Abstract
Dopamine is a major regulator of proximal tubule salt reabsorption and is a modulator of renin release. Dopamine has been reported to stimulate renin release in vitro through activation of D1-like receptors. However, previous studies investigating dopamine regulation of renin release in vivo have provided contradictory results, indicating stimulation, inhibition or no effect. We have previously reported that macula densa cyclooxygenase-2 (COX-2) is suppressed by dopamine. Since macula densa COX-2 stimulates renal renin expression, our current studies investigated dopamine regulation of renal renin release and synthesis in vivo. Acute treatment with a D1-like receptor agonist, fenoldopam, significantly inhibited renin release, as did acute inhibition of proximal tubule salt reabsorption with acetazolamide. In catechol-O-methyl transferase knockout (COMT-/-) mice, which have increased kidney dopamine levels due to deletion of the major intrarenal dopamine metabolizing enzyme, there was attenuation in response to a low salt diet of the increases of renal cortical COX-2 and renin expression and renin release. A high salt diet led to significant decreases in renal renin expression, but much less significant decreases in COMT-/- mice than wild type mice, resulting in higher renal renin expression in COMT-/- mice. In high salt treated wild type mice or COX-2 knockout mice on a normal salt diet, fenoldopam stimulated renal renin expression. These results suggest that dopamine predominantly inhibits renal renin expression and release by inhibiting macula densa COX-2, but suppression of renal cortical COX-2 activity reveals a contrasting effect of dopamine to stimulate renal renin expression through activation of D1-like receptors.
Keywords: dopamine, renin, kidney, COX-2, salt reabsorption, proximal tubule, macula densa
Although dopamine is an essential neurotransmitter, it also serves important physiologic functions in the mammalian kidney. Dopamine is a major regulator of mammalian proximal tubule salt and water reabsorption 1. In mammalian kidney, dopamine is primarily produced in the proximal tubule. The dopamine precursor L-dihydroxyphenylalanine (L-DOPA) is filtered at the glomerulus and is taken up by the proximal tubule via luminal transporters and converted to dopamine by aromatic L-amino acid decarboxylase (AADC), which is highly expressed in the proximal tubule. In the kidney, dopamine is metabolized predominantly by catechol-O-methyl-transferase (COMT), with a smaller contribution by monoamine oxidase. Through activation of D1-like receptors, locally produced dopamine in the proximal tubule acts as an autocrine/paracrine natriuretic hormone by inhibiting activity of both apical (e.g., Na/H exchange and chloride-bicarbonate exchange and Na-P cotransport) and basolateral (e.g., Na-K-ATPase and Na-HCO3 cotransport) transporters 2-5.
The renin angiotensin system plays a pivotal role in the control of electrolyte and fluid balance and blood pressure. Under normal physiologic conditions, renal renin release and synthesis are tightly regulated. In the kidney, renin is primarily expressed in the juxtaglomerular cells of afferent arterioles, and its release and expression are regulated by numerous factors, including variations in dietary salt intake, renal arteriolar tone, macula densa-derived signals, sympathetic nervous system activity and hormones 6, 7. The macula densa regulates afferent arteriolar tone and renin production and release by sensing alterations in luminal NaCl 8, 9.
Intrarenal dopamine has been proposed to be a modulator of renal renin release, but the effects of dopamine on renal renin release are still incompletely understood. Dopamine has been shown to increase renin release in vitro in cultured juxtaglomerular cells or kidney cortical slices through activation of D1-like receptors 10-12. However, the effect of dopamine on renin release in vivo is far from resolved. Different in vivo studies have reported that dopamine can increase, decrease, or have no effect on renin release 13-19. The contradictory effects of dopamine on renin release in vivo may be related to differences in experimental design. Theoretically, dopamine could regulate renal renin release in vivo through at least 3 different mechanisms: 1) direct stimulation via activation of D1-like receptors as demonstrated in the in vitro studies 10-12; 2) indirect stimulation by decreasing blood pressure and/or afferent arteriolar tone 20, 21; or 3) indirect inhibition due to inhibiting proximal tubule salt reabsorption, with a subsequent increase of tubular NaCl delivery to the macula densa 8, 9.
Recent studies have suggested that cyclooxygenase-2 (COX-2) may represent another locally regulated system mediating renal salt and water homeostasis 6. In adult kidney cortex, COX-2 is predominantly restricted to the macula densa and adjacent cortical thick ascending limbs. Macula densa COX-2 stimulates renal renin expression and release 6, 20, 22.
Crosstalk exists between intrarenal dopaminergic and COX-2 systems. Our previous studies suggested that intrarenal dopamine tonically suppresses COX-2 expression in the macula densa via modulation of salt and fluid reabsorption in the proximal tubule 23. Therefore, our current studies were undertaken to investigate 1) dopamine regulation of renal renin release and synthesis in vivo and 2) the potential role of macula densa COX-2 in dopamine regulation of renal renin synthesis and release. Our results suggest that dopamine predominantly inhibits renal renin expression and release, but there is a residual stimulation of renal renin expression through activation of D1-like receptors when renal cortical COX-2 activity is suppressed.
