Abstract
Reelin, an extracellular protein that signals through the Dab1 adapter protein, and Lis1 regulate neuronal migration and cellular layer formation in the brain. Loss of Reelin and reduction in Lis1 activity in mice or humans results in the disorganization of cortical structures. Lis1, the product of the Pafah1b1 gene associates with Alpha1 (the product of the Pafah1b3 gene) and Alpha2 (the product of the Pafah1b2 gene) to form the Pafah1b heterotrimeric complex. This complex interacts biochemically and genetically with the Reelin pathway, however, the role of Alpha1 and Alpha2 in brain development is poorly understood. We previously demonstrated that compound mutations of Pafah1b1 with genes in Reelin pathway result in layering defects and the appearance of hydrocephalus in double mutant mice. Here we generate triple mouse mutants to investigate the effect of individual Pafah1b Alpha subunits on cellular layer formation and hydrocephalus. We found that Pafah1b3 mutations exacerbate the layering defects, whereas Pafah1b2 mutations strongly suppress the hydrocephalus phenotype of compound mutant mice. The data indicate that the two Pafah1b Alpha subunits have profoundly different effects on brain development and interact in a significantly different manner with the Reelin signaling pathway.
Keywords: reeler, neuronal migration, Disabled-1, lis1, platelet activating factor acetylhydrolase, neocortex
INTRODUCTION
The platelet activating factor (PAF) acetyl hydrolase 1b (Pafah1b) complex is an enzyme composed of two Alpha subunits that catalyze PAF hydrolysis and a non-catalytic Beta regulatory subunit [14]. The catalytic subunits Alpha1 (encoded by the Pafah1b3 gene) and Alpha2 (encoded by the Pafah1b2 gene) are highly related, whereas the regulatory subunit Lis1 (encoded by the Pafah1b1 gene) is distinct [12]. Pafah1b inactivates PAF, a lipid involved in a variety of physiological processes [5], however its physiological role in brain development is not well understood. Pafah1b structurally resembles G protein signaling complexes raising the possibility that it may function independently of its enzymatic activity [17]. The regulatory subunits Lis1 is clearly implicated in brain development. Contiguous gene deletions including the human PAFAH1B1 gene result in Miller-Dieker syndrome, whereas heterozygous mutations involving only PAFAH1B1 result in type 1 lissencephaly, a disease characterized by impaired neuronal migration and reduction in the number of cortical gyri [13, 25]. Homozygous mutations in the mouse Pafah1b1 gene are embryonic lethal, whereas heterozygous or compound hypomorph/null mutations cause neuronal migration defects [16]. The migration defects in Pafah1b1 mutants may be mediated through interactions with proteins other than the Pafah1b subunits. Indeed, Lis1 is known to interact with the microtubule-associated cytoplasmic dynein/dynactin-motor complex [11, 26] and with Dab1 [3], an essential transducer of the Reelin signal that is crucially involved in the control of neuronal migration [8].
Homozygous mutations in Reln, the gene encoding Reelin, in reeler mutant mice results in the disruption of cellular layers of the brain. Reelin [10] is an extracellular protein that binds to two receptors, VLDLR and ApoER2 [9, 15]. In the presence of Reelin, these receptors cluster [27] and activate src-family kinases, which in turn phosphorylate Dab1 on specific tyrosine residues [2, 6, 19, 20]. PhosphoDab1 then binds a variety of intracellular proteins involved in cytoskeletal dynamics [4, 7, 24], including Lis1 [3], and ultimately results in the formation of brain cellular layers. Heterozygous mutations in Pafah1b1, compound with mutations of genes in the Reelin pathway, result in further migration defects and a high frequency of hydrocephalus [3]. These results suggested that Reelin and Lis1 cooperate in the control of unknown factors that influence hydrocephalus development, possibly affecting brain structure organization, ependymal layer formation, or the production or circulation of the cerebrospinal fluid.
