Abstract
Background
Many older patients who recover from an episode of major depression continue to suffer from depressed mood, anxiety, and sleep problems. Our study assesses the impact of these residual symptoms on the risk of recurrence during maintenance treatment of late-life depression.
Method
We analyzed data from a randomized clinical trial of maintenance treatment in patients with unipolar depression aged ≥70, 116 of whom remitted and remained stable during open pharmacotherapy and interpersonal psychotherapy (IPT) and were randomized to clinical management/pharmacotherapy; clinical management/placebo; monthly maintenance IPT/pharmacotherapy; or monthly maintenance IPT/placebo. We assessed the impact of overall residual symptoms (based on the Hamilton Depression Rating Scale (HAM-D) total score) and of specific residual symptom clusters – mood symptoms (depressed mood, guilt, suicidality, energy/interests), sleep disturbance (early, middle, late insomnia), and anxiety (agitation, psychic and somatic anxiety, hypochondriasis) measured at randomization. Sleep disturbance was also assessed with the Pittsburgh Sleep Quality Index (PSQI). We used Cox proportional hazards regression models controlling for antidepressant medication versus placebo to identify predictors of recurrence.
Results
Residual anxiety and residual sleep disturbance (as measured by the PSQI but not the HAM-D) independently predicted early recurrence.
Limitations
Use of HAM-D clusters to define residual symptoms; analysis limited to completers of acute and continuation treatment.
Conclusions
In patients with late-life depression who have remitted with pharmacotherapy and psychotherapy, the deleterious effect of residual symptoms is due to persisting anxiety and, possibly, residual sleep disturbance.
Keywords: depression, geriatric, aged, symptoms, residual symptoms, sleep, anxiety, mood, recurrence
INTRODUCTION
Many patients whose major depressive episode has remitted continue to suffer from some symptoms of depression. In longitudinal studies in mixed-age samples, the presence of these residual symptoms consistently predicts increased risk of recurrence (Judd et al., 1998; Kanai et al., 2003; Karp et al., 2004; Paykel et al., 1995; Ramana et al., 1995; Van Londen et al., 1998). However, little is known about which particular residual symptoms impact long-term outcmes in late-life depression. Persisting anxiety has been associated with higher recurrence rates in older patients treated with nortriptyline (Flint and Rifat, 1997), or untreated (Meyers et al., 1996). Similarly, residual sleep disturbance (poorer subjective sleep quality and higher REM density) has been shown to predict recurrence during maintenance treatment of late-life depression (Buysse et al., 1996). However, to our knowledge, no study has assessed the relative impact of several groups of residual symptoms. Furthermore, the role of persisting anxiety and sleep disturbance has not been evaluated in patients treated with selective serotonin reuptake inhibitors (SSRIs), now the mainstay of late-life depression treatment.
Thus, we analyzed prospectively gathered data from a randomized clinical trial of maintenance paroxetine and interpersonal therapy (IPT) in late-life depression. We assessed the impact of overall burden of residual symptoms and of specific symptom clusters – core mood symptoms, sleep disturbance, and anxiety symptoms – on recurrence during two-year maintenance treatment. We hypothesized that participants with higher overall levels of residual symptoms, and persisting anxiety and sleep disturbance in particular, would be more likely to suffer a recurrence.
METHOD
Between 1999 and 2003, the Maintenance Treatment for Late-Life Depression-2 (MTLD-2) (Reynolds et al., 2006) study recruited patients aged ≥70 with current, non-psychotic, non-bipolar major depression, recurrent or single episode, diagnosed by SCID/DSM-IV criteria. Study participants were required to have a score of 15 or higher on the 17-item Hamilton Depression Rating Scale (HAM-D) (Hamilton, 1960), and a score of 17 or higher on the Folstein Mini-Mental State Exam (MMSE) (Folstein et al., 1975). All participants provided written informed consent.
