Abstract
Objectives
This study examines the tolerability and efficacy of methylphenidate (MPH) and behavior modification therapy (BMOD) in children with attention-deficity/hyperactivity disorder (ADHD) and severe mood dysregulation (SMD).
Methods
Children (ages 5–12) from a summer program for ADHD were screened for SMD and additional manic-like symptoms using structured assessments and direct clinical interview with the Young Mania Rating Scale (YMRS). The SMD group was comprised of 33 subjects with SMD and elevated YMRS scores (mean = 23.7). They underwent weekly mood assessments plus the daily ADHD measures that are part of the program. The comparison group (n = 68) was comprised of the rest of the program participants. Using a crossover design, all subjects in both groups were treated with three varying intensities of BMOD (no, low, high) each lasting 3 weeks, with MPH dose (placebo, 0.15 mg/kg t.i.d., 0.3mg/kg t.i.d., and 0.6mg/kg t.i.d.) varying daily within each behavioral treatment.
Results
Groups had comparable ADHD symptoms at baseline, with the SMD group manifesting more oppositional defiant disorder/conduct disorder (ODD/CD) symptoms (p < 0.001). Both groups showed robust improvement in externalizing symptoms (p < 0.001). There was no evidence of differential treatment efficacy or tolerability. Treatment produced a 34% reduction in YMRS ratings in SMD subjects (p − 0.001). However, they still exhibited elevated YMRS ratings, more ODD/CD symptoms (p < 0.001), and were more likely to remain significantly impaired at home than non-SMD subjects (p < 0.05).
Conclusions
MPH and BMOD are tolerable and effective treatments for children with ADHD and SMD, but additional treatments may be needed to optimize their functioning.
Introduction
Over the past decade there has been a 40-fold increase in the diagnosis of bipolar disorder (BP) in children (Moreno et al. 2007), particularly among those diagnosed with attention-deficit/hyperactivity disorder (ADHD) (Pavuluri et al. 2005; Pliszka et al. 2006; Blader and Carlson 2007). As the reported prevalence of BP increases, so does the controversy surrounding the disorder. At the center of the controversy is the child who has both ADHD and chronically dysregulated moods but lacks distinct cycles of mania and depression. In the Multimodal Treatment of ADHD (MTA) and other longitudinal ADHD studies, 10% of ADHD subjects exhibited manic-like symptoms, such as marked irritability and emotional dysregulation (Carlson et al. 1998; Galanter et al. 2003). Biederman reported that 17% of ADHD youths drawn from psychiatric clinics meet full criteria for BP (Biederman et al. 2004). It has been speculated that the rapid increase in the prevalence of pediatric BP is fueled by the inclusion of these emotionally dysregulated ADHD children into the bipolar spectrum (Blader and Carlson, 2007; Moreno et al. 2007). However, it remains to be determined if they have genuine bipolar spectrum illness or a severe externalizing behavior disorder.
A National Institute of Mental Health (NIMH) work group addressed this controversy by creating the diagnostic label of “severe mood dysregulation” (SMD) to refer to children with persistent irritability, hyperarousal, and emotional reactivity that lack other mania specific symptoms (Leibenluft et al. 2003; Brotman et al. 2006). SMD is a relatively new construct that overlaps significantly with oppositional defiant disorder (ODD). However, it has been posited that many children with ODD will not meet full criteria for SMD (Rich et al. 2007). In addition, SMD is associated with deficits in emotional processing not seen in youths with only externalizing behavior disorders (Guyer et al. 2007), suggesting SMD may represent a distinct diagnostic entity.
Unfortunately, there have been no controlled treatment studies of children with SMD, and the primary pharmacological treatments for BP and ADHD are quite different. It is presently unclear if the initial treatment for children with ADHD and dysregulated moods should be stimulants and behavior therapy for ADHD or mood-stabilizing medications for affective instability.
Further complicating this issue are concerns that stimulants may induce mania in children with or at risk for BP, leading some to conclude that children with ADHD and any manic-like symptoms should not be prescribed stimulants (Papolos and Papolos 1999; Vitiello 2001; Ross 2006). The Food and Drug Administration (FDA) recently added a warning label to all stimulants regarding their potential to produce “new or worse bipolar illness” (FDA 2006). Despite these concerns, many children diagnosed with BP are initially treated with stimulants for ADHD (Tillman et al. 2005), stressing the need to define better the mood altering effects of stimulants in children.
Several retrospective analyses have found a connection between stimulant exposure and the course of BP (Delbello et al. 2001; Faedda et al. 2004; Reichart 2004), whereas others have not (Biederman et al. 1999; Carlson et al. 2000; Carlson and Mick 2003). Any retrospective observations linking stimulants and BP must control for the confounding effects of ADHD symptom severity because most children with prepubertal BP first exhibit prominent ADHD symptoms, leading to stimulant treatment at an early age. There are only two published controlled trials of stimulants in children with well-defined BP, totaling 46 youths. In these trials of children already on a stabilized on antimanic medications, methylphenidate (MPH) and mixed amphetamine salts (MAS) significantly improved ADHD symptoms and were well tolerated, except for one case of worsening manic symptoms on MAS (Scheffer et al. 2005; Findling et al. 2007).
Some improvement to stimulant treatment alone has been documented in ADHD youths with manic symptoms. Carlson and Kelly (1998) noted as much improvement in children with ADHD and manic symptoms as those with ADHD without manic symptoms. More recently, Galanter reviewed the profiles of all children completing the MTA's 4-week medication titration using a proxy screen consisting of the Diagnostic Interview Schedule for Children (DISC) and the Child Behavior Checklist (CBCL) to identify subjects with manic-like symptoms (Galanter et al. 2003). While children diagnosed with BP were excluded from the MTA, 11% of the subjects exhibited manic-like symptoms based on significant elevations in the attention, aggression, and anxiety/depression subtest scores on the CBCL. Although these subjects were more severely impaired at baseline than those without manic symptoms, MPH was equivalently effective and well tolerated in both groups. Although these baseline symptom differences persisted for the 14 months of assigned treatment, the groups exhibited comparable responses to MPH and behavior modification therapy (BMOD), with no evidence of differential drug tolerability between groups (Galanter et al. 2005). MTA findings were limited by the poor agreement between the DISC and the CBCL proxies, the lack of mania-specific assessment tools, and the retrospective nature of the review. In contrast, other trials have found that youths with affective co-morbidity have a reduced response to ADHD medications (Biederman et al. 1998; Daviss et al. 2001; Gadow et al. 2002).
Behavioral therapy, the other evidence-based treatment for pediatric ADHD, has found to be effective for children with a range of co-morbidities (Pelham and Fabiano, 2008). In the MTA, BMOD improved ADHD symptoms, and adding BMOD to stimulants improved symptoms of conduct disorder (CD), ODD, and parent–child and peer relations (Jensen et al. 2001; Swanson et al. 2001). The combination of stimulants and BMOD were most effective for MTA subjects with both internalizing and externalizing co-morbidities (Jensen et al. 2001), suggesting that youths with SMD may be excellent candidates for combination therapies.
Behavioral therapies are also an indicated treatment for ADHD in children with bipolar spectrum illness, but other than Galanter's MTA analyses, where behavioral treatment was especially useful for the ADHD–mania proxy youths, there is no controlled data on the efficacy of these therapies in children with BP or SMD (Kowatch et al. 2005). None of published psychosocial trials for prepubertal BP specifically addressed ADHD symptoms (Pavuluri et al. 2004; Fristad 2006).
The alternative to ADHD treatments for children with ADHD and SMD is the use of mood-stabilizing medication. However, these agents have limited efficacy for ADHD and more worrisome side-effect profiles than stimulants (Correll and Carlson 2006; Delbello and Kowatch 2006), making them an unpalatable initial choice for children with ADHD who are not exhibiting discrete manic episodes. Hence, there are no evidence-based treatments for SMD that have been found to reduce ADHD symptoms effectively without risk of exacerbating mood symptoms.
In summary, there has been an increasing recognition that a subset of children with ADHD also exhibit severe mood dysregulation that is difficult to distinguish from mania. Because most of these children do not exhibit discrete mood cycles that would fulfill Diagnostic and Statistical Manual for Mental Disorders (DSM) criteria for BD, it is debatable as to whether they have genuine bipolar spectrum illness. These diagnostic uncertainties have hindered the development of evidence-based treatments for these children who are clearly impaired across multiple settings.
We attempted to address this void by identifying a subset of ADHD youth with SMD from a study of the synergistic effects stimulants and behavior modification therapy for ADHD. The primary aim was to compare the efficacy and tolerability of these two proven ADHD treatments in children with ADHD plus SMD versus those with ADHD but not SMD or other affective illness to determine if it is safe and advisable to treat ADHD in a child with SMD before addressing the mood dysregulation. It was not our goal to determine if these children have genuine BP, because definitive diagnostic criteria and biological markers for this disorder have yet to be defined.
Because stimulants and BMOD are established therapies for ADHD but not mood disorders, we hypothesized that MPH and BMOD would improve externalizing symptoms but not lead to reductions in depressive or manic-like symptoms in youths with SMD. Due to their additional symptom burden and prior work suggesting that co-morbid affective illness may reduce responsiveness to stimulants, it was hypothesized that children with SMD would have a diminished therapeutic response to MPH and BMOD compared to ADHD subjects not meeting SMD criteria.
Methods
Participants
All study participants were drawn from the 2003 or 2004 University of Buffalo (UB) Summer Treatment Program (STP). The STP is an ADHD research program for children ages 5–12 in the form of an intensive 9-week therapeutic summer camp (Pelham et al. 1997; Pelham et al. 2005a; Fabiano et al. 2007). From 2002 to 2004, the STP was the site for a double-blind study of MPH and BMOD (MH62946) designed to assess the relative efficacy and synergistic effects of differential doses of these interventions. To accomplish this goal, STP subjects were randomly assigned to three intensities of BMOD that varied every 3 weeks and four different doses of MPH that varied daily within each 3-week BMOD cell (see Fig. 1). In 2003 and 2004, the current protocol designed to assess the efficacy and tolerability of BMOD and MPH in children with SMD was incorporated into the larger STP study by adding SMD measures to the existing STP assessments.
FIG. 1.
Study methodology for 2002–2004 Summer Treatment Program. Three, 3-week behavior modification conditions assigned randomly. BMOD = behavior modification therapy; MPH = methylphenidate; mg = milligrams; kg = kilograms.
