Abstract
Objective
Acute hyperglycemia is independently associated with larger myocardial infarct size (IF) in both diabetic and non-diabetic patients. We hypothesized that the oxidative stress imposed by acute hyperglycemia contributes to the exacerbation of IF during reperfusion.
Methods
C57BL/6 mice underwent 30 min of LAD occlusion followed by 60 min of reperfusion. Acute hyperglycemia was induced with an i.p. injection of dextrose (2g/kg body weight) 30 min prior to LAD occlusion. An antioxidant, N-2-mercaptopropionyl glycine (MPG), was injected i.v. 2 min before the onset of reperfusion at a dose of 20 mg/kg. A NADPH oxidase inhibitor, apocynin (50 mg/kg), was applied either before or after the induction of hyperglycemia.
Results
Blood glucose level before LAD occlusion was 153±19 mg/dl in control mice and 444±26 mg/dl in hyperglycemic (HG) mice (p<0.05). Plasma lipid peroxidation product (malondialdehyde, MDA) was significantly increased in both control and HG mice at 1 hour post-reperfusion, and levels of MDA in HG mice were higher than that in control mice (3.38±0.21 versus 2.33±0.12 μM, p<0.05). MPG administered just before reperfusion significantly reduced MDA levels in both control and HG mice (1.21±0.06 and 1.03±0.24 μM). Acute hyperglycemia increased IF (% of risk region) from 34.0±2.7% to 49.4±1.6% (p<0.05). MPG reduced IF to 19.5±2.3 in control mice, and to 26.2±2.9 in HG mice. Apocynin also reduced MDA levels and IF in HG mice if administered 5 minutes before injection of dextrose, but not before reperfusion.
Conclusion
Acute hyperglycemia enhances oxidative stress and exacerbates myocardial infarction in mice through activation of NADPH oxidase.
Introduction
Reperfusion injury following cardioplegic arrest exists even with optimal cardioprotective strategy. Hyperglycemia is commonly present in the perioperative period in patients undergoing cardiac surgery1–3. Hyperglycemia during cardiopulmonary bypass is an independent risk factor for mortality and morbidity in both diabetic (DM) and non-diabetic patients3. However, the significance of hyperglycemia in the nondiabetic cardiac surgical patient and the potential benefits of glucose-insulin-potassium infusion are still the subject of intense debate1. An increasing body of clinical evidence has shown that acute hyperglycemia (or stress hyperglycemia) is independently associated with larger myocardial infarct size and impaired left ventricular function in both DM and non-diabetic patients4, 5. Acute hyperglycemia is common in patients with myocardial infarction (MI), even in the absence of a history of DM. In one recent study of 1664 patients with acute MI, over 25% had acute hyperglycemia upon admission but over 30% of these patients had no previous history of DM6. Moreover, animal studies have shown that the size of MI increases in response to elevations in blood glucose levels7, and that acute hyperglycemia counteracts the cardioprotective effects of ischemic and pharmacological preconditioning8, 9. While the hyperglycemic exacerbation of MI has become the subject of increasing research, little is known about the mechanisms underlying this phenomenon.
Acute hyperglycemia is known to decrease endothelial nitric oxide production thus impairing normal vascular function10–12, adversely impacting coronary microcirculation13, and markedly attenuating signal transduction pathways critical to endogenous cardioprotective responses8. A recent study shows that pharmacologic preconditioning against MI in the setting of acute hyperglycemia can be restored by N-acetylcysteine (a potent antioxidant)14, suggesting a possible role for oxidative stress in the acute hyperglycemic exacerbation of MI.
In the current study, acute hyperglycemia was created in mice by intra-peritoneal (i.p.) injection of concentrated dextrose prior to the scheduled ischemia/reperfusion injury. We hypothesized that the exacerbation of myocardial infarction by acute hyperglycemia is due to oxidative stress resulting from the activation of NADPH oxidase.
Materials and Methods
This study conformed to the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health (NIH publication No. 85–23, revised 1985) and was conducted under protocols approved by the University of Virginia’s Institutional Animal Care and Use Committee.
Agents and chemicals
Triphenyl tetrazolium chloride (TTC), N-2-mercaptopropionyl glycine (MPG) and apocynin (APO) were purchased from Sigma-Aldrich (St. Louis, MO). Phthalo blue was purchased from Heucotech Ltd. (Fairless Hills, PA).