We recently showed that both Pafah1b Alpha subunits bind specifically to the Reelin receptor VLDLR [29]. Since Lis1 also binds Dab1, these data indicate that the entire Pafah1b complex may interact with the Reelin signaling machinery. In this study we examined the genetic interactions of individual Pafah1b Alpha subunits with the Reelin pathway and Lis1. We generated triple mutant mice carrying mutations in Pafah1b1, Reln or Dab1, and either Pafah1b3 or Pafah1b2, and examined traits such as the hydrocephalus phenotype and layer formation in the brain. We found that mutations in the Pafah1b3 gene exacerbate the layer defects of compound mice whereas mutations in the Pafah1b2 gene specifically suppress the hydrocephalus phenotype without affecting cortical layering. These data point to differential roles of the Pafah1b Alpha subunits in brain development.
MATERIALS AND METHODS
Mouse colonies
All efforts were made to minimize animal pain and discomfort. Experiments involving mice were carried out in accordance with protocols approved by an Institutional committee at Baylor College of Medicine, and with the National Institute of Health Guide for the Care and Use of Laboratory Animals (revised 1996). Reeler mutant mice were obtained from The Jackson Laboratories on a C57BL/6 x C3H hybrid background and genotyped by PCR [23]. Dab1 knock out mice were backcrossed on the C57BL/6 genetic background for several generations and genotyped by PCR [18]. Both, reeler and Dab1 knock out mice were intercrossed with Pafah1b1 heterozygous mutants maintained on a C57BL/6 x 129S6/SvEv mixed background. Pafah1b1 mice utilized in this study are null (Pafah1b1neo), and were genotyped by PCR [16]. Pafah1b3 and Pafah1b2 knock out mice were obtained and genotyped by Southern blot [28]. Generation of the animals used in this occurred over a period of five years.
Histology and Immunohistochemistry
Animals were anesthetized and perfused with 4% paraformaldehyde. Brains were embedded in paraffin and sectioned (5 μm thickness). Some sections were processed for immunohistochemistry using antibodies anti Calbindin (Sigma). Images were obtained with a Nikon E800 microscope using a Coolsnap camera (Roper) and Metaview Software (Universal Imaging).
Western blot analysis
Brain lysates from mutant mice were subjected to Western blot analysis using anti-Lis1 (Santa Cruz) or anti-Dab1 (Novus) antibodies. Blots were reprobed with β-Tubulin (Sigma) antibodies as internal control for protein loading, and analyzed as previously described [3].
RESULTS
Pafah1b2 mutations rescue the hydrocephalus phenotype of double Pafah1b1;Reln or Pafah1b1;Dab1 mutant mice
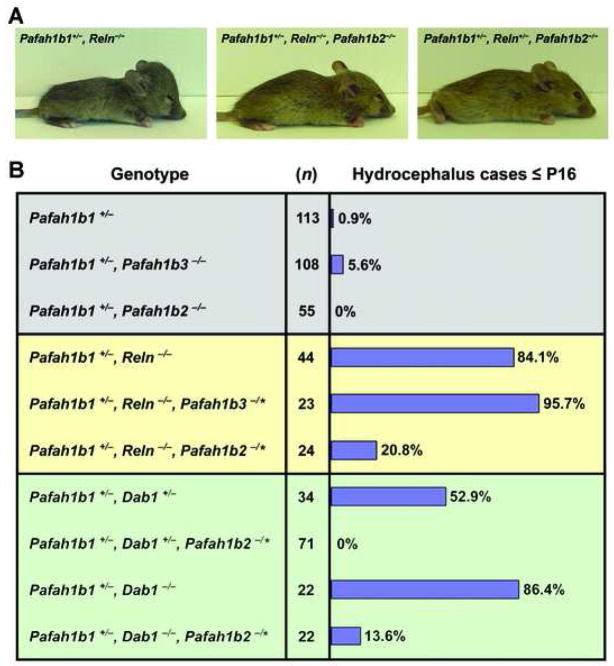
We investigated the effects of Pafah1b3 or Pafah1b2 null mutations on the frequency of hydrocephalus of single Pafah1b1+/− or double Pafah1b1;Reln, and the effects of Pafah1b2 mutations on the frequency of hydrocephalus of double Pafah1b1;Dab1 mutant mice. The presence of hydrocephalus, visible in intact animals as an enlargement of the skull, was recorded at or before postnatal day 16 in a large cohort (n=516 mice) maintained on similar genetic background (Fig. 1B). A representative case of