Participants were recruited via referrals from schools and health-care providers and radio and print advertisements. Parents and children provided informed consent, and the UB Health Sciences IRB approved the protocol. Subjects were required to stop all psychotropic medication 1 week prior to intake. All STP participants met full Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV) (American Psychiatric Association 1994) diagnostic criteria for ADHD. Diagnoses were made using a combination of ratings from parents and the primary school teacher on the Disruptive Behavior Disorders Rating Scale (DBD) (Pelham et al. 1992) and parent report on the DISC (Shaffer et al. 2000). Symptoms were counted as positive if they were endorsed on either measure. In addition, ADHD related impairment in at least two realms using the Parent and Teacher versions of the Impairment Rating Scale (IRS) was required for entry into the STP (Fabiano et al. 2006). The majority (92%) met criteria for the combined ADHD subtype. Participants were required to have a full-scale intelligence quotient (IQ) >80 and not to have a documented serious adverse reaction to MPH. Children with significant developmental delays, psychotic symptoms, or autistic spectrum illness were not enrolled. Children actively using psychotropic medication for disorders besides ADHD, including the use of antidepressants or mood-stabilizing medications, were also excluded because subjects were required to stop all psychotropics before STP entry. Subjects newly identified with major depressive disorder (MDD) or BP on the DISC were directly assessed by a M.D./Ph.D.-level clinician during the intake procedure. Subjects found to meet full criteria for the narrow-phenotype criteria of BP (Leibenluft et al. 2003) or any subject in need of urgent psychiatric treatment (active suicidal ideation) were not enrolled in the STP but instead were referred for appropriate treatment.
SMD criteria
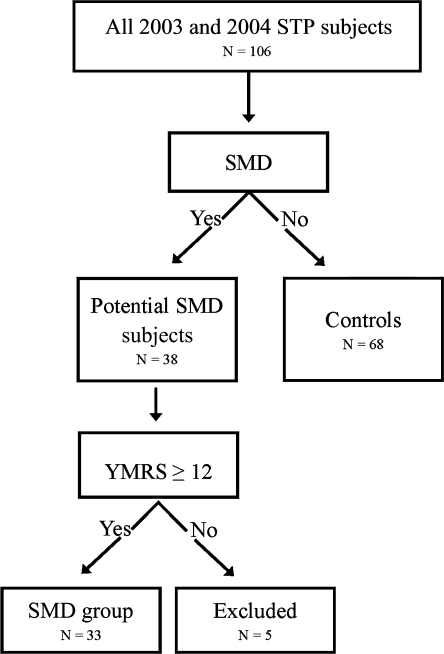
All subjects enrolled in the larger STP study were screened for SMD to identify a subgroup of mood dysregulated ADHD youths. ADHD subjects not meeting criteria for SMD comprised the control or non-SMD group (n = 68). Information was gathered from children, parents, and teachers, because children exhibiting mood symptoms in multiple settings are more impaired than those with symptoms confined to one setting (Carlson and Youngstrom 2003), and cross-domain impairment is required for a diagnosis of SMD (Leibenluft et al. 2003) (Fig. 2).
FIG. 2.
Severe mood dysregulation inclusion criteria. SMD = severe mood dysregulation; YMRS = Young Mania Rating Scale; STP = Summer Treatment Program.
First, all STP subjects were screened for SMD using the NIMH criteria (Leibenluft et al. 2003; Brotman et al. 2006). We used the two mania proxy measures from the MTA (parent version of the DISC and parent and teacher versions of the CBCL) to define the SMD criterion of “persistently abnormal mood” (Galanter et al. 2003). Parents/teachers needed to endorse severe irritability (coded as answering yes to the irritability lead-in question and the follow-up severity question on the mania assessment) and 1+ additional manic symptom on the DISC or endorse t-scores of 70+ on the aggression and anxious/depressed categories on the CBCL (Achenbach et al. 1983; Shaffer et al. 2000). Identical thresholds for manic symptoms were employed in the MTA analyses. The SMD criterion of “increased reactivity to emotional stimuli” was evaluated during a semistructured interview of parent and child by a graduate-level clinician that is part of the STP intake process. This interview, designed to identify the child's primary behavioral impairments, specifically inquires about extended temper tantrums, verbal rages, and aggression toward others and property, all of which are used in the definition of SMD (Leibenluft et al. 2003; Brotman et al. 2006). The “hyperarousal” criterion was waived because almost all STP subjects met full criteria for the combined subtype of ADHD and exhibit constant evidence of hyperarousal sufficient to meet SMD criteria (Brotman et al. 2006). Symptoms must have been present for at least 12 months with no symptom-free periods longer than 60 days (Leibenluft et al. 2003).
Because many children with ODD would meet criteria for SMD, a second assessment step was added in an attempt to identify children with manic-like mood dysregulation, as they are the SMD patients for whom there would be the greatest reservations about the safety of stimulants. A child psychiatrist (J.W.) experienced in the diagnosis of pediatric BP directly interviewed all subjects and their parents who met SMD criteria using the Young Mania Rating Scale (YMRS), the Children's Depression Rating Scale Revised (CDRS-R), and Clinical Global Impressions Scale (CGI) for MDD and BP (Guy 1976; Young et al. 1978; Poznanski and Mikos 1996). Subjects were only enrolled in the SMD group if they had an YMRS score based on last month's behavior of 12+ and CGI mania severity score of 3+ (mild or worse symptoms). Depressive symptoms were assessed as they commonly co-occur with manic-like symptoms (Pavuluri et al. 2005), but elevated CDRS-R scores were not required for entry in the SMD group.
The YMRS is the most widely used measure of manic symptomatology in pediatric clinical trials (Pavuluri et al. 2005). Youths with elevated scores on this scale present with a mix of irritability and mood lability beyond that seen with ODD or ADHD (Findling et al. 2005). Moreover, improvement in ADHD symptoms does not significantly improve YMRS scores in youths with BP (Scheffer et al. 2005; Findling et al. 2007). Therefore, children with elevated YMRS scores may comprise the subset of SMD youths most likely to be distinct from ODD. A score of 12 for the YMRS was selected, because it is the defined set point for syndromal remission for pediatric BP (Patel et al. 2007) and has been employed elsewhere to identify youths at risk for BP (Baumer et al. 2006; Delbello et al. 2007). Most children with uncomplicated ADHD do not score above this threshold (Fristad et al. 1992).
A total of 33 STP subjects (31% of the 2003/2004 STP participants) comprised the SMD group. While these subjects manifested elevated scores on the YMRS (mean = 23.7) and met all SMD criteria, none met full criteria for BP, mostly due to the lack of discrete mood cycles or insufficient criterion B symptoms. Depressive symptoms (CDRS-R >28) were present in 24/33 (72%) of the SMD group. Five subjects met SMD criteria but had an YMRS <12 and were therefore excluded from both the SMD and non-SMD groups. The non-SMD group consisted of the other 68 STP subjects from the 2003/2004 STP who met criteria for ADHD but not SMD. The groups were similar in demographics except SMD subjects were younger (8.0 vs. 8.7 years) and trended toward higher math achievement (Table 1).
Table 1.
Subject Characteristics
| SMD (SD) | Non-SMD (SD) | |
|---|---|---|
| N | 33 | 68 |
| Age** mean (SD) | 8 (2.1) | 8.7 (2) |
| % male | 82 | 81 |
| % minority | 21 | 21 |
| IQ | 107 (17) | 104 (14) |
| Reading achievement | 104 (14.5) | 102 (12.5) |
| Math achievement* | 106.5 (21) | 102 (14) |
| % with family history of mood disorder*/bipolar | 71%/21% | 53%/18% |
| DISC diagnosis of ODD** | 72% | 46% |
| DISC diagnosis of CD** | 24% | 6% |
| DBD teacher inattention | 17.9 (8.5) | 20.5 (7.7) |
| DBD teacher hyp/imp | 18.7 (7.4) | 18 (7.3) |
| DBD teacher ODD** | 12.3 (8.1) | 9.4 (6.7) |
| DBD teacher conduct** | 10.4 (12) | 5.4 (7.2) |
| YMRS | 23 (3.5) | NA |
| CDRS-R | 35 (7.7) | NA |
| CGI severity MDD | 2.8 (1) | NA |
| CGI severity BP | 3.5 (.57) | NA |
| IRS | 5.0 (.8) | 4.9 (1.1) |
df = 99; ** < 0.10.
SMD = severe mood dysregulation; SD = standard deviation; DISC = Diagnostic Interview Schedule for Children; ODD = oppositional defiant disorder; CD = conduct disorder; DBD = Disruptive Behavior Disorder Scale; YMRS = Young Mania Rating Scale; CDRS-R = Children's Depression Rating Scale–Revised; CGI = Clinical Global Impressions; MDD = major depressive disorder; BP = bipolar disorder; IRS = Impairment Rating Scale.
As part of the STP screening, parents reported on diagnosed mental illness in all first- and second-degree relatives using a structured rating form designed at our center that specifically inquires about BP and MDD. In the SMD group, 20/28 (71%) subjects had a family history of a mood disorder (5 had unknown family histories), including 6 (21%) that had a first- or second-degree relative with BP. For the non-SMD group with known family histories, 32/60 (53%) had familial affective illness including 11 (18%) with a first- or second-degree relative with BP. There was a trend toward a greater percentage of familial affective illness in the SMD group (odds ratio [OR] = 2.4, 0.92–6.3, p = 0.07).
Setting and design
STP participants were placed in groups of 12 according to age and supervised by five counselors per group. The STP lasted 9 hours per day, Monday through Friday for 9 weeks, totaling 45 days. Children spent 2 hours a day in academic settings and the remainder of the day in recreational activities. Parents attended a weekly parent-training course in which they learned to implement behavioral management skills at home (Cunningham et al. 1998).
The primary goal of the larger STP study (MH62946) was to evaluate the interactions between varying doses of behavioral and pharmacological treatments for ADHD. The study consisted of two within-subjects factors with order counterbalanced across group and year: medication (placebo, 0.15 mg/kg t.i.d., 0.3 mg/kg t.i.d., 0.6 mg/kg MPH t.i.d.) and behavior modification (no behavior modification [NBM], low-intensity behavior modification [LBM], high-intensity behavior modification [HBM]). Behavioral treatment varied every 3 weeks with the order (NBM vs. LBM vs. HBM) randomized across subjects irrespective of mood status. In each 3-week behavioral cell, subjects received each of the four medication doses (placebo, 0.15 mg/kg, 0.3 mg/kg, 0.6 mg/kg MPH t.i.d.), with the dose assigned randomly on a daily basis so that each subject received all 12 treatment combinations (i.e., HBM × 0.3 mg/kg MPH t.i.d., etc.) for 3–4 days apiece, totaling 45 days of treatment. The use of placebos and the NBM condition allowed for assessment of the individual efficacy of each intervention. Children, parents, and STP staff were blind to medication conditions. Clinicians rating mood symptoms were blind to MPH dose but not BMOD status, because some were STP staff. Staff who rated ADHD symptoms were blind to SMD status.