Animals and experimental protocol
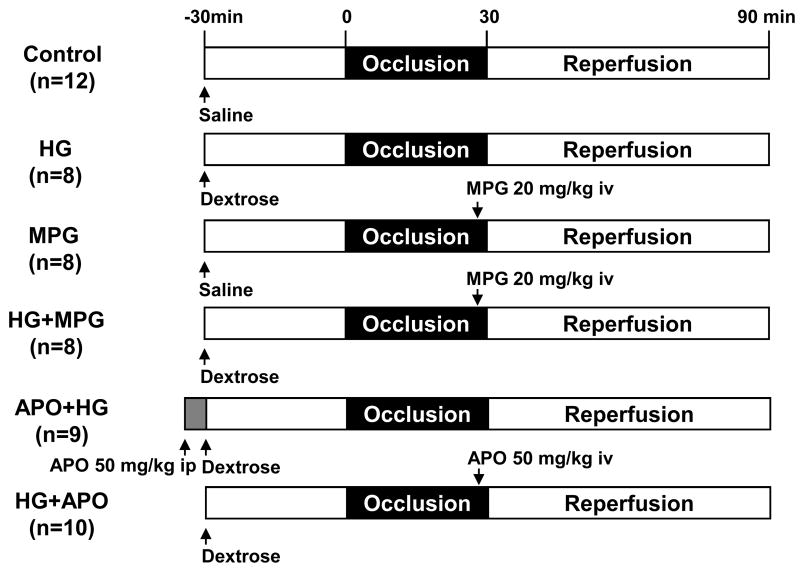
Sixty-three C57BL/6 mice (8–12 weeks old, purchased from Jackson Laboratories) were used in this study. Of these mice, 5 were used for dextrose dosing study in order to create acute hyperglycemia. Another 3 mice underwent left thoracotomy as sham controls. The rest of the mice were assigned to 6 different groups and underwent 30 minutes of ischemia and 60 minutes of reperfusion as shown in Figure 1. Acute hyperglycemia was induced by i.p. injection of 20% dextrose 30 minutes prior to LAD occlusion at a dose of 10 μl/g body weight. In control mice, normal saline was given by i.p. injection 30 min prior to LAD occlusion at a dose of 10 μl/g body weight. In MPG-treated mice, MPG (10 mg/ml in PBS) was administered 2 minutes before reperfusion by i.v. injection at a dose of 2 μl/g. In apocynin-treated mice, apocynin (5 mg/ml in 5% ethanol) was administered at a dose of 2 μl/g either i.v. 5 minutes before reperfusion in HG+APO mice or i.p. 5 min before induction of hyperglycemia in APO+HG mice (Figure 1).
Figure 1.
Study groups and experiment protocols.
Myocardial ischemia/reperfusion injury and measurement of infarct size
Mice were subjected to 30 minutes of coronary occlusion followed by 60 minutes of reperfusion and then euthanized to evaluate myocardial infarct by TTC-Phthalo blue staining. A standard protocol was used as detailed previously15, 16. Briefly, mice were anesthetized with sodium pentobarbital (100 mg/kg i.p.) and orally intubated. Artificial respiration was maintained with a FiO2 of 0.80, 100 strokes per minute, and a 2- to 3-mL stroke volume. The heart was exposed through a left thoracotomy, and coronary artery occlusion was achieved by passing a suture beneath the left anterior descending coronary artery (LAD) and tightening over a piece of PE-60 tubing for 30 minutes. Reperfusion was induced by removal of the PE-60 tubing. ECG was monitored perioperatively using PowerLab instrumentation (ADInstruments, Colorado Springs, CO). The mice were euthanized 60 minutes after reperfusion, and the hearts were cannulated through the ascending aorta for perfusion with 3 to 4 ml of 1.0% TTC. The LAD was then re-occluded with the same suture used for coronary occlusion prior to 10% Phthalo blue perfusion to determine risk region (RR). The left ventricle was then cut into 5 to 7 transverse slices that were weighed and digitally photographed to determine infarct size as a percent of RR. Reperfusion for 60 min is selected because the infarct size appears homogeneous at that time and does not become larger with longer reperfusion periods17.
Measurement of plasma malondialdehyde (MDA)
At the end of 1-hour reperfusion, blood was collected by puncturing then right ventricle and plasma was obtained after centrifuging the blood at 500 g for 8 minutes. MDA was measured with a commercial assay kit (Cayman Chemical Company, Ann Arbor, MI).
Statistical analysis
All data are presented as the mean ± SEM (standard error of the mean). Peri-ischemic heart rate changes were analyzed using a repeated measures ANOVA followed by Bonferroni pairwise comparisons. All other data were compared using one-way ANOVA followed by t-test for unpaired data with Bonferroni correction.
Results
Perioperative heart rate changes
Table 1 shows changes in heart rate before, during and after LAD occlusion. Heart rate was increased significantly after LAD occlusion and remained tachycardiac until early reperfusion. There was no significant difference in heart rate between control and treated mice.
Table 1.
Blood glucose levels before LAD occlusion and perioperative heart rates.
| Groups | Blood glucose (mg/dl) |
Heart Rate (beats per minutes) |
||
|---|---|---|---|---|
| Before occlusion | During occlusion | Reperfusion | ||
| Control | 153±19 | 406±14 | 443±9# | 445±10# |
| HG | 444±26 * | 407±15 | 466±18# | 448±20 |
| MPG | 124±13 | 408±12 | 458±11# | 428±10 |
| HG+MPG | 480±21* | 404±6 | 453±16# | 445±13 |
| HG+APO | 422±24 * | 393±9 | 451±12# | 472±15# |
| APO+HG | 496±16 * | 395±12 | 439±7# | 430±7# |
HG: hyperglycemia, MPG: N-2-mercaptopropionyl glycine, APO: apocynin.
p<0.05 vs. Control
p<0.05 vs. before occlusion.