METHODS
Animals
All animal experiments were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee of Vanderbilt University. Male Sprague-Dawley rats (4-6 wk old) were used, as renal cortical COX-2 expression is still relatively high at this age compared with adult animals 24. COX-2-/- mice on the 129/B6 background were originally generated by Dinchuk et al 25, and heterozygous breeding pairs were obtained from Jackson Laboratories (Bar Harbor, ME). Mice were genotyped by PCR as noted in our previous reports 26. Wild type and COMT-/- mice on the 129J/sv background were obtained from Dr. Maria Karayiorgou at Rockefeller University 27. Animals on low salt diets received a single injection of furosemide (1 mg/kg i.p.) before being placed on the low salt diet (0.02-0.03% NaCl, ICN Biochemicals, Irvine, CA). Other animals were maintained on high salt diets (8% NaCl, Research Diets, New Brunswick, NJ). The dopamine precursor L-DOPA was given in the drinking water (0.5 mg/ml) containing 0.1% L-ascorbic acid to prevent oxidation of L-DOPA (Sigma-Aldrich). The selective D1-like receptor agonist fenoldopam was given at a dose of 1 mg/kg via i.p. injection for 1 h treatment or at a dose of 2 mg/kg/day via osmotic minipump (2001, Alzet, Palo Alto, CA) implanted subcutaneously under sterile conditions and ether anesthesia for 1 week treatment 28. In addition, a subset of fenoldopam-treated COX-2-/- mice was given 0.5% NaCl in the drinking water to prevent potential volume depletion resulting from D1-like receptor-mediated natriuresis and diuresis. Acetazolamide has been reported to effectively inhibit bicarbonate reabsorption in the proximal tubule at a dose of 20 mg/kg (i.p.){DuBose, 1983 #484}. The selective COX-2 inhibitor SC58236 was a gift from Searle Monsanto Co. and was given at a dose of 2 mg/kg via i.p. injection.
Measurement of Kidney Dopamine
Dopamine and its metabolites were measured by HPLC coupled with electrochemical detection by the Neurochemistry Core Laboratory at Vanderbilt University's Center for Molecular Neuroscience Research 29.
Immunohistochemistry
At the termination of an experiment, one kidney from each animal was removed for western blot analysis and the other was perfused with fixative in situ for histology. Under deep anesthesia with Nembutal (70 mg/kg i.p.), the animal was first exsanguinated with heparinized saline (0.9% NaCl, 2 U/ml heparin, 0.02% sodium nitrite) through a transcardial aortic cannula and then fixed with 3.7% formaldehyde in an acidic solution (pH 4.5) containing phosphate, periodate, acetate, and sodium chloride as previously described 30. The fixed kidney was dehydrated through a graded series of ethanols, embedded in paraffin, sectioned (4 μm), and mounted on glass slides. Internal controls and comparisons were facilitated by creating compound blocks with multiple specimens that were sectioned and stained together. The kidney sections were immunostained with affinity-purified rabbit anti-murine COX-2 antibody (160126, Cayman Chemicals, Ann Arbor, MI) diluted to 2.5 μg/ml, or with rabbit anti-renin antiserum (1: 6,000 dilution, a generous gift from Prof. T. Inagami, Vanderbilt University). Vectastain ABC-Elite was used to localize the primary antibodies with a chromogen of oxidized diaminobenzidine, followed by a light toluidine blue counterstain.
Quantitative Image Analysis
Based on the distinctive density and color of renin-immunoreactivity in video images, the number, size, and position of stained cells were quantified using the BIOQUANT true-color windows system (R & M Biometrics, Nashville, TN) equipped with digital stage encoders that allow high-magnification images to be mapped to global coordinates throughout the whole section 24. Whole renal cortices from each section were quantified at 160× magnification. Sections from at least three regions of each kidney were analyzed, and their average immunoreactive renin area/cortex area was used as data from one animal sample.
Immunoblotting
Renal cortex was homogenized in 30 mmol/L Tris-HCl, pH 8.0, and 100 mmol/L phenylmethylsulfonyl fluoride (1:9 wt/vol). After a 10-min centrifugation at 10,000 g, the supernatant was centrifuged for 60 min at 110, 000 g to prepare microsomes, as described previously 31. The microsomes were resuspended in SDS-sample buffer and heated to 100°C for 5 min, and the protein was separated on 8% SDS-PAGE gels under reducing conditions and transferred to Immobilon-P transfer membranes. The blot was blocked overnight with 100 mmol/L Tris-HCl, pH 7.4, containing 5% nonfat dry milk and 0.1% Tween-20, followed by incubation for 16 h with 1 μg/ml affinity-purified rabbit anti-murine COX-2 antibody. The primary antibodies were detected with peroxidase-labeled goat anti-rabbit IgG (Santa Cruz Biotechnology) and exposed on film by using enhanced chemiluminescence (ECL, Amersham).
Blood Collection and Determination of Plasma Renin Activity (PRA)
Blood was taken from conscious mice via the femoral vein in the morning between 9:00 am to 11:00 am and collected into a microvette containing 2 μl 125 mM EDTA in its tip. The plasma was separated and frozen at -80°C until assayed. PRA was determined by radioimmunoassay (Gammacoat, DiaSorin, Stillwater, MN) as the generation of angiotensin I (ANG I) 20. Plasma samples were incubated for 1 h with excess exogenous renin substrate (plasma from rats nephrectomized 48 h before collection) to generate ANG I.
