Abstract
The ex vivo induction of alloantigen-specific hyporesponsiveness by costimulatory pathway blockade or exposure to immunoregulatory cytokines has been shown to inhibit proliferation, IL-2 production, and the GVHD capacity of adoptively transferred T-cells. We hypothesized that inhibition of the intracellular NF-κB pathway in alloreactive T-cells, which is critical for T cell activation events including IL-2 transcription, could lead to alloantigen hyporesponsiveness and loss of GVHD capacity. We demonstrate that treatment of mixed lymphocyte reaction (MLR) cultures with PS1145, a potent inhibitor of NF-κB activation, can induce T cell hyporesponsiveness to alloantigen in primary and secondary responses while preserving in vitro responses to potent mitogenic stimulation. GVHD lethality in recipients of ex vivo PS1145-treated cells was profoundly inhibited. Parking of control- or PS1145- treated MLR cells in syngeneic Rag−/− recipients resulted in intact contact hypersensitivity responses. However, GVHD lethality capacity also was restored, suggesting that lymphopenic expansion uncoupled alloantigen hyporesponsiveness. These results indicate that the NF-κB pathway is a critical regulator of alloresponses and provide a novel small molecule inhibitor based approach that is effective in preventing early post-transplant GVHD lethality but that also permits donor T cell responses to recover after a period of lymphopenic expansion.
Keywords: immune tolerance, alloantigens, allogeneic, nuclear factor - kappa B (NF-kB), allograft tolreance, alloreactive T cells, allorecognition, allotransplantation, T cell, bone marrow transplantation, CD4+ T cells, T cell signalling, donor T-cell, transplantation tolerance, graft-versus-host disease (GVHD)
Introduction
Graft-versus-host disease (GVHD) remains a significant cause of morbidity and mortality following allogeneic hematopoietic cell transplantation (HCT). Despite conventional immunosuppressive approaches used to prevent GVHD, the incidence of clinically significant acute GVHD remains high (30–75%), especially for recipients with matched unrelated donors (1–4). While T cell depletion of the donor graft reduces GVHD lethality, it can also result in increased relapse rate, graft failure, and susceptibility to opportunistic infections (5–7). Thus, there is a clear need to develop new strategies that increase the safety of allogeneic HCT without compromising immune reconstitution.
As an alternative to conventional therapies in which donor T-cells are either globally suppressed in vivo by pharmacological agents or removed in vitro, alloreactive donor T-cells may be specifically rendered hyporesponsive to host alloantigens prior to in vivo infusion. Alloantigen-reactive T-cells are present in a low frequency and can be rendered hyporesponsive when exposed to alloantigen-bearing cells in a mixed lymphocyte reaction (MLR) under tolerizing conditions (8, 9). Ex vivo tolerance induction strategies have shown promise in limiting GVHD lethality in murine models and in human clinical trials (8–15). During the process of tolerance induction, the remaining non-alloreactive T-cells, such as anti-viral T-cells, are not functionally altered as tolerization requires T cell receptor (TCR) ligation. Thus, ex vivo tolerance induction, may be used to prevent GVHD while leaving donor T-cells that do not participate in GVHD available to respond to tumor and foreign antigens.
A fully functional T cell response requires ligation of the antigen-specific TCR and the additional supplementary or costimulatory signals typically provided by antigen-presenting cells (APCs) (16). Following TCR ligation and CD28 costimulation of normal T cell activation, T-cells become activated and produce IL-2 (16). Ex vivo tolerance induction therapies are based on the observation that suboptimal TCR stimulation, which fails to induce IL-2 gene transcription or cell cycle progression, will render such T-cells unable to be restimulated by the same antigen (17–19). Previously described approaches for inducing tolerance ex vivo for GVHD protection have relied on costimulatory blockade (9, 10). The biochemical connection between CD28 costimulation and IL-2 transcription is well defined, as the promoter of the IL-2 gene contains a CD28 response element with binding sites for several transcriptional regulators including NF-κB (20). Thus, pharmacologic blockade of NF-κB signaling in TCR activated cells would mimic the signaling defect induced by costimulatory blockade and serve as a direct means of tolerance induction in antigen-activated alloreactive T-cells.
Activation and nuclear translocation of NF-κB via CD28-dependent pathways requires phosphorylation of IκB by the IκB kinase (IKK) complex (21–26). Human mutations in IKK complex genes result in several clinical manifestations, including T cell immunodeficiency (27–29). Because this step is critical and non-redundant in the activation of NF-κB, we chose to block NF-κB activation with PS1145, a small molecule inhibitor of IKK. PS1145 has previously been shown to inhibit NF-κB activation in multiple myeloma cells through inhibition of IκB phosphorylation (30).
We hypothesized that treatment with PS1145 during activation of donor T-cells with recipient alloantigen would result a reduced donor T cell capacity for causing GVHD, while permitting responses to nominal antigen exposure. Our data supports this hypothesis and identifies a critical role for NF-κB signaling during allogeneic T cell responses. Furthermore, strategies that selectively the NF-κB pathway in pathogenic T-cells have potential clinical application for the prevention of GVHD and other T cell mediated diseases.
Methods
Mice
B6.C.H2bm12/KhEg (bm12), CBySmn.CB17-PrkdcSCID/J (BALB/c SCID, B6.CB17-PrkdcSCID/SzJ (B6 SCID), C3H SCID and B6.Rag-1−/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME). BALB/c SCID mice were bred with B6 SCID mice to generate (BALB/c × B6 SCID) F1 (CB6F1) mice. BALB/c and C57BL/6 (B6) mice were purchased from the National Institutes of Health (Bethesda, MD). All mice were housed in a specific pathogen-free facility according to NIH guidelines.
Mixed lymphocyte reactions
CD4+ T-cells were isolated as previously described (31). Purity of CD4+ T-cells was routinely ≥95%. Whole T-cells were isolated from lymph nodes using PE-labeled antibodies for CD19, DX5 and γδ TCR followed by anti-PE bead incubation and magnetic bead separation (Miltenyi Biotech, Bergisch-Gladbach, Germany). CD25+ T-cells were depleted with PE-labeled anti-CD25 (PC61) mAb (Pharmingen), followed by incubation with anti-PE beads (Miltenyi), and removal by MACS LS columns (Miltenyi). CD4+CD25− T cell purity was ≥98%. Responder T-cells were mixed with irradiated (30 Gy), T- and NK-cell depleted splenic stimulators, prepared as described previously (31). Responders and stimulators were cultured at 37°C and 10% CO2 for 4–9 days at a final concentration of 0.57×106/mL in 24-well plates (Costar, Acton, MA) in DMEM (BioWhittaker, Walkersville, MD) complete media (32). 20 mM stocks of PS1145 (Millennium Pharmaceuticals, Cambridge, MA) were prepared in dimethyl sulfoxide (DMSO) and added to cultures at final concentrations as indicated. In some experiments, cells were pre-treated with 6 or 10 µM PS1145 for 30 minutes at 4°C and washed extensively before establishing cultures. To monitor primary and secondary responses, 105 responders and stimulators per well were cultured in 96-well round-bottom microtiter plates (Costar) and recombinant human IL-2 was added as indicated (Amgen). In some experiments, In other experiments, dead and dying cells were removed using an Annexin V microbead kit (Miltenyi). Where indicated, anti-CD3ε/anti-CD28 mAb-coated microspheres (33) (Dr. Carl June, Philadelphia, PA) were used to stimulate recovered T-cells after treatment with vehicle or PS1145 in a primary MLR culture. Cells were pulsed with tritiated thymidine (1 µCi/well) (Amersham Life Sciences, Buckinghamshire, United Kingdom) on the indicated days for 16 to 18 hours prior to harvesting and counted in the absence of scintillation fluid on a β-plate reader (Packard Instrument Company, Meriden, CT) and analyzed in at least triplicate.