hydrocephalus in a double Pafah1b1+/−;Reln−/− mutant is shown in Fig. 1A. We found a modest increase in the frequency of hydrocephalus in double Pafah1b1+/−;Pafah1b3 −/− mice (5.6%) compared to single Pafah1b1+/− mice (0.9%), and a complete absence of hydrocephalus in double Pafah1b1+/−;Pafah1b2−/− mice (0%) (Fig. 1B). Next, we crossed Pafah1b3 or Pafah1b2 knock out mice with double Pafah1b1;Reln mutant mice, and Pafah1b2 knock out mice with double Pafah1b1;Dab1 mutants. Since the effect of heterozygous or homozygous mutations (−/*) in Pafah1b3 or Pafah1b2 was very similar we grouped the data resulting from triple mutants. As previously noted [3], double Pafah1b1+/−;Reln−/− displayed a high frequency of hydrocephalus (84.1%). The addition of Pafah1b3 mutations resulted in a slight increase in the occurrence of this phenotype (95.7%), whereas the addition of Pafah1b2 mutations resulted in a considerable decrease (20.8%). A representative triple Pafah1b1+/−;Reln−/−;Pafah1b2 −/* mouse lacking hydrocephalus, but retaining the runt appearance and the abnormal posture typical of reeler homozygous mice, is shown in Fig. 1A. Control Pafah1b1+/−;Reln+/−;Pafah1b2 −/* appeared completely normal (Fig. 1A), as were single Pafah1b1+/−, single Reln+/− or double Pafah1b1+/−;Reln+/− heterozygous mice (not shown).
Figure 1. Pafah1b2 mutations suppress the hydrocephalus in compound mutants.
A, representative images of mice in our mutant cohort. A double Pafah1b1+/−;Reln−/− mutant (left panel) presents with a dome shaped skull indicative of hydrocephalus and the runt appearance typical of reeler mice. A triple Pafah1b1+/−;Reln−/−;Pafah1b2 −/− mouse (middle panel) lacks hydrocephalus, but retains the runt appearance of reeler mice. A triple Pafah1b1+/−;Reln+/−;Pafah1b2 −/− mouse (right panel) lacks hydrocephalus and exhibits the normal appearance of a heterozygous reeler mouse or heterozygous Pafah1b1+/− mice. B, frequency of hydrocephalus in our mutant cohort. The plots indicate the frequency of hydrocephalus in mice carrying the Pafah1b1+/− mutation alone or in combination with either a homozygous mutation in the Pafah1b2 or the Pafah1b3 gene (lavender sector), double Pafah1b1+/−;Reln−/− mutations in combination with either a mutation in the Pafah1b2 or the Pafah1b3 gene (yellow sector), and double Pafah1b1+/−;Dab1+/− or double Pafah1b1+/−;Dab1−/− mutations in combination with a mutation in the Pafah1b2 gene (green sector). All compound mice carrying a mutation in the Pafah1b2 gene displayed reduced frequency of hydrocephalus compared to their controls. n = number of mice examined at or before postnatal day 16.
Heterozygous and homozygous Dab1 mutations result in a high frequency of hydrocephalus when combined with Pafah1b1+/− mutations [3]. In this cohort, double Pafah1b1+/−;Dab1+/− mutants exhibited a 52.9%, frequency of hydrocephalus, whereas double Pafah1b1+/−;Dab1−/− mutants exhibited an 86.4% frequency (Fig. 1B). The addition of Pafah1b2 mutations completely suppressed the phenotype of double heterozygous (0%) and dramatically reduced that of double Pafah1b1+/−;Dab1−/− (13.6%) (Fig. 1B) without affecting the ataxic phenotype of Dab1 homozygous mutants (not shown).