Acute hyperglycemia in mice
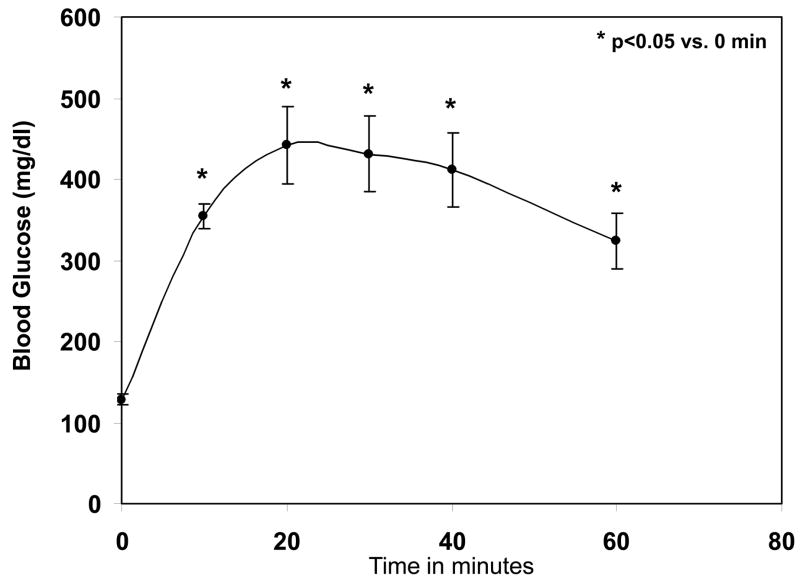
A preliminary dose range study conducted with a conventional glucose meter (iTest, Auto Control Med. Inc., Canada) indicated that a single bolus injection of 20% dextrose (10 μl/g or 2 g dextrose/kg body weight) sufficed to achieve transient blood glucose levels between 400–500 mg/dl. A time course of blood glucose levels was then evaluated at this dose in a group of 5 C57Bl/6J mice. Before injection, blood glucose in unfasted mice ranged from 117–150 mg/dl (mean=129±8 mg/dl). After injection, blood glucose increased rapidly to a peak at 20 min post-injection of 442±48 mg/dl, and then gradually declined while retaining mean levels of over 400 mg/dl for a period of 20 min (Fig. 2). Blood glucose returned to baseline 2 hrs after dextrose injection. Table 1 shows the blood glucose levels before LAD occlusion in all of the experimental groups, demonstrating that the hyperglycemic mice had significantly higher blood glucose levels than the other groups.
Figure 2.
Time course of blood glucose levels after single i.p. injection of 20% dextrose (10 μl/g body weight). *p<0.05 vs. baseline (time 0).
Acute hyperglycemia enhances oxidative stress and exacerbates myocardial infarction
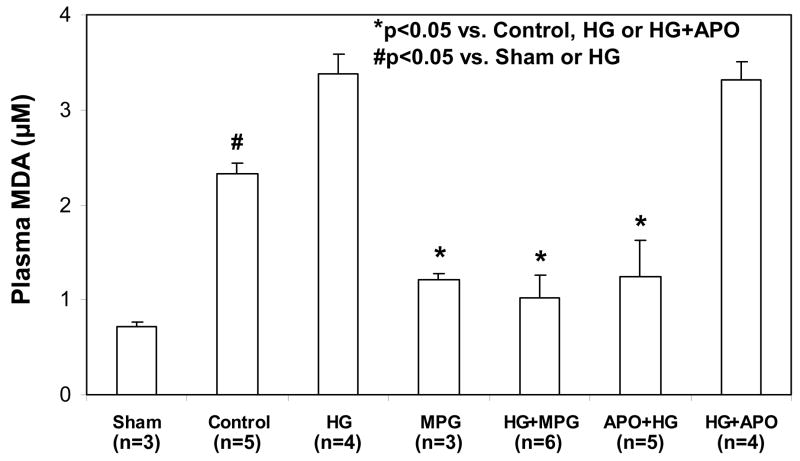
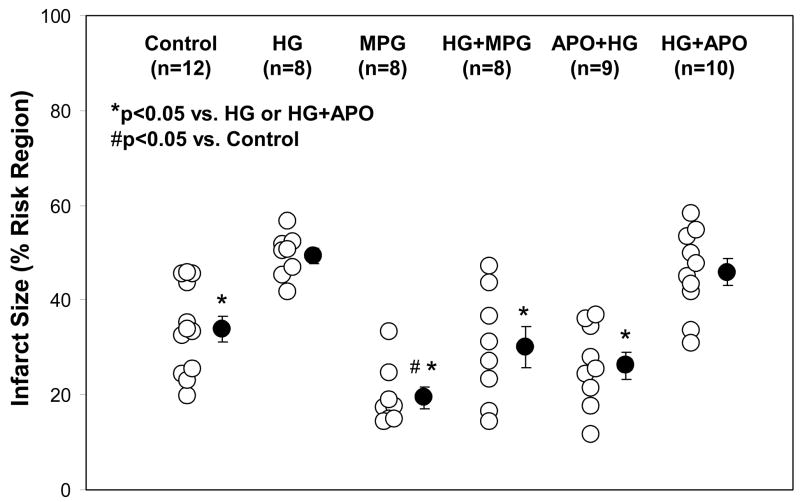
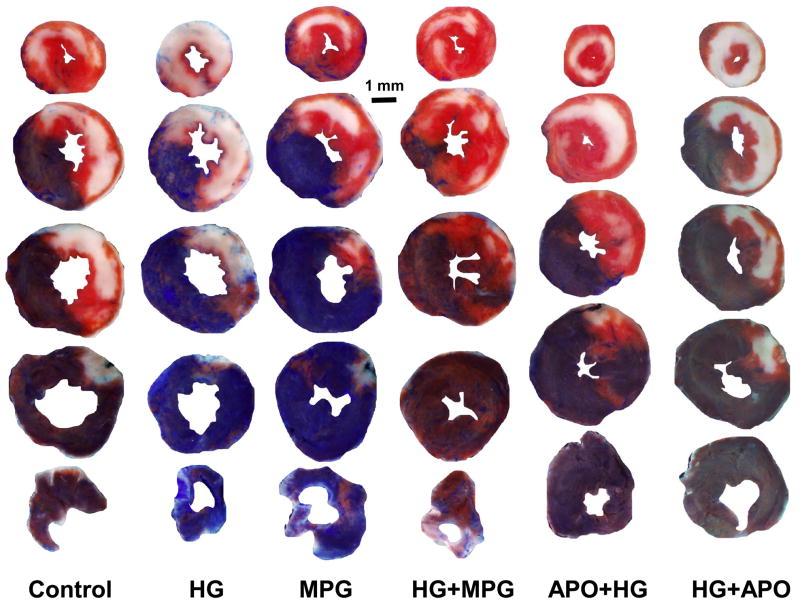
Four groups of mice (Fig 1, top 4 groups) underwent 30 min of LAD occlusion followed by 60 min of reperfusion, and an additional sham group underwent thoracotomy without LAD occlusion. A potent antioxidant, MPG, was administered in MPG-treated groups at a dose of 20 mg/kg 2 min before reperfusion. The mean blood glucose levels before LAD occlusion were 135±11 mg/dl in control or 124±13 in MPG-treated (MPG group) and 444±26 mg/dl in untreated hyperglycemic mice (p<0.05, Table 1). In control mice, plasma MDA was significantly increased during reperfusion compared to sham-operated mice (2.33±0.12 versus 0.71±0.05 μM, p<0.05). Acute HG further increased plasma MDA to 3.38±0.21 μM (p<0.05 versus Control or Sham). Treatment with MPG significantly reduced plasma MDA in both control and HG mice to 1.21±0.24 μM and 1.03±0.06 μM, respectively (p<0.05 versus either MPG or HG groups, Fig 3). Correspondingly, myocardial infarct size was enhanced by acute hyperglycemia. There were no statistical differences in