Abstract
Objective:
Transient ischemic attacks (TIA) predict future stroke. However, there are no sensitive and specific diagnostic criteria for TIA and interobserver agreement regarding the diagnosis is poor. Diffusion-weighted MRI (DWI) demonstrates acute ischemic lesions in approximately 30% of TIA patients; the yield of perfusion-weighted MRI (PWI) is unclear.
Methods:
We prospectively performed both DWI and PWI within 48 hours of symptom onset in consecutive patients admitted with suspected hemispheric TIAs of <24 hours symptom duration. Two independent raters, blinded to clinical features, assessed the presence and location of acute DWI and PWI lesions. Lesions were correlated with suspected clinical localization and baseline characteristics. Clinical features predictive of a PWI lesion were assessed.
Results:
Forty-three patients met the inclusion criteria. Thirty-three percent had a PWI lesion and 35% had a DWI lesion. Seven patients (16%) had both PWI and DWI lesions and 7 (16%) had only PWI lesions. The combined yield for identification of either a PWI or a DWI was 51%. DWI lesions occurred in the clinically suspected hemisphere in 93% of patients; PWI lesions in 86%. PWI lesions occurred more frequently when the MRI was performed within 12 hours of symptom resolution, in patients with symptoms of speech impairment, and among individuals younger than 60 years.
Conclusions:
The combination of early diffusion-weighted MRI and perfusion-weighted MRI can document the presence of a cerebral ischemic lesion in approximately half of all patients who present with a suspected hemispheric transient ischemic attack (TIA). MRI has the potential to improve the accuracy of TIA diagnosis.
GLOSSARY
- ACA
= anterior cerebral artery;
- CI
= confidence interval;
- DWI
= diffusion-weighted MRI;
- ICA
= internal carotid artery;
- MCA
= middle cerebral artery;
- MRA
= magnetic resonance angiography;
- MTT
= mean transit time;
- OR
= odds ratios;
- PCA
= posterior cerebral artery;
- PWI
= perfusion-weighted MRI;
- RR
= risk ratios;
- TIA
= transient ischemic attacks;
- TOAST
= Trial of Org 10172 in Acute Stroke Treatment.
Transient ischemic attacks (TIA) are defined as sudden, temporary attacks (<24 hours) with focal symptoms attributable to dysfunction of one artery of the brain. The risk of stroke after a TIA is up to 10% in the first week following symptom onset.1 Recent studies demonstrated that a prompt etiologic investigation followed by the initiation of an appropriate prevention strategy can substantially reduce this risk.2,3 Hence, confirmation of the diagnosis of TIA is a major challenge since in most cases the clinical deficit has resolved at the time of evaluation and conventional brain imaging does not detect most conditions that masquerade as a TIA.4
In multimodal MRI, diffusion-weighted imaging (DWI) demonstrates the presence of severe cytotoxic ischemic injury within minutes, which provides an estimate of the ischemic core,5,6 and perfusion-weighted imaging (PWI) provides an assessment of cerebral hemodynamics.7 Since 1999, several studies have confirmed the sensitivity of DWI for the detection of an acute ischemic lesion among cases with suspected TIA.8–18 These studies were recently compiled in a systematic review.19 The rate of positive DWI varied from 16 to 67% among suspected TIA cases. Therefore, DWI appears to be a valuable tool to confirm an ischemic etiology in patients with transient neurologic focal symptoms. PWI has the potential to both confirm the ischemic nature of DWI hyperintensities20 as well as to reveal hypoperfusion in patients without DWI lesions. Therefore, PWI could increase the yield of MRI for the confirmation of TIA diagnosis.
Two studies, one retrospective21 and one prospective,22 have investigated the yield of combined DWI and PWI among TIA patients. While they found the same rate of positive PWI (32%), the percentage of cases with positive PWI and negative DWI differed (14% vs 3%). At present, there are inadequate data regarding the yield and clinical relevance of PWI findings in TIA patients. We investigated the yield of combined DWI and PWI among TIA patients scanned by multimodal MRI within 48 hours of symptom onset.
METHODS
Study group.