These data demonstrate that Pafah1b2 mutations significantly suppress the Lis1-, Reln- and Dab1-dependent hydrocephalus phenotype in vivo, and suggest the Alpha2 subunit plays a unique role in the expression of this trait in compound mutants.
Pafah1b3, but not Pafah1b2 mutations exacerbate cellular layering defects in compound Pafah1b1+/−;Reln−/− mutant mice
To examine the effect of Pafah1b2 or Pafah1b3 mutations on layer formation we focused on the hippocampus, a structure where cortical layer abnormalities are readily observed. Unlike homozygous Reln or Dab1 mutations, disruptions of Pafah1b2 (encoding Alpha2) or Pafah1b3 (encoding Alpha1) genes did not lead to cellular layer abnormalities. Intact pyramidal and dentate granule cell layers similar to wild type (Fig. 2A) were observed in the hippocampus of Pafah1b2 (Fig. 2B) or Pafah1b3 (Fig. 2C) homozygous mice. A modest split in the hippocampal pyramidal layer is routinely observed in single Pafah1b1+/− mice (Fig. 2D). The addition of Pafah1b2 (not shown) or Pafah1b3 mutations (Fig. 2E) does not affect this phenotype. Homozygous loss of Reln in reeler mice results in the complete disruption of all hippocampal layers (Fig. 2F). This well-known dramatic phenotype overshadows the modest layer abnormality due to Lis1 reduction in double Pafah1b1+/−;Reln−/− mice, as the hippocampus of these mutants appears indistinguishable from that of reeler mice (Fig. 2G). Compound Pafah1b2 mutations in triple mutants also did not affect the reeler hippocampal layering defect (Fig. 2H), despite the dramatic suppression of the hydrocephalus described above in Pafah1b1+/−;Reln−/−;Pafah1b2 */− mice. Similar results were observed in the neocortex of compound mutants (not shown). Pafah1b3 mutations, on the other hand, resulted in the nearly complete loss of all hippocampal structures in triple Pafah1b1+/−;Reln−/−;Pafah1b3 −/− mice (Fig. 2I). These data suggest that Alpha1, but not Alpha2, may contribute to the control of forebrain layer formation in a manner that is additive to the activity Reelin.
Figure 2. Hippocampal structures in single and compound mutant mice.
Sagittal sections of the hippocampus were obtained from adult mice of the indicated genotype and were stained with cresyl violet. Compact cellular layers are present in the hippocampus and dentate gyrus of wild type mice (A). Single homozygous Pafah1b2 (B) or Pafah1b3 (C) mutations have no effect, whereas heterozygous Pafah1b1 mutations result in a split of the pyramidal layer (D). The loss of Pafah1b3 compounded with Pafah1b1 mutations has no obvious further effect (E). All cellular layers are severely disrupted in single Reln−/− (F) and double Pafah1b1+/−;Reln−/− (G) mice, The addition of Pafah1b2 mutations in triple Pafah1b1+/−;Reln−/−; Pafah1b2−/− mutants (H) has no further deleterious effect, whereas the addition of Pafah1b3 mutation (I) results in the almost complete loss of hippocampal structures. Scale bar = 500μm.
Pafah1b2 mutations do not affect forebrain cell layering in compound Pafah1b1+/−;Dab1*/− mutant mice
To further investigate the effect of Pafah1b2 mutations on cellular layer formation we examined hippocampal and cortical structures of single, double or triple mutant mice carrying mutations in the Dab1 and Pafah1b1 genes. Forebrain structures in Dab1+/− mice are phenotypically normal (Fig. 3A and E), whereas Pafah1b1+/− mice present a modest split in hippocampal cellular layers [3, 16] (Fig. 3B and F). As we previously reported (3), double heterozygous mutations in Dab1 and Pafah1b1 result in an enhanced disruption of hippocampal layers (Fig. 3C), and the appearance of a phenotype in the neocortex characterized by the abnormal dispersion of Calbindin-positive neurons in lower cortical layers (Fig. 3G). When disruption of the Pafah1b2 gene was further introduced in triple heterozygous mutant mice we did not observe any change in the appearance of hippocampal (Fig. 3D) or cortical layers (Fig. 3H) compared to double Pafah1b1+/−;Dab1+/− mice. These data suggest that Alpha2 does not affect the regulation of forebrain layer formation by the Reelin pathway.
