SUMMARY
When dietary carbohydrate is unavailable, glucose required to support metabolism in vital tissues is generated via gluconeogenesis in the liver. Expression of phosphoenolpyruvate carboxykinase (PEPCK), commonly considered the control point for liver gluconeogenesis, is normally regulated by circulating hormones to match systemic glucose demand. However, this regulation fails in diabetes. Because other molecular and metabolic factors can also influence gluconeogenesis, the explicit role of PEPCK protein content in the control of gluconeogenesis was unclear. In this study, metabolic control of liver gluconeogenesis was quantified in groups of mice with varying PEPCK protein content. Surprisingly, livers with a 90% reduction in PEPCK content showed only a ~40% reduction in gluconeogenic flux, indicating a lower than expected capacity for PEPCK protein content to control gluconeogenesis. However, PEPCK flux correlated tightly with TCA cycle activity, suggesting that under some conditions in mice, PEPCK expression must coordinate with hepatic energy metabolism to control gluconeogenesis.
INTRODUCTION
Gluconeogenesis from lactate and amino acids is important for the maintenance of circulating glucose levels during fasting (Chandramouli et al., 1997) or strenuous activity (Petersen et al., 2004). This process occurs predominantly in liver under normal conditions and ensures a continuous supply of glucose for red blood cells and the central nervous tissues, systems that are incapable of metabolizing fatty acids. Normally, gluconeogenesis is under exquisite control to ensure that rates of glucose production precisely match whole-body glucose requirements, but this regulation appears to fail in diabetes (Boden et al., 2001; Wajngot et al., 2001). For this reason, the mechanisms by which gluconeogenesis is controlled have been widely studied, yet they remain incompletely understood.
Gluconeogenesis can be considered the reversal of glycolysis (Pilkis and Granner, 1992), with the exception of a few specific steps in which unique enzymes are used either to bypass a thermodynamically unfavorable step or to avoid uncontrolled futile cycling (Pilkis and Granner, 1992). For example, the last step in glycolysis is the conversion of phosphoenolpyruvate (PEP) to pyruvate, a thermodynamically favorable reaction catalyzed by pyruvate kinase (PK). Under typical cellular conditions, the liver PK reaction is irreversible, so an alternate pathway for converting pyruvate back into PEP is required for continual glucose production. This is accomplished in two enzymatic steps: conversion of pyruvate to oxaloacetate (OAA) catalyzed by pyruvate carboxylase (PC), followed by conversion of OAA to PEP catalyzed by phosphoenolpyruvate carboxykinase (PEPCK) (Hanson and Garber, 1972). The latter step is commonly considered rate controlling in gluconeogenesis (Rognstad, 1979).
The concept that PEPCK expression is rate controlling for gluconeogenesis has prompted numerous investigations of the mechanisms that control PEPCK gene expression, as previously reviewed (Chakravarty et al., 2005; Granner and Pilkis, 1990; Hanson and Reshef, 1997). These studies have revealed that the PEPCK promoter has binding sites for transcription factors directly related to glucose homeostasis. For example, CREB (Leahy et al., 1999), C/EBPα (Roesler et al., 1989), and Foxo1 (Schmoll et al., 2000; Zhang et al., 2006) are important in the induction of hepatic PEPCK expression in response to fasting or stress and its attenuation during feeding as reflected by changes in circulating levels of insulin, glucagon, and glucocorticoids. Accordingly, PEPCK is overexpressed in all forms of diabetes, in which gluconeogenesis is inappropriately high (Veneziale et al., 1983), and a 7-fold overexpression of PEPCK in mice results in hyperglycemia (Valera et al., 1994). As PEPCK expression is exquisitely coordinated and appears to parallel whole-body glucose demand, PEPCK expression is widely used as an indicator of changes in gluconeogenic flux in response to molecular or pharmacologic interventions (Chakravarty et al., 2005).
Although robust control of PEPCK gene expression is consistent with the concept that PEPCK is rate controlling for gluconeogenesis, metabolic control theory suggests that regulation of such a critical pathway by a single enzyme is unlikely (Fell, 1997), and metabolic control analysis of gluconeogenesis in hepatocytes provides evidence to the contrary (Argaud et al., 1991; Groen et al., 1986; Rigoulet et al., 1987). Adaptive regulation of other enzymes in the gluconeogenic pathway, by either gene expression or allosteric modification of activity, also high-lights the importance of the entire network in regulation of gluconeogenic flux, but it does not explicitly exclude a dominant role for PEPCK. Recently, a number of mouse models have been generated with impaired PEPCK gene expression, allowing a detailed study of the role of PEPCK protein content in gluconeogenesis (She et al., 2000). Surprisingly, changes in PEPCK expression fail to dramatically impact fasting glycemia, while, for instance, alteration in the activity of glucokinase, a rate-limiting enzyme for glycolysis, has profound effects (Magnuson et al., 2003). Data from these mouse models suggest that liver PEPCK plays a role more complex than simply regulating gluconeogenesis. Disruption of hepatic energy metabolism in these mice (Burgess et al., 2004; She et al., 2000) and in an independent mouse model lacking cytosolic PEPCK (Hakimi et al., 2005) suggests that PEPCK flux may interact with energy generation in the hepatic TCA cycle. In addition, Hanson and coworkers (Chakravarty et al., 2005) identified PEPCK as a critical enzyme for the synthesis of glycerol-3-phosphate, used in esterification of fatty acids to triglycerides. These new data prompted us to examine the capacity of PEPCK protein content to control hepatic gluconeogenesis and to determine whether altered PEPCK flux correlates with changes in hepatic energy production in intact livers from mice with graded PEPCK protein content. PEPCK protein content was found to weakly influence flux through the pathway, while hepatic energy production strongly correlated with the rate of gluconeogenesis.
