Abstract
Background
During vertebrate embryogenesis, somites are generated at regular intervals, the temporal and spatial periodicity of which is governed by a gradient of fibroblast growth factor (FGF) and/or Wnt signaling activity in the presomitic mesoderm (PSM) in conjunction with oscillations of gene expression of components of the Notch, Wnt and FGF signaling pathways.
Principal Findings
Here, we show that the expression of Sprouty4, which encodes an FGF inhibitor, oscillates in 2-h cycles in the mouse PSM in synchrony with other oscillating genes from the Notch signaling pathway, such as lunatic fringe. Sprouty4 does not oscillate in Hes7-null mutant mouse embryos, and Hes7 can inhibit FGF-induced transcriptional activity of the Sprouty4 promoter.
Conclusions
Thus, periodic expression of Sprouty4 is controlled by the Notch segmentation clock and may work as a mediator that links the temporal periodicity of clock gene oscillations with the spatial periodicity of boundary formation which is regulated by the gradient of FGF/Wnt activity.
Introduction
During vertebrate development, the most prominent metameric structures are the somites, which give rise to the vertebrae, the ribs, the skeletal muscles and the dermis [1]. Somites are generated as epithelial spheres of cells that sequentially bud off at regular intervals from the anterior extremity of the presomitic mesoderm (PSM) in an anterior-to-posterior direction with a strict periodicity, which is controlled by the segmentation clock [2]. The first evidence of an oscillator coupled to somite segmentation was provided by the finding of the oscillatory expression of the basic helix–loop–helix (bHLH) gene c-hairy1 in the chick PSM [3]. The expression of c-hairy1 oscillates in a synchronous manner among neighboring cells of the chick PSM, where the expression displays cyclic wave-like propagation patterns in a caudal-to-rostral direction by gradual phase delay. It has since been shown that several genes exhibit such a cyclic behavior in a variety of vertebrate species, including fish, chick and mouse, and some of them are evolutionarily conserved among the species [4]. Among the clock genes identified from the Notch pathway, the hairy related or homologous genes in mouse and fish, namely Hes and her, also cycle [5]–[9]. However, cyclic PSM expression of lunatic fringe (Lfng), which encodes a glycosyl-transferase that modulates activity of the Notch receptor, is detected in mouse and chick [10]–[12], but not in fish [13], [14]. Some genes in the Wnt and FGF pathway display oscillatory gene expression in the mouse PSM, such as Axin2 and Nkd1, both inhibitors of Wnt signaling, Sprouty2 and Dusp4, both inhibitors of FGF signaling, and Snail1, a transcriptional repressor [15]–[19], but cyclic behavior of their homologues in other vertebrate species have not been reported, other than Snail2 in chick.
In the PSM, a complex gene network that includes several feedback loops could elicit highly dynamic gene expression to form the robust segmentation clock. In mouse, oscillating Hes7 represses Lfng and its own transcription periodically and establishes a feedback loop, which is essential for cyclic gene expression and participates in the mechanism of the segmentation clock [20]. Lfng modulates Notch activity periodically and forms a negative feedback loop, which provides cyclic Notch activity in the chick PSM [21]. A negative feedback of Axin2 also generates cyclic Wnt signaling in mouse PSM, which is essential for somite formation [17].
FGF and Wnt signaling are both crucial to determine the position of somite boundary specification [17], [22], [23]. Both the FGF8 and Wnt3A ligands establish posterior-to-anterior gradients of expression in the PSM [24]. The position of the determination front demarcates the region where the PSM cells are able to embark on their segmentation program and the temporal periodicity of oscillatory gene expression becomes converted to the spatial periodicity of the somites. Several levels of crosstalk between these pathways and the segmentation clock have been reported. Thus, FGF signaling initiates the oscillation of Hes7 in the mouse PSM [18]. An FGF downstream gene, her13.2 is required for the auto-repression of her, which is the key mechanism of the segmentation clock in zebrafish [25]. However, it largely remains to be elucidated how the segmentation clock cooperates with the Wnt and FGF pathways to generate regular interval pattern.
We have shown that the FGF target and negative regulator, Sprouty4, shows oscillatory gene expression in the mouse PSM. Sprouty4 cycles in phase with other Notch regulated clock genes, such as Lfng and Hes7, and its oscillation depends on Hes7, which is one of the core factors of the mouse segmentation clock mechanism. Thus, Sprouty4 could be one of the candidates for the mediator that integrates spatiotemporal information in somitogenesis. We further find the cyclic expression of Sprouty4 is not evolutionarily conserved since it does not oscillate in the zebrafish PSM.