This study was a predefined substudy of an NIH-funded prospective clinical evaluation of the diagnostic utility of MRI in consecutive patients presenting with symptoms of cerebrovascular disease from July 16, 2001, to May 3, 2005. The purpose of this substudy was to evaluate the yield of the combination of DWI and PWI within 48 hours of symptom onset in suspected TIA patients. Patients who had transient neurologic symptoms (<24 hours in duration) and had both a DWI and PWI sequence performed at the baseline scan were eligible. The inclusion criteria for this substudy included the following:
Men and nonpregnant women, at least 18 years of age.
Admission to the Stanford Stroke Service within 48 hours of the onset of stroke-like symptoms.
Complete resolution of symptoms within 24 hours.
Technically adequate PWI and DWI sequences performed during the baseline MRI scan within 48 hours of symptom onset.
Clinical symptoms most likely referable to one of the cerebral hemispheres based on the attending stroke neurologist’s initial clinical evaluation (patients with clinical symptoms referable to the posterior fossa were excluded because the perfusion images did not provide complete coverage of the brainstem and cerebellum).
Exclusion criteria were as follows:
Patients receiving any thrombolytic agent or an investigational drug therapy prior to MRI scanning.
Level of consciousness score of 2 or greater as defined by the NIH Stroke Scale.
Symptoms likely related to psychoactive drugs.
Psychiatric or substance abuse disorder or dementia that could interfere with evaluation or interpretation of the neurologic assessment.
Informed consent could not be obtained either directly from the patient or from a legally authorized representative.
Severe coexisting or terminal systemic disease that limited life expectancy or that could interfere with the conduct of the study.
Symptoms most likely related to a nonischemic diagnosis such as psychiatric disorder, seizure, or migraine.
Image acquisition.
MRI scans were acquired using the 1.5 T GE Signa Horizon scanners equipped with enhanced gradient systems (GE Medical Systems, Waukesha, WI). For this study the following sequences were evaluated: dynamic susceptibility PWI after a bolus of IV gadolinium (12 slices, 40 phases, 128 × 128 matrices, field of view = 240 mm, slice thickness/gap = 5.0/2.0 mm, repetition time/echo time = 2,000/60 msec, flip angle = 60° gradient recall echoplanar imaging, 3 mL/sec of 0.1 mmol/kg Gd, 20 mL saline flash); DWI (20 slices, 256 × 256 matrices, field of view = 240 mm, slice thickness/gap = 7.0/0.0 mm spin echo echoplanar imaging); three-dimensional time-of-flight magnetic resonance angiography (MRA) of the intracranial and internal carotid artery (ICA) circulation.
Data processing.
Mean transit time (MTT) quantifies the time that the bolus of contrast takes to traverse the voxel. We used in-house developed software (UCLA/Stanford Image Processing Program) based on the deconvolution algorithms of Ostergaard et al.23,24 Arterial input functions from the proximal middle cerebral artery in both hemispheres were identified semiautomatically25 and used to generate perfusion maps.
Study design.
MR imaging protocol and acquisition of clinical data were approved by the local Institutional Review Board. All patients provided informed consent. Both the baseline DWI and PWI studies were independently assessed by two stroke neurologists (G.W.A. and M.L.) who were blinded to all clinical information (other than the knowledge that all patients had clinical features suggestive of a TIA). Focal regions of hyperintensity on DWI, assessed by the rater to be compatible with brain ischemia, were considered to be lesions with no specific size or intensity criteria required. MTT maps were chosen for all PWI analyses based on preliminary data indicating a greater sensitivity for detecting PWI lesions in TIA patients. The DWI scans were read separately from the PWI scans during different reading sessions and in random order. Readers did not view any other MRI sequences than DWI and PWI MTT scans. The readers were asked to identify if an acute lesion was present on the corresponding sequence and if so, in which hemisphere (or hemispheres) it was located. After both readers completed their assessment of all DWI and PWI scans, each scan where there was any disagreement between the two readers was evaluated in a joint session. Blinded to their original readings, the two readers were asked to come to a consensus regarding the presence or absence of a lesion and hemisphere involvement.
Separately, the MRA scans of both the extracranial and intracranial part of the ICA and the middle cerebral artery (MCA), the posterior cerebral artery (PCA), and the anterior cerebral artery (ACA) were assessed by the third stroke neurologist (J.M.O.) who was blinded to all clinical and imaging data, including other MRI sequences. An arterial lesion was defined as 1) an ICA stenosis >70%—a threshold at which a hemodynamic effect on cerebral flow is likely produced26; 2) decreased flow or occlusion of the M1 or the M2 segment of the MCA, the A1 segment of the ACA, or the P1 segment of the PCA.
