Abstract
Place cells in hippocampal area CA1 are essential for spatial learning and memory. Here, we examine whether daily exposure to a previously unexplored environment can alter place cell properties. We demonstrate two previously unreported slowly developing plasticities in mouse place fields: both the spatial tuning and the trial-to-trial reproducibility of CA1 place fields improve over days. We asked whether these two components of improved spatial coding rely on the α-isoform of the calcium/calmodulin-dependent protein kinase II (αCaMKII) autophosphorylation, an effector mechanism of NMDA receptor-dependent long-term potentiation and an essential molecular process for spatial memory formation. We show that, in mice with deficient autophosphorylation of αCaMKII, the spatial tuning of place fields is initially similar to that of wild-type mice, but completely fails to show the experience-dependent increase over days. In contrast, place field reproducibility in the mutants, although impaired, does show the experience-dependent increase over days. Consequently, the progressive improvement in spatial coding in new hippocampal place cell maps depends on the existence of two molecularly dissociable, experience-dependent processes.
Keywords: CaMKII, hippocampus, LTP, place cells, plasticity, spatial memory
Introduction
Place cells are hippocampal pyramidal neurons that fire selectively when the animal is in a particular location within a particular environment (O'Keefe and Dostrovsky, 1971; O'Keefe and Nadel, 1978). Previous research in the rat has shown that, although some place field activity is present immediately in a novel environment, place field activity also undergoes significant development over the first few minutes of environmental experience (Hill, 1978; Wilson and McNaughton, 1993; Frank et al., 2004). The present study demonstrates two previously unreported slowly developing plasticities in place fields of the mouse, spatial tuning and spatial reproducibility, both of which improve over several days. We further show that they are differentially dependent on intracellular molecular mechanisms, spatial tuning being dependent on the switching properties of the α-isoform of the calcium/calmodulin-dependent protein kinase II (αCaMKII) and spatial reproducibility being independent of this property.
αCaMKII plays a prominent role in plasticity at hippocampal glutamatergic synapses and in spatial memory formation (Elgersma et al., 2004). Crucially, αCaMKII can undergo autophosphorylation at threonine 286, allowing its kinase activity to switch from Ca2+ dependence to Ca2+ independence (Miller and Kennedy, 1986). This molecular switch has been proposed to subserve synaptic information storage (Lisman et al., 2002) or memory formation (Irvine et al., 2006). Knock-in αCaMKIIT286A mutant (mut) mice, which have a targeted point mutation that inactivates the αCaMKII autophosphorylation, do not show NMDA receptor-dependent long-term potentiation (LTP) in hippocampal area CA1 (Giese et al., 1998; Yasuda et al., 2003; Lengyel et al. 2004; Cooke et al., 2006) and show profound behavioral deficits in the hippocampus-dependent, hidden-platform version of the Morris water maze task (Morris et al. 1982; Giese et al. 1998; Need and Giese, 2003).
Previous unit-recording studies have indicated the importance of αCaMKII functionality for the spatial properties of hippocampal place cells (Rotenberg et al., 1996; Cho et al., 1998). Neither study, however, addressed the temporal development of spatial firing. For instance, Cho et al. (1998) reported that αCaMKIIT286A mutant place cells showed decreased spatial selectivity and stability, in an already-familiar radial arm maze, using a recording period of 30 min. This methodology could not distinguish between baseline deficits in spatial selectivity and reproducibility, and changes in these measures as a function of plasticity-dependent mechanisms. To address this question, we tracked place cell activity during the initial exposure to the recording enclosure and during the subsequent 3 d experience.
The present results show that, in wild-type (wt) mice, both the spatial tuning and reproducibility of CA1 place cells improve with experience over this 4 d period. In contrast, in the αCaMKIIT286A mutants, only the reproducibility of CA1 place fields improves but not their spatial tuning.
Materials and Methods
Animals.
Subjects were maintained on a 12 h light/dark schedule (lights off at 3:00 P.M.). Animals weighed 25–35 g at surgery. After surgery, they were maintained at 90% of free-feeding weight. Male homozygous αCaMKIIT286A (n = 7) mutants and control male wild-type (n = 4) littermates were obtained in the 129B6F2,3 background by intercrosses of heterozygous mutants. PCR genotyping was performed as described previously (Giese et al., 1998). All experiments were performed blind to genotype and in accordance with the United Kingdom Animals (Scientific Procedures) Act 1986.