Treatment, participant flow and characteristics
As described in detail elsewhere (Reynolds et al., 2006), 195 participants received acute open pharmacological treatment and weekly IPT; 151 achieved sustained response as defined by a score of 10 or less on the Hamilton scale for 3 consecutive weeks and received 16 weeks of continuation pharmacotherapy and IPT every other week; 116 participants remained stable during continuation and were randomized to two-year maintenance with [1] clinical management/pharmacotherapy; [2] clinical management/placebo; [3] monthly maintenance IPT/pharmacotherapy; or [4] monthly maintenance IPT/placebo. Of the 116 participants randomized to maintenance treatment, 65% were women, 93% were Caucasian; mean (SD) education was 12.9 (2.9) years, 41% suffered from recurrent depression and 26% had a lifetime diagnosis of one or more anxiety disorders (including Agoraphobia Without History of Panic Disorder, Anxiety Disorder NOS, Generalized Anxiety Disorder, Obsessive-Compulsive Disorder, Panic Disorder With Agoraphobia, Panic Disorder Without Agoraphobia, Post-Traumatic Stress Disorder, and Social Phobia). The mean HAM-D score at randomization was 20.1 (3.3), and the mean MMSE score was 27.9 (2.5). As described elsewhere (Whyte et al., 2004), all patients had received acute pharmacotherapy with paroxetine (median final dose: 30 mg/day); 69 had also received augmentation with nortriptyline, bupropion, or lithium. Participants randomly assigned to maintenance pharmacotherapy received the medications at doses associated with sustained response. Participants randomly assigned to placebo had their medications slowly tapered.
Measures of outcome and residual symptoms
Patients were assessed monthly and all assessments included the HAM-D. Recurrence was defined as a HAM-D score of ≥15 and meeting DSM-IV criteria for a major depressive episode during a SCID interview, confirmed in an interview with a geriatric psychiatrist. Assessors were blind to treatment assignment. We measured residual symptoms at randomization, after participants completed acute and continuation treatment. Similarly to other recent studies of residual symptoms (Nelson et al., 2005), we used subscales derived from the HAM-D to measure core mood symptoms (depressed mood, guilt, suicidality, energy/interests), sleep disturbance (early, middle, late insomnia), and anxiety (agitation, psychic and somatic anxiety, hypochondriasis). This grouping is consistent with a recent meta-analysis of the HAM-D factor structure (Shafer, 2006). As we found previously, HAM-D factors, from which these subscales were derived, explain s imilar proportions of overall symptom variability; the subscales possess high interrater reliability and moderate internal consistency (Dombrovski et al., 2006). These subscales were only weakly inter-correlated; thus, we examined them as independent variables. In addition to the HAM-D subscales, sleep disturbance was also assessed by the Pittsburgh Sleep Quality Index (Buysse et al., 1989) at randomization.
Data analysis
We included data on all 116 randomized participants. We used Cox proportional hazards regression models controlling for antidepressant medication to examine the relationship of residual symptoms level (HAM-D total score at the time of randomization) and residual core mood, sleep disturbance, and anxiety symptoms (HAM-D subscales and PSQI score at the time of randomization) to subsequent recurrence. Drug assignment was dummy-coded. We assessed for the interaction of these selected predictors with drug assignment, which if present would indicate moderation of drug effect. Next, we tested whether identified predictors would remain significant after accounting for other known predictors of recurrence using a multiple Cox proportional hazards regression model. In the present study, these included subjective sleep quality measured by PSQI and medical burden measured by CIRS-G (Reynolds et al., 2006).