Medication was randomly assigned using double-blind methodology with the dose changing daily. Medication was administered at 7:45 am, 11:45 am, and 3:45 pm. Placebo and MPH were packed in identical opaque capsules to maintain blinding. Average MPH doses were 5, 10, and 18 mg for the 0.15, 0.3, and 0. mg/kg doses, respectively. HBM resembled the behavior intervention used in the summer component of the MTA (MTA 1999). It included daily social skills training and a comprehensive point system with reward and cost components. Children earned daily social rewards for high point totals, and earned daily and weekly rewards for meeting point goals. In the classroom, behavior was managed through a modified version of the point system. Time-out procedures were used when children exhibited defiant behavior. Children received daily report cards (DRCs) evaluating their performance on individualized target behaviors. DRCs were reviewed with parents at the end of the day and used as criteria for rewards at home. Any time the standard procedures were not sufficient to produce the desired behavioral changes, individualized behavioral programs were developed (Pelham et al. 1997; Pelham et al. 2005b).
LBM resembled traditional interventions implemented in community mental health settings or special education classrooms. The primary difference between the two was the use of daily (HBM) versus weekly (LBM) contingency rewards and social skills training sessions as well as the use of individualized behavior plans only in HBM. In the NBM condition, the behavior modification system was suspended to emulate a typical classroom or camp setting. Staff members recorded all point system behaviors but did not award or take away points. The structure and content of the activities remained the same. Children did not receive DRCs in the NBM condition, and social skills training, problem-solving discussions, and time-out procedures were not used. Children earned daily and weekly STP reinforcers noncontingently.
When the BMOD intensity changed, it was explained to parents and subjects that new rules were in effect, with specific examples provided describing changes to the existing procedures (time outs, point system, etc.). The point system reset weekly within each BMOD cell after the reward was earned or not on Fridays, so that the subjects were accustomed to starting a new point system each week, minimizing the transition between the different BMOD intensities. STP rules were reviewed daily with subjects at the beginning and end of each camp day and during individual activities, so there was ample opportunity to adjust to any rule changes. Staff adherence to the assigned BMOD cell was monitored daily by senior STP staff using treatment integrity and fidelity procedures as outlined in the STP manual (Pelham et al. 1997). Staff members were given immediate feedback on their performance and their adherence to each of the three behavioral treatment conditions. Parents were reminded daily about what parenting practices were allowed in each of the BMOD cells and were asked to record the frequency of specific BMOD practices used at home (time out, use of contingency rewards, etc.) to monitor adherence to the assigned BMOD cell. Similar crossover designs using treatment conditions intermixed with nontreatment conditions have been widely employed in behavioral treatments studies (Fabiano et al. 2007; Pelham and Fabiano, 2008). For more details on the specific components of the BMOD arms and treatment fidelity procedures, see Fabiano et al. (2007) or Pelham et al. (2005a).
ADHD outcome measures
Frequency counts of externalizing behavioral problems exhibited at the STP were completed daily by counselors using a well-validated and reliable observational coding scheme (Pelham et al. 2005b). Using a similar system, teachers recorded daily rates of classroom behaviors and academic productivity. Daily totals for percentage of time following activity rules (FAR), noncompliance with staff requests (NC), and the percentage of seatwork completed (SC) served as the primary dependent variables. These measures have been shown to detect treatment effects of pharmacological and behavioral interventions for ADHD and have adequate interrater reliability (Pelham et al. 1993; Pelham et al. 1999; Pelham et al. 2005a). The parent-rated DBD, which rates all DSM 3R and IV symptoms for ODD, CD, and ADHD rated on a 0–3 Likert scale, was administered at baseline and end point (Pelham et al. 1992).
Mood outcome measures
For the SMD group, the clinician-rated YMRS and CDRS-R were completed during a weekly interview with parents and subject by child psychiatrists experienced in the treatment of pediatric mood disorders and ADHD. Both scales are the current standards for measuring affective symptoms in children and were employed in the two published controlled trials of stimulants in children with BP (Carlson et al. 2003; Scheffer et al. 2005; Findling et al. 2007). The CDRS has established psychometrics, including acceptable levels of interrater reliability (Poznanski et al. 1984). The YMRS has been found to be a valid measure of mood symptoms in children ages 5–11 with acceptable interrater reliability (kappa > 0.85) (Youngstrom et al. 2002). It has been demonstrated that children with ADHD and no affective co-morbidity typically score <12 on the YMRS distinguishes them from children with BP (Fristad et al. 1992). Parents also completed a version of the YMRS (PYMRS) on the first day for each of the 12 different dosing combination of MPH × BMOD (total of 12 times) to detect any acute exacerbation of manic-like symptoms from the end of the STP day to drop-off the following morning. It has been shown to distinguish manic from ADHD symptoms and is a sensitive measure of change in manic symptoms (Gracious et al. 2002; Youngstrom et al. 2004).
Subjects in the non-SMD group were carefully screened at study entry for symptoms of SMD, MDD, and BP. They were not reassessed for these symptoms during the STP because the primary focus of the STP was to assess the synergistic effects of BMOD and MPH on change in ADHD symptoms, not mood symptoms.
Side effects
The Pittsburgh Side Effect Rating Scale (PSERS), which rates common stimulant-related adverse events as none (0), mild (1), moderate (2), or severe (3), was completed daily by camp staff and parents (Pelham 1993).
Global Impairment Rating
Parents completed the IRS at baseline and end point to assess global functioning at home. The IRS converts narrative descriptions of functioning across five key domains into a global impairment rating using a 7-point scale (0 = no impairment, 6 = severe impairment). It has established psychometrics and has been used in several pediatric ADHD trials (Pelham et al. 2005a; Fabiano et al. 2006; Fabiano et al. 2007).
Data analysis
Independent sample t-tests were conducted to evaluate between-group differences in baseline characteristics and measures of tolerability (PSERS ratings). Pairwise t-tests were used in the SMD group to compare pre- and postdifferences in CDRS-R total score, YMRS total and cluster scores, as well as PSERS and PYMRS ratings from low-dose MPH to high-dose MPH. All t-tests were two tailed with significance set at p < 0.05. A Bonferroni correction was made for multiple comparisons (PSERS ratings and YMRS clusters). Chi-squared analyses were completed using cross-tabulation procedures to compute ORs comparing the groups on categorical variables, such as family history, and IRS (impaired or not). For the change in parent-rated DBD, a 4 (MPH dose) by 3 (BMOD dose) repeated-measures multivariate analysis of variance was performed using the general linear model procedure (SPSS Inc., Chicago IL).
The STP behavioral frequency counts for the outcome variables were measured as percentages (FAR and SC) were analyzed by fitting a binomial mixed model with a logit link. The between-subject variables (SMD status), within-subject variables (BMOD, MPH), and their interactions were included as independent variables in the model. A Poisson mixed model with a log link was fitted to explain the variability in noncompliance (NC), which was measured as a raw count, accounted by SMD status, MPH, BMOD, and their interactions. The NLMIXED procedure of SAS 9.1 was used to perform this analysis. Effect sizes of each dose of MPH and BMOD were then computed for FAR, SC, and NC by calculating mean differences between treatment and no-treatment (placebo, NBM), divided by the no-treatment SD. Independent sample t-tests were used to compare differences in effect sizes between the SMD and non-SMD groups.
Results
Externalizing symptoms
Groups had comparable baseline ADHD scores by teacher (Table 1, DBD ADHD scales, all p values > 0.1) and parent report (Table 2, DBD ADHD scales, all p values > 0.1). The SMD group had elevated baseline ODD (parent, t(98) = 5.1, p < 0.001; teacher, t(96) = 1.9), p < 0.05) and CD ratings (parent, t(98) = 2.6, p < 0.001; teacher, t(96) = 2.6, p < 0.01) on the DBD.
Table 2.
Parent Disruptive Behavior Disorder Scale Before and After Treatment
| |
SMD Group n = 33 |
Non-SMD Group n = 68 |
|
|
|
||
|---|---|---|---|---|---|---|---|
| Pre | Post | Pre | Post | Time | Group | Time × group | |
| Attention Mean (SD) | 18 (5.3) | 12.9 (5) | 17 (5.8) | 13.2 (5.8) | F = 84.8, p < .01 | F = .13 p < .71 | F = .055 p < .82 |
| Hyperactivity/impulsivity | 18.8 (5.3) | 11.7 (4.7) | 18 (6) | 11.7 (5.3) | F = 90.4 p < .01 | F = .06 p < .80 | F = .295 p < .59 |
| ODD | 16 (4.5) | 10.4 (4.9) | 11 (5.1) | 6.8 (3.5) | F = 88.2 p < .01 | F = 30.7 p < .01 | F = 1.43 p < .24 |
| CD | 7.6 (6.1) | 2.7 (2.6) | 5.1 (2.3) | 1.6 (1.9) | F = 52.3 p < .01 | F = 8.5 p < .01 | F = 2.3 p < .13 |
SMD = severe mood dysregulation; ODD = oppositional defiant disorder; CD = conduct disorder.
There was a multivariate main effect of time for all participants on total level of parent-rated externalizing behaviors on the DBD (F(1, 91) = 29.4, p < 0.001). As hypothesized, there was a significant reduction over time in ADHD, ODD, and CD symptoms (Table 2). Group differences in levels of ODD (F(1,91) = 30.7, p < 0.001) and CD symptoms (F(1,91) = 8.5, p < 0.0005) persisted. However, in contrast to what was expected, the group by time interaction was not significant for the ODD, CD, and ADHD subscales of the DBD.
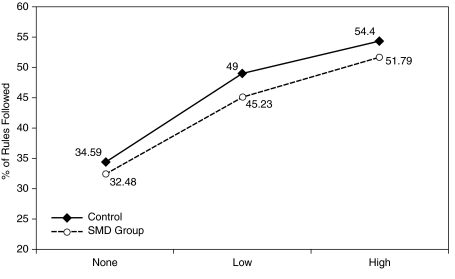
Similar results were found using the behavioral observations from the STP. In the mixed model, there was a significant effect of each MPH dose on FAR, F(3, 96) = 140; SC, F(3, 96) = 56.8; and NC, F(3, 96) = 360.1; all p values < 0.01. BMOD also significantly improved FAR, F(2, 96) = 673.8, SC F(2, 96) = 185 and NC, F(2, 96) = 414.22; all p values < 0.01. However, the interaction of SMD × MPH and SMD × BMOD was not significant in all cases except for SMD × MPH for NC where there was a low-intensity difference in the 0.3 mg/kg MPH dose that was not evidenced for placebo or the other two MPH doses. There were no significant group differences in the slope of the dose–response curves for BMOD or MPH for all three variables, suggesting that SMD status did not significantly alter the effectiveness of either treatment (Figs. 3 and 4).