Results and Discussion
The expression of Sprouty4 oscillates in the mouse PSM
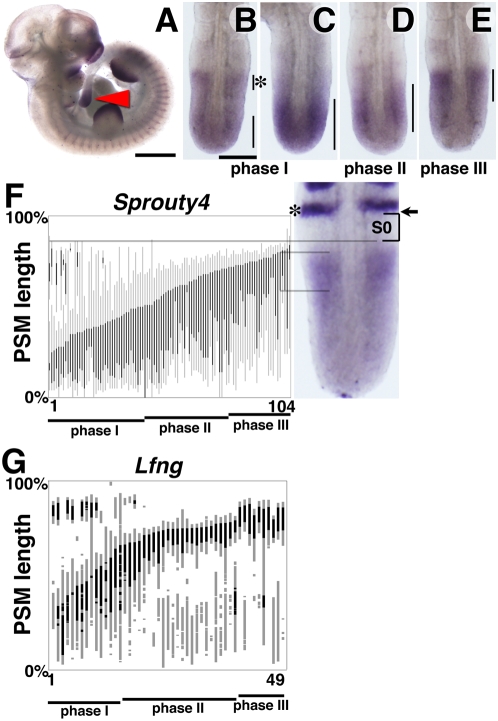
The mRNA expression of Sprouty4 coincides with regions of FGF signaling activity in the mouse embryo at embryonic day (E) 10.5, including the PSM, the somites, the limb buds and the frontonasal processes as previously reported (Figure 1A) [26], [27]. Among a group of stage matched E10.5 embryos, the expression pattern of Sprouty4 in the PSM varied considerably. The expression patterns can be grouped into 3 phases [28]. In some embryos, the expression domain extends throughout the posterior PSM and tail bud region (Figure 1B,C, n = 42/104, phase I). The second group of embryos displayed a broad pattern of expression with the strongest signal in the middle part of the PSM (Figure 1D, n = 36/104, phase II). The last group of embryos showed the highest intensity of expression in the anterior part of the PSM with no expression in the posterior PSM and tail bud region (Figure 1E, n = 26/104, phase III). In order to more precisely determine the dynamic expression profile of Sprouty4 in the PSM, we measured the domains of Sprouty4 expression in the PSM of individual embryos, scored the distance between the boundary of the newest somite and the anterior limit of PSM expression and represented these measurements graphically in order of increasing length of expression domain from the posterior end of the PSM (Figure 1F) [21]. The stacked expression patterns of embryos (n = 104) indicate that Sprouty4 mRNA expression changes as a continuous progressive wave from the posterior to the anterior end of the PSM. This expression profile is similar to that of other cyclic genes such as Lfng in the mouse PSM (Figure 1G) and Lfng, c-hairy1, c-hairy2 in the chick PSM [21], [29]. The only difference is that while the rate of progression of the wave of expression of these other clock genes is much faster in the posterior than the anterior PSM, that of Sprouty4 appears uniform along the whole length of the mouse PSM (Figure 1F).
Figure 1. Cyclic expression pattern of Sprouty4 in mouse PSM.
(A–E) Expression pattern of Sprouty4 at E10.5. Sprouty4 mRNA is expressed in the caudal region of somites, limb buds, and frontonasal processes as well as the PSM (red arrowhead) (A), in which various expression patterns were observed, categorized as phase I (B,C), II (D) and III (E). (F,G) Analysis of Sprouty4 and Lfng expression in the mouse PSM. Each vertical bar represents one embryo. Black stripes represent stronger expression domains as compared to gray bars. We examined 104 embryos for Sprouty4 (E), and 49 embryos for Lfng (F). We used Uncx4.1 probe as a positional marker (asterisk in E). The position of S0 (bracket), the forming somite, and the boundary of the newest somite (arrow) are indicated, respectively. Scale bars: A, 1 mm; B, 200 µm.
Some embryos in phase I, in addition to the caudal expression domain also display a narrow band of expression in the anterior part of the PSM (Figure 1B, asterisk), which most likely corresponds to the domain of expression in phase III, suggesting the expression of Sprouty4 in the mouse PSM may be cyclic. We found that 42, 35 and 29 embryos display Phase I, II and III, respectively and thus the ratio of embryo number in each phase indicates a roughly equal distribution in each phase which suggests each phase lasts roughly an equivalent time. In the case of Lfng, 15, 24 and 10 embryos were classified as Phase I, II and III, respectively (n = 49). These results suggest that the expression of Sprouty4 is cyclic and displays similar pattern to that of Lfng in the mouse PSM.
The expression of Sprouty4 oscillates in 2-h cycles in phase with the Notch regulated cyclic gene, Lfng
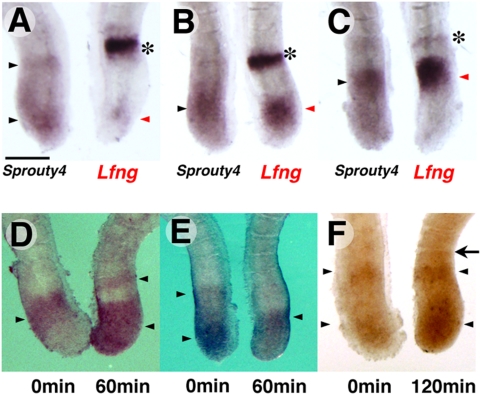
Previous reports clearly demonstrated that, in the mouse PSM, the expression of Lfng shows a cyclic pattern, which coincides with the periodicity of somite formation [11], [12], and that oscillating genes belonging to the Notch and FGF pathways cycle synchronously [19]. However, some cyclic genes in the Wnt pathway, including Axin2, oscillate out of phase with the Notch clock genes [17], [19]. To investigate whether the expression of Sprouty4 oscillates and , if so, to investigate that it oscillates in or out of phase with Lfng, we bisected the posterior region of E10.5 mouse embryos and analysed Lfng or Sprouty4 mRNA in respective halves of each embryo. In all embryos, both were expressed in the same regions of the PSM (n = 23, Figure 2A–2C), although Sprouty4 was never expressed in the anterior most region as a narrow and sharp band, which is seen in the Lfng expression profile (asterisks in Figure 2A–2C).
Figure 2. The expression of Sprouty4 oscillates in 2-h cycles in phase with Lfng.