RESULTS
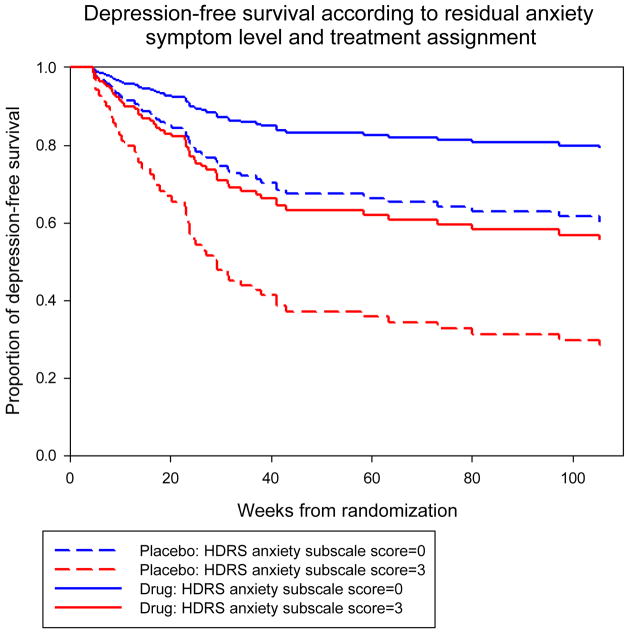
Total burden of residual symptoms (hazard ratio [HR]: 1.15, confidence interval [CI]: 1.04–1.28, p=0. 009) and persisting anxiety (HR: 1.36, CI: 1.09–1.69, p=0.007) predicted recurrence. Sleep disturbance measured by the PSQI (HR: 1.11, CI: 1.03–1.20, p=0.01) but not the HAM-D subscale (HR: 1.26, CI: 0.98–1.64, p=0.08) was also a significant predictor, while the level of core mood symptoms was not (HR: 1.12, CI: 0.92–1.38, p=0.26). Survival curves for patients with high and low residual anxiety are presented in Figure 1. As predicted by the corrected group prognosis method (Ghali et al., 2001), in the placebo group the probability of two-year depression-free survival was 60% for a patient with a score of 0 on the HAM-D anxiety subscale at randomization (no residual anxiety symptoms) and 28%, for a patient with a score of 3 (significant residual anxiety symptoms). In the pharmacotherapy group, it was 79% and 56%, respectively. We did not detect any moderation effects. Residual anxiety (HR: 1.386, CI: 1.096–1.754, p=0.0065) and PSQI residual sleep disturbance (HR: 1.091, CI: 1.007–1.181, p=0.0422) remained significant predictors in a multiple Cox regression model, controlling for assignment to paroxetine or placebo and pre-treatment medical burden – the only other predictors of recurrence identified in our study.
Figure 1.
Survival curves generated from the Cox model using the group prognostic method with values of 0 and 1 for placebo and drug respectively, and Hamilton Depression Rating Scale anxiety subscale scores of 0 (no residual anxiety) and 3 (significant residual anxiety).
Having identified residual anxiety as an independent predictor of recurrence, we examined in a post-hoc analysis its relationship with patients’ lifetime anxiety disorder history. Of the 116 patients, 30 (26%) met DSM-IV criteria for one or more lifetime anxiety disorders at baseline. Participants with a lifetime anxiety disorder diagnosis had more residual anxiety symptoms than those without anxiety disorder history (mean [SD] HAM-D anxiety: 2.50 versus 1.97, T=2.03, df=114, p=0.045); no such difference was seen for PSQI sleep disturbance (p=0.62). Our SCID assessment did not include lifetime sleep disorders.
DISCUSSION
We found that in older depressed patients a higher level of residual anxiety and possibly residual sleep disturbance predicted shorter time to recurrence on SSRI treatment and on placebo. Not surprisingly, residual anxiety was more prominent in patients with a lifetime anxiety disorder history. Thus, in agreement with previously published findings (Flint and Rifat, 1997; Lenze et al., 2001), persisting anxiety emerges as a robust predictor of poorer long-term outcomes in late-life depression, and may represent the “active ingredient” of residual symptoms in depression.
