Abstract
Cellular elements of the neurovascular unit are essential for the physiological functioning of brain vessels. If any of these vascular elements are disturbed the consequences can be dire. Cerebral Amyloid Angiopathy (CAA), a disorder caused by the accumulation of amyloid in cerebral vessels, provides a case study of progressive neurovascular unit dysfunction leading to failure of vascular reactivity, smooth muscle cell loss, and eventual frank breakdown of vessel integrity resulting in recurrent and sometimes fatal intracerebral hemorrhage.
Introduction
By far the most common form of CAA is the Aβ type, defined by the accumulation of aggregated β-amyloid peptide (Aβ) in small cerebral vessels – most prominently the penetrating arterioles of the cortex. Aβ is derived from the amyloid precursor protein (APP) through a series of enzymatic cleavages, and is found in parenchymal plaques of patients with Alzheimer’s Disease (AD). The 42-amino acid length Aβ (Aβ42) is principally found in plaques, while the shorter Aβ40 is the major form found in CAA. Though CAA is a very common finding in brains of AD patients and is recognized as a histopathological hallmark of the disease, it is also commonly found in the elderly without AD1. One of the most widely recognized complications of CAA is spontaneous intracerebral hemorrhage (ICH), usually involving the cortex or subcortical white matter (“lobar hemorrhage”). This is believed to be a late consequence of disease. More recently, accumulating evidence suggests that CAA may also have effects on vascular reactivity, even at very early stages of the disease. Moreover, there are suggestions that this failure of vascular reactivity may result in chronic ischemia of the white matter within the watershed zone of the most prominently affected penetrating arterioles of the cortex.
Aβ-induced cerebral vessel dysfunction
The first clues that Aβ may cause substantial vascular impairment stemmed from clinical observations that cerebrovascular abnormalities occur not only in early stages of AD2 but also in genetically predisoposed patients prior to the development of AD3. Subsequent experimental data strongly support this notion. First, soluble Aβ – prior to its deposition as CAA – was found to be strongly vasoactive (particularly Aβ40). For example, physiologically relevant levels of soluble Aβ40 led to dose-dependent vasoconstriction of isolated cerebral arteries4,5, and potent vasoactivity was noted when soluble Aβ40 was topically applied to the neocortex of wild-type (WT) mice5. Moreover, young mutant APP mice having elevated levels of soluble Aβ (but no CAA) were shown to have marked cerebrovascular abnormalities, including reduced resting cerebral blood flow (CBF), attenuated CBF response to endothelium-dependent vasodilators, and impaired cerebral autoregulation5. Subsequent studies demonstrated that acute Aβ depletion via γ-secretase inhibition restored cerebrovascular function in young APP mice, further implicating soluble Aβ as the causative factor underlying these in vivo cerebrovascular abnormalities6.
Even greater degrees of vascular impairment have been shown when aggregated Aβ deposits within cerebral vessels, as is seen with CAA (Fig. 1). Christie and colleagues7 were the first to demonstrate this finding – noting severe vasodilatory dysfunction in older APP mice having CAA as compared to young APP mice with no CAA. Others have noted similar age-dependent cerebrovascular deficits in APP mice6,8. In addition, we have recently demonstrated a dose response between extent of vascular amyloid deposition and degree of impaired vasomotor function, further implicating CAA in Aβ-induced vessel dysfunction6. Surprisingly, this dose response data also documented that very little CAA (<20% coverage) was required to produce profound vessel dysfunction6. Taken together, these animal data suggest that CAA impairs vascular function to a greater degree than soluble Aβ alone. Accordingly, clinical data have associated CAA with multiple clinical indicators of disease including cognitive impairment on neurological examination9, white matter changes on radiographic studies10, and cortical infarcts on pathological analyses11, while no such associations have thus far been identified for soluble Aβ.