Figure 3. Hippocampal and cortical structures in single and compound Pafah1b1;Dab1 heterozygous mice.

Sagittal sections of the hippocampus were obtained from adult mice of the indicated genotype and were stained with cresyl violet (A–D). Neocortical sections from the same mice were processed for immunohistochemistry using Calbindin antibodies (E–H). Single heterozygous Dab1 mice are phenotypically normal as they exhibit compact cellular layers in the hippocampus (A) and Calbidin-positive cortical neurons correctly positioned mostly in upper layers (E). Single heterozygous Pafah1b1 mice exhibit a modest split in the hippocampal pyramidal layer (B) but normal-appearing cortical layers (F). Double heterozygous Pafah1b1;Dab1 exhibit enhanced disruption of hippocampal (C) and cortical (G) layers. Triple mutants (D and H) also carrying a mutation in Pafah1b2 appeared similar to double mutants. Scale bar = 500μm.
Loss of Pafah1b2 does not alter Lis1 or Dab1 protein expression in the brain
Male homozygous Pafah1b2 mice are infertile due to disruptions in spermatogenesis, and display elevated levels of testicular Lis1 protein [21, 28]. Excessive Lis1 activity in homozygous Pafah1b2 mice appears to be directly responsible for defective sperm development since reduction of Pafah1b1 gene dosage in double Pafah1b1+/−;Pafah1b2−/− mice restores fertility. To determine whether compensatory changes in gene expression also occur in the brain of Pafah1b2−/− mutant mice, which could explain the observed suppression of the hydrocephalus phenotype, we examined Lis1 and Dab1 protein levels at different stages of development. Western blot analysis demonstrated that Lis1 (Fig. 4A) as well as Dab1 (Fig. 4B) protein levels are unaltered in the brain of Pafah1b2+/− or Pafah1b2−/− mutant mice at any of the examined ages of development. This data suggest that the mechanism underlying the suppression of the hydrocephalus phenotype in the brain is distinct from that underlying the suppression of sterility in the testis.
Figure 4. Pafah1b2 mutations do not alter Lis1 or Dab1 protein expression in the developing brain.

Western blot analysis of protein extracts obtained from the brain of wild type, heterozygous or homozygous Pafah1b2 mutant mice at the indicated embryonic (E) or postnatal (P) ages were probed with anti-Lis1 (A) or Dab1 (B) antibodies. The blots were reprobed with β-tubulin (β-Tub) antibodies as an internal control to ensure that equal amounts of proteins were loaded in each lane.
DISCUSSION
Reelin and Lis1 activities are important for neuronal migration and the formation of cellular layers. Simultaneous disruption of the Reelin signaling pathway and Lis1also leads to a high frequency of hydrocephalus [3]. Here we demonstrated that the Pafah1b Alpha2 subunit encoded by the Pafah1b2 gene does not affect cortical layer formation but is critically involved in the development of hydrocephalus in double mutants defective in Reelin and Lis1 signaling. Our genetic studies revealed that Pafah1b2 mutations strongly suppress the phenotype of double Pafah1b1;Reln or Pafah1b1;Dab1 mutants. The suppression is not due to a compensatory increase in Lis1 or Dab1 expression in the brain of Pafah1b2 mutants. The exact molecular mechanisms underlying the development of hydrocephalus in double mutants or the suppression of this phenotype by Pafah1b2 null mutations are not presently clear. One possibility is that elevated Alpha2 activity in double mutants may lead to the development of hydrocephalus, and thus its reduction or loss may rescue the phenotype. Elevated Alpha2 activity may be present in double mutant mice possibly due to partial loss of Alpha2 binding partners such as Lis1 or VLDLR [3, 29]. Consistent with this hypothesis, the frequency of hydrocephalus is higher in double Pafah1b1;Vldlr mutants compared to double Pafah1b1;Apoer2 mutants [3]. However, further studies will be necessary to firmly establish whether upregulated Alpha2 activity directly underlies the development of hydrocephalus, and to determine whether this putative activity is related to its ability to catalyze PAF or to interact with yet unidentified signaling molecules.