To our knowledge, this is the first report from a randomized double-blind placebo-controlled maintenance trial on the effect of several groups of residual symptoms on long-term outcomes. However, selecting patients who benefited the most from acute and continuation treatment may underestimate the level and impact of risk factors such as residual anxiety since those who did not remit or dropped out before randomization may be more prone to adverse outcomes. The use of HAM-D subscales to measure residual symptoms is another potential limitation of our study, however, a recent meta-analysis of the HAM-D factor structure (Shafer, 2006) suggests a very similar factor solution, and earlier studies strongly support the existence of an anxiety factor (Bagby et al., 2004; Cole et al., 2004). Finally, our multiple regression model did not include pre-treatment self-rated anxiety, which also predicted early recurrence in this trial (Andreescu et al., (in press); Reynolds et al., 2006), to avoid co-linearity with residual anxiety.
It is noteworthy that the burden of anxiety symptoms, and not of core mood symptoms, predicts recurrence of a major depressive episode. Flint and Rifat suggested incomplete resolution of depressive illness as the explanation for the high risk of recurrence in patients with persistent anxiety (Flint and Rifat, 1997). This hypothesis, however, fails to explain the selective nature of anxiety as a risk factor as opposed to other depressive symptoms. Alternatively, anxiety persisting after remission of the depressive episode may reflect Cluster C personality features (Morse et al., 2005). A related hypothesis states that residual symptoms in depression represent co-occurring anxiety disorders (Boulenger, 2004). Our finding of higher residual anxiety in patients with a lifetime history of anxiety disorder seems to indicate that these symptoms may capture a chronic aspect of anxiety disorders that does not respond completely to pharmacotherapy and interpersonal therapy, increasing the risk of further depressive episodes.
While residual sleep disturbance measured by the PSQI reliably predicted depressive recurrence, the HAM-D sleep subscale was not a significant predictor (p=0.08). The difference is probably due a more comprehensive assessment of sleep quality by the PSQI, with items rating daytime effects of insomnia and problems that interfere with sleep (Buysse et al., 1989). Our results replicate previous findings of association between poor sleep quality and depressive recurrence (Buysse et al., 1996). Thus, we conclude that in older depressed patients clinicians need to assess broader sleep quality and not simply insomnia. Would a more aggressive treatment of sleep problems make a difference? Sedating antidepressants such as nefazodone (Manber et al., 2003) are known to impact disturbed sleep in depressed patients beyond the improvements associated with recovery from depression. It is conceivable that augmentation with nefazodone or trazodone, would help those patients with persisting sleep disturbance who can tolerate it.
We do not know whether increasing the SSRI dose in patients with persisting anxiety, as the Expert Consensus Guidelines for the Treatment of Geriatric Depression recommend (Alexopoulos et al., 2001), would have been beneficial. Future studies need to test pharmacological approaches beyond higher-dose SSRIs, including augmentation with GABA-ergic agents that have some controlled data to support their efficacy in anxiety disorders, such as clonazepam (Davidson and Moroz, 1998) or anticonvulsants like gabapentin (Pande et al., 1999; Pande et al., 2000), pregabalin (Rickels et al., 2005), and lamotrigine (Hertzberg et al., 1999).
On the other hand, augmentation with anxiety-specific psychotherapy may have more lasting effects and be better tolerated in older patients. Interpersonal therapy was not successful in preventing recurrence of depression in MTLD-2 (Reynolds et al., 2006). Anxiety and panic symptoms in particular have been associated with poorer response to interpersonal therapy in midlife depressed patients (Frank et al., 2000), with lower levels of stress sensitivity representing an independent predictor of poor response. Anxious patients may feel particularly overwhelmed by interpersonal stress and may avoid problems instead of actively tackling them. Thus, co-occurring anxiety and avoidance behaviors may interfere with the active resolution of interpersonal problems as prescribed by interpersonal psychotherapy. Adaptations of psychotherapy that incorporate anxiety-specific psychoeducation and behavioral interventions, such as interpersonal psychotherapy for depression with panic spectrum symptoms (Cyranowski et al., 2005) hold promise. Cognitive behavioral therapy for older patients with anxiety syndromes (Wetherell et al., 2003; Wetherell et al., 2005) provides an alternative approach.