FIG. 3.
Change in following activity rules with increasing methylphenidate (MPH) dose.
FIG. 4.
Change in following activity rules with increasing behavior modification therapy.
Effect sizes of each dose of MPH and BMOD were computed for FAR, SC, and NC. Because of the large daily variability in FAR as compared to SC and NC, subjects more than 2 standard deviations (SDs) beyond the group mean for FAR on each dose of MPH/BMOD were excluded from the effect size analysis.1 Within-subject effect sizes for both groups were robust with no significant differences between groups (Table 3). In the SMD group, 85% (28/33) showed a 50% or greater reduction in FAR, SC, and NC at their optimal combination of BMOD and MPH. For 55% (19/33) of the SMD group, their optimal combination consisted of the low or medium dose medication crossed with one of the active BMOD conditions.
Table 3.
Effect Sizes for STP Observed Behavior
| SMD M (SD) | Non-SMD M (SD) | t | |
|---|---|---|---|
| % of activity rules followed | |||
| LBM* | 0.52 (2.0) | 0.43 (2.9) | 0.16 |
| HBM* | 0.48 (3.1) | 0.70 (3.3) | −0.33 |
| .15* | 0.77 (1.0) | 0.87 (1.1) | −0.43 |
| .3* | 1.30 (0.8) | 1.70 (1.6) | −1.20 |
| .6* | 1.80 (1.1) | 2.10 (1.4) | −1.20 |
| Noncompliance with staff | |||
| LBM | 1.00 (1.8) | 0.94 (1.5) | 0.40 |
| HBM | 1.50 (1.8) | 1.10 (2.0) | 1.00 |
| .15 | 0.50 (1.8) | 0.70 (1.3) | −0.55 |
| .3 | 1.00 (1.7) | 1.30 (1.8) | −0.76 |
| .6 | 1.40 (1.7) | 1.30 (1.6) | 0.40 |
| % of seatwork completed | |||
| LBM | 0.65 (0.14) | 0.65 (0.14) | 0.13 |
| HBM | 0.67 (0.17) | 0.71 (0.10) | −1.10 |
| .15 | 0.60 (0.17) | 0.61 (0.14) | −0.69 |
| .3 | 0.65 (0.17) | 0.65 (0.14) | 0.01 |
.15 = 0.15 mg/kg per dose of methylphenidate (MPH); .3 = 0.3 mg/kg dose of MPH; .6 = 0.6 mg/kg dose of MPH.
Participants with values > 2 SDs beyond the mean were excluded from the analysis. All t-test values were nonsignificant.
SMD = severe mood dysregulation; SD = standard deviation; STP = Summer Treatment Program; LBM = low behavior modification; HBM = high behavior modification.
Tolerability
One SMD subject, who was discovered to have prominent PDD traits, was withdrawn after 3 weeks due to the mutual agreement of staff and family that the STP was not an appropriate treatment venue. There was no evidence of manic activation seen in this case. One non-SMD subject was withdrawn due to tic-like movements on medication. Neither parents nor staff reported any cases of manic activation, and no subjects required additional psychotropic medication during the STP.
MPH was well tolerated, with appetite suppression, insomnia, tics, and anxiety being the most common side effects. Parents reported more side effects than counselors. Side effects were most frequent at the 0.6mg/kg dose, with 11 subjects requiring reductions of this dose (2 SMD and 9 non-SMD subjects). Parent and staff PSERS ratings were analyzed for evidence of treatment-induced mood exacerbations by assessing change in the PSERS items seen in manic/depressed states (anxiety, tearful/depressed mood, withdrawn, hallucinations, trouble sleeping, and irritability). In SMD subjects, there was no significant increase in these side effects with increasing MPH dose except for trouble sleeping (t(31)= 2.6, p < 0.02) and appearing withdrawn (t(31) = 2.5, p < 0.02). Irritability ratings on the PSERS improved as MPH dose increased (t(31) = 2.8, p < 0.001). After correcting for multiple comparisons, there was no longer a difference in ratings of appearing withdrawn or trouble sleeping, but the improvements in irritability with increasing doses remained significant.
Parent PSERS ratings were averaged across days within each MPH dose to assess for differential tolerability between groups. There were significantly higher rates of anxiety (t(99) = 2.4, p < 0.05), irritability (t(99) = 3.0, p < 0.05), and tearfulness (t(99) = 2.1, p < 0.01) in SMD versus non-SMD subjects for all three MPH doses. However, all means were in the mild range (<0.5 on the 0 to 3 PSERS scale), and the differences persisted on placebo days.
Mood symptoms
Baseline mood ratings were consistent with a moderate level of manic-like symptoms and a mild level of depressive symptoms for SMD subjects. In the SMD group, 9 weeks of multimodal treatment was associated with a 34% improvement in YMRS scores (23.7 to 15.4 (SD = 5.8); t(32) = 8.2, p < 0.001) and a 31% improvement in CDRS-R scores (35 to 24 (SD = 6.4); t(32) = 9.8, p < 0.001). YMRS scores gradually declined as the STP progressed, with only 31% of the improvement occurring in the first week, increasing to 45% after 2 weeks. We divided the YMRS into three clusters to see which types of YMRS items were most responsive to treatment: The ODD cluster (irritability and disruptive/aggressive), the ADHD cluster (increased activity, speech and language), and the mania cluster based on the symptoms identified by Geller to be specific to BP versus ADHD (elevated mood, sexual interest, sleep, and content) (Geller et al. 2002). Correcting for multiple comparisons, there was a significant improvement in all three clusters (see Table 4). Change in the ODD cluster was responsible for 47% of the 8.3-point improvement in total YMRS score, while 23% was attributable to changes in the ADHD cluster and 25% of the total change occurred in the mania cluster. The remaining two items (appearance and insight) accounted for less than 5% of the total change.
Table 4.
Change in YMRS Scores from Baseline to End Point (N = 33)
| |
Baseline |
End point |
|
|
|
||||
|---|---|---|---|---|---|---|---|---|---|
| YMRS items | Mean | SD | % of total score | Mean | SD | % of total score | df | Test Value | p* |
| Total score | 23.7 | 3.53 | 15.4 | 6.15 | 32 | 8.3 | <.001 | ||
| ADHD items | 8.32 | 1.65 | 35% | 6.49 | 2.74 | 42% | 32 | 3.8 | <.001 |
| ODD items | 8.24 | 1.47 | 35% | 4.39 | 2.45 | 29% | 32 | 9.0 | <.001 |
| Mania specific items | 6.48 | 1.45 | 27% | 4.27 | 2.09 | 28% | 32 | 5.8 | <.001 |
Attention-deficit/hyperactivity disorder items are increased motor activity (#2), speech (#6), and language (#7).
Oppositional defiant disorder items are irritability (#5) and disruptive aggression behaviors (#9).
Mania-specific items are elevated mood (#1), sexual interest (#3), sleep (#4), and content (#8).
p set at 0.0125 to correct for multiple comparisons.
YMRS = Young Mania Rating Scale; SD = standard deviation; ADHD = attention-deficit/hyperactivity disorder; ODD = oppositional defiant disorder.
Unlike ADHD symptoms, which were measured daily at the STP, the individual effects of BMOD and MPH on YMRS and CDRS-R scores could not be assessed as they were completed weekly while MPH dose changed daily and BMOD varied every 3 weeks. Therefore, parents of SMD subjects completed the parent version of the YMRS (PYMRS) once for each of the 12 combinations of MPH × BMOD to assess for signs of manic activation in the 24 hours following a dose. There was no significant change in PYMRS scores with increasing MPH dose (p values > 0.1).
Global improvement
Baseline parent ratings on the IRS were similar between the groups (Table 1). Parents rated improvements in overall impairment for both groups at study completion (SMD group, t(32)= 4.6, p < 0.01; non-SMD group, t(63) = 6.2, p < 0.01). The mean end point IRS rating (0 = no impairment, 6 = severe impairment) for the SMD group was 4.1 (1.1) versus 3.6 (1.3) for non-SMD group, with a trend toward the SMD group being more impaired (t(95) = 1.4, p = 0.08). Using a categorical definition of remission (IRS <3), parents rated only 6% (2/31) of SMD subjects versus 27% (18/66) of non-SMD subjects as unimpaired after completion of the STP (OR = 4.7, 95% CI = 1.3–17.2, p = 0.03).
Discussion
Externalizing symptoms
There has been increasing debate surrounding the diagnostic relevancy of SMD and other manic-like symptoms in children with ADHD. However, there has been little investigation into the impact of these symptoms on the efficacy of ADHD treatments or into the development of treatments specifically for SMD. The evidence base to guide clinicians on whether children with SMD and ADHD should first receive stimulants and behavior therapy for ADHD or mood stabilizers for mood dysregulation is small. When such children manifest discrete mood episodes meeting criteria for BP, there is little debate that initial treatment should target mood stabilization. Yet more youths presenting for mental health evaluations will meet criteria for ADHD and SMD than BP (Lewinsohn et al. 1995; Kowatch et al. 2005; Pavuluri et al. 2005; Brotman et al. 2006). We attempted to address this dilemma by assessing the efficacy and tolerability BMOD and MPH, the two primary treatments for ADHD, in children with ADHD and manic-like SMD to determine if they are appropriate first-line interventions.
Both treatments were efficacious and well tolerated in children with SMD and elevated YMRS ratings. Given their additional symptom burden, we expected the SMD subjects to exhibit a reduced treatment response versus subjects with uncomplicated ADHD. Even though the SMD group exhibited higher levels of ODD and CD symptoms at baseline, the groups manifested similarly robust improvement across settings and assessment methods, comparable to prior STP studies combining BMOD and stimulants (Pelham et al. 1993; Pelham et al. 1999; Pelham et al. 2005a; Fabiano et al. 2007). The large within-subject effect sizes suggest that the study was sufficiently powered to detect any meaningful group differences. Of the 5 (15%) SMD subjects not displaying significant improvements at the STP, 4 demonstrated significant improvements in externalizing symptoms at home. Three of these subjects exhibited very low rates of externalizing symptoms at camp while the fourth had a highly variable response to BMOD, limiting the ability to detect treatment response. The fifth subject was withdrawn from the trial after 3 weeks, unrelated to mood symptoms.
