Abstract
Preclinical studies have suggested that opioid exposure may induce a paradoxical decrease in the nociceptive threshold, commonly referred as opioid-induced hyperalgesia (OIH). While OIH may have implications in acute and chronic pain management, its clinical features remain unclear. Using an office-based quantitative sensory testing (QST) method, we compared pain threshold, pain tolerance, and the degree of temporal summation of the second pain in response to thermal stimulation among three groups of subjects: those with neither pain nor opioid therapy (Group 1), with chronic pain but without opioid therapy (Group 2), and with both chronic pain and opioid therapy (Group 3). We also examined the possible correlation between QST responses to thermal stimulation and opioid dose, opioid treatment duration, opioid analgesic type, pain duration, or gender in group 3 subjects. As compared with both group 1 (n = 41) and group 2 (n = 41) subjects, group 3 subjects (n = 58) displayed a decreased heat pain threshold and exacerbated temporal summation of the second pain to thermal stimulation. In contrast, there were no differences in cold or warm sensation among three groups. Among clinical factors, daily opioid dose consistently correlated with the decreased heat pain threshold and exacerbated temporal summation of the second pain in group 3 subjects. These results indicate that decreased heat pain threshold and exacerbated temporal summation of the second pain may be characteristic QST changes in subjects with opioid therapy. The data suggest that QST may be a useful tool in the clinical assessment of OIH.
INTRODUCTION
Opioid analgesics are effective for the management of moderate to severe pain. Similar to other pain medications, opioids have well-documented side effects such as nausea, constipation, and sedation. With continuation of opioid therapy, some opioid-related side effects such as sedation may subside. On the other hand, a prolonged course of opioid therapy may lead to tolerance to and physical dependence on opioid analgesics. A notable feature of physical dependence on opioid is the hyperalgesic response during opioid withdrawal [3,8,14,19,34,37]. This phenomenon suggests that opioid exposure may activate a pro-nociceptive process, which could be unmasked upon the withdrawal from opioids. Recently, hyperalgesia has also been demonstrated in the absence of overt opioid withdrawal in preclinical models of opioid exposure [5,22,26], indicating a paradoxical phenomenon of opioid-induced hyperalgesia (OIH). A growing body of evidence supports the notion that OIH is likely to be mediated through the neural and molecular mechanisms similar to those of neuropathic pain [27,35], suggesting that OIH could have implications in clinical opioid therapy [2,4,24].
Several lines of clinical evidence indicate that OIH may play a role in opioid therapy for pain management. (1) Patients receiving a high spinal or intravenous dose of morphine can develop the hyperalgesic response and myoclonus [1,11,12,32,33]. (2) The opioid analgesic effect decreases following a course of opioid treatment [15,20,23,36], although no changes in the opioid analgesic effect under similar clinical conditions have been reported [10]. (3) A short-term intravenous remifentanil (a potent μ-opioid analgesic) infusion in human subjects induces hyperalgesia similar to that from the naloxone-precipitated opioid withdrawal [21,31]. (4) Pain sensitivity increases in opioid addicts as compared with normal subjects [9,13,17,28]. (5) OIH is suspected in patients receiving escalating doses of opioid analgesics [16] and hyperalgesia is detected by a cold pressor test after one month of oral morphine therapy in prior opioid-naïve patients [7].
To date, clinical diagnosis of OIH remains difficult due to the lack of evidence for clinical characteristics of OIH [25]. For example, it is unclear whether responses to noxious stimulation would differ in chronic pain subjects with or without opioid therapy. In this study, we examined a variety of responses to innocuous or noxious heat and cold stimulation using an office-based quantitative sensory testing (QST) method in order to achieve three objectives: 1) to compare and contrast QST responses, including pain threshold, pain tolerance, and temporal summation of the second pain, among subjects with or without opioid therapy, 2) to identify characteristic QST responses in chronic pain subjects with opioid therapy that would be distinguishable from chronic pain subjects without opioid therapy, and 3) to examine possible correlations between QST responses and several clinical factors related to opioid therapy such as opioid dose, opioid treatment duration, opioid type, pain duration, and gender.
METHODS
Study subjects
This study was approved through our Institutional Research Board. Study subjects were recruited from the Massachusetts General Hospital (MGH) and local community through advertisement and physician referrals. Three groups of subjects were recruited: those with neither pain nor opioid therapy (Group 1), with chronic pain but not on opioid therapy (Group 2), and with chronic pain and on opioid therapy (Group 3). We estimated that a sample size of 31 subjects in each group would have 80% power to detect a 1.2-degree (°C) difference in mean heat pain threshold with a standard deviation of less than 3 degrees (°C).
All subjects were between ages 18 and 65 years. The following inclusion criteria were used: 1) Group 1 subjects have had no pain and no opioid treatment for at least six months (naïve controls); 2) Group 2 subjects have had a stable pain condition (e.g., low back pain) but without opioid treatment for at least three months; 3) Group 3 subjects have had a stable pain condition for at least three months and also have been on opioid therapy for at least past three months; 4) Opioid therapy was defined as taking at least 30 mg daily morphine equivalent dose without opioid dose changes during the past month. For the standardized data analysis, we used the following conversion ratios between an oral dose of morphine and other opioid analgesics (1 mg morphine = 0.33 mg oxycodone, 0.25 mg hydromorphone, 0.33 mg hydrocodone, 0.33 mg methadone, 4 mg codeine, or 0.21 μg transdermal fentanyl); and 5) Since it is difficult to recruit subjects with pain but not on any pain medications, group 2 and group 3 subjects may have been taking non-opioid pain medications but without recent (within one month) dose changes.