The clinically symptomatic hemisphere was determined based on the history obtained by the attending stroke neurologist prior to reviewing the MRI scan results. Clinical characteristics of each patient were prospectively collected on case report forms. The most likely etiology was determined at hospital discharge by a stroke neurologist using the Trial of Org 10172 in Acute Stroke Treatment (TOAST) classification.27 Patients considered at discharge to have a nonischemic etiology were included in the “uncertain/other” category. Any recurrent stroke or TIA within the first 30 days was recorded.
Statistical analysis.
We calculated descriptive statistics based on the presence or absence of lesions on DWI or PWI including frequencies and mean values of clinical characteristics. We compared groups to analyze clinical factors possibly associated with the presence of a perfusion abnormality using t test and Mann-Whitney U test for continuous and ordinal variables; χ2 and Fisher exact tests for independent proportions. We estimated odds ratios (OR) or risk ratios (RR) of perfusion abnormalities for categorical variables. We assessed inter-rater agreement between the two raters by calculating Cohen kappa. Statistical analysis was performed using SPSS 16.0 (SPSS, Inc., Chicago, IL).
RESULTS
During the study, 108 patients were enrolled with transient neurologic symptoms lasting less than 24 hours that were initially thought to be caused by brain ischemia. Of these, 89 had an MRI scan obtained within 48 hours of symptom onset. Thirty-five of the remaining patients were excluded because the PWI sequence was not performed (n = 30) or was technically inadequate (n = 5). Eleven cases with symptoms attributed to posterior fossa TIA were also excluded. There was no statistical difference between the clinical characteristics of the included and excluded cohorts: age 71 ± 13 vs 70 ± 16 (p = 0.796); symptom duration in both cohorts 1–3 hours (interquartile range <1 hour to 3–12 hours, p = 0.915); time to MRI 23.2 ± 12.5 vs 26.5 ± 11.8 hours (p = 0.209); proportion of DWI positive cases 35% vs 30% (p = 0.655).
The demographic characteristics of patients included in this study are described in table 1; table 2 summarizes the PWI and DWI findings. Fourteen (33%) patients had a perfusion lesion (95% confidence interval [CI] = 19–47%), 15 patients (35%) had an acute DWI lesion (95% CI = 21–49%). Seven patients (16%) had an isolated PWI lesion (95% CI = 5–27%), 7 (16%) had both DWI and PWI lesions (95% CI = 5–27%), and 8 (19%) had an isolated DWI lesion (95% CI = 7–30%). Therefore, the total yield for detecting either a DWI or PWI lesion was 51% (95% CI = 36–66%). Figure 1 illustrates a case with a positive PWI and a negative DWI, while figure 2 shows a case with both positive DWI and PWI.
Table 1 Comparison of clinical characteristics among transient ischemic attack (TIA) patients with and without a perfusion-weighted MRI (PWI) lesion
Table 2 Summary of perfusion-weighted MRI (PWI) and diffusion-weighted MRI (DWI) findings
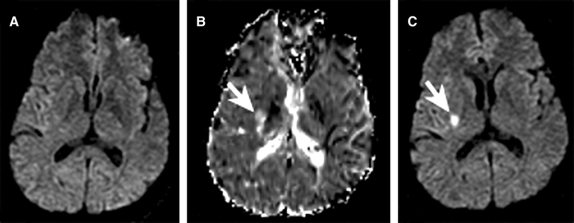
Figure 1 Diffusion-weighted MRI (DWI) and perfusion-weighted MRI (PWI) scans from a 48-year-old man with transient left face weakness, left hemisensory loss, dysarthria, headache, and dizziness
Baseline DWI, acquired 11.5 hours from symptom onset, did not reveal a diffusion abnormality (A), while the baseline mean transit time map revealed a perfusion lesion near the posterior limb of the right internal capsule (B). Follow-up DWI 48 hours later revealed a diffusion lesion in the same location as the baseline perfusion lesion (C).
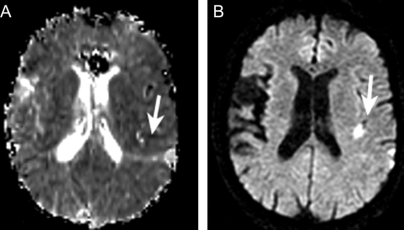
Figure 2 Diffusion-weighted MRI (DWI) and perfusion-weighted MRI (PWI) scans from a 72-year-old woman with transient aphasia, right arm weakness, and right hemisensory loss
Baseline DWI and PWI, acquired 13.5 hours from symptom onset, revealed both a perfusion deficit (A) and diffusion abnormality (B) in the left frontal/parietal region.
The location of the PWI abnormalities corresponded to the expected hemisphere based on clinical symptoms among 12 of the 14 cases (86%) with a positive PWI. Both of the patients in whom the PWI lesion did not correspond with the clinically suspected hemisphere also had an unsuspected DWI lesion in the same location. The DWI lesions corresponded to the expected hemisphere in 14/15 cases (93%). Two patients also had additional unexpected acute DWI lesions in the contralateral hemisphere. The two raters agreed on both the existence and location of a PWI lesion in 35 (PWI lesion present in 10 and absent in 25) out of 43 cases (81%) resulting in moderate inter-rater agreement (kappa = 0.58; 95% CI = 0.31–0.84). For DWI lesions, the raters agreed in 41/43 cases (98%) resulting in a very good strength of agreement (kappa = 0.95; 95% CI = 0.84–1.0).
Adequate quality MRA scans of both the ICA and intracranial vessels were available in 42 of the 43 included patients in this study (one missing ICA MRA). MRA lesions were detected in 17 (40%) patients. Six were ipsilateral to the symptomatic hemisphere. Among the 14 patients with a PWI lesion, 5 (36%) had an ipsilateral arterial lesion (5 intracranial and 0 extracranial). Among the 15 cases with a DWI lesion, 3 (20%) had an ipsilateral arterial lesion (2 intracranial and 1 extracranial).
We found that an MRI performed before 12 hours after symptom resolution was associated with the occurrence of a PWI lesion (table 1): OR = 5.6 (95% CI = 1.4–22.6). In addition, PWI lesions were associated with symptoms of speech impairment (OR = 5.6, 95% CI = 1.1–29.6); age <60 y (OR = 7.5, 95% CI = 1.2–45.6); and TOAST etiologic subtype (p = 0.021). In particular, patients with the large artery atherothrombosis TOAST etiologic subtype were more likely to have PWI lesions compared to other subtypes (RR = 3.9, 95% CI = 2.3–6.7). Finally, we found a trend toward a higher likelihood of PWI lesions in patients with multiple episodes: OR = 3.8 (95% CI = 1.0–15.2).
Symptom duration more than 12 hours was associated with occurrence of a DWI lesion: OR = 7.3 (95% CI = 1.5–35.0). We also found a trend toward association of DWI lesions with higher ABCD2 score1 (median [IQR] = 6 [5–6] vs 5 [4–6], p = 0.079) and reporting weakness as one of the clinical symptoms (80% vs 54%, p = 0.087).
None of the patients had recurrent TIA or stroke at 30 days.
DISCUSSION
In an unselected series of consecutive patients thought to have suffered a hemispheric TIA, we found that approximately half had objective evidence of brain ischemia on either DWI or PWI. PWI lesions occurred most frequently among patients who were scanned within 12 hours of symptom resolution, had symptoms of speech impairment, and were younger than 60 years of age. Our results suggest that PWI increases the overall yield of MRI for confirming an ischemic etiology for transient neurologic symptoms and that the yield may be even greater than 50% among TIA patients who are scanned urgently.
The overall rate of acute ischemic lesion detection on DWI (35%) and the association with symptom duration found in our study concurred with the result of a recent systematic review.19 Other factors previously associated with DWI lesions, including speech impairment, atrial fibrillation, and ICA stenosis ≥50%, were not confirmed in our study.
The percentage of TIA patients with PWI lesions (33%) concurred with previous studies. We found that half of the cases with a positive PWI had a negative DWI. This rate is similar to that documented in one previous retrospective study,21 but differs from a study of patients with speech or motor transient ischemic attacks scanned prospectively with a 3 T MRI that found only a 10% yield.22 The selection of cases and the use of a 3 T MRI, which was associated with a positive DWI in 46% compared to 35% in our study, may explain this discrepancy. This previous study also found that PWI lesion occurrence was associated with a reduced onset to MRI time.22 We found that scanning the patients within the 12 hours of symptom resolution significantly increased the occurrence of PWI lesions.