(A–C) Comparison of the expression of Sprouty4 (left) and Lfng (right) in bisected caudal portions of E10.5 mouse embryos. The expression patterns are categorized into three phases: phase I (A), phase II (B) and phase III (C). The expression of Sprouty4 (black arrowhead) is similar to that of Lfng (red arrowhead) in each phase, except the Sprouty4 profile does not include the anterior band of the Lfng profile (asterisks). (D–F) The caudal portions of E10.5 mouse embryos were bisected. One half (left) was fixed immediately, and the other half (right) was cultured for 60 min (D,E) or 120 min (F), before fixation. The expression patterns of Sprouty4 (arrowheads) of the cultured halves were different from the other halves when they were cultured for 60 min. However, when the cultured halves were incubated for 120 min, the patterns were similar to the control halves and they had a new boundary (arrow). Scale bar: A, 200 µm.
To clarify whether the expression of Sprouty4 oscillates further, we carried out an explant culture experiment. The posterior region of embryos was bisected and one half was fixed immediately and the other half was cultured for 60 min or 120 min before fixation. When one half was cultured for 60 min, the expression patterns of Sprouty4 were different from those of the uncultured half (Figure 2D,E, n = 10). By contrast, when we cultured one half for 120 min, the expression patterns of Sprouty4 were very similar to those of uncultured half (Figure 2F, n = 4), and we observed a newly formed boundary in the explants (Figure 2F, arrow). Taken together, these results indicate that the expression of Sprouty4 oscillates in 2-h cycles in the mouse PSM in synchrony with the Notch regulated clock genes.
Hes7 controls the cyclic expression of Sprouty4 via transcriptional inhibition
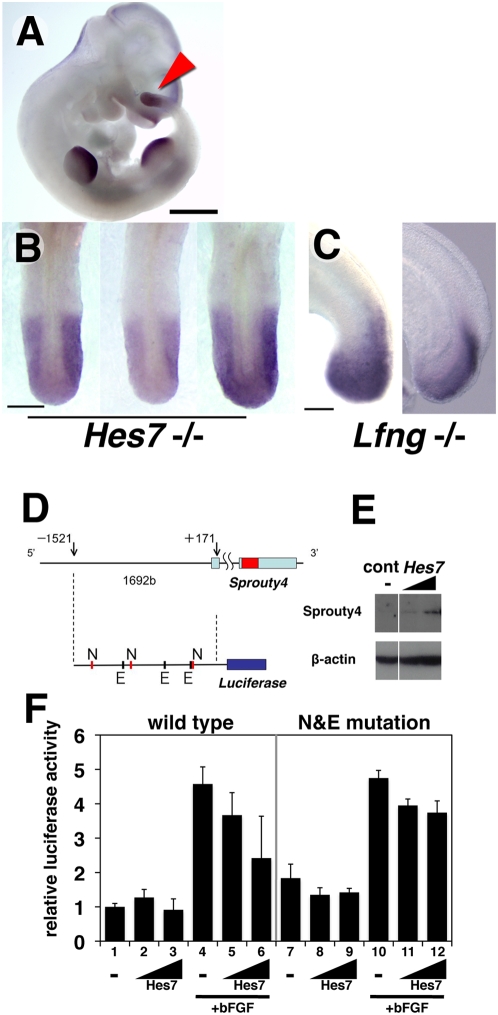
The cyclic expression of Lfng, as well as that of Nkd1 and Hes7 itself, is controlled by Hes7 via periodic transcriptional repression [15], [20]. In order to examine whether the cyclic expression of Sprouty4 is also under the control of Hes7, we analysed Sprouty4 mRNA expression in the absence of Hes7 [5]. The expression of Sprouty4 is not affected in Hes7-null mutant mouse embryos in tissues other than the PSM, although the metameric pattern in the somites is faded (Figure 3A). This result is consistent with the previous observations, in which the expression pattern of Hes7 is restricted to the PSM [30] and the phenotype of Hes7-null mutant mice embryos whose somites are severely disorganized [5]. In all mutants examined (n = 24), Sprouty4 is uniformly expressed throughout the PSM (Figure 3B). Interestingly, although the phenotype of Lfng-null mutant mice closely resembles that of Hes7-null mice in somitogenesis [31], [32], Sprouty4 still oscillates in the PSM of Lfng-null mutant mice (Figure 3C). Thus, the cyclic expression of Sprouty4 depends on Hes7.
Figure 3. Cyclic expression of Sprouty4 depends on Hes7.
(A–C) Expression pattern of Sprouty4 in Hes7-null or Lfng-null mutant embryos. In E10.5 Hes7-/- mutant embryos, Sprouty4 is expressed in similar tissues as seen in wild-type embryos (A). All Hes7-/- mutant embryos display a uniform expression in the PSM (n = 24) (B). Lfng-/- mutant embryos display various expression patterns in the PSM (n = 5) (C). Scale bars: A, 1 mm; B, 200 µm; C, 100 µm. (D–F) Promoter analysis of Sprouty4. (D) The 1.7 kb Sprouty4 promoter, which contains 3 consensus sequences of N-box and 3 consensus sequences of E-box, was inserted upstream of luciferase. (E) Hes7 protein was specifically detected by Western blotting with anti-Hes7 antibody in the Hes7 transfected cells. β-actin were detected in Hes7 transfected and control cells. (F) Luciferase activity under the control of the Sprouty4 promoter was not significantly suppressed in the presence of Hes7 (lane 2,3). However, the activity induced by bFGF (lane 4) was inhibited in the presence of Hes7 (lane 5,6). Luciferase activity under the control of the Sprouty4 promoter with mutations of N-box and E-box was not significantly suppressed in the presence of Hes7 again (lane 8,9). The activity induced by bFGF (lane 10) was not significantly inhibited in the presence of Hes7 (lane 11,12).
