Abstract
Mosquito swarms are poorly understood mating aggregations. In the malaria vector Anopheles gambiae Giles, they are known to depend on environmental conditions, such as the presence of a marker on the ground, and they may be highly relevant to reproductive isolation. We present quantitative measurements of individual An. gambiae positions within swarms from Donéguébougou, Mali, estimated by stereoscopic video image analysis. Results indicate that swarms in this species are approximately spherical, with an unexpectedly high density of individuals close to the swarm centroid. This high density may be the result of individual males maximizing their probability of encountering a female or a product of mosquito orientation through cues within the swarm. Our analysis also suggests a difference in swarm organization between putative incipient species of An. gambiae with increasing numbers of males. This may be related to a difference in marker use between these groups, supporting the hypothesis that swarming behavior is a mechanism of mate recognition and ultimately reproductive isolation.
Keywords: Anopheles gambiae, swarm, mate recognition, three-dimensional localization, stereoscopic image analysis
Mosquito swarms have long been observed and described, but with few exceptions (such as Gibson 1985), their physical organization and dynamics remain poorly understood, especially in the field. In this paper, we address two fundamental issues as they relate to swarms of the malaria vector Anopheles gambiae Giles. The first is how swarms of males are organized in this species. The second is what role this organization might play in mate choice and reproductive isolation within the An. gambiae species complex.
Part of the reason little is known about mosquito swarms is that they are generally difficult to study in a natural setting. Locating the swarms can be difficult for many species (Service 1993), and even once found, it is difficult to gather quantitative measurements (although not impossible; see Yuval and Bouskila 1993). Previous studies on insect swarming generally used image acquisition and processing techniques to examine swarms (Okubo et al. 1981, Riley 1993). Stereoscopic image analysis in particular has been used to study mosquito swarms, resulting in some sophisticated statistical methods for localizing individuals (Ikawa and Okabe 1997). However, these approaches have yielded limited biological insight into the organization and dynamics of mosquito swarms, probably because of the technical difficulty involved in image acquisition and analysis.
Anopheles gambiae swarms are known to be composed almost entirely of males (Diabaté et al. 2006), and are often, although not always, found over “swarming markers” (Marchand 1984, Charlwood et al. 2002). As in other dipterans, they are thought to be mating aggregations (Downes 1969, Sullivan 1981). They probably fit the strict definition of a lek (an area where males congregate to secure mates), especially because they represent non–resource-based aggregations. There may be competition for more advantageous positions within the aggregation enabling better access to mates, as observed in other lekking species (Höglund and Alatalo 2007). Supporting the idea of competition within anopheline swarms, previous work has shown an effect of body size on mating success in Anopheles freeborni Aitken (Yuval and Bouskila 1993) (although this was not detected in An. gambiae; Charlwood et al. 2002) and physical combat for females within swarms has been observed in other genera (Sullivan 1981).
Swarms of males may serve various purposes with respect to mating. They may reduce the risk to an individual mosquito of searching for a female or make the probability of encountering one higher because the range of attraction is short (Charlwood and Jones 1979). They may also help ensure that males do not expend reproductive effort on females that have already been inseminated. These advantages would depend on females not entering the swarm randomly but instead entering specifically to mate.
Especially if females enter nonrandomly, the swarm of males may operate as an attractant to females and may contain cues to indicate if the males in the swarm are con- or heterospecific. Differences between sibling species or populations in swarming behaviors have been hypothesized (Charlwood and Jones 1980, Sullivan 1981) but are not yet supported by data. Such variation would make swarming a characteristic of the mosquito life history where reproductive isolation may evolve between sympatric taxa through the existence of mono-specific male swarms segregated by marker choice, differences in swarm organization, or temporal differences in swarming time.
Incipient speciation between the M and S molecular forms of An. gambiae has been widely discussed and studied (della Torre et al. 2002). These two taxonomic groups are distinguished by differences in ribosomal DNA (rDNA) sequences (Favia et al. 2001), but they have not exhibited barriers to hybridization in the laboratory (Diabaté et al. 2007a). In nature, however, hybrids are very rare (della Torre et al. 2001), as are heterospecific matings (Tripet et al. 2001). This indicates that the molecular forms are separated by prezygotic reproductive barriers. Such barriers could easily occur if there are differences in the structure, timing, or location of male swarms. The existence of such barriers is supported by evidence that molecular forms swarm assortatively (Diabaté et al. 2006).
It has been suggested that there are systematic differences in swarm marker choice between the molecular forms in our study area (A. D., unpublished data). Specifically, the M molecular form tends to swarm over markers of horizontal contrast, such as those formed between a grassy area and a footpath, over a small tree, or over a well. The S molecular form in this same area is almost invariably found over bare ground, with no marker discernible under the swarm. This agrees with suggestions of spatial segregation between subtaxa in the An. gambiae complex (Gilles and De Meillon 1968). If there are differences in marker use or nonuse, these might be reflected in swarm structure or dynamic.
Materials and Methods
Study Site
The swarms analyzed in this study were all filmed in the village of Donéguébougou, Mali (12°48′38″ N, 7°59′05″ W) between 29 August and 27 October 2007. Donéguébougou is a village of 1,345 inhabitants situated ≈25 km north of Bamako, in a typical Sudan Savana habitat of low grasslands on rolling hills. The area receives between 500 and 1,000 mm of rainfall annually in a highly seasonal pattern, with almost all the precipitation occurring between May and October. There is an alternating dry season from November to April. Malaria transmission in this area is seasonal, coincident with rains and higher vector densities from June to November (Dicko et al. 2004). Further details about the environment and human activities are given in Touré et al. (1998).