In summary, we found that older patients recovered from depression with psychotherapy and pharmacotherapy are more likely to suffer another depressive episode if they have persisting anxiety symptoms and poor subjective sleep quality.
Acknowledgments
Supported by: grants P30MH52247, P30MH071944, R37MH43832, R01MH37869, R25MH60473, K24MH069430, K23MH070471, and the John A. Hartford Foundation. GlaxoSmithKline Inc. provided paroxetine for the study.
Footnotes
DECLARATION OF INTEREST
Alexandre Y. Dombrovski, Mary Amanda Dew, and Carmen Andreescu do not have any potential conflict to acknowledge. Eric J. Lenze has received grant support from Forest Laboratories, Pfizer Inc., and Johnson & Johnson Co. Benoit Mulsant has received honoraria and/or research support from Bristol-Myers Squibb, Eli Lilly, Forest Laboratories, Glaxo SmithKline, Lundbeck, and Pfizer. Bruce G. Pollock has received honoraria and/or research support from Janssen Pharmaceutica, Forest Laboratories, GlaxoSmithKline, and Solvay and is on the speakers’ bureau for Forest Pharmaceuticals and Sepracor. Charles F. Reynolds III has received research support from GlaxoSmithKline, Pfizer Inc., Eli Lilly and Co., Bristol Meyers Squibb, and Forest Pharmaceuticals.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexopoulos GS, Katz IR, Reynolds CF, 3rd, Carpenter D, Docherty JP, Ross RW. Pharmacotherapy of depression in older patients: a summary of the expert consensus guidelines. J Psychiatr Pract. 2001;7:361–376. doi: 10.1097/00131746-200111000-00003. [DOI] [PubMed] [Google Scholar]
- Andreescu C, Lenze E, Dew MA, Begley A, Mulsant BH, Dombrovski AY, Pollock BG, Stack J, Miller MD, Reynolds CF. Comorbid anxiety delays treatment response and increases relapse risk in late-life depression: a controlled study. Br J Psychiatry. doi: 10.1192/bjp.bp.106.027169. (in press) [DOI] [PubMed] [Google Scholar]
- Bagby RM, Ryder AG, Schuller DR, Marshall MB. The Hamilton Depression Rating Scale: has the gold standard become a lead weight? American Journal of Psychiatry. 2004;161:2163–2177. doi: 10.1176/appi.ajp.161.12.2163. [DOI] [PubMed] [Google Scholar]
- Boulenger JP. Residual symptoms of depression: clinical and theoretical implications. Eur Psychiatry. 2004;19:209–213. doi: 10.1016/j.eurpsy.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Hoch CC, Houck PR, Kupfer DJ, Mazumdar S, Frank E. Longitudinal effects of nortriptyline on EEG sleep and the likelihood of recurrence in elderly depressed patients. Neuropsychopharmacology. 1996;14:243–252. doi: 10.1016/0893-133X(95)00114-S. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cole JC, Motivala SJ, Dang J, Lucko A, Lang N, Levin MJ, Oxman MN, Irwin MR. Structural Validation of the Hamilton Depression Rating Scale. Journal of Psychopathology and Behavioral Assessment. 2004;26:241–254. [Google Scholar]
- Cyranowski JM, Frank E, Shear MK, Swartz H, Fagiolini A, Scott J, Kupfer DJ. Interpersonal psychotherapy for depression with panic spectrum symptoms: a pilot study. Depress Anxiety. 2005;21:140–142. doi: 10.1002/da.20069. [DOI] [PubMed] [Google Scholar]
- Davidson JR, Moroz G. Pivotal studies of clonazepam in panic disorder. Psychopharmacol Bull. 1998;34:169–174. [PubMed] [Google Scholar]