Since Hes7 works as a transcriptional repressor that binds to E-box and N-box sequences [30] and transcription of Lfng and Hes7 are periodically repressed by Hes7 to generate their oscillatory gene expression profile [20], we next examined whether Hes7 can inhibit the expression of Sprouty4 by a promoter analysis. Because the 1.7 kb upstream region of human Sprouty4 is sufficient for the transcription in response to FGF signaling[33], we took the 1.7 kb promoter region of mouse Sprouty4 upstream of a luciferase reporter gene (Figure 3D). When we transfected the reporter carrying the Sprouty4 promoter into the mouse cell line, the stimulation with basic FGF (bFGF) increased the reporter activitiy (Figure 3F, left), suggesting that the mouse Sprouty4 promoter, which we used here could response to bFGF, similar to the human promoter [33]. When we co-transfected Hes7 with the reporter (Figure 3E), Hes7 could suppress the bFGF-dependent transcriptional activity, but did not inhibit the basal level of the promoter activity (Figure 3F, left). This observation is consistent with previous results showing Hes7 inhibits Hes7 promoter activity induced by Notch signaling but it does not effectively inhibit the baseline activity of the Hes7 promoter [20], which is periodically suppressed by Hes7 itself in the mouse PSM. Since the promoter region contains several Hes7 responsive elements, E-box and N-box consensus sequences [30], we generated the Sprouty4 promoter without E-box and N-box sequences by multiple mutagenesis [34], [35]. Although the Sprouty4 promoter without E-box and N-box was normally activated by bFGF, the inhibition of bFGF-dependent activation by Hes7 was partially canceled (Figure 3F, right), suggesting that E-box and N-box sequences act as the Hes7-responsive elements. Thus, in vitro situation, Hes7 can inhibit the expression of Sprouty4 through the Hes7-responsive elements in the Sprouty4 promoter.
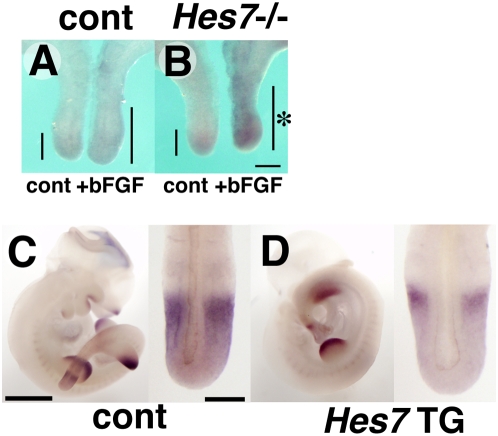
To examine whether Hes7 inhibits Sprouty4 expression in vivo, we performed two experiments: an explant culture of the PSM of Hes7-null mutant embryos and an overexpression of Hes7 in the PSM by transient transgenic analysis. In former experiments, we bisected the posterior region of Hes7-null or wild-type embryo, and treated with bFGF for 4h only in one half. In the PSM of wild-type embryo, which normally expresses Hes7, treatment of bFGF slightly induced Sprouty4 expression (Figure 4A, n = 10). However, in the PSM of Hes7-null mutant, treatment of bFGF dramatically increased Sprouty4 expression (Figure 4B, n = 3). Conversely, in the latter experiments, overexpression of Hes7 specifically in the PSM by using Hes7 transgenic mice inhibited Sprouty4 expression just in the PSM (Figure 4D, n = 6/10), compared to the transgene-negative littermates (Figure 4C, n = 11), although both showed similar patterns and levels of Sprouty4 expression in tissue other than the PSM. These data indicate that Hes7 can inhibit Sprouty4 expression in the PSM. Taken together, it is likely that cyclic Hes7 inhibits the Sprouty4 promoter periodically in the mouse PSM, resulting in the oscillatory expression profile of Sprouty4.
Figure 4. Hes7 can inhibit the expression of Sprouty4 in the PSM.
(A,B) Explant culture with Hes7-null embryo. (A) The expression of Sprouty4 (bars) was slightly induced by 4-h exposure of bFGF in the wild-type PSM. (B) In the PSM of Hes7-null embryos, 4-h exposure of bFGF dramatically induced Sprouty4 expression in the PSM (asterisk). (C,D) Over expressed Hes7 inhibits the expression of Sprouty4 in the PSM. The expression of Sprouty4 was detected in the transgenic embryos overexpressing Hes7 from the 5 kb Hes7 promoter (D) and wild-type embryos (C). In the PSM of transgenic embryos, the expression of Sprouty4 was exclusively down-regulated. Scale bars: B, 200 µm; C, 1mm (left), 200 µm (right).