The following exclusion criteria were used in all groups: 1) Subject has sensory deficits at the QST site resulting from such medical conditions as diabetes, alcoholic neuropathy, AIDS neuropathy, severe thyroid, liver or kidney diseases; 2) Subject has scar tissue, infection, or acute injury at the QST site; 3) Subject has had interventional pain management procedures that may alter QST responses including neuraxial or local anesthetic block within the last eight weeks; 4) Subject has a major psychiatric disorder requiring a recent (within one month) hospitalization, such as major depression, bipolar disorder, schizophrenia, anxiety disorder, and psychosis; and 5) Subject is taking illicit drug detected through a urine toxicology screen.
Study procedure
Potential subjects were first screened through a phone interview according to the inclusion and exclusion criteria. Those subjects who passed the phone interview were scheduled to visit the MGH Center for Translational Pain Research. Group 3 subjects were asked to take their routine opioid dose between 4 and 6 hours before the scheduled visit to minimize the variation and avoid potential opioid withdrawals. Upon arrival, the informed consent was obtained and co-signed by an investigator.
Each subject filled out a modified McGill Pain Questionnaire containing information on the demographic data, clinical pain inventory (pain location, pain intensity on visual analogue scale, pain pattern, pain duration, clinical diagnosis), and medications including non-opioid and opioid analgesics. A urine sample was obtained for a urine toxicology screen. The urine toxicology screen was used to detect illicit drug use and opioid analgesics. To verify that sensory deficits did not exist, each subject underwent a focused physical examination, which included vital signs and sensory examination at the site of QST (one of the forearms unrelated to the dermatome distribution of the subject’s pre-existing pain). Sensory examination included responses to alcohol swab, cotton swab, and tuning fork.
QST parameters
Quantitative thermal testing
QST responses to thermal stimulation were examined using Medoc Thermal Sensory Analyzer described previously [29, 39]. Each QST session was carried out in a quiet room maintained at 25 ± 2 °C. A contact thermode (3 × 3 cm) was gently attached and secured with a band onto the ventromedial part of a forearm in each subject. Temperature at the thermode changed at 10 °C/sec from a neutral temperature of 32 °C to a cutoff temperature of either 53 °C (heat stimulation) or 0 °C (cold stimulation). By pressing a computer mouse button, each subject was able to stop stimulation at any time during a session. Four categories of thermal QST parameters were examined in the following sequence: cold and warm sensation, cold and heat pain threshold, cold and heat pain tolerance, and temporal summation of the second pain to heat stimulation. Each test was repeated for three times with a 3-min interval.
To test cold and warm sensation, subjects were instructed to stop stimulation when they first perceived cold or warm sensation as temperature changed from the neural temperature (32 °C).
To detect cold and heat pain threshold, subjects were instructed to stop stimulation when they first perceived painful sensation, as temperature descended (cold pain) or ascended (heat pain) from the neural temperature of 32 °C. The temperature at which the subject stopped the stimulation was recorded as threshold temperature (°C).
To examine pain tolerance, two protocols were used. (a) To detect the maximal tolerable temperature (°C) for cold or heat pain, subjects were asked to tolerate the stimulation beyond their cold or heat pain threshold until it reached the maximal tolerable level. The maximum temperature was preset at 53 °C and 0 °C for heat pain and cold pain, respectively, to avoid tissue injury. (b) To detect the duration (in seconds) of tolerance to supra-threshold heat pain stimulation, subjects were asked to tolerate, as long as he/she could, heat stimulation preset at 47 °C for a maximum of 60 seconds. Since the heat pain threshold in normal subjects is about 45 °C and subjects on opioid therapy were expected to have a lower than normal heat pain threshold, this preset supra-threshold heat stimulation was above the heat pain threshold for the vast majority of subjects in all three groups (see Table 2) in order to make valid comparisons.
The temporal summation of the second pain is a characteristic psychophysical response in human subjects correlating with the electrophysiological response of nociceptive neurons in the spinal cord dorsal horn (windup) [29]. To examine the temporal summation of the second pain, a train of four identical stimuli at 47 °C, separated by a 2.2-second interval between stimuli, was applied to the subject’s forearm. Subjects were asked to rate their pain by visual analogue scale following each of four stimuli.
Table 2.