risk region size (RR, % of LV) among the 4 groups (Control: 42.7±1.7, HG: 41.6±1.3, MPG: 37.9±1.8, HG+MPG: 38.9±1.5). In control mice, infarct size (% of RR) was 34.0±2.7%. Infarct size in HG mice increased by 45% to 49.4±1.6% (p<0.05 versus control). Administration of MPG (MPG group) reduced infarct size to 19.5±2.3, (43% reduction from control). Administration of MPG to HG+MPG mice reduced IF to 30.0±4.3%, a 39% reduction (p<0.05 versus HG mice, Fig. 4 & 5).
Figure 3.
Plasma levels of malondialdehyde (MDA) after 60 minutes of reperfusion following 30 minutes of LAD occlusion. *p<0.05 vs. Control, HG or HG+APO; #p<0.05 vs. Sham or HG
Figure 4.
Myocardial infarct size after 30 minutes of LAD occlusion and 60 minutes of reperfusion. Acute hyperglycemia exacerbates infarct size. The hyperglycemic exacerbation of infarct size was completely abrogated by MPG administered at reperfusion or apocynin administered prior to hyperglycemia. *p<0.05 vs. HG or HG+APO; #p<0.05 vs. Control
Figure 5.
TTC- and phthalo blue–stained, short-axis tissue sections of left ventricles from representative mice from the six groups summarized in Figures 1 and 4. Blue areas are nonischemic tissue, yellowish-white areas are infarcted tissue, and red areas represent salvaged (viable) tissue within the previously ischemic myocardium (risk region).
NADPH oxidase contributes to acute hyperglycemic exacerbation of MI
Apocynin, a specific NADPH oxidase inhibitor, was administered at a bolus dose of 50 mg/kg body weight either shortly before the onset of reperfusion intravenously (HG+APO) or before induction of hyperglycemia intra-peritoneally (APO+HG, Fig 1). LAD ligation caused comparable risk regions in apocynin-treated mice as with other groups (APO+HG: 37.2±1.4, HG+APO: 39.3±2.5; p=NS versus control or HG group). Administration of apocynin before reperfusion (HG+APO) had no effect on plasma MDA levels (3.32±0.18 μM, p=NS versus HG group); and correspondingly had no effect on infarct size of hyperglycemic mice (45.9±2.8%, p=NS versus HG group, p<0.05 vs. control, Figs 3 to 5). However, administration of apocynin before the induction of hyperglycemia (APO+HG) significantly reduced MDA levels to 1.25±0.46 μM (p<0.05 vs. HG or HG+APO) and abrogated the hyperglycemia-induced infarct expansion and significantly reduced infarct size to 26.2±2.9% (p<0.05 vs. HG, p=NS vs. control, Figs 3 to 5).
