Abstract
The temperature-dependence of the crystalline phase of (nitrosyl)(tetraphenylpor-phinato)iron(II), [Fe(TPP)(NO)], has been explored over the temperature range of 33 to 293 K. The crystalline complex is found in the tetragonal crystal system at higher temperatures and in the triclinic crystal system at lower temperatures. In the tetragonal system, the axial ligand is strongly disordered with the molecule having crystallographically required 4/m symmetry leading to eight distinct positions of the single nitrosyl oxygen atom. The phase transition to the triclinic crystal system leads to a partial ordering with the molecule now having inversion symmetry and disorder of the axial nitrosyl ligand over only two positions. The increase in ordering allows subtle molecular geometry features to be observed, in particular an off-axis tilt of the Fe–NNO bond from the heme normal is apparent. The transition of the reversible phase change begins at about 250 K. This transition has been confirmed by both X-ray diffraction studies and a differential scanning calorimetry study.
Introduction
The perception and understanding of nitric oxide has changed from that of merely a toxic gas to that of an essential cellular signaling agent.1 NO coordination to heme proteins has many physiological consequences. It has been implicated in a number of fundamental processes including smooth muscle relaxation,2, 3 platelet deaggregation,4 neuronal communication,5 aspects of myocardial function, and numerous other physiological functions.6 NO is synthesized by nitric oxide synthase through a heme-mediated oxidation of L-arginine7, 8, 9 or from .10 In many of these biochemical processes, the binding of NO to a heme protein and the labilization or loss of the ligand trans to the NO or another rearrangement is the significant signalling event resulting in a five-coordinate heme-NO active complex.
Five-coordinate [Fe(TPP)(NO)]11 was originally characterized in this laboratory in 1975.12 Although this was more than a decade before the known biological importance of NO, this investigation provided the first insight into the coordination chemistry of NO to heme. Some of the structural features were obscured as a result of the 4/m symmetry of the molecular center in the I4/m crystal system. The molecule had an eight-fold disorder of the NO oxygen, an Fe–NNO bond exactly perpendicular to the porphyrin plane, and phenyl rings rotated exactly 90° from the porphyrin plane. Subsequent structural investigations of well-resolved heme-NO complexes utilizing octaethylporphyrin more precisely detailed the geometrical parameters of the heme-NO complex.13, 14 Some of the more salient observations were the off-axis tilting of the Fe–NNO vector from the heme normal. An equatorial asymmetry observed in the Fe–Np distances with a short pair of bonds bracketing the projection of the tilted and bent NO ligand onto the porphyrin core. These observations were further noted and the electronic basis for the tilt and the equatorial asymmetry was subsequently recognized in several DFT calculations.15, 16, 17, 18.
In solid-state vibrational studies utilizing the NRVS technique,19 a substantial temperature-dependent variation in the Fe–NNO stretch was noted.20 A low-temperature crystallographic study of [Fe(TPP)(NO)] showed that there was an apparent phase change. This led to the substantial temperature-dependent study of the phase change and the resulting phenomenon that accompanies the phase change. The resulting order-disorder phase change, which is completely reversible, is reported herein. The phase change also leads to the observation that, at low temperatures, the structure of [Fe(TPP)(NO)] shows the off-axis tilting of the Fe–NNO bond seen in a number of other iron nitrosyl species.
Experimental Section
General Information
All reactions were carried out under anaerobic conditions using standard Schlenk techniques under an argon atmosphere. All solvents were freeze/pump/thaw degassed (×3) prior to use. Methanol (Acros) and pyridine (Fisher) were used as received. Nitric oxide (Mittler Specialty Gases) was purified by passing it through a trap containing 4 Å molecular sieves immersed in an ethanol/dry ice slurry.21 Free base porphyrin [H2TPP] was prepared according to Adler et al.22 [Fe(TPP)(Cl)] was prepared according to the metalation procedure of Adler et al.23 IR measurements were taken on a Nicolet Nexus 670 FT-IR spectrometer. Selected single crystals were ground/mixed minimally with KBr. An approximately 50:1 (KBr:[Fe(TPP)(NO)]) mixture was formed into a pellet using a hydraulic press.
Synthesis of [Fe(TPP)(NO)]
[Fe(TPP)(NO)] was prepared using a modification of the previously reported synthesis.12 A solution of 100 mg of [Fe(TPP)(Cl)], 3 mL of chloroform, 0.2 mL of methanol and 0.05 mL of pyridine was placed in a Schlenk flask and freeze/pump/thaw degassed (×3). The solution was then purged with argon for 5 min followed by bubbling of NO for approximately 5 min. Upon addition of NO the solution turns from brown to brown-orange and the final solution may contain some undissolved material. [Fe(TPP)(NO)] was then precipitated by addition of 100 mL of methanol with thorough stirring. The [Fe(TPP)(NO)] precipitate was isolated by filtration onto sintered glass. Crystals of [Fe(TPP)(NO)] were prepared by dissolving twenty-five milligrams (0.04 mmol) of the isolated solid in 7 mL of chloroform and carefully layering the solution in 8 mm glass tubes with degassed methanol followed by flame sealing. X-ray quality crystals of [Fe(TPP)(NO)] were isolated after 10 d at room temperature. IR νNO in KBr: 1700 cm−1. Note: if KBr is not thoroughly dried, to > 130 °C, an additional νNO band is present at 1677 cm−1. This band is irreversibly converted on heating of the KBr pellet (to the 1700 peak).
X-Ray Crystallographic Studies
Four distinct single crystals were indexed at 8 temperatures in ascending and descending order using a Bruker D8 Apex II system, with graphite-monochromated Mo Kα (λ = 0.71073 Å) radiation from 33–293 K (CRYO Industries or 700 Series Oxford Cryostream).24 Crystals at 273, 290 and 293 K could be indexed as tetragonal (I-centered), whereas crystals at 33, 90, 100, 130 and 180 K could be indexed in the triclinic crystal system in the space group P1̄. Cell parameters and crystal domains were further investigated using cell now.26 All examples of the triclinic phase are composed of four domains related by ~90 or ~180° rotations about the primitive triclinic [1, 0, 0] axis.27.
Eleven data sets were collected and the programs SADABS28 or TWINABS29 were applied for absorption correction. All structures were solved using direct methods as implemented in XS30 and refined using XL.30 All atoms were found after successive full-matrix least-squares refinement cycles on F2 and refined with anisotropic thermal parameters. Hydrogen atoms were placed at calculated geometries and allowed to ride on the position of the parent atom. Hydrogen thermal parameters were set to 1.2 × the equivalent isotropic U of the parent atom.