QST Results by Group
| Group 1 (n=41) | Group 2 (n=41) | Group 3 (n=58) | ANOVA (F- value; P-value) | |
|---|---|---|---|---|
| CS | 27.4±0.4 °C [20.3–31.2 (27.8)] |
27.9±0.4 °C [20.7–31.1 (28.5)] |
28.2±0.4 °C [14.6–31.5 (28.7)] |
0.94; 0.39 |
| WS | 35.4±0.3 °C [32.5–40.2 (34.7)] |
34.8±0.2 °C [33.1–40.8 (34.5)] |
34.9±0.2 °C [32.9–42.6 (34.5)] |
1.64; 0.19 |
| CPTh | 8.4±1.2 °C [0–25.1 (5.2)] |
10.0±1.3 °C [0–26.9 (6.3)] |
10.9±1.2 °C [0–27.7 (6.1)] |
0.99; 0.38 |
| HPTh | 45.3±0.5 °C [36.7–50.8 (45.7)] |
45.4±0.4 °C [38.3–49.6 (46.0)] |
43.8±0.4 °C [34.3–49.9 (44.5)] |
3.91; 0.02 * |
| CPTol | 0.5±0.2 °C [0–7.1 (0)] |
2.6±0.8 °C [0–23.9 (0)] |
3.3±0.7 °C [0–24.8 (0)] |
4.32; 0.01 * |
| HPTol | 50.1±0.2 °C [47.3–52.9 (49.7)] |
49.5±0.3 °C [42.3–53.0 (49.6)] |
48.6±0.3 °C [36.3–53.0 (49.0)] |
5.85; 0.01 * |
| HPDur | 47.6±3.0 sec (n=35) [60–60 (60)] |
42.4±3.4 sec (n=35) [4–60 (60)] |
35.2±2.7 sec (n=54) [4–60 (41)] |
4.25; 0.02 * |
CS: cold sensation; WS: warm sensation; CPTh: cold pain threshold; HPTh: heat pain threshold; CPTol: cold pain tolerance; HPTol: heat pain tolerance; HPDur: duration of tolerance to supra-threshold heat pain stimulation. The quantitative data are shown as mean ± standard error.
P< 0.05, repeated measure one-way ANOVA as compared among three groups. Of note, those numbers shown in a parenthesis under group mean ± standard error represent the range and median number of a QST parameter in each group. For the duration of heat pain tolerance, the number is smaller than the total number of its respective group because a small number of subjects in each group had the heat pain threshold above 47 °C and were not included in this analysis.
von Frey filament and pinprick testing
A set of standard von Frey filaments was used to examine the response to mechanical stimulation. A filament was perpendicularly applied to the subject’s forearm (the same site for QST) until it bent. The up-and-down method with different sizes of filaments (ranging from 26 to 300 grams of force, i.e., size #16 to #20) was used to detect a mechanical pain threshold. The mechanical pain threshold was defined as a painful response, reported by a subject, to at least one out of three stimuli using a filament. This test was mainly to detect the presence of mechanical allodynia among these subjects. The pinprick test was performed by briefly striking the subject’s forearm twice using a standard desk pin. The subject’s response to this stimulation was documented as painful or non-painful. This test was mainly to detect the presence of mechanical hyperalgesia among these subjects. The von Frey filament and pinprick test was always performed before thermal QST.
Statistical analysis
The following statistical analyses were performed. (1) For the non-parametric analysis, the Mann Whitney U test was used to analyze the data from the von Frey filament test. (2) For the parametric analysis, the data from each QST test in a subject were first averaged to yield a mean response. Repeated one-way analysis of variance (ANOVA) was then used to examine differences among groups. When a main effect was detected, the post hoc Tukey test was used to determine the source(s) of differences. (3) Pearson’s correlation analysis was used to examine the relationship between a clinical factor (e.g., opioid dose) and a QST response. (4) To compare the degree of temporal summation of the second pain among groups, the percent change in the response to the second, third, and fourth stimulation over the response to the first (baseline) stimulation was calculated and analyzed using repeated one-way ANOVA followed by the Tukey test. For each statistical test, the significance level was set at P < 0.05.
RESULTS
A total of 311 potential subjects were interviewed over the phone. Of these interviewees, 41 subjects were recruited into group 1, 41 subjects into group 2, and 67 subjects into group 3. Of them, 9 subjects were excluded from the overall analysis secondary to the unsatisfactory urine test (e.g., detection of illicit drugs) or incomplete QST sessions. Overall, there were no statistical differences in age among three groups (P> 0.05; Table 1). Pain conditions were comparable between group 2 and group 3 subjects as well, and there were no differences in the mean duration of clinical pain between these two groups (P> 0.05; Table 1). In addition, the percentage of subjects who were taking non-opioid pain medications as well as the type of non-opioid medications was also similar between group 2 and group 3 subjects (Table 1).
Table 1.
General Information on Study Subjects
| Group 1 (n=41) | Group 2 (n=41) | Group 3 (n=58) | |
|---|---|---|---|
| Age (year) | 39.2 ± 4.5 | 43.4 ± 3.4 | 47.7 ± 3.3 |
| Gender (M/F) | 13/28 | 15/26 | 27/31 |
| DOP (year) | N/A | 7.9 ± 5.6 | 7.6 ± 5.1 |
| DOM (year) | N/A | N/A | 2.7 ± 0.5 |
| Clinical pain | N/A | Back pain (76%), pelvic pain (2%), leg/knee pain (15%), others (7%) | Back pain (78%), pelvic pain (1%), leg/knee pain (13%), others (8%) |
| Other pain medications | N/A | antidepressant (8%), anticonvulsant (2%), NSAIDs (27%), gabapentin (10%), muscle relaxant (5%), no other pain medication (48%) | antidepressant (12%), anticonvulsant (2%), NSAIDs (7%), gabapentin (10%), muscle relaxant (18%), no other pain medication (51%) |
| Opioid types | N/A | N/A | Morphine, Hydromorphone, Hydrocodone, fentanyl, codeine |
DOP: Duration of pain; DOM: Duration of opioid treatment. The quantitative data are shown as mean ± standard error. Unlisted other clinical pain conditions include complex regional pain syndrome in lower extremity or facial pain.