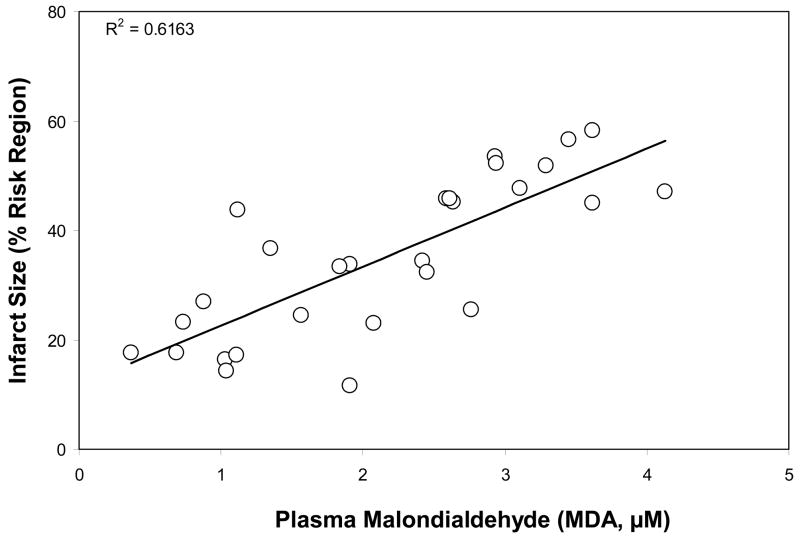
Correlation between plasma MDA level and myocardial infarct size
By pooling the data from all six groups of mice, a strong correlation was found between MDA levels and myocardial infarct size at 1 hour post-reperfusion, R2=0.62 (Fig. 6).
Figure 6.
Correaltion between plasma MDA levels and myocardial infarct size by pooling the data from different groups. R2=0.62.
Discussion
Through administration of concentrated dextrose, acute hyperglycemia was created in non-diabetic mice. Acute hyperglycemia significantly increases the production of reactive oxygen species (ROS) during reperfusion as characterized by lipid peroxidation products (MDA) in plasma and thereby exacerbates myocardial infarct size in mice. The efficacy of MPG in reducing infarct size in hyperglycemic mice when administered only minutes prior to reperfusion demonstrates that oxidative stress contributes importantly to the hyperglycemic exacerbation of infarct size. MPG is a potent ROS scavenger and thus acts on the end products (ROS) of oxidative stress. Apocynin, a selective NADPH oxidase inhibitor, similarly inhibited oxidative stress and protected the heart against the hyperglycemic exacerbation of infarct size, but only when it was applied before the hyperglycemic insults, indicating that the hyperglycemic oxidative stress involves NADPH oxidase. Taken together, the results indicate that acute hyperglycemia induced in non-diabetic mice shortly before myocardial ischemia significantly increases oxidative stress and exacerbates myocardial infarction in mice through the activation of NADPH oxidase.
Compelling evidence has shown that acute (or stress) hyperglycemia is an independent predictor of cardiovascular morbidity and mortality, independent of diabetes3,18–20. Stress hyperglycemia shares many properties with hyperglycemia associated with type 2 diabetes, including increased oxidative stress21–24, inflammation25–28, and activation of stress-responsive kinase20,26. Infarcts are usually larger in patients with stress or diabetes-related hyperglycemia4,5,20,29, and animals with acute hyperglycemia sustain dramatically larger infarcts following experimental ischemia-reperfusion than do euglycemic controls7–9. Increased sensitivity to ischemia-reperfusion injury and more severe myocardial damage is one reason for the poor prognosis of acute MI patients with stress hyperglycemia.
Although the mechanisms underlying acute or stress hyperglycemia may differ between patients and animal models8,29, hyperglycemia has proven to be an independent risk factor for larger infarct size4,5,7,8 and this hyperglycemic infarct exacerbation is not related to increased osmolality7. In order to simulate the stress hyperglycemia seen in human patients, acute hyperglycemia was similarly induced in mice in the current study by i.p. injection of concentrated dextrose. Sustained hyperglycemia ensued lasting over 30 minutes. LAD occlusion and reperfusion were performed during this period of the hyperglycemia. Blood glucose levels were 3-fold greater in hyperglycemic mice than in non-hyperglycemic mice. There was no difference in preoperative heart rate between hyperglycemic and non-hyperglycemic mice (Table). Myocardial ischemia and reperfusion induced oxidative stress that was documented after reperfusion. Acute hyperglycemia exacerbated this oxidative stress. Correspondingly, a significantly larger infarct size was found in hyperglycemic mice. This oxidative stress is positively correlated with myocardial infarct size (R2=0.62, Fig 6). A ROS scavenger, MPG, significantly reduced oxidative stress in both control and hyperglycemic mice and completely abrogated the hyperglycemic exacerbation of infarct size (Figure 3–5). In order to further define the source of the ROS responsible for the hyperglycemic exacerbation of infarct size, a selective inhibitor of NADPH oxidase30 (apocynin) was applied to hyperglycemic mice. Apocynin proved to be as effective as MPG in completely abrogating the hyperglycemic oxidative stress and abolishing the hyperglycemic exacerbation of infarct size, provided it was administered prior to the induction of hyperglycemia. However, the cardioprotective effect of apocynin was lost when its application was delayed until after the induction of hyperglycemia (Fig 4 & 5).