The molecular and chromosomal form composition in this area is known to shift with changes in climatic conditions between wet and dry seasons. The M molecular form (Mopti chromosomal form) is more prevalent together with Anopheles arabiensis Patton during the dry season, but they are gradually replaced by the S molecular form (first Savana then Bamako chromosomal forms) as precipitation increases (Manoukis 2006). This region has the M and S molecular forms in sympatry, and for our study period, both were present.
Donéguébougou has been the site of previous research on An. gambiae swarms (Diabaté et al. 2007b). That work has shown that An. gambiae males tend to swarm at the same locations throughout the season and that particular locations are consistently associated with swarms of a single molecular form. We used this information to attempt to film swarms of both molecular forms.
Filming Procedures
Approximately 40 min before sunset, twin Sony HDR-HC7 high-definition digital camcorders (Sony, Tokyo, Japan) were mounted on a horizontal metal bar affixed to a tripod. The lenses were aligned to be as close to parallel as possible by using a ruler against the front of both barrels simultaneously to ensure that they were flush. The zoom setting was set to minimum before filming began. All the analyzed footage was captured in infrared (IR) filming mode with illumination from a pair of IRLamp6 external IR light sources (Wildlife Engineering, Tempe, AZ), because there is some evidence that An. gambiae can detect light in the red range but not in IR (Gibson 1995). The cameras were controlled through a LANC synchronizer (Rob Crockett, Ledametrix-.com, Grass Valley CA) that started and stopped the cameras together, permitting films recorded by the camera pair to be accurately synchronized.
Each evening we recorded a calibration sequence. This entailed filming a one-half frame of PVC piping with markings in the horizontal and vertical directions every 10 cm. The alignment of both the frame and the tripod was checked with bubble levels. The frame was measured at distances of 1, 2, 3, and 4 m from the cameras.
Approximately 20 min after sunset, swarms began to appear. As soon as males were visibly congregating at the target location or a nearby site, we began filming and attempted to continue filming as long as there were males present. During filming, occasionally camera position or orientation had to be changed to accommodate the movement of the swarm. Toward the end of filming, we sampled flying males with a hand net to evaluate the molecular form of the individuals in the swarm.
Image Processing and Mosquito Localization
MPEG compressed high-definition movies from the cameras were processed with a series of free software packages (see Appendix). Grayscale stereomovies were ultimately produced, which could be extracted to a series of still images for measurement with ImageJ (Abramoff et al. 2004). Further details on the image processing and method used for localization are given in the Appendix.
We detected some image distortion, based on the analysis of calibration images, where the bias in position estimates was negative at small distances and positive at longer distances. This is probably because of inaccuracies in the camera model geometry (see Appendix) or lens distortion. To correct for this effect, we fit a second-degree polynomial regression model to a set of calibration data points at distances from 1 to 4 m, which we used to adjust the estimates of individual locations in swarm footage (see Results).
Calibration
We verified the accuracy of localization using our protocol by making measurements on calibration images. To do this, we measured four 10-cm intervals marked on footage of the calibration PVC frame with horizontal and vertical arms, taken on different days and at four known distances from the camera (1, 2, 3, and 4 m). Two of the 10-cm distances were along the horizontal plane of the image and two along the vertical plane.
We used four such sequences (16 stereo images) to fit a polynomial correction model (a total of 64 measurements of 10-cm intervals). This can be considered a training dataset. We tested our ability to estimate position and distances between points by measuring the same intervals in a different set of three sequences (12 stereo images, 12 distances estimated at each depth, total = 48 distances estimated).
From this testing dataset, we found the mean estimate (x̄) of the 10-cm distance at all depths from the camera to be 10.13 ± 1.36 (SD) cm. The accuracy of these estimates was lower with greater distance, and there was evidence of some remaining systematic change in bias with distance (1 m: x̄ = 10.88 ± 0.58 cm. 2m: x̄ = 10.22 ± 1.02 cm. 3 m: x̄ = 9.80 ± 1.41 cm. 4m: x̄ = 9.67 ± 1.87 cm), although these were much reduced over the uncorrected images. We note that the mean distance from the cameras to individual mosquitoes used in the analysis was 2.61 ± 0.73 m.
Data Analysis
We estimated the locations of all visible individual mosquitoes in one image about every 15 s of footage. At each of these points, we confirmed that we were measuring the position of swarming mosquitoes by stepping forward and backward in the image sequence to follow individuals and ensure that they were not passing through the swarm or that they were unlikely to be An. gambiae.
The data include a high degree of nesting, which we examined and took into account in our statistical tests: We have positions of individuals within an image (time point), several time points within a swarm, and several swarms per molecular form.
In several of the analyses, we calculated the swarm centroid, often to recenter the positions of individuals to be comparable between images. The centroid is defined as the mean of the {X,Y,Z} positions of mosquitoes seen in the swarm.
Results
Spatial and Temporal Sampling
Overall, we estimated >5,000 positions of individual mosquitoes seen in 376 stereographic images. These images were taken from footage of 12 An. gambiae swarms from all areas of the village, each filmed on a different date between August and October 2007. An overview of the data and coverage is given in Table 1.
Table 1.