Responses to quantitative thermal testing
Seven QST parameters in response to thermal stimulation were examined in all subjects (Table 2). Statistically significant differences were detected among three groups in four out of seven parameters including heat pain threshold, cold and heat pain tolerance (maximum tolerable temperature to cold or heat stimulation), and duration of tolerance to the supra-threshold heat stimulation at 47 °C (ANOVA, each P< 0.05, Table 2). The post-hoc analysis showed that significant differences in these four QST parameters were present between group 1 and group 3 subjects, but not between group 1 and group 2 subjects, (Tukey, each P < 0.05). Neither cold nor warm sensation differed among three groups (ANOVA, P> 0.05; Table 2), indicating that the observed differences in thermal QST responses is not due to changes in baseline cold and warm sensation in these subjects. The range and median number of each QST parameter are also displayed in Table 2 for each group in order to show the within-group variation among the subjects.
Since the detected difference in QST changes between group1 (naïve control) and group 3 (subjects with pain and opioid therapy) could be due to the influence from a pre-existing clinical pain condition, further comparisons were made between group 2 (subjects with pain but not on opioid therapy) and group 3 subjects. In this analysis, heat pain threshold was the only QST parameter that significantly differed between group 2 and group 3 subjects (Tukey, P< 0.05). This result indicates that, despite the differences in several QST parameters between group 1 and group 3 subjects, a decreased heat pain threshold is a characteristic QST change in chronic pain subjects on opioid therapy, which is independent of their pre-existing pain conditions.
Response to von Frey filament and pinprick stimulation
There were no differences among three groups in the mechanical threshold force (180 to 300 g) that elicited a painful response to von Frey filament stimulation at the same site for QST (Mann Whitney, P> 0.05). Subjects in all three groups also demonstrated a similar painful response to the pinprick test at the QST site, without the exacerbated painful response (mechanical hyperalgesia). In addition, all subjects demonstrated an intact response to the cotton swab and tuning folk stimulation. These results indicate that no mechanical allodynia or hyperalgesia was present at the QST site in all three groups of subjects.
Temporal summation of the second pain
Temporal summation of the second pain (windup) to heat stimulation was examined in 100 subjects (n = 25, 25, 50 for group 1, 2, and 3, respectively). Of note is that the number of subjects included in this analysis is smaller than that for the above analysis, because 1) some subjects did not have chance to participate in this test and 2) a small number of subjects in each group had a heat pain threshold above 47 °C (see Table 2) and these subjects were not included in this analysis.
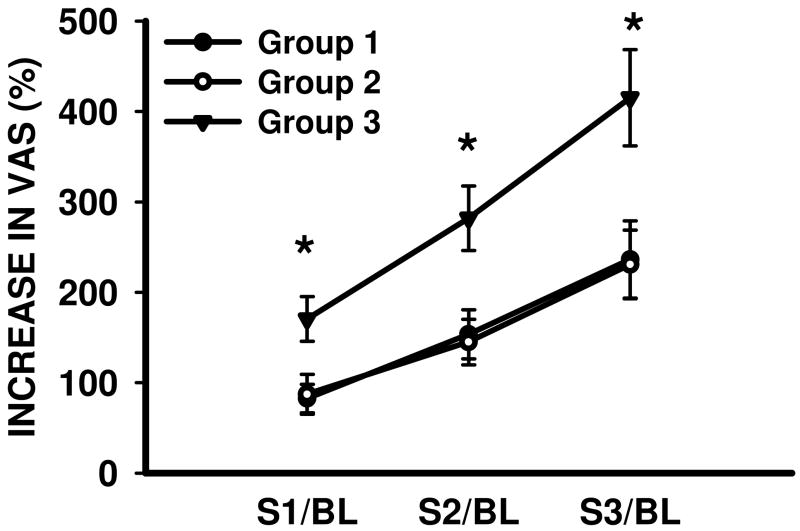
Consistent with the known psychophysical phenomenon of windup, a train of four identical supra-threshold heat stimuli (47 °C) resulted in a progressive increase in pain intensity (visual analogue scale) in subjects from all three groups (Fig. 1, ANOVA P < 0.05). However, the degree of temporal summation of the second pain was significantly exacerbated in group 3 subjects as compared with either group 1 or group 2 subjects (Fig. 1; Tukey, P< 0.05). In contrast, there were no differences in the degree of temporal summation of the second pain between group 1 and group 2 subjects (Fig. 1; Tukey, P> 0.05). These results indicate that exacerbation of temporal summation of the second pain is another characteristic QST change in group 3 subjects, which is distinguishable from either normal subjects (group 1) or subjects with pain but not on opioid therapy (group 2).
Fig. 1. Exacerbated temporal summation of the second pain in group 3 subjects.
* P< 0.05, as compared with group 1 and group 2 subjects. S1/BL, S2/BL, S3/BL: the percent increase in VAS (visual analogue scale) score in response to the second, third, and fourth stimulation over that of the first stimulation in a train of four noxious heat (47 °C) stimuli.