Surgery and electrodes.
Mice under deep anesthesia were chronically implanted with microdrives loaded with four tetrodes. Details of surgical procedure were similar to Cacucci et al. (2004), adapted for mice. Mice were allowed a 1 week postoperative recovery, after which microelectrodes were advanced ventrally by 30 μm/d. When hippocampal pyramidal cells were found, recording sessions began. After the experiments, the mice were perfused with 4% paraformaldehyde, the brain was sliced coronally into 40 μm sections and was stained with cresyl violet, for electrode localization.
Behavioral testing.
Experiments were conducted in a black-curtained, circular testing arena 1.7 m in diameter Animals were given five recording trials per day for 4 d. All trials were 20 min duration with an intertrial interval of 20 min. At all other times, mice were kept on a holding platform outside the arena. Recording environments were two identical circular-walled, light-gray wooden boxes (diameter, 48 cm; height, 36 cm), placed on a black platform that was washed between every trial. An external cue card (100 cm high; 80 cm wide) suspended inside the curtains provided directional constancy. During trials, the mouse searched for chocolate flakes randomly thrown into the environment. During cue rotation trials (fourth trial of days 2 and 4), the cue card was rotated by 180° relative to the testing arena. All other cues remained constant.
Spike sorting.
Isolation of single units from multiunit data were performed manually, blind to genotype, on the basis of peak-to-trough amplitude, using custom software (TINT; Neil Burgess, University College London, London, UK). Analysis of cluster quality, following Schmitzer-Torbert et al. (2005), ruled out unit isolation as a source of difference between the groups (supplemental Fig. 1, available at www.jneurosci.org as supplemental material).
Quantitative analysis of place fields.
Position data were sorted into 1.5 × 1.5 cm bins. Firing-rate maps were constructed as described by Cacucci et al. (2004). On each trial, cells were included in the analysis if they had (1) spike width (time between peak and trough) of >300 μs and (2) peak firing rate of >1 Hz. In general, different cells were recorded on each day. Occasionally, cells recorded on different days were judged to be the same, on the basis of stable spike clusters. In this case, only data from the recording of the first day were used.
Spatial information is a measure of the extent to which the firing of a cell can be used to predict the position of the animal. The estimate of the rate of information I(R|X) between firing rate R and location X proposed by Skaggs et al. (1993) is as follows:
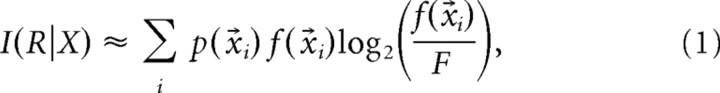 |
where p(x⃗i) is the probability for the animal being at location x⃗i, f(x⃗i) is the firing rate observed at x⃗i, and F is the overall firing rate of the cell. To obtain a measure of spatial information expressed in bits/spike, we divided the value obtained from Equation 1 by the overall mean firing rate F. The spatial information for each cell was the mean value for the first three trials of the day. Place field reproducibility was measured by correlating rate values of spatially corresponding bins from two consecutive trials, using only those bins in which firing rate >0 Hz in at least one trial. The reproducibility score for each cell was the mean r value from the correlation of the first versus the second trial, and the second versus the third trial, of the day. The spatial coherence for each firing-rate map was computed as the mean correlation between the firing rate of each bin with the aggregate rate of the 24 nearest bins.
Results
Changes in place cell firing with repeated exposure to an environment
With repeated exposures to a cylindrical environment, place cells in wild-type mice increase their spatial tuning and reproducibility. Figure 1A shows the firing of three cells recorded over 4 consecutive days. On the first day, each cell has a hotspot of elevated firing, but there is a tendency for diffuse firing to occur over large parts of the environment. Over days, the area of the field tends to decrease and for some cells (e.g., cell 3) there is a marked increase in firing in the field. A measure that takes both these variables into account is the Skaggs information measure (Skaggs et al., 1993), which is shown beneath each firing-rate map in Figure 1A and plotted in B. At the same time, the reproducibility of the field location also increases (Fig. 1C). Although both measures are increasing over time and are correlated (r = 0.372, p < 0.001 for the whole-cell population), it is clear that large increases in spatial information are compatible with small increases in reproducibility (e.g., cell 1, days 1–2) and vice versa (e.g., cell 1, days 2–3). This raises the question as to whether both types of plasticity are attributable to the same underlying mechanisms. We asked whether one or both types of plasticity rely on the autophosphorylation switch of αCaMKII by comparing the development of place fields in the αCaMKIIT286A mouse with those of wt mice.