Micrography
Bright-field images from a Leitz Orthoplan microscope with DVC digital RGB video camera were digitized by the BIOQUANT image analysis system and saved as computer files. Contrast and color level adjustment (Adobe Photoshop) were performed for the entire image; i.e., no region- or object-specific editing or enhancements were performed.
Statistical Analysis
Values are presented as means ± s.d. ANOVA and Bonferroni t-test were used for statistical analysis, and differences were considered significant when P < 0.05.
RESULTS
Acute Fenoldopam Treatment Inhibits Renin Release
To investigate dopamine regulation of renal renin release, adult mice (male, 129/B6) were treated with vehicle (water) or the D1-like receptor agonist, fenoldopam (1 mg/kg, i.p.) for 1h, and then blood samples were collected for measurement of PRA 20. As shown in Figure 1A, acute fenoldopam treatment significantly reduced renal renin release (PRA: 1405 ± 230 vs. 2579 ± 455 ng ANG I/ml/hr of vehicle, P < 0.05, n = 5). Acute fenoldopam treatment also inhibited renin release in mice with low salt diets for a week [PRA (ng ANG I/ml/hr): normal salt: 2363 ± 473; low salt: 5853 ± 1825, P < 0.05 vs. normal salt; low salt plus fenoldopam: 3295 ± 232 P < 0.05 vs. normal salt and low salt groups, n = 4] (Figure 1B). One potential mechanism of fenoldopam-mediated inhibition of renin release is inhibition of proximal tubule salt reabsorption. In this regard, acute inhibition of proximal tubule salt reabsorption was induced by administering acetazolamide. As shown in Figure 1A, acute acetazolamide treatment (1 h, 20 mg/kg, i.p.) mimicked fenoldopam to reduce renin release (PRA: 1236 ± 400 vs. 2579 ± 455 ng ANG I/ml/hr of vehicle, P < 0.05, n = 4). To investigate whether fenoldopam or acetazolamide had additive effects, a subset of mice was treated with acetazolamide (20 mg/kg) and fenoldopam (1 mg/kg). As shown in Figure 1A, acetazolamide and fenoldopam reduced renin release to similar levels seen in mice treated with acetazolamide or fenoldopam alone (PRA: 1325 ± 153 ng ANG I/ml/hr, P > 0.05 vs. acetazolamide or fenoldopam treated mice, n = 4). In addition, inhibition of COX-2 activity with SC58236 (2 mg/kg) did not affect acetazolamide or fenoldopam induced inhibition of renin release (data not shown).
Figure 1.
Acute fenoldopam treatment inhibited plasma renin activity. A: Acute treatment with fenoldopam or acetazolamide alone or both of them inhibited plasma renin activity (PRA) similarly. *: P <0.05 vs. vehicle. B: Acute fenoldopam treatment also inhibited PRA in low salt treated animals. *: P < 0.05 vs. normal salt; †: P < 0.05 vs. low salt.
COMT-/- Mice Have Increased Endogenous Dopamine Levels in the Kidneys
In contrast to dopamine regulation of renin release, dopamine regulation of renal renin expression has been largely ignored. In our previous studies, we determined that either administration of the dopamine precursor, L-DOPA, or the D1-like agonist, SKF-81297, would inhibit macula densa COX-2 expression in the rats 23. Similar to COX-2 inhibition, our pilot studies indicated that renal renin expression was also inhibited in the rats treated with L-DOPA or SKF-81297 (data not shown). However, we were not able to rule out potential systemic or off target actions of these pharmacologic manipulations. COMT-/- mice, in which the major intrarenal dopamine-metabolizing enzyme has been deleted, provide an alternative experimental system 27, 32. As shown in Figure 2, COMT-/- mice had increased renal dopamine levels (184 ± 14 vs. 106 ± 18 ng/mg protein of wild type, P < 0.01, n = 6) and increased urinary dopamine excretion (7132 ± 999 vs. 3811 ± 1038 ng/24 h of wild type, P < 0.01, n = 6), along with decreased renal levels of 3-MT, the major dopamine metabolite in the kidney (12 ± 4 vs. 35 ± 8 ng/mg protein of wild type, P < 0.01, n = 6). Plasma dopamine concentrations, however, were comparable between wild type and COMT-/- mice (0.43 ± 0.11 vs. 0.38 ± 0.10 pg/ml of wild type, P > 0.05, n = 6). Therefore, the observed elevation of renal dopamine levels in COMT-/- mouse kidney are the result of absent COMT metabolism of dopamine in the kidney.
Figure 2.
COMT-/- mice exhibited increased renal dopamine levels. Compared with ageand sex-matched wild type mice, COMT-/- mice had increased kidney dopamine levels (A) and urinary dopamine excretion (B), but decreased renal levels of the dopamine metabolite, 3-MT (C). Plasma dopamine concentrations were similar among wild type and COMT-/- mice (D). *: P < 0.01 vs. wild type.