- Dombrovski AY, Blakesley-Ball RE, Mulsant BH, Mazumdar S, Houck PR, Szanto K, Reynolds CF. Speed of improvement in sleep disturbance and anxiety compared to core mood symptoms during acute treatment of depression in old age. American Journal of Geriatric Psychiatry. 2006;14:550–554. doi: 10.1097/01.JGP.0000218325.76196.d1. [DOI] [PubMed] [Google Scholar]
- Flint AJ, Rifat SL. Two-year outcome of elderly patients with anxious depression. Psychiatry Res. 1997;66:23–31. doi: 10.1016/s0165-1781(96)02964-2. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Frank E, Shear MK, Rucci P, Cyranowski JM, Endicott J, Fagiolini A, Grochocinski VJ, Houck P, Kupfer DJ, Maser JD, Cassano GB. Influence of panic-agoraphobic spectrum symptoms on treatment response in patients with recurrent major depression. Am J Psychiatry. 2000;157:1101–1107. doi: 10.1176/appi.ajp.157.7.1101. [DOI] [PubMed] [Google Scholar]
- Ghali WA, Quan H, Brant R, van Melle G, Norris CM, Faris PD, Galbraith PD, Knudtson ML. Comparison of 2 methods for calculating adjusted survival curves from proportional hazards models. Jama. 2001;286:1494–1497. doi: 10.1001/jama.286.12.1494. [DOI] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertzberg MA, Butterfield MI, Feldman ME, Beckham JC, Sutherland SM, Connor KM, Davidson JR. A preliminary study of lamotrigine for the treatment of posttraumatic stress disorder. Biol Psychiatry. 1999;45:1226–1229. doi: 10.1016/s0006-3223(99)00011-6. [DOI] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, Paulus MP, Kunovac JL, Leon AC, Mueller TI, Rice JA, Keller MB. Major depressive disorder: a prospective study of residual subthreshold depressive symptoms as predictor of rapid relapse. J Affect Disord. 1998;50:97–108. doi: 10.1016/s0165-0327(98)00138-4. [DOI] [PubMed] [Google Scholar]
- Kanai T, Takeuchi H, Furukawa TA, Yoshimura R, Imaizumi T, Kitamura T, Takahashi K. Time to recurrence after recovery from major depressive episodes and its predictors. Psychol Med. 2003;33:839–845. doi: 10.1017/s0033291703007827. [DOI] [PubMed] [Google Scholar]
- Karp JF, Buysse DJ, Houck PR, Cherry C, Kupfer DJ, Frank E. Relationship of variability in residual symptoms with recurrence of major depressive disorder during maintenance treatment. Am J Psychiatry. 2004;161:1877–1884. doi: 10.1176/ajp.161.10.1877. [DOI] [PubMed] [Google Scholar]
- Lenze EJ, Mulsant BH, Shear MK, Alexopoulos GS, Frank E, Reynolds CF., 3rd Comorbidity of depression and anxiety disorders in later life. Depress Anxiety. 2001;14:86–93. doi: 10.1002/da.1050. [DOI] [PubMed] [Google Scholar]
- Manber R, Rush AJ, Thase ME, Amow B, Klein D, Trivedi MH, Korenstein SG, Markowitz JC, Dunner DL, Munsaka M, Borian FE, Martin Keller B. The effects of psychotherapy, nefazodone, and their combination on subjective assessment of disturbed sleep in chronic depression. Sleep. 2003;26:130–136. doi: 10.1093/sleep/26.2.130. [DOI] [PubMed] [Google Scholar]
- Meyers BS, Gabriele MS, Kakuma T, Ippolito L, Alexopolous G. Anxiety and Depression as Predictors of Recurrence in Geriatric Depression: A Preliminary Report. American Journal of Geriatric Psychiatry. 1996;4:252–257. doi: 10.1097/00019442-199622430-00009. [DOI] [PubMed] [Google Scholar]