Multiple different PWI sequences and analyses techniques are available to assess cerebral perfusion.28,29 Previous studies used Time to Peak21 and MTT22 to detect persistent perfusion abnormalities following TIA. We used non-thresholded MTT maps generated by deconvolution of the tissue concentration over time curve using an arterial input function from the middle cerebral artery. Different analysis techniques may produce different results. Future studies should compare various analysis techniques to clarify how PWI acquisition and analysis methods affect the overall yield in TIA patients.
We found that the DWI and PWI lesions were consistently located in the clinically suspected hemisphere or found in conjunction with other MRI findings that confirmed the presence of acute brain ischemia. PWI lesions were often small and the inter-rater agreement for detecting PWI lesions was not as high as for DWI lesions (kappa 0.58 vs 0.95); however, their correspondence with the suspected hemisphere of the acute symptoms provides reassurance that these lesions are likely to be closely linked with the clinical events. Therefore, we propose that detection of either a DWI or PWI lesion in the setting of symptoms compatible with a TIA provides objective confirmation that brain ischemia is the likely etiology for the patient’s acute symptoms as well as evidence for the involved vascular territory or territories.
Several studies have suggested that PWI lesions are most likely to be detected in TIA patients who have significant carotid stenosis or an intracranial occlusion that can be visualized on MRA.22,30,31 However, in our study only a minority of PWI-positive patients had an ipsilateral internal carotid or intracranial lesion on MRA. Therefore, MRA findings do not appear to be an adequate surrogate for PWI in TIA patients. The presence of a PWI lesion in the absence of a MRA lesion in TIA patients is not unexpected. Most of the PWI lesions detected were small and may reflect occlusion of distal intracranial vessels that are not well visualized on MRA. In addition, despite recanalization of intracranial vessels, PWI deficits may persist because of abnormalities of tissue microperfusion (no reflow phenomenon).32 Nonetheless, we found that all the cases with large artery atherothrombosis (n = 4) had a positive PWI. Among these cases, MRA detected ipsilateral intracranial stenosis in two of them, and a relevant ICA stenosis (>50%) in the other two. Therefore, among TIA patients with positive PWI scans, MRA appears to have a high yield for detection of potentially relevant lesions.
This exploratory study has several limitations. It has a small sample size and nearly one third of the potentially eligible patients were excluded because they did not undergo both DWI and PWI imaging, typically because of limitations on obtaining research sequences in acute TIA patients that were in place at our institution during the time of this study. Multiple clinical factors were assessed for association with PWI and DWI lesions; however, a larger sample size is required for a multivariable analysis to assess which features are independently associated. The PWI scans were not analyzed in real time, as post-processing was required to generate the PWI maps, so we could not evaluate the influence of PWI information on subsequent clinical management or diagnosis. Software is now available to perform rapid deconvolution algorithms with minimal operator interaction allowing quantitative maps of PWI images to be produced rapidly and easily. New parallel imaging acquisition techniques are now available that may be more accurate and reliable than the PWI techniques used for this study.33 In this study, follow-up imaging to confirm the presence or absence of a new infarct in the territory of the acute DWI or PWI lesions was not routinely performed. Therefore, the fate of the tissue involved with these early lesions could not be determined. In addition, it was not possible to determine if some of the PWI lesions were chronic.
In the future, both DWI and PWI should be incorporated into larger prospective studies of TIA patients to clarify the independent prognostic value of these techniques as well as their associations with clinical characteristics.
AUTHOR CONTRIBUTIONS
Michael Mlynash, MD, MS Epidemiology, MS Computer Science, conducted the statistical analysis.
Supplementary Material
Address correspondence and reprint requests to Dr. Gregory W. Albers, Department of Neurology and Neurological Sciences, Stanford Stroke Center, 701 Welch Road, Suite B325, Palo Alto, CA 94304 albers@stanford.edu
Editorial, page 1118
e-Pub ahead of print on December 17, 2008, at www.neurology.org.
The DIAGNOSIS study was funded by the NIH R01 NS34866, Principal Investigator Michael Moseley. Additional funding for this substudy was provided by K24 NS044848, Principal Investigator Gregory W. Albers, and K23 NS051372, Principal Investigator Maarten G. Lansberg. Jean-Marc Olivot received research funding from Philippe Foundation (New York, NY) and Fondation pour la Recherche Medicale (Paris, France).
Disclosure: The authors report no disclosures.
Received June 30, 2008. Accepted in final form October 7, 2008.
REFERENCES
- 1.Johnston SC, Rothwell PM, Nguyen-Huynh MN, et al. Validation and refinement of scores to predict very early stroke risk after transient ischaemic attack. Lancet 2007;369:283–292. [DOI] [PubMed] [Google Scholar]
- 2.Rothwell PM, Giles MF, Chandratheva A, et al. Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (express study): a prospective population-based sequential comparison. Lancet 2007;370:1432–1442. [DOI] [PubMed] [Google Scholar]
- 3.Lavallee PC, Meseguer E, Abboud H, et al. A transient ischaemic attack clinic with round-the-clock access (SOS-TIA): feasibility and effects. Lancet Neurol 2007;6:953–960. [DOI] [PubMed] [Google Scholar]
- 4.Fazekas F, Fazekas G, Schmidt R, Kapeller P, Offenbacher H. Magnetic resonance imaging correlates of transient cerebral ischemic attacks. Stroke 1996;27:607–611. [DOI] [PubMed] [Google Scholar]
- 5.Moseley ME, Kucharczyk J, Mintorovitch J, et al. Diffusion-weighted MR imaging of acute stroke: correlation with T2-weighted and magnetic susceptibility-enhanced MR imaging in cats. AJNR Am J Neuroradiol 1990;11:423–429. [PMC free article] [PubMed] [Google Scholar]
- 6.Hjort N, Christensen S, Solling C, et al. Ischemic injury detected by diffusion imaging 11 minutes after stroke. Ann Neurol 2005;58:462–465. [DOI] [PubMed] [Google Scholar]
- 7.Shih LC, Saver JL, Alger JR, et al. Perfusion-weighted magnetic resonance imaging thresholds identifying core, irreversibly infarcted tissue. Stroke 2003;34:1425–1430. [DOI] [PubMed] [Google Scholar]
- 8.Ay H, Oliveira-Filho J, Buonanno FS, et al. ‘Footprints’ of transient ischemic attacks: a diffusion-weighted MRI study. Cerebrovasc Dis 2002;14:177–186. [DOI] [PubMed] [Google Scholar]
- 9.Kidwell CS, Alger JR, Di Salle F, et al. Diffusion MRI in patients with transient ischemic attacks. Stroke 1999;30:1174–1180. [DOI] [PubMed] [Google Scholar]
- 10.Engelter ST, Provenzale JM, Petrella JR, Alberts MJ. Diffusion MR imaging and transient ischemic attacks. Stroke 1999;30:2762–2763. [DOI] [PubMed] [Google Scholar]
- 11.Takayama H, Mihara B, Kobayashi M, Hozumi A, Sadanaga H, Gomi S. [Usefulness of diffusion-weighted MRI in the diagnosis of transient ischemic attacks.] No To Shinkei 2000;52:919–923. [PubMed] [Google Scholar]
- 12.Rovira A, Rovira-Gols A, Pedraza S, Grive E, Molina C, Alvarez-Sabin J. Diffusion-weighted MR imaging in the acute phase of transient ischemic attacks. AJNR Am J Neuroradiol 2002;23:77–83. [PMC free article] [PubMed] [Google Scholar]
- 13.Kamal AK, Segal AZ, Ulug AM. Quantitative diffusion-weighted mr imaging in transient ischemic attacks. AJNR Am J Neuroradiol 2002;23:1533–1538. [PMC free article] [PubMed] [Google Scholar]
- 14.Crisostomo RA, Garcia MM, Tong DC. Detection of diffusion-weighted MRI abnormalities in patients with transient ischemic attack: correlation with clinical characteristics. Stroke 2003;34:932–937. [DOI] [PubMed] [Google Scholar]
- 15.Winbeck K, Bruckmaier K, Etgen T, von Einsiedel HG, Rottinger M, Sander D. Transient ischemic attack and stroke can be differentiated by analyzing early diffusion-weighted imaging signal intensity changes. Stroke 2004;35:1095–1099. [DOI] [PubMed] [Google Scholar]
- 16.Inatomi Y, Kimura K, Yonehara T, Fujioka S, Uchino M. DWI abnormalities and clinical characteristics in TIA patients. Neurology 2004;62:376–380. [DOI] [PubMed] [Google Scholar]
- 17.Lamy C, Oppenheim C, Calvet D, et al. Diffusion-weighted MR imaging in transient ischaemic attacks. Eur Radiol 2006;16:1090–1095. [DOI] [PubMed] [Google Scholar]