Overview of the images analyzed, molecular form composition, and environmental conditions for the swarms used in the analysis
| Date | No. (mean/range)a | N images | Timeb | Mc | Sd | Swarm marker | Gust speede |
|---|---|---|---|---|---|---|---|
| 09 Oct. | 13.7/7–19 | 16 | 19.7–24.7 | 0 | 17 | Bare ground | 0.00 |
| 01 Oct. | 13.8/8–18 | 21 | 21.0–27.2 | 0 | 3 | Bare ground | 0.38 |
| 27 Sep. | 13.6/5–26 | 27 | 22.3–31.2 | 0 | 2 | Bare ground | 0.38 |
| 21 Sep. | 12.8/6–21 | 13 | 31.6–35.0 | 0 | 13 | Bare ground | 0.00 |
| 11 Sep. | 13.4/6–18 | 25 | 30.0–36.7 | 0 | 4 | Bare ground | 0.00 |
| 04 Sep. | 27.6/21–33 | 9 | 17.0–19.5 | 0 | 2 | Bare ground | 4.95 |
| 29 Aug. | 19.7/5–32 | 35 | 27.1–38.6 | 0 | 11 | House contruction | 7.23 |
| 27 Oct. | 7.4/2–11 | 57 | 32.4–50.0 | 5 | 0 | Garbage | 0.00 |
| 24 Oct. | 7.1/3–13 | 26 | 19.3–26.2 | 2 | 0 | Wall | 0.00 |
| 19 Oct. | 4.7/2–8 | 31 | 24.8–33.6 | 2 | 0 | Vegetation | 0.00 |
| 16 Sep. | 15.4/4–40 | 52 | 30.0–45.3 | 3 | 0 | Well | 0.00 |
| 08 Sep. | 33.2/22–47 | 28 | 21.6–31.3 | (M)f | 0 | Vegetation | 1.14 |
All swarms were filmed in 2007.
The no. of mosquitoes visible per image.
Minutes after sunset (data from U.S. Naval Observatory).
Number of M molecular form mosquitoes sampled from the swarm.
Number of S molecular form mosquitoes sampled from the swarm.
In meters per second. Data obtained from a weather station in Banambani, 2 km from Dongbougou.
No samples were available for typing from this swarm. However, samples were taken at this same location of a different date and were found to be the M molecular form.
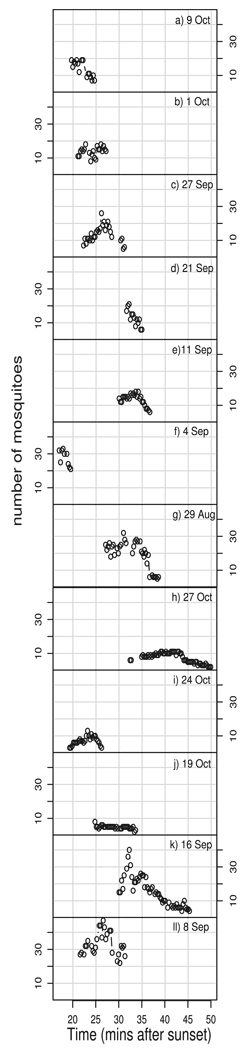
The number of visible mosquitoes per swarm varied over time as shown in Fig. 1. Although we were usually not able to capture the beginning of the swarms, we often did film while numbers were still increasing, and until swarming ended. We qualitatively examined all our response variables for systematic effects of time but found that there were none that could be distinguished from those that could be attributed to changes in the number of individuals, a variable we explicitly analyzed.
Fig. 1.
Number of mosquitoes over time for each of the swarms. (a–g) S molecular form swarms. (h–l) M molecular form swarms.
Distribution of Individuals Within Swarm
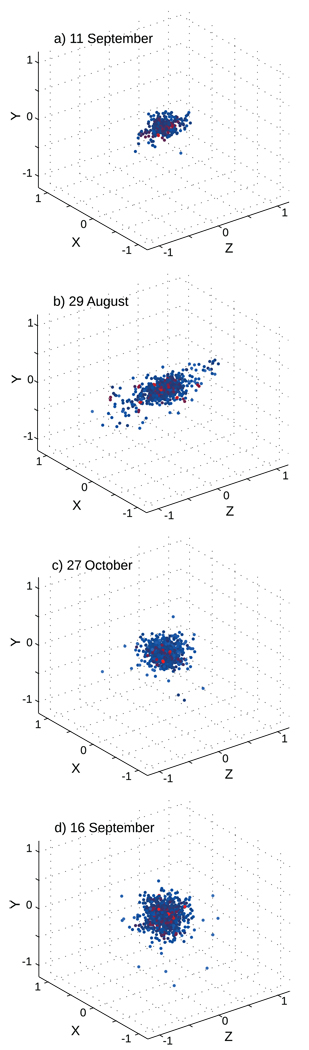
We performed a qualitative examination of the distribution of individuals within swarms, samples of which are shown in Fig. 2. For each swarm, the positions of all individuals seen throughout the filming are shown, with the color of each point representing the distance to the nearest neighbor at the moment the individual was observed (hereafter DNN). To compare positions from different times, we normalized all positions based on the swarm centroid at the moment when the individual was detected; this was accomplished by setting the swarm centroid to always be at position {X,Y,Z} = {0,0,0}. Doing so eliminated deformation of the swarm shape caused by camera or overall swarm movement. Interactive and animated visualizations of the swarms are given in the Supplemental Information.
Fig. 2.
A sampling of cumulative three-dimensional locations of individuals detected in each swarm. All positions are given in meters relative to the swarm centroid at the moment of detection to control for the effect of camera movement. Colors indicate the natural logarithm of the distance to nearest neighbor (DNN): red = closer, blue = further (scale is consistent between panels). Axis orientation relative to camera position is as follows: y = vertical axis, x = horizontal, left/right, and z = horizontal, distance from the camera. (a and b) Swarms of the S molecular form. (c and d) M form swarms. Note that the swarm represented in b was filmed durng conditions of high wind, which might explain the atypically horizontally elongated swarm shape.