Cyclic expression of Sprouty4 is not observed in the zebrafish PSM
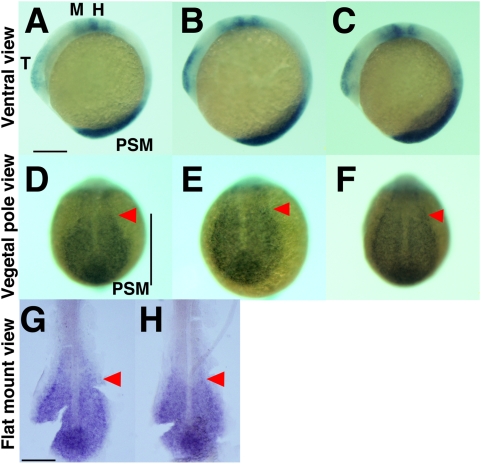
Finally, we examined the expression profile of the orthologue of Sprouty4 in the zebrafish PSM. Some genes in the Notch pathway including her1, her7 and deltaC show cyclic expression in the zebrafish [6]–[8], [36], but other genes which cycle in the mouse PSM do not display cyclic expression or have not been well examined in zebrafish [14]. These observations mean that the cyclic expression of some but not all genes is conserved among species. In zebrafish, Sprouty4 is expressed in the region of FGF signaling, including the PSM, the midbrain-hindbrain boundary, the heart and the brachial arch primordia at the 10 somite stage (Figure 5A–5C). This expression pattern is highly conserved with that of mouse (Figure 1A) [26], [27]. However, we did not find evidence of any cyclic expression of Sprouty4 in the zebrafish PSM (n = 12) (Figure 5D–5H). These results suggest that the oscillatory behavior of Sprouty4 in the mouse is not conserved in zebrafish.
Figure 5. Sprouty4 is expressed in zebrafish PSM but not cyclic.
(A–C) Whole mount embryos show expression of Sprouty4 in the PSM, telencephalon T), midbrain-hindbrain boundary (M), heart and brachial arch primordia (H) in lateral views of 6 somite stage zebrafish embryos. Lateral view and anterior to the top. (D–F) The expression patterns of Sprouty4 in the zebrafish PSM do not vary among embryos. Dorsal view and anterior to the top. The stained embryos were flat mounted by removing the yolk (G, H). Red arrowheads indicate the anterior limit of the Sprouty4 positive region. Scale bars: A, G, 200 µm.
Sprouty4 is a strong candidate for the mediator that integrates spatiotemporal information during somitogenesis
In the PSM, FGF signaling establishes a posterior-to-anterior gradient, which is involved in the positioning of somite boundaries [22], [23]. Although the precise target is unclear, Sprouty4 inhibits the FGFsignaling [37], [38]. Our results in this study revealed that the expression of Sprouty4 oscillates in phase with Lfng and hence also with Hes7 in the mouse PSM, and that this cyclic expression depends on Hes7. Thus, the periodic expression of Sprouty4 potentially acts to periodically repress FGF signaling and thereby periodically modulate spatial information in the mouse PSM (Figure 6). Therefore, we propose that Sprouty4 could be one of the candidates for the mediator that integrates spatiotemporal information during somitogenesis. Of course, other feedback inhibitors that show cyclic expression in the PSM, including Sprouty2 and Dusp4, are also candidates for this putative mediator activity. Most probably, these factors work together redundantly during somitogenesis, since for example, no obvious abnormalities in somite segmentation have been reported in the null mutant of Sprouty2 [39], [40] or Sprouty4 [41], or in the double mutant of the two genes [42], although a detailed investigation into the segmental patterns in these mutants is still required. FGF inhibitors, acting as putative mediators, may periodically suppress FGF signaling in the middle to anterior PSM around the position of the determination front in order to generate a discrete pattern, by which the position of somite boundaries is determined.
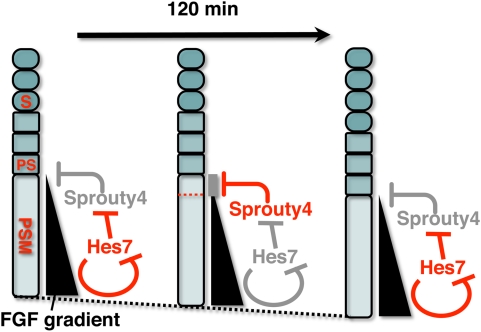
Figure 6. A Putative Model for the role of Sprouty4 as a mediator that links the segmentation clock to the gradient of FGF signaling.
In the PSM, FGF signaling establishes a posterior-to-anterior gradient, which is involved in the positioning of presumptive somite boundaries. Cyclic Sprouty4, which is controlled by the Notch segmentation clock, the mechanism of which includes negative feedback loop of Hes7, may inhibit the FGF signaling possibly around the anterior border of the FGF signaling positive area, where the FGF signaling is close to its threshold. Thus, the FGF signaling may be periodically inhibited by Sprouty4, by which temporal periodicity of Notch segmentation clock may be translated to spatial periodicity of the array of somites. S, somite; PS, presumptive somite.
Materials and Methods
In Situ Hybridization and Graphical Analysis
Whole mount in situ hybridization were carried out as previously described[30], [43]. The following regions were used as probes; mouse Sprouty4, nucleotide residues -25-1177; Lfng, 17-1382; Uncx4.1, -14-1680, zebrafish Sprouty4, 32-830. We detected expression of Sprouty4 or Lfng in E10.5 mouse PSM by BCIP/NBT. The expression pattern was digitally scored Sion image software. The relative positions in the PSM were normalized by the lengths between the newest somite boundary (100%), for which we used Uncx4.1 probe as a marker, and posterior end of embryo (0%). The values from each assessment were analyzed graphically.
Experiments were approved by the Animal Care Committee of Nara Institute of Science and Technology and were conducted in accordance with guidelines established by the Science Council of Japan.
Explant Culture
The posterior part of embryo was bisected at E10.5. One half was fixed immediately, and the other half was cultured in 10% FBS-DMEM/F12 for 60 or 120 min before fixation. Hes7-null mutants and its littermates were bisected and cultured for 4 h in 1% FBS-DMEM/F12 with or without bFGF (20 nanogram/ml). After fixation, the expression of Sprouty4 was detected by in situ hybridization.