Crystal structure data collected at 273, 290, and 293 K were refined in the I4/m space group where the molecule has required 4/m symmetry. The iron and nitrosyl nitrogen atoms are disordered over two positions equally occupied as required by the mirror plane that lies in the porphyrin plane. The nitrosyl oxygen atom is disordered over eight positions, four positions above and four positions below the porphyrin plane, equally occupied as required by the fourfold axis along the Fe–NNO vector. The porphyrin phenyl groups are rotated 90° from the porphyrin plane with required mirror symmetry.
Crystal structure data collected at 33, 90, 100, 130, and 180 K were refined in P1̄, Z = 1. However, alternatively they may be described in a nonstandard setting, I1̄, Z = 2,27 to more conveniently compare with the structures described in I4/m. The Fe–N–O unit is disordered over two positions equally occupied as required by the inversion symmetry. The four domains of the triclinic crystals are related by rotations about the [1, 0, 0] axis, which is coincident with the original Fe–NNO bond.
Powder Diffraction Studies
Powder X-ray Diffraction (XRD) patterns were obtained on a Bruker Smart Apex I CCD diffractometer with graphite-monochromated Cu Kα (λ = 1.54178 Å) radiation from 100–300 K (700 Series Oxford Cryostream). Samples were mulled in Apiezon M vacuum grease, mounted on a glass fiber, and transferred to the cold gas stream of the diffractometer. Samples were equilibrated for 5 min at each temperature before data was collected. Data were collected with the 2D Apex detector fixed at 100 mm, 2θ = 12°, ϕ = 0°, and ω scanned between 0–20° over a 5 min. interval. Data were collected with both increasing and decreasing temperature. Data were integrated using the XRD2 plug-in in the APEX II suite of programs31 and further analyzed using the program EVA.32.
DSC Measurements
The differential scanning calorimetry (DSC) measurements were performed at 150 to 400 K with 10 K/min heating rate using a Netzsch STA 404 DSC. Cooling was provided by liquid nitrogen boil-off. Temperature calibration was performed at the melting points of n-hexane, cyclohexane, octane, triply deionized water, gallium, naphthalene, indium, tin, and zinc. The scans were carried out in an atmosphere of flowing He (40 mL/min). A standard 40 µL aluminum crucible with a lid, which could be sealed hermetically with a crucible sealing press, was used for all experiments. Crucibles and lids were heated before experiments at about 570 K for 15 minutes to convert any hydroxides of aluminium that had been formed on the crucible surface to aluminum oxide. All DSC measurements were performed on 8–12 mg samples sealed under atmospheric conditions. Onset temperatures and enthalpies were deter-mined from 2 individual measurements using a software package supplied by the manufacturer. Reproducibility was confirmed using Setaram DSC 111.