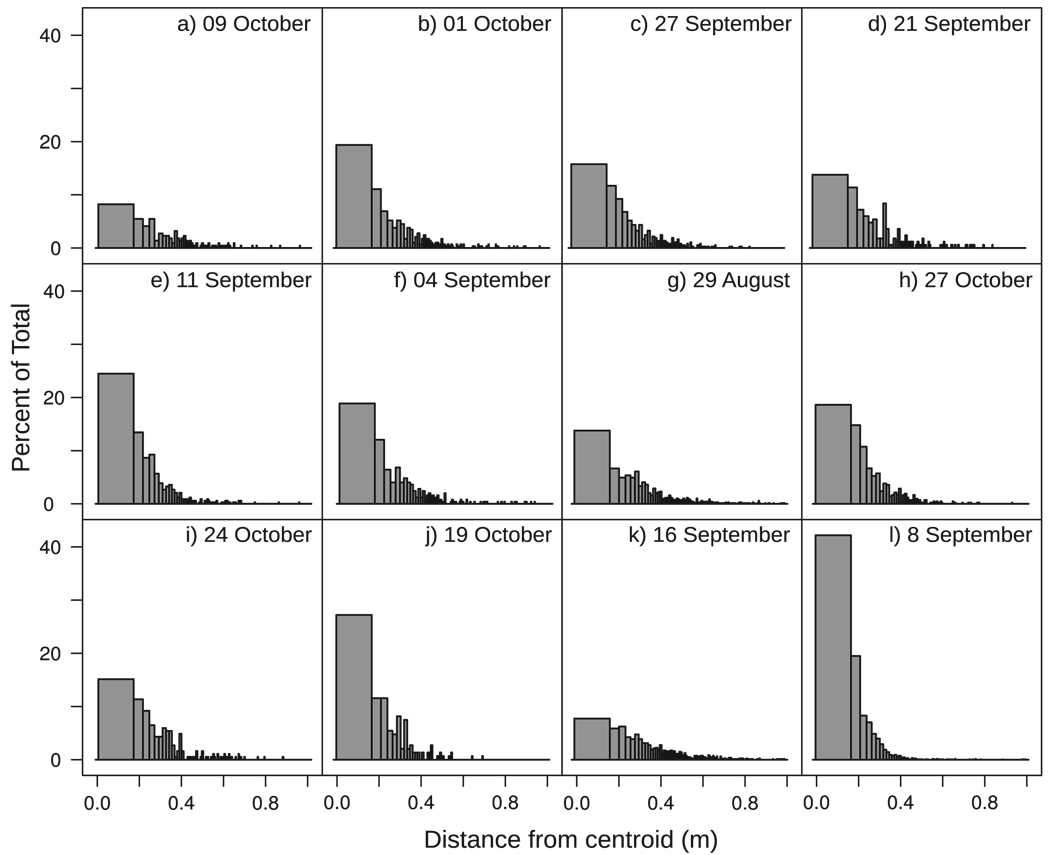
Mosquito density decreases with increasing distance from the swarm centroid, often quite quickly (Fig. 3). The data shown in Fig. 3 might be misleading, however, because they are a composite of all observations of individual mosquitoes over all images analyzed per swarm. They do not include any information on how density might have changed over time.
Fig. 3.
Distributions of distances from the centroid over all images by swarm. Intervals represent successive equal volumes of 0.02m3 (20 liters) around the swarm centroid. Only observations within a 1-m radius of the centroid are included for clarity (98.5% of the data are in this range).
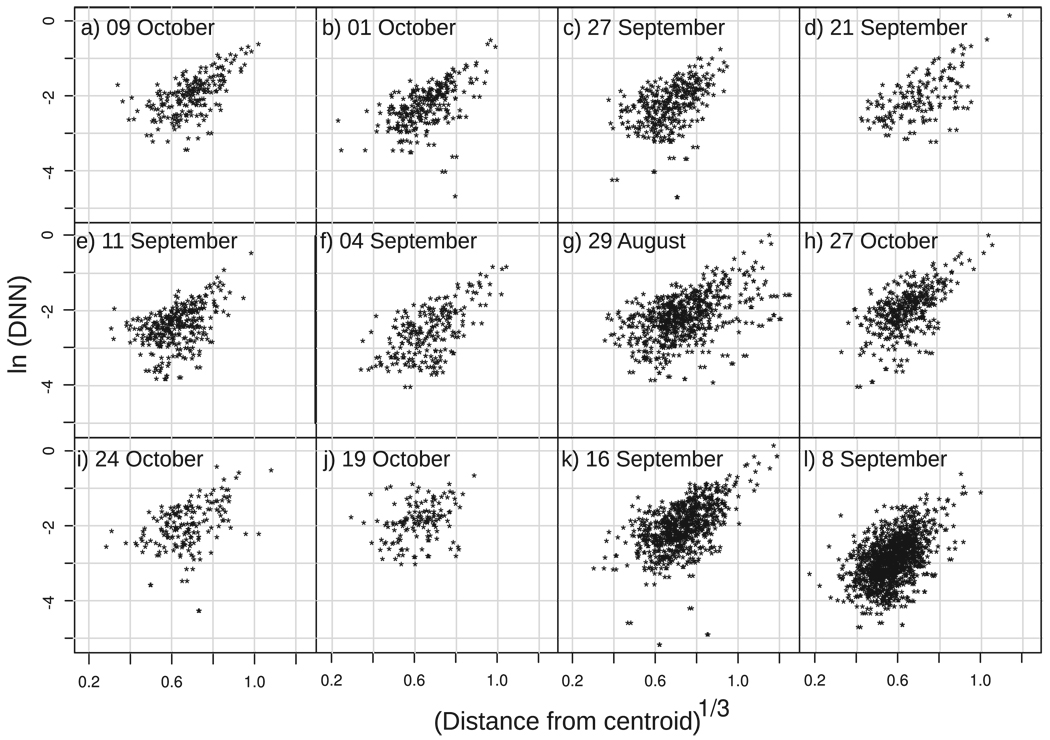
A more stringent test of the effect of position in the swarm on density is shown in Fig. 4. For each swarm, a positive relationship between distance from the swarm centroid and DNN is evident. Fig. 4 preserves information about changes that may have occurred over time, because DNN is calculated for the moment when each individual was observed. We tested whether there was any statistical relationship between the distance from the swarm centroid and the natural logarithm of DNN using a linear mixed effect model (Pinheiro and Bates 2000), with the cube root of the distance from swarm centroid and molecular form composition of the swarms as fixed effects and swarm identity as a random variable. Natural logarithm transformations were used to reduce heteroskedasticity in the data, whereas the cubed root transformation was used to model the increase in volume with distance from the swarm centroid. This analysis showed a significant effect of distance from the centroid on the distance to nearest neighbor, taking into account random differences between swarms and that this effect did not vary between molecular forms (Table 2).