Figure 1.
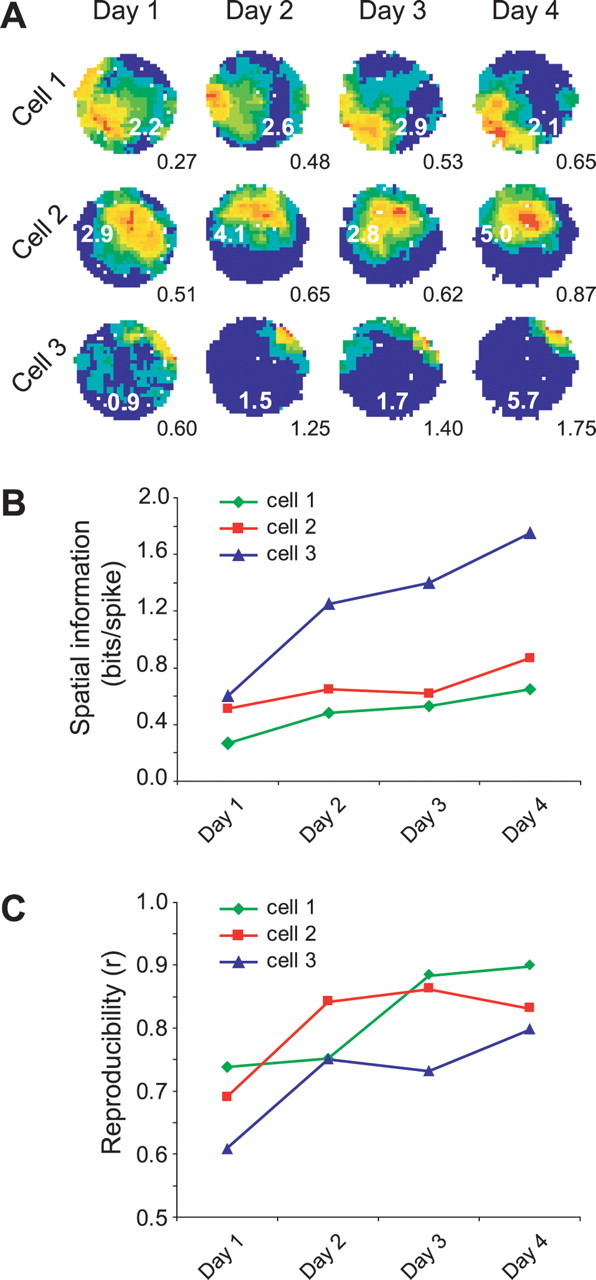
Two types of plastic changes in CA1 place cells of a wild-type mouse. A, Place fields of three cells simultaneously recorded over 4 d in a wild-type mouse. Spatial information increases across days. Firing rates shown as false color maps in which each color represents 10% of the peak firing rate. Numbers in white on each false-color map are peak firing rate, and numbers in black below are the spatial information scores. Data were taken from the third trial of each day. B, Graph of spatial information over the 4 d for the three cells shown in A. C, Graph of the spatial reproducibility measure over 4 d. Data were taken from the correlation of the second versus the third trial of each day.
The effects of experience on two spatial measures (spatial information and spatial reproducibility) were examined during repeated exposures to the same cylindrical enclosure for 4 consecutive days. The total dataset consists of 226 mutant and 98 wt cells recorded from seven mutant and four wt mice (littermate controls).
Hippocampal place cell spatial information in αCaMKIIT286A mice fails to improve with experience
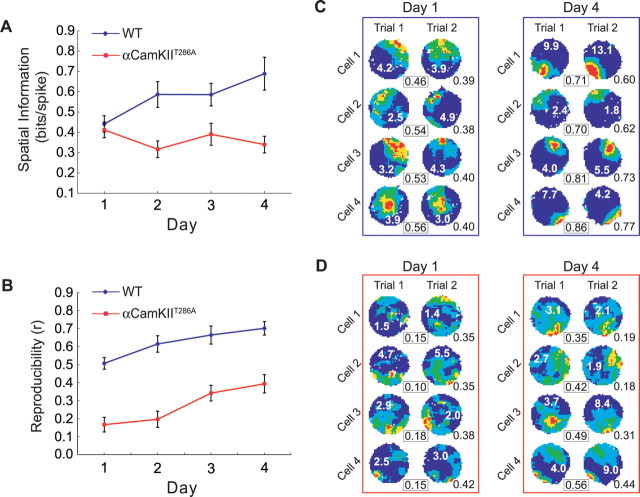
As shown in Figure 2A, place cell spatial information increased across days in wt mice, confirming at the population level what we had seen for the individual cells shown in Figure 1. In contrast, spatial information did not change in the mutant mice during the same experience (two-way ANOVA, main effect of genotype, F(1,314) = 29.69, p < 0.001; genotype by day interaction, F(3,314) = 3.17, p = 0.025). The increase in spatial information over time in the wt mice is not likely to be attributable to nonspatial contributions to this measure. An ANOVA on the mean running speeds revealed no difference in genotype (F(1,36) = 2.14; p > 0.15) or genotype by day interactions (F(3,36) = 0.3; p > 0.83), ruling out differences in running speed between the groups. We performed a similar analysis on total path length and find wt mice did not change the amount they sampled the environment across days (total path length, F(3,13) = 0.16, p > 0.92), no difference between genotypes (F(1,36) = 2.22; p > 0.15), and no genotype by day interaction (F(3,36) = 0.35; p > 0.79). Moreover, no differences were observed across genotype or training day, in running speed variance (genotype, F(1,36) = 2.0, p = 0.17; day, F(3,36) = 0.01, p = 0.99) or angular head velocity (genotype, F(1,36) = 2.09, p = 0.16; day, F(3,36) = 0.85, p = 0.48).
Figure 2.
A, Spatial information increases over days in wild-type cells but not in mutant cells. Note that values are similar on day 1. B, Between-trial spatial reproducibility increases over exposure days in both wt and αCaMKIIT286A mice, although absolute levels of reproducibility are impaired in αCaMKIIT286A mice. Error bars indicate SEM. C, D, Representative examples of wt (C) and αCaMKIIT286A (D) place cells recorded during two consecutive trials on days 1 and 4 (different cell populations on different days). Reproducibility values for trial 1 versus trial 2 comparison are shown in black boxes between the maps. Numbers in white on each false-color map are peak firing rates. Numbers at the bottom right of the trial 2 maps refer to the spatial information carried by the place cell (mean value for trial 1 and 2). Note how between-trial reproducibility is low in both mutants and wt on day 1 and higher on day 4. Spatial information content is higher on day 4 than on day 1 in the wt cells but fails to improve in the αCaMKIIT286A cells.
However, we did find a significant overall difference in firing rate between genotypes (F(1,314) = 3.71; p = 0.05) but no interaction between genotype and days (F(3,314) = 0.13; p > 0.94), ruling out any systematic differential changes in firing rates as a causal explanation for our findings. Indeed, recalculating spatial information after deleting random spikes, to match mean firing rate across genotypes, does not alter the results (supplemental Fig. 2, available at www.jneurosci.org as supplemental material).
The experience-dependent effect on spatial information emerged by the second day. That is, although there was no difference in place cell spatial information in wt and mutants on day 1 (wt, 0.44 ± 0.04 bits/spike; mut, 0.41 ± 0.04 bits/spike; one-tailed t test, t = 0.43, p = 0.33), there was a difference on day 2 (wt, 0.59 ± 0.06 bits/spike; mut, 0.32 ± 0.04 bits/spike; t = 3.58, p < 0.001).
Because the effects described relate to differences across groups of animals, an alternative statistical approach is to analyze the data on a by-animal basis. Using this approach, the trends described above are still apparent (supplemental Fig. 3A, available at www.jneurosci.org as supplemental material). There is a main effect of genotype (F(1,36) = 20.74; p < 0.001) and an effect of day on the wild-type spatial information (F(1,13) = 4.56; p < 0.029). However, there is no longer a significant genotype by day interaction (F(1,36) = 1.87; p = 0.15).
Place field reproducibility is impaired in αCaMKIIT286A mice but improves with experience
Both wt and mutant place cells were tested for reproducibility of their firing patterns (Fig. 2B). Overall, mutant place fields show significantly lower trial-to-trial reproducibility scores than wild-type place fields when firing patterns recorded from temporally adjacent trials are compared (wt, 0.62 ± 0.02; αCaMKIIT286A, 0.27 ± 0.02; main effect of genotype: F(1,224) = 98.87, p < 0.001). This indicates that place field reproducibility is compromised in the αCaMKIIT286A mice. In addition, however, there was also a significant effect of day on place field trial-to-trial reproducibility (main effect of day: F(3,224) = 7.62, p < 0.001) and no interaction between genotype and days (F(3,224) = 0.50; p > 0.68). This latter indicates that the reproducibility of both wt and mutant place fields increased in parallel over exposure days (wt: F(3,76) = 4.33, p = 0.007; mutant: F(3,148) = 6.05, p = 0.001), indicating that one form of plasticity is being spared in the αCaMKIIT286A mice (Fig. 2B,D). When spatial reproducibility is analyzed by animal, the trends described above are still apparent (supplemental Fig. 3B, available at www.jneurosci.org as supplemental material). There is still a main effect of genotype (F(1,36) = 45.21; p < 0.001); however, there is no longer a significant effect of day (F(1,36) = 1.81; p = 0.16).
Interestingly, spatial coherence, which is a measure of the local smoothness of place fields, although significantly impaired in αCaMKIIT286A place cells, improves over training, in a very similar manner to that observed for intertrial spatial reproducibility (supplemental Fig. 4, available at www.jneurosci.org as supplemental material).
αCaMKIIT286A place field impairments are not attributable to deficits in the head direction system
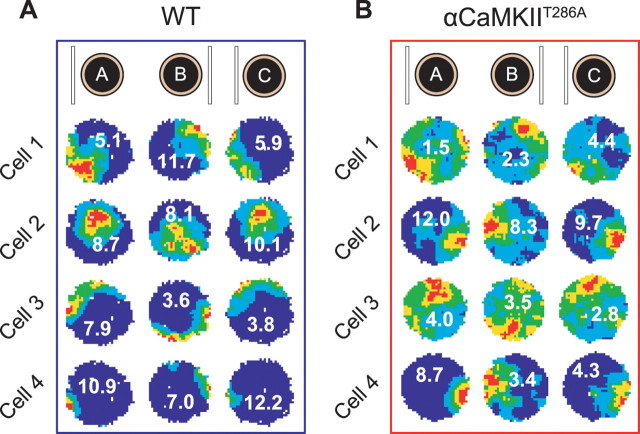
To test whether the spatial impairments observed in mutant place cells are caused by deficits of the head direction system or the anchoring of the place cells to the head direction system, cells were recorded while the cue card was rotated by 180° (see Materials and Methods). As shown in Figure 3, although place fields recorded from the αCaMKIIT286A mice appear less organized, they still follow the cue card rotation, like those recorded from the wt mice. This indicates that the mutant head direction system is still capable of correctly processing visual information and of using it as an orientation landmark.
Figure 3.
A, B, Four simultaneously recorded cells from one wt (A) and one αCaMKIIT286A mouse (B) during a cue rotation experiment. Both sets of place fields rotate by a similar amount to the cue card during both the 180° rotation (trial B) and the return to baseline position (trial C).
The degree to which place cells were under the control of the cue card was assessed by comparing the angular displacement between sessions in 21 wild-type cells and 53 αCaMKIIT286A mutant cells. Cue rotation firing-rate maps were rotated in 6° steps and correlated against baseline rate maps: the angular displacement was the rotation of maximum correlation. Place field angular displacement did not differ across genotype (wt, mean vector ± circular SD, 179.6 ± 16.3°; αCaMKIIT286A, 167.9 ± 59.2°; Watson–Williams F test, F(1,73) = 1.01, p = 0.32).
An additional indication that the impaired reproducibility of αCaMKIIT286A place cells cannot be attributed to deficits in the head direction system was that, when rate maps from baseline trials were rotated as an ensemble, to maximize reproducibility, the reproducibility deficit observed in αCaMKIIT286A mice was not rescued (main effect of rotation in the αCaMKIIT286A mice, F(1,296) = 2.21, p = 0.14) (supplemental Fig. 5, available at www.jneurosci.org as supplemental material).
Discussion
The present study shows that, on exposure to a previously unexplored environment, both spatial information and spatial reproducibility of CA1 place cells increase over days in wild-type mice. We also observed this progressive increase in both spatial measures in place cells in a different mouse strain to that used in the present study (C57BL/6) (F. Cacucci, unpublished observations). An additional novel finding is that these two plasticities are dissociable. In the absence of αCaMKII autophosphorylation at threonine 286, despite many repeated exposures to the same environment, hippocampal CA1 place fields do not show any increase in spatial information, but do show an increase in the place field reproducibility and spatial coherence.
The data were examined using either cells or animals as the experimental unit, the former assessing the effects of training and genotype on place cells as a population, the latter, their effects on the two groups of animals. The by-animal results are similar to the by-cell results (Fig. 2; supplemental Fig. 3, available at www.jneurosci.org as supplemental material), although some effects do not reach statistical significance, possibly because of low statistical power. Unfortunately, the practical constraints of in vivo physiological recordings in mutant mice militate against the collection of datasets sufficiently large for satisfactory by-animal analysis (for additional discussion of the choice of experimental unit, see Leger and Didrichsons, 1994).
Few studies have addressed the temporal development of hippocampal place cell firing after repeated exposures to the same environment over days. Hill (1978) reported that most place fields emerged immediately. Wilson and McNaughton (1993) found that the reconstruction of the rat's position in a novel portion of an open field environment from CA1 place cell firing was more accurate during the second 10 min compared with the first 10 min of exposure. Kentros et al. (1998) showed that NMDA receptor (NMDAR)-dependent mechanisms were required for CA1 spatial maps formed in a novel environment to be stable on the second day in that environment. Lee et al. (2004) suggested that CA1 might be less plastic than CA3 on the basis of their finding that the center of mass of CA3 place fields shifts rapidly within the first day of exposure to a novel environment, whereas in CA1 the center-of-mass shift only occurs on the second day of exposure. One study closely comparable with the present one is Frank et al. (2004), in which rats were exposed to the same novel environment for 3 d. The greatest changes to place fields were on day 1, but changes also occurred on day 2 if the rat was in the novel environment for <4 min on day 1. If day 1 exposure was >5 min, place fields were stable on day 2. In the present study, in contrast, place fields changed over 4 d, despite 100 min of training each day. Although these few studies in different recording paradigms do not permit firm conclusions, they broadly indicate the importance of within-day plasticities in normal rat place cell function. The current study indicates that, in the mouse, CA1 place cell properties change over days. However, in the absence of a directly comparable study in the rat, it is not possible to conclude whether there is a species difference in the timescale of place field development. It would be interesting to test whether in the mouse this process might be modulated by the behavioral demands of the spatial task in which the animals are engaged (Kentros et al., 2004).
In an important comparable exposure-to-novelty mouse study, Nakazawa et al. (2003) did not observe any progressive increase in CA1 place cell tuning in wild-type mice. The discrepancy could be attributable to the fact that their novel and familiar linear track environments shared extramaze cues, whereas in the present study, the curtained recording arena provided a complete set of novel cues.
The present study is broadly consistent with previous mouse studies indicating the importance of αCaMKII functionality in spatial tuning and reproducibility of hippocampal place cells (Rotenberg et al., 1996; Cho et al., 1998). Place cells in mice expressing a constitutively active version of αCaMKII (αCaMKII-Asp286) showed reduced spatial coherence and impaired stability (Rotenberg et al., 1996) similarly to this study (Fig. 2; supplemental Fig. 4, available at www.jneurosci.org as supplemental material). Cho et al. (1998) showed impaired spatial selectivity in αCaMKIIT286A mice during exposures to a familiar radial maze. An interesting aspect of the present results is that spatial coherence and across-trial reproducibility follow very similar trends, which may indicate that both measures are assessing the spatial reliability of firing, either across trial (spatial reproducibility) or within trial (spatial coherence). Perhaps both reflect a common neural mechanism.