Results
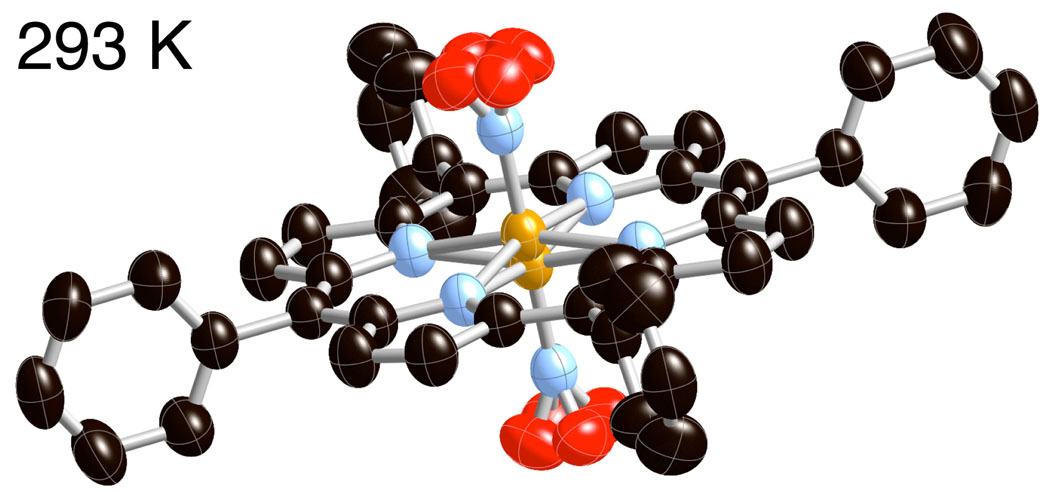
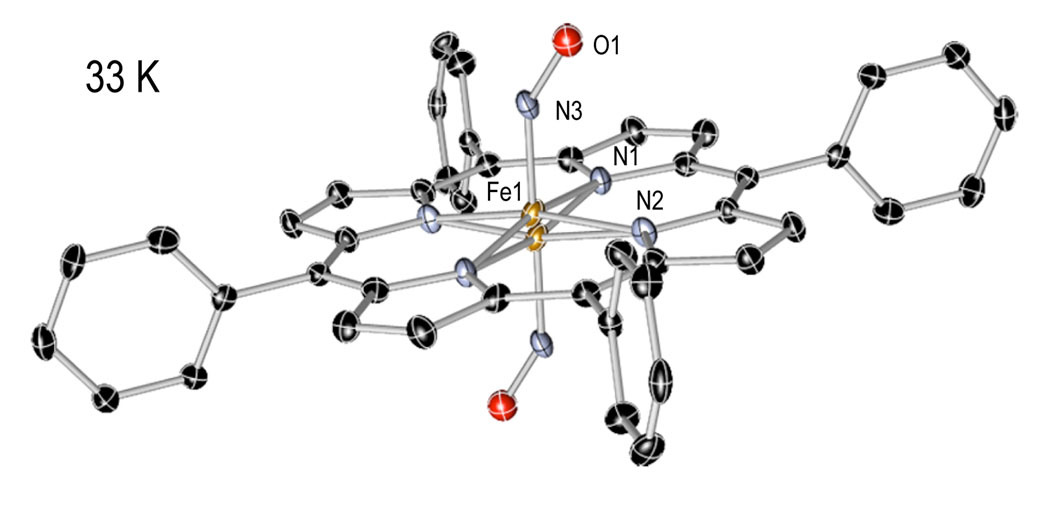
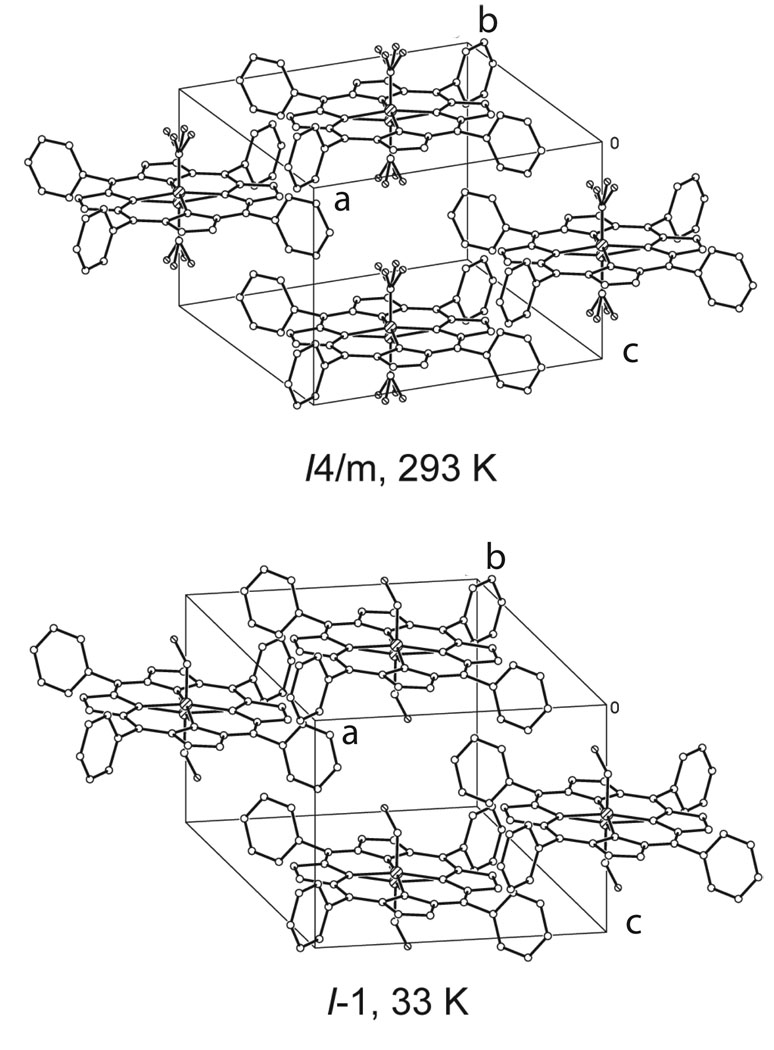
Crystal and molecular structures of [Fe(TPP)(NO)] have been obtained at 33, 90, 100, 130, 180, 273, 290, and 293 K on four different crystalline specimens. A reversible phase change from the tetragonal crystal system to the the triclinic crystal system was identified. Crystal lographic details for all eleven structure determinations are summarized in Table 3. Thermal ellipsoid plots of [Fe(TPP)(NO)] at 33 K and 290 K are illustrated in Figure 1 and Figure 2. At 293 K [Fe(TPP)(NO)] has eight equally occupied NO orientations consistent with the previously reported structure at 298 K.12 At 33 K [Fe(TPP)(NO)] has two equally occupied NO orientations consistent with the triclinic system. A number of diffraction experiments confirm the phase change that is also characterized by differential scanning calorimetry. Figure 3 gives representations of the I-centered cells at 33 and 293 K.
Table 3.
Molecule center to center distances and symmetry operators for [Fe(TPP)(NO)] in the I1̄ (33 K) and I4/m (293 K) phases from crystal 1.
| symmetry position |
x, y, z |
I1̄ Ct⋯Ct Å |
I4/m Ct⋯Ct Å |
Difference Å |
|---|---|---|---|---|
| x, y, z | 1/2, 1/2, 0 | 0 | 0 | 0 |
| x, y, z + 1 | 1/2, 1/2, 1 | 9.745 | 9.769 | −0.024 |
| x, y, z − 1 | 1/2, 1/2, −1 | 9.745 | 9.769 | −0.024 |
| −x, −y, −z | 1, 1, 1/2 | 9.867 | 10.707 | −0.840 |
| −x − 1, −y − 1, −z − 1 | 0, 0, −1/2 | 9.867 | 10.707 | −0.840 |
| −x, −y −1, −z | 1, 0, 1/2 | 10.973 | 10.707 | 0.266 |
| −x − 1, −y, −z − 1 | 0, 1, −1/2 | 10.973 | 10.707 | 0.266 |
| −x − 1, −y, −z | 0, 1, 1/2 | 10.375 | 10.707 | −0.332 |
| −x, −y −1,−z − 1 | 1, 0, −1/2 | 10.375 | 10.707 | −0.332 |
| −x − 1, −y − 1, −z | 0, 0, 1/2 | 11.256 | 10.707 | 0.549 |
| −x, −y, −z − 1 | 1, 1, −1/2 | 11.256 | 10.707 | 0.549 |
| x + 1, y, z | 3/2, 1/2, 0 | 13.373 | 13.475 | −0.102 |
| x − 1, y, z | −1/2, 1/2, 0 | 13.373 | 13.475 | −0.102 |
| x, y + 1, z | 1/2, 3/2, 0 | 13.353 | 13.475 | −0.122 |
| x, y − 1, z | 1/2, −1/2, 0 | 13.353 | 13.475 | −0.122 |
(Ct⋯Ct in I1̄) − (Ct⋯Ct in I4/m).
Figure 1.
Thermal ellipsoid plot of [Fe(TPP)(NO)] at 293 K (50% probability ellipsoids). Hydrogen atoms are omitted for clarity.
Figure 2.
Thermal ellipsoid plot of [Fe(TPP)(NO)] at 33 K (50% probability ellipsoids). Hydrogen atoms are omitted for clarity.
Figure 3.
Diagrams of the tetragonal and triclinic I-centered cells showing the effects of the partial order-disorder phase transition. At 33 K the Fe–NO group retains the disorder with respect to crystallographic inversion but the disorder of the NO group due to fourfold symmetry has been removed. In the tetragonal phase the porphyrin plane is perpendicular to the c axis, but is tipped by 9.3° from the perpendicular to the c axis in the triclinic phase.