Low Salt-Induced Renal Cortical COX-2 Elevation Is Attenuated in COMT-/- Mice
COX-2 was not detectable by immunostaining in kidney cortex in adult control wild type and COMT-/- mice on a normal salt diet, consistent with previous reports 30, 33. After low salt treatment for 3 wk, macula densa COX-2 expression was significantly higher in low salt treated wild type mice than low salt treated COMT-/- mice (Figure 3A), consistent with our previous report that low salt-induced cortical COX-2 elevation was attenuated by increased intrarenal dopamine activity in the rats 23. Immunoblotting confirmed that renal cortical COX-2 expression was higher in low salt treated wild type than low salt treated COMT-/- mice (Figure 3B).
Figure 3.
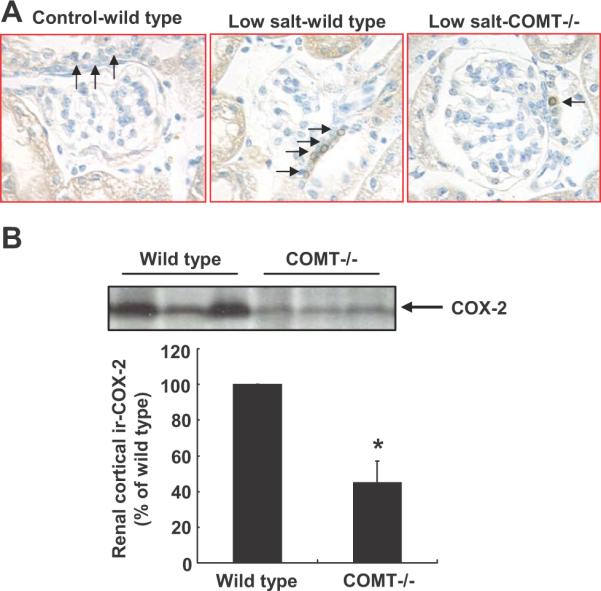
Low salt-induced increases in renal cortical COX-2 were attenuated in COMT-/- mice. A: On low salt diets, more epithelial cells of the macula densa were COX-2-positive in wild type mouse kidney than COMT-/- mouse kidney. Arrow: epithelial cells of the macula densa. Original magnification: 400×. B: Immunoblotting indicated higher renal cortical COX-2 expression in wild type than COMT-/- mice on low salt diets. *: P < 0.01, n = 3.
Low Salt-Induced Increases in Renal Renin Synthesis and Release Are Attenuated in COMT-/- Mice
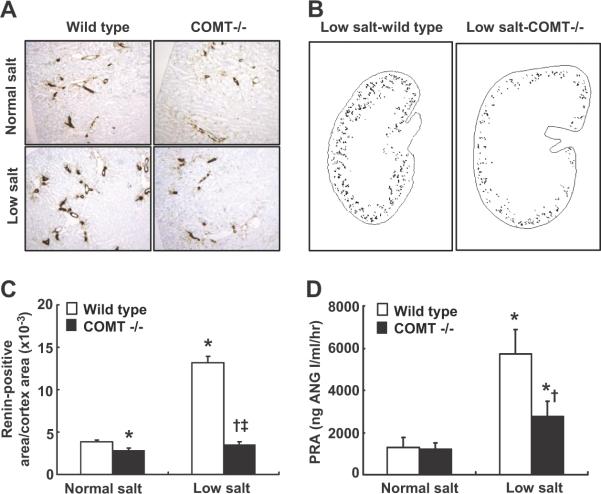
Renal renin expression was determined by immunostaining and was quantified with an image analysis system 24. As indicated in Figure 4 A&C, in mice on a normal salt diet, renal renin expression was significantly lower in COMT-/- than age- and gender-matched wild type mice (renin-positive area/cortex area ×10-3: 2.84 ± 0.22 vs. 3.84 ± 0.29 of wild type, P < 0.05, n = 4), indicating that renal renin expression may be tonically suppressed by the intrarenal dopaminergic system. Low salt treatment for 3 wk led to significant increases in expression of renal renin (250%) in wild type mice (renin-positive area/cortex area ×10-3: 13.16 ± 0.77, P < 0.01 vs. control wild type, n = 4), but less significant increases (30%) in COMT-/- mice (renin-positive area/cortex area ×10-3: 3.53 ± 0.30, P < 0.05 vs. control COMT-/- and P < 0.01 vs. low salt treated wild type, n = 4), resulting in 270% increase in renal renin expression in low salt treated wild type than low salt treated COMT-/- mice (Figure 4 A-C). In wild type mice, low salt induced increases in renal renin expression were attenuated by fenoldopam treatment for a week (renin-positive area/cortex area ×10-3: 8.25 ± 0.48, P < 0.05 vs. control and low salt treated wild type, n = 4). Similarly, low salt treatment also led to significant increases in plasma renin levels (340%) in wild type mice (PRA: 5703 ± 1177 vs. 1303 ± 475 ng ANG I/ml/hr of control, P < 0.01, n = 6), but less significant increases (130%) in COMT-/- mice (PRA: 2744 ± 747 vs. 1203 ± 309 ng ANG I/ml/hr of control, P < 0.01 vs. control COMT-/- and low salt treated wild type, n = 6) (Figure 4D). Therefore, low salt-induced increases in renal renin synthesis and release, as well as increases in renal cortical COX-2 expression, were attenuated in COMT-/- mice.
