium in controls (Abdelwahid et al., 2001) (Fig. 5E, control), and downregulated in the myocardium abutting the chambers in mutants (Fig. 5E, Bmp2Nkx2.5del). Taken together, expansion of Nppa and Smpx and absence of Tbx2 and Msx2 expression suggest that chamber myocardium is specified at the expense of AVC myocardium in Bmp2-deficient embryos.
Fig. 5.
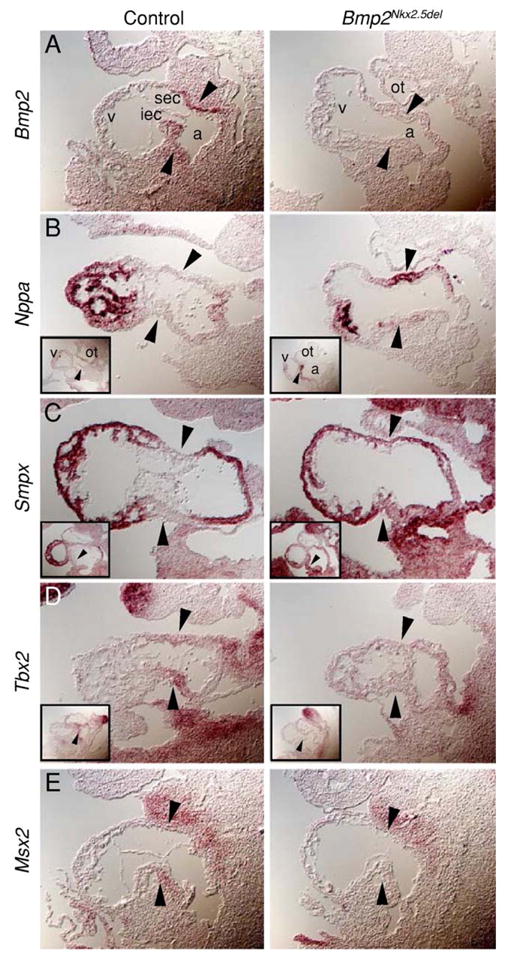
Bmp2 is required for the AV canal myocardium to retain its identity as primary myocardium. (A–E) Section in situ hybridization for control and Bmp2Nkx2.5del hearts showing that the atrioventricular canal fails to be specified in the absence of Bmp2. Note that AVC restricted transcripts are absent in Bmp2Nkx2.5del hearts: Bmp2 (A), Tbx2 (D), Msx2 (E) and the upregulation of the chamber specific transcripts Nppa (B) and Smpx (C) to the putative atrioventricular canal myocardium. Black arrowheads indicate atrioventricular canal myocardium. Insets in (B–D) are sections through the outflow portion of the same heart. Magnification 200×. a, atria; v, ventricle; ot, outflow tract; sec, superior endocardial cushion; iec, inferior endocardial cushion.
Bmp2 maintains the molecular competence of the cardiac endothelium, which is required to form heart-valve mesenchyme
Because AVC myocardium induces the adjacent endothelium to undergo EMT and form the valves, we examined the expression of Id1, Notch1, and Gata4, genes that are either present in AVC endothelium during EMT (Jen et al., 1996; Timmerman et al., 2004; Zeisberg et al., 2005) or have been implicated in the process (Timmerman et al., 2004) (Figs. 6A–C, control). Expression of Id1, a negative regulator of basic helix–loop–helix transcription factors (Jen et al., 1996), was absent from Bmp2Nkx2.5del heart endothelium compared to controls (Fig. 6A, Bmp2Nkx2.5del), suggesting BMP2 regulates Id1 expression in AVC endothelium. This finding is consistent with other studies demonstrating that Id1 is induced by BMP2 in osteoblast-like and myoblastic cells (Katagiri et al., 1994; Ogata et al., 1993) and is a direct target of BMP in embryonic stem cells (Hollnagel et al., 1999).