RESULTS
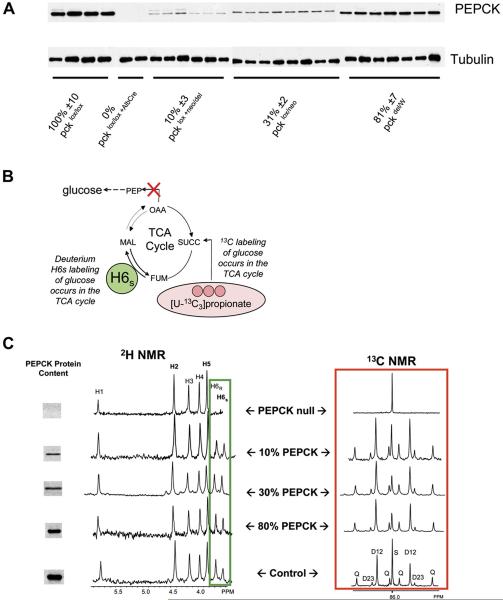
Postperfusion assay of PEPCK protein content revealed that livers from pckdel/W, pcklox/neo, and pcklox + neo/del mice had 80%, 30%, and 10% PEPCK protein levels (respectively) compared to control pcklox/lox mice, and pcklox/lox + AlbCre mice had undetectable levels of PEPCK protein compared to the control mice (Figure 1A). Metabolic fluxes through gluconeogenesis, PEPCK, pyruvate cycling, and TCA cycle oxidation were measured using 2H2O and [U-13C3]propionate tracers. These measurements are based on the fact that glucose cannot become 2H enriched in position H6s or labeled with 13C in any position without PEPCK activity (Figure 1B) (Burgess et al., 2004). NMR was used to detect the appearance of these tracers in glucose, and, as anticipated, glucose produced by livers lacking cytosolic PEPCK had extremely low deuterium enrichment in the H6s position and close to naturalabundance 13C in all carbon resonances (Figure 1C, top), as previously reported (Burgess et al., 2004). In contrast, livers with as little as 10% of normal PEPCK protein content produced glucose with substantial 2H and 13C labeling in the H6s and C2 positions, respectively, consistent with gluconeogenesis from substrates passing through the TCA cycle (Figure 1C).
Figure 1. Effect of PEPCK Protein Content on 2H and 13C Tracer Incorporation into Glucose.
(A) Western blots for PEPCK and tubulin protein content. Relative PEPCK content was determined from the PEPCK/tubulin ratio normalized to 100% for the control strain (pcklox/lox). Mean ± SEM is reported.
(B) The carbons used for gluconeogenesis originate in the TCA cycle. Glucose cannot become 2H enriched at position H6s or labeled with 13C in any position without PEPCK activity. NMR can be used to measure these enrichments and combined with total glucose production to determine flux through the TCA cycle and gluconeogenesis.
(C) Decreasing the PEPCK protein content in mice causes only small changes in the 2H enrichment of H6s (left box) or the C2 13C multiplets (right box). This indicates significant rates of gluconeogenesis despite low levels of PEPCK protein content. Only glucose from the PEPCK null livers had dramatically reduced 2H or 13C enrichment in these positions, indicating that those livers produced glucose from glycerol rather than the TCA cycle (Burgess et al., 2004). 13C multiplets are labeled Q (quartet), D12 (doublet 12), D34 (doublet 34), and S (singlet). The areas of these multiplets represent the isotopomer distribution of 13C in glucose and are used to determine fluxes associated with the TCA cycle (Jones et al., 1997).
There were no significant differences in the percent of glucose synthesized from PEP in livers with 80% or even 30% PEPCK protein content compared to controls (Table 1). Livers with 10% PEPCK content had ~15% less glucose coming from PEP and a corresponding increase in glucose coming from glycerol. 13C NMR of liver effluent glucose reported a decline in the relative fluxes through PEPCK, pyruvate cycling, and gluconeogenesis (relative to TCA cycle flux) only in livers with 30% or less of normal PEPCK content (Table 1). Total glucose production dropped by ~25% when PEPCK content was 10% of control and remained at ~43% in livers completely lacking PEPCK. This indicates that even PEPCK null livers retain an ability to synthesize glucose from source besides PEP; 2H NMR showed clearly that virtually all of the remaining glucose production was accounted for by gluconeogenesis from glycerol. Oxygen consumption was relatively insensitive to changes in PEPCK content, except in the null liver, which showed a 20% decrease (Table 2).
Table 1.