Loss of Pafah1b2, alone or in combination with mutations in the Reelin pathway and/or Lis1, does not affect neuronal migration, which occurs mostly at embryonic ages in the forebrain, but it rescued the hydrocephalus, a phenotype that develops postnatally. On the contrary, Pafah1b3 mutations exacerbated the layering phenotype of double mutants, but did not rescue the hydrocephalus. This functional pattern correlates to some extent with the developmental expression pattern of these subunits. Alpha1 expression is abundant in the embryonic brain at a time when neuronal migration occurs, but declines rapidly in the postnatal brain, whereas Alpha2 levels are low in the prenatal brain but become elevated after birth [1, 22]. Thus, the effect of Pafah1b2 and Pafah1b3 mutations on brain development may reflect, at least in part, their different gene expression pattern. However, since some forebrain structures such the dentate gyrus continue to develop postnatally when Alpha2 is expressed at high levels, it seems unlikely that the different time course of gene expression alone can explain the differential genetic effects on cellular lamination and hydrocephalus described in this study. Further studies are ongoing to determine whether distinct biochemical interactions contribute to the specificity of Pafah1b2 and Pafah1b3 mutations on brain development.
Acknowledgments
This work was supported in part by NIH/NINDS R01 NS042616 (G.D.). We thank J. Cooper for Dab1 knock out mice, G. Eichele for Pafah1b3 mutant mice, B.A. Antalffy and the MRDDRC at Baylor College of Medicine for histology support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Albrecht U, Abu-Issa R, Ratz B, Hattori M, Aoki J, Arai H, Inoue K, Eichele G. Platelet-activating factor acetylhydrolase expression and activity suggest a link between neuronal migration and platelet-activating factor. Dev Biol. 1996;180:579–593. doi: 10.1006/dbio.1996.0330. [DOI] [PubMed] [Google Scholar]
- 2.Arnaud L, Ballif BA, Forster E, Cooper JA. Fyn tyrosine kinase is a critical regulator of disabled-1 during brain development. Curr Biol. 2003;13:9–17. doi: 10.1016/s0960-9822(02)01397-0. [DOI] [PubMed] [Google Scholar]
- 3.Assadi AH, Zhang G, Beffert U, McNeil RS, Renfro AL, Niu S, Quattrocchi CC, Antalffy BA, Sheldon M, Armstrong DD, Wynshaw-Boris A, Herz J, D’Arcangelo G, Clark GD. Interaction of reelin signaling and Lis1 in brain development. Nat Genet. 2003;35:270–276. doi: 10.1038/ng1257. [DOI] [PubMed] [Google Scholar]
- 4.Ballif BA, Arnaud L, Arthur WT, Guris D, Imamoto A, Cooper JA. Activation of a Dab1/CrkL/C3G/Rap1 pathway in Reelin-stimulated neurons. Curr Biol. 2004;14:606–610. doi: 10.1016/j.cub.2004.03.038. [DOI] [PubMed] [Google Scholar]
- 5.Bazan NG, Rodriguez de Turco EB. Platelet-activating factor is a synapse messenger and a modulator of gene expression in the nervous system. Neurochem Int. 1995;26:435–441. doi: 10.1016/0197-0186(94)00138-k. [DOI] [PubMed] [Google Scholar]