Fig. 6.
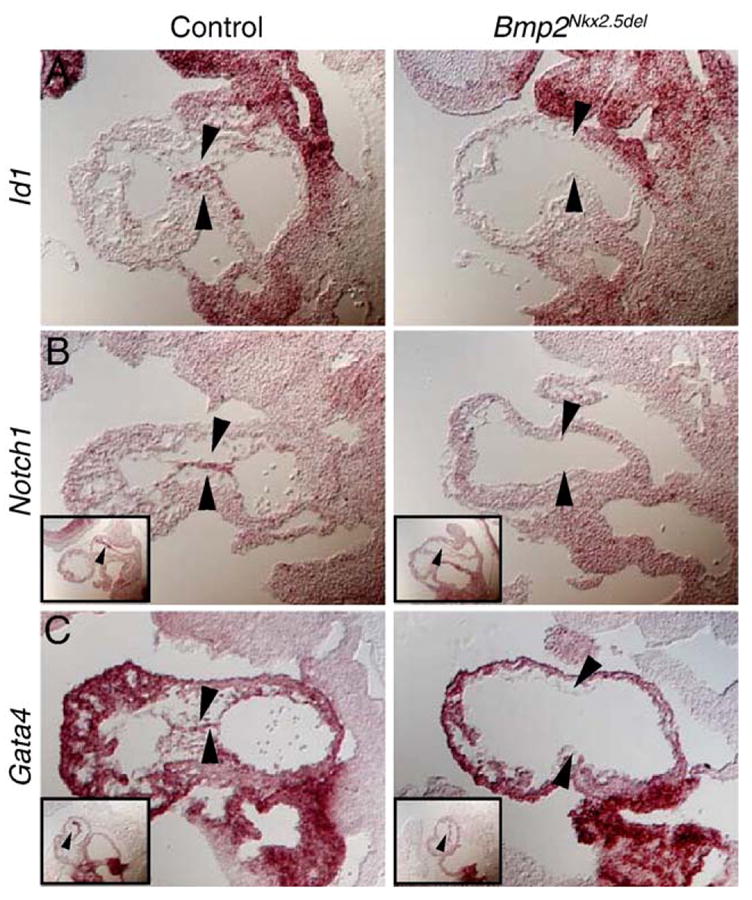
Bmp2 is necessary to induce the endothelial cell competence to transform. (A–C) Section in situ hybridization for control and Bmp2Nkx2.5del hearts showing that Id1 (A), Notch1 (B), and Gata4 (C), endothelial specific markers, are downregulated from the putative endocardial endothelium. Black arrowheads indicate atrioventricular canal endocardium. Insets in panels B and C are sections through the outflow portion of the same heart. Magnification 200×.
Since the Notch1 signal transduction pathway has been found to directly regulate genes important for endocardial EMT (Timmerman et al., 2004), and deletion of Notch1 results in hypocellular ECs (Timmerman et al., 2004), we examined if Notch1 expression is altered in the absence of Bmp2. While control hearts exhibit robust Notch1 expression in AVC endothelium and mesenchyme (Fig. 6B, control), Bmp2Nkx2.5del endothelium completely lacks Notch1 transcripts (Fig. 6B, Bmp2Nkx2.5del). Although Notch1 makes endocardial endothelium competent to form heart-valve mesenchyme, we find that Bmp2 is necessary for Notch1 expression and inducing this competence.