Protein Content from Western Blotting and Relative Fluxes from NMR Analysis in Isolated Perfused Livers
| Strain | pcklox/lox | pckdel/W | pcklox/neo | pcklox + neo/del | pcklox/lox + AlbCre |
|---|---|---|---|---|---|
| PEPCK protein content | 100% ± 10% | 81% ± 7%# | 31% ± 2%# | 10% ± 3%# | 0% (bd)# |
| Data from 2H NMR: sources of effluent glucose (%) | |||||
| Glycogen | 6 ± 5 | 0 ± 2 | 9 ± 9 | 0 ± 3 | 5 ± 6* |
| Glycerol | 48 ± 15 | 5 ± 5 | 35 ± 5 | 60 ± 5 | 88 ± 7* |
| PEP | 46 ± 11 | 48 ± 4 | 56 ± 5 | 40 ± 4 | 7 ± 2* |
| Data from 13C NMR: fluxes relative to citrate synthase flux | |||||
| PEPCK | 5.9 ± 0.6 | 6.5 ± 0.4 | 4.5 ± 0.2* | 4.3 ± 0.4* | 2.00 ± 0.3* |
| Pyruvate cycling (PK+ME) | 2.1 ± 0.4 | 2.7 ± 0.2 | 1.5 ± 0.2 | 1.4 ± 0.1* | 0.16 ± 0.04* |
| Gluconeogenesis from PEP | 3.8 ± 0.23 | 3.8 ± 0.2 | 3.0 ± 0.01* | 2.9 ± 0.3* | 1.86 ± 0.3* |
| n = | 5 | 7 | 5 | 5 | 5 |
Livers from fasted mice were perfused with nonrecirculating media. Data are presented as mean ± SEM.
p < 0.05 versus control (pcklox/lox)
p < 0.05 versus column to the left. bd, below detection limits.
Table 2.
Glucose Production, Oxygen Consumption, and Absolute Fluxes
| Strain | pcklox/lox | pckdel/W | pcklox/neo | pcklox + neo/del | pcklox/lox + AlbCre |
|---|---|---|---|---|---|
| Substrate uptake and production (μmol/min/grams wet weight) | |||||
| Glucose production | 0.47 ± 0.10 | 0.35 ± 0.07 | 0.31 ± 0.06 | 0.35 ± 0.01* | 0.20 ± 0.10* |
| O2 consumption | 2.20 ± 0.17 | 2.40 ± 0.34 | 2.00 ± 0.31 | 1.90 ± 0.18 | 1.74 ± 0.24* |
| Flux of substrate to glucose (μmol/min/grams wet weight) | |||||
| Glycogen (GLY) | 0.02 ± 0.01 | 0.00 ± 0.01 | 0.03 ± 0.01 | 0.00 ± 0.03 | 0.01 ± 0.02 |
| Glycerol (GNGglycerol) | 0.47 ± 0.13 | 0.39 ± 0.09 | 0.23 ± 0.09 | 0.42 ± 0.02 | 0.35 ± 0.18 |
| PEP (GNGPEP) | 0.43 ± 0.12 | 0.31 ± 0.05 | 0.33 ± 0.05 | 0.28 ± 0.04 | 0.03 ± 0.02* |
| Fluxes associated with the TCA cycle (μmol/min/grams wet weight) | |||||
| PEPCK | 0.65 ± 0.15 | 0.54 ± 0.08 | 0.49 ± 0.04 | 0.41 ± 0.04 | 0.03 ± 0.02* |
| Pyruvate cycling (PK+ME) | 0.22 ± 0.05 | 0.22 ± 0.03 | 0.16 ± 0.02* | 0.13 ± 0.01 | 0.00 ± 0.08* |
| TCA cycle (citrate synthase) | 0.12 ± 0.04 | 0.09 ± 0.02 | 0.11 ± 0.02 | 0.10 ± 0.01 | 0.02 ± 0.01* |
Livers from fasted mice were perfused with nonrecirculating media. Absolute fluxes were determined from the NMR data and rate of glucose production (see Supplemental Experimental Procedures). Data are presented as mean ± SEM.
p < 0.05 versus control (pcklox/lox).
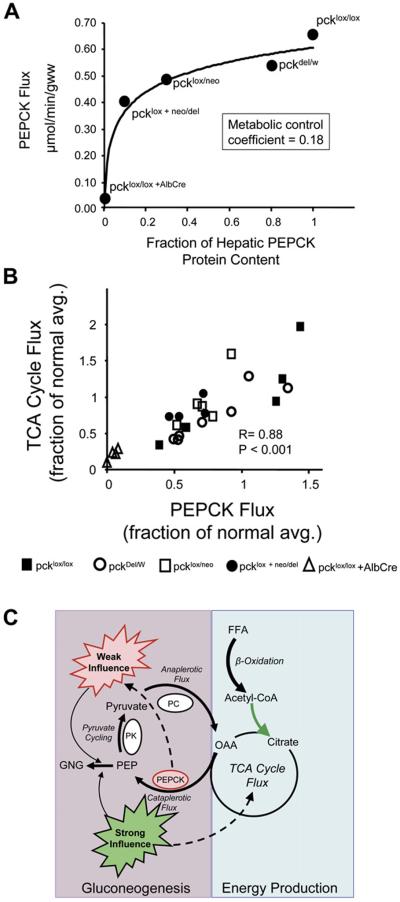
Absolute fluxes through multiple pathways contributing to glucose production were evaluated by combining NMR data with measured glucose production (Burgess et al., 2004, 2006; Hausler et al., 2006). This analysis showed that in livers with PEPCK content reduced to 30% and 10% of normal, PEPCK flux was reduced by 25% and 40%, respectively (Figure 2A). A metabolic control analysis (Fell, 1997) of these data indicated that in the intact isolated mouse liver, PEPCK has a metabolic control coefficient of 0.18 for gluconeogenesis from the level of the TCA cycle. According to metabolic control theory, a rate-controlling enzyme should have a control coefficient approaching 1, while an enzyme with no influence on flux through a pathway will have a control coefficient approaching 0 (Fell, 1997). These results indicate that liver PEPCK protein content has limited control over gluconeogenesis from the TCA cycle in the isolated perfused liver; a result that is inconsistent with the standard textbook teaching that gluconeogenesis is regulated by PEPCK expression.