Clinical factors and QST responses to thermal stimulation
Five clinical factors were analyzed in group 3 subjects in relation to thermal QST responses, including opioid dose, duration of opioid treatment, opioid analgesic type, duration of pain, and gender. The majority of these clinical factors had no correlation with QST responses in our study subjects (Table 3; Pearson’s correlation, each P> 0.05). In addition, short-acting or long-acting opioid analgesics did not independently influence thermal QST responses in group 3 subjects, nor were there differences in thermal QST responses between group 3 subjects who have been on methadone (n=11) or other opioid analgesics (n= 39) (ANOVA, P> 0.05).
Table 3.
Pearson’s Correlation between QST Response and Clinical Factor
| CPTh | HPTh | CPTol | HPTol | HPDur | |
|---|---|---|---|---|---|
| MED | 0.15 | −0.36* | 0.08 | 0.02 | 0.11 |
| DOT | −0.08 | 0.02 | −0.07 | −0.14 | 0.03 |
| DOP | −0.12 | −0.12 | 0.12 | −0.17 | 0.07 |
MED: daily morphine equivalent dose; DOT: duration of opioid treatment; DOP: duration of pain; CPTh: cold pain threshold; HPTh: heat pain threshold; CPTol: cold pain tolerance; HPTol: heat pain tolerance; HPDur: duration of tolerance to supra-threshold heat pain stimulation. All values are Pearson’s correlation coefficients.
P< 0.05.
However, daily morphine equivalent dose (MED) consistently correlated with the degree of heat pain threshold (Table 3; Pearson’s correlation, P< 0.05), such that a higher MED was correlated with a lower heat pain threshold but not with other thermal QST parameters. A similar correlation was observed when comparing the average MED with the degree of temporal summation of the second pain in group 3 subjects. The degree of temporal summation of the second pain was significantly greater (P< 0.05) in group 3 subjects on 75 mg or higher MED (mean dose: 155.1±15.3 mg; n = 37) than those on less than 75 mg MED (mean dose: 53.6±4.2 mg; n=13), although the duration of opioid therapy was comparable between these two subgroups (higher or lower than 75 mg MED). Collectively, these results indicate that MED is a clinical factor that is related to characteristic QST changes (decreased heat pain threshold and exacerbated temporal summation of the second pain) in group 3 subjects.
DISCUSSION
Our results demonstrate that, although significant changes in thermal QST responses were detectable in chronic pain patients on opioid therapy, including decreased heat pain threshold, decreased cold and heat pain tolerance, reduced tolerance to the supra-threshold heat pain stimulation, and exacerbated temporal summation of the second pain, only the decreased heat pain threshold and exacerbated temporal summation of the second pain were distinguishable between group 3 subjects and both group 1 and group 2 subjects. These results suggest that these two QST findings may be characteristic clinical indications of OIH. The data also suggest that the clinical relevance of QST findings should be evaluated by extracting non-specific influences such as pre-existing pain condition. Of interest to note is that our results appear to indicate that thermal hyperalgesia is a primary presentation in group 3 subjects because neither mechanical allodynia nor mechanical hyperalgesia were present in our study subjects. While the exact mechanism for these findings is unclear, the data appear to be consistent with the findings in animal studies using the paw-withdrawal from radiant heat stimulation [26].
Methodological considerations
Except for one negative report [30], changes in pain threshold and/or pain tolerance have been observed in subjects with opioid therapy [7] or opioid addiction [9,13] using various techniques. In the present study, several methodological issues were considered. Firstly, a battery of seven thermal QST parameters was used, including cold and warm sensation, cold and heat pain threshold, and cold and heat pain tolerance, which allowed us to make distinctions between changes in cold and heat pain sensation and non-specific variations in baseline cold and warm sensation. Secondly, temporal summation of the second pain (windup) was used as an important QST parameter because both windup and OIH share common cellular mechanisms that are also contributory to neuropathic pain, both of which have been shown to be at least partially medicated through the spinal glutamatergic mechanism [27,29]. Thirdly, normal subjects (with neither pain nor opioid therapy) were recruited and their baseline QST responses were compared with those of pain subjects with or without opioid therapy. These comparisons allowed us to analyze differences between QST changes related to pre-existing pain or opioid therapy. Fourthly, a urine toxicology screen was conducted in all subjects and group 3 subjects on opioid therapy were instructed to take their routine opioid dose within 4–6 hours before QST to avoid possible opioid withdrawals. Finally, the site of QST was chosen away from the dermatome distribution of pre-existing pain because OIH induced by systemic opioid analgesics, if present, should be detectable independent of pre-existing pain [24].
However, several methodological limitations should be noted as well. First, since the QST data was compared as group means, it is possible that the within-group individual variation may be masked. In table 2, group means for each QST parameter as well as their range and median number were included for comparisons. These two sets of data appear to be largely consistent, although the possible influence of the individual variation on the group outcome could not be entirely ruled out. Moreover, it is difficult to determine a reference number for each QST parameter due to individual variations. Therefore, the average value for each parameter reported in this study should not be used as a simple reference to determine whether a subject has OIH in the clinical setting. Second, it is possible that our study cohorts may not represent actual clinical populations despite the randomization process through the phone interview, which is a notable limitation of a cross-section study like this one. Moreover, both age and gender could influence the detection of nociceptive threshold using QST [39]. Although there were no significant differences in our demographic data regarding subjects’ age and gender among these groups, the influence of age and gender on the QST response could not be ruled out and should be further investigated in larger cohorts. In addition, the study did not specifically examine whether a shorter pain tolerance time would reflect the lack of motivation to tolerate suprathreshold pain in some subjects. Third, other factors such as clinical pain condition, duration of pain, clinical comorbidity, non-opioid pain medications could also influence the data analysis between group 2 and group 3 subjects, although, as illustrated in table 1, these clinical factors were comparable between these two groups. Nonetheless, the data from this study should be viewed with the caveats of these methodological limitations.