Figure 4.
Increases in renal renin expression and release in response to a low salt diet were attenuated in COMT-/- mice. A: Renal renin immunostaining in wild type and COMT-/- mice on normal salt or low salt diets. B: Representative topographic maps of renin immunostaining in kidneys from wild type and COMT-/- mice on low salt diets. C: Renal renin expression was lower in COMT-/- mice than wild type on normal salt diets. Renal renin elevation in response to a low salt diet was attenuated in COMT-/- mice. *: P < 0.05 vs. control wild type; †: P < 0.05 vs. control COMT-/-; ‡: P < 0.01 vs. low salt wild type. D: Low salt-induced PRA elevation was attenuated in COMT-/- mice. *: P < 0.01 vs. control wild type; †: P < 0.01 vs. low salt wild type.
Intrarenal Dopamine Stimulates Renal Renin Expression in High Salt Treated Animals
To investigate whether dopamine regulation of renal renin expression is influenced by dietary salt intake, COMT-/- and wild type mice were treated with a high salt diet for 2 wk and renal renin expression was measured. High salt treatment led to significant decreases in renal renin expression in wild type mice (renin-positive area/cortex area ×10-3: 0.86 ± 0.03 vs. 3.84 ± 0.29 of control wild type, P < 0.05, n = 4), but less significant decreases in COMT-/- mice (renin-positive area/cortex area ×10-3: 1.51 ± 0.25 vs. 2.84 ± 0.22 of control COMT-/-, P < 0.05, n = 4), resulting in 75% increase in renal renin expression in high salt treated COMT-/- mice than high salt treated wild type mice (Figure 5A).
Figure 5.
Dopamine stimulated renal renin expression when renal cortical COX-2 was inhibited. A: Renal renin immunostaining and topographic maps of renin immunostaining in wild type and COMT-/- mice on high salt diets. High salt led to significant decreases in renal renin in wild type mice, but less significant decreases in COMT-/- mice. *: P <0.05 vs. control wild type; †: P < 0.05 vs. control COMT-/-; ‡: P < 0.05 vs. high salt wild type. B: The dopamine D1-like receptor agonist fenoldopam inhibited renin expression in mice on a normal salt diet but increased renal renin expression in mice on a high salt diet. *: P <0.05 vs. normal salt diet; †: P < 0.05 vs. high salt diet. C: Renal renin expression was higher in fenoldopam treated COX-2-/- mice compared with untreated COX-2-/- mice, but was still much lower than untreated wild type mice. *: P <0.05 vs. untreated wild type; †: P < 0.05 vs. untreated COX-2-/-.
In further studies, wild type mice on a normal salt diet or wild type mice on a high salt diet for the second week were treated with fenoldopam for 7 d. As indicated in Figure 5B, fenoldopam treatment inhibited renal renin expression in mice on a normal salt diet, but increased renal renin expression in high salt treated mice (renin-positive area/cortex area ×10-3: control: 6.04 ± 1.60; fenoldopam: 3.8 ± 0.32, P < 0.05 vs. control; high salt: 1.56 ± 0.36, P < 0.05 vs. control; high salt plus fenoldopam: 3.32 ± 0.24, P < 0.05 vs. control and high salt, n = 4), suggesting that dopamine may stimulate renal renin expression via activation of D D1-like receptor in volume expanded conditions. Immunoreactive renal cortical COX-2 was undetectable in both control and fenoldopam treated animals on the high salt diet (data not shown).
Activation of D1-like Receptor Stimulates Renal Renin Expression in COX-2-/- Mice
The observation that fenoldopam stimulates renal renin expression in the setting of suppressed COX-2 expression and activity (high salt treatment) was investigated further using COX-2-/- mice. As shown in Figure 5C, renal renin expression was much lower at baseline in COX-2-/- than corresponding wild type mice on a normal salt diet. However, renal renin expression was significantly stimulated by fenoldopam in COX-2-/- mice, although levels were still significantly lower compared to those seen in wild type mice (renin-positive area/cortex area ×10-3: wild type: 8.61 ± 0.89; fenoldopam: 5.6 ± 1.0, P < 0.05 vs. wild type; COX-2-/-: 0.27 ± 0.12, P < 0.05 vs. wild type; fenoldopam treated COX-2-/-: 2.34 ± 0.21, P < 0.05 vs. wild type and untreated COX-2-/-, n = 4). To minimize any volume-depleting effect of fenoldopam, which could stimulate renin release 20, another group of fenoldopam-treated COX-2-/- mice had 0.5% NaCl added to the drinking water; this did not alter the fenoldopaminduced stimulation of renal renin expression.
DISCUSSION
The current studies were undertaken to investigate the effects of the intrarenal dopaminergic system on renal release and synthesis. The major findings include: 1) acute treatment with the D1-like agonist fenoldopam inhibited renin release; 2) increases in renal cortical COX-2 and renin expression and renin release in response to a low salt diet were attenuated in COMT-/- mice, which have increased intrarenal dopamine levels; 3) renal renin expression was higher in COMT-/- than wild type mice after high salt treatment; and 4) the D1-like agonist fenoldopam stimulated renal renin expression in high salt treated wild type mice or in COX-2-/- mice on a normal salt diet. Therefore, these results indicate that dopamine predominantly inhibits renal renin synthesis and release through its effects to inhibit COX-2 expression and activity, but prior inhibition of renal cortical COX-2 activity uncovers a smaller effect of dopamine to stimulate renin expression and release.