Gata4, a transcription factor essential for heart formation (Kuo et al., 1997; Molkentin et al., 1997), is robustly expressed at 9.5 dpc in control AVC endocardium and EC mesenchyme, which is mostly derived from AVC endocardium (Kisanuki et al., 2001; Zeisberg et al., 2005) (Fig. 6C, control). Interestingly, myocardial-specific ablation of Gata4 results in the loss of endocardial Gata4 expression, indicating a paracrine signal derived from myocardium regulates its endocardial expression (Zeisberg et al., 2005). In Bmp2Nkx2.5del hearts, myocardial expression of Gata4 remains intact while the expression in AV endothelium was lost (Fig. 6C, Bmp2Nkx2.5del). Based on this finding, we speculate that this paracrine signal is Bmp2. Taken together, absence of Id1, Notch1, and Gata4 expression from Bmp2-deficient endothelium suggests that these mutants failed to acquire endothelial cell competence for transformation. It also places Id1, Notch1, and Gata4 epistatic to Bmp2 during heart morphogenesis. Moreover, putative AVC endothelium in Bmp2Nkx2.5del hearts has the molecular signature of chamber endothelium. Thus, this region in Bmp2Nkx2.5del hearts has acquired chamber fates at the expense of primary myocardium progenitors.
Conclusion
The regional specificity of induction and the instructive potential of Bmp2 in the myocardium of the AVC initiate a cascade of events that establish EMT competence in the endothelium. Despite the promiscuity of BMP ligand/receptor binding (Ebisawa et al., 1999; ten Dijke et al., 1994), we find that one signaling molecule, Bmp2, is essential and non-redundant for specification of the heart-valve-forming region. Hence, our results place Bmp2 at the top of a genetic hierarchy essential for formation of the AVC and, consequently, for formation of the heart valves.
Acknowledgments
We are grateful to Vicki Rosen for the use of the Bmp2 floxed allele prior to publication and for the recombinant BMP2 protein. We thank Richard Harvey for the Nkx2.5::Cre line, Jenna L. Galloway for critical reading of the manuscript, members of the laboratories of CJT, C. Cepko, S. Dymecki, and J. and C. Seidman for the reagents, discussions, and advice. Many thanks to Michael Peterson and Dane Loeliger for help with the last rounds of genotyping. Heart development research in CJT’s laboratory is supported by the NIH (HD0045499). JR-F was supported by a predoctoral National Research Service Award from the NIH.
References
- Abdelwahid E, Rice D, Pelliniemi LJ, Jokinen E. Overlapping and differential localization of Bmp-2, Bmp-4, Msx-2 and apoptosis in the endocardial cushion and adjacent tissues of the developing mouse heart. Cell Tissue Res. 2001;305:67–78. doi: 10.1007/s004410100399. [DOI] [PubMed] [Google Scholar]
- Benezra R, Davis RL, Lockshon D, Turner DL, Weintraub H. The protein Id: a negative regulator of helix–loop–helix DNA binding proteins. Cell. 1990;61:49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- Bolender DL, Markwald RR. Epithelial–mesenchymal transformation in chick atrioventricular cushion morphogenesis. Scan Electron. 1979:313–321. [PubMed] [Google Scholar]
- Brent AE, Schweitzer R, Tabin CJ. A somitic compartment of tendon progenitors. Cell. 2003;113:235–248. doi: 10.1016/s0092-8674(03)00268-x. [DOI] [PubMed] [Google Scholar]