Fig. 4.
Plots of the natural logarithm of distance to nearest neighbor (abcissa) versus the cube root of Euclidean distance from the swarm centroid (ordinate) for each swarm.
Table 2.
Linear mixed-effects model of the effect of the cube root of Euclidean distance from the swarm centroid () and molecular form composition on the natural logarithm of distance to nearest neightbor
| Source | Value | SE | df | t | P |
|---|---|---|---|---|---|
| Intercept | −3.754 | 0.162 | 5196 | −23.257 | <0.0001 |
| 2.461 | 0.152 | 5196 | 16.205 | <0.0001 | |
| Molecular form | −0.180 | 0.128 | 10 | −1.405 | 0.1903 |
Random effect = swarm identity.
We further tested if there was any difference in the swarm structure along the horizontal versus the vertical directions by comparing the extent of swarm size along these directions. Wilcoxon signed-rank tests of paired observations of swarm width (X) and height (Y) taken from each image were performed per swarm. After Boneferroni correction, 4 of the 12 swarms had significant differences in width versus height (we denote mean difference as x̄), but these differences were neither consistent nor very large (29 August X < Y: x̄ = 0.16 ± 0.11; 11 September X > Y: x̄ = 0.11 ± 0.07; 16 September X > Y: x̄ = 0.19 ± 0.14; 19 October X < Y: x̄ = 0.18 ± 0.14).
Effect of Numbers of Individuals on Swarm Density
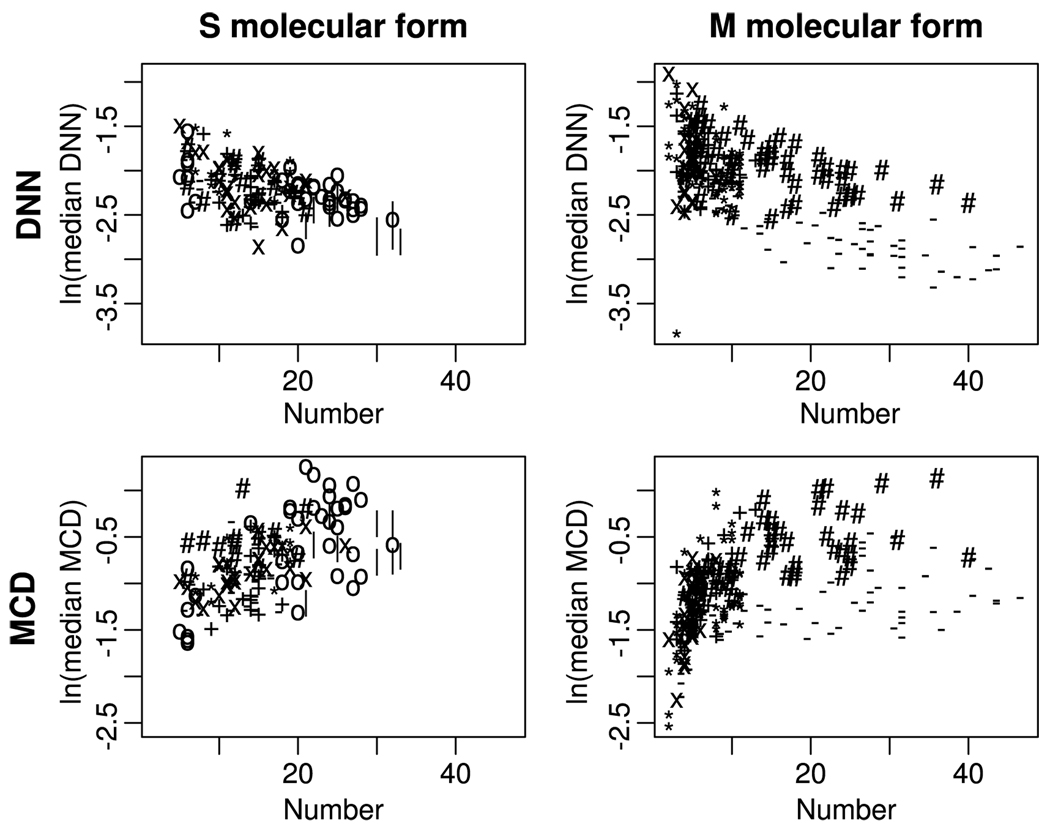
To examine the effect of numbers of individuals on swarm organization, we analyzed each image, and not each individual mosquito, as a sample. We used the number of visible mosquitoes as a predictor of median DNN and overall swarm size as estimated by the maximum observed distance from the swarm centroid (hereafter MCD).