- 6.Bock HH, Herz J. Reelin activates SRC family tyrosine kinases in neurons. Curr Biol. 2003;13:18–26. doi: 10.1016/s0960-9822(02)01403-3. [DOI] [PubMed] [Google Scholar]
- 7.Bock HH, Jossin Y, Liu P, Forster E, May P, Goffinet AM, Herz J. PI3-Kinase interacts with the adaptor protein Dab1 in response to Reelin signaling and is required for normal cortical lamination. J Biol Chem. 2003;278:38772–38779. doi: 10.1074/jbc.M306416200. [DOI] [PubMed] [Google Scholar]
- 8.D’Arcangelo G. The reeler mouse: anatomy of a mutant. In: Dhossche DM, editor. International Review of Neurobiology. Vol. 17. Elsevier Inc.; San Diego, CA: 2005. pp. 383–417. [DOI] [PubMed] [Google Scholar]
- 9.D’Arcangelo G, Homayouni R, Keshvara L, Rice DS, Sheldon M, Curran T. Reelin is a ligand for lipoprotein receptors. Neuron. 1999;24:471–479. doi: 10.1016/s0896-6273(00)80860-0. [DOI] [PubMed] [Google Scholar]
- 10.D’Arcangelo G, Miao GG, Chen SC, Soares HD, Morgan JI, Curran T. A protein related to extracellular matrix proteins deleted in the mouse mutant reeler. Nature. 1995;374:719–723. doi: 10.1038/374719a0. [DOI] [PubMed] [Google Scholar]
- 11.Faulkner NE, Dujardin DL, Tai CY, Vaughan KT, O’Connell CB, Wang Y, Vallee RB. A role for the lissencephaly gene LIS1 in mitosis and cytoplasmic dynein function. Nat Cell Biol. 2000;2:784–791. doi: 10.1038/35041020. [DOI] [PubMed] [Google Scholar]
- 12.Hattori M, Adachi H, Tsujimoto M, Arai H, Inoue K. The catalytic subunit of bovine brain platelet-activating factor acetylhydrolase is a novel type of serine esterase. J Biol Chem. 1994;269:23150–23155. [PubMed] [Google Scholar]
- 13.Hattori M, Adachi H, Tsujimoto M, Arai N, Inoue K. Miller-Dieker lissencephaly gene encodes a subunit of brain platelet-activating factor acetylhydrolase. Nature. 1994;370:216–218. doi: 10.1038/370216a0. [DOI] [PubMed] [Google Scholar]
- 14.Hattori M, Arai H, Inoue K. Purification and characterization of bovine brain platelet-activating factor acetylhydrolase. J Biol Chem. 1993;268:18748–18753. [PubMed] [Google Scholar]
- 15.Hiesberger T, Trommsdorff M, Howell BW, Goffinet AM, Mumby MC, Cooper JA, Herz J. Direct binding of Reelin to VLDL receptor and ApoE receptor 2 induces tyrosine phosphorylation of Disabled-1 and modulates Tau phosphorylation. Neuron. 1999;24:481–489. doi: 10.1016/s0896-6273(00)80861-2. [DOI] [PubMed] [Google Scholar]
- 16.Hirotsune S, Fleck MW, Gambello MJ, Bix GJ, Chen A, Clark GD, Ledbetter DH, McBain CJ, Wynshaw-Boris A. Graded reduction of Pafah1b1 (Lis1) activity results in neuronal migration defects and early embryonic lethality. Nat Genet. 1998;19:333–339. doi: 10.1038/1221. [DOI] [PubMed] [Google Scholar]
- 17.Ho YS, Swenson L, Derewenda U, Serre L, Wei Y, Dauter Z, Hattori M, Adachi T, Aoki J, Arai H, Inoue K, Derewenda ZS. Brain acetylhydrolase that inactivates platelet-activating factor is a G-protein-like trimer. Nature. 1997;385:89–93. doi: 10.1038/385089a0. [DOI] [PubMed] [Google Scholar]
- 18.Howell BW, Hawkes R, Soriano P, Cooper JA. Neuronal position in the developing brain is regulated by mouse disabled-1. Nature. 1997;389:733–736. doi: 10.1038/39607. [DOI] [PubMed] [Google Scholar]