Dose–response curves for BMOD and MPH were comparable between groups, implying that traditional ADHD dosing paradigms may be employed in children with ADHD and SMD. Similar to past STP trials and the MTA, combining BMOD with MPH led to reductions in optimal medication dosage without reducing efficacy (MTA 1999; Pelham et al. 2005a; Fabiano et al. 2007).
Because judicious use of stimulants is recommended for children with BP and ADHD (Kowatch et al. 2005; Delbello and Kowatch 2006), BMOD may be especially valuable for children with BP or those at increased risk for the disorder, because it reduces the stimulant dosage needed for optimal response.
Internalizing symptoms
While the change in YMRS and CDRS-R ratings was less than that seen in trials of antimanic medication in children with BP (Delbello et al. 2005; Delbello et al. 2006; Tohen et al. 2007), SMD subjects exhibited clinically relevant declines in YMRS (8 points) and CDRS-R scores (11 points), suggesting that the mood dysregulation seen in SMD may be responsive to ADHD-based treatments. The design of the STP makes it difficult to determine if it was time, BMOD, or MPH that led to the improvement in these scores. For example, the 8-point decline in YMRS scores matches that experienced by the placebo group in two double-blind trials of adolescent mania (Wagner et al. 2006; Tohen et al. 2007). However, YMRS scores improved gradually over the course of the STP, with less than half of the 8-point YMRS decline occurring in the first 2 weeks of camp. In contrast, almost all of the placebo response in medication trials for pediatric BP occurs in the first 1–2 weeks, suggesting that the STP treatments may have been the cause of the improvement (Wagner et al. 2006; Chang et al. 2007; Pandina et al. 2007).
In a recently published trial of olanzapine for pediatric BP, the aggression and irritability items improved by 20% in the placebo group versus 43% in the medication group (Tohen et al. 2007). In our study, aggression and irritability were the YMRS items (the ODD cluster in Table 4)showing the greatest change, improving by 45%, comparable to that seen with olanzapine. Hence, the degree of improvement during the STP for these two YMRS items was double that seen with placebo, suggesting a direct effect of treatment on irritability and aggression. Whether YMRS scores improved as a result of the provided therapies or from time, results suggest that the use of mood-stabilizing medications as a first-line treatment may not be necessary to achieve improvement in children with ADHD and SMD.
There is theoretical justification for the STP's intensive behavioral treatments producing improvements in mood because they target peer relations and incorporate CBT principles found to be helpful for pediatric depression and BP (Compton et al. 2004; Pavuluri et al 2004; Fristad 2006). In the MTA, subjects with ADHD, ODD, and internalizing symptoms, as well as those with manic-like symptoms, were most responsive to treatments integrating pharmacological and behavioral modalities (Jensen et al. 2001; Galanter et al. 2005).
Depressive symptoms essentially normalized, falling below the cutoff for remission on the CDRS-R. These results are consistent with published findings that mild depressive symptoms respond to supportive treatments (Birmaher and Brent 2007) and the recommendation to treat ADHD before depression, except in cases where the depressive symptoms are severe (Pliszka et al. 2006).
Tolerability
Similar to the MTA findings (Galanter et al. 2003; Galanter et al. 2005), ADHD treatments were well tolerated with no evidence of differential tolerability between the groups or cases of manic activation. The most common adverse events were traditional stimulant side effects such as appetite loss and sleep delay. Irritability, tearfulness, and anxiety reactions were more common in SMD subjects, but the between-group differences were quite small and persisted on placebo days. Dose reductions were not more frequent in SMD subjects, and PSERS irritability ratings improved with increasing MPH dose. These results suggest that side effect differences were likely due to greater baseline depressive and oppositional symptoms in SMD subjects versus differential treatment tolerability. In this protocol, MPH doses could vary from 0 to 1.8 mg/kg per day in a 24-hour span. If these marked dose fluctuations failed to produce manic activation, it is unlikely that the gradual MPH titrations employed in clinical practice would produce acute manic activation in children with no history of discrete manic episodes.
Group differences
The lack of appreciable group differences in the efficacy or tolerability of ADHD treatments suggests that SMD represents the severe end of the externalizing behavior spectrum rather than a distinct mood disorder. In support of this conclusion, one of the primary group differences was that SMD subjects manifested greater ODD/CD symptoms than non-SMD subjects. Other studies have found that most children with SMD also meet criteria for ODD and ADHD (Brotman et al. 2007; Dickstein et al. 2007; Guyer et al. 2007; Rich et al. 2007), making it difficult to determine if SMD represents a distinct diagnostic construct. Given the phenotypic similarities between severe ADHD + ODD, SMD, and the BP spectrum, investigators have searched for neuropsychological differences between the groups, but findings have been mixed. Dickstein found that children with SMD have deficits in selective attention seen in children with ADHD but not the impairments in cognitive flexibility seen in youths with the narrow BP phenotype (Dickstein et al. 2007). In contrast, Pine observed comparable impairments in social cognition and reciprocity between SMD and BP (Pine et al. 2008). Guyer also found that SMD and BP youths had similar deficits in emotional processing that were not seen in youths with ADHD, half of which had co-morbid ODD/CD (Guyer et al. 2007), suggesting that there may be additional impairments associated with SMD beyond that seen in ADHD + ODD. Family genetic studies have also produced mixed results, finding elevated rates of MDD but not BP in parents of SMD youths (Brotman et al. 2007). It remains unclear if children with SMD should be viewed as most comparable to ODD + ADHD or BP or neither, and thus it is necessary to develop treatment algorithms specifically for SMD. This study represents a first step in these efforts.
There were some findings in support of the SMD group having genuine affective co-morbidity. The SMD group tended toward higher rates of familial affective illness (71%) versus the non-SMD group (53%). The overall rate of familial affective illness in the SMD group was comparable to that found in the rigorously defined BP NOS subjects from the Course and Outcome Bipolar Youth Study (COBY) (Birmaher et al. 2006). Despite similar baseline impairment ratings and a comparably robust improvement in ADHD/ODD symptoms, SMD subjects were almost five times more likely than non-SMD subjects to remain significantly impaired at home after completion of the STP. While depressive symptoms normalized, manic symptoms (YMRS = 15) remained elevated above the level reported in typical ADHD samples (Fristad et al. 1992). Hence, the persistence of manic-like symptoms was associated with ongoing impairment at home. When the end point YMRS score was subdivided into the ODD, ADHD, and mania specific clusters (see Table 4), all three clusters contributed a sizable percentage to the total score. In addition, end point parent ratings of ADHD on the DBD (see Table 2)were not different between the groups, with both groups declining to a mild intensity of ADHD and ODD symptoms. These results suggest that the elevated impairment levels in the SMD group were not simply due to ADHD symptoms rebounding at home after MPH had worn off.
Because the STP provided an intensive 9-week multimodal intervention targeting both children and parents, it is unlikely that more ADHD treatments would lead to significant additional improvements. Regardless of their propensity for bipolarity, treatments beyond core ADHD therapies are likely needed to optimize functioning in children with ADHD and SMD.
Limitations
The primary limitation of the study is that it is unknown if the elevated YMRS scores in the SMD group were driven by ODD/CD symptoms or represent distinct impairments in mood regulation not seen in youths with pure externalizing behavior disorders. All STP subjects were thoroughly screened for SMD, but only those meeting SMD criteria were administered the YMRS and CDRS during the STP due to time and staffing constraints (it takes a trained clinician 60 minutes to complete this battery). Therefore, it is unknown if children with ADHD and ODD not meeting SMD criteria would also exhibit elevated YMRS ratings. However, prior work suggests this is unlikely (Fristad et al. 1992; Findling et al. 2005). The YMRS was selected as the mania symptom measure because it is the most commonly used efficacy scale in pediatric BP trials and reliably detects treatment effects (Carlson et al. 2003; Pavuluri et al. 2005; Scheffer et al. 2005; Findling et al. 2007). While the YMRS has normative data in ADHD populations showing that most children with ADHD will not score in the elevated range (Gracious et al. 2002; Fristad et al. 1992; Youngstrom et al. 2002), it was not designed to identify manic symptoms in children with ADHD. Most rating scales of pediatric BP are plagued by similar limitations due to the inherent difficulties with separating ODD + ADHD from BP at a single time point (Pavuluri et al. 2006; Youngstrom et al. 2006).
To address this limitation, additional criteria were added to the SMD definition to maximize the probability that SMD subjects exhibited mood symptoms beyond the irritability seen in ODD. Unlike the MTA analysis (Galanter et al. 2003), we used disease-specific ratings scale for depression and mania (CDRS-R, YMRS) administered by trained clinicians and collected ratings from children, parents and teachers. Subjects were only included in the SMD group if they scored 12+ on the YMRS and were identified on the CGI as having at least mild manic-like symptoms. Among all STP subjects diagnosed with ODD/CD on the DISC (see Table 1), only 48% met criteria for SMD, suggesting that SMD may represent more than ODD + ADHD. Our SMD subjects exhibited a mix of internalizing and externalizing symptoms resembling BP NOS and prodromal bipolar states described in other studies (Fergus et al. 2003; Leibenluft et al. 2003; Findling et al. 2005; Birmaher et al. 2006). However, their mood ratings were milder than subjects from pediatric BP trials (Delbello et al. 2005; Delbello et al. 2006; Wagner et al. 2006; Tohen et al. 2007).
Results should not be interpreted as promoting MPH and BMOD as effective treatments for BP because no SMD subjects met full criteria for BP, primarily due to the lack of sustained mood cycles that are thought to be a primary distinguishing feature of BP from ADHD (Geller et al. 2000; Leibenluft et al. 2003). While elevated scores on the YMRS have been identified as a risk factor for BP (Findling et al. 2007), our findings suggest that additional evidence is required before making a definitive diagnosis of BP in children with ADHD, because all of our subjects had elevated YMRS ratings yet none met criteria full for BP.
Improvements in ADHD symptoms did not appear to be the source of the YMRS improvements as only 23% of the total YMRS change occurred in items overlapping with ADHD symptoms (motor activity, speech, concentration). However, the YMRS heavily weights irritability and aggression, and our SMD group had almost 100% co-morbidity with ODD/CD. Almost 50% of the total YMRS improvement occurred in these two items, while 25% was in symptoms reported to be specific to mania over ADHD (elated mood, hypersexuality, decreased sleep, content) (Geller et al. 2002), suggesting that improvement in ODD/CD symptoms may have accounted for the majority of the YMRS change. Our results are similar to those of a recently published controlled trial of olanzapine in adolescents with BP I where only 24% of the YMRS improvement occurred in core mania items other than sleep (sleep excluded because of the sedating effects of olanzapine) (Tohen et al. 2007). These findings suggest that for children, it may be harder to induce or detect sizable changes in core manic symptoms than in irritability/aggression, regardless of the prescribed treatment. These limitations do not negate the finding that children with ADHD, SMD, and elevated YMRS scores appear to respond positively to stimulants and behavior therapy.