Clinical factors and QST responses
It has been suggested that opioid dose, treatment duration, opioid type, and gender may influence OIH [2,18,24]. Accordingly, we examined the relationship between various clinical factors and QST responses in group 3 subjects. Our data indicate that MED is an important factor that is related to characteristic QST responses including the decreased heat pain threshold and exacerbated temporal summation of the second pain in group 3 subjects, although the relationship between MED and these QST responses showed a relatively weak but statistically significant correlation. This finding is consistent with the previous data showing that OIH is likely to be present following even a short course of intravenous infusion with remifentanil (a potent μ-opioid agonist) [21,31]. It should be noted that, since our inclusion criteria required group 3 subjects to have been on opioid therapy for at least three months, the influence of opioid treatment duration on QST responses was not adequately examined in this study. Likewise, the exact relationship between opioid type and QST changes should be further evaluated despite the fact that the influence of opioid type (short-acting versus long-acting opioids; methadone versus other opioids) on QST changes was not detected in the present study. Collectively, the present data suggest that characteristic QST responses indicative of OIH may be influenced by certain clinical factors, one of which is MED with a treatment course of at least three months.
Clinical implications
Apparent clinical opioid tolerance refers to the diminished opioid analgesic effect during an opioid therapy. Besides a worsening pain state and/or pharmacological opioid tolerance (e.g., desensitization of opioid receptors), OIH may be an indication of neuroplastic changes in the nociceptive process contributory to apparent clinical opioid tolerance [6]. Clinically, resolving apparent clinical opioid tolerance would depend on the proper differential diagnosis between OIH, pharmacological opioid tolerance, and a worsening pain state. While opioid dose escalation may improve a worsening pain state and/or pharmacological opioid tolerance, supervised opioid tapering, opioid rotation, as well as adjunctive medications including ketamine have been shown to improve OIH [20,23,33]. Thus, recognizing characteristic QST responses may help formulate clinical plans for managing difficult cases of opioid therapy.
It should be emphasized that detection of QST changes in chronic pain subjects with opioid therapy does not necessarily indicate that OIH has contributed to the clinical phenomenon of apparent opioid tolerance. What it does indicate is that these subjects have altered responses to a standard battery of noxious stimulation. While the impact of these findings on clinical pain management remains to be evaluated, we suggest that the following categories of clinical factors could be considered in the differential diagnosis of possible OIH in a clinical setting (table 4). These clinical indicators may include characteristic QST responses, relevant clinical factors, changes in pain pattern and location, and other factors (e.g., gender, opioid type). Moreover, these indicators should be further considered along with additional clinical information such as the use of adjunctive non-opioid pain medications and the presence of comorbidities. Future studies should also address the relationship between 1) OIH and clinical pain intensity, 2) OIH and satisfaction with opioid therapy, and 3) improvement of OIH and clinical opioid therapy. Nonetheless, the present data suggest that detecting characteristic changes in QST responses may be a useful step towards the clinical assessment of OIH. Additional steps in this line of research may include a chronological follow up (e.g., before and after a course of opioid therapy) to assess QST changes and the use of a ketamine test in QST sessions.
Table 4.
Clinical Factors Implicated in the Differential Diagnosis of OIH
| Clinical factor | Clinical finding |
|---|---|
| Temporal summation of second pain | Exacerbated (QST) |
| Pain threshold (cold or heat) | Decreased (QST) - particularly heat pain |
| Pain tolerance (cold or heat) | Decreased (QST) |
| Supra-threshold stimulation | Decreased duration of tolerance (QST) |
| Average opioid dose | ≥ 75 mg daily morphine equivalent dose |
| Duration of opioid therapy | ≥ 3 months |
| Opioid dose escalation | Limited improvement or normalization on QST responses |
| Opioid dose reduction | Improved opioid analgesia [38] |
| Pain quality | Burning, diffuse pain, and spontaneous pain similar to those seen with neuropathic pain |
| Pain location | At and/or beyond the dermatome distribution of a pre-existing pain condition |
| Pain intensity | Similar or greater than pre-existing pain |
| Opioid type to be considered | Short-acting vs. long acting; methadone versus other opioid analgesics |
| Gender | Female vs. male? |
Acknowledgments
This study is supported by NIH RO1 grant DA22576 (J.M.). We would like to thank Drs. James Rathmell, Gary Brenner, Milan Stojanovic, Shihab Ahmed, Padma Gulur, and Gary Polykoff and Tina Toland, B.A., M.H.A. of the Massachusetts General Hospital Center for Pain Medicine for their help in the subject recruitment.