- Morse JQ, Pilkonis PA, Houck PR, Frank E, Reynolds CF., 3rd Impact of cluster C personality disorders on outcomes of acute and maintenance treatment in late-life depression. Am J Geriatr Psychiatry. 2005;13:808–814. doi: 10.1176/appi.ajgp.13.9.808. [DOI] [PubMed] [Google Scholar]
- Nelson JC, Portera L, Leon AC. Residual symptoms in depressed patients after treatment with fluoxetine or reboxetine. J Clin Psychiatry. 2005;66:1409–1414. doi: 10.4088/jcp.v66n1110. [DOI] [PubMed] [Google Scholar]
- Pande AC, Davidson JR, Jefferson JW, Janney CA, Katzelnick DJ, Weisler RH, Greist JH, Sutherland SM. Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol. 1999;19:341–348. doi: 10.1097/00004714-199908000-00010. [DOI] [PubMed] [Google Scholar]
- Pande AC, Pollack MH, Crockatt J, Greiner M, Chouinard G, Lydiard RB, Taylor CB, Dager SR, Shiovitz T. Placebo-controlled study of gabapentin treatment of panic disorder. J Clin Psychopharmacol. 2000;20:467–471. doi: 10.1097/00004714-200008000-00011. [DOI] [PubMed] [Google Scholar]
- Paykel ES, Ramana R, Cooper Z, Hayhurst H, Kerr J, Barocka A. Residual symptoms after partial remission: an important outcome in depression. Psychol Med. 1995;25:1171–1180. doi: 10.1017/s0033291700033146. [DOI] [PubMed] [Google Scholar]
- Ramana R, Paykel ES, Cooper Z, Hayhurst H, Saxty M, Surtees PG. Remission and relapse in major depression: a two-year prospective follow-up study. Psychol Med. 1995;25:1161–1170. doi: 10.1017/s0033291700033134. [DOI] [PubMed] [Google Scholar]
- Reynolds CF, 3rd, Dew MA, Pollock BG, Mulsant BH, Frank E, Miller MD, Houck PR, Mazumdar S, Butters MA, Stack JA, Schlernitzauer MA, Whyte EM, Gildengers A, Karp J, Lenze E, Szanto K, Bensasi S, Kupfer DJ. Maintenance treatment of major depression in old age. N Engl J Med. 2006;354:1130–1138. doi: 10.1056/NEJMoa052619. [DOI] [PubMed] [Google Scholar]
- Rickels K, Pollack MH, Feltner DE, Lydiard RB, Zimbroff DL, Bielski RJ, Tobias K, Brock JD, Zornberg GL, Pande AC. Pregabalin for treatment of generalized anxiety disorder: a 4-week, multicenter, double-blind, placebo-controlled trial of pregabalin and alprazolam. Arch Gen Psychiatry. 2005;62:1022–1030. doi: 10.1001/archpsyc.62.9.1022. [DOI] [PubMed] [Google Scholar]
- Shafer AB. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. J Clin Psychol. 2006;62:123–146. doi: 10.1002/jclp.20213. [DOI] [PubMed] [Google Scholar]
- Van Londen L, Molenaar RP, Goekoop JG, Zwinderman AH, Rooijmans HG. Three- to 5-year prospective follow-up of outcome in major depression. Psychol Med. 1998;28:731–735. doi: 10.1017/s0033291797006466. [DOI] [PubMed] [Google Scholar]
- Wetherell JL, Gatz M, Craske MG. Treatment of generalized anxiety disorder in older adults. J Consult Clin Psychol. 2003;71:31–40. doi: 10.1037//0022-006x.71.1.31. [DOI] [PubMed] [Google Scholar]
- Wetherell JL, Sorrell JT, Thorp SR, Patterson TL. Psychological interventions for late-life anxiety: a review and early lessons from the CALM study. J Geriatr Psychiatry Neurol. 2005;18:72–82. doi: 10.1177/0891988705276058. [DOI] [PubMed] [Google Scholar]
- Whyte EM, Basinski J, Farhi P, Dew MA, Begley A, Mulsant BH, Reynolds CF. Geriatric depression treatment in nonresponders to selective serotonin reuptake inhibitors. J Clin Psychiatry. 2004;65:1634–1641. doi: 10.4088/jcp.v65n1208. [DOI] [PubMed] [Google Scholar]