The trial lasted only 9 weeks and could not address the long-term risks of stimulants. However, this trial was twice as long as other stimulant trials in children with BP (Scheffer et al. 2005; Finding et al. 2007). We are continuing to monitor the SMD subjects for the emergence of BP to address this limitation. Moreover, a recent analysis of a different subject pool of 364 young adults with ADHD found no significant association between childhood stimulant usage and the development of BP, despite finding an elevated prevalence of BP in the ADHD subjects (Waxmonsky et al. 2007).
Whereas combined treatment led to improvements in mood with no cases of manic activation, the design of the larger STP trial prevented us from parceling out the individual effects of MPH and BMOD on mood symptoms because they were rated weekly while MPH varied daily and BMOD changed every 3 weeks. It was deemed impractical to complete mood assessments on a daily basis. Moreover, pediatric mood ratings are usually not measured daily due to their high degree of temporal variability. As in the Treatment of Adolescent Depression Study (TADS), it is possible that the behavioral treatments may have suppressed negative emotional outcomes precipitated by medication (March et al. 2004). Sarampote reported successful treatment of an affectively labile 7-year-old girl with combined ADHD treatments after stimulants had failed (Sarampote et al. 2002), suggesting that mood-dysregulated ADHD youths may do best with combination therapies. Nonetheless, these tolerability findings for MPH cannot be safely generalized to the treatment of SMD youths not engaged in behavioral therapies.
The STP design also complicated interpretation of the effects of BMOD on mood ratings because subjects were in different BMOD intensities at end point, with a third ending the STP in the “No BMOD” condition. This methodological issue may have limited the ability to detect the full impact of BMOD on mood ratings. In addition, treating subjects with three different intensities of BMOD during the course of the STP may have reduced the impact on mood symptoms versus continuous use of high-intensity BMOD. However, we observed a significant improvement in mood ratings despite this limitation.
Last, like many pediatric ADHD studies, subjects were predominantly Caucasian and middle class, limiting the ability to generalize findings.
Clinical implications
Many children with ADHD are also identified as having problems with SMD. The debate over whether SMD belongs in the bipolar spectrum versus the severe end of the externalizing behavior spectrum has led to significant controversy as to what should constitute the initial treatment for children with ADHD and SMD: Stimulants and behavior therapy for ADHD or mood-stabilizing medications for affective lability? No controlled-treatment studies in children with SMD have been completed that would address this question. Therefore, we evaluated the tolerability and efficacy of stimulants and behavior therapy in this population.
We found that BMOD and MPH were tolerable and effective treatments in children with SMD. There was no evidence that SMD moderated the efficacy or tolerability of either treatment. Multimodal treatment significantly reduced the entire spectrum of externalizing symptoms and improved ratings of depression and mania. Although additional treatments may be needed to optimize functioning in the home, there appeared to be little acute risk of either treatment precipitating manic states in these children.
Results suggest that in children with ADHD and severe mood dysregulation, but otherwise not meeting full criteria for BP, it is reasonable to initiate pharmacological and behavioral treatments for ADHD and reassess the severity of mood dysregulation after ADHD treatments have been optimized. Similar strategies have been recommended for the treatment of ADHD youths with anxiety and depression (Greenhill et al. 2002; Pliszka et al. 2006). We strongly caution against interpretation of results as promoting behavior modification or stimulants as safe and effective treatments in children with BP because it is unknown if children with SMD have genuine bipolar spectrum illness. Whenever BP is clearly present, it should be treated prior to any attempts to address ADHD pharmacologically, because these treatments could produce dramatically different and detrimental results in bipolar youths. However, further studies of multimodal ADHD treatments in youths with bipolar spectrum illness are warranted given these promising results.
Footnotes
Omission of outliers for the FAR analyses led to exclusion of 5 subjects for LBM, 4 for HBM, 9 for 0.15 mg/kg MPH, 6 for 0.3 mg/kg MPH, and 8 for 0.6 mg/kg MPH. Inclusion of outliers in the FAR analyses impacted only the 0.6 mg/kg dose where the adjusted effect size was 1.77 for SMD subjects and 2.81 for the non-SMD group (t = −1.7, p < 0.05) vs. 1.80 and 2.10 respectively (p > 0.1) when outliers were excluded (see Table 3).
This study was funded by National Institute of Mental Health (NIMH) grant MH62946 to Dr. Pelham and a Klingenstein Third Generation Foundation Fellowship in Child and Adolescent Depression Research to Dr. Waxmonsky.
Findings from this study have previously been presented at the 52nd Annual Meeting of the American Academy of Child and Adolescent Psychiatry, October, 2005, Toronto, Canada.
Disclosures
Dr. Waxmonsky serves on the Speaker's Bureau for Novartis, received honoraria from Shire and Scepter, and has received research support from Eli Lilly and Shire Laboratories. Dr. Pelham has served as a consultant for Shire, McNeil, Noven, Celltech/Medeva, Novartis, and Abbott Laboratories; received honoraria from Shire and research support from Shire, Alza, Eli Lilly, Noven, and Cephalon; and holds common stock in Abbott Laboratories. Dr. Hoffman has received research support from Shire and also serves on their advisory board and speaker's bureau. Dr. Waschbusch has received research support from Eli Lilly. Drs. Cummings, Majumdar, Massetti, Burrowns-MacLean, Fabiano, and Chacko and Ms. Gnagy, Ms. O'Connor, Ms. Verley, Ms. Arnold, Ms. Walker, Ms. Garefino, and Ms. Robb have no conflicts of interest or financial ties to report.
Several authors have relocated since completion of the study. Dr. Chacko is now affiliated with the Department of Psychiatry at the Mt. Sinai School of Medicine. Dr Massetti is now with the Centers for Disease Control and Prevention. Dr. Majumdar is now a full time employee of Bristol Meyers Squib.
References
- Achenbach TM. Edelbrock CS. Manual for Child Behavior Checklist and Revised Child Behavior Profile. Burlington, VT: University of Vermont Department of Psychiatry; 1983. [Google Scholar]
- American Psychiatric Associatio. Diagnostic and Statistical Manual of Mental Disorders. 4th. Washington (DC): American Psychiatric Association; 1994. [Google Scholar]
- Baumer FM. Howe M. Gallelli K. Simenova DI. Hallymeyer J. Chang KD. A pilot study of antidepressant induced mania in pediatric bipolar disorder: Characteristics, risk factors and the serotonin transporter gene. Biol Psychiatry. 2006;60:1005–12. doi: 10.1016/j.biopsych.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Biederman J. Mick E. Bostic JQ. Prince J. Daly J. Wilens TE. Spencer T. Garcia-Jetton J. Russell R. Wozniak J. Faraone SV. The naturalistic course of pharmacologic treatment of children with manic-like symptoms: A systematic chart review. J Clin Psych. 1998;59:628–637. doi: 10.4088/jcp.v59n1111. [DOI] [PubMed] [Google Scholar]
- Biederman J. Mick E. Prince J. Bostic JQ. Wilens TE. Spencer TS. Wozniak JW. Faraone SV. Systematic chart review of the pharmacologic treatment of comorbid attention-deficit/hyperactivity disorder in youth with bipolar disorder. J Child Adolesc Psychopharmacol. 1999;9:247–256. doi: 10.1089/cap.1999.9.247. [DOI] [PubMed] [Google Scholar]
- Biederman J. Mick E. Faraone SV. Van Patten S. Burback M. Wozniak J. A prospective follow-up study of pediatric bipolar disorder in boys with attention-deficit/hyperactivity disorder. J Affect Disord. 2004;82s:S17–S23. doi: 10.1016/j.jad.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Birmaher B. Brent D. Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry. 2007;46:1503–1526. doi: 10.1097/chi.0b013e318145ae1c. [DOI] [PubMed] [Google Scholar]
- Birmaher B. Axelson D. Strober M. Gill MK. Valeri S. Chiappetta L. Ryan N. Leonard H. Hunt J. Iyengar S. Keller M. Clinical course of children and adolescents with bipolar spectrum disorders. Arch Gen Psychiatry. 2006;63:175–186. doi: 10.1001/archpsyc.63.2.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blader JC. Carlson GA. Increased rates of bipolar disorder diagnoses among U.S. child, adolescent, and adult inpatients, 1996–2004. Biol Psychiatry. 2007;62:107–114. doi: 10.1016/j.biopsych.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman MA. Schmajuk M. Rich B. Dickstein D. Guyer Costello J. Egger H. Angold A. Leibenluft A. Prevalence, clinical correlates and longitudinal course of severe mood dysregulation in children. Biol Psychiatry. 2006;60:991–997. doi: 10.1016/j.biopsych.2006.08.042. [DOI] [PubMed] [Google Scholar]
- Brotman MA. Kassem L. Reising MM. Guyer AE. Dickstein DP. Rich BA. Towbin KE. Pine DS. McMahon FJ. Leibenluft E. Parental diagnoses in youth with narrow phenotype bipolar disorder or severe mood dysregulation. Am J Psych. 2007;164:1238–1241. doi: 10.1176/appi.ajp.2007.06101619. [DOI] [PubMed] [Google Scholar]