Discussion
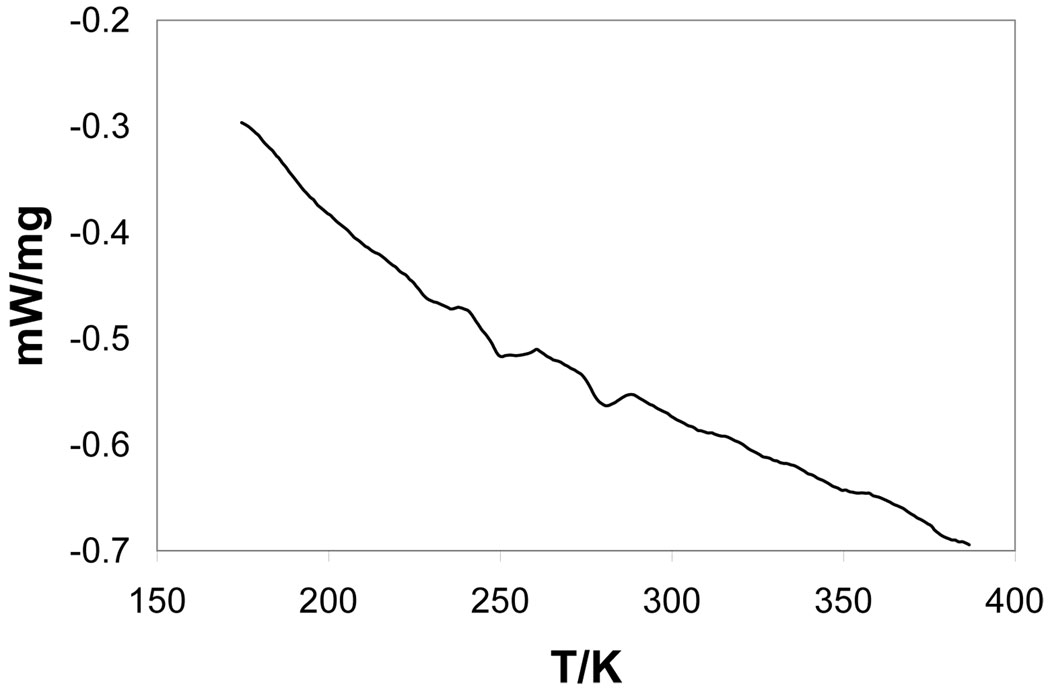
Crystalline [Fe(TPP)(NO)] displays temperature-dependent phases that change from a tetragonal I-centered room-temperature phase to a triclinic phase at reduced temperatures. The triclinic phase can be described in terms of an I-centered triclinic cell that is a nonstandard crystallographic choice or as a standard primitive triclinic cell. The transition from room temperature to reduced temperature has been followed by differential scanning calorimetry. The DSC scan is illustrated in Figure 4. Two endotherms are observed that are centered at 280.4 K and 249.2 ± 0.2 K. The lower temperature transition is the one associated with the crystallo graphic phase change. The basis of the higher temperature transition is unclear; perhaps it is associated with a modest change in the porphyrin ring conformation. However, the structure determinations (at 273 and 293 K) that bracket this endotherm do not show any distinct, different features. We are thus unable to offer a conclusive explanation for this higher temperature transition.
Figure 4.
DSC scan for [Fe(TPP)(NO)]. The endotherms are centered at 280.4 and 249.2 ± 0.2 K. The lower temperature transition corresponds to the observed crystallographic phase change.
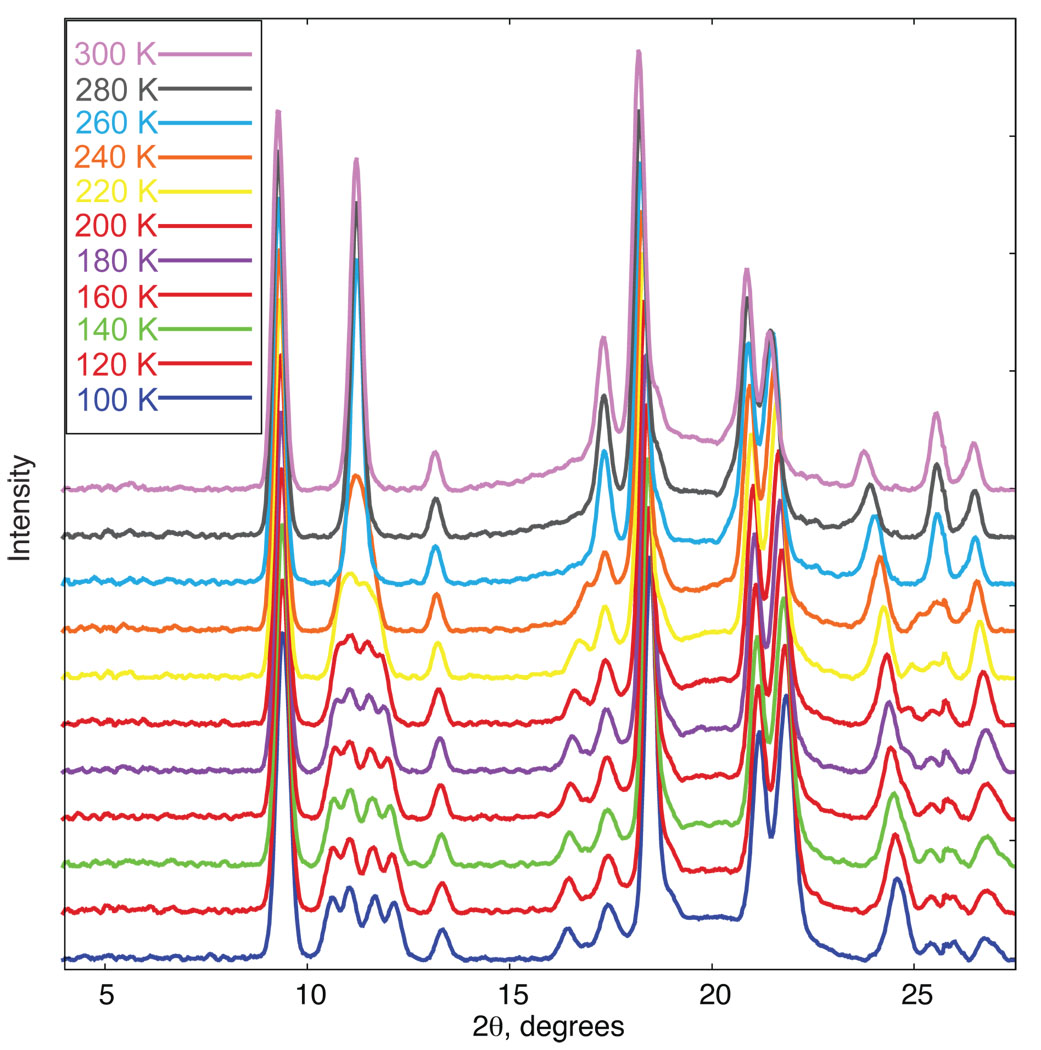
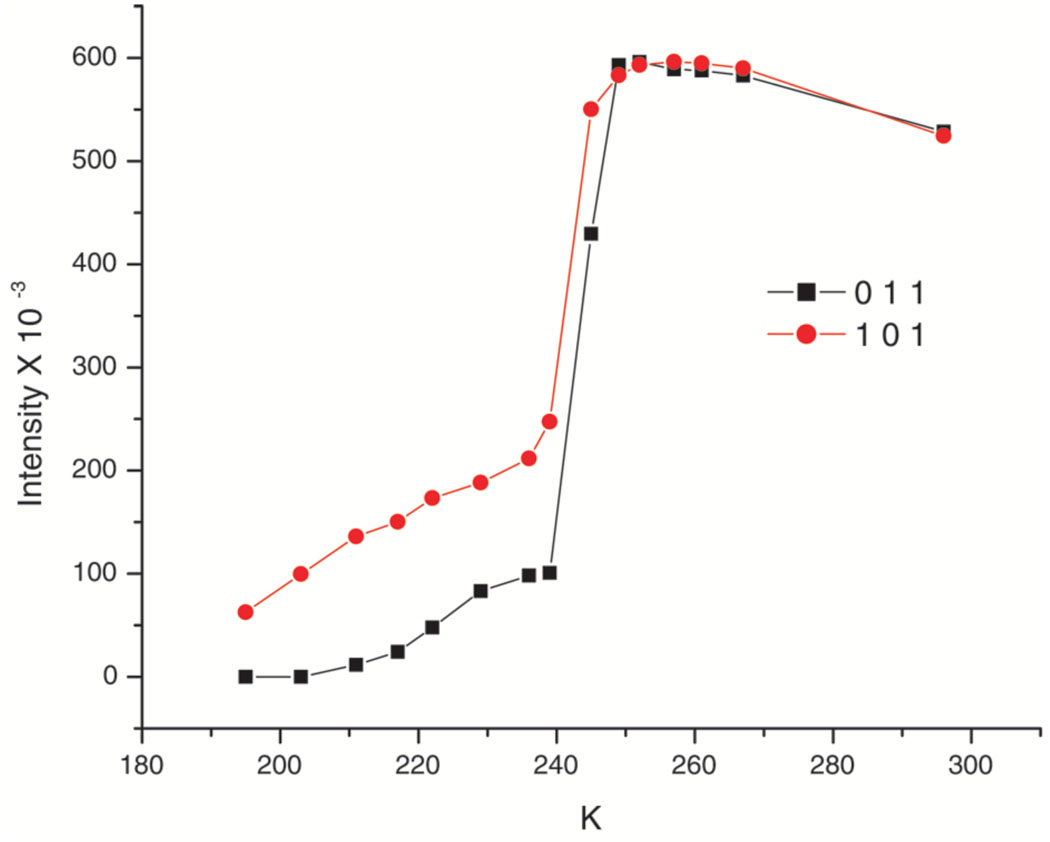
We have also followed the phase change by examining the diffraction pattern over a range of temperatures. Figure S1 displays the predicted powder diffraction patterns at 100 and 290 K (Cu Kα radiation) that are based on the cell constants obtained from single crystals at those temperatures. The tetragonal reflection 101 (at 11.25° 2θ) cleanly splits into four resolved reflections on the decrease in crystal system symmetry. Accordingly, we focused on that region in a temperature-dependent powder diffraction measurement. Figure 5 displays the actual powder diffraction traces observed every 20° from 300 to 100 K. Figure S2 displays the change in intensity at the 11.25° 2θ position for both ascending and descending temperature and Figure 6 shows the intensity change for two triclinic reflections that are Laue equivalents in the tetragonal phase. Although the transition begins sharply at about 249 K, there does appear to be an asymmetry in the transition as the temperature is decreased which approaches the final complete change asymptotically. This behavior is also seen in two other regions where peaks in the two phases are sufficiently resolved to allow a measurement. These are the tetragonal reflection 231 (2θ = 25.57°) and 121 (2θ = 17.36°). These are also illustrated in Figure S2 of the SI. The calculation of the inflection point, also shown on the figure, are all at essentially the same temperature (243 to 248 K). A single-crystal data collection taken at 242 K, could not be indexed solely in terms of either the triclinic cell or the tetragonal cell but rather only in terms of both lattices. There may also be a slight asymmetry in the phase change between ascending and descending temperatures, but the complete reversibility is to be emphasized.
Figure 5.
X-ray powder diffraction spectra of [Fe(TPP)NO)] from 100 to 300 K at 20 degree increments.
Figure 6.
A plot of measured intensities on a single crystal of Fe(TPP)(NO). Above the transition point the reflections (0 1 1) and (1 0 1) are Laue equivalents in the tetragonal cell and the quality of the data sets for the two phases appear similar. They become inequivalent at the phase change near 24 °C. The decrease in the intensities at the higher temperature can be ascribed to the effects of thermal motion.