Analysis of covariance (ANCOVA) of the effect of numbers of individuals and molecular form identity on the median DNN and MCD are given in Table 3. The number of individuals in the swarm had a significant negative relationship on proximity to neighbors (median DNN) and a significant positive relationship on swarm size (MCD), although the latter relationship was different depending on the molecular form of the swarm. These relationships are shown graphically in Fig. 5.
Table 3.
ANCOVA of the effect of the no. of individuals, swarm identity, and molecular form composition on proximity between mosquitoes [log(DNN)] and overall swarm size [log(MCD)]
| Source | Partial SS | df | F | P |
|---|---|---|---|---|
| Response: log(median DNN) |
||||
| Number | 29.087 | 1 | 340.19 | <0.001 |
| Swarm | 8.291 | 10 | 9.70 | <0.001 |
| Form | 0.004 | 1 | 0.04 | 0.840 |
| Residual | 31.038 | 363 | MS = 0.086 | |
| Response: log(MCD) | ||||
| Number | 13.750 | 1 | 126.18 | <0.001 |
| Swarm | 19.449 | 10 | 17.85 | <0.001 |
| Form | 5.746 | 1 | 52.73 | <0.001 |
| Residual | 39.557 | 363 | MS = 0.109 | |
Fig. 5.
Effect of the number of mosquitoes in a swarm on DNN (top row) and MCD (bottom row) by molecular form (left column = S form, right column = M form). Plotting point types indicate swarm identity: * = 09 Oct. (S) or 27 Oct. (M); + = 01 Oct. (S) or 24 Oct. (M);x = 27 Sep. (S) or 19 Oct. (M); # = 21 Sep. (S) or 16 Sep. (M); − = 11 Sep. (S) or 8 Sep. (M); = 04 Sep. (S); o = 29 Aug. (S).
Discussion
Our analysis showed the basic characteristics of An. gambiae male swarm structure, including novel information on swarm size, shape, temporal extent, and spatial patterns of density. We also analyzed an aspect of swarm dynamics—the effect of increasing numbers on swarm organization—which suggests a difference between incipient species, possibly driven by fundamental insect perception mechanisms.
Fig. 2 qualitatively shows that swarms were often spherical, although occasionally flattened along the horizontal axis. Flattening may have been caused by wind (see swarm of 29 August). Perhaps the most striking feature of the distribution of individuals within a swarm is the very high density of individuals around the swarm centroid, a feature that was seen in all samples.
The high density at the swarm center deviates from what would be expected under random movement through a predefined spherical volume. If mosquitoes were moving in this manner, we would expect to find a roughly uniform distribution of locations where individuals were observed throughout the volume. The equivolumnar histograms shown in Fig. 3 would produce bars of approximately the same height. Instead, we see massive aggregation around the center of the volume. This result is further supported by the significant positive effect of distance from the swarm centroid on DNN.
There are several hypotheses that could explain why individual An. gambiae are seen excessively often near the swarm centroid. One possibility is that mosquitoes actually prefer this location because it allows them the quickest access to any part of the swarm periphery, should a female enter the aggregation. This ability might be advantageous if females do not enter from any particular direction or height. Alternatively, females may simply be more likely to pass through the center of the swarm, making this location advantageous to waiting males. Females passing through the centroid may result from their flying toward the swarm based on sound; possibly, this cue leads them more often to pass through the center.
Another possibility is that the concentration of individuals near the swarm centroid is the product of mosquito perception of the swarm marker. If individuals are trying to remain directly over the marker so as not to lose contact with the swarm, a pattern such as the one observed might emerge. Although it is well known that An. gambiae use swarm markers on the ground (Charlwood and Jones 1980), one half of the swarms in this study were observed to form over bare ground, so this explanation should not hold for them.
Male mosquito perception in the swarm may not be limited to the marker on the ground, however. The males can detect the flight tone of other males and indeed may use this at short range to differentiate between males and females (Gibson and Russell 2006) and probably also to avoid collisions while swarming. Detecting other males makes it possible that swarming An. gambiae try to maximize their proximity to other males as a way to remain in the region of the swarm most likely to be detected and entered by a female. Such a mechanism could be similar to the “Boids” flocking model (Reynolds 1987) or elements of it, where agents use three simple rules (separation, alignment, and cohesion) to organize themselves. We note that swarm organization using perception of other males is not exclusive of marker-based methods.
Changes in the distribution of individuals within a swarm over time could not be distinguished from those that may have been caused by changes in the number of mosquitoes. The number of mosquitoes in a swarm did have a significant negative relationship with the distances between them and a significant positive effect on the size of the swarm. In the case of the former, we also detected a significant difference between molecular forms: the M molecular form swarms increased in size with number and apparently plateau to a maximum that varied between swarms. The S molecular form swarms appeared to grow in size without this constraint.
This observation may be indicative of the role of a swarm marker in organizing the males in the M form: if individuals perceive the marker, they may not be able to stray too far from it, leading to an effective ceiling on the maximum volume a swarm may occupy. The S molecular form individuals found in this study were almost always over bare ground, so this may not have been a constraint in their case.
Taken together, the results of this study support the idea that swarming males are aware of both other males and swarm markers, when these are used. A difference in the weighting given to each might have led to spatial segregation of the molecular forms of An. gambiae in Donéguébogou, a situation that would have made swarming behavior a natural target for mate recognition mechanisms to arise in females and may lead to reproductive isolation.
Future studies on male swarming in this species using video stereoscopy could focus on the many topics that remain completely unexplored: movement of the entire swarm over a marker or over bare ground, orientation of individuals with respect to wind, and where females actually encounter males to mate with within the swarm volume, to name a few. Moreover, we are working on extending this method to tracking individual trajectories though space, which should make possible other research questions and more sophisticated analytical approaches (Okubo et al. 1981). We also note that stereo sound–based localization of swarms could provide novel insight into the swarming behavior of An. gambiae.
Acknowledgments
We thank the residents of Donéguébougou for their kind permission to conduct the filming in their village; S. Doumbia, S. F. Traoré , and R. Sakai of the Malaria Research and Training Center (MRTC), University of Bamako, for invaluable logistical assistance and discussion of this work; M. B. Touré and the GIS team at MRTC for the weather data from Banambani; R. Gwadz and F. Tokumasu of the Laboratory of Malaria and Vector Research, NIAID, for assistance with planning, support, and discussion; C. Reynolds for discussions on a visual approach to these data; C. Struchiner of the Fundação Oswaldo Cruz and P. McQueen of the NIH for statistical advice; and C. Taylor and D. Earl of UCLA for valuable discussions. We acknowledge the support of the Bioinformatics and Scientific Information Technology Program (BSIP), NIAID OTIS for hosting materials related to this work. This research was supported by the Intramural Research Program of the NIH, NIAID.
Appendix
Estimating the position of an individual mosquito in video footage involved (1) preparing a stereo image from the movies of two cameras, (2) measuring the location of the individual in the images, and (3) using simple geometry to obtain the estimate of position in three dimensions. Figure 6a shows an illustration of the camera orientations and how they are related to world coordinates. We will use that notation through-out the following discussion.
Fig. 6.
Method used for estimating positions (see text). (a) Camera and axis orientation. (b) Representation of the pin-hole camera model.
The camera lenses are aligned to present a parallel projection. We could identify individual mosquitoes (correspond) using the epipolar constraint method (meaning that the two images of the same mosquito should be aligned vertically; see Hartley and Zisserman 2004) and by stepping through the images confirm that both points did indeed represent a single individual by observing that they were moving in similar directions. We made our measurements on stereo images, which are superimpositions of the images captured by each camera at the same moment. We were able to produce these images and make measurements on them using freely available third party software packages and some computer code written for this purpose (details below).
Localization of individual mosquitoes in three-dimensional space once stereoimages were available consisted of two discrete steps. First, we estimated the distance from the cameras along the Z axis (Fig. 6a) of the mosquito using triangulation on measurements taken from the stereoscopic image. Second, we used a pin-hole camera model on one of the images from the stereo pair to determine the ray along which the mosquito lay. Using the estimate of distance and knowing the direction, we could obtain an estimate of the position of the individual in three dimensions. First we describe the camera model, because this is fundamental to the remainder of the discussion.
The camera model is shown graphically in Fig. 6b. We require f, the focal length of the lens in millimeters, the size of the sensor (U = horizontal size in millimeters, V = vertical size in millimeters), the position of a mosquito in the image ({up,υ p}, in pixels), and the number of pixels in the image horizontally and vertically (h and w, respectively). We first calculate {u,υ} in millimeters, as follows; note that u is relative to the image center, which is defined as {h/2,w/2}:
| [1] |
Distance from the camera (Z; Fig. 6b) was estimated using simple triangulation, as shown below:
| [2] |
where b is the baseline (distance between the optic centers of each camera, in m), f is the focal length (in meters), and ul and ur are the size of u for the left and right cameras, respectively, in meters. We note that, because the projections are parallel, υl = υr (where υl and υr are υ for the right and left cameras, respectively). Once Z is known, X and Y were estimated as follows:
| [3] |
We used the right camera for u and υ, as shown above, but the left also could have been used.
Software to facilitate the collection of data from stereoimages and estimate positions from those measurements is available at http://exon.niaid.nih.gov/3dswarms. This site also includes time lapse and manipulable three-dimensional visualizations of the swarms analyzed in this paper.
References Cited
- Abramoff M, Magelhaes P, Ram S. Image processing with ImageJ. Biophotonics Int. 2004;11:36–42. [Google Scholar]
- Charlwood JD, Jones MDR. Mating behavior in the mosquito Anopheles gambiae sl. 1. close range and contact-behavior. Physiol. Entomol. 1979;4:111–120. [Google Scholar]
- Charlwood JD, Jones MDR. Mating in the mosquito Anopheles gambiae sl. 2. swarming behavior. Physiol. Entomol. 1980;5:315–320. [Google Scholar]