- Carlson GA. Kelly KL. Manic symptoms in psychiatrically hospitalized children—what do they mean ? J Affect Disord. 1998;51:123–135. doi: 10.1016/s0165-0327(98)00211-0. [DOI] [PubMed] [Google Scholar]
- Carlson GA. Mick E. Drug-induced disinhibtion in psychiatrically hospitalized children. J Child Adolesc Psychopharm. 2003;13:153–163. doi: 10.1089/104454603322163871. [DOI] [PubMed] [Google Scholar]
- Carlson GA. Youngstrom EA. Clinical Implications of pervasive manic symptoms in children. Biol Psychiatry. 2003;53:1050–1058. doi: 10.1016/s0006-3223(03)00068-4. [DOI] [PubMed] [Google Scholar]
- Carlson GA. Loney J. Salisbury H. Volpe RJ. Young referred boys with DICA-P manic symptoms vs. two comparison groups. J Affect Disord. 1998;121:113–121. doi: 10.1016/s0165-0327(98)00210-9. [DOI] [PubMed] [Google Scholar]
- Carlson GA. Loney J. Salisbury H. Kramer JR. Arthur C. Stimulant treatment in young boys with symptoms suggesting childhood mania: A report from a longitudinal study. J Child Adolesc Psychopharm. 2000;10:175–184. doi: 10.1089/10445460050167287. [DOI] [PubMed] [Google Scholar]
- Carlson GA. Jensen PS. Findling RL. Meyer RE. Calabrese J. DelBello MP. Emslie G. Flynn L. Goodwin F. Hellander M. Kowatch R. Kusumakar V. Laughren T. Leibenluft E. McCracken J. Nottelmann E. Pine D. Sachs G. Shaffer D. Simar R. Strober M. Weller EB. Wozniak J. Youngstrom EA. Methodological issues and controversies in clinical trials with child and adolescent patients with bipolar disorder: Report of a consensus conference. J Child Adolesc Psychopharmacology. 2003;13:13–27. doi: 10.1089/104454603321666162. [DOI] [PubMed] [Google Scholar]
- Chang KD. Nylias M. Aurang C. Johnson B. Jin N. Marcus R. Forbes RA. Carson WH. Kahn A. Findling RL. Efficacy of aripiprazole in children with mania. Scientific Proceedings of the 2007 Annual Meeting of the American Academy of Child and Adolescent Psychiatry; Boston MA. 2007. [Google Scholar]
- Compton SN. March JS. Brent D. Albano AM. Weersing R. Curry J. Cognitive-behavioral psychotherapy for anxiety and depressive disorders in children and adolescents: An evidence-based medicine review. J Am Acad Child Adolesc Psychiatry. 2004;43:930–959. doi: 10.1097/01.chi.0000127589.57468.bf. [DOI] [PubMed] [Google Scholar]
- Correll CU. Carlson HE. Endocrine and metabolic adverse effects of psychotropic medications in children and adolescents. J Am Acad Child Adolesc Psychiatry. 2006;45:771–91. doi: 10.1097/01.chi.0000220851.94392.30. [DOI] [PubMed] [Google Scholar]
- Cunningham CE. Bremner R. Secord-Gilbert M. The community parent education (cope) program: A school-based family systems oriented course for parents of children with disruptive behavior disorders. Chedoke-McMaster Hospitals and McMaster University; 1998. [Google Scholar]
- Daviss WB. Bentivoglio P. Racusin R. Brown KM. Bostic JQ. Wiley L. Bupropion sustained release in adolescents with comorbid attention-deficit/hyperactivity disorder and depression. J Am Acad Child Adolesc Psychiatry. 2001;40:307–314. doi: 10.1097/00004583-200103000-00010. [DOI] [PubMed] [Google Scholar]
- Delbello M. Kowatch R. Pharmacological interventions for bipolar youth: Developmental considerations. Dev Psychopathol. 2006;18:1231–1246. doi: 10.1017/S0954579406060597. [DOI] [PubMed] [Google Scholar]
- Delbello MP. Soutullo CA. Hendricks W. Niemeier RT. McElroy SL. Strakowski SM. Prior stimulant treatment in adolescents with bipolar disorder: Association with age at onset. Bipolar Disord. 2001;3:53–57. doi: 10.1034/j.1399-5618.2001.030201.x. [DOI] [PubMed] [Google Scholar]
- Delbello MP. Findling RL. Kushner S. Wang D. Olson WH. Capece JA. Fazzio L. Rosenthal NR. A pilot controlled trial of topiramate for mania in children and adolescents with bipolar disorder. J Am Acad Child Adolesc. 2005;44:539–547. doi: 10.1097/01.chi.0000159151.75345.20. [DOI] [PubMed] [Google Scholar]
- DelBello MP. Kowatch RA. Adler CM. Stanford KE. Welge JA. Barzman DH. Nelson E. Strakowski SM. A double-blind randomized pilot study comparing quetiapine and divalproex for adolescent mania. J Am Acad Child Adolesc. 2006;45:305–313. doi: 10.1097/01.chi.0000194567.63289.97. [DOI] [PubMed] [Google Scholar]
- Delbello MP. Adler CM. Whitsel RM. Stanford SE. Strakowski SM. A 12 week single blind trial for quetiapine for the treatment of mood symptoms in adolescents at high risk for developing Bipolar I Disorder. J Clin Psychiatry. 2007;68:789–795. doi: 10.4088/jcp.v68n0520. [DOI] [PubMed] [Google Scholar]
- Dickstein DP. Nelson EE. McClure EB. Grimley ME. Knopf L. Brotman MA. Rich BA. Pine DS. Leibenluft E. Cognitive flexibility in phenotypes of pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:341–455. doi: 10.1097/chi.0b013e31802d0b3d. [DOI] [PubMed] [Google Scholar]
- Fabiano GA. Pelham WE. Gnagy EM. Waschbusch DA. Lahey BB. Chronis AM. Onyango AN. Kipp H. Williams A. Burrows-McLean L. A practical measure of impairment: Psychometric properties of the Impairment Rating Scale in samples of children with attention deficit hyperactivity disorder and two school-based samples. J Clin Child Adolesc Psych. 2006;35:369–385. doi: 10.1207/s15374424jccp3503_3. [DOI] [PubMed] [Google Scholar]
- Fabiano G. Pelham WE. Gnagy EM. Waschbusch DA. Lahey BB. Chronis AM. Onyango AN. Kipp H. Williams A. The single and combined effects of multiple intensities of behavior modification and methylphenidate for children with attention deficit hyperactivity disorder in a classroom setting. School Psych Review. 2007;36:195–216. [Google Scholar]
- Faedda GL. Baldessarini RJ. Glovinsky IP. Austin NB. Treatment-emergent mania in pediatric bipolar disorder: A retrospective case review. J Affect Disord. 2004;82:149–158. doi: 10.1016/j.jad.2003.12.011. [DOI] [PubMed] [Google Scholar]
- FDA. FDA warning for stimulants in patients with bipolar disorders. http://www.fda.gov/medwatch/safety/2006/Dexedrine_DHCP_Letter.pdf-08-21-2006/ http://www.fda.gov/medwatch/safety/2006/Dexedrine_DHCP_Letter.pdf-08-21-2006/
- Fergus EL. Miller RB. Luckenbaugh DA. Leverich GS. Findling RL. Speer A. Post RM. Is there progression from irritability/dyscontrol to major depressive, manic symptoms? A retrospective community survey of parents of bipolar children. J Affect Disord. 2003;77:71–78. doi: 10.1016/s0165-0327(02)00176-3. [DOI] [PubMed] [Google Scholar]
- Findling RL. Youngstrom EA. McNamara NK. Stansbrey RJ. Demeter CA. Bedoya D. Kahana SY. Calabrese JR. Early symptoms of mania and the role of parental risk. Bipolar Disord. 2005;7:623–634. doi: 10.1111/j.1399-5618.2005.00260.x. [DOI] [PubMed] [Google Scholar]
- Findling RL. Short EJ. McNamara NK. Demeter CA. Stansbrey RJ. Gracious BL. Whipkey R. Manos MJ. Calabrese JR. Methylphenidate in the treatment of children and adolescents with bipolar disorder and attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2007;46:1445–1453. doi: 10.1097/chi.0b013e31814b8d3b. [DOI] [PubMed] [Google Scholar]
- Fristad MA. Psychoeducational treatment for school-aged children with bipolar disorder. Dev Psychopathol. 2006;18:1289–1306. doi: 10.1017/S0954579406060627. [DOI] [PubMed] [Google Scholar]
- Fristad MA. Weller EB. Weller RA. The Mania Rating Scale: Can it be used in children? A preliminary report. J Am Acad Child Adolesc Psychiatry. 1992;31:252–257. doi: 10.1097/00004583-199203000-00011. [DOI] [PubMed] [Google Scholar]
- Gadow KD. Nolan EE. Sverd J. Sprafkin J. Schwartz J. Anxiety and depression symptoms and response to methylphenidate in children with attention-deficit hyperactivity disorder and tic disorder. J Clin Psychopharmacol. 2002;22:267–274. doi: 10.1097/00004714-200206000-00007. [DOI] [PubMed] [Google Scholar]
- Galanter CA. Carlson GA. Jensen PS. Greenhill LL. Davies M. Li W. Chuang SZ. Elliott GR. Arnold LE. March JS. Hechtman L. Pelham WE. Swanson JM. Response to methylphenidate in children with attention deficit hyperactivity disorder and manic symptoms in the multimodal treatment study of children with attention deficit hyperactivity disorder titration trial. J Child Adolesc Psychopharmacol. 2003;13:123–136. doi: 10.1089/104454603322163844. [DOI] [PubMed] [Google Scholar]
- Galanter CA. Pagar DL. Davies M. Li W. Carlson G. Abikoff HB. Arnold LE. Bukstein OG. Pelham W. Elliott GR. Hinshaw S. Epstein JN. Wells K. Hechtman L. Newcorn JH. Greenhill L. Wigal T. Swanson JM. Jensen PS. ADHD and manic symptoms: Diagnostic and treatment implications. Clin Neurosci Res. 2005;5:283–294. [Google Scholar]
- Geller B. Zimmerman B. Williams M. Bolhofner K. Craney JL. Delbello MP. Soutullo CA. Diagnostic characteristics of 93 cases of prepubertal, early adolescent bipolar disorder phenotype by gender puberty and comorbid ADHD. J Child Adolesc Psychopharmacol. 2000;10:157–164. doi: 10.1089/10445460050167269. [DOI] [PubMed] [Google Scholar]
- Geller B. Zimmerman B. Williams M. Delbello M. Bolhofner K. Craney J. Frazier J. Beringer L. Nickelsburg M. DSM IV mania symptoms in prepubertal and early adolescent bipolar disorder phenotype compared to attention deficit hyperactive and normal controls. J Child Adolesc Psychopharmacol. 2002;12:11–25. doi: 10.1089/10445460252943533. [DOI] [PubMed] [Google Scholar]
- Gracious BL. Youngstrom EA. Findling RL. Calabrese JR. Discriminative validity of a parent version of the Young Mania Rating Scale. J Am Acad Child Adolesc Psychiatry. 2002;41:1350–1359. doi: 10.1097/00004583-200211000-00017. [DOI] [PubMed] [Google Scholar]