Myocardial ischemia/reperfusion injury is also associated with oxidative stress in the non-diabetic subjects as shown in the current study (Fig 3) and others31,32. However, the role of ROS scavenger or NADPH inhibition in reducing myocardial infarction remains controversial33. Acute hyperglycemic exacerbation of myocardial ischemia/reperfusion injury has been documented in both clinical and animal studies, but the role of antioxidant therapy in this setting is lacking.
It is well known that acute or stress hyperglycemia is associated with oxidative stress and inflammatory responses10,21,23,24,26–28. The degree of oxidative stress correlates most closely with acute, not chronic, glucose fluctuations24. Oxidative stress is defined in general as excess formation and/or insufficient removal of highly reactive molecules such as reactive oxygen species (ROS) and reactive nitrogen species (RNS). There are multiple potential sources of oxidative stress during hyperglycemia; including nonenzymatic, enzymatic and mitochondrial sources34. Nonenzymatic sources of oxidative stress originate from auto-oxidation of glucose or formation of advanced glycation end products. Enzymatic sources for the augmented generation of oxidative stress during hyperglycemia include nitric oxide synthase (NOS), NADPH oxidase and xanthine oxidase. The mitochondrial respiratory chain is another potential source of oxidative stress in this setting. Although not fully understood, evidence is mounting to suggest that NADPH oxidase plays an important role in mediating acute hyperglycemic oxidative stress23,27,34.
In the current study, acute hyperglycemia-induced oxidative stress was clearly defined during reperfusion and this oxidative stress, as well as the exacerbation in infarct size, was completely abolished with using a potent ROS scavenger or NADPH oxidase inhibitor. Interestingly, neither MPG nor apocynin could reduce infarct size in hyperglycemic mice back to the size found in MPG-treated euglycemic mice (Fig 4). This may reflect the deleterious effects of hyperglycemia on the endogenous defense system of cardiomyocytes, which makes them sensitive to ischemic injury7,8,20. Thus antioxidant therapy appears to abrogate the exacerbation of reperfusion injury, but not ischemic injury.
Apocynin inhibits NADPH oxidase by acting as an irreversible inhibitor of the p47phox subunit. Apocynin prevents the translocation of cytosolic p47phox to Nox2 in the membrane of leukocytes, monocytes and endothelial cells35–38. However, the compound requires activation by myeloperoxidase and/or H2O238 to be effective. For this reason, the inhibitory action of the compound appears to be most effective in leukocytes and then only after a lag period37,38. Although the current experiment did not explore the possible cellular sources of oxidative stress, an increasing body of evidence indicates that acute or stress hyperglycemia activates leukocytes which mediate the oxidative stress and inflammatory responses5,23,26. The cardioprotective effect of apocynin against the hyperglycemic exacerbation of infarct size is thus probably due to its action on the NADPH oxidases in leukocytes.
In the current experiments, the anti-oxidant and cardioprotective effects of apocynin against the hyperglycemic exacerbation of infarct size disappeared when it was administered just prior to reperfusion. The reason for this may be two-folds: 1) acute hyperglycemia caused the translocation of p47phox and activation of NADPH oxidase prior to the administration of apocynin, and 2) the lag time required for activation of the compound made it less effective against hyperglycemia-induced p47phox translocation. Treatment with apocynin prior to the induction of hyperglycemia in the “APO+HG” protocol provides adequate lag time for the activation of apocynin, thus enabling it to be effective in the inhibition of NADPH oxidase.
Significance and limitation of the study
The present study clearly shows that acute hyperglycemia enhances oxidative stress and exacerbates myocardial infarction. The cardioprotective effect of MPG is manifest even when the compound is given at the time of reperfusion. This has important clinical implications because it demonstrates that it may be possible to intervene with antioxidants to reduce infarct size in cardiac patients undergoing cardiopulmonary bypass or in medical patients with acute myocardial infarction undergoing percutaneous coronary intervention or thrombolysis by the simple administration of a potent antioxidant. Although NADPH oxidase plays a critical role in hyperglycemia-induced oxidative stress, the clinical application of inhibitor of NADPH oxidase is not practical since it has to be used before the onset of hyperglycemia. However, further experiments are needed to define its role when it is administered before or early during ischemia.
Conclusion
The current study indicates that acute hyperglycemia enhances oxidative stress during reperfusion, which in turn exacerbates myocardial infarct size in non-diabetic mice. Potent antioxidants can protect the heart against the hyperglycemic exacerbation of infarct size. The efficacy of apocynin indicates that hyperglycemia-induced oxidative stress is mediated by the activation of NADPH oxidase. Finally, this study provides direct evidence showing that the deleterious effects of hyperglycemia on infarct size can be completely reversed in a practical and clinically relevant manner.