Transgenic mice
For transient transgenic experiment, we used a vector containing the 5.4 kb Hes7 promoter followed by exonic regions of Hes7 with IRES-Venus and SV40 polyadenylation signal [18].
Luciferase Assays
For promoter analysis, a luciferase reporter (GL3, Promega, 50 nanogram) under the control of the Sprouty4 promoter (−1521 to +171) were transfected into NIH3T3 cells, which were plated in 24-well plates, with 0, 25, 50 nanogram of expression vector (pCI, Promega) for Hes7 as previously described[30]. The vector for Renilla luciferase gene under the control of the SV40 promoter (5 nanogram) was co-transfected as an internal standard to normalize the transfected efficacy. After 24 h, bFGF (10 nanogram/ml) were added to the culture media and incubate for 24 h. Then, the cells were harvested and luciferase activities were measured. To introduce mutations to the promoter of Sprouty4, site-directed and semi-random mutagenesis was performed as previously described[44]. Primer sequences (underlines show mutated nucleotide). N-box1 Mutant: CTATGAAGGCCAAACCATGGCAAGATAGATCTATC, E-box1 Mutant: CTGCTCCACCCATCTGCTCAGCTCATTCTCCCTAT, N-box2 Mutant: AAAGGGGAGAGGGCCCATGGAATACAAAGGCCTGG, E-box2 Mutant: CCACGCAGCTAAGCTGGTCACTGCAGTCGCCGCCG, E-box3 and N-box3 Mutant: GCGCGCACGGGGTTGGTCGACCCCACCCATTCATA.
Western Blotting
NIH 3T3 cells were transfected with 0, 25, 50 nanogram of Hes7 expression vector. After 48 hour, they were lysed in lysis buffer (50 mM Tris pH 8.0, 300 mM NaCl, 2 mM EDTA, 1%NP-40, 0.1% SDS and 0.5% Deoxycolate). The lysates were sonicated for 30second interval and 10second and it was repeated five times, and were separated by SDS-PAGE and transferred to polyvinylidene difluoride membranes. Then, the membrane was incubated with 1/500 anti-Hes7 antibody[20], followed by applying anti-guinea pig IgG antibody conjugated peroxidase.
Zebrafish
Wild type (AB) was used in this work.
Acknowledgments
We thank Michiko Saito, Kazuaki Takahashi and Wataru Sakamoto for generation of transgenic mice, Akihiko Yoshimura and Koji Taniguchi for materials and helpful comments, and Ryoichiro Kageyama for encouragement and advice.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by KAKENHI (Grant-in-Aid for Scientific Research) on Priority Areas “Systems Genomics”, KAKENHI(B), and WAKATE(B) from the Ministry of Education, Culture, Sports, Science and Technology (MEXT), Japan, and by the UEHARA Memorial Foundation. This work was also supported in part by Global COE Program in NAIST (Frontier Biosciences: strategies for survival and adaptation in a changing global environment), MEXT, Japan. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Pourquie O. Vertebrate somitogenesis. Annu Rev Cell Dev Biol. 2001;17:311–350. doi: 10.1146/annurev.cellbio.17.1.311. [DOI] [PubMed] [Google Scholar]
- 2.Dequeant ML, Pourquie O. Segmental patterning of the vertebrate embryonic axis. Nat Rev Genet. 2008;9:370–382. doi: 10.1038/nrg2320. [DOI] [PubMed] [Google Scholar]
- 3.Palmeirim I, Henrique D, Ish-Horowicz D, Pourquie O. Avian hairy gene expression identifies a molecular clock linked to vertebrate segmentation and somitogenesis. Cell. 1997;91:639–648. doi: 10.1016/s0092-8674(00)80451-1. [DOI] [PubMed] [Google Scholar]
- 4.Bessho Y, Kageyama R. Oscillations, clocks and segmentation. Curr Opin Genet Dev. 2003;13:379–384. doi: 10.1016/s0959-437x(03)00083-2. [DOI] [PubMed] [Google Scholar]
- 5.Bessho Y, Sakata R, Komatsu S, Shiota K, Yamada S, et al. Dynamic expression and essential functions of Hes7 in somite segmentation. Genes Dev. 2001;15:2642–2647. doi: 10.1101/gad.930601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holley SA, Geisler R, Nüsslein-Volhard C. Control of her1 expression during zebrafish somitogenesis by a Delta-dependent oscillator and an independent wave-front activity. Genes Dev. 2000;14:1678–1690. [PMC free article] [PubMed] [Google Scholar]
- 7.Oates AC, Ho RK. Hairy/E(spl)-related (Her) genes are central components of the segmentation oscillator and display redundancy with the Delta/Notch signaling pathway in the formation of anterior segmental boundaries in the zebrafish. Development. 2002;129:2929–2946. doi: 10.1242/dev.129.12.2929. [DOI] [PubMed] [Google Scholar]
- 8.Henry CA, Urban MK, Dill KK, Merlie JP, Page MF, et al. Two linked hairy/Enhancer of split-related zebrafish genes, her1 and her7, function together to refine alternating somite boundaries. Development. 2002;129:3693–3704. doi: 10.1242/dev.129.15.3693. [DOI] [PubMed] [Google Scholar]