- Greenhill LL. Pliszka S. Dulcan MK. Bernet W. Arnold V. Beitchman J. Benson RS. Bukstein O. Kinlan J. McClellan J. Rue D. Shaw JA. Stock S. American Academy of Child and Adolescent Psychiatry. Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adoles Psychiatry. 2002;41(2 Suppl):26S–49S. doi: 10.1097/00004583-200202001-00003. [DOI] [PubMed] [Google Scholar]
- Guy W. ECDEU Assessment Manual for Psychopharmacology. Washington, DC: U.S. Department of Health, Education and Welfare; 1976. [Google Scholar]
- Guyer AE. McClure EB. Adler AD. Brotman MA. Rich BA. Kimes AS. Pine DS. Ernst M. Leibenluft E. Specificity of facial expression labeling deficits in childhood psychopathology. J Child Psychol Psychiatry Allied Disciplines. 2007;48:863–871. doi: 10.1111/j.1469-7610.2007.01758.x. [DOI] [PubMed] [Google Scholar]
- Jensen PS. Hinshaw SP. Kraemer HC. Lenora N. Newcorn JH. Abikoff HB. March JS. Arnold EL. Cantwell DS. Conners CK. Elliott GR. Greenhill LL. Hechtman L. Hoza B. Pelham WE. Severe JB. Swanson JM. Wells KC. Wigal T. Vitiello B. ADHD comorbidity findings from the MTA study: Comparing comorbid subgroups. J Am Acad Child Adolesc Psychiatry. 2001;40:147–158. doi: 10.1097/00004583-200102000-00009. [DOI] [PubMed] [Google Scholar]
- Kowatch RA. Fristad M. Birmaher B. Wagner KD. Findling RL. Hellander M. Treatment guidelines for children and adolescents with bipolar disorder: Child Psychiatric Workgroup on Bipolar Disorder. J Am Acad Child Adolesc Psychiatry. 2005;44:213–235. doi: 10.1097/00004583-200503000-00006. [DOI] [PubMed] [Google Scholar]
- Leibenluft E. Chaney D. Towbin KE. Bhangoo RK. Pine DS. Defining clinical phenotypes of juvenile mania. Am J Psychiatry. 2003;160:430–437. doi: 10.1176/appi.ajp.160.3.430. [DOI] [PubMed] [Google Scholar]
- Lewinsohn PM. Kelin DN. Seeley JR. Bipolar disorders in a community sample of older adolescents: Prevalence, phenomenology, comorbidity, and course. J Am Acad Child Adolesc Psychiatry. 1995;34:454–463. [PubMed] [Google Scholar]
- March J. Silva S. Petrycki S. Curry J. Wells K. Fairbank J. Burns B. Domino M. McNulty S. Vitiello B. Severe J. Treatment for Adolescents With Depression Study (TADS) Team (2004), fluoxetine, cognitive-behavioral therapy, and their combination for adolescents with depression: Treatment for Adolescents With Depression Study (TADS) JAMA. 2004;292:807–820. doi: 10.1001/jama.292.7.807. [DOI] [PubMed] [Google Scholar]
- Moreno C. Laje G. Blanco C. Jiang H. Schmidt AB. Olfson M. National trends in the outpatient diagnosis and treatment of bipolar disorder in youth. Arch Gen Psychiatry. 2007;64:1032–1039. doi: 10.1001/archpsyc.64.9.1032. [DOI] [PubMed] [Google Scholar]
- MTA Cooperative Group. 14-month randomized clinical trial of treatment strategies for attention deficit hyperactivity disorder. Arch Gen Psychiatry. 1999;56:1073–1086. doi: 10.1001/archpsyc.56.12.1073. [DOI] [PubMed] [Google Scholar]
- Pandina G. Delbello M. Kushner S. Van Hove I. Augustyns I. Kusumakar V. Hass M. Risperidone for the treatment of acute mania in bipolar youth. Scientific Proceedings of the 2007 Annual Meeting of the American Academy of Child and Adolescent Psychiatry; Boston MA. 2007. [Google Scholar]
- Papolos DF. Papolos J. The Bipolar Child. New York: Broadway Books; 1999. [Google Scholar]
- Patel NC. Patrick DM. Youngstrom EA. Strakowski SM. Delbello MP. Response and remission in adolescent mania: Signal detection analyses of the Young Mania Rating Scale. J Am Acad Child Adolesc Psych. 2007;46:628–635. doi: 10.1097/chi.0b013e3180335ae4. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN. Graczyk PA. Henry DB. Carbray JA. Heidenreich JL. Miklowitz DJ. Child and family focused cognitive-behavioral therapy for pediatric bipolar disorder: Development and preliminary results. J Am Acad Child Adolesc Psychiatry. 2004;43:528–537. doi: 10.1097/00004583-200405000-00006. [DOI] [PubMed] [Google Scholar]
- Pavuluri MN. Birmaher B. Naylor MW. Pediatric bipolar disorder: A Review of the past 10 years. J Am Acad Child Adolesc Psychiatry. 2005;44:846–871. doi: 10.1097/01.chi.0000170554.23422.c1. [DOI] [PubMed] [Google Scholar]
- Pelham W. Pharmacotherapy for children with ADHD. School Psych Review. 1993;22:199–227. [Google Scholar]
- Pelham WE. Fabiano GA. Evidence- Based psychosocial treatments for attention-deficit hyperactivity disorder. J Clin Child Adolesc Psych. 2008;37:184–214. doi: 10.1080/15374410701818681. [DOI] [PubMed] [Google Scholar]
- Pelham WE. Gnagy EM. Greenslade KE. Milich R. Teacher ratings of DSM-III-R symptoms of the disruptive behavior disorders. J Am Acad Child Adolesc Psych. 1992;31:210–218. doi: 10.1097/00004583-199203000-00006. [DOI] [PubMed] [Google Scholar]
- Pelham WE. Carlson C. Sams SE. Vallano G. Dixon MJ. Hoza B. Separate and combined effects of methylphenidate and behavior modification on boys with ADHD in the classroom. J Consult Clin Psychiatry. 1993;61:506–515. doi: 10.1037/0022-006X.61.3.506. [DOI] [PubMed] [Google Scholar]
- Pelham WE. Greiner A. Gnagy EM. Children's Summer Treatment Program Manual. Buffalo, NY: Comprehensive Treatment for Attention Disorders, Inc; 1997. [Google Scholar]
- Pelham WE. Aronoff HR. Midlam JK. Shapiro CJ. Gnagy EM. Chronis AM. Onyango AN. Forehand G. Nguyen A. Waxmonsky J. A comparison of Ritalin and Adderall: Efficacy, time-course in children with attention-deficit/hyperactivity disorder. Pediatrics. 1999;103:e43. doi: 10.1542/peds.103.4.e43. [DOI] [PubMed] [Google Scholar]
- Pelham WE. Burrows-Maclean L. Gnagy EM. Fabiano GA. Coles EK. Tresco KE. Chacko A. Wymbs BT. Wienke AL. Walker KS. Hoffman MT. Transdermal methylphenidate, behavioral, and combined treatment for children with ADHD. Exp Clin Psychopharmacol. 2005a;13:111–26. doi: 10.1037/1064-1297.13.2.111. [DOI] [PubMed] [Google Scholar]
- Pelham WE. Fabiano GA. Gnagy EM. Greiner A. Hoza B. Comprehensive psychosocial treatment for ADHD. In: Hibbs E, editor; Jensen P, editor. Psychosocial Treatments for Child and Adolescent Disorders: Empirically Based Strategies for Clinical Practice. Washington, DC: American Psychological Association; 2005b. pp. 377–410. [Google Scholar]
- Pine DS. Guyer AE. Goldwin M. Towbin K. Leibenluft E. Autism spectrum disorder scale scores in pediatric mood and anxiety disorders. J Am Acad Child Adoles Psychiatry. 2008;47:652–661. doi: 10.1097/CHI.0b013e31816bffa5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pliszka SR. Crismon ML. Hughes CW. Conners CK. Emslie GJ. Jensen PS. McCracken JT. Swanson JM. Lopez M. The Texas Consensus Panel on Pharmacotherapy on Attention-Deficit/Hyperactivity Disorder, The Texas Children's Medication Algorithm Project: Revision of the algorithm for pharmacotherapy for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2006;45:642–657. doi: 10.1097/01.chi.0000215326.51175.eb. [DOI] [PubMed] [Google Scholar]
- Poznanski EO. Grossman JA. Buchsbaum Y. Banegas M. Freeman L. Gibbons R. Preliminary studies of the reliability and validity of the children's depression rating scale. J Am Acad Child Adolesc Psychiatry. 1984;23:191–197. doi: 10.1097/00004583-198403000-00011. [DOI] [PubMed] [Google Scholar]
- Poznanski EO. Mikos H. Children's Depression Rating Scale Revised (CDRS-R) Los Angeles: Western Psychological Services; 1996. [Google Scholar]
- Reichart CG. Nolen WA. Earlier onset of bipolar disorder in children by antidepressants or stimulants? A hypothesis. J Affect Disord. 2004;78:81–84. doi: 10.1016/s0165-0327(02)00180-5. [DOI] [PubMed] [Google Scholar]
- Rich BA. Schmajuk M. Perez-Edgar KE. Pine DS. Leibenluft E. Different psychophysiological and behavioral responses elicited by frustration in pediatric bipolar disorder and severe mood dysregulation. Am J Psych. 2007;164:309–317. doi: 10.1176/ajp.2007.164.2.309. [DOI] [PubMed] [Google Scholar]
- Ross RG. Psychotic and manic-like symptoms during stimulant treatment of attention deficit hyperactivity disorder. Am J Psych. 2006;163:1149–1152. doi: 10.1176/ajp.2006.163.7.1149. [DOI] [PubMed] [Google Scholar]
- Sarampote CS. Efron LA. Robb AS. Pearl PL. Stein MA. Can stimulant rebound mimic pediatric bipolar disorder? J Child Adolesc Psychopharmacol. 2002;12:63–67. doi: 10.1089/10445460252943588. [DOI] [PubMed] [Google Scholar]
- Scheffer RE. Kowatch RA. Carmody T. Rush J. Randomized, placebo controlled trial of mixed amphetamine salts for symptoms of comorbid ADHD in pediatric bipolar disorder after mood stabilization with divalproex sodium. Am J Psychiatry. 2005;162:58–64. doi: 10.1176/appi.ajp.162.1.58. [DOI] [PubMed] [Google Scholar]
- Shaffer D. Fisher P. Lucas CP. Dulcan MK. Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): Description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Soutullo CA. DelBello MP. Ochsner JE. McElroy SL. Taylor SA. Strakowski SM. Keck PE. Severity of bipolarity in hospitalized manic adolescents with history of stimulant or antidepressant treatment. J Affect Disord. 2002;70:323–327. doi: 10.1016/s0165-0327(01)00336-6. [DOI] [PubMed] [Google Scholar]
- Swanson JM. Kraemer HC. Hinshaw SP. Arnold LE. Conners CK. Abikoff HB. Clevenger W. Davies M. Elliott GR. Greenhill LL. Hechtman L. Hoza B. Jensen PS. March JS. Newcorn JH. Owens EB. Pelham WE. Schiller E. Severe JB. Simpson S. Vitiello B. Wells K. Wigal T. Wu M. Clinical relