Abstract
To define the role of free radical formation and potential energy depletion in noise induced hearing loss (NIHL), we measured the effectiveness of tempol (free radical scavenger) and creatine (enhances cellular energy storage) alone and in combination to attenuate NIHL. Guinea pigs were divided into four treatment groups: controls, 3 % creatine diet (2 weeks prior to noise exposure), tempol (3 mM in drinking water 2 weeks prior to exposure), and creatine plus tempol and exposed to 120 dB SPL one-octave band noise centered at 4 kHz for 5 h. The noise-only control group showed frequency-dependent auditory threshold shifts (measured by auditory brainstem response, ABR) of up to 73 dB (16 kHz) on day 1, and up to 50 dB (8 kHz) on day 10. Creatine-treated subjects had significantly smaller ABR threshold shifts on day 1 and on day 10. Tempol alone significantly reduced ABR threshold shifts on day 10 but not on day 1. ABR shifts after combination treatment were similar to those in the creatine group. Hair cell loss on day 10 was equally attenuated by creatine and tempol alone or in combination. Our results indicate that the maintenance of ATP levels is important in attenuating both temporary and permanent NIHL, while the scavenging of free radicals provides protection from permanent NIHL.
Keywords: Guinea pigs, ATP, reactive oxygen species, antioxidant, inner ear, hair cells
1. Introduction
Exposure to high-intensity sound triggers a decrease in local blood flow in the inner ear, especially at very high sound exposure levels of 120 – 155 dB (Perlman and Kimura, 1962; Quirk et al., 1992; Miller et al., 1996), while metabolic activity remains elevated well above basal levels (Canlon and Schacht, 1983). At very high sound exposure levels, the reduction in cochlear blood flow (CBF) and local vasoconstriction can subject the cochlea to severe hypoperfusion in the presence of high energy demands. Impaired energy status can lead to activation of excitatory amino acid receptors (Brown and Borutaite, 2002), increasing intracellular calcium (White et al., 2000), and the generation of free radicals (Lang-Rollin et al., 2003), which are all potentially damaging to the inner ear. If the initial step in the development of noise trauma is a depletion of cellular energy stores, then agents that can buffer cellular energy stores may be protective. Creatine kinase is a key enzyme involved in regulating energy metabolism in cells with intermittently high and fluctuating energy requirements (Weiss et al., 2005), including the inner ear (Spicer and Schulte, 1992). The enzyme catalyzes the transfer of high-energy phosphate from phosphocreatine to ADP to generate ATP. Several cytoplasmic and mitochondrial isoforms have been identified that, with the substrates creatine and phosphocreatine, constitute an intricate cellular energy buffering and transport system connecting sites of energy production to sites of energy consumption (Hemmer and Wallimann, 1993). We tested the hypothesis that compounds that increase the cochlear energy reserve will be protective by assessing if oral administration of creatine attenuates noise-induced hearing loss.
Another putative cause of noise-induced hair cell death is oxidative stress. The formation of reactive oxygen species (ROS) results from high levels of mitochondrial activity induced by noise (Balaban et al., 2005; Gourlay and Ayscough, 2005; Yamashita et al., 2004; Ohinata et al., 2000), and is exacerbated by reduction in CBF, which accompanies intense sound exposure. Consequently, ROS scavengers and inhibitors attenuate NIHL (Yamasoba et al., 1999; Henderson et al., 1999; Kopke et al., 2000; Ohinata et al., 2003). Tempol (4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl) is a water-soluble analogue of the spin label TEMPO, widely employed in electron spin resonance spectroscopy (Saito et al., 2003). Tempol permeates biological membranes and acts as a spin trap for superoxide radicals. It can reduce superoxide-related injury in ischemia/reperfusion, inflammation, and radiation-induced damage (Thiemermann, 2003). In the present study we assessed the efficacy of tempol to prevent NIHL.
Creatine and tempol should act on different pathways associated with NIHL. Several studies have shown that an additive protection against NIHL can be achieved by combining several agents acting against different damaging mechanisms (Hight et al., 2003; Kopke et al., 2000; Yamashita et al., 2005; for recent reviews, see Miller et al., 2006 and LePrell et al., 2006). To provide further insights into the independence of these factors and the potential efficacy of a combination of agents, we examine the effectiveness of creatine or tempol alone and in combination on auditory threshold shifts and hair cell damage induced by noise exposure.
2. Results
ABR
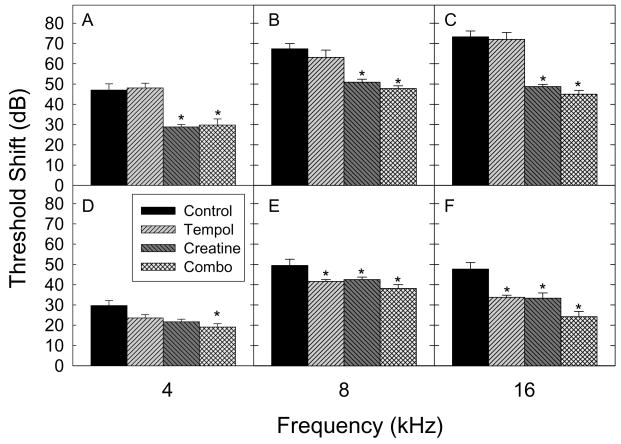
Temporary noise-induced threshold shifts (TTS), measured one-day post noise exposure, were significantly reduced by treatment with creatine, or a combination of creatine plus tempol. Permanent noise-induced hearing loss measured 10 days post noise was significantly reduced by treatment with creatine, tempol, or a combination thereof at 4 and 8 kHz (see Figure 2). Only the combination of creatine and tempol provided a significant attenuation of the noise-induced threshold shift at 4 kHz 10 days following exposure. Group differences were statistically reliable for treatment (saline, tempol, creatine, or a combination of tempol and creatine) and time (pre, 1 day post, or 10 days post noise); the interaction of treatment and time was also statistically reliable.
Fig. 2. Noise-induced ABR threshold shifts.
ABR threshold shifts (mean ± S.D., n = 6 animals per group) were assessed at each test frequency (4, 8, and 16 kHz) following noise exposure. On Day 1, the group treated with creatine or both creatine and tempol showed significantly smaller threshold shifts at each frequency (panels A–C) compared to that of the normal-diet group (control). In contrast, tempol alone did not reduce the threshold shift on day 1 at any frequency. On day 10, treatment with each of creatine, tempol, or both agents together attenuate noise-induced threshold shifts at 8 (panel E) and 16 kHz (panel F); while only the combination treatment of creatine and tempol was sufficient to produce a statistically significant attenuation at 4 kHz (panel D). All asterisks indicate significance relative to control group. Statistical analysis was by two way repeated measures ANOVA.
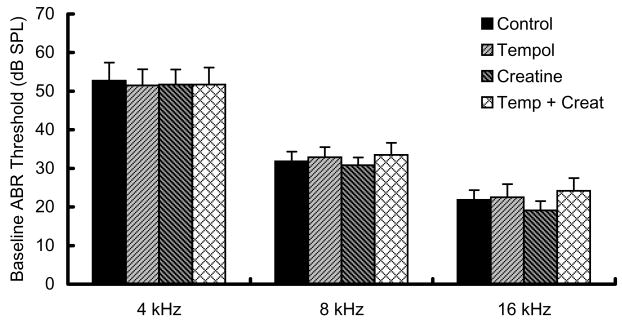
Pair-wise comparisons revealed the following key results. First, there were no differences in baseline measures of threshold sensitivity for any groups at any test frequency (Fig 1). Second, within each treatment group, thresholds measured either 1 or 10 days post-noise were significantly elevated relative to pre-noise baseline threshold. Third, all groups showed statistically reliable functional improvement from day 1 to day 10 post-noise (i.e., day 10 thresholds were significantly better than day 1 thresholds, with the exception of 4 kHz data for creatine treated animals).
Fig. 1. Baseline ABR threshold.
Baseline ABR threshold was assessed bilaterally before treatment. Pre-treatment baseline ABR thresholds did not differ significantly between the four assigned treatment groups, mean ± SD.
Key differences among the treatment groups included the following. First, treatment groups that received creatine (alone, or in combination with tempol) had lower TTS than control animals or animal treated with tempol alone. Reducing TTS did not improve protection against permanent threshold shifts (PTS); the therapeutic effects of creatine and tempol were equivalent when permanent hearing loss was evaluated 10 days post noise. Second, the combination of creatine and tempol reliably and significantly reduced PTS at all test frequencies, although neither creatine nor tempol alone resulted in statistically reliable reduction of PTS at 4 kHz. Third, treatment with a combination of creatine and tempol provided the greatest reduction in PTS at 16 kHz; protection was statistically improved relative to tempol alone. Taken together, although additive effects of the combined agents were limited to two of the three frequencies tested, these results provide evidence that synergistic effects can be achieved via treatments that include agents intervening at different points in the traumatic cascade.
Hair cell loss
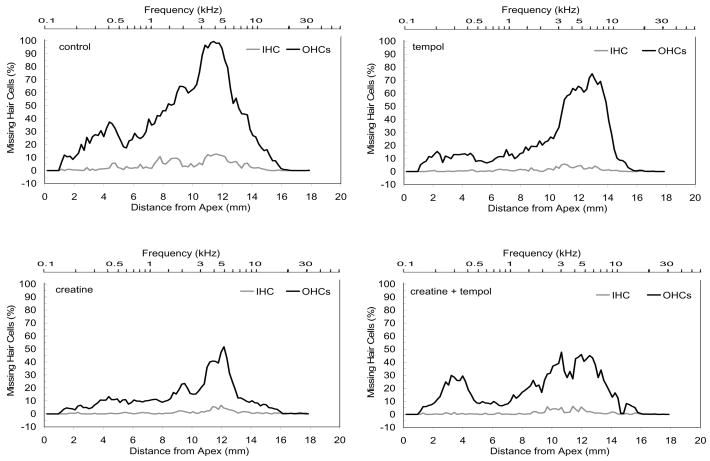
Missing hair cells were counted in rhodamine phalloidin-labeled surface preparations, and the percentage of IHC and OHC loss was quantitatively evaluated and graphed as the mean loss for each treatment group (fig. 3). In the normal-diet group the damage extended from approximately 5 to 15 mm from the apex. In contrast, the animals receiving either creatine, tempol, or both had less hair cell loss and narrower noise-induced lesions. This is most compelling in the creatine treated group, in which damage is largely restricted to the region 10 – 12 mm from the apex, the region primarily affected by the noise exposure. However, each treated group shows a reduction in the percent OHC missing in this 10 – 12 mm region and a more or less symmetrical reduction in the extent of the damage both apically and basally. IHC loss was minimal in all conditions and no significant change in the extent of IHC loss was observed across treatment groups.
Fig. 3. Noise-induced hair cell loss, cytocochleograms.
Average cytocochleograms for each group (n = 6 animals per group) across the whole cochlea. These cytocochleograms show the mean percent hair cell loss for IHCs and OHCs rows 1–3 along the length of the cochlea. In the normal-diet group (control) the damaged region extends from approximately 5 to 15 mm from the apex. In contrast, the animals receiving either creatine, tempol, or both had less hair cell loss and narrower noise-induced lesions. Distance from apex is indicated on axis below. Equivalent frequency is indicated on horizontal axis above each figure (Viberg and Canlon, 2004).
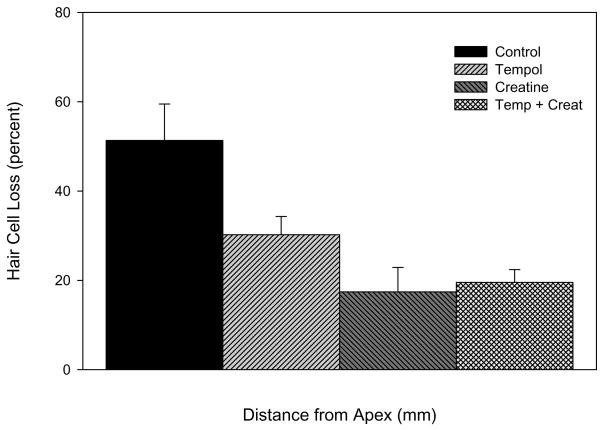
Mean hair cell loss on day 10 within the region extending from 5–15 mm from the apex is quantified in Figure 4. The noise-only group showed the greatest OHC loss of 51%. Treatment with either tempol (30% OHC loss), creatine (17% loss), or both (19.5% loss) significantly reduced OHC death compared to untreated controls (p < 0.01) but there were no significant differences between any of the treated groups. We previously showed that hair cell loss, which increased over days following noise exposure, stabilized on days 7–10 (Yamashita et al., 2004). In agreement with this finding, no significant increase in cell loss occurred between days 10 and 14 or 10 and 21.
Fig. 4. Noise-induced hair cell loss.
Average OHC loss for each group (n = 6 animals per group) within the region of the cochlea spanning 5–15 mm from the apex (± S.E.). OHC loss on day 10 was significantly attenuated in the tempol, creatine, and combination groups compared to control animals exposed to noise; but there were no significant differences between the treated groups using a one-way ANOVA. However, a t-test showed a significant difference between tempol treated vs. the control group as well.
3. Discussion
Creatine can reduce both temporary (TTS) and permanent (PTS) threshold shifts, while the antioxidant tempol attenuates the PTS only. At 16 kHz we also observed that the combination of these two agents provided a significantly greater attenuation of the noise-induced threshold shift than tempol alone. This additivity, however, was not observed at lower test frequencies and was not supported by histological assessment.
The creatine-mediated reduction in both temporary and permanent ABR threshold shifts indicates that maintaining energy homeostasis during the noise exposure reduces the immediate stress-induced pathophysiology that leads to a TTS as well as those longer-term events that lead to permanent pathology. Another possible interpretation of the findings is that creatine-induced attenuation of the immediate stress-induced events essentially prevents the initiation of the subsequent events leading to permanent pathology. Improved cellular energy reduces formation of free radicals (Matthews et al., 1998) which may otherwise induce cell death by destruction of vital cell components (Barnham et al., 2004) or by acting as signaling molecules for apoptosis (Mattson, 2000). Creatine may reduce PTS through stabilization of the energy state and an associated reduction in the formation of free radicals.
Creatine functions as an energy buffer through creatine kinase (Persky et al., 2003), which is abundant in marginal cells of the cochlear stria vascularis (Spicer and Schulte, 1992). Creatine kinase in these cells can supply ATP for the Na/K-ATPase that maintains the high K+ of endolymph (Spicer and Schulte, 1998; Thorne et al., 2002; Housley et al., 2002). In addition to its role as an energy substrate, extracellular ATP is a cochlear neuromodulator acting on purinergic receptors in the organ of Corti and spiral ganglion (Housley et al., 1999). Noise exposure increases ATP secretion into the endolymphatic and perilymphatic compartments of the cochlea (Munoz et al., 2001) changing the ATP homeostasis in the cochlea.
Given the differences in susceptibility to noise and hypoxic insult between the base and apex of the cochlea, a difference may have been expected between the extent of protection (differences in hair cell counts) along the cochlea. However, tempol and creatine appeared broadly effective throughout the 5 – 15 mm region from the apex that was affected by noise (see control group Fig 3) and within this region there were no clear apical-basal differences.
The development of TTS in response to high levels of noise is influenced by a wide variety of parameters including oxygen tension (Patchett, 1980), sympathectomy (Hildesheimer et al., 1991), glutamate receptor antagonist (Khan et al., 2000), local administration of ATP (Sugahara et al., 2004). TTS is thought to arise mainly from various reversible changes in the cochlea affecting the mechanical compliance of the basilar membrane (LePage, 1989), decreased stiffness of the hair cell stereocilia (Saunders et al., 1986), or afferent terminal excitotoxicity (Pujol and Puel, 1999). Noise-induced attenuation of the endocochlear potential could contribute to acute threshold shifts that resolve to permanent threshold shifts (Hirose and Liberman, 2003; Ide and Morimitsu, 1990). While our study does not allow us to identify the target of creatine-mediated protection from TTS, creatine may contribute to the maintenance of the endocochlear K+ level, thus reducing this immediate effect of noise.
ROS and RNS can directly and indirectly modulate noise-induced cell death (Jacono et al., 1998). Following intense noise, superoxide anion and hydroxyl radicals (as well as their reaction products with lipids, proteins and nucleotides) are generated in the cochlea (Yamane et al., 1995; Ohlemiller et al., 1999; Yamashita et al., 2004; Ohinata et al., 2000), and protective glutathione and glutathione-related enzymes decrease (Yamasoba et al., 1998). Conversely, antioxidants attenuate NIHL and noise-induced hair cell death (Ohinata et al., 2003; Yamasoba et al., 1999; Henderson et al., 1999; Kopke et al., 2000). Tempol is a membrane-permeable radical scavenger that interferes with the formation or the effects of many radicals (Thiemermann, 2003) and its protective effect on NIHL is consistent a key role of free radicals in initiation of cell death. Tempol may be of clinical utility in the prevention of noise-induced hearing loss. Preclinical studies suggest that tempol may be useful in the therapy of ischemia-reperfusion injury, shock, and inflammation (Cuzzocrea et al., 2004). It has been used to treat humans topically (Metz et al., 2004) but not systemically, and side effects of oral consumption in humans are currently unknown.
To date, of all studies aimed at the prevention of noise-induced hearing loss, none propose that it is entirely preventable, other than by avoiding the exposure. This may be due to the fact that noise damage in part results from direct mechanical trauma to cell structures. On the other hand, it may be due to our inability to prevent all metabolically induced stress-related events including prevention of free radical formation, excitotoxic events, and noise-induced modulation of inner ear blood perfusion. In such a scenario, multi-drug therapy may be more effective in attenuating noise damage than any single agent. However, this was not the case for tempol and creatine except for a small improvement at one frequency (16 kHz) which was not supported by histological assessment of OHC loss. The inability of combination therapy to provide a larger protective effect than the individual drugs could indicate a ceiling effect for the protection offered by the individual drugs. Alternatively, maintenance of the energy status of the cell by creatine could reduce free radical formation and thereby obviate the effect of tempol. Moreover, while we selected a concentration and duration of treatment based upon data from the literature (Ipsiroglu et al., 2001; Schnackenberg et al., 1999; Nishiyama et al., 2003), there is no direct data on the dosing that may produce optimal treatment of the inner ear. Both dose response studies for systemic administration as well as studies of the effect of direct (cannula-osmotic pump) administration of these agents locally into the scala tympani would be of value in understanding the efficacy of these agents. Our data indicates that with oral administration creatine and tempol can cross the blood-perilymph barrier, reach the tissues of the cochlea and attenuate noise-induced hearing loss. The protection of hearing loss was less than the apparent protection of hair cells from noise-induced death. This discrepancy may simply reflect an inability to judge hair cell function from hair cell morphology. In conclusion, this is the first report providing evidence of creatine-mediated protection of NIHL. It indicates that the preservation of ATP levels is important in attenuating both temporary and permanent NIHL, while the scavenging of free radicals contributes mostly to protect from permanent NIHL.
4. Experimental Procedure
Materials and methods
Experimental design
Pigmented male guinea pigs (200–400 g, 2 – 4 weeks old; Elm Hill Breeding Labs, Chelmsford, MA) were used in this study. Creatine and tempol were obtained from Sigma (St. Louis, MO). Creatine was administered orally to the guinea pigs in their food at doses of 3 % of the diet. Tempol was administered at 3 mM in drinking water. Controls received unsupplemented but otherwise identical diets and drinking water. The diets were administered over a total of 24 days beginning 14 days before noise exposure, to provide sufficient time for the agents to reach a stable organ and tissue level (Ipsiroglu et al., 2001; Schnackenberg et al., 1999). Animals were divided into four experimental groups (n = 6 each): 1) untreated controls; 2) animals fed a 3 % creatine diet; 3) animals receiving 3 mM tempol; and 4) animals treated with creatine + tempol. Auditory brainstem response (ABR) was assessed bilaterally before treatment to assure normal hearing and reassessed 1 and 10 days after noise exposure. Following final ABR recordings, the animals were euthanized for histologic assessment. The experimental protocol was approved by the Animal Care and Use Committee at the University of Michigan and conformed to the National Research Council’s Guidelines for the Care and Use of Laboratory Animals.
Auditory brainstem response
Animals were anesthetized with an intramuscular injection of xylazine (10 mg/kg) and ketamine (40 mg/kg). The external ear canals and tympanic membranes were inspected using an operating microscope to assure the ear canal was free of wax, that there was no canal deformity, no inflammation of tympanic membrane, and no effusion of the middle ear. During the ABR measurement, to avoid an anesthetic-induced reduction in body temperature a Deltaphase Isothermal pad was used. A differential active needle electrode was placed subcutaneously below the test ear, a reference electrode at the vertex, and a ground electrode below the contralateral ear. The sound stimulus consisted of 15 ms tone bursts, with a rise-fall time of 1 ms at frequencies of 4, 8 and 16 kHz, and was generated by Tucker-Davis Technologies (Alachua, FL) BioSig© program. The stimuli were presented to the external auditory meatus in a closed acoustic system through a tube connected to a transducer (Beyer DT-48, Beyer Dynamic, Farmingdale, NY). The sound source was calibrated by coupling with a B & K ¼ inch microphone through a 0.6 cc rubber tube. Initial stimuli were presented at a level of 100–105 dB SPL, which consistently evoked a clear and robust ABR; sound intensity was initially decreased in 10 to 20 dB steps until the amplitude of the response indicated that threshold was near, thereafter sound intensity was decreased in 5-dB decrements, until “threshold” was defined. One thousand and twenty-four tone presentations, delivered at 10 times per second, were averaged to obtain a waveform, using a Tucker–Davis data acquisition system with computer and custom software. Hearing threshold was defined as the lowest intensity of stimulation that yielded a repeatable waveform with an identifiable peak 3 or 4 in the ABR waveform.
Noise exposure
Animals were exposed to one-octave band noise (OBN) centered at 4 kHz, at 120 dB SPL, for 5 hr in a ventilated sound exposure chamber. The sound chamber was fitted with speakers (Model 2450H; JBL) driven by a noise generator (ME 60 Micrographic equalizer; Rane) and power amplifier (HCA-1000 high current power amplifier; Parasound Products). Sound levels were calibrated (Type 2203 precision sound level meter, Type 4134 microphone; Bruel and Kjaer Instruments) at multiple locations within the sound chamber to ensure uniformity of the stimulus. The stimulus intensity varied by a maximum of 3 dB across measured sites within the exposure chamber. During noise exposure, noise levels were monitored using a sound level meter, a preamplifier, and a condenser microphone. The microphone was positioned above the cages but calibrated to record at the level of the animal’s head during the noise exposure.
Hair cell count
Following final ABR recordings, animals were sacrificed under xylazine and ketamine anesthesia. The temporal bones were immediately removed and placed in 4% paraformaldehyde in 0.01 M phosphate-buffered saline (PBS, pH 7.4). Under a dissecting microscope, the round and oval windows and the bone near the apex were opened, followed by gentle local perfusion of 4% paraformaldehyde from the apex through the cochlea. The tissue was kept in the fixative overnight. After removal of the bony capsule and the lateral wall tissues, the modiolar core, including the organ of Corti, was dissected from the temporal bone. Following permeabilization with 0.3% Triton X-100 for 5 min, the organ of Corti was stained for F-actin with rhodamine phalloidin for 40 min to visualize hair cells and their stereocilia. After washing with PBS, the organ of Corti was dissected and slide mounted as a surface preparation. The tissues were observed under fluorescence microscopy and missing inner hair cells (IHC) and outer hair cells (OHC) were determined in 0.19 mm sections from apex to base. Missing hair cells were apparent as dark spots and/or the typical phalangeal scar of supporting cells (Raphael and Altschuler, 1991). Counting was begun 0.95 mm from apex, omitting the irregular apical part of the cochlea spiral. The percent of IHC and OHC loss per 0.19 mm counted distance was calculated by comparison to an existing normative laboratory database. The mean of OHC loss for all 3 rows per 0.19 mm counted distance were calculated. Then the mean for each group was calculated per 0.19 mm counted distance. Cytocochleograms were generated displaying the mean percentage loss of IHCs and OHCs along the cochlear length (as described in Ekborn et al., 2003). Differences across groups in the region 0.95 – 18.05 mm from the apex were evaluated for statistical significance.
Statistical methods
Statistical differences among the different groups were evaluated using two-way repeated measures ANOVA for each frequency followed by Student–Newman–Keul’s as a post hoc test. A p value of <0.05 was considered significant. Hair cell data was analyzed performing a one-way ANOVA.
Acknowledgments
Supported by NIH DC 04058 and DC 05188, General Motors Corp., United Autoworkers Union, and the Ruth and Lynn Townsend Professorship of Communication Disorders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell. 2005;120:483–495. doi: 10.1016/j.cell.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Barnham KJ, Masters CL, Bush AI. Neurodegenerative diseases and oxidative stress. Nat Rev Drug Discov. 2004;3:205–214. doi: 10.1038/nrd1330. [DOI] [PubMed] [Google Scholar]
- Brown GC, Borutaite V. Nitric oxide inhibition of mitochondrial respiration and its role in cell death. Free Radic Biol Med. 2002;33:1440–1450. doi: 10.1016/s0891-5849(02)01112-7. [DOI] [PubMed] [Google Scholar]
- Canlon B, Schacht J. Acoustic stimulation alters deoxyglucose uptake in the mouse cochlea and inferior colliculus. Hear Res. 1983;10:217–226. doi: 10.1016/0378-5955(83)90055-2. [DOI] [PubMed] [Google Scholar]
- Cuzzocrea S, Pisano B, Dugo L, Ianaro A, Patel NS, Caputi AP, Thiemermann C. Tempol reduces the activation of nuclear factor-kappaB in acute inflammation. Free Radic Res. 2004;38:813–819. doi: 10.1080/10715760410001710829. [DOI] [PubMed] [Google Scholar]
- Ekborn A, Laurell G, Ehrsson H, Miller J. Intracochlear administration of thiourea protects against cisplatin-induced outer hair cell loss in the guinea pig. Hear Res. 2003;181:109–115. doi: 10.1016/s0378-5955(03)00181-3. [DOI] [PubMed] [Google Scholar]
- Gourlay CW, Ayscough KR. The actin cytoskeleton: a key regulator of apoptosis and aging? Nat Rev Mol Cell Biol. 2005;6:583–589. doi: 10.1038/nrm1682. [DOI] [PubMed] [Google Scholar]
- Hemmer W, Wallimann T. Functional aspects of creatine kinase in brain. Dev Neurosci. 1993;15:249–260. doi: 10.1159/000111342. [DOI] [PubMed] [Google Scholar]
- Henderson D, McFadden SL, Liu CC, Hight N, Zheng XY. The role of antioxidants in protection from impulse noise. Ann N Y Acad Sci. 1999;28:368–380. doi: 10.1111/j.1749-6632.1999.tb08655.x. [DOI] [PubMed] [Google Scholar]
- Hight NG, McFadden SL, Henderson D, Burkard RF, Nicotera T. Noise-induced hearing loss in chinchillas pre-treated with glutathione monoethylester and R-PIA. Hear Res. 2003;179:21–32. doi: 10.1016/s0378-5955(03)00067-4. [DOI] [PubMed] [Google Scholar]
- Hildesheimer M, Sharon R, Muchnik C, Sahartov E, Rubinstein M. The effect of bilateral sympathectomy on noise induced temporary threshold shift. Hear Res. 1991;51:49–53. doi: 10.1016/0378-5955(91)90006-u. [DOI] [PubMed] [Google Scholar]
- Hirose K, Liberman MC. Lateral wall histopathology and endocochlear potential in the noise-damaged mouse cochlea. J Assoc Res Otolaryngol. 2003;4:339–352. doi: 10.1007/s10162-002-3036-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Housley GD, Jagger DJ, Greenwood D, Raybould NP, Salih SG, Jarlebark LE, Vlajkovic SM, Kanjhan R, Nikolic P, Munoz DJ, Thorne PR. Purinergic regulation of sound transduction and auditory neurotransmission. Audiol Neurootol. 2002;7:55–61. doi: 10.1159/000046865. [DOI] [PubMed] [Google Scholar]
- Housley GD, Kanjhan R, Raybould NP, Greenwood D, Salih SG, Jarlebark L, Burton LD, Setz VC, Cannell MB, Soeller C, Christie DL, Usami S, Matsubara A, Yoshie H, Ryan AF, Thorne PR. Expression of the P2X(2) receptor subunit of the ATP-gated ion channel in the cochlea: implications for sound transduction and auditory neurotransmission. J Neurosci. 1999;19:8377–8388. doi: 10.1523/JNEUROSCI.19-19-08377.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ide M, Morimitsu T. Long term effects of intense sound on endocochlear DC potential. Auris Nasus Larynx. 1990;17:1–10. doi: 10.1016/s0385-8146(12)80014-9. [DOI] [PubMed] [Google Scholar]
- Ipsiroglu OS, Stromberger C, Ilas J, Hoger H, Muhl A, Stockler-Ipsiroglu S. Changes of tissue creatine concentrations upon oral supplementation of creatine-monohydrate in various animal species. Life Sci. 2001;69:1805–1815. doi: 10.1016/s0024-3205(01)01268-1. [DOI] [PubMed] [Google Scholar]
- Jacono AA, Hu B, Kopke RD, Henderson D, Van De Water TR, Steinman HM. Changes in cochlear antioxidant enzyme activity after sound conditioning and noise exposure in the chinchilla. Hear Res. 1998;117:31–38. doi: 10.1016/s0378-5955(97)00214-1. [DOI] [PubMed] [Google Scholar]