- 9.Jouve C, Palmeirim I, Henrique D, Beckers J, Gossler A, et al. Notch signalling is required for cyclic expression of the hairy-like gene HES1 in the presomitic mesoderm. Development. 2000;127:1421–1429. doi: 10.1242/dev.127.7.1421. [DOI] [PubMed] [Google Scholar]
- 10.McGrew MJ, Dale JK, Fraboulet S, Pourquie O. The lunatic fringe gene is a target of the molecular clock linked to somite segmentation in avian embryos. Curr Biol. 1998;8:979–982. doi: 10.1016/s0960-9822(98)70401-4. [DOI] [PubMed] [Google Scholar]
- 11.Aulehla A, Johnson RL. Dynamic expression of lunatic fringe suggests a link between notch signaling and an autonomous cellular oscillator driving somite segmentation. Dev Biol. 1999;207:49–61. doi: 10.1006/dbio.1998.9164. [DOI] [PubMed] [Google Scholar]
- 12.Forsberg H, Crozet F, Brown NA. Waves of mouse Lunatic fringe expression, in four-hour cycles at two-hour intervals, precede somite boundary formation. Curr Biol. 1998;8:1027–1030. doi: 10.1016/s0960-9822(07)00424-1. [DOI] [PubMed] [Google Scholar]
- 13.Elmasri H, Liedtke D, Lucking G, Volff JN, Gessler M, et al. her7 and hey1, but not lunatic fringe show dynamic expression during somitogenesis in medaka (Oryzias latipes). Gene Expr Patterns. 2004;4:553–559. doi: 10.1016/j.modgep.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 14.Prince VE, Holley SA, Bally-Cuif L, Prabhakaran B, Oates AC, et al. Zebrafish lunatic fringe demarcates segmental boundaries. Mech Dev. 2001;105:175–180. doi: 10.1016/s0925-4773(01)00398-7. [DOI] [PubMed] [Google Scholar]
- 15.Ishikawa A, Kitajima S, Takahashi Y, Kokubo H, Kanno J, et al. Mouse Nkd1, a Wnt antagonist, exhibits oscillatory gene expression in the PSM under the control of Notch signaling. Mech Dev. 2004;121:1443–1453. doi: 10.1016/j.mod.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Dale JK, Malapert P, Chal J, Vilhais-Neto G, Maroto M, et al. Oscillations of the snail genes in the presomitic mesoderm coordinate segmental patterning and morphogenesis in vertebrate somitogenesis. Dev Cell. 2006;10:355–366. doi: 10.1016/j.devcel.2006.02.011. [DOI] [PubMed] [Google Scholar]
- 17.Aulehla A, Wehrle C, Brand-Saberi B, Kemler R, Gossler A, et al. Wnt3a plays a major role in the segmentation clock controlling somitogenesis. Dev Cell. 2003;4:395–406. doi: 10.1016/s1534-5807(03)00055-8. [DOI] [PubMed] [Google Scholar]
- 18.Niwa Y, Masamizu Y, Liu T, Nakayama R, Deng CX, et al. The initiation and propagation of Hes7 oscillation are cooperatively regulated by Fgf and notch signaling in the somite segmentation clock. Dev Cell. 2007;13:298–304. doi: 10.1016/j.devcel.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 19.Dequeant ML, Glynn E, Gaudenz K, Wahl M, Chen J, et al. A complex oscillating network of signaling genes underlies the mouse segmentation clock. Science. 2006;314:1595–1598. doi: 10.1126/science.1133141. [DOI] [PubMed] [Google Scholar]
- 20.Bessho Y, Hirata H, Masamizu Y, Kageyama R. Periodic repression by the bHLH factor Hes7 is an essential mechanism for the somite segmentation clock. Genes Dev. 2003;17:1451–1456. doi: 10.1101/gad.1092303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dale JK, Maroto M, Dequeant ML, Malapert P, McGrew M, et al. Periodic notch inhibition by lunatic fringe underlies the chick segmentation clock. Nature. 2003;421:275–278. doi: 10.1038/nature01244. [DOI] [PubMed] [Google Scholar]
- 22.Sawada A, Shinya M, Jiang YJ, Kawakami A, Kuroiwa A, et al. Fgf/MAPK signalling is a crucial positional cue in somite boundary formation. Development. 2001;128:4873–4880. doi: 10.1242/dev.128.23.4873. [DOI] [PubMed] [Google Scholar]
- 23.Dubrulle J, McGrew MJ, Pourquie O. FGF signaling controls somite boundary position and regulates segmentation clock control of spatiotemporal Hox gene activation. Cell. 2001;106:219–232. doi: 10.1016/s0092-8674(01)00437-8. [DOI] [PubMed] [Google Scholar]
- 24.Dubrulle J, Pourquie O. fgf8 mRNA decay establishes a gradient that couples axial elongation to patterning in the vertebrate embryo. Nature. 2004;427:419–422. doi: 10.1038/nature02216. [DOI] [PubMed] [Google Scholar]
- 25.Kawamura A, Koshida S, Hijikata H, Sakaguchi T, Kondoh H, et al. Zebrafish hairy/enhancer of split protein links FGF signaling to cyclic gene expression in the periodic segmentation of somites. Genes Dev. 2005;19:1156–1161. doi: 10.1101/gad.1291205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Maximy AA, Nakatake Y, Moncada S, Itoh N, Thiery JP, et al. Cloning and expression pattern of a mouse homologue of drosophila sprouty in the mouse embryo. Mech Dev. 1999;81:213–216. doi: 10.1016/s0925-4773(98)00241-x. [DOI] [PubMed] [Google Scholar]