Footnotes
The authors claim no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ali NM. Hyperalgesic response in a patient receiving high concentrations of spinal morphine. Anesthesiology. 1986;65:449. doi: 10.1097/00000542-198610000-00028. [DOI] [PubMed] [Google Scholar]
- 2.Angst MS, Clark JD. Opioid-induced hyperalgesia: a qualitative systematic review. Anesthesiology. 2006;104:570–587. doi: 10.1097/00000542-200603000-00025. [DOI] [PubMed] [Google Scholar]
- 3.Angst MS, Koppert W, Pahl I, Clark DJ, Schmelz M. Short-term infusion of the mu-opioid agonist remifentanil in humans causes hyperalgesia during withdrawal. Pain. 2003;106:49–57. doi: 10.1016/s0304-3959(03)00276-8. [DOI] [PubMed] [Google Scholar]
- 4.Ballantyne JC, Mao J. Opioid therapy for chronic pain. N Engl J Med. 2003;349:1943–1953. doi: 10.1056/NEJMra025411. [DOI] [PubMed] [Google Scholar]
- 5.Celerier E, Laulin JP, Corcuff JB, Le Moal M, Simonnet G. Progressive enhancement of delayed hyperalgesia induced by repeated heroin administration: a sensitization process. J Neurosci. 2001;21:4074–4080. doi: 10.1523/JNEUROSCI.21-11-04074.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang G, Chen L, Mao J. Opioid tolerance and hyperalgesia. Med Clin North Am. 2007;91:199–211. doi: 10.1016/j.mcna.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Chu LF, Clark DJ, Angst MS. Opioid tolerance and hyperalgesia in chronic pain patients after one month of oral morphine therapy: a preliminary prospective study. J Pain. 2006;7:43–48. doi: 10.1016/j.jpain.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Compton P, Athanasos P, Elashoff D. Withdrawal hyperalgesia after acute opioid physical dependence in nonaddicted humans: a preliminary study. J Pain. 2003;4:511–519. doi: 10.1016/j.jpain.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 9.Compton P, Charuvastra VC, Ling W. Pain intolerance in opioid-maintained former opiate addicts: effect of long-acting maintenance agent. Drug Alcohol Depend. 2001;63:139–146. doi: 10.1016/s0376-8716(00)00200-3. [DOI] [PubMed] [Google Scholar]
- 10.Cortinez LI, Brandes V, Munoz HR, Guerrero ME, Mur M. No clinical evidence of acute opioid tolerance after remifentanil-based anaesthesia. Br J Anaesth. 2001;87:866–869. doi: 10.1093/bja/87.6.866. [DOI] [PubMed] [Google Scholar]
- 11.De Conno F, Caraceni A, Martini C, Spoldi E, Salvetti M, Ventafridda V. Hyperalgesia and myoclonus with intrathecal infusion of high-dose morphine. Pain. 1991;47:337–339. doi: 10.1016/0304-3959(91)90225-M. [DOI] [PubMed] [Google Scholar]
- 12.Devulder J. Hyperalgesia induced by high-dose intrathecal sufentanil in neuropathic pain. J Neurosurg Anesthesiol. 1997;9:146–148. doi: 10.1097/00008506-199704000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Doverty M, White JM, Somogyi AA, Bochner F, Ali R, Ling W. Hyperalgesic responses in methadone maintenance patients. Pain. 2001;90:91–96. doi: 10.1016/s0304-3959(00)00391-2. [DOI] [PubMed] [Google Scholar]
- 14.Dunbar SA, Pulai IJ. Repetitive opioid abstinence causes progressive hyperalgesia sensitive to N-methyl-D-aspartate receptor blockade in the rat. J Pharmacol Exp Ther. 1998;284:678–686. [PubMed] [Google Scholar]
- 15.Guignard B, Bossard AE, Coste C, Sessler DI, Lebrault C, Alfonsi P, Fletcher D, Chauvin M. Acute opioid tolerance: intraoperative remifentanil increases postoperative pain and morphine requirement. Anesthesiology. 2000;93:409–417. doi: 10.1097/00000542-200008000-00019. [DOI] [PubMed] [Google Scholar]
- 16.Heger S, Maier C, Otter K, Helwig U, Suttorp M. Morphine induced allodynia in a child with brain tumour. BMJ. 1999;319:627–629. doi: 10.1136/bmj.319.7210.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ho A, Dole VP. Pain perception in drug-free and in methadone-maintained human ex-addicts. Proc Soc Exp Biol Med. 1979;162:392–395. doi: 10.3181/00379727-162-40689. [DOI] [PubMed] [Google Scholar]
- 18.Holtman JR, Jr, Wala EP. Characterization of morphine-induced hyperalgesia in male and female rats. Pain. 2005;114:62–70. doi: 10.1016/j.pain.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 19.Hood DD, Curry R, Eisenach JC. Intravenous remifentanil produces withdrawal hyperalgesia in volunteers with capsaicin-induced hyperalgesia. Anesth Analg. 2003;97:810–815. doi: 10.1213/01.ANE.0000078811.80093.88. [DOI] [PubMed] [Google Scholar]
- 20.Joly V, Richebe P, Guignard B, Fletcher D, Maurette P, Sessler DI, Chauvin M. Remifentanil-induced postoperative hyperalgesia and its prevention with small-dose ketamine. Anesthesiology. 2005;103:147–155. doi: 10.1097/00000542-200507000-00022. [DOI] [PubMed] [Google Scholar]