- Khan MJ, Seidman MD, Quirk WS, Shivapuja BG. Effects of kynurenic acid as a glutamate receptor antagonist in the guinea pig. Eur Arch Otorhinolaryngol. 2000;257:177–181. doi: 10.1007/s004050050218. [DOI] [PubMed] [Google Scholar]
- Kopke RD, Weisskopf PA, Boone JL, Jackson RL, Wester DC, Hoffer ME, Lambert DC, Charon CC, Ding DL, McBride D. Reduction of noise-induced hearing loss using L-NAC and salicylate in the chinchilla. Hear Res. 2000;149:138–146. doi: 10.1016/s0378-5955(00)00176-3. [DOI] [PubMed] [Google Scholar]
- Lang-Rollin IC, Rideout HJ, Noticewala M, Stefanis L. Mechanisms of caspase-independent neuronal death: energy depletion and free radical generation. J Neurosci. 2003;23:11015–11025. doi: 10.1523/JNEUROSCI.23-35-11015.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LePage EL. Functional role of the olivo-cochlear bundle: a motor unit control system in the mammalian cochlea. Hear Res. 1989;38:177–198. doi: 10.1016/0378-5955(89)90064-6. [DOI] [PubMed] [Google Scholar]
- Le Prell CG, Yamashita D, Minami S, Yamasoba T, Miller JM. Mechanisms of noise-induced hearing loss indicate multiple methods of prevention. Hear Res. 2006 doi: 10.1016/j.heares.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews RT, Yang L, Jenkins BG, Ferrante RJ, Rosen BR, Kaddurah-Daouk R, Beal MF. Neuroprotective effects of creatine and cyclocreatine in animal models of Huntington’s disease. J Neurosci. 1998;18:156–163. doi: 10.1523/JNEUROSCI.18-01-00156.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol. 2000;1:120–129. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- Metz JM, Smith D, Mick R, Lustig R, Mitchell J, Cherakuri M, Glatstein E, Hahn SM. A phase I study of topical Tempol for the prevention of alopecia induced by whole brain radiotherapy. Clin Cancer Res. 2004;10:6411–6417. doi: 10.1158/1078-0432.CCR-04-0658. [DOI] [PubMed] [Google Scholar]
- Miller JM, Ren T-Y, Dengerink HA, Nuttall AL. Cochlear blood flow changes with short sound stimulation. In: Axelsson A, Borchgrevink H, Hamernik RP, Hellstrom P-A, Henderson D, Salvi RJ, editors. Scientific Basis of Noise-Induced Hearing Loss. Thieme; New York: 1996. pp. 95–109. [Google Scholar]
- Miller J, Yamashita S, Minami S, Yamasoba T, LePrell C. Mechanisms and prevention of noise induced hearing loss. Otol Jpn. 2006;16 (2):139–153. [Google Scholar]
- Munoz DJ, Kendrick IS, Rassam M, Thorne PR. Vesicular storage of adenosine triphosphate in the guinea-pig cochlear lateral wall and concentrations of ATP in the endolymph during sound exposure and hypoxia. Acta Otolaryngol. 2001;121:10–15. doi: 10.1080/000164801300006209. [DOI] [PubMed] [Google Scholar]
- Nishiyama A, Kobori H, Fukui T, Zhang GX, Yao L, Rahman M, Hitomi H, Kiyomoto H, Shokoji T, Kimura S, Kohno M, Abe Y. Role of angiotensin II and reactive oxygen species in cyclosporine A-dependent hypertension. Hypertension. 2003;42:754–60. doi: 10.1161/01.HYP.0000085195.38870.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlemiller KK, Wright JS, Dugan LL. Early elevation of cochlear reactive oxygen species following noise exposure. Audiol Neurootol. 1999;4:229–236. doi: 10.1159/000013846. [DOI] [PubMed] [Google Scholar]
- Ohinata Y, Miller JM, Altschuler RA, Schacht J. Intense noise induces formation of vasoactive lipid peroxidation products in the cochlea. Brain Res. 2000;878:163–173. doi: 10.1016/s0006-8993(00)02733-5. [DOI] [PubMed] [Google Scholar]
- Ohinata Y, Miller JM, Schacht J. Protection from noise-induced lipid peroxidation and hair cell loss in the cochlea. Brain Res. 2003;966:265–273. doi: 10.1016/s0006-8993(02)04205-1. [DOI] [PubMed] [Google Scholar]
- Patchett RF. The effect of oxygen inhalation on temporary threshold shift in humans. J Aud Res. 1980;20:227–231. [PubMed] [Google Scholar]
- Perlman HB, Kimura R. Cochlear blood flow and acoustic trauma. Acta Otolaryngol. 1962;54:99–119. doi: 10.3109/00016486209126927. [DOI] [PubMed] [Google Scholar]
- Persky AM, Brazeau GA, Hochhaus G. Pharmacokinetics of the dietary supplement creatine. Clin Pharmacokinet. 2003;42:557–574. doi: 10.2165/00003088-200342060-00005. [DOI] [PubMed] [Google Scholar]
- Pujol R, Puel JL. Excitotoxicity, synaptic repair, and functional recovery in the mammalian cochlea: a review of recent findings. Ann NY Acad Sci. 1999;884:249–254. doi: 10.1111/j.1749-6632.1999.tb08646.x. [DOI] [PubMed] [Google Scholar]