- 19.Howell BW, Herrick TM, Cooper JA. Reelin-induced tyrosine phosphorylation of Disabled 1 during neuronal positioning. Genes Dev. 1999;13:643–648. doi: 10.1101/gad.13.6.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keshvara L, Benhayon D, Magdaleno S, Curran T. Identification of reelin-induced sites of tyrosyl phosphorylation on disabled 1. J Biol Chem. 2001;276:16008–16014. doi: 10.1074/jbc.M101422200. [DOI] [PubMed] [Google Scholar]
- 21.Koizumi H, Yamaguchi N, Hattori M, Ishikawa TO, Aoki J, Taketo MM, Inoue K, Arai H. Targeted disruption of intracellular type I platelet activating factor-acetylhydrolase catalytic subunits causes severe impairment in spermatogenesis. J Biol Chem. 2003;278:12489–12494. doi: 10.1074/jbc.M211836200. [DOI] [PubMed] [Google Scholar]
- 22.Manya H, Aoki J, Watanabe M, Adachi T, Asou H, Inoue Y, Arai H, Inoue K. Switching of platelet-activating factor acetylhydrolase catalytic subunits in developing rat brain. J Biol Chem. 1998;273:18567–18572. doi: 10.1074/jbc.273.29.18567. [DOI] [PubMed] [Google Scholar]
- 23.Niu S, Renfro A, Quattrocchi CC, Sheldon M, D’Arcangelo G. Reelin promotes hippocampal dendrite development through the VLDLR/ApoER2-Dab1 pathway. Neuron. 2004;41:71–84. doi: 10.1016/s0896-6273(03)00819-5. [DOI] [PubMed] [Google Scholar]
- 24.Pramatarova A, Ochalski PG, Chen K, Gropman A, Myers S, Min KT, Howell BW. Nck beta interacts with tyrosine-phosphorylated disabled 1 and redistributes in Reelin-stimulated neurons. Mol Cell Biol. 2003;23:7210–7221. doi: 10.1128/MCB.23.20.7210-7221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Reiner O, Carrozzo R, Shen Y, Wehnert M, Faustinella F, Dobyns WB, Caskey CT, Ledbetter DH. Isolation of a Miller-Dieker lissencephaly gene containing G protein beta-subunit-like repeats. Nature. 1993;364:717–721. doi: 10.1038/364717a0. [DOI] [PubMed] [Google Scholar]
- 26.Smith DS, Niethammer M, Ayala R, Zhou Y, Gambello MJ, Wynshaw-Boris A, Tsai LH. Regulation of cytoplasmic dynein behaviour and microtubule organization by mammalian Lis1. Nat Cell Biol. 2000;2:767–775. doi: 10.1038/35041000. [DOI] [PubMed] [Google Scholar]
- 27.Strasser V, Fasching D, Hauser C, Mayer H, Bock HH, Hiesberger T, Herz J, Weeber EJ, Sweatt JD, Pramatarova A, Howell B, Schneider WJ, Nimpf J. Receptor clustering is involved in Reelin signaling. Mol Cell Biol. 2004;24:1378–1386. doi: 10.1128/MCB.24.3.1378-1386.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yan W, Assadi AH, Wynshaw-Boris A, Eichele G, Matzuk MM, Clark GD. Previously uncharacterized roles of platelet-activating factor acetylhydrolase 1b complex in mouse spermatogenesis. Proc Natl Acad Sci U S A. 2003;100:7189–7194. doi: 10.1073/pnas.1236145100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang G, Assadi AH, McNeil RS, Beffert U, Wynshaw-Boris A, Herz J, Clark GD, D’Arcangelo G. The Pafah1b Complex Interacts with the Reelin Receptor VLDLR. PLoS ONE. 2007;2:e252. doi: 10.1371/journal.pone.0000252. [DOI] [PMC free article] [PubMed] [Google Scholar]