Dopamine receptors are divided into two subclasses: D1-like and D2-like receptors. D1-like receptors (D1 and D5) are coupled to Gs and stimulate adenylate cyclase, while D2-like receptors (D2, D3, and D4) are coupled to Gi and inhibit adenylate cyclase 2-5. Previous studies indicated that dopamine could increase renin release in cultured juxtaglomerular cells or renal cortical slices through activation of D1-like receptors 10-12. However, the role of dopamine in regulation of renin release in vivo is contradictory. Acute fenoldopam treatment has been reported to increase renin release in volunteers and in anesthetized dog 19, 21. In these experiments, increased renin release in response to acute fenoldopam treatment was associated with decreased blood pressure, suggesting that decreased blood pressure after acute fenoldopam treatment contributes to increased renin release. In contrast, in normal volunteers, acute gludopa (synthetic dipeptide γ-L-glutamyl-L-DOPA) treatment led to dramatic increases in urinary dopamine and sodium excretion without affecting blood pressure and led to significant suppression of renin release 13. Gludopa is enzymatically converted to L-DOPA locally by γ-glutamyltranspeptidase (γ-GT), an abundant enzyme in the brush border membrane of proximal tubule, and the L-DOPA is transported across renal brush border membrane and then further converted to dopamine by AADC 34.
Our previous studies indicated that macula densa COX-2 expression is suppressed by the intrarenal dopaminergic system 23. It is also noteworthy that we have previously reported that acetazolamide treatment inhibits macula densa COX-2 expression, even in the face of significant natriuresis and diuresis 23. In the current studies, macula densa COX-2 expression was also found to be significantly lower after low salt treatment in COMT-/- mice than wild type mice. Acetazolamide's diuretic actions are due to inhibition of carbonic anhydrase activity in the proximal tubule, which results in decreases in the proximal tubule salt reabsorption and increases in tubular NaCl delivery to the macula densa. Since acute acetazolamide treatment inhibited renin release, similar to what was observed with fenoldopam and in the COMT-/- mice, the overall inhibitory effect on renin release in mice with increased intrarenal dopamine production or by administration of a selective D1-like agonist underscores the importance of dopamine-mediated inhibition of proximal tubule salt reabsorption in renin release and indicates that dopamine inhibits renal renin expression by inhibiting macula densa COX-2 expression.
In contrast, in response to a high salt diet, COMT-/- mice had significantly less inhibition of renal renin compared with wild type mice, leading to a higher renal renin expression in high salt treated COMT-/- mice than high salt treated wild type mice. In high salt treated wild type mice, fenoldopam also stimulated renal renin expression. Furthermore, fenoldopam stimulated renal renin expression in COX-2-/- on a normal salt diet. These studies indicate that dopamine can stimulate renal renin expression under conditions in which renal cortical COX-2 activity is already inhibited.
Therefore, our data suggest that the intrarenal dopaminergic system may regulate renal renin expression through at least two different mechanisms: indirect inhibition via decreasing proximal salt reabsorption and modulating macula densa COX-2 expression and activity 23 and direct stimulation via activation of D1-like receptors 10-12. The overall effect of dopamine on renal renin expression may be a balance between the indirect inhibitory effects and direct stimulatory effects, with indirect inhibition being predominant in normal or volume depleted conditions but a small direct stimulation becoming evident in volume expanded conditions.
Clinical Perspectives
These studies point up the complexity and the tightly integrated control of renal regulation of net salt and water excretion and the renin-angiotensin II system. The predominant effect of the intrarenal dopaminergic system is to depress the renin-angiotensin system secondary to decreased proximal reabsorption and inhibition of macula densa-derived signals for renin expression and release. However, these studies elucidated a second effect of dopamine to stimulate renin, presumably by direct activation of juxtaglomerular renin production and release that is masked under normal conditions. Thus the intrarenal effects of dopamine are in many ways a mirror image to that of angiotensin II, which stimulates proximal reabsorption and therefore decreases NaCl delivery to the macula densa, but also directly feeds back on the juxtaglomerular apparatus to inhibit renin.
Acknowledgments
Sources of Funding These studies were supported in part by grants from the National Institutes of Health (DK62794, DK079341) and funds from the Veterans Administration.
Footnotes
Disclosures None.