- 18.Schulz UG, Briley D, Meagher T, Molyneux A, Rothwell PM. Diffusion-weighted MRI in 300 patients presenting late with subacute transient ischemic attack or minor stroke. Stroke 2004;35:2459–2465. [DOI] [PubMed] [Google Scholar]
- 19.Redgrave JNE, Coutts SB, Schulz UG, Briley D, Rothwell PM. Systematic review of associations between the presence of acute ischemic lesions on diffusion-weighted imaging and clinical predictors of early stroke risk after transient ischemic attack. Stroke 2007;38:1482–1488. [DOI] [PubMed] [Google Scholar]
- 20.Warach S, Kidwell CS. The redefinition of TIA: the uses and limitations of DWI in acute ischemic cerebrovascular syndromes. Neurology 2004;62:359–360. [DOI] [PubMed] [Google Scholar]
- 21.Restrepo L, Jacobs MA, Barker PB, Wityk RJ. Assessment of transient ischemic attack with diffusion- and perfusion-weighted imaging. AJNR Am J Neuroradiol 2004;25:1645–1652. [PMC free article] [PubMed] [Google Scholar]
- 22.Krol AL, Coutts SB, Simon JE, Hill MD, Sohn CH, Demchuk AM. Perfusion MRI abnormalities in speech or motor transient ischemic attack patients. Stroke 2005;36:2487–2489. [DOI] [PubMed] [Google Scholar]
- 23.Ostergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn Reson Med 1996;36:715–725. [DOI] [PubMed] [Google Scholar]
- 24.Ostergaard L, Sorensen AG, Kwong KK, Weisskoff RM, Gyldensted C, Rosen BR. High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part II: Experimental comparison and preliminary results. Magn Reson Med 1996;36:726–736. [DOI] [PubMed] [Google Scholar]
- 25.Mlynash M, Eyngorn I, Bammer R, Moseley M, Tong DC. Automated method for generating the arterial input function on perfusion-weighted MR imaging: validation in patients with stroke. AJNR Am J Neuroradiol 2005;26:1479–1486. [PMC free article] [PubMed] [Google Scholar]
- 26.van Everdingen KJ, Klijn CJ, Kappelle LJ, Mali WP, van der Grond J. MRA flow quantification in patients with a symptomatic internal carotid artery occlusion. The Dutch EC-IC bypass study group. Stroke 1997;28:1595–1600. [DOI] [PubMed] [Google Scholar]
- 27.Adams HP Jr, Bendixen BH, Kappelle LJ, et al. Classification of subtype of acute ischemic stroke: definitions for use in a multicenter clinical trial: TOAST Trial of Org 10172 in Acute Stroke Treatment. Stroke 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 28.Calamante F, Thomas DL, Pell GS, Wiersma J, Turner R. Measuring cerebral blood flow using magnetic resonance imaging techniques. J Cereb Blood Flow Metab 1999;19:701–735. [DOI] [PubMed] [Google Scholar]
- 29.Calamante F, Gadian DG, Connelly A. Quantification of perfusion using bolus tracking magnetic resonance imaging in stroke: assumptions, limitations, and potential implications for clinical use. Stroke 2002;33:1146–1151. [DOI] [PubMed] [Google Scholar]
- 30.Maeda M, Yuh WT, Ueda T, et al. Severe occlusive carotid artery disease: hemodynamic assessment by MR perfusion imaging in symptomatic patients. AJNR Am J Neuroradiol 1999;20:43–51. [PubMed] [Google Scholar]
- 31.Chaves CJ, Staroselskaya I, Linfante I, Llinas R, Caplan LR, Warach S. Patterns of perfusion-weighted imaging in patients with carotid artery occlusive disease. Arch Neurol 2003;60:237–242. [DOI] [PubMed] [Google Scholar]
- 32.del Zoppo GJ, Mabuchi T. Cerebral microvessel responses to focal ischemia. J Cereb Blood Flow Metab 2003;23:879–894. [DOI] [PubMed] [Google Scholar]
- 33.Newbould RD, Skare ST, Jochimsen TH, et al. Perfusion mapping with multiecho multishot parallel imaging EPI. Magn Reson Med 2007;58:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.