We have also measured the reflection profile of the (3 1 0) reflection on a point detector diffractometer (ω-scans). As the temperature is lowered, the peak begins to broaden between 252 and 248 K. The peak begins to split at 245 K and at 235 is well resolved into two peaks. Little further change occurs below 212 K. These ω-scan plots are given in Figure S3 of the SI. Single-crystal data collected at 180 K or lower are readily indexed in the triclinic cell and that at 273 K or higher in the tetragonal cell and the quality of the data sets appears to be similar in both crystal systems. Before describing the differences in the crystal structure between the two phases, we first describe the structure of [Fe(TPP)(NO)] in the two phases.
Molecular Structure of [Fe(TPP)(NO)] - I4/m Phase
The molecular structure of the room temperature phase of [Fe(TPP)(NO)] has been previously reported.12 The complex’s molecular structure agrees well with that previously reported. Complete details of all structure determinations in the tetragonal phase are given in the SI.
As displayed in Figure 1, the NO ligand is disordered over eight positions. The 4/m symmetry at the molecular center necessitates equal occupation of each of the eight positions. One common feature of iron(II) heme nitrosyls is the tilting of the Fe–NNO vector.13, 14 However, the nitrogen atom of NO is located on the 4-fold axis making any evaluation of ligand tilt impossible and the angle is nominally equal to 0°. Past experience suggests that if the off-axis tilts were similar to those observed previously,14 the displacement of the NO nitrogen atom is just at the limit of leaving a trace in the thermal ellipsoid of the N atom. Any small changes in the position of nitrogen caused by the eight orientations make it very difficult to resolve a nitrogen position where the Fe–N vector is tilted off the heme normal. The uncertainties in the N–O bond length and FeNO bond angle are further accentuated by the uncertainty in the nitrogen position. The porphyrin ring is required to be precisely planar with the peripheral phenyl group making a dihedral angle of 90° with the central plane.
Molecular Structure of [Fe(TPP)(NO)] - P1̄ Phase
The low-temperature phase of [Fe(TPP)(NO)] was refined in the P1̄ setting. The fourfold symmetry observed in the tetragonal phase is reduced to inversion symmetry in the triclinic phase and hence, the NO oxygen is disordered only over two positions related by inversion symmetry. An ORTEP diagram of [Fe(TPP)(NO)] at 33 K is given in Figure 2. The ordering allows for a better description of the iron coordination parameters. The elimination of the NO rotational disordering reveals one hallmark feature of Fe(II) nitrosyl porphyrinates.33 This feature is the off-axis tilting of the Fe–NNO from the heme normal in the direction of the NO oxygen atom. At 33 K, the Fe–NNO vector is tilted 6.3° off the heme normal in the direction of the NO oxygen atom. The Fe–NNO off-axis tilts and several additional bonding parameters for [Fe(Por)(NO)] and related complexes are given in Table 2.34–37 The tilt is also usually associated with an asymmetry in the equatorial Fe-Np bonds. The two Fe–Np bonds that bracket the NO ligand are shorter than the average whereas the other two are longer. This asymmetry is clearly a bonding effect. There is only a hint of this asymmetry for triclinic [Fe(TPP)(NO)]. At 33 K, the two short Fe–Np bonds are 1.995(9) Å and 1.997(9) Å, whereas the long Fe–Np bonds are 2.000(9) Å and 2.004(9) Å. The presence of the inversion center is likely to have led to obscuring of any in-plane asymmetry.