CFSE staining and flow cytometry
Freshly purified T-cells were stained with 1 µM CFSE (Molecular Probes, Eugene, OR) at room temperature for 2 minutes with shaking, followed by washing 2X with cold 2% FCS in PBS. CD4 and CD8 mAbs were purchased from Pharmingen (San Diego, CA) and staining was done according to manufacturer’s protocol. Determination of cell viability was performed using Annexin V (Pharmingen) according to the manufacturer’s protocol. All flow cytometric experiments were done using a FACSCalibur (Becton Dickinson, San Jose, CA).
Confocal Microscopy
Purified CD4+ T-cells were stimulated with anti-CD3ε/anti-CD28 mAb-coated microspheres at a 1:1 ratio for 24 hours. After washing, cells were dried on microscope slides with a Cytospin 2 centrifuge (Shandon), fixed with 4% paraformaldehyde for 5 minutes and permeabilized with 0.5% Tween for 15 minutes. After blocking for 30 minutes with 10% normal donkey serum in PBS cells were stained with 1:50 rabbit anti-p65 mAb (Santa Cruz) for one hour at room temperature, washed, and incubated with Cy3-conjugated donkey anti-rabbit mAb (1:500 in PBS/0.5% Tween). For acquisition, a Melles Griot 543 nm HeNe laser and Olympus BX61 laser scan confocal microscope equipped with a 100x oil immersion objective was used.
Electrophoretic Mobility Shift Assay (EMSA)
Nuclear extracts were prepared from day 7 MLR cultures, incubated with α-32P-labeled NF-κB consensus sequence probe and EMSA was performed as previously described (34).
Induction of GVHD
To test B6 anti-bm12 CD4+ T cell tolerance induction, bm12 recipients were sublethally irradiated (6 Gy total body irradiation (TBI)) and 105 freshly isolated B6 CD4+ T-cells or 105 viable day 7 cultured cells were injected into the lateral tail vein. To test Balb/c anti-B6 whole T cell tolerance induction, NK cell-depleted CB6 F1 SCID recipients were irradiated (2.1 Gy TBI) and 105 viable day 5 cultured cells were injected into the lateral tail vein. NK cell depletion was continued 2×/week throughout the experiment using a purified NK1.1 mAb (PK136, 10 µg/g).
Parking of MLR-cultured cells and assessment of immune responses
2 × 106 freshly isolated B6 CD4+ T-cells or day 7 B6 anti-bm12 control- or PS1145 (10 µM)-treated MLR cells were injected into B6.Rag-1−/−mice. After 30 days, mice were sensitized with 0.15 ml 3% 4-ethoxymethylene-2-phenyloxazolone (oxazolone) (Sigma; St. Louis, MO) in ethanol:acetone (3:1) on shaved thoracic and abdominal skin and then rechallenged 6 days later on the right ear with 0.025 ml 1% oxazolone in acetone:olive oil (4:1) to induce a contact hypersensitivity (CH) response (35). 24 and 96 hours after challenge, swelling in the right ear was measured with a digital microcaliper (Fowler; Newton, MA). In other studies, splenic CD4+ T-cells (3 × 104) were adoptively transferred into 6 Gy TBI conditioned bm12 or NK-depleted, 1.6 TBI conditioned third party C3H SCID recipients.
Statistics
Group comparisons were made by Student's t test. Survival data were analyzed by lifetable methods and actuarial survival rates are shown. Probability (P) values < 0.05 were considered significant.
Results
PS1145 inhibits NF-κB activation and proliferation of alloreactive T-cells in MLR cultures
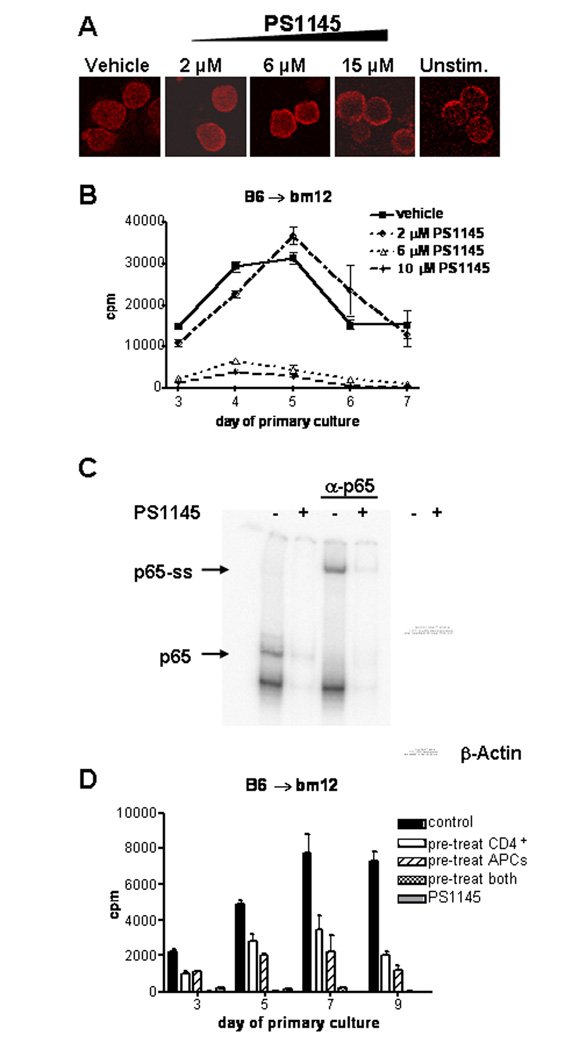
PS1145 has been shown to be an inhibitor of the NF-κB pathway in tumor cells. To determine the effect of various concentrations of PS1145 on NF-κB localization in primary T-cells, we used confocal fluorescence microscopy to assess nuclear localization of NF-κB following mitogenic stimulation in the presence or absence of PS1145. Nuclear localization of p65, a subunit of NF-κB expressed in T-cells, was observed in anti-CD3/anti-CD28 mAb stimulated CD4+ T-cells untreated or treated with 2 µM PS1145, but was inhibited at concentrations greater than 6 µM as evidenced by increasing localization of p65 to the cytoplasmic space (Figure 1A). To determine whether PS1145 could inhibit alloreactive T cell responses in vitro, highly purified B6 CD4+ T-cells were cultured with T cell-depleted, irradiated splenic stimulators from MHC class II-disparate bm12 mice in the presence of 2, 6, or 10 µM PS1145 or vehicle control (0.05% DMSO). As compared to a control without vehicle, the vehicle alone had no effect on proliferation (data not shown). Whereas 2 µM PS1145 did not significantly inhibit peak T cell proliferation, 6 and 10 µM PS1145 inhibited T cell proliferation at all time points measured during the 7 day primary MLR (Figure 1B). Peak proliferation (at day 5) was inhibited by more than 86% for 6 µM treated cultures and 90% for 10 µM treated cultures. These data suggested a threshold effect for PS1145 of > 2 µM and ≤ 6 µM. Therefore, PS1145 concentrations of 6–10 µM were chosen for further study. PS1145 treatment (10 µM) also significantly reduced the amount of the T cell effector cytokines IL-2 and IFN-γ as well as cytokines released by activated APCs, including IL-6 and GM-CSF, in the supernatant of d5 MLR cultures (data not shown).
Figure 1.
PS1145 inhibits NF-κB activation and proliferation of alloreactive T-cells in MLR cultures. (A) p65 nuclear localization is inhibited with increasing concentrations of PS1145. p65 nuclear localization was assessed in anti-CD3/anti-CD28 mAb stimulated CD4+ T-cells that were treated with vehicle or PS1145. Rabbit anti-p65 mAb and Cy3-conjugated donkey anti-rabbit mAb were used to visualize p65 by confocal microscopy. (B) B6 anti-bm12 MLR cultures were established with vehicle control or the indicated concentrations of PS1145. Proliferation was measured by 3H-thymidine incorporation on the indicated days. Control versus PS1145 (6 and 10 µM) p<0.05. Each data point represents mean ± SEM. Data are representative of 3 experiments. (C) p65 binding is inhibited in PS1145-treated (10 µM) primary MLR cultures. Nuclear extracts from day 7 MLR cultures were incubated with labeled NF-κB consensus sequence probe. DNA-protein complexes were separated by PAGE and visualized by autoradiography using a p65 specific Ab or whole protein content in nuclear extracts were visualized by Coomassie blue staining. β-actin Western blot analysis was used as an additional control. (D) Maximal inhibition of alloresponses requires treatment PS1145 treatment of CD4+ T-cells and APCs. Purified CD4+ T-cells and APCs were pre-treated with 10 µM PS1145 or vehicle separately for 30 minutes and washed prior to initiation of MLR cultures. Proliferation was measured on the indicated days and expressed as mean CPM ± SEM.
To verify that PS1145 treatment was functionally inhibiting NF-κB activity in MLR cultured cells, EMSA was performed on nuclear extracts prepared from MLR cultured cells that had been treated or not with PS1145 (10 µM). As shown in Figure 1C, binding of nuclear extracts from PS1145-treated cultures to an NF-κB consensus sequence probe is significantly less than that observed in vehicle-cultured cells. Similar amounts of nuclear extracts of vehicle treated and PS1145-treated culture were loaded as shown by Coomassie staining and Western blot of β-actin (figure 2C right panel).
Figure 2.
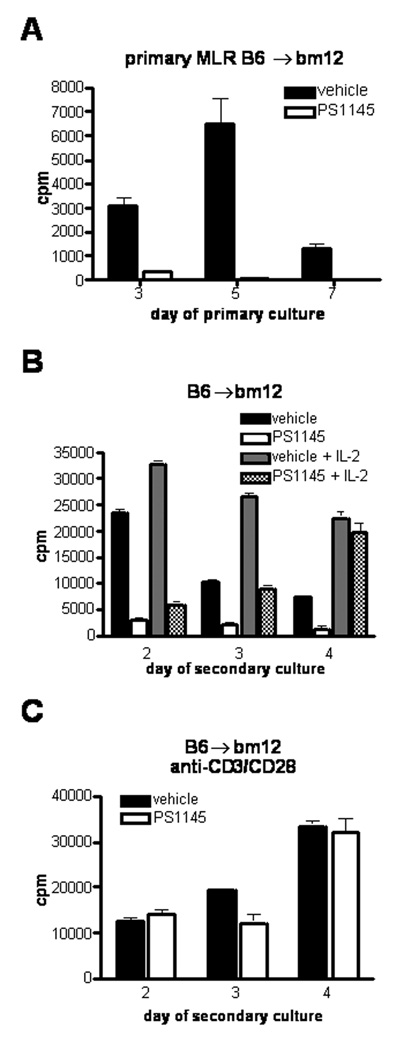
PS1145 induces alloantigen-specific tolerance in CD4+ T-cells in a MHC class II mismatch model that is reversed by the addition of exogenous IL-2. (A) B6 anti-bm12 MLR cultures were established and treated with vehicle or 6 µM PS1145. Proliferation was measured by 3H-thymidine incorporation on the indicated days. Day 7 MLR cultured cells were extensively washed and equal numbers of viable cells were re-stimulated with irradiated bm12 stimulators in the absence or presence of 100 U/ml recombinant human IL-2 (B) or anti-CDε/anti-CD28 coated beads (C) at a 1:1 ratio. At all time points measured, secondary alloresponses were inhibited by at least 80% (p<0.0001). Data are from one of three replicate experiments with similar results.
Because the NF-κB pathway can regulate APC function, including the expression of MHC and costimulatory molecules (36), experiments were performed to determine whether the capacity of PS1145 to inhibit in vitro alloresponses was due to its effects on T-cells, APCs or both. Prior to MLR cultures, responder and stimulator cells were pre-loaded separately with PS1145 and then washed free of excess drug. Cell viability was unaffected by PS1145 pre-treatment (data not shown). PS1145 pre-treatment of responder or stimulator cells resulted in inhibition of alloresponses at all time points observed (Figure 1D). Although no significant differences between pretreatment of responder or stimulator cells alone were observed on day 3, pre-treatment of APCs was modestly more potent at inhibiting alloproliferation on days 5 and 7 of culture (Figure 1D). Maximal inhibition of the alloresponse at all time points required pre-treatment of both responder and stimulator cells prior to initiation of culture. These data indicate that PS1145 treatment has direct effects on stimulator APCs and responder T-cells, and both effects are required for optimal MLR inhibition.
PS1145 induces alloantigenic hyporesponsiveness as assessed in vitro
T-cells activated with suboptimal costimulation are hyporesponsive and remain hyporesponsive upon restimulation. To determine whether inhibition of alloresponses in primary CD4+ MLR cultures by PS1145 (6 µM) treatment would result in hyporesponsiveness to alloantigen after restimulation, cells were recovered on day 7 of a primary MLR (Figure 2A), extensively washed free of PS1145 or vehicle, and equal numbers of viable cells were restimulated with freshly prepared allogeneic bm12 splenic stimulators. Control cells responded vigorously to restimulation at levels that exceeded the primary response and peaked on day 2, indicative of allopriming. In contrast, cells recovered from PS1145-treated MLR cultures were profoundly hyporesponsive to alloantigen restimulation (Figure 2B). At all time points measured, secondary alloresponses of PS1145-treated cells were inhibited by at least 80% even when measured as late as 10 days of culture (data not shown). Addition of exogenous IL-2 reversed tolerance in PS1145-treated CD4 T cell MLR cultures on day 4 (Figure 2B). Since the restoration of proliferation of PS1145-treated cells to alloantigen restimulation in the presence of exogenous IL-2 occurred later than control cultured cells, these data suggest either that the precursor frequency of responding CD4 T-cells was reduced or there was a time period during which PS1145 continued to exert its action after removal from primary cultures.
Because secondary alloresponses of PS1145-treated cells were potently inhibited, we sought to determine whether PS1145-treated cells could respond to non-alloantigenic stimulation through the TCR. Cells recovered from PS1145 or vehicle treated cultures were stimulated with anti-CD3ε/anti-CD28 mAb-coated beads. Although responses of PS1145-treated cells to alloantigenic restimulation were inhibited (Figure 2B), PS1145-treated cells responded vigorously to stimulation with anti-CD3ε and anti-CD28 mAbs (Figure 2C). These results indicate that in vitro treatment of MLR cultured responder cells with PS1145 results in potent CD4+ hyporesponsiveness upon alloantigen restimulation, while only modestly reducing non-specific mitogenic responses.
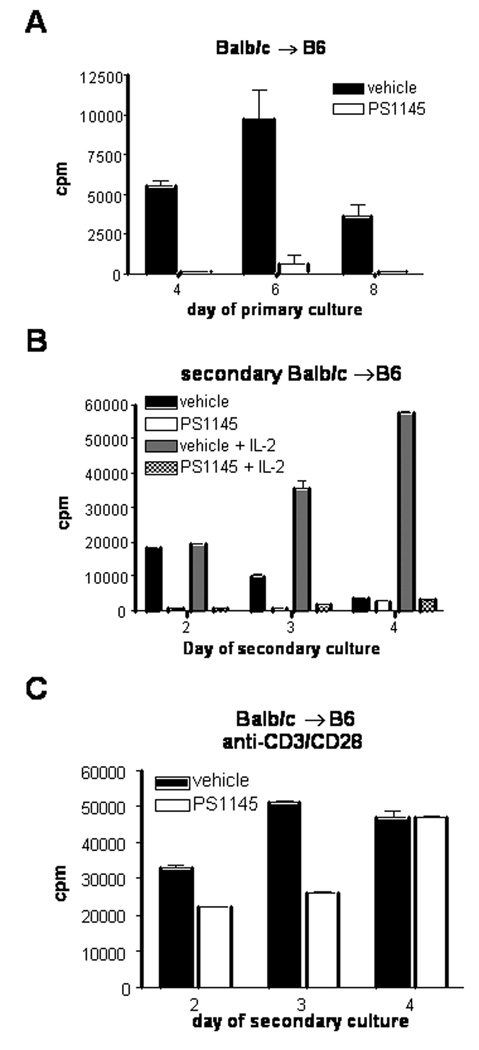
To assess whether alloantigenic hyporesponsiveness by PS1145 treatment of CD4+ T cell containing MLR cultures is similarly effective for MLR cultures initated with both CD4+ and CD8+ T-cells, similar experiments were performed using whole Balb/c T cell responders and T cell-depleted irradiated B6 splenic stimulators. As shown in Figure 3A, primary MLR responses were significantly inhibited in the presence of PS1145 (10 µM). Secondary MLR cultures from day 5 vehicle treated primary cultures responded vigorously to alloantigen restimulation, whereas PS1145-treated cultures showed significant reduction in proliferation at early time points (days 2 and 3) with only low levels of proliferation by day 4 (Figure 3B). CD4 to CD8 ratios of control and PS1145-treated primary cultures were similar on day 5 (CD4 T-cells: 83% vs 85% and CD8 T-cells: 14% vs 12 % for vehicle and PS1145-treated cultures, respectively). Interestingly, in contrast to CD4 T cell MLR cultures exogenous IL-2 could not restore proliferation of PS1145-treated whole T cell cultures to control proliferation (Figure 2B). Although there was a modest early decrease in proliferative responses to stimulation with anti-CD3ε and anti-CD28 mAbs as compared to controls, by day 4 responses were comparable and peak response of the PS1145-treated cells achieved levels of 92% of control responses (Figure 3C). Therefore despite the profound inhibition of MLR responses, mitogenic responses were only modestly and transiently reduced. Thus, PS1145 treatment of whole T cell containing MLR cultures resulted in alloantigen hypoproliferation that was not restored by exogenous IL-2, suggesting immune deviation or loss of alloreactive T-cells.
Figure 3.
PS1145 induces alloantigen-specific tolerance in combined CD4+ and CD8+ T cell cultures in a complete MHC mismatch model. (A) Balb/c anti-B6 MLR cultures were established and treated with vehicle or 10 µM PS1145. Proliferation was measured by 3H-thymidine incorporation on the indicated days. (B) Day 5 MLR cultured cells were extensively washed and equal numbers of viable cells were re-stimulated with irradiated B6 stimulators at a 1:1 ratio the absence or presence of 100 U/ml recombinant human IL-2 or (C) anti-CD3ε/anti-CD28 coated beads at a 1:3 ratio. Representative data from three experiments are shown.
PS1145 preferentially induces hyporesponsiveness and apoptosis in antigen-activated T-cells
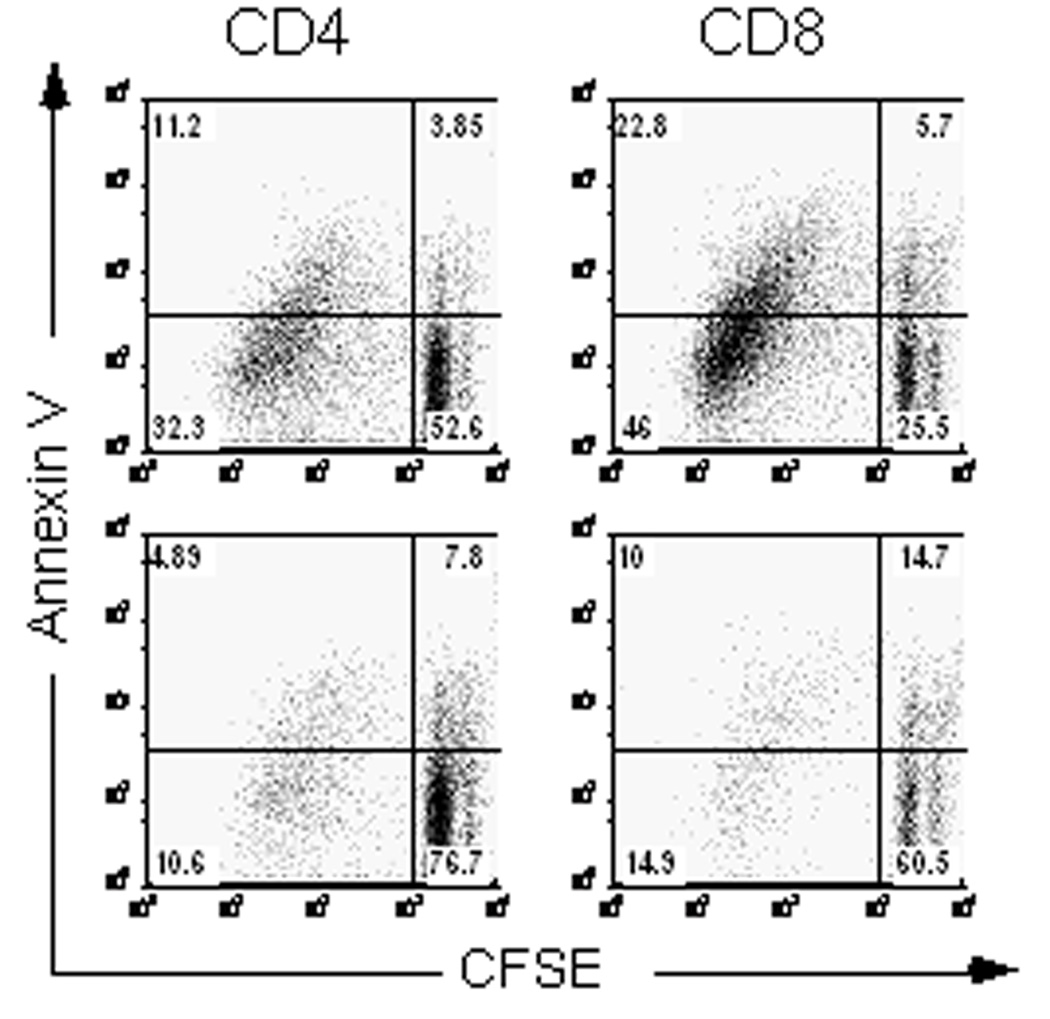
To determine whether deletion of antigen-activated cells contributes to the observed alloantigen hyporesponsiveness, we performed analysis of cell division and induction of apoptosis by CFSE and Annexin V staining, respectively. Analysis of day 6 BALB/c anti-B6 MLR cultures indicated that more CD4+ and CD8+ cells had undergone multiple cell divisions (CFSElo quadrants) in control cultures (43.5% and 68.8%, respectively), compared to CD4+ and CD8+ cells in PS1145-treated cultures (15.5% and 24.9%, respectively) (Figure 4). Simultaneous Annexin V staining indicated that the proportion of apoptotic (Annexin Vhi) CFSElo cells was higher in PS1145-treated cultures than control cultures (31.5% vs. 25.7% and 40.2% vs. 33.1% for CD4+ and CD8+ T-cells, respectively). Thus, PS1145 limits cell division of alloreactive T-cells but also sensitizes antigen-activated CD4+ and CD8+ T-cells to undergo deletion which likely results in a T cell population that is enriched for non-alloreactive T-cells. Consistent with this hypothesis, the removal of dead and dying cells using Annexin V microbeads prior to performing secondary MLR cultures did not restore hypoproliferation of PS1145-treated T-cells to alloantigen disparate stimulators (n = 2 experiments; data not shown). A similar increase in Annexin V binding in CFSElo responder CD4+ T-cells was seen in PS1145-treated polyclonal B6 anti-bm12 cultures (data not shown).
Figure 4.
PS1145 selectively induces hyporesponsiveness and apoptosis in antigen-activated T-cells. Balb/c anti-B6 MLR cultures were established using CFSE-labeled responder T-cells and assessed for Annexin V binding on day 6 of MLR. Representative data from two experiments are shown.
PS1145 treatment of alloreactive T-cells significantly reduces their capacity for GVHD lethality
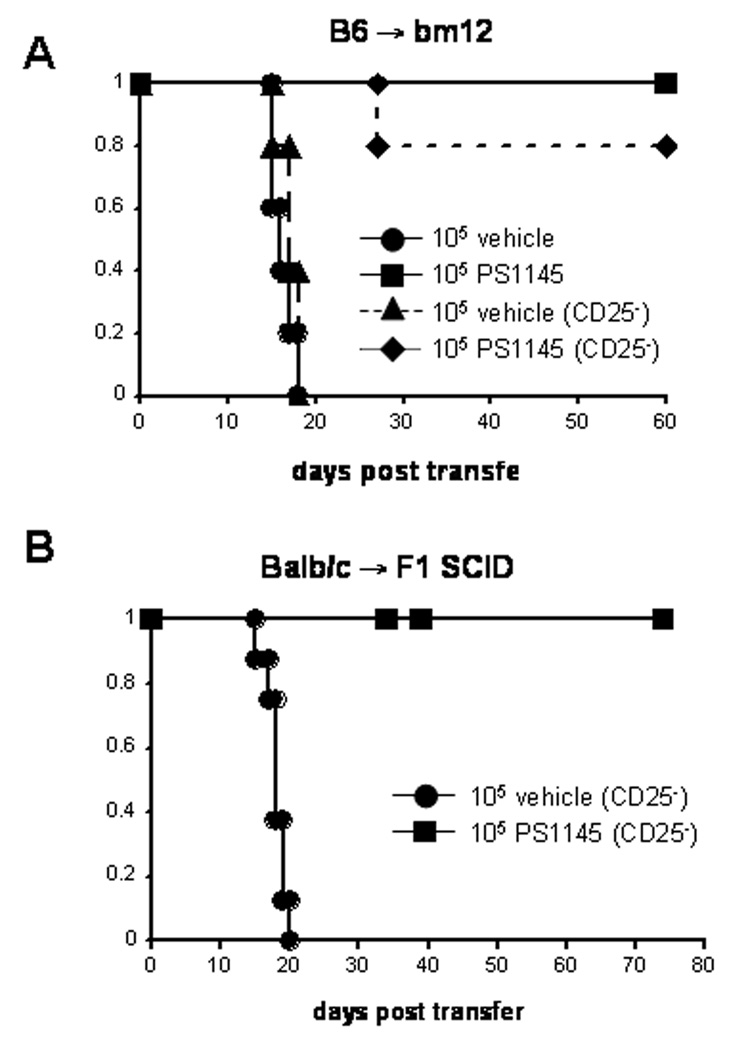
Our data showed that PS1145 treatment of MLR cultures inhibited primary and secondary alloresponses in vitro. To determine whether PS1145 treatment of alloreactive T-cells during activation would confer reduced capacity for GVHD lethality, sublethally irradiated bm12 recipients were given an adoptive transfer of 105 vehicle or PS1145 cultured (10 µM) B6 CD4+ T-cells. Since previous work from our laboratory has demonstrated a requirement for CD4+CD25+ regulatory T-cells (Tregs) during ex vivo tolerization protocols by costimulatory blockade for protection from GVHD (37), we wanted to determine whether PS1145 induced tolerance also required the presence of Tregs. Studies were performed with MLR cultures established with either whole CD4+ T-cells or CD4+CD25− T-cells. Uniform lethality was observed in bm12 recipients by day 18 following adoptive transfer with vehicle treated CD4+ or CD4+25− T-cells obtained from day 7 MLR cultures (Figure 5A). In contrast, transfer of the same number of viable PS1145-treated CD4+ or CD4+25− T-cells recovered from 7-day MLR cutures resulted in 100% or 80% long-term survival rates, respectively (Figure 5A). These results show that ex vivo PS1145 treatment of alloreactive T-cells during activation can result in long-term alloantigen tolerance in vivo that is sufficient to prevent GVHD lethality, irrespective of the presence of CD4+CD25+ T-cells during tolerization.
Figure 5.
PS1145 treatment of alloreactive T-cells significantly reduces their capacity for GVHD lethality in primary host-type recipients. (A) 105 vehicle-cultured cells or PS1145 cultured (10 µM) cells recovered from B6 anti-bm12 day 7 from MLR cultures established with whole CD4+ T-cells or CD4+CD25− T-cells and injected into sublethally irradiated bm12 recipients (n=5 to 10/group). (B) 105 vehicle-cultured cells or PS1145-cultured (10 µM) cells recovered on Balb/c anti-B6 day 5 from MLR cultures established with whole CD25− T-cells and injected into sublethally irradiated CB6F1 SCID recipients (vehicle n=8, PS1145-treated n=16, p=9.24×10−7).
As shown in Figure 3, whole T-cells could also be tolerized using PS1145 treatment as assessed in primary and secondary MLR cultures. To determine if PS1145-treated whole T cell cultures have decreased GVHD lethality, 105 vehicle or PS1145 (10 µM)-treated CD25− T-cells obtained from Balb/c anti-B6 day 5 MLR cultures were injected into sublethally irradiated, NK cell-depleted CB6F1 SCID recipients. As shown in Figure 5B, vehicle-treated MLR cultures induced GVHD lethality by day 20 post-transfer whereas PS1145-treated cultures lost their GVHD inducing capability. Thus, PS1145 treatment of MLR cultured cells was highly effective in preventing GVHD lethality mediated by CD4+, CD4+25− or CD25− whole T-cells.
PS1145-treated cells have intact immune responses after parking for one month in lymphopenic syngeneic recipients
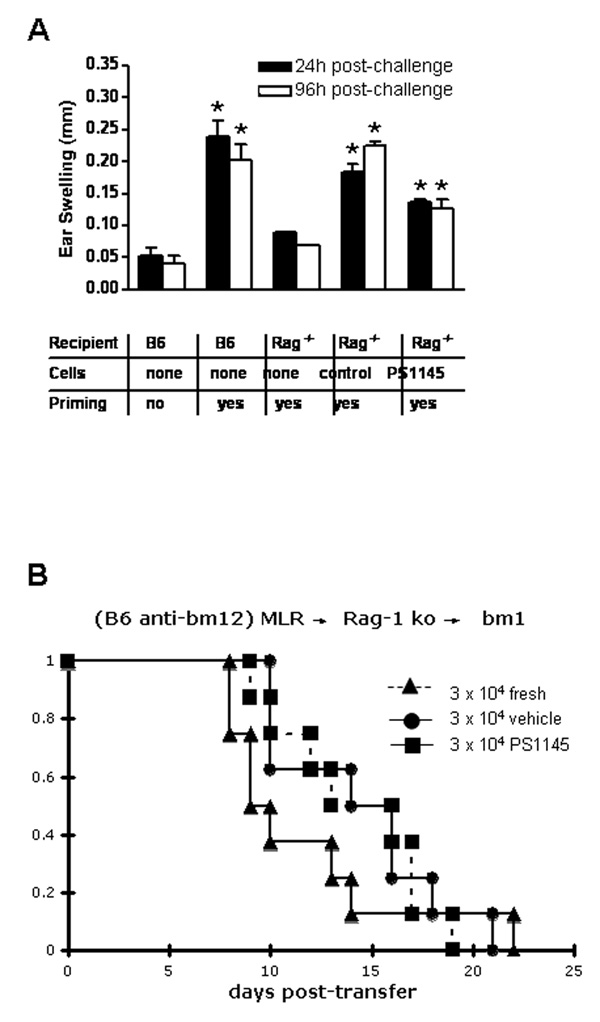
A major advantage of ex vivo tolerance induction approaches is the specific targeting of alloreactive T-cells without affecting non-allospecific T-cells. Because PS1145-treated cells had impaired secondary alloresponses but intact non-specific mitogen responses in vitro, we sought to determine whether such tolerized cells could mount an in vivo response to non-alloantigenic stimulation. Syngeneic Rag-1−/− recipient mice were injected with 2 × 106 vehicle-treated or PS1145-treated (10 µM) CD4+ T-cells obtained from B6 anti-bm12 day 7 MLR cultures. Under these conditions, the only T-cells that would be available for an immune response in the recipient would be derived from the MLR cultured cells. After 30 days to allow for T cell repopulation and for cells to become quiescent after homeostatic expansion has been completed, recipient mice were sensitized with 3% oxazolone. Six days later, mice were rechallenged with 1% oxazolone on the right ear pinna to induce a contact hypersensitivity (CHS) response. As shown in Figure 6A, Rag-1−/− recipients of PS1145-treated CD4+ T-cells exhibited significantly more ear swelling than challenged, but unsensitized B6 control mice or Rag-1−/− recipients without transfer of MLR cells. Because the CHS response requires T cell activation and recruitment to the site of challenge, this assay indicates that PS1145-treated cells have retained the capacity to mediate a CD4+ response in vivo, despite their loss in GVHD lethality capacity. These results indicate that PS1145 treatment and selective targeting of the NF-κB pathway has the capacity to induce generation of CD4+ T-cells tolerant to alloantigen in vivo but capable of other CD4+ T cell functions.
Figure 6.
Contact hypersensitive responses and GVHD lethality capacity of PS1145-treated day 7 MLR (B6 anti-bm12) cultured cells is intact after one month parking in syngeneic, lymphopenic Rag-1−/− recipients. (A) 2 × 106 day 7 MLR cultured (10 µM) CD4+ cells were injected into syngeneic Rag-1−/− recipient mice. After 30 days, recipient mice were sensitized with 3% oxazolone on thoracic and abdominal skin. Mice were re-challenged 6 days later on the right ear with 1% oxazolone to induce CHS. Swelling in the right ear was measured at 24 and 96 hours post-challenge. Results of one of two similar experiments are shown (3–5 mice/experiment). * denotes significance compared to unprimed control (p<0.05); (B) 2 × 106 fresh, control- or PS1145 (10 µM)-treated, day 7 MLR (B6 anti-bm12) cultured CD4+ cells were injected into syngeneic Rag-1−/− recipient mice. After 30 days, splenic CD4+ T cells were isolated and given to 6 Gy TBI conditioned bm12 recipients (n = 6–8/group). No significant differences were noted in the survival rates between groups.
Although alloantigen hyporesponsiveness was seen in secondary MLR cultures, it is possible that the response to potent mitogenic stimuli in vitro were not indicative of alloantigen-specific tolerance. Therefore, it is possible that the observed CHS responses are due to a restoration of T cell responses after lymphopenic expansion. To determine if a prolonged state of alloantigen-specific hyporesponsiveness could be seen, 2 × 106 freshly isolated, control- or PS1145-treated day 7 MLR cells (98% inhibition of primary MLR) were adoptively transferred into Rag-1−/− recipients. After one month, splenic CD4+ T-cells from each group of mice were transferred into sublethally irradiated bm12 (Figure 6B) or NK-depleted, sublethally irradiated C3H SCID mice (not shown). All bm12 recipients succumbed to GVHD induced lethality by day 22 post-transfer. In third party C3H SCID recipients of control- vs PS1145-treated MLR cells, survival rates were significantly lower (day 23: 66% vs 100%, respectively; p = 0.05) and there was a statistical trend (p = 0.07) toward higher mean weight curves. Together, these data indicate that lymphopenic expansion permits the restoration of immune responses in PS1145-treated MLR cells and suggests that immune deviation and/or suppression contributes to the reduced GVHD lethality capacity of freshly infused day 7, PS1145-treated MLR cells given to host-type recipients.
Discussion
Our studies indicate that ex vivo inhibition of the NF-κB pathway is a new target that impairs alloreactivity of donor T-cells but can preserve other T cell functions in vitro and in vivo. These data suggest that the preferential effect of PS1145 on alloreactive versus naïve non-alloresponsive CD4+ T-cells is dependent upon T cell activation during PS1145 exposure. Because TCR activation pathways are linked to cytokine production and cell survival pathways via NF-κB signaling, selective inhibition of NF-κB activation in alloreactive cells that are stimulated by antigen in MLR cultures results in impaired cytokine production and increased susceptibility to apoptosis. In addition, disconnecting TCR activation from proliferation (cell cycle progression) and cytokine production is thought to result in anergy (18). Activated alloreactive cells that are able to survive the minimal cytokine (especially IL-2 containing) environment and restricted proliferation that follows PS1145 treatment therefore likely become hyporesponsive. Secondary responses of PS1145 MLR cultures prepared from CD4+ T-cells could be restored to control responses at later time points using exogenous IL-2 (Figure 2B). These findings suggest that PS1145 treatment induces hyporesponsiveness in CD4+ T-cells that can be reversed by exogenous IL-2. In contrast, the addition of IL-2 to secondary whole T cell MLR cultures did not increase proliferation in PS1145-treated cells. Thus, cultures that also include CD8+ T-cells have a different mechanism for hyporesponsiveness that may result from efficient deletion of alloreactive T-cells, immune deviation, or residual PS1145 biological effects in T-cells that persist in the priming phase of secondary MLR cultures.
The fraction of T-cells in the MLR culture that do not recognize alloantigen and thus do not have TCR activation appears largely unaffected by PS1145 treatment as measured by responses to mitogenic stimuli in vitro and nominal antigen challenge in vivo. These cells likely remain unaffected because they do not have a biochemical requirement for NF-κB induced cytokine production and survival pathways that would occur in T-cells that have received an initial potent TCR signal. Alternatively, the potent mitogenic stimuli used in these assays (anti-3/CD28 mAb coated microbeads) was sufficient to overcome the hyporesponsiveness of PS1145 treatment. Consistent with the latter hypothesis, CD4+ T cell responses could be intermittently observed in secondary MLR cultures despite profound primary MLR responses (data not shown), which we hypothesize may be due to differences in intracellular PS1145 metabolism or the TCR signal strength that may vary with allo-MLR stimulator cells between experiments. More importantly, the one month parking of control- or PS1145- treated MLR cells in lymphopenic syngeneic recipients not only resulted intact CHS responses but permitted the uniform GVHD lethality capacity of splenic CD4+ T-cells adoptively transferred into sublethally irradiated secondary recipients (Figure 6B). These data are consistent with studies demonstrating that homeostatic proliferation results in a dominant resistance to tolerance induction (38) and suggest that full tolerance induction by PS1145 treatment of MLR cells may require a resting state in which T-cells are not driven to expand by inflammatory stimuli and the accumulation of cytokines that signal T-cells via the IL-2R common gamma chain. Alternatively, the transfer of day 7, PS1145-treated MLR cells may result in a temporary state of immune deviation or suppression due to intracellular concentrations of PS1145 sufficient to transiently inhibit NF-κB translocation and blunt the immune response, which is restored sometime during the one month parking period in syngeneic lymphopenic recipients. Such an approach still may be advantageous at preventing early GVHD induced lethality, while subsequently permitting mature T cell immune responses to return after the acute period of GVHD risk.
The effects of PS1145 on antigen-activated cells are similar to that of the proteasome inhibitor Bortezomib, which may inhibit NF-κB pathway activation due to impaired degradation of IκB. It is tempting to speculate that specific inhibition of the NF-κB pathway in vivo during the early post-transplant stage when alloreactive donor T-cells become activated would also result in GVHD inhibition. Consistent with this hypothesis, administration of Bortezomib resulted in apoptosis of activated alloreactive cells which was sufficient to prevent GVHD in mice following BMT while anti-tumor responses and donor engraftment were maintained (12). However, there is a narrow time window for Bortezomib administration in rodent GVHD models such that the delayed administration of Bortezomib can markedly increase, rather than reduce, GVHD lethality (12, 39, 40). How the timing of Bortezomib administration in rodent models will precisely translate to human allogeneic BMT is unknown. Moreover, although one study reported that PS1145 administration early post-BMT protected mice against GVHD lethality (41), in vivo PS1145 administration is subject to the same critical post-BMT timing issues as Bortezomib (JSS, unpublished data). If the acute toxicities seen with extended Bortezomib or PS1145 treatment are due to the release of pro-inflammatory cytokines and tissue injury caused by the conditioning regimen, it is possible that Bortezomib and NF-κB inhibitors given in vivo later post-BMT with delayed lymphocyte infusions would be highly effective at preventing GVHD-induced lethality when these insults would have subsided. Nonetheless, because of the difficulty in monitoring tolerance induction, controlling antigenic exposure during tolerization and drug-related toxicities, an efficient ex vivo tolerance induction approach would be favorable to an in vivo administration of pharmacological agents for prevention of GVHD in the early post-BMT period.
Previous work by our group and others have shown that activation of alloreactive cells in the presence of costimulatory blockade or immunesuppressive cytokines may provide a means to generate a tolerized population of alloreactive T-cells which are impaired in causing GVHD (9, 10). These approaches required the presence of donor CD4+CD25+ Tregs for tolerance induction (37), whereas PS1145 induced hyporesponsiveness does not. In certain clinical settings the presence or availability of functional donor Tregs may be limiting such as following chemotherapy treatment of the recipient or the use of denileukin diftitox to reduce Tregs in vivo. Therefore, strategies that directly induce tolerance in CD4+ T-cells may be advantageous by being independent of the presence of Tregs and perhaps by being more stable after in vivo adoptive transfer, when Tregs and responding T-cells may no longer be in physical contact.
In summary, we have shown that targeting of the NF-κB pathway can be a highly effective means of inducing donor anti-host hyporesponsiveness. Ex vivo tolerization of donor T-cells with PS1145 can induce long-term alloantigen-specific tolerance that is sufficient to significantly decrease GVHD lethality across MHC barriers while a non-alloreactive CD4+ T cell response can be maintained in vivo. The use of drugs to inhibit NF-κB activation in this setting may therefore have clinical advantages over more globally immunosuppressive strategies. Thus, PS1145 and other drugs designed to specifically inhibit NF-κB pathway activation in alloreactive cells provide a new clinically applicable strategy for the prevention of GVHD.
Acknowledgments
Supported by National Institutes of Health Grants R01 AI 34495 and 2R37HL56067 to B.R.B. M.J.O. was supported by NIH Medical Scientist Training Grant T32 GM08244-15.
References
- 1.Nademanee A, Schmidt GM, Parker P, Dagis AC, Stein A, Snyder DS, et al. The outcome of matched unrelated donor bone marrow transplantation in patients with hematologic malignancies using molecular typing for donor selection and graft-versus-host disease prophylaxis regimen of cyclosporine, methotrexate, and prednisone. Blood. 1995;86(3):1228–1234. [PubMed] [Google Scholar]
- 2.Davies SM, Shu XO, Blazar BR, Filipovich AH, Kersey JH, Krivit W, et al. Unrelated donor bone marrow transplantation: influence of HLA A and B incompatibility on outcome. Blood. 1995;86(4):1636–1642. [PubMed] [Google Scholar]
- 3.Nash RA, Antin JH, Karanes C, Fay JW, Avalos BR, Yeager AM, et al. Phase 3 study comparing methotrexate and tacrolimus with methotrexate and cyclosporine for prophylaxis of acute graft-versus-host disease after marrow transplantation from unrelated donors. Blood. 2000;96(6):2062–2068. [PubMed] [Google Scholar]
- 4.Ratanatharathorn V, Nash RA, Przepiorka D, Devine SM, Klein JL, Weisdorf D, et al. Phase III study comparing methotrexate and tacrolimus (prograf, FK506) with methotrexate and cyclosporine for graft-versus-host disease prophylaxis after HLA-identical sibling bone marrow transplantation. Blood. 1998;92(7):2303–2314. [PubMed] [Google Scholar]
- 5.Ho VT, Soiffer RJ. The history and future of T-cell depletion as graft-versus-host disease prophylaxis for allogeneic hematopoietic stem cell transplantation. Blood. 2001;98(12):3192–3204. doi: 10.1182/blood.v98.12.3192. [DOI] [PubMed] [Google Scholar]
- 6.Butturini A, Gale RP. T cell depletion in bone marrow transplantation for leukemia: current results and future directions. Bone marrow transplantation. 1988;3(3):185–192. [PubMed] [Google Scholar]
- 7.Martin PJ, Hansen JA, Torok-Storb B, Durnam D, Przepiorka D, O'Quigley J, et al. Graft failure in patients receiving T cell-depleted HLA-identical allogeneic marrow transplants. Bone marrow transplantation. 1988;3(5):445–456. [PubMed] [Google Scholar]
- 8.Zeller JC, Panoskaltsis-Mortari A, Murphy WJ, Ruscetti FW, Narula S, Roncarolo MG, et al. Induction of CD4+ T cell alloantigen-specific hyporesponsiveness by IL-10 and TGF-beta. J Immunol. 1999;163(7):3684–3691. [PubMed] [Google Scholar]
- 9.Blazar BR, Taylor PA, Noelle RJ, Vallera DA. CD4(+) T-cells tolerized ex vivo to host alloantigen by anti-CD40 ligand (CD40L:CD154) antibody lose their graft-versus-host disease lethality capacity but retain nominal antigen responses. The Journal of clinical investigation. 1998;102(3):473–482. doi: 10.1172/JCI3741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gribben JG, Guinan EC, Boussiotis VA, Ke XY, Linsley L, Sieff C, et al. Complete blockade of B7 family-mediated costimulation is necessary to induce human alloantigen-specific anergy: a method to ameliorate graft-versus-host disease and extend the donor pool. Blood. 1996;87(11):4887–4893. [PubMed] [Google Scholar]
- 11.Godfrey WR, Krampf MR, Taylor PA, Blazar BR. Ex vivo depletion of alloreactive cells based on CFSE dye dilution, activation antigen selection, and dendritic cell stimulation. Blood. 2004;103(3):1158–1165. doi: 10.1182/blood-2003-04-1098. [DOI] [PubMed] [Google Scholar]
- 12.Sun K, Welniak LA, Panoskaltsis-Mortari A, O'Shaughnessy MJ, Liu H, Barao I, et al. Inhibition of acute graft-versus-host disease with retention of graft-versus-tumor effects by the proteasome inhibitor bortezomib. Proceedings of the National Academy of Sciences of the United States of America; 2004. pp. 8120–8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cavazzana-Calvo M, Stephan JL, Sarnacki S, Chevret S, Fromont C, de Coene C, et al. Attenuation of graft-versus-host disease and graft rejection by ex vivo immunotoxin elimination of alloreactive T-cells in an H-2 haplotype disparate mouse combination. Blood. 1994;83(1):288–298. [PubMed] [Google Scholar]
- 14.Solomon SR, Mielke S, Savani BN, Montero A, Wisch L, Childs R, et al. Selective depletion of alloreactive donor lymphocytes: a novel method to reduce the severity of graft-versus-host disease in older patients undergoing matched sibling donor stem cell transplantation. Blood. 2005;106(3):1123–1129. doi: 10.1182/blood-2005-01-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guinan EC, Boussiotis VA, Neuberg D, Brennan LL, Hirano N, Nadler LM, et al. Transplantation of anergic histoincompatible bone marrow allografts. The New England journal of medicine. 1999;340(22):1704–1714. doi: 10.1056/NEJM199906033402202. [DOI] [PubMed] [Google Scholar]
- 16.Jenkins MK, Taylor PS, Norton SD, Urdahl KB. CD28 delivers a costimulatory signal involved in antigen-specific IL-2 production by human T-cells. J Immunol. 1991;147(8):2461–2466. [PubMed] [Google Scholar]
- 17.DeSilva DR, Urdahl KB, Jenkins MK. Clonal anergy is induced in vitro by T cell receptor occupancy in the absence of proliferation. J Immunol. 1991;147(10):3261–3267. [PubMed] [Google Scholar]
- 18.Jenkins MK. The role of cell division in the induction of clonal anergy. Immunology today. 1992;13(2):69–73. doi: 10.1016/0167-5699(92)90137-V. [DOI] [PubMed] [Google Scholar]
- 19.Jenkins MK, Schwartz RH. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. The Journal of experimental medicine. 1987;165(2):302–319. doi: 10.1084/jem.165.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ghosh P, Tan TH, Rice NR, Sica A, Young HA. The interleukin 2 CD28-responsive complex contains at least three members of the NF kappa B family: c-Rel, p50, and p65. Proceedings of the National Academy of Sciences of the United States of America; 1993. pp. 1696–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang D, Matsumoto R, You Y, Che T, Lin XY, Gaffen SL, et al. CD3/CD28 costimulation-induced NF-kappaB activation is mediated by recruitment of protein kinase C-theta, Bcl10, and IkappaB kinase beta to the immunological synapse through CARMA1. Molecular and cellular biology. 2004;24(1):164–171. doi: 10.1128/MCB.24.1.164-171.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang D, You Y, Case SM, McAllister-Lucas LM, Wang L, DiStefano PS, et al. A requirement for CARMA1 in TCR-induced NF-kappa B activation. Nature immunology. 2002;3(9):830–835. doi: 10.1038/ni824. [DOI] [PubMed] [Google Scholar]
- 23.Ruland J, Duncan GS, Elia A, del Barco Barrantes I, Nguyen L, Plyte S, et al. Bcl10 is a positive regulator of antigen receptor-induced activation of NF-kappaB and neural tube closure. Cell. 2001;104(1):33–42. doi: 10.1016/s0092-8674(01)00189-1. [DOI] [PubMed] [Google Scholar]
- 24.Coudronniere N, Villalba M, Englund N, Altman A. NF-kappa B activation induced by T cell receptor/CD28 costimulation is mediated by protein kinase C-theta. Proceedings of the National Academy of Sciences of the United States of America; 2000. pp. 3394–3399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin X, O'Mahony A, Mu Y, Geleziunas R, Greene WC. Protein kinase C-theta participates in NF-kappaB activation induced by CD3-CD28 costimulation through selective activation of IkappaB kinase beta. Molecular and cellular biology. 2000;20(8):2933–2940. doi: 10.1128/mcb.20.8.2933-2940.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun Z, Arendt CW, Ellmeier W, Schaeffer EM, Sunshine MJ, Gandhi L, et al. PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature. 2000;404(6776):402–407. doi: 10.1038/35006090. [DOI] [PubMed] [Google Scholar]
- 27.Janssen R, van Wengen A, Hoeve MA, ten Dam M, van der Burg M, van Dongen J, et al. The same IkappaBalpha mutation in two related individuals leads to completely different clinical syndromes. The Journal of experimental medicine. 2004;200(5):559–568. doi: 10.1084/jem.20040773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Doffinger R, Smahi A, Bessia C, Geissmann F, Feinberg J, Durandy A, et al. X-linked anhidrotic ectodermal dysplasia with immunodeficiency is caused by impaired NF-kappaB signaling. Nature genetics. 2001;27(3):277–285. doi: 10.1038/85837. [DOI] [PubMed] [Google Scholar]
- 29.Courtois G, Smahi A, Reichenbach J, Doffinger R, Cancrini C, Bonnet M, et al. A hypermorphic IkappaBalpha mutation is associated with autosomal dominant anhidrotic ectodermal dysplasia and T cell immunodeficiency. The Journal of clinical investigation. 2003;112(7):1108–1115. doi: 10.1172/JCI18714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hideshima T, Chauhan D, Richardson P, Mitsiades C, Mitsiades N, Hayashi T, et al. NF-kappa B as a therapeutic target in multiple myeloma. The Journal of biological chemistry. 2002;277(19):16639–16647. doi: 10.1074/jbc.M200360200. [DOI] [PubMed] [Google Scholar]
- 31.Chen ZM, O'Shaughnessy MJ, Gramaglia I, Panoskaltsis-Mortari A, Murphy WJ, Narula S, et al. IL-10 and TGF-beta induce alloreactive CD4+CD25- T-cells to acquire regulatory cell function. Blood. 2003;101(12):5076–5083. doi: 10.1182/blood-2002-09-2798. [DOI] [PubMed] [Google Scholar]
- 32.O'Shaughnessy MJ, Chen ZM, Gramaglia I, Taylor PA, Panoskaltsis-Mortari A, Vogtenhuber C, et al. Elevation of intracellular cyclic AMP in alloreactive CD4(+) T-cells induces alloantigen-specific tolerance that can prevent GVHD lethality in vivo. Biol Blood Marrow Transplant. 2007;13(5):530–542. doi: 10.1016/j.bbmt.2007.01.071. [DOI] [PubMed] [Google Scholar]
- 33.Brice GT, Riley JL, Villinger F, Mayne A, Hillyer CD, June CH, et al. Development of an animal model for autotransfusion therapy: in vitro characterization and analysis of anti-CD3/CD28 expanded cells. J Acquir Immune Defic Syndr Hum Retrovirol. 1998;19(3):210–220. doi: 10.1097/00042560-199811010-00002. [DOI] [PubMed] [Google Scholar]
- 34.Mayo MW, Norris JL, Baldwin AS. Ras regulation of NF-kappa B and apoptosis. Methods in enzymology. 2001;333:73–87. doi: 10.1016/s0076-6879(01)33046-x. [DOI] [PubMed] [Google Scholar]
- 35.Taube M, Svensson L, Carlsten H. T lymphocytes are not the target for estradiol-mediated suppression of DTH in reconstituted female severe combined immunodeficient (SCID) mice. Clinical and experimental immunology. 1998;114(2):147–153. doi: 10.1046/j.1365-2249.1998.00700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Saemann MD, Kelemen P, Bohmig GA, Horl WH, Zlabinger GJ. Hyporesponsiveness in alloreactive T-cells by NF-kappaB inhibitor-treated dendritic cells: resistance to calcineurin inhibition. Am J Transplant. 2004;4(9):1448–1458. doi: 10.1111/j.1600-6143.2004.00547.x. [DOI] [PubMed] [Google Scholar]
- 37.Taylor PA, Noelle RJ, Blazar BR. CD4(+)CD25(+) immune regulatory cells are required for induction of tolerance to alloantigen via costimulatory blockade. The Journal of experimental medicine. 2001;193(11):1311–1318. doi: 10.1084/jem.193.11.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu Z, Bensinger SJ, Zhang J, Chen C, Yuan X, Markmann JF, et al. Homeostatic proliferation is a barrier to transplantation tolerance. Nature Medicine. 2004;10(1):87–92. doi: 10.1038/nm965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun K, Wilkins DE, Anver MR, Sayers TJ, Panoskaltsis-Mortari A, Blazar BR, et al. Differential effects of proteasome inhibition by bortezomib on murine acute graft-versus-host disease (GVHD): delayed administration of bortezomib results in increased GVHD-dependent gastrointestinal toxicity. Blood. 2005;106(9):3293–3299. doi: 10.1182/blood-2004-11-4526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sun K, Sayers TJ, Welniak LA, Murphy WJ. Differential effects of donor T-cell cytokines on outcome with continuous bortezomib administration after allogeneic bone marrow transplantation. Blood. 2008;112(4):1522–1529. doi: 10.1182/blood-2008-03-143461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vodanovic-Jankovic S, Hari P, Jacobs P, Komorowski R, Drobyski WR. NF-kappaB as a target for the prevention of graft-versus-host disease: comparative efficacy of bortezomib and PS-1145. Blood. 2006;107(2):827–834. doi: 10.1182/blood-2005-05-1820. [DOI] [PMC free article] [PubMed] [Google Scholar]