Figure 2. Influence of PEPCK Protein Content and TCA Cycle Flux on PEPCK Flux in Isolated Livers.
(A) PEPCK flux versus liver PEPCK protein content. The data indicate that large changes in PEPCK content are required to modulate even small changes in flux through PEPCK. Metabolic control analysis of these data provided a control coefficient of 0.18, indicating that under these conditions, PEPCK content is not rate limiting for gluconeogenesis.
(B) Plot of PEPCK flux versus TCA cycle flux (relative to the average of pcklox/lox) for all 27 livers studied. There was a strong correlation (p < 0.001) between TCA cycle flux and PEPCK flux. These data directly link energy produced in the hepatic TCA cycle to the energy-consuming process of gluconeogenesis.
(C) Under the conditions studied here, hepatic PEPCK protein content has only a weak influence on the rate of PEPCK flux and gluconeogenesis, while the rate of energy produced by the hepatic TCA cycle is strongly correlated with the rate of hepatic PEPCK flux (and gluconeogenesis), providing a potential mechanism by which gluconeogenesis and the energy required for this process are coordinated.
Interestingly, TCA cycle flux determined from NMR data (Table 2) is linearly proportional to PEPCK flux, with a slope near 1 (Figure 2B). This observation suggests that as gluconeogenesis doubles, oxidative flux through the TCA cycle must also approximately double. Oxygen consumption also correlated with glucose production in these livers, but a doubling of glucose production required only a 30% increase in O2 consumption (Table 2). This is likely because the majority of fasting hepatic energy consumption (as reflected by O2 uptake) is provided by β-oxidation and ketogenesis (especially in the presence of octanoate) rather than terminal oxidation in the TCA cycle (McGarry and Foster, 1980). Nonetheless, the responsiveness of the TCA cycle to gluconeogenesis suggests that energy generated in the hepatic TCA cycle during fasting is closely associated with the rate of gluconeogenesis.
DISCUSSION
Early experiments on isolated hepatocytes treated with pharmacological inhibitors of PEPCK activity yielded conflicting results concerning the role of PEPCK in control of gluconeogenesis. Rognstad (1979) concluded that PEPCK is the rate-controlling enzyme for gluconeogenesis based upon inhibition of hepatocyte glucose production from lactate in the presence of the PEPCK inhibitor mercaptopicolinate. This conclusion was later disputed by Groen et al. (1982) and others, who used both inhibitor methods and elasticities to show that PEPCK was only weakly controlling in isolated hepatocytes (Argaud et al., 1991; Groen et al., 1986; Rigoulet et al., 1987). These groups concluded that, depending on hormone exposure, pyruvate carboxylase and/or pyruvate kinase were more important enzymes in controlling the rate of gluconeogenesis (Groen et al., 1986). More recently, similar results were obtained by a combination of GC/MS-based metabolomic and mass isotopomer analysis in perfused rat liver treated with mercaptopicolinate (Yang et al., 2006).
When PEPCK flux was measured as a function of PEPCK protein content in the intact isolated mouse liver, the results suggested only a weak relationship between PEPCK protein content and flux through the enzyme. One important consideration is whether gradations in PEPCK protein levels in the present study are representative of typical physiological levels in response to hormonemediated PEPCK expression. Presumably, PEPCK protein content in 24 hr-fasted control mice represents the highest normal hepatic PEPCK content. The 90% reduction of PEPCK found in the pcklox + neo/del mouse is sub-stantially more dramatic than the 50% reduction found after hyperinsulinemic clamp in mice (Sun et al., 2002), yet hyperinsulinemic clamp dramatically suppresses gluconeogenesis, while PEPCK deficiency alone only decreases it by 40%. This suggests that other factors such as peripheral substrate supply or insulin-mediated effects on other gluconeogenic enzymes and/or hepatic energy metabolism must coordinate with PEPCK expression to attenuate gluconeogenesis.