- Bruneau BG, Bao ZZ, Fatkin D, Xavier-Neto J, Georgakopoulos D, Maguire CT, Berul CI, Kass DA, Kuroskide Bold ML, de Bold AJ, Conner DA, Rosenthal N, Cepko CL, Seidman CE, Seidman JG. Cardiomyopathy in Irx4-deficient mice is preceded by abnormal ventricular gene expression. Mol Cell Biol. 2001;21:1730–1736. doi: 10.1128/MCB.21.5.1730-1736.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai CL, Liang X, Shi Y, Chu PH, Pfaff SL, Chen J, Evans S. Isl1 identifies a cardiac progenitor population that proliferates prior to differentiation and contributes a majority of cells to the heart. Dev Cell. 2003;5:877–889. doi: 10.1016/s1534-5807(03)00363-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai CL, Zhou W, Yang L, Bu L, Qyang Y, Zhang X, Li X, Rosenfeld MG, Chen J, Evans S. T-box genes coordinate regional rates of proliferation and regional specification during cardiogenesis. Development. 2005;132:2475–2487. doi: 10.1242/dev.01832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr, Kubalak S, Klewer SE, McDonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. J Clin Invest. 2000;106:349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camenisch TD, Molin DG, Person A, Runyan RB, Gittenbergerde Groot AC, McDonald JA, Klewer SE. Temporal and distinct TGFbeta ligand requirements during mouse and avian endocardial cushion morphogenesis. Dev Biol. 2002a;248:170–181. doi: 10.1006/dbio.2002.0731. [DOI] [PubMed] [Google Scholar]
- Camenisch TD, Schroeder JA, Bradley J, Klewer SE, McDonald JA. Heart-valve mesenchyme formation is dependent on hyaluronan-augmented activation of ErbB2-ErbB3 receptors. Nat Med. 2002b;8:850–855. doi: 10.1038/nm742. [DOI] [PubMed] [Google Scholar]
- Christoffels VM, Habets PE, Franco D, Campione M, de Jong F, Lamers WH, Bao ZZ, Palmer S, Biben C, Harvey RP, Moorman AF. Chamber formation and morphogenesis in the developing mammalian heart. Dev Biol. 2000;223:266–278. doi: 10.1006/dbio.2000.9753. [DOI] [PubMed] [Google Scholar]
- Christoffels VM, Hoogaars WM, Tessari A, Clout DE, Moorman AF, Campione M. T-box transcription factor Tbx2 represses differentiation and formation of the cardiac chambers. Dev Dyn. 2004;229:763–770. doi: 10.1002/dvdy.10487. [DOI] [PubMed] [Google Scholar]
- Dor Y, Camenisch TD, Itin A, Fishman GI, McDonald JA, Carmeliet P, Keshet E. A novel role for VEGF in endocardial cushion formation and its potential contribution to congenital heart defects. Development. 2001;128:1531–1538. doi: 10.1242/dev.128.9.1531. [DOI] [PubMed] [Google Scholar]
- Ebisawa T, Tada K, Kitajima I, Tojo K, Sampath TK, Kawabata M, Miyazono K, Imamura T. Characterization of bone morphogenetic protein-6 signaling pathways in osteoblast differentiation. J Cell Sci. 1999;112 (pt 20):3519–3527. doi: 10.1242/jcs.112.20.3519. [DOI] [PubMed] [Google Scholar]
- Fishman MC, Chien KR. Fashioning the vertebrate heart: earliest embryonic decisions. Development. 1997;124:2099–2117. doi: 10.1242/dev.124.11.2099. [DOI] [PubMed] [Google Scholar]
- Franco D, Markman MM, Wagenaar GT, Ya J, Lamers WH, Moorman AF. Myosin light chain 2a and 2v identifies the embryonic outflow tract myocardium in the developing rodent heart. Anat Rec. 1999;254:135–146. doi: 10.1002/(SICI)1097-0185(19990101)254:1<135::AID-AR17>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Gaussin V, Van de Putte T, Mishina Y, Hanks MC, Zwijsen A, Huylebroeck D, Behringer RR, Schneider MD. Endocardial cushion and myocardial defects after cardiac myocyte-specific conditional deletion of the bone morphogenetic protein receptor ALK3. Proc Natl Acad Sci U S A. 2002;99:2878–2883. doi: 10.1073/pnas.042390499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habets PE, Moorman AF, Clout DE, van Roon MA, Lingbeek M, van Lohuizen M, Campione M, Christoffels VM. Cooperative action of Tbx2 and Nkx2.5 inhibits ANF expression in the atrioventricular canal: implications for cardiac chamber formation. Genes Dev. 2002;16:1234–1246. doi: 10.1101/gad.222902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan BL. Bone morphogenetic proteins in development. Curr Opin Genet Dev. 1996;6:432–438. doi: 10.1016/s0959-437x(96)80064-5. [DOI] [PubMed] [Google Scholar]