- 27.Minowada G, Jarvis LA, Chi CL, Neubuser A, Sun X, et al. Vertebrate Sprouty genes are induced by FGF signaling and can cause chondrodysplasia when overexpressed. Development. 1999;126:4465–4475. doi: 10.1242/dev.126.20.4465. [DOI] [PubMed] [Google Scholar]
- 28.Pourquie O, Tam PP. A nomenclature for prospective somites and phases of cyclic gene expression in the presomitic mesoderm. Dev Cell. 2001;1:619–620. doi: 10.1016/s1534-5807(01)00082-x. [DOI] [PubMed] [Google Scholar]
- 29.Maroto M, Dale JK, Dequeant ML, Petit AC, Pourquie O. Synchronised cycling gene oscillations in presomitic mesoderm cells require cell-cell contact. Int J Dev Biol. 2005;49:309–315. doi: 10.1387/ijdb.041958mm. [DOI] [PubMed] [Google Scholar]
- 30.Bessho Y, Miyoshi G, Sakata R, Kageyama R. Hes7: a bHLH-type repressor gene regulated by Notch and expressed in the presomitic mesoderm. Genes Cells. 2001;6:175–185. doi: 10.1046/j.1365-2443.2001.00409.x. [DOI] [PubMed] [Google Scholar]
- 31.Zhang N, Gridley T. Defects in somite formation in lunatic fringe-deficient mice. Nature. 1998;394:374–377. doi: 10.1038/28625. [DOI] [PubMed] [Google Scholar]
- 32.Evrard YA, Lun Y, Aulehla A, Gan L, Johnson RL. lunatic fringe is an essential mediator of somite segmentation and patterning. Nature. 1998;394:377–381. doi: 10.1038/28632. [DOI] [PubMed] [Google Scholar]
- 33.Ding W, Bellusci S, Shi W, Warburton D. Genomic structure and promoter characterization of the human Sprouty4 gene, a novel regulator of lung morphogenesis. Am J Physiol Lung Cell Mol Physiol. 2004;287:L52–59. doi: 10.1152/ajplung.00430.2003. [DOI] [PubMed] [Google Scholar]
- 34.Takebayashi K, Sasai Y, Sakai Y, Watanabe T, Nakanishi S, et al. Structure, chromosomal locus, and promoter analysis of the gene encoding the mouse helix-loop-helix factor HES-1. Negative autoregulation through the multiple N box elements. J Biol Chem. 1994;269:5150–5156. [PubMed] [Google Scholar]
- 35.Ikeda K, Nagano K, Kawakami K. Anomalous interaction of Sp1 and specific binding of an E-box-binding protein with the regulatory elements of the Na,K-ATPase alpha 2 subunit gene promoter. Eur J Biochem. 1993;218:195–204. doi: 10.1111/j.1432-1033.1993.tb18365.x. [DOI] [PubMed] [Google Scholar]
- 36.Holley SA, Julich D, Rauch GJ, Geisler R, Nüsslein-Volhard C. her1 and the notch pathway function within the oscillator mechanism that regulates zebrafish somitogenesis. Development. 2002;129:1175–1183. doi: 10.1242/dev.129.5.1175. [DOI] [PubMed] [Google Scholar]
- 37.Mason JM, Morrison DJ, Basson MA, Licht JD. Sprouty proteins: multifaceted negative-feedback regulators of receptor tyrosine kinase signaling. Trends Cell Biol. 2006;16:45–54. doi: 10.1016/j.tcb.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 38.Sasaki A, Taketomi T, Kato R, Saeki K, Nonami A, et al. Mammalian Sprouty4 suppresses Ras-independent ERK activation by binding to Raf1. Nat Cell Biol. 2003;5:427–432. doi: 10.1038/ncb978. [DOI] [PubMed] [Google Scholar]
- 39.Shim K, Minowada G, Coling DE, Martin GR. Sprouty2, a mouse deafness gene, regulates cell fate decisions in the auditory sensory epithelium by antagonizing FGF signaling. Dev Cell. 2005;8:553–564. doi: 10.1016/j.devcel.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 40.Taketomi T, Yoshiga D, Taniguchi K, Kobayashi T, Nonami A, et al. Loss of mammalian Sprouty2 leads to enteric neuronal hyperplasia and esophageal achalasia. Nat Neurosci. 2005;8:855–857. doi: 10.1038/nn1485. [DOI] [PubMed] [Google Scholar]
- 41.Klein T, Arias AM. Interactions among Delta, Serrate and Fringe modulate Notch activity during Drosophila wing development. Development. 1998;125:2951–2962. doi: 10.1242/dev.125.15.2951. [DOI] [PubMed] [Google Scholar]
- 42.Taniguchi K, Ayada T, Ichiyama K, Kohno R, Yonemitsu Y, et al. Sprouty2 and Sprouty4 are essential for embryonic morphogenesis and regulation of FGF signaling. Biochem Biophys Res Commun. 2007;352:896–902. doi: 10.1016/j.bbrc.2006.11.107. [DOI] [PubMed] [Google Scholar]
- 43.Matsui T, Raya A, Kawakami Y, Callol-Massot C, Capdevila J, et al. Noncanonical Wnt signaling regulates midline convergence of organ primordia during zebrafish development. Genes Dev. 2005;19:164–175. doi: 10.1101/gad.1253605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sawano A, Miyawaki A. Directed evolution of green fluorescent protein by a new versatile PCR strategy for site-directed and semi-random mutagenesis. Nucleic Acids Res. 2000;28:E78. doi: 10.1093/nar/28.16.e78. [DOI] [PMC free article] [PubMed] [Google Scholar]