References
- 1.Cheng HF, Becker BN, Harris RC. Dopamine decreases expression of type-1 angiotensin II receptors in renal proximal tubule. J Clin Invest. 1996;97:2745–2752. doi: 10.1172/JCI118729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aperia AC. Intrarenal dopamine: a key signal in the interactive regulation of sodium metabolism. Annu Rev Physiol. 2000;62:621–647. doi: 10.1146/annurev.physiol.62.1.621. [DOI] [PubMed] [Google Scholar]
- 3.Jose PA, Eisner GM, Felder RA. Dopamine and the kidney: a role in hypertension? Curr Opin Nephrol Hypertens. 2003;12:189–194. doi: 10.1097/00041552-200303000-00010. [DOI] [PubMed] [Google Scholar]
- 4.Pedrosa R, Jose PA, Soares-da-Silva P. Defective D1-like receptor-mediated inhibition of the Cl-/HCO3- exchanger in immortalized SHR proximal tubular epithelial cells. Am J Physiol Renal Physiol. 2004;286:F1120–1126. doi: 10.1152/ajprenal.00433.2003. [DOI] [PubMed] [Google Scholar]
- 5.Carey RM. Theodore Cooper Lecture: Renal dopamine system: paracrine regulator of sodium homeostasis and blood pressure. Hypertension. 2001;38:297–302. doi: 10.1161/hy0901.096422. [DOI] [PubMed] [Google Scholar]
- 6.Harris RC, Zhang MZ, Cheng HF. Cyclooxygenase-2 and the renal renin-angiotensin system. Acta Physiol Scand. 2004;181:543–547. doi: 10.1111/j.1365-201X.2004.01329.x. [DOI] [PubMed] [Google Scholar]
- 7.Kim SM, Chen L, Faulhaber-Walter R, Oppermann M, Huang Y, Mizel D, Briggs JP, Schnermann J. Regulation of Renin Secretion and Expression in Mice Deficient in {beta}1- and {beta}2-Adrenergic Receptors. Hypertension. 2007;50:103–109. doi: 10.1161/HYPERTENSIONAHA.107.087577. [DOI] [PubMed] [Google Scholar]
- 8.Schnermann J. Juxtaglomerular cell complex in the regulation of renal salt excretion. Am J Physiol. 1998;274:R263–279. doi: 10.1152/ajpregu.1998.274.2.R263. [DOI] [PubMed] [Google Scholar]
- 9.Schnermann J, Traynor T, Yang T, Arend L, Huang YG, Smart A, Briggs JP. Tubuloglomerular feedback: new concepts and developments. Kidney Int Suppl. 1998;67:S40–45. doi: 10.1046/j.1523-1755.1998.06708.x. [DOI] [PubMed] [Google Scholar]
- 10.Kurtz A, Della Bruna R, Pratz J, Cavero I. Rat juxtaglomerular cells are endowed with DA-1 dopamine receptors mediating renin release. J Cardiovasc Pharmacol. 1988;12:658–663. doi: 10.1097/00005344-198812000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Antonipillai I, Broers MI, Lang D. Evidence that specific dopamine-1 receptor activation is involved in dopamine-induced renin release. Hypertension. 1989;13:463–468. doi: 10.1161/01.hyp.13.5.463. [DOI] [PubMed] [Google Scholar]
- 12.Yamaguchi I, Yao L, Sanada H, Ozono R, Mouradian MM, Jose PA, Carey RM, Felder RA. Dopamine D1A Receptors and Renin Release in Rat Juxtaglomerular Cells. Hypertension. 1997;29:962–968. doi: 10.1161/01.hyp.29.4.962. [DOI] [PubMed] [Google Scholar]
- 13.Jeffrey RF, MacDonald TM, Marwick K, Lee MR. The effect of carbidopa and indomethacin on the renal response to gamma-L-glutamyl-L-dopa in normal man. Br J Clin Pharmacol. 1988;25:195–201. doi: 10.1111/j.1365-2125.1988.tb03291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Worth DP, Harvey JN, Brown J, Worral A, Lee MR. Domperidone treatment in man inhibits the fall in plasma renin activity induced by intravenous gamma-L-glutamyl-L-dopa. Br J Clin Pharmacol. 1986;21:497–502. doi: 10.1111/j.1365-2125.1986.tb02831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaulhausen H, Oney T, Leyendecker G. Inhibition of the renin--aldosterone axis and of prolactin secretion during pregnancy by L-dopa. Br J Obstet Gynaecol. 1982;89:483–488. doi: 10.1111/j.1471-0528.1982.tb03642.x. [DOI] [PubMed] [Google Scholar]
- 16.Vikse A, Bugge J, Dahl E, Kiil F. Dissociation between renal prostaglandin E2 and renin release. Effects of glucagon, dopamine and cyclic AMP in dogs. Acta Physiol Scand. 1985;125:619–626. doi: 10.1111/j.1748-1716.1985.tb07763.x. [DOI] [PubMed] [Google Scholar]
- 17.Harvey JN, Worth DP, Brown J, Lee MR. The effect of oral fenoldopam (SKF 82526-J), a peripheral dopamine receptor agonist, on blood pressure and renal function in normal man. Br J Clin Pharmacol. 1985;19:21–27. doi: 10.1111/j.1365-2125.1985.tb02608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mizoguchi H, Dzau VJ, Siwek LG, Barger AC. Effect of intrarenal administration of dopamine on renin release in conscious dogs. Am J Physiol. 1983;244:H39–45. doi: 10.1152/ajpheart.1983.244.1.H39. [DOI] [PubMed] [Google Scholar]