- Hollnagel A, Oehlmann V, Heymer J, Ruther U, Nordheim A. Id genes are direct targets of bone morphogenetic protein induction in embryonic stem cells. J Biol Chem. 1999;274:19838–19845. doi: 10.1074/jbc.274.28.19838. [DOI] [PubMed] [Google Scholar]
- Jacobs JJ, Keblusek P, Robanus-Maandag E, Kristel P, Lingbeek M, Nederlof PM, van Welsem T, van de Vijver MJ, Koh EY, Daley GQ, van Lohuizen M. Senescence bypass screen identifies TBX2, which represses Cdkn2a (p19 (ARF)) and is amplified in a subset of human breast cancers. Nat Genet. 2000;26:291–299. doi: 10.1038/81583. [DOI] [PubMed] [Google Scholar]
- Jen Y, Manova K, Benezra R. Expression patterns of Id1, Id2, and Id3 are highly related but distinct from that of Id4 during mouse embryogenesis. Dev Dyn. 1996;207:235–252. doi: 10.1002/(SICI)1097-0177(199611)207:3<235::AID-AJA1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Jiao K, Kulessa H, Tompkins K, Zhou Y, Batts L, Baldwin HS, Hogan BL. An essential role of Bmp4 in the atrioventricular septation of the mouse heart. Genes Dev. 2003;17:2362–2367. doi: 10.1101/gad.1124803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127:1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley DM, Bland AE, Grubber JM, Marker PC, Russell LB, Copeland NG, Jenkins NA. The mouse short ear skeletal morphogenesis locus is associated with defects in a bone morphogenetic member of the TGF beta superfamily. Cell. 1992;71:399–410. doi: 10.1016/0092-8674(92)90510-j. [DOI] [PubMed] [Google Scholar]
- Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-Cre transgenic mice: a new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- Kuo CT, Morrisey EE, Anandappa R, Sigrist K, Lu MM, Parmacek MS, Soudais C, Leiden JM. GATA4 transcription factor is required for ventral morphogenesis and heart tube formation. Genes Dev. 1997;11:1048–1060. doi: 10.1101/gad.11.8.1048. [DOI] [PubMed] [Google Scholar]
- Lyons KM, Pelton RW, Hogan BL. Organogenesis and pattern formation in the mouse: RNA distribution patterns suggest a role for bone morphogenetic protein-2A (BMP-2A) Development. 1990;109:833–844. doi: 10.1242/dev.109.4.833. [DOI] [PubMed] [Google Scholar]
- McFadden DG, Olson EN. Heart development: learning from mistakes. Curr Opin Genet Dev. 2002;12:328–335. doi: 10.1016/s0959-437x(02)00306-4. [DOI] [PubMed] [Google Scholar]
- Mjaatvedt CH. Mechanisms of segmentation, septation, and remodeling of the tubular heart: endocardial cushion fate and cardiac looping. In: Harvey RP, Rosenthal N, editors. Heart Development. Academic Press; San Diego: 1999. pp. 159–174. [Google Scholar]
- Mjaatvedt CH, Markwald RR. Induction of an epithelial–mesenchymal transition by an in vivo adheron-like complex. Dev Biol. 1989;136:118–128. doi: 10.1016/0012-1606(89)90135-8. [DOI] [PubMed] [Google Scholar]
- Mjaatvedt CH, Lepera RC, Markwald RR. Myocardial specificity for initiating endothelial–mesenchymal cell transition in embryonic chick heart correlates with a particulate distribution of fibronectin. Dev Biol. 1987;119:59–67. doi: 10.1016/0012-1606(87)90206-5. [DOI] [PubMed] [Google Scholar]