- Quirk WS, Avinash G, Nuttall AL, Miller JM. The influence of loud sound on red blood cell velocity and blood vessel diameter in the cochlea. Hear Res. 1992;63:102–108. doi: 10.1016/0378-5955(92)90079-3. [DOI] [PubMed] [Google Scholar]
- Raphael Y, Altschuler RA. Reorganization of cytoskeletal and junctional proteins during cochlear hair cell degeneration. Cell Motil Cytoskel. 1991;18:215–227. doi: 10.1002/cm.970180307. [DOI] [PubMed] [Google Scholar]
- Saito K, Takeshita K, Ueda J, Ozawa T. Two reaction sites of a spin label, TEMPOL (4-hydroxy-2,2,6,6-tetramethylpiperidine-N-oxyl), with hydroxyl radical. J Pharm Sci. 2003;92:275–280. doi: 10.1002/jps.10304. [DOI] [PubMed] [Google Scholar]
- Saunders JC, Canlon B, Flock A. Changes in stereocilia micromechanics following overstimulation in metabolically blocked hair cells. Hear Res. 1986;24:217–225. doi: 10.1016/0378-5955(86)90020-1. [DOI] [PubMed] [Google Scholar]
- Schnackenberg CG, Wilcox CS. Two-week administration of tempol attenuates both hypertension and renal excretion of 8-Iso prostaglandin f2alpha. Hypertension. 1999;33:424–428. doi: 10.1161/01.hyp.33.1.424. [DOI] [PubMed] [Google Scholar]
- Spicer SS, Schulte BA. Evidence for a medial K+ recycling pathway from inner hair cells. Hear Res. 1998;118:1–12. doi: 10.1016/s0378-5955(98)00006-9. [DOI] [PubMed] [Google Scholar]
- Spicer SS, Schulte BA. Creatine kinase in epithelium of the inner ear. J Histochem Cytochem. 1992;40:185–192. doi: 10.1177/40.2.1313059. [DOI] [PubMed] [Google Scholar]
- Sugahara K, Shimogori H, Okuda T, Takemoto T, Hashimoto M, Yamashita H. Cochlear administration of adenosine triphosphate facilitates recovery from acoustic trauma (temporary threshold shift) ORL J Otorhinolaryngol Relat Spec. 2004;66:80–84. doi: 10.1159/000077800. [DOI] [PubMed] [Google Scholar]
- Thiemermann C. Membrane-permeable radical scavengers (tempol) for shock, ischemia-reperfusion injury, and inflammation. Crit Care Med. 2003;31:S76–84. doi: 10.1097/00003246-200301001-00011. [DOI] [PubMed] [Google Scholar]
- Thorne PR, Munoz DJ, Nikolic P, Mander L, Jagger DJ, Greenwood D, Vlajkovic S, Housley GD. Potential role of purinergic signalling in cochlear pathology. Audiol Neurootol. 2002;7:180–184. doi: 10.1159/000058307. [DOI] [PubMed] [Google Scholar]
- Viberg A, Canlon B. The guide to plotting a cochleogram. Hear Res. 2004;197:1–10. doi: 10.1016/j.heares.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Weiss RG, Gerstenblith G, Bottomley PA. ATP flux through creatine kinase in the normal, stressed, and failing human heart. Proc Natl Acad Sci U S A. 2005;102(3):808–813. doi: 10.1073/pnas.0408962102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White BC, Sullivan JM, DeGracia DJ, O’Neil BJ, Neumar RW, Grossman LI, Rafols JA, Krause GS. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J Neurol Sci. 2000;179:1–33. doi: 10.1016/s0022-510x(00)00386-5. [DOI] [PubMed] [Google Scholar]
- Yamane H, Nakai Y, Takayama M, Iguchi H, Nakagawa T, Kojima A. Appearance of free radicals in the guinea pig inner ear after noise-induced acoustic trauma. Eur Arch Otorhinolaryngol. 1995;252:504–508. doi: 10.1007/BF02114761. [DOI] [PubMed] [Google Scholar]
- Yamashita D, Jiang HY, Schacht J, Miller JM. Delayed production of free radicals following noise exposure. Brain Res. 2004;1019:201–209. doi: 10.1016/j.brainres.2004.05.104. [DOI] [PubMed] [Google Scholar]
- Yamashita D, Jiang HY, Le Prell CG, Schacht J, Miller JM. Post-exposure treatment attenuates noise-induced hearing loss. Neuroscience. 2005;134:633–642. doi: 10.1016/j.neuroscience.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Yamasoba T, Schacht J, Shoji F, Miller JM. Attenuation of cochlear damage from noise trauma by an iron chelator, a free radical scavenger and glial cell line-derived neurotrophic factor in vivo. Brain Res. 1999;815:317–325. doi: 10.1016/s0006-8993(98)01100-7. [DOI] [PubMed] [Google Scholar]
- Yamasoba T, Harris C, Shoji F, Lee RJ, Nuttall AL, Miller JM. Influence of intense sound exposure on glutathione synthesis in the cochlea. Brain Res. 1998;804:72–78. doi: 10.1016/s0006-8993(98)00660-x. [DOI] [PubMed] [Google Scholar]