Failure of spatial information to increase over a 4 d period in the mutant animals could be interpreted in terms of a deficit in NMDA-mediated plasticity. Conversely, the preserved increase in reproducibility and coherence suggests that some plasticity might be taking place at the level of CA1 place cells in the αCaMKIIT286A mice. One possibility is that these processes are supported at different synaptic loci within the hippocampus. For instance, NMDAR-dependent LTP at CA3–CA1 synapses is abolished in αCaMKIIT286A mice (Giese et al., 1998; Cooke et al., 2006), implying that any intrahippocampal learning mechanisms reliant on NMDAR plasticity in CA3–CA1 circuits would be disrupted. However, medial entorhinal-dentate gyrus LTP is intact in αCaMKIIT286A mice (Cooke et al., 2006), allowing the reproducibility and coherence increases to be supported by plasticity at this synaptic site. Alternative possibilities are that the plasticities underlying spatial tuning and place field reproducibility/coherence take place at the same synaptic loci, but depend on different intracellular mechanisms, or that they might be supported by extrahippocampal mechanisms perhaps in the medial entorhinal grid cell system (Fyhn et al., 2004).
The cue card rotation experiment supports the view that the deficits observed in αCaMKIIT286A place cells are not attributable to an impaired head direction system. Cue rotation is preserved in mutants, showing that the αCaMKIIT286A mouse head direction cells can use sensory stimuli as spatial landmarks, that the head direction system can exert control over the place cells. It further suggests that there are no gross visual sensory impairments in the αCaMKIIT286A mouse.
In summary, this study demonstrates the existence of two slowly developing plasticities in mouse place fields, spatial tuning and spatial reproducibility, both of which improve over several days. Our findings indicate that deficient autophosphorylation of αCaMKII completely prevents the improvement in spatial tuning but, while impairing spatial reliability, does not prevent its experience-dependent improvement over days.
Footnotes
This work was supported by Biotechnology and Biological Sciences Research Council project grants and by a Wellcome Trust program grant. We thank Stephen Burton and Jim Donnett for technical support and Neil Burgess, Alastair McClelland, and Ming Yi for helpful discussions. We thank Elaine E. Irvine for genotyping of the mice and Anna Need for providing them.
References
- Cacucci F, Lever C, Wills TJ, Burgess N, O'Keefe J. Theta-modulated place-by-direction cells in the hippocampal formation in the rat. J Neurosci. 2004;24:8265–8277. doi: 10.1523/JNEUROSCI.2635-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho YH, Giese KP, Tanila H, Silva AJ, Eichenbaum H. Abnormal hippocampal spatial representations in alphaCaMKIIT286A and CREBalphaDelta- mice. Science. 1998;279:867–869. doi: 10.1126/science.279.5352.867. [DOI] [PubMed] [Google Scholar]
- Cooke SF, Wu J, Plattner F, Errington M, Rowan M, Peters M, Hirano A, Bradshaw KD, Anwyl R, Bliss TV, Giese KP. Autophosphorylation of alphaCaMKII is not a general requirement for NMDA receptor-dependent LTP in the adult mouse. J Physiol (Lond) 2006;574:805–818. doi: 10.1113/jphysiol.2006.111559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgersma Y, Sweatt JD, Giese KP. Mouse genetic approaches to investigating calcium/calmodulin-dependent protein kinase II function in plasticity and cognition. J Neurosci. 2004;24:8410–8415. doi: 10.1523/JNEUROSCI.3622-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank LM, Stanley GB, Brown EN. Hippocampal plasticity across multiple days of exposure to novel environments. J Neurosci. 2004;24:7681–7689. doi: 10.1523/JNEUROSCI.1958-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fyhn M, Molden S, Witter MP, Moser EI, Moser MB. Spatial representation in the entorhinal cortex. Science. 2004;305:1258–1264. doi: 10.1126/science.1099901. [DOI] [PubMed] [Google Scholar]