Table 2.
Notable Bonding Parameters for [Fe(TPP)(NO)] and related compounds.
| Complex, temp., and crystal |
Fe–XYa | X–Ya | Fe–X–Yb | <Fe–Np¿a | ΔFed | Fe–N, tiltb |
νX–Yc | ref |
|---|---|---|---|---|---|---|---|---|
| [Fe(TPP)(NO)], 33 K, 1 | 1.739(6) | 1.163(5) | 144.4(5) | 1.999(4) | 0.20 | 6.3 | tw | |
| [Fe(TPP)(NO)], 90 K, 1 | 1.740(5) | 1.153(4) | 145.6(4) | 1.997(13) | 0.20 | 5.8 | tw | |
| [Fe(TPP)(NO)], 180 K, 1 | 1.737(5) | 1.131(4) | 147.3(4) | 2.002(14) | 0.21 | 4.7 | tw | |
| [Fe(TPP)(NO)], 293 K, 1 | 1.720(6) | 1.107(11) | 149.5(7) | 2.0005 | 0.23 | e | 1670/1700f | tw |
| [Fe(TPP)(NO)] | 1.722(2) | 1.167(3) | 144.4(2) | 2.004(15) | 0.29 | e | 1670f | 12 |
| [Fe(TPPBr4)(NO)](A′) | 1.734(8) | 1.119(11) | 147.9(8) | 2.01(3) | 0.37 | 5.6 | 1678g | 14 |
| [Fe(TPPBr4)(NO)](A″) | 1.726(9) | 1.144(12) | 146.9(9) | 2.00(2) | 0.32 | 7.1 | 1678g | 14 |
| [Fe(TPPBr4)(NO)](B) | 1.691(11) | 1.145(16) | 145(1) | 1.95(3) | 0.29 | 1681g | 14 | |
| [Fe(OEP)(NO)](A) | 1.722(2) | 1.167(3) | 144.4(2) | 2.004(15) | 0.29 | 6.5 | 1666g | 13,14 |
| [Fe(OEP)(NO)](B) | 1.7307(7) | 1.1677(11) | 142.74(8) | 2.009(12) | 0.27 | 8.2 | 1673g | 13,14 |
| [Fe(oxoOEC)(NO)] | 1.7320(13) | 1.1696(19) | 143.11(15) | 2.009(9) | 0.26 | 7.1 | 1690g | 14 |
| [Fe(OETAP)(NO)] | 1.721(4) | 1.155(5) | 143.7(4) | 1.932(9) | 0.31 | 7.6 | 1666g | 34 |
| [Fe(OEP)(NO)]+ | 1.6528(13) | 1.140(2) | 173.19(13) | 1.994(5) | 0.32 | 4.6 | 1838g | 35 |
| [Fe(OEP)(NO)]+ | 1.644(3) | 1.112(4) | 176.9(3) | 1.994(1) | 0.29 | 0.6 | 1868g | 36 |
| [Fe(OEP)(CO)] | 1.7140(11) | 1.1463(12) | 177.20(8) | 1.988(2) | 0.20 | 3.8 | 1944g | 37 |
| [Fe(OEP)(CO)]·C6H6 | 1.7077(13) | 1.1259(16) | 177.20(11) | 1.984(3) | 0.22 | 2.4 | 1948g | 37 |
| [Fe(OEP)(CO)2] | 1.8558(10) | 1.1216(13) | 173.95(9) | 2.0133(7) | 0.00 | 5.9 | 2021g | 37 |
Å
degrees.
cm−1.
Displacement from 24-atom mean plane.
Value not determined, obscured by crystallographic symmetry.
KBr pellet.
Nujol mull.
The porphyrin plane is required to be precisely planar in the tetragonal phase; in the triclinic phase there is no such requirement, but the core remains effectively planar with the largest core atom displacement from the best 24-atom plane only 0.03 Å (at 33 K). The out-of-plane displacement of iron is 0.20 Å, slightly less than seen in the tetragonal phase. The peripheral phenyl group, required to have exactly orthogonal dihedral angles in the tetragonal phase, have rotated slightly with dihedral angles between the phenyl plane and the core plane of 83.1 and 81.4°.
Phase Change
Why does the phase change occur? The phase change does not appear to lead to a substantial increase in the solid-state packing efficiency. The decrease in cell volume of ~3.5% between 90 and 293 K is similar to those seen in a number of other complexes over a similar temperature change and for which no phase change is observed.38 Rather, the phase change appears to result from the fact that the [Fe(TPP)(NO)] molecule is intrinsically not as symmetric as it appears (and is required) in the tetragonal phase. This is partly, if not entirely, the consequence of the intrinsic off-axis tilt of the Fe–NNO vector that has been observed in all well-ordered (nitrosyl)iron porphyrinates. One explanation of the phase change is that it leads to an order–disorder transition that is sometimes observed on crystal cooling.39 In this view, the intrinsically lower symmetry of the molecule is favored even though its solid-state structure does not pack with significantly larger efficiency. Nonetheless, the solid-state interaction of the porphyrin molecules display significant changes, as shown below.
An alternative idea is that the axial ligand distortion plays a fundamental role. As the temperature is decreased the Fe–NO off-axis tilt become increasingly favored. When the distor-tion occurs, the entire lattice will make small adjustments in the intermolecular packing that leads to the descent to triclinic symmetry and a single Fe–NO orientation above the porphyrin plane. That the NO tilting is driving the phase change is consistent with the observation of four crystal domains, with relative rotations of either 90 or 180° with respect to the major domain. The rotations are along the original Fe–NO axis, consistent with the four possible tilt direction choices. Of course a similar change must occur on the other side of the porphyrin plane since that side of the porphyrin plane is also populated in the tetragonal phase. The reasons for the preservation of an inversion center at the porphyrin ring center is that it is the most symmetric of the possible orientations of the FeNO group and leads to the preservation of the intrinsic NO-induced asymmetry of the Fe interaction with the porphyrin nitrogen atoms (core asymmetry).