It remains unclear whether compensatory mechanisms in hepatic metabolism due to chronic PEPCK deficiency play a role in the apparent low control strength of PEPCK over hepatic gluconeogenesis or whether PEPCK might even have a different control strength in vivo. These questions linger mainly because the low control strength for PEPCK over its own flux is difficult to rationalize in light of the exquisite hormonal control of PEPCK expression and the fact that several interventions of PEPCK expression have demonstrated remarkable effects on systemic glucose metabolism in mice. A 7-fold overexpression of PEPCK results in hyperglycemia (Valera et al., 1994), while a 2-fold overexpression results in insulin resistance (Sun et al., 2002). The complexity of transcriptional regulation of gluconeogenesis was nicely illustrated in the latter study, where a point mutation in PEPCK expression also resulted in overexpression of glucose-6-phosphatase and underexpression of glucokinase and GLUT2, all conditions that naturally contribute to increased gluconeogenic flux. The opposite was illustrated by Perales and coworkers (Gomez-Valades et al., 2006), who used PEPCK RNA silencing to suppress PEPCK expression in diabetic mice, resulting in correction of hyperglycemia. Interestingly, they also noted that inhibiting PEPCK expression in diabetic mice decreased circulating fatty acids and increased circulating ketones, suggesting an interaction between PEPCK expression and hepatic energy metabolism. On the other hand, a variety of studies in mice demonstrate important modulations in hepatic glucose metabolism even in the absence of altered PEPCK expression (Burgess et al., 2006; Kersten et al., 1999) or altered PEPCK expression without significant effect on gluconeogenesis (Xu et al., 2006). This disconnection might occur because other factors, such as hepatic energy metabolism (Pryor et al., 1987), can also project substantial control over the rate of gluconeogenesis. The present data indicate that changes in PEPCK content alone may be insufficient to modulate gluconeogenesis; thus, taken in combination with prior knowledge regarding the exquisite regulation of this enzyme, it seems likely that PEPCK expression must coordinate with other mechanisms to regulate gluconeogenesis.
The finding that PEPCK flux is intimately linked to hepatic energy metabolism as well as gluconeogenesis fits with earlier observations that flux through PEPCK is necessary for normal energy generation in the hepatic TCA cycle (Burgess et al., 2004; Hakimi et al., 2005). In the complete absence of hepatic PEPCK, livers accumulate triglycerides (She et al., 2000) secondary to decreased fat oxidation in the TCA cycle (Burgess et al., 2004; Hakimi et al., 2005). Moreover, when hepatic TCA cycle activity is limited by decreased TCA cycle and electron transport chain enzyme expression, PEPCK flux and gluconeogenesis are also impaired (Burgess et al., 2006). Together, these data suggest a bidirectional feedback between cataplerosis and energy generated in the hepatic TCA cycle (Figure 2C), a mechanism which may allow appropriate coordination of energy production and anabolism, specifically gluconeogenesis. This coordination may be facilitated by PC flux since this enzyme is under allosteric control by acetyl-CoA, whose levels are responsive to the energy state of the hepatocyte via β-oxidation and oxidation in the TCA cycle. The ostensibly separate pathways of hepatic energy production and gluconeogenesis are in fact linked transcriptionally by a number of coactivators, such as PGC-1 (Lin et al., 2005), TORC (Koo et al., 2005), and the forkhead family of transcription factors (Wolfrum et al., 2004; Zhang et al., 2006), that act as “master regulators” controlling the expression of multiple enzymes of gluconeogenesis and fatty acid oxidation in parallel. In this way, the liver is well equipped to coordinate the energy-consuming pathway of gluconeogenesis with the energy-producing pathway of fat oxidation. Results presented here suggest that one mechanism by which the metabolic fluxes of gluconeogenesis and fat oxidation interact involves oxidative flux through the TCA cycle, leading to stimulation of anaplerosis, cataplerotic flux through PEPCK, and ultimately gluconeogenesis (Figure 2C).
In summary, contrary to the widely held view that PEPCK is the primary control point for gluconeogenesis, the present results show that hepatic PEPCK content alone only weakly influences gluconeogenesis. In light of the robust response of PEPCK expression to physiology, these data suggest a broader role for PEPCK, perhaps in integrating hepatic energy metabolism and gluconeogenesis. In agreement with this role, we observed that PEPCK flux correlates strongly with energy generated in the hepatic TCA cycle. We hypothesize that in mice, hormone-regulated PEPCK expression is generally controlled in parallel with hepatic energy production, and these two factors cooperate (perhaps with other factors as well) to determine the rate of gluconeogenesis. Finally, these findings demonstrate that metabolic flux is subject to regulation by the entire metabolic network and that changes in the expression, content, or activity of individual enzymes do not always predictably affect flux through the intact pathway.
EXPERIMENTAL PROCEDURES
Chemicals
[U-13C3] propionate (99%) and 2H2O (99%) were purchased from Cambridge Isotopes. Other common chemicals were purchased from Sigma unless otherwise noted.
Animals
Liver-specific PEPCK-deficient (pckdel/W, pcklox/neo, pcklox + neo/del, pcklox/lox + AlbCre) and control (pcklox/lox) mice were generated as previously described (She et al., 2000).
Liver Perfusion Experiments
All protocols were approved by the UTSWMC Institutional Animal Care and Use Committee. Livers from various strains of mice were isolated after a 24 hr fast and perfused without recirculation for 60 min as previously detailed (Burgess et al., 2004, 2006; Hausler et al., 2006). The perfusion media consisted of Krebs-Henseleit bicarbonate buffer containing 1.5 mM lactate, 0.15 mM pyruvate, 0.25 mM glycerol, 0.2 mM octanoate, 0.5 mM [U-13C3]propionate, and 3% v/v D2O. Effluent perfusate was collected for assays of glucose production and oxygen consumption as well as isolation of glucose for NMR analysis as previously described (Burgess et al., 2004, 2006; Hausler et al., 2006).