- Giese KP, Fedorov NB, Filipkowski RK, Silva AJ. Autophosphorylation at Thr286 of the alpha calcium-calmodulin kinase II in LTP and learning. Science. 1998;279:870–873. doi: 10.1126/science.279.5352.870. [DOI] [PubMed] [Google Scholar]
- Hill AJ. First occurrence of hippocampal spatial firing in a new environment. Exp Neurol. 1978;62:282–297. doi: 10.1016/0014-4886(78)90058-4. [DOI] [PubMed] [Google Scholar]
- Irvine EE, von Hertzen LS, Plattner F, Giese KP. alphaCaMKII autophosphorylation: a fast track to memory. Trends Neurosci. 2006;29:459–465. doi: 10.1016/j.tins.2006.06.009. [DOI] [PubMed] [Google Scholar]
- Kentros C, Hargreaves E, Hawkins RD, Kandel ER, Shapiro M, Muller RV. Abolition of long-term stability of new hippocampal place cell maps by NMDA receptor blockade. Science. 1998;280:2121–2126. doi: 10.1126/science.280.5372.2121. [DOI] [PubMed] [Google Scholar]
- Kentros C, Agnihotri NT, Streater S, Hawkins RD, Kandel ER. Increased attention to spatial context increases both place field stability and spatial memory. Neuron. 2004;42:183–185. doi: 10.1016/s0896-6273(04)00192-8. [DOI] [PubMed] [Google Scholar]
- Lee I, Rao G, Knierim JJ. A double dissociation between hippocampal subfields: differential time course of CA3 and CA1 place cells for processing changed environments. Neuron. 2004;42:803–815. doi: 10.1016/j.neuron.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Leger DW, Didrichsons IA. An assessment of data pooling and some alternatives. Anim Behav. 1994;48:823–832. [Google Scholar]
- Lengyel I, Voss K, Cammarota M, Bradshaw K, Brent V, Murphy KP, Giese KP, Rostas JA, Bliss TV. Autonomous activity of CaMKII is only transiently increased following the induction of long-term potentiation in the rat hippocampus. Eur J Neurosci. 2004;20:3063–3072. doi: 10.1111/j.1460-9568.2004.03748.x. [DOI] [PubMed] [Google Scholar]
- Lisman J, Schulman H, Cline H. The molecular basis of CaMKII function in synaptic and behavioural memory. Nat Rev Neurosci. 2002;3:175–190. doi: 10.1038/nrn753. [DOI] [PubMed] [Google Scholar]
- Miller SG, Kennedy MB. Regulation of brain type II Ca2+/calmodulin-dependent protein kinase by autophosphorylation: a Ca2+-triggered molecular switch. Cell. 1986;44:861–870. doi: 10.1016/0092-8674(86)90008-5. [DOI] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Sun LD, Quirk MC, Rondi-Reig L, Wilson MA, Tonegawa S. Hippocampal CA3 NMDA receptors are crucial for memory acquisition of one-time experience. Neuron. 2003;38:305–315. doi: 10.1016/s0896-6273(03)00165-x. [DOI] [PubMed] [Google Scholar]
- Need AC, Giese KP. Handling and environmental enrichment do not rescue learning and memory impairments in alphaCaMKII(T286A) mutant mice. Genes Brain Behav. 2003;2:132–139. doi: 10.1034/j.1601-183x.2003.00020.x. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34:171–175. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford: Clarendon; 1978. [Google Scholar]
- Rotenberg A, Mayford M, Hawkins RD, Kandel ER, Muller RU. Mice expressing activated CaMKII lack low frequency LTP and do not form stable place cells in the CA1 region of the hippocampus. Cell. 1996;87:1351–1361. doi: 10.1016/s0092-8674(00)81829-2. [DOI] [PubMed] [Google Scholar]
- Schmitzer-Torbert N, Jackson J, Henze D, Harris K, Redish AD. Quantitative measures of cluster quality for use in extracellular recordings. Neuroscience. 2005;131:1–11. doi: 10.1016/j.neuroscience.2004.09.066. [DOI] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL, Gothard KM, Markus EJ. An information-theoretic approach to deciphering the hippocampal code. Neural Inf Process Syst. 1993;5:1030–1037. [Google Scholar]
- Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Barth AL, Stellwagen D, Malenka RC. A developmental switch in the signaling cascades for LTP induction. Nat Neurosci. 2003;6:15–16. doi: 10.1038/nn985. [DOI] [PubMed] [Google Scholar]