The phase change leads to asymmetry in the environment of an individual [Fe(TPP)(NO)] molecule. The I-centered triclinic cell constants are a = 13.373(3), b = 13.353(4), c = 9.745(2) Å, α = 99.310(17), β = 93.651(15), and γ = 90.64(2)° and are clearly similar to those of the tetragonal phase. This has been shown in Figure 3. In the triclinic system the porphyrin planes are in the xy plane, and form parallel layers of porphyrin molecules comparable to the planes parallel to the xy plane in the tetragonal phase. However, in the tetragonal phase the porphyrin layers are exactly superimposed, but in the triclinic phase the molecules are no longer directly above each other as a result of the 9.3° tilt of the porphyrin planes from the c axis. Distances between molecular centers reflect this change. The fourteen closest intermolecular center⋯center distances in both crystal systems are given in Table 3. The closest pair of contacts to the target molecule remain the pair related by a translation perpendicular to the molecular array direction. The next eight closest contacts are all equal in the tetragonal system. In the triclinic system, the analogously related intermolecular distances are either shorter or longer and fall along the the body diagonals in pairs and are summarized in Table 3. One of the consequences of the resulting asymmetry is that the intermolecular contacts now constrain the FeNO group to a single allowed orientation on each side of the porphyrin plane in the triclinic phase. This also allows the observation of the off-axis tilt of the Fe–NO vector in the triclinic phase that, if present in the tetragonal system, will be obscured by the crystallographically required symmetry.
Summary
Crystalline [Fe(TPP)(NO)] is found to undergo a reversible temperature-dependent phase change from tetragonal to triclinic. In the lower symmetry crystal system, the intrinsic off-axis tilt of the Fe–NO vector from the porphyrin plane becomes apparent. Two suggestions for the origin of the phase change are given.
Supplementary Material
Figure S1, simulated powder diffraction data based on single-crystal diffraction experiments, Figure S2, single-reflection intensity/temperature-dependence plots and the first derivative of temperature-dependence plots, Figure S3, reflection profiles. Figures S4–S14, thermal ellipsoid plots. Figure S15, formal diagrams displaying the perpendicular displacement of core atoms from the 24-atom mean planes at all measured temperatures; Figure S16, stereo diagrams of the two cells; Tables S1–S66, give complete crystallographic details, atomic coordinates, bond distances and angles, anisotropic temperature factors, and hydrogen positions for [Fe(TPP)(NO)] at all measured temperatures. Crystallographic data is available as CIF files. This material is available free of charge via the Internet at http://pubs.acs.org.
Table 1.
Summary of Crystallographic Data for [Fe(TPP)(NO)] at several temperatures.
| temp, K, cryst. # |
a, Å | b, Å | c, Å | α, deg | β, deg | γ, deg | V/Z, Å3 | unique data |
obs. dataa |
final R |
final wR2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 33, 1b | 9.745(2) | 9.867(2) | 10.375(3) | 82.66(2) | 66.01(2) | 70.05(2) | 856.7(4) | 4221 | 2476 | 0.0486 | 0.0884 |
| 90, 1b | 9.754(2) | 9.943(2) | 10.394(3) | 82.25(2) | 65.78(2) | 69.44(2) | 855.81(4) | 4337 | 2843 | 0.0476 | 0.1084 |
| 100, 2b | 9.7310(8) | 9.9238(8) | 10.3902(6) | 82.195(1) | 65.693(4) | 69.384(5) | 855.8(1) | 6497 | 4766 | 0.0449 | 0.1213 |
| 100, 3b | 9.734(3) | 9.960(3) | 10.391(3) | 82.100(3) | 65.63(2) | 69.25(2) | 858.0(4) | 5092 | 4085 | 0.0788 | 0.1979 |
| 100, 4b | 9.7201(7) | 9.9105(6) | 10.3715(6) | 82.304(4) | 65.802(4) | 69.546(4) | 853.81(9) | 6741 | 4480 | 0.0638 | 0.1543 |
| 130, 4b | 9.7394(10) | 9.9634(10) | 10.3998(10) | 82.078(4) | 65.661(4) | 69.225(4) | 859.65(15) | 4103 | 3204 | 0.0537 | 0.1354 |
| 180, 1b | 9.765(2) | 10.120(2) | 10.466(2) | 81.35(2) | 65.23(2) | 68.12(2) | 871.4(4) | 4628 | 2579 | 0.0481 | 0.0981 |
| 273, 4c | 13.4152(2) | 13.4152(2) | 9.7151(3) | 90 | 90 | 90 | 874.2(7) | 1101 | 918 | 0.0391 | 0.1132 |
| 290, 2c | 13.4466(3) | 13.4466(3) | 9.7498(5) | 90 | 90 | 90 | 881.43(5) | 1155 | 973 | 0.0340 | 0.0996 |
| 293, 1c | 13.4748(12) | 13.4748(12) | 9.7686(18) | 90 | 90 | 90 | 886.9(2) | 1081 | 842 | 0.0351 | 0.0989 |
| 293, 2c | 13.4502(3) | 13.4502(3) | 9.7533(4) | 90 | 90 | 90 | 882.23(5) | 1108 | 881 | 0.0372 | 0.1024 |
| 293,0c,d | 13.468(4) | 13.468(4) | 9.755(8) | 90 | 90 | 90 | 951 | 0.044 |
Acknowledgments
We thank the National Institutes of Health for support of this research under Grant GM-38401 (W.R.S) and the NSF for X-ray instrumentation support through Grant CHE-0443233. M.M.O. thanks the the University of California., Davis for the He diffraction setup. We acknowledge Olga Trofymluk, Department of Chemistry, Thermochemistry Facility, University of California, Davis for providing assistance with the DSC results. The thoughtful remarks of the reviewers helped significantly in the final form of this paper.
References and Notes
- 1.Culotta E, Koshland DE. Science. 1992;258:1862. doi: 10.1126/science.1361684. [DOI] [PubMed] [Google Scholar]
- 2.Rapoport RM, Murad F. Circ. Res. 1983;52:352. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- 3.Ignarro LJ, Adams JB, Horwitz PM, Wood KS. J. Biol. Chem. 1986;261:4997. [PubMed] [Google Scholar]
- 4.Azuma H, Ishikawa M, Sekizaki S. Br. J. Pharmacol. 1986;88:411. doi: 10.1111/j.1476-5381.1986.tb10218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Furlong B, Henderson AH, Lewis MJ, Smith JA. Br. J. Pharmacol. 1987;90:687. doi: 10.1111/j.1476-5381.1987.tb11221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]; Radomski MW, Palmer RMJ, Moncada S. Br. J. Pharmacol. 1987;92:639. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garthwaite J. Trends Neurosci. 1991;14:60. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- 6.Koshland DE., Jr Science. 1992;258:1861. doi: 10.1126/science.1470903. [DOI] [PubMed] [Google Scholar]; Butler AR, Williams DLH. Chem. Soc. Rev. 1993:233. [Google Scholar]; Moncada S, Palmer RMJ, Higgs EA. Pharmacol. Rev. 1991;43:109. [PubMed] [Google Scholar]
- 7.Marletta MA. J. Biol. Chem. 1993;17:12231. [PubMed] [Google Scholar]
- 8.Griffith OW, Stuehr DJ. Annu. Rev. Physiol. 1995;57:707. doi: 10.1146/annurev.ph.57.030195.003423. [DOI] [PubMed] [Google Scholar]
- 9.Alderton WK, Cooper CE, Knowles RG. Biochem. J. 2001;357:593. doi: 10.1042/0264-6021:3570593. [DOI] [PMC free article] [PubMed] [Google Scholar]; Griffith OW, Stuehr DJ. Annu. Rev. Physiol. 1995;57:707. doi: 10.1146/annurev.ph.57.030195.003423. [DOI] [PubMed] [Google Scholar]
- 10.Averill BA. Chem. Rev. 1996;96:2951. doi: 10.1021/cr950056p. [DOI] [PubMed] [Google Scholar]; Eich RF, Li T, Lemon DD, Doherty DH, Curry SR, Aitken JF, Mathews AJ, Johnson KA, Smith RD, Phillips GN, Jr., Olson JS. Biochemistry. 1996;35:6976. doi: 10.1021/bi960442g. [DOI] [PubMed] [Google Scholar]
- 11.The following abbreviation ts used in this paper. OEP, dianion of octaethylporphyrin; oxoOEC dianion of oxooctaethylchlorin, (3,3,7,8,12,13,17,18-octaethyl-(3H)-porphin-2-onato(2-); OETAP, dianion of octaethyltetraphenylporphyrin; TPP, dianion of meso-tetraphenylporphyrin; TPPBr4, dianion of 2,3,12,13-tetrabromortetraahenylporhyrin; Np, porphinato nitrogen atom.