Since there are no known allosteric activators of PEPCK (Hanson and Patel, 1994), relative PEPCK protein content was taken as PEPCK activity. Livers were freeze clamped after the perfusion period, and PEPCK protein content was measured with standard western blotting techniques. Tubulin protein content was used as an internal control and was assumed to be unchanged in livers from various genotypes. Tubulin monoclonal antibody was obtained from Sigma, and PEPCK polyclonal antibody was obtained from Cayman Chemical. Four to eight samples per strain were used to determine protein levels by comparing western blot densities. PEPCK and tubulin western blots were run on the same gel, and relative PEPCK content was determined by dividing the PEPCK density by the same sample's tubulin density. The PEPCK/tubulin ratio was set to 100% for the control strain (pcklox/lox), and the other strains were normalized accordingly by dividing their PEPCK/tubulin ratio by the control ratio. Relative PEPCK protein content is reported as a percent of control (mean ± SEM).
NMR Analysis
Glucose purified from the effluent perfusion media was converted to the 1,2-diisopropylidene glucofuranose derivative (monoacetone glucose, MAG) and analyzed by NMR at 14.1 T to determine the positional enrichment of 2H and 13C as previously described (Burgess et al., 2004, 2006; Hausler et al., 2006).
Metabolic Profile
Briefly (see also Supplemental Experimental Procedures), the relative deuterium enrichments in glucose H2, H5, and H6s were determined by 2H NMR, and these values were used to determine the rates of gluconeogenesis and glycogenolysis as previously described (Burgess et al., 2003; Landau et al., 1995). Pathways intersecting the TCA cycle were evaluated by 13C isotopomer analysis of glucose C2 (or glutamate for PEPCK null livers). The 13C NMR multiplets in glucose or glutamate generated by the tracer [U-13C3]propionate were evaluated to determine flux through PEPCK and pyruvate cycling (Jones et al., 1997). Fractional glycogenolysis and gluconeogenesis measured by 2H NMR were combined with PEPCK, pyruvate cycling and gluconeogenesis relative to TCA cycle flux measured by 13C NMR, and the absolute rate of glucose production (μmol/min/g wet liver tissue) to yield the absolute fluxes through each of these pathways (Burgess et al., 2004).
Metabolic control analysis was performed as described by Fell (1997). In brief, the natural log of relative PEPCK protein content was plotted versus the natural log of PEPCK flux (for pckdel/W, pcklox/neo, pcklox + neo/del, and pcklox/lox mice), and the slope was taken as the metabolic control coefficient. This correlation gave a slope = 0.18 with r = 0.94 (data not shown).
Statistical Methods
Statistical analyses were performed using SigmaStat 3.0 (SPSS, Inc.). Differences between two groups were evaluated using unpaired t tests (means) or Mann-Whitney rank-sum tests (medians). One-way ANOVA or ANOVA based on ranks, followed by multiple pairwise comparisons, was used for multigroup comparisons. Correlations between variables were determined using the Pearson product-moment test. p < 0.05 was considered statistically significant.
ACKNOWLEDGMENTS
S.C.B. is the recipient of American Diabetes Association Junior Faculty Award 1-50-JF-05. These studies were supported in part by National Institutes of Health grants RR-02584, HL-34557, and DK59632. We are grateful to C. Storey and A. Milde for their expertise in performing the isolated liver perfusions.
Footnotes
Supplemental Data Supplemental Data include Supplemental Experimental Procedures and Supplemental References and can be found with this article online at http://www.cellmetabolism.org/cgi/content/full/5/4/313/DC1/.
REFERENCES
- Argaud D, Halimi S, Catelloni F, Leverve XM. Inhibition of gluconeogenesis in isolated rat hepatocytes after chronic treatment with phenobarbital. Biochem. J. 1991;280:663–669. doi: 10.1042/bj2800663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boden G, Chen X, Stein TP. Gluconeogenesis in mod-erately and severely hyperglycemic patients with type 2 diabetes mel-litus. Am. J. Physiol. Endocrinol. Metab. 2001;280:E23–E30. doi: 10.1152/ajpendo.2001.280.1.E23. [DOI] [PubMed] [Google Scholar]
- Burgess SC, Nuss M, Chandramouli V, Hardin DS, Rice M, Landau BR, Malloy CR, Sherry AD. Analysis of gluconeogenic pathways in vivo by distribution of 2H in plasma glucose: comparison of nuclear magnetic resonance and mass spectrometry. Anal. Biochem. 2003;318:321–324. doi: 10.1016/s0003-2697(03)00158-1. [DOI] [PubMed] [Google Scholar]