- Molkentin JD, Lin Q, Duncan SA, Olson EN. Requirement of the transcription factor GATA4 for heart tube formation and ventral morphogenesis. Genes Dev. 1997;11:1061–1072. doi: 10.1101/gad.11.8.1061. [DOI] [PubMed] [Google Scholar]
- Ogata T, Wozney JM, Benezra R, Noda M. Bone morphogenetic protein 2 transiently enhances expression of a gene, Id (inhibitor of differentiation), encoding a helix–loop–helix molecule in osteoblast-like cells. Proc Natl Acad Sci U S A. 1993;90:9219–9222. doi: 10.1073/pnas.90.19.9219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer S, Groves N, Schindeler A, Yeoh T, Biben C, Wang CC, Sparrow DB, Barnett L, Jenkins NA, Copeland NG, Koentgen F, Mohun T, Harvey RP. The small muscle-specific protein Csl modifies cell shape and promotes myocyte fusion in an insulin-like growth factor 1-dependent manner. J Cell Biol. 2001;153:985–998. doi: 10.1083/jcb.153.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierpont ME, Markwald RR, Lin AE. Genetic aspects of atrioventricular septal defects. Am J Med Genet. 2000;97:289–296. [PubMed] [Google Scholar]
- Runyan RB, Markwald RR. Invasion of mesenchyme into three-dimensional collagen gels: a regional and temporal analysis of interaction in embryonic heart tissue. Dev Biol. 1983;95:108–114. doi: 10.1016/0012-1606(83)90010-6. [DOI] [PubMed] [Google Scholar]
- Singh MK, Christoffels VM, Dias JM, Trowe MO, Petry M, Schuster-Gossler K, Burger A, Ericson J, Kispert A. Tbx20 is essential for cardiac chamber differentiation and repression of Tbx2. Development. 2005;132:2697–2707. doi: 10.1242/dev.01854. [DOI] [PubMed] [Google Scholar]
- Stanley EG, Biben C, Elefanty A, Barnett L, Koentgen F, Robb L, Harvey RP. Efficient Cre-mediated deletion in cardiac progenitor cells conferred by a 3′UTR-ires-Cre allele of the homeobox gene Nkx2-5. Int J Dev Biol. 2002;46:431–439. [PubMed] [Google Scholar]
- Stennard FA, Costa MW, Lai D, Biben C, Furtado MB, Solloway MJ, McCulley DJ, Leimena C, Preis JI, Dunwoodie SL, Elliott DE, Prall OW, Black BL, Fatkin D, Harvey RP. Murine T-box transcription factor Tbx20 acts as a repressor during heart development, and is essential for adult heart integrity, function and adaptation. Development. 2005;132:2451–2462. doi: 10.1242/dev.01799. [DOI] [PubMed] [Google Scholar]
- Sugi Y, Yamamura H, Okagawa H, Markwald RR. Bone morphogenetic protein-2 can mediate myocardial regulation of atrioventricular cushion mesenchymal cell formation in mice. Dev Biol. 2004;269:505–518. doi: 10.1016/j.ydbio.2004.01.045. [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Yamashita H, Sampath TK, Reddi AH, Estevez M, Riddle DL, Ichijo H, Heldin CH, Miyazono K. Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J Biol Chem. 1994;269:16985–16988. [PubMed] [Google Scholar]
- Timmerman LA, Grego-Bessa J, Raya A, Bertran E, Perez-Pomares JM, Diez J, Aranda S, Palomo S, McCormick F, Izpisua-Belmonte JC, de la Pompa JL. Notch promotes epithelial–mesenchymal transition during cardiac development and oncogenic transformation. Genes Dev. 2004;18:99–115. doi: 10.1101/gad.276304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeisberg EM, Ma Q, Juraszek AL, Moses K, Schwartz RJ, Izumo S, Pu WT. Morphogenesis of the right ventricle requires myocardial expression of Gata4. J Clin Invest. 2005;115:1522–1531. doi: 10.1172/JCI23769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977–2986. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]