- 12.Scheidt WR, Frisse ME. J. Am. Chem. Soc. 1975;97:17. doi: 10.1021/ja00834a005. [DOI] [PubMed] [Google Scholar]
- 13.Ellison MK, Scheidt WR. J. Am. Chem. Soc. 1997;119:7404. [Google Scholar]
- 14.Scheidt WR, Duval HF, Neal TJ, Ellison MK. J. Am. Chem. Soc. 2000;122:4651. [Google Scholar]
- 15.Leu BM, Zgierski MZ, Wyllie GRA, Scheidt WR, Sturhahn W, Alp EE, Durbin SM, Sage JT. J. Am. Chem. Soc. 2004;126:4211. doi: 10.1021/ja038526h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Praneeth VKK, Näther C, Peters G, Lehnert N. Inorg. Chem. 2006;45:2795. doi: 10.1021/ic050865j. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh A, Wondimagegn T. J. Am. Chem. Soc. 2000;122:8101. [Google Scholar]; Ghosh A. Acc. Chem. Res. 2005;38:943. doi: 10.1021/ar050121+. [DOI] [PubMed] [Google Scholar]
- 18.Cheng L, Novozhilova I, Kim C, Kovalevsky A, Bagley KA, Coppens P, Richter-Addo GB. J. Am. Chem. Soc. 2000;122:7142. [Google Scholar]
- 19.Scheidt WR, Durbin SM, Sage JT. J. Inorg. Biochem. 2005;99:60. doi: 10.1016/j.jinorgbio.2004.11.004. [DOI] [PubMed] [Google Scholar]
- 20.Silvernail NJ, Sage JT, Alp EE, Sturhahn W, Zhang Z, Scheidt WR. There does not appear to be a relationship in these phenomena. work in progress. [Google Scholar]
- 21.Dodd RE, Robinson PL. Experimental Inorganic Chemistry. New York: Elsevier; 1957. p. 253. [Google Scholar]
- 22.Adler AD, Longo FR, Finarelli JD, Goldmacher J, Assour J, Korsakoff L. J. Org. Chem. 1967;32:476. [Google Scholar]
- 23.Adler AD, Longo FR, Kampus F, Kim J. J. Inorg. Nucl. Chem. 1970;32:2443. [Google Scholar]
- 24.The Oxford control unit is calibrated at the factory using the phase change of Rochelle’s salt at 109 K.25 The factory calibration was checked in our laboratory with a well-insulated iron-constantan thermocouple and an Omega 199 temperature meter. All reported temperatures are believed accurate to within 2 K.
- 25.Tomaszewski PE. Phase Trans. 1992;38:127. [Google Scholar]
- 26.Sheldrick GM. cell_now. Madison, WI: Bruker-Nonius AXS; 2001. [Google Scholar]
-
27.The transformation matrix (M) converts P1̄, Z = 1 to I1̄, Z = 2.
The rotations in the triclinic I-centered cell are about the c-axis. - 28.Sheldrick GM. SADABS. Göttingen, Germany: Universität Göttingen; 2006. [Google Scholar]
- 29.Sheldrick GM. TWINABS. Göttingen, Germany: Universität Göttingen; 2006. [Google Scholar]
- 30.Sheldrick GM. Acta Cryst. 2008;A64:112. [Google Scholar]
- 31.APEX-II. Madison, WI: Bruker-Nonius AXS; 2004. [Google Scholar]
- 32.EVA. Madison, WI: Bruker-Nonius AXS; 2002. [Google Scholar]
- 33.Wyllie GRA, Scheidt WR. Chem. Rev. 2002;102:1067. doi: 10.1021/cr000080p. [DOI] [PubMed] [Google Scholar]
- 34.Bohle DS, Debrunner P, Fitzgerald J, Hansert B, Hung C-H, Thompson AJ. J. Chem. Soc., Chem. Commun. 1997:91. [Google Scholar]
- 35.Ellison MK, Schulz CE, Scheidt WR. Inorg. Chem. 2000;39:5102. doi: 10.1021/ic000789e. [DOI] [PubMed] [Google Scholar]
- 36.Scheidt WR, Lee YJ, Hatano K. J. Am. Chem. Soc. 1984;106:3191. [Google Scholar]
- 37.Silvernail NJ, Noll BC, Scheidt WR. Inorg. Chem. 2006;45:7050. doi: 10.1021/ic0613356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silvernail NJ, Pavlik JW, Noll BC, Schulz CE, Scheidt WR. Inorg. Chem. 2008;47:912. doi: 10.1021/ic701700p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kubicki M. Acta Crystallogr., Sect. B. 2004;B60:333. doi: 10.1107/S0108768104005853. and references therein. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1, simulated powder diffraction data based on single-crystal diffraction experiments, Figure S2, single-reflection intensity/temperature-dependence plots and the first derivative of temperature-dependence plots, Figure S3, reflection profiles. Figures S4–S14, thermal ellipsoid plots. Figure S15, formal diagrams displaying the perpendicular displacement of core atoms from the 24-atom mean planes at all measured temperatures; Figure S16, stereo diagrams of the two cells; Tables S1–S66, give complete crystallographic details, atomic coordinates, bond distances and angles, anisotropic temperature factors, and hydrogen positions for [Fe(TPP)(NO)] at all measured temperatures. Crystallographic data is available as CIF files. This material is available free of charge via the Internet at http://pubs.acs.org.