- Burgess SC, Hausler N, Merritt M, Jeffrey FMH, Storey C, Milde A, Koshy S, Lindner J, Magnuson MA, Malloy CR, Sherry AD. Impaired tricarboxylic acid cycle activity in mouse livers lacking cytosolic phosphoenolpyruvate carboxykinase. J. Biol. Chem. 2004;279:48941–48949. doi: 10.1074/jbc.M407120200. [DOI] [PubMed] [Google Scholar]
- Burgess SC, Leone TC, Wende AR, Croce MA, Chen Z, Sherry AD, Malloy CR, Finck BN. Diminished hepatic gluconeogenesis via defects in tricarboxylic acid cycle flux in peroxisome proliferator-activated receptor gamma coactivator-1alpha (PGC-1alpha)-deficient mice. J. Biol. Chem. 2006;281:19000–19008. doi: 10.1074/jbc.M600050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty K, Cassuto H, Reshef L, Hanson RW, Cox MM. Factors that control the tissue-specific transcription of the gene for phosphoenolpyruvate carboxykinase-C. Crit. Rev. Biochem. Mol. Biol. 2005;40:129–154. doi: 10.1080/10409230590935479. [DOI] [PubMed] [Google Scholar]
- Chandramouli V, Ekberg K, Schumann WC, Kalhan SC, Wahren J, Landau BR. Quantifying gluconeogenesis during fasting. Am. J. Physiol. Endocrinol. Metab. 1997;273:E1209–E1215. doi: 10.1152/ajpendo.1997.273.6.E1209. [DOI] [PubMed] [Google Scholar]
- Fell DA. Understanding the Control of Metabolism. Portland Press; London: 1997. [Google Scholar]
- Gomez-Valades AG, Vidal-Alabro A, Molas M, Boada J, Bermu-dez J, Bartrons R, Perales JC. Overcoming diabetes-induced hyperglycemia through inhibition of hepatic phosphoenolpyruvate carboxykinase (GTP) with RNAi. Mol. Ther. 2006;13:401–410. doi: 10.1016/j.ymthe.2005.08.026. [DOI] [PubMed] [Google Scholar]
- Granner D, Pilkis S. The genes of hepatic glucose metabolism. J. Biol. Chem. 1990;265:10173–10176. [PubMed] [Google Scholar]
- Groen AK, van der Meer R, Westerhoff HV, Wanders RJ, Acker-boom TPM, Tager JM. Control of metabolic fluxes. In: Seis H, editor. Metabolic Compartmentation. Academic Press; London: 1982. pp. 9–37. [Google Scholar]
- Groen AK, van Roermund CW, Vervoorn RC, Tager JM. Control of gluconeogenesis in rat liver cells. Flux control coefficients of the enzymes in the gluconeogenic pathway in the absence and presence of glucagon. Biochem. J. 1986;237:379–389. doi: 10.1042/bj2370379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakimi P, Johnson MT, Yang J, Lepage DF, Conlon RA, Kal-han SC, Reshef L, Tilghman SM, Hanson RW. Phosphoenolpyruvate carboxykinase and the critical role of cataplerosis in the control of hepatic metabolism. Nutr. Metab. (Lond.) 2005;2:33. doi: 10.1186/1743-7075-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson RW, Garber AJ. Phosphoenolpyruvate carboxykinase. I. Its role in gluconeogenesis. Am. J. Clin. Nutr. 1972;25:1010–1021. doi: 10.1093/ajcn/25.10.1010. [DOI] [PubMed] [Google Scholar]
- Hanson RW, Patel YM. Phosphoenolpyruvate carboxykinase (GTP): The gene and the enzyme. Adv. Enzymol. Relat. Areas Mol. Biol. 1994;69:203–281. doi: 10.1002/9780470123157.ch6. [DOI] [PubMed] [Google Scholar]
- Hanson RW, Reshef L. Regulation of phosphoenolpyruvate carboxykinase (GTP) gene expression. Annu. Rev. Biochem. 1997;66:581–611. doi: 10.1146/annurev.biochem.66.1.581. [DOI] [PubMed] [Google Scholar]
- Hausler N, Browning J, Merritt M, Storey C, Milde A, Jeffrey FM, Sherry AD, Malloy CR, Burgess SC. Effects of insulin and cytosolic redox state on glucose production pathways in the isolated perfused mouse liver measured by integrated 2H and 13C NMR. Biochem. J. 2006;394:465–473. doi: 10.1042/BJ20051174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JG, Naidoo R, Sherry AD, Jeffrey FMH, Cottam GL, Malloy CR. Measurement of gluconeogensis and pyruvate recycling in the rat liver: a simple analysis of glucose and glutamate isotopomers during metabolism of [1,2,3-13C3]propionate. FEBS Lett. 1997;412:131–137. doi: 10.1016/s0014-5793(97)00764-3. [DOI] [PubMed] [Google Scholar]
- Kersten S, Seydoux J, Peters JM, Gonzalez FJ, Desvergne B, Wahli W. Peroxisome proliferator-activated receptor alpha mediates the adaptive response to fasting. J. Clin. Invest. 1999;103:1489–1498. doi: 10.1172/JCI6223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo S-H, Flechner L, Qi L, Zhang X, Screaton RA, Jeffries S, Hedrick S, Xu W, Boussouar F, Brindle P, et al. The CREB coactivator TORC2 is a key regulator of fasting glucose metabolism. Nature. 2005;437:1109–1111. doi: 10.1038/nature03967. [DOI] [PubMed] [Google Scholar]
- Landau BR, Wahren J, Chandramouli V, Schumann WC, Ek-berg K, Kalhan SC. Use of 2H2O for estimating rates of gluconeogenesis. Application to the fasted state. J. Clin. Invest. 1995;95:172–178. doi: 10.1172/JCI117635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy P, Crawford DR, Grossman G, Gronostajski RM, Hanson RW. CREB binding protein coordinates the function of multiple transcription factors including nuclear factor i to regulate phosphoenolpyruvate carboxykinase (GTP) gene transcription. J. Biol. Chem. 1999;274:8813–8822. doi: 10.1074/jbc.274.13.8813. [DOI] [PubMed] [Google Scholar]
- Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Magnuson MA, She P, Shiota M. Gene-altered mice and metabolic flux Control. J. Biol. Chem. 2003;278:32485–32488. doi: 10.1074/jbc.R300020200. [DOI] [PubMed] [Google Scholar]
- McGarry JD, Foster DW. Regulation of hepatic fatty acid oxidation and ketone body production. Annu. Rev. Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Price TB, Bergeron R. Regulation of net hepatic glycogenolysis and gluconeogenesis during exercise: impact of type 1 diabetes. J. Clin. Endocrinol. Metab. 2004;89:4656–4664. doi: 10.1210/jc.2004-0408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilkis SJ, Granner DK. Molecular physiology of the regulation of hepatic gluconeogenesis and glycolysis. Annu. Rev. Physiol. 1992;54:885–909. doi: 10.1146/annurev.ph.54.030192.004321. [DOI] [PubMed] [Google Scholar]
- Pryor HJ, Smyth JE, Quinlan PT, Halestrap AP. Evidence that the flux control coefficient of the respiratory chain is high during gluconeogenesis from lactate in hepatocytes from starved rats. Implications for the hormonal control of gluconeogenesis and action of hypoglycaemic agents. Biochem. J. 1987;247:449–457. doi: 10.1042/bj2470449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigoulet M, Leverve XM, Plomp PJ, Meijer AJ. Stimulation by glucose of gluconeogenesis in hepatocytes isolated from starved rats. Biochem. J. 1987;245:661–668. doi: 10.1042/bj2450661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesler W, Vandenbark G, Hanson R. Identification of multiple protein binding domains in the promoter-regulatory region of the phosphoenolpyruvate carboxykinase (GTP) gene. J. Biol. Chem. 1989;264:9657–9664. [PubMed] [Google Scholar]
- Rognstad R. Rate-limiting steps in metabolic pathways. J. Biol. Chem. 1979;254:1875–1878. [PubMed] [Google Scholar]
- Schmoll D, Walker KS, Alessi DR, Grempler R, Burchell A, Guo S, Walther R, Unterman TG. Regulation of glucose-6-phosphatase gene expression by protein kinase Balpha and the forkhead transcription factor FKHR. J. Biol. Chem. 2000;275:36324–36333. doi: 10.1074/jbc.M003616200. [DOI] [PubMed] [Google Scholar]
- She P, Shiota M, Shelton KD, Chalkley R, Postic C, Magnu-son MA. Phosphoenolpyruvate carboxykinase is necessary for the integration of hepatic energy metabolism. Mol. Cell. Biol. 2000;20:6508–6517. doi: 10.1128/mcb.20.17.6508-6517.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Liu S, Ferguson S, Wang L, Klepcyk P, Yun JS, Friedman JE. Phosphoenolpyruvate carboxykinase overex-pression selectively attenuates insulin signaling and hepatic insulin sensitivity in transgenic mice. J. Biol. Chem. 2002;277:23301–23307. doi: 10.1074/jbc.M200964200. [DOI] [PubMed] [Google Scholar]
- Valera A, Pujol A, Pelegrin M, Bosch F. Transgenic mice overexpressing phosphoenolpyruvate carboxykinase develop non-insulin-dependent diabetes mellitus. Proc. Natl. Acad. Sci. USA. 1994;91:9151–9154. doi: 10.1073/pnas.91.19.9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veneziale C, Donofrio J, Nishimura H. The concentration of P-enolpyruvate carboxykinase protein in murine tissues in diabetes of chemical and genetic origin. J. Biol. Chem. 1983;258:14257–14262. [PubMed] [Google Scholar]
- Wajngot A, Chandramouli V, Schumann WC, Ekberg K, Jones PK, Efendic S, Landau BR. Quantitative contributions of gluconeogenesis to glucose production during fasting in type 2 diabetes mellitus. Metabolism. 2001;50:47–52. doi: 10.1053/meta.2001.19422. [DOI] [PubMed] [Google Scholar]
- Wolfrum C, Asilmaz E, Luca E, Friedman JM, Stoffel M. Foxa2 regulates lipid metabolism and ketogenesis in the liver during fasting and in diabetes. Nature. 2004;432:1027–1032. doi: 10.1038/nature03047. [DOI] [PubMed] [Google Scholar]
- Xu J, Gowen L, Raphalides C, Hoyer KK, Weinger JG, Renard M, Troke JJ, Vaitheesyaran B, Lee WNP, Saad MF, et al. Decreased hepatic futile cycling compensates for increased glucose disposal in the pten heterodeficient mouse. Diabetes. 2006;55:3372–3380. doi: 10.2337/db06-0002. [DOI] [PubMed] [Google Scholar]
- Yang L, Kasumov T, Yu L, Jobbins KA, David F, Previs SF, Kelleher JK, Brunengraber H. Metabolomic assays of the concentration and mass isotopomer distribution of gluconeogenic and citric acid cycle intermediates. Metabolomics. 2006;2:85–94. [Google Scholar]
- Zhang W, Patil S, Chauhan B, Guo S, Powell DR, Le J, Klotsas A, Matika R, Xiao X, Franks R, et al. FoxO1 regulates multiple metabolic pathways in the liver. J. Biol. Chem. 2006;281:10105–10117. doi: 10.1074/jbc.M600272200. [DOI] [PubMed] [Google Scholar]