- Charlwood JD, Pinto J, Sousa CA, Madsen H, Ferreira C, do Rosario VE. The swarming and mating behaviour of Anopheles gambiae s.s. (Diptera: Culicidae) from São Tomé Island. J. Vector Ecol. 2002;27:178–183. [PubMed] [Google Scholar]
- della Torre A, Fanello C, Akogbeto M, Dossou-yovo J, Favia G, Petrarca V, Coluzzi M. Molecular evidence of incipient speciation within Anopheles gambiae s.s. in West Africa. Insect Mol. Biol. 2001;10:9–18. doi: 10.1046/j.1365-2583.2001.00235.x. [DOI] [PubMed] [Google Scholar]
- della Torre A, Costantini C, Besansky NJ, Caccone A, Petrarca V, Powell JR, Coluzzi M. Speciation within Anopheles gambiae: the glass is half full. Science. 2002;298:115–117. doi: 10.1126/science.1078170. [DOI] [PubMed] [Google Scholar]
- Diabaté A, Dabire RK, Kengne P, Brengues C, Baldet T, Ouari A, Simard F, Lehmann T. Mixed swarms of the molecular M and S forms of Anopheles gambiae (Diptera: Culicidae) in sympatric area from Burkina Faso. J. Med. Entomol. 2006;43:480–483. doi: 10.1603/0022-2585(2006)43[480:msotmm]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Diabaté A, Dabire RK, Millogo N, Lehmann T. Evaluating the effect of postmating isolation between molecular forms of Anopheles gambiae (Diptera: Culicidae) J. Med. Entomol. 2007a;44:60–64. doi: 10.1603/0022-2585(2007)44[60:eteopi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Diabaté A, Dao A, Yaro A, Alpha A, Traoré SC, Gonzalez R, Gwadz R, Lehmann T. Swarm segregation is the main mechanism that prevents mating between sympatric molecular forms of Anopheles gambiae. Am. J. Trop. Med. Hyg. 2007b;77(5 Suppl):200. [Google Scholar]
- Dicko A, Klion A, Thera M, Sangara I, Yalcouye D, Niambele M, Sogoba M, Dolo G, Dao A, Diallo D, Doumbo O, Miller L. The etiology of severe anemia in a village and a periurban area in Mali. Blood. 2004;104:1198–1200. doi: 10.1182/blood-2003-11-3884. [DOI] [PubMed] [Google Scholar]
- Downes JA. Swarming and mating flight of Diptera. Annu. Rev. Entomol. 1969;14:271–298. [Google Scholar]
- Favia G, Lanfrancotti A, Spanos L, Siden-Kiamos I, Louis C. Molecular characterization of ribosome DNA polymorphisms discriminating among chromosomal forms of Anopheles gambiae s.s. Insect Mol. Biol. 2001;10:19–23. doi: 10.1046/j.1365-2583.2001.00236.x. [DOI] [PubMed] [Google Scholar]
- Gibson G. Swarming behavior of the mosquito Culex pipiens-quinquefasciatus—a quantitative analysis. Physiol. Entomol. 1985;10:283–296. [Google Scholar]
- Gibson G. A behavioral test of the sensitivity of a nocturnal mosquito, Anopheles gambiae, to dim white, red and infrared light. Physiol. Entomol. 1995;20:224–228. [Google Scholar]
- Gibson G, Russell I. Flying in tune: sexual recognition in mosquitoes. Curr. Biol. 2006;16:1311–1316. doi: 10.1016/j.cub.2006.05.053. [DOI] [PubMed] [Google Scholar]
- Gilles MT, De Meillon B. The Anophelinae of Africa, south of Sahara (Ethiopian Zoogrographical Region) 2nd ed. Johannesburg, South Africa: South African Institute of Medical Research; 1968. [Google Scholar]
- Hartley RI, Zisserman A. Multiple view geometry in computer vision. 2nd ed. Cambridge, United Kingdom: Cambridge University Press; 2004. [Google Scholar]
- Höglund J, Alatalo R. Leks. Princeton, NJ: Princeton University Press; 2007. [Google Scholar]
- Ikawa T, Okabe H. Three-dimensional measurements of swarming mosquitoes. In: Parish JK, Hamner WM, editors. Animal groups in three dimensions. Cambridge, United Kingdom: Cambridge University Press; 1997. pp. 90–103. [Google Scholar]
- Manoukis NC. PhD dissertation. Los Angeles, CA: University of California; 2006. Studies on the ecology and adaptation of Anopheles gambiae in Mali and their impacts on malaria transmission and control. [Google Scholar]
- Marchand RP. Field observations on swarming and mating in Anopheles gambiae mosquitos in Tanzania. Neth. J. Zool. 1984;34:367–387. [Google Scholar]
- Okubo A, Bray DJ, Chiang HC. Use of shadows for studying the 3-dimensional structure of insect swarms (Anarete pritchardi Kim) (Cecidomiidae, Diptera) Ann. Entomol. Soc. Am. 1981;74:48–50. [Google Scholar]
- Pinheiro JC, Bates DM. Mixed effects models in S and S-Plus. New York: Springer; 2000. [Google Scholar]
- Reynolds CW. Flocks, herds, and schools: a distributed behavioral model, in computer graphics; SIGGRAPH ’87 Conf. Proc; 1987. pp. 25–34. [Google Scholar]
- Riley J. Flying insects in the field. In: Wratten SD, editor. Video techniques in animal ecology and behavior. London, United Kingdom: Chapman & Hall; 1993. pp. 1–15. [Google Scholar]
- Service M. Mosquito ecology: field sampling methods. 2nd ed. London, United Kingdom: Elsevier Applied Science Publishers; 1993. [Google Scholar]
- Sullivan RT. Insect swarming and mating. Fla. Entomol. 1981;64:44–65. [Google Scholar]
- Touré YT, Dolo G, Petrarca V, Traoré SF, Bouaré M, Dao A, Carnahan J, Taylor CE. Mark-release-recapture experiments with Anopheles gambiae sl in Banambani village, Mali, to determine population size and structure. Med. Vet. Entomol. 1998;12:74–83. doi: 10.1046/j.1365-2915.1998.00071.x. [DOI] [PubMed] [Google Scholar]
- Tripet F, Touré YT, Taylor CE, Norris DE, Dolo G, Lanzaro GC. DNA analysis of transferred sperm reveals significant levels of gene flow between molecular forms of Anopheles gambiae. J. Mol. Evol. 2001;10:1725–1732. doi: 10.1046/j.0962-1083.2001.01301.x. [DOI] [PubMed] [Google Scholar]
- Yuval B, Bouskila A. Temporal dynamics of mating and predation in mosquito swarms. Oecologia (Berl.) 1993;95:65–69. doi: 10.1007/BF00649508. [DOI] [PubMed] [Google Scholar]