Acknowledgments
Funding sources: This study was funded by NIH R01 HL 058582 to BAF
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lazar HL. Hyperglycemia during cardiac surgery. J Thorac Cardiovasc Surg. 2006;131:11–13. doi: 10.1016/j.jtcvs.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 2.Quinn DW, Pagano D, Bonser RS, et al. Improved myocardial protection during coronary artery surgery with glucose-insulin-potassium: a randomized controlled trial. J Thorac Cardiovasc Surg. 2006;131:34–42. doi: 10.1016/j.jtcvs.2005.05.057. [DOI] [PubMed] [Google Scholar]
- 3.Doenst T, Wijeysundera D, Karkouti K, et al. Hyperglycemia during cardiopulmonary bypass is an independent risk factor for mortality in patients undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2005;130:1144. doi: 10.1016/j.jtcvs.2005.05.049. [DOI] [PubMed] [Google Scholar]
- 4.Ishihara M, Inoue I, Kawagoe T, et al. Impact of acute hyperglycemia on left ventricular function after reperfusion therapy in patients with a first anterior wall acute myocardial infarction. American Heart Journal. 2003;146:674–678. doi: 10.1016/S0002-8703(03)00167-4. [DOI] [PubMed] [Google Scholar]
- 5.Marfella R, Siniscalchi M, Esposito K, et al. Effects of stress hyperglycemia on acute myocardial infarction: role of inflammatory immune process in functional cardiac outcome. Diabetes Care. 2003;26:3129–3135. doi: 10.2337/diacare.26.11.3129. [DOI] [PubMed] [Google Scholar]
- 6.Wahab NN, Cowden EA, Pearce NJ, et al. Is blood glucose an independent predictor of mortality in acute myocardial infarction in the thrombolytic era? J Am Coll Cardiol. 2002;40:1748–1754. doi: 10.1016/s0735-1097(02)02483-x. [DOI] [PubMed] [Google Scholar]
- 7.Kersten JR, Toller WG, Gross ER, et al. Diabetes abolishes ischemic preconditioning: role of glucose, insulin, and osmolality. American Journal of Physiology - Heart & Circulatory Physiology. 2000;278:H1218–1224. doi: 10.1152/ajpheart.2000.278.4.H1218. [DOI] [PubMed] [Google Scholar]
- 8.Kersten JR, Schmeling TJ, Orth KG, et al. Acute hyperglycemia abolishes ischemic preconditioning in vivo. American Journal of Physiology. 1998;275:H721–725. doi: 10.1152/ajpheart.1998.275.2.H721. [DOI] [PubMed] [Google Scholar]
- 9.Kersten JR, Montgomery MW, Ghassemi T, et al. Diabetes and hyperglycemia impair activation of mitochondrial K(ATP) channels. American Journal of Physiology - Heart & Circulatory Physiology. 2001;280:H1744–1750. doi: 10.1152/ajpheart.2001.280.4.H1744. [DOI] [PubMed] [Google Scholar]
- 10.Giugliano D, Marfella R, Coppola L, et al. Vascular effects of acute hyperglycemia in humans are reversed by L-arginine. Evidence for reduced availability of nitric oxide during hyperglycemia. Circulation. 1997;95:1783–1790. doi: 10.1161/01.cir.95.7.1783. [DOI] [PubMed] [Google Scholar]
- 11.Bohlen HG, Nase GP. Arteriolar nitric oxide concentration is decreased during hyperglycemia-induced betaII PKC activation. American Journal of Physiology - Heart & Circulatory Physiology. 2001;280:H621–627. doi: 10.1152/ajpheart.2001.280.2.H621. [DOI] [PubMed] [Google Scholar]
- 12.Beckman JA, Goldfine AB, Gordon MB, et al. Inhibition of protein kinase Cbeta prevents impaired endothelium-dependent vasodilation caused by hyperglycemia in humans.[see comment] Circulation Research. 2002;90:107–111. doi: 10.1161/hh0102.102359. [DOI] [PubMed] [Google Scholar]
- 13.Fujimoto K, Hozumi T, Watanabe H, et al. Acute hyperglycemia induced by oral glucose loading suppresses coronary microcirculation on transthoracic Doppler echocardiography in healthy young adults. Echocardiography. 2006;23:829–834. doi: 10.1111/j.1540-8175.2006.00325.x. [DOI] [PubMed] [Google Scholar]
- 14.Kehl F, Krolikowski JG, Weihrauch D, et al. N-acetylcysteine restores isoflurane-induced preconditioning against myocardial infarction during hyperglycemia. Anesthesiology. 2003;98:1384–1390. doi: 10.1097/00000542-200306000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Yang Z, Day Y-J, Toufektsian M-C, et al. Infarct-sparing effect of A2A-adenosine receptor activation is due primarily to its action on lymphocytes. Circulation. 2005;111:2190–2197. doi: 10.1161/01.CIR.0000163586.62253.A5. [DOI] [PubMed] [Google Scholar]
- 16.Yang Z, Day Y-J, Toufektsian M-C, et al. Myocardial infarct-sparing effect of adenosine A2A receptor activation is due to its action on CD4+ T lymphocytes. Circulation. 2006;114:2056–2064. doi: 10.1161/CIRCULATIONAHA.106.649244. [DOI] [PubMed] [Google Scholar]
- 17.Schwarz ER, Somoano Y, Hale SL, et al. What is the required reperfusion period for assessment of myocardial infarct size using triphenyltetrazolium chloride staining in the rat? J Thromb Thrombolysis. 2000;10:181–187. doi: 10.1023/a:1018770711705. [DOI] [PubMed] [Google Scholar]
- 18.Capes SE, Hunt D, Malmberg K, et al. Stress hyperglycaemia and increased risk of death after myocardial infarction in patients with and without diabetes: a systematic overview.[see comment] Lancet. 2000;355:773–778. doi: 10.1016/S0140-6736(99)08415-9. [DOI] [PubMed] [Google Scholar]