- 21.Koppert W, Angst M, Alsheimer M, Sittl R, Albrecht S, Schuttler J, Schmelz M. Naloxone provokes similar pain facilitation as observed after short-term infusion of remifentanil in humans. Pain. 2003;106:91–99. doi: 10.1016/s0304-3959(03)00294-x. [DOI] [PubMed] [Google Scholar]
- 22.Laulin JP, Maurette P, Corcuff JB, Rivat C, Chauvin M, Simonnet G. The role of ketamine in preventing fentanyl-induced hyperalgesia and subsequent acute morphine tolerance. Anesth Analg. 2002;94:1263–9. doi: 10.1097/00000539-200205000-00040. table of contents. [DOI] [PubMed] [Google Scholar]
- 23.Luginbuhl M, Gerber A, Schnider TW, Petersen-Felix S, Arendt-Nielsen L, Curatolo M. Modulation of remifentanil-induced analgesia, hyperalgesia, and tolerance by small-dose ketamine in humans. Anesth Analg. 2003;96:726–32. doi: 10.1213/01.ANE.0000048086.58161.18. table of contents. [DOI] [PubMed] [Google Scholar]
- 24.Mao J. Opioid-induced abnormal pain sensitivity: implications in clinical opioid therapy. Pain. 2002;100:213–217. doi: 10.1016/S0304-3959(02)00422-0. [DOI] [PubMed] [Google Scholar]
- 25.Mao J. Opioid-induced abnormal pain sensitivity. Curr Pain Headache Rep. 2006;10:67–70. doi: 10.1007/s11916-006-0011-5. [DOI] [PubMed] [Google Scholar]
- 26.Mao J, Price DD, Mayer DJ. Thermal hyperalgesia in association with the development of morphine tolerance in rats: roles of excitatory amino acid receptors and protein kinase C. J Neurosci. 1994;14:2301–2312. doi: 10.1523/JNEUROSCI.14-04-02301.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mao J, Price DD, Mayer DJ. Mechanisms of hyperalgesia and morphine tolerance: a current view of their possible interactions. Pain. 1995;62:259–274. doi: 10.1016/0304-3959(95)00073-2. [DOI] [PubMed] [Google Scholar]
- 28.Martin JE, Inglis J. Pain tolerance and narcotic addiction. Br J Soc Clin Psychol. 1965;4:224–229. doi: 10.1111/j.2044-8260.1965.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 29.Price DD, Mao J, Frenk H, Mayer DJ. The N-methyl-D-aspartate receptor antagonist dextromethorphan selectively reduces temporal summation of second pain in man. Pain. 1994;59:165–174. doi: 10.1016/0304-3959(94)90069-8. [DOI] [PubMed] [Google Scholar]
- 30.Reznikov I, Pud D, Eisenberg E. Oral opioid administration and hyperalgesia in patients with cancer or chronic nonmalignant pain. Br J Clin Pharmacol. 2005;60:311–318. doi: 10.1111/j.1365-2125.2005.02418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt S, Bethge C, Forster MH, Schafer M. Enhanced postoperative sensitivity to painful pressure stimulation after intraoperative high dose remifentanil in patients without significant surgical site pain. Clin J Pain. 2007;23:605–611. doi: 10.1097/AJP.0b013e318122d1e4. [DOI] [PubMed] [Google Scholar]
- 32.Singla A, Stojanovic MP, Chen L, Mao J. A differential diagnosis of hyperalgesia, toxicity, and withdrawal from intrathecal morphine infusion. Anesth Analg. 2007;105:1816–9. doi: 10.1213/01.ane.0000290338.39037.38. table of contents. [DOI] [PubMed] [Google Scholar]
- 33.Sjogren P, Jonsson T, Jensen NH, Drenck NE, Jensen TS. Hyperalgesia and myoclonus in terminal cancer patients treated with continuous intravenous morphine. Pain. 1993;55:93–97. doi: 10.1016/0304-3959(93)90188-U. [DOI] [PubMed] [Google Scholar]
- 34.Tilson HA, Rech RH, Stolman S. Hyperalgesia during withdrawal as a means of measuring the degree of dependence in morphine dependent rats. Psychopharmacologia. 1973;28:287–300. doi: 10.1007/BF00429309. [DOI] [PubMed] [Google Scholar]
- 35.Vanderah TW, Ossipov MH, Lai J, Malan TP, Jr, Porreca F. Mechanisms of opioid–zinduced pain and antinociceptive tolerance: descending facilitation and spinal dynorphin. Pain. 2001;92:5–9. doi: 10.1016/s0304-3959(01)00311-6. [DOI] [PubMed] [Google Scholar]
- 36.Vinik HR, Kissin I. Rapid development of tolerance to analgesia during remifentanil infusion in humans. Anesth Analg. 1998;86:1307–1311. doi: 10.1097/00000539-199806000-00033. [DOI] [PubMed] [Google Scholar]
- 37.Von Voigtlander PF, Lewis RA. A withdrawal hyperalgesia test for physical dependence: evaluation of mu and mixed partial opioid agonists. J Pharmacol Methods. 1983;10:277–282. doi: 10.1016/0160-5402(83)90022-0. [DOI] [PubMed] [Google Scholar]
- 38.Vorobeychik Y, Chen L, Bush MC, Mao J. Improved opioid analgesic effect after opioid dose reduction. Pain Med. 2008;9:724–727. doi: 10.1111/j.1526-4637.2008.00501.x. [DOI] [PubMed] [Google Scholar]
- 39.Yarnitsky D. Quantitative sensory testing. Muscle Nerve. 1997;20:198–204. doi: 10.1002/(sici)1097-4598(199702)20:2<198::aid-mus10>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]