- 19.Montier F, Katchadourian P, Pratz J, Cavero I. Increase in plasma renin activity evoked by fenoldopam in dogs is directly mediated by dopamine1 receptor stimulation. J Cardiovasc Pharmacol. 1989;13:739–747. [PubMed] [Google Scholar]
- 20.Kim SM, Chen L, Mizel D, Huang YG, Briggs JP, Schnermann J. Low plasma renin and reduced renin secretory responses to acute stimuli in conscious COX-2-deficient mice. Am J Physiol Renal Physiol. 2007;292:F415–422. doi: 10.1152/ajprenal.00317.2006. [DOI] [PubMed] [Google Scholar]
- 21.Harvey JN, Worth DP, Brown J, Lee MR. Studies with fenoldopam, a dopamine receptor DA1 agonist, in essential hypertension. Br J Clin Pharmacol. 1986;21:53–61. doi: 10.1111/j.1365-2125.1986.tb02822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peti-Peterdi J, Komlosi P, Fuson AL, Guan Y, Schneider A, Qi Z, Redha R, Rosivall L, Breyer MD, Bell PD. Luminal NaCl delivery regulates basolateral PGE2 release from macula densa cells. J Clin Invest. 2003;112:76–82. doi: 10.1172/JCI18018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang MZ, Yao B, McKanna JA, Harris RC. Cross talk between the intrarenal dopaminergic and cyclooxygenase-2 systems. Am J Physiol Renal Physiol. 2005;288:F840–845. doi: 10.1152/ajprenal.00240.2004. [DOI] [PubMed] [Google Scholar]
- 24.Zhang MZ, Wang JL, Cheng HF, Harris RC, McKanna JA. Cyclooxygenase-2 in ratnephron development. Am J Physiol. 1997;273:F994–1002. doi: 10.1152/ajprenal.1997.273.6.F994. [DOI] [PubMed] [Google Scholar]
- 25.Dinchuk JE, Car BD, Focht RJ, Johnston JJ, Jaffee BD, Covington MB, Contel NR, Eng VM, Collins RJ, Czerniak PM, Gorry SA, Trzaskos JM. Renal abnormalities and an altered inflammatory response in mice lacking cyclooxygenase II. Nature. 1995;378:406–409. doi: 10.1038/378406a0. [DOI] [PubMed] [Google Scholar]
- 26.Cheng HF, Wang JL, Zhang MZ, Wang SW, McKanna JA, Harris RC. Genetic deletion ofCOX-2 prevents increased renin expression in response to ACE inhibition. Am J Physiol Renal Physiol. 2001;280:F449–456. doi: 10.1152/ajprenal.2001.280.3.F449. [DOI] [PubMed] [Google Scholar]
- 27.Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D, Karayiorgou M. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphi cchanges in catecholamine levels and behavior. Proc Natl Acad Sci USA. 1998;95:9991–9996. doi: 10.1073/pnas.95.17.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Murphy MB, Murray C, Shorten GD. Fenoldopam--A Selective Peripheral Dopamine-Receptor Agonist for the Treatment of Severe Hypertension. N Engl J Med. 2001;345:1548–1557. doi: 10.1056/NEJMra010253. [DOI] [PubMed] [Google Scholar]
- 29.Perez FA, Palmiter RD. Parkin-deficient mice are not a robust model of parkinsonism. Proc Natl Acad Sci U S A. 2005;102:2174–2179. doi: 10.1073/pnas.0409598102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang MZ, Yao B, Cheng HF, Wang SW, Inagami T, Harris RC. Renal cortical cyclooxygenase 2 expression is differentially regulated by angiotensin II AT(1) and AT(2) receptors. Proc Natl Acad Sci U S A. 2006;103:16045–16050. doi: 10.1073/pnas.0602176103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harris RC, McKanna JA, Akai Y, Jacobson HR, Dubois RN, Breyer MD. Cyclooxygenase-2 is associated with the macula densa of rat kidney and increases with salt restriction. J Clin Invest. 1994;94:2504–2510. doi: 10.1172/JCI117620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Helkamaa T, Mannisto PT, Rauhala P, Cheng ZJ, Finckenberg P, Huotari M, Gogos JA, Karayiorgou M, Mervaala EM. Resistance to salt-induced hypertension in catechol-O-methyltransferase-gene-disrupted mice. J Hypertens. 2003;21:2365–2374. doi: 10.1097/00004872-200312000-00026. [DOI] [PubMed] [Google Scholar]
- 33.Wagner C, Vitzthum H, Castrop H, Schumacher K, Bucher M, Albertin S, Coffman TM, Arendshorst WJ, Kurtz A. Differential regulation of renin and Cox-2 expression in the renal cortex of C57Bl/6 mice. Pflugers Arch. 2003;447:214–222. doi: 10.1007/s00424-003-1157-1. [DOI] [PubMed] [Google Scholar]
- 34.de Toledo FG, Thompson MA, Bolliger C, Tyce GM, Dousa TP. gamma-L-glutamyl-L-DOPA inhibits Na(+)-phosphate cotransport across renal brush border membranes and increases renal excretion of phosphate. Kidney Int. 1999;55:1832–1842. doi: 10.1046/j.1523-1755.1999.00419.x. [DOI] [PubMed] [Google Scholar]