- 19.Ishihara M, Kojima S, Sakamoto T, et al. Acute hyperglycemia is associated with adverse outcome after acute myocardial infarction in the coronary intervention era. American Heart Journal. 2005;150:814–820. doi: 10.1016/j.ahj.2004.12.020. [DOI] [PubMed] [Google Scholar]
- 20.Webster KA. Stress hyperglycemia and enhanced sensitivity to myocardial infarction. Curr Hypertens Rep. 2008;10:78–84. doi: 10.1007/s11906-008-0015-0. [DOI] [PubMed] [Google Scholar]
- 21.Hu Y, Block G, Norkus EP, et al. Relations of glycemic index and glycemic load with plasma oxidative stress markers. American Journal of Clinical Nutrition. 2006;84:70–76. doi: 10.1093/ajcn/84.1.70. quiz 266–267. [DOI] [PubMed] [Google Scholar]
- 22.Kawano H, Motoyama T, Hirashima O, et al. Hyperglycemia rapidly suppresses flow-mediated endothelium-dependent vasodilation of brachial artery. Journal of the American College of Cardiology. 1999;34:146–154. doi: 10.1016/s0735-1097(99)00168-0. [DOI] [PubMed] [Google Scholar]
- 23.Mohanty P, Hamouda W, Garg R, et al. Glucose challenge stimulates reactive oxygen species (ROS) generation by leucocytes. J Clin Endocrinol Metab. 2000;85:2970–2973. doi: 10.1210/jcem.85.8.6854. [DOI] [PubMed] [Google Scholar]
- 24.Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 25.Sudic D, Razmara M, Forslund M, et al. High glucose levels enhance platelet activation: involvement of multiple mechanisms. British Journal of Haematology. 2006;133:315–322. doi: 10.1111/j.1365-2141.2006.06012.x. [DOI] [PubMed] [Google Scholar]
- 26.Aljada A, Friedman J, Ghanim H, et al. Glucose ingestion induces an increase in intranuclear nuclear factor kappaB, a fall in cellular inhibitor kappaB, and an increase in tumor necrosis factor alpha messenger RNA by mononuclear cells in healthy human subjects. Metabolism: Clinical & Experimental. 2006;55:1177–1185. doi: 10.1016/j.metabol.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 27.Dhindsa S, Tripathy D, Mohanty P, et al. Differential effects of glucose and alcohol on reactive oxygen species generation and intranuclear nuclear factor-kappaB in mononuclear cells. Metabolism: Clinical & Experimental. 2004;53:330–334. doi: 10.1016/j.metabol.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 28.Iwasaki Y, Kambayashi M, Asai M, et al. High glucose alone, as well as in combination with proinflammatory cytokines, stimulates nuclear factor kappa-B-mediated transcription in hepatocytes in vitro. Journal of Diabetes & its Complications. 2007;21:56–62. doi: 10.1016/j.jdiacomp.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 29.Zarich SW, Nesto RW. Implications and Treatment of Acute Hyperglycemia in the Setting of Acute Myocardial Infarction. Circulation. 2007;115:e436–439. doi: 10.1161/CIRCULATIONAHA.105.535732. [DOI] [PubMed] [Google Scholar]
- 30.Ximenes VF, Kanegae MP, Rissato SR, et al. The oxidation of apocynin catalyzed by myeloperoxidase: proposal for NADPH oxidase inhibition. Arch Biochem Biophys. 2007;457:134–141. doi: 10.1016/j.abb.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 31.Horwitz LD, Kong Y, Robertson AD. Timing of treatment for myocardial reperfusion injury. J Cardiovasc Pharmacol. 1999;33:19–29. doi: 10.1097/00005344-199901000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Zhu X, Zuo L, Cardounel AJ, et al. Characterization of in vivo tissue redox status, oxygenation, and formation of reactive oxygen species in postischemic myocardium. Antioxid Redox Signal. 2007;9:447–455. doi: 10.1089/ars.2006.1389. [DOI] [PubMed] [Google Scholar]
- 33.Hoffmeyer MR, Jones SP, Ross CR, et al. Myocardial ischemia/reperfusion injury in NADPH oxidase-deficient mice. Circ Res. 2000;87:812–817. doi: 10.1161/01.res.87.9.812. [DOI] [PubMed] [Google Scholar]
- 34.Johansen JS, Harris AK, Rychly DJ, et al. Oxidative stress and the use of antioxidants in diabetes: linking basic science to clinical practice. Cardiovasc Diabetol. 2005;4:5. doi: 10.1186/1475-2840-4-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barbieri SS, Cavalca V, Eligini S, et al. Apocynin prevents cyclooxygenase 2 expression in human monocytes through NADPH oxidase and glutathione redox-dependent mechanisms. Free Radic Biol Med. 2004;37:156–165. doi: 10.1016/j.freeradbiomed.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 36.Johnson DK, Schillinger KJ, Kwait DM, et al. Inhibition of NADPH oxidase activation in endothelial cells by ortho-methoxy-substituted catechols. Endothelium. 2002;9:191–203. doi: 10.1080/10623320213638. [DOI] [PubMed] [Google Scholar]
- 37.Heumuller S, Wind S, Barbosa-Sicard E, et al. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 38.Stolk J, Hiltermann TJ, Dijkman JH, et al. Characteristics of the inhibition of NADPH oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol. 1994;11:95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]