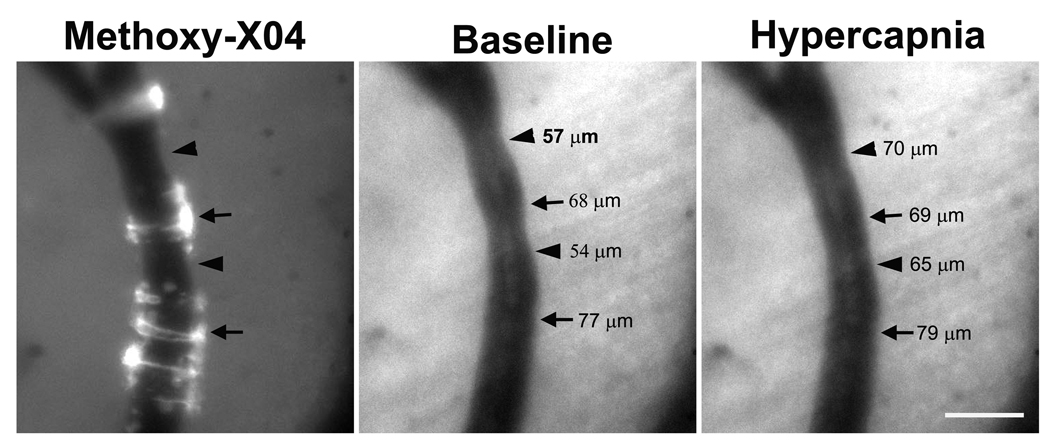
Figure 1. Severely impaired vasodilation in CAA-affected vessels.
Live images of the leptomeningeal arterioles of a 12 month-old Tg2576 mouse brain were taken before (baseline) and 4 min after induced hypercapnia. Methoxy-X04-positive CAA deposits were imaged in the same vessel. Hypercapnia-induced vasodilation was noted in vessel segments without CAA (arrowheads), while minimal vascular response was noted in vessel segments with CAA (arrows), as determined by vessel caliber. Scale bar: 100 µm.
Regarding potential underlying mechanism, several key observations have been made. First, CAA-induced vessel dysfunction is not simply the result of amyloid-induced structural changes to the tunica media or frank loss of vascular smooth muscle cells (VSMCs)6,7. Second, the neurovascular cell type responsible for Aβ-induced vessel dysfunction has been identified in the case of soluble Aβ, with several studies documenting dysfunctional endothelial cell responses to endothelium-dependent vasodilators but normal VSMC responses to endothelium-independent vasodilators5. In the case of CAA, it is far less clear, with some studies implicating endothelial cell dysfunction8 and others VSMC dysfunction6,7. Third, the molecular effectors by which Aβ induces vessel dysfunction have recently been investigated. In the case of soluble Aβ, both reactive oxygen species (ROS)5,12 and proinflammatory cascades4 have been implicated. The data supporting ROS is particularly strong, as multiple pharmacologic and genetic approaches towards counteracting the effects of ROS – including genetic inactivation of a catalytic subunit of NADPH oxidase – have been shown to reduce or eliminate Aβ-induced cerebrovascular dysfunction5,12,13. In the case of CAA, molecular studies have only just begun, but early results suggest that ROS and NADPH oxidase may again be significant effectors8,12.
Amyloid cytotoxicity and disruption of the ECM
Considerable attention has focused on the neurotoxic properties of Aβ and its aggregates, which has provided support for the amyloid hypothesis of AD. In addition to neurons, numerous in vitro studies suggest that Aβ is toxic VSMCs14, human brain pericytes15, and cerebral endothelial cells (CECs)16. Aβ1–40 was noted to be more toxic than Aβ1–42 in CECs17, but the opposite was found for SMCs18, and Aβ with the Dutch mutation (E22Q) was more toxic to SMCs than native Aβ19. One feature of Aβ which appeared to be related to its toxicity, was its ability to aggregate as insoluble amyloid fibrils on the membranes of SMCs. More recent evidence suggests that soluble oligomeric intermediates may also be responsible for cytotoxic activity.
Although in vitro studies indicate that vascular cells are vulnerable to Aβ toxicity, neuropathological and in vivo animal model studies suggests that profound vascular consequences occur even before CAA-related cytotoxicity is observed. Initially, amyloid accumulates at the outer basal lamina in close proximity to SMCs20,21. At this stage, there are no structural changes to the tunica media and no SMC loss, but progressive and often profound vessel dysfunction can occur (see above). As the disease progresses, amyloid deposits extend into the SMC layer20, 21, leading to significant structural alterations, early SMC loss (Fig. 2), and complete vessel shutdown (i.e. no functional response to vasoactive stimuli) (see above). With advanced CAA, the media eventually becomes completely replaced by amyloid and becomes devoid of surviving smooth muscle cells22. While endothelial cells have abnormal appearance (atrophic cell bodies, irregular nuclei), cell degeneration is not observed until very late in the disease23. With end-stage disease, amyloid appears to disrupt the basement membrane (BM) and spread to adjacent neuropil forming dyshoric vessels24.
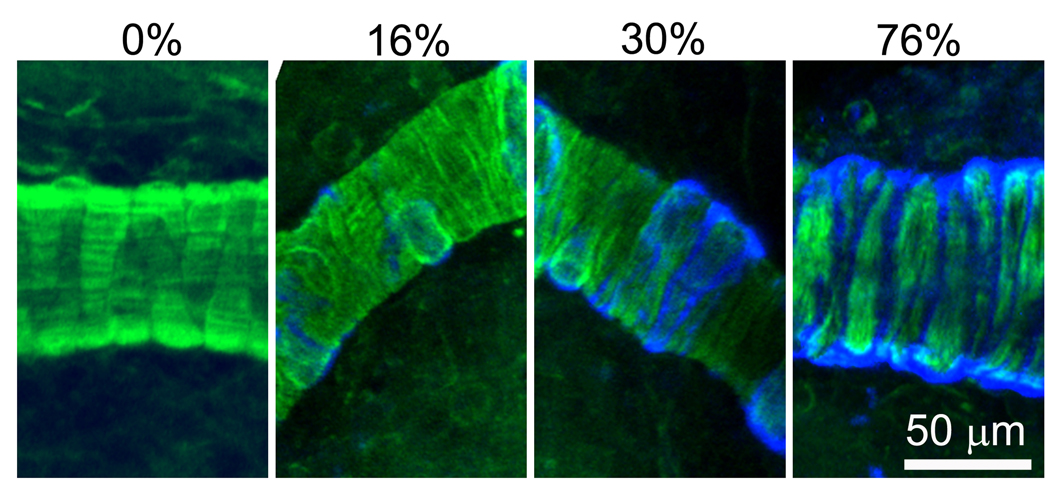
Figure 2. Pathological changes in CAA-affected vessels.
Amyloid deposition and vascular smooth muscle cells (VSMCs) in leptomeningeal vessels of 12–15 month old Tg2576 mice were stained with methoxy-X04 (blue) and phalloidin-Alexa 488 (green), respectively, and imaged with a two-photon microscope. In vessel segments without CAA, VSMCs were arranged closely in parallel (A). In vessel segments with CAA, extent of structural disruption and VSMC loss varied according to severity of CAA. With very mild CAA (<20%), no disruption and no VSMC loss were noted (B). With moderate CAA (20–40% coverage), amyloid deposits were associated wtih early disruption of VSMC arrangement, but no significant VSMC loss (C). With advanced CAA (>40% coverage), severe disruption of VSMC arrangement and obvious VSMC loss were noted (D). Scale bar: 50 µm.
Advanced CAA is often associated with microvascular abnormalities, including microaneurysms with thinning and disruption of the tunica media, as well as fibrinoid necrosis in amyloid-laden vessels25. Frequently, affected vessels demonstrate a “double barrel” lumen, suggestive of a weakened vascular extracellular matrix (ECM) resulting in the separation of intima from media during tissue preparation26. Many of these microvascular abnormalities are confined to amyloid-laden segments, and observed in the vicinity of hemorrhagic lesions27. While these vasculopathies suggest breakdown of vascular ECM and weakening of the vessel wall, the pathogenesis of CAA-related ICH is poorly understood.
Proteases, amyloid, and intracerebral hemorrhage
It has been known for some time that Aβ induces MMP-9 protein and activity in CECs in vitro28. In addition, recent studies report that mutant Aβ (Dutch mutation) stimulated the expression of uPA and its receptor uPAR29, and induced MT1-MMP expression in human CECs30. Both uPA and MT1-MMP are upstream proteases which are known to activate MMP-9, through a cascade of proteolytic cleavages. We have examined MMP-9 expression in aged APP mice (Tg2576 mice). MMP-9 immunostaining was undetectable in young Tg2576 mice and in aged WT mice. In contrast, increased immunohistochemical staining of MMP-9 was found in amyloid-laden cerebral vessels in aged Tg2576 mice. Furthermore, the vast majority of amyloid-laden vessels that had evidence of prior microhemorrhage demonstrated MMP-9 immunostaining31. Given the role of MMP-9 in hemorrhagic transformation following ischemic stroke, these findings raise the possibility that Aβ induces vascular MMP-9 activity which contributes to the development of CAA-related spontaneous hemorrhage32.
Though this hypothesis suggests that inhibition of MMP-9 activity as a potential therapeutic target for the prevention of CAA-related hemorrhage, more recent evidence suggests that MMP-9 may play a more complex role than initially thought. In mice, MMP-9 was found to degrade soluble Aβ in the brains of mice; knockout of the gene results in increased levels of brain Aβ33. Furthermore, it appears that MMP-9 is capable of degrading aggregated amyloid fibrils and is expressed in activated astrocytes surround amyloid plaques in Tg2576 mice34. These activities suggest that MMP-9 may play a role in regulating brain Aβ levels and in limiting amyloid plaque growth. Thus, inhibiting its activity may have both salutary as well as detrimental effects.
Conclusion
CAA is a neurovascular degenerative disease resulting in progressive dysfunction of the neurovascular unit with increasingly severe clinical consequences (Table 1). Recent findings support the idea that severe neurovascular dysfunction occurs prior to significant changes in vascular structure and may lead to chronic white matter ischemia. However, the complication of vascular rupture and spontaneous hemorrhage appears to be a consequence of advanced disease, occurring only after significant cell loss and compromise in structural integrity of the affected arterioles. Understanding the relationship between amyloid accumulation and the dysfunction of elements of the neurovascular unit will be important for identifying potential therapeutic targets to prevent these clinical consequences.
Table 1.
Progression of CAA
| Elevated soluble Aβ |
Early CAA | Moderate CAA | Advanced CAA | Dyshoric vessels |
|
|---|---|---|---|---|---|
|
Vascular Pathology |
None | None | Altered VSMC architecture |
Amyloid replacement of VSMCs |
Fibrinoid necrosis, microaneurysms |
| Cell death | None | None | VSMCs (early loss) |
VSMCs (severe loss) |
VSMCs, CECs, pericytes |
|
Vascular Function |
Impaired reactivity (moderate) |
Impaired reactivity (severe) |
Absent reactivity |
Absent reactivity |
Absent reactivity |
|
Brain Pathology |
None | White matter disease and cortical infarcts | Frank hemorrhage |
||
| Clinical | ? | Cognitive dysfunction? | Major M&M | ||
VSMCs, vascular smooth muscle cells
CECs, cerebral endothelial cells
Acknowledgements
This work was supported by NIH R01 NS048283, P01 NS032636, a grant from the American Health Assistance Foundation and the Hope Center for Neurological Disorders (J-M.L.), and NIH K08 NS053899, PO1 NS032636, and a grant from the American Health Assistance Foundation (G.J.Z.).
Footnotes
Conflicts of Interest Disclosure
None.
References
- 1.Vinters HV. Cerebral amyloid angiopathy. A critical review. Stroke. 1987;18:311–324. doi: 10.1161/01.str.18.2.311. [DOI] [PubMed] [Google Scholar]
- 2.Prohovnik I, Mayeux R, Sackeim HA, Smith G, Stern Y, Alderson PO. Cerebral perfusion as a diagnostic marker of early alzheimer's disease. Neurology. 1988;38:931–937. doi: 10.1212/wnl.38.6.931. [DOI] [PubMed] [Google Scholar]
- 3.Smith CD, Andersen AH, Kryscio RJ, Schmitt FA, Kindy MS, Blonder LX, Avison MJ. Altered brain activation in cognitively intact individuals at high risk for alzheimer's disease. Neurology. 1999;53:1391–1396. doi: 10.1212/wnl.53.7.1391. [DOI] [PubMed] [Google Scholar]
- 4.Paris D, Humphrey J, Quadros A, Patel N, Crescentini R, Crawford F, Mullan M. Vasoactive effects of a beta in isolated human cerebrovessels and in a transgenic mouse model of alzheimer's disease: Role of inflammation. Neurol Res. 2003;25:642–651. doi: 10.1179/016164103101201940. [DOI] [PubMed] [Google Scholar]
- 5.Iadecola C. Cerebrovascular effects of amyloid-beta peptides: Mechanisms and implications for alzheimer's dementia. Cell Mol Neurobiol. 2003;23:681–689. doi: 10.1023/A:1025092617651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Han BH, Abousaleh F, Koenigsknecht-Talboo J, Brendza RP, Edeh SN, Dietrich HH, Holtzman DM, Zipfel GJ. Cerebrovascular dysfunction precedes structural disruption of vascular smooth muscle cells in a mouse model of cerebral amyloid angiopathy. Society for Neuroscience. 2007 [Google Scholar]
- 7.Christie R, Yamada M, Moskowitz M, Hyman B. Structural and functional disruption of vascular smooth muscle cells in a transgenic mouse model of amyloid angiopathy. Am J Pathol. 2001;158:1065–1071. doi: 10.1016/S0002-9440(10)64053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park L, Zhou P, Pitstick R, Capone C, Anrather J, Norris EH, Younkin L, Younkin S, Carlson G, McEwen BS, Iadecola C. Nox2-derived radicals contribute to neurovascular and behavioral dysfunction in mice overexpressing the amyloid precursor protein. Proc Natl Acad Sci U S A. 2008;105:1347–1352. doi: 10.1073/pnas.0711568105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfeifer LA, White LR, Ross GW, Petrovitch H, Launer LJ. Cerebral amyloid angiopathy and cognitive function: The haas autopsy study. Neurology. 2002;58:1629–1634. doi: 10.1212/wnl.58.11.1629. [DOI] [PubMed] [Google Scholar]
- 10.Holland CM, Smith EE, Csapo I, Gurol ME, Brylka DA, Killiany RJ, Blacker D, Albert MS, Guttmann CR, Greenberg SM. Spatial distribution of white-matter hyperintensities in alzheimer disease, cerebral amyloid angiopathy, and healthy aging. Stroke. 2008;39:1127–1133. doi: 10.1161/STROKEAHA.107.497438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenberg SM. Cerebral amyloid angiopathy and vessel dysfunction. Cerebrovasc Dis. 2002;13 Suppl 2:42–47. doi: 10.1159/000049149. [DOI] [PubMed] [Google Scholar]
- 12.Hamel E, Nicolakakis N, Aboulkassim T, Ongali B, Tong XK. Oxidative stress and cerebrovascular dysfunction in mouse models of alzheimer's disease. Exp Physiol. 2008;93:116–120. doi: 10.1113/expphysiol.2007.038729. [DOI] [PubMed] [Google Scholar]
- 13.Park L, Anrather J, Zhou P, Frys K, Pitstick R, Younkin S, Carlson GA, Iadecola C. Nadph-oxidase-derived reactive oxygen species mediate the cerebrovascular dysfunction induced by the amyloid beta peptide. J Neurosci. 2005;25:1769–1777. doi: 10.1523/JNEUROSCI.5207-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davis-Salinas J, Saporito-Irwin SM, Cotman CW, Van Nostrand WE. Amyloid beta-protein induces its own production in cultured degenerating cerebrovascular smooth muscle cells. J Neurochem. 1995;65:931–934. doi: 10.1046/j.1471-4159.1995.65020931.x. [DOI] [PubMed] [Google Scholar]
- 15.Verbeek MM, de Waal RM, Schipper JJ, Van Nostrand WE. Rapid degeneration of cultured human brainpericytes by amyloid beta protein. J Neurochem. 1997;68:1135–1141. doi: 10.1046/j.1471-4159.1997.68031135.x. [DOI] [PubMed] [Google Scholar]
- 16.Thomas T, Thomas G, McLendon C, Sutton T, Mullan M. Beta-amyloid-mediated vasoactivity and vascular endothelial damage. Nature. 1996;380:168–171. doi: 10.1038/380168a0. [DOI] [PubMed] [Google Scholar]
- 17.Sutton ET, Hellermann GR, Thomas T. Beta-amyloid-induced endothelial necrosis and inhibition of nitric oxide production. Exp Cell Res. 1997;230:368–376. doi: 10.1006/excr.1996.3440. [DOI] [PubMed] [Google Scholar]
- 18.Van Nostrand WE, Davis-Salinas J, Saporito-Irwin SM. Amyloid beta-protein induces the cerebrovascular cellular pathology of alzheimer's disease and related disorders. Ann N Y Acad Sci. 1996;777:297–302. doi: 10.1111/j.1749-6632.1996.tb34436.x. [DOI] [PubMed] [Google Scholar]
- 19.Davis J, Van Nostrand WE. Enhanced pathologic properties of dutch-type mutant amyloid beta-protein. Proc Natl Acad Sci U S A. 1996;93:2996–3000. doi: 10.1073/pnas.93.7.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Herzig MC, Winkler DT, Burgermeister P, Pfeifer M, Kohler E, Schmidt SD, Danner S, Abramowski D, Sturchler-Pierrat C, Burki K, van Duinen SG, Maat-Schieman ML, Staufenbiel M, Mathews PM, Jucker M. Abeta is targeted to the vasculature in a mouse model of hereditary cerebral hemorrhage with amyloidosis. Nat Neurosci. 2004;7:954–960. doi: 10.1038/nn1302. [DOI] [PubMed] [Google Scholar]
- 21.McGowan E, Pickford F, Kim J, Onstead L, Eriksen J, Yu C, Skipper L, Murphy MP, Beard J, Das P, Jansen K, Delucia M, Lin WL, Dolios G, Wang R, Eckman CB, Dickson DW, Hutton M, Hardy J, Golde T. Abeta42 is essential for parenchymal and vascular amyloid deposition in mice. Neuron. 2005;47:191–199. doi: 10.1016/j.neuron.2005.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wisniewski HM, Wegiel J. Beta-amyloid formation by myocytes of leptomeningeal vessels. Acta Neuropathol (Berl) 1994;87:233–241. doi: 10.1007/BF00296738. [DOI] [PubMed] [Google Scholar]
- 23.Kalaria RN, Cohen DL, Greenberg BD, Savage MJ, Bogdanovic NE, Winblad B, Lannfelt L, Adem A. Abundance of the longer a beta 42 in neocortical and cerebrovascular amyloid beta deposits in swedish familial alzheimer's disease and down's syndrome. Neuroreport. 1996;7:1377–1381. doi: 10.1097/00001756-199605310-00009. [DOI] [PubMed] [Google Scholar]
- 24.Coria F, Rubio I. Cerebral amyloid angiopathies. Neuropathol Appl Neurobiol. 1996;22:216–227. [PubMed] [Google Scholar]
- 25.Okazaki H, Reagan TJ, Campbell RJ. Clinicopathologic studies of primary cerebral amyloid angiopathy. Mayo Clin Proc. 1979;54:22–31. [PubMed] [Google Scholar]
- 26.Gilbert JJ, Vinters HV. Cerebral amyloid angiopathy: Incidence and complications in the aging brain. I. Cerebral hemorrhage. Stroke. 1983;14:915–923. doi: 10.1161/01.str.14.6.915. [DOI] [PubMed] [Google Scholar]
- 27.Vonsattel JP, Myers RH, Hedley-Whyte ET, Ropper AH, Bird ED, Richardson EP., Jr Cerebral amyloid angiopathy without and with cerebral hemorrhages: A comparative histological study. Ann Neurol. 1991;30:637–649. doi: 10.1002/ana.410300503. [DOI] [PubMed] [Google Scholar]
- 28.Deb S, Gottschall PE. Increased production of matrix metalloproteinases in enriched astrocyte and mixed hippocampal cultures treated with beta-amyloid peptides. J Neurochem. 1996;66:1641–1647. doi: 10.1046/j.1471-4159.1996.66041641.x. [DOI] [PubMed] [Google Scholar]
- 29.Davis J, Wagner MR, Zhang W, Xu F, Van Nostrand WE. Amyloid β-protein stimulates the expression of urokinase-type plasminogen activator (upa) and its receptor (upar) in human cerebrovascular smooth muscle cells. J Biol Chem. 2003;278:19054–19061. doi: 10.1074/jbc.M301398200. [DOI] [PubMed] [Google Scholar]
- 30.Jung SS, Zhang W, Van Nostrand WE. Pathogenic abeta induces the expression and activation of matrix metalloproteinase-2 in human cerebrovascular smooth muscle cells. J Neurochem. 2003;85:1208–1215. doi: 10.1046/j.1471-4159.2003.01745.x. [DOI] [PubMed] [Google Scholar]
- 31.Lee J-M, Yin KJ, Hsin I, Chen SW, Fryer JD, Hotlzman DM, Hsu CY, Xu J. Matrix metalloproteinase-9 and spontaneous hemorrhage in an animal model of cerebral amyloid angiopathy. Ann Neurol. 2003 doi: 10.1002/ana.10671. in press. [DOI] [PubMed] [Google Scholar]
- 32.Lee J-M, Yin KJ, Hsin I, Chen SW, Fryer JD, Holtzman DM, Hsu CY, Xu J. Matrix metalloproteinase-9 in cerebral amyloid angiopathy-related hemorrhage. J Neurol Sci. 2005;229–230:249–254. doi: 10.1016/j.jns.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 33.Yin KJ, Cirrito JR, Yan P, Hu X, Xiao Q, Pan X, Bateman R, Song H, Hsu FF, Turk J, Xu J, Hsu CY, Mills JC, Holtzman DM, Lee J-M. Matrix metalloproteinases expressed by astrocytes mediate extracellular amyloid-beta peptide catabolism. J Neurosci. 2006;26:10939–10948. doi: 10.1523/JNEUROSCI.2085-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yan P, Hu X, Song H, Yin KJ, Bateman R, Cirrito JR, Xiao Q, Hsu FF, Turk J, Xu J, Hsu CY, Holtzman DM, Lee J-M. Matrix metalloproteinase-9 degrades amyloid-beta fibrils in vitro and compact plaques in situ. J Biol Chem. 2006;281:24566–24574. doi: 10.1074/jbc.M602440200. [DOI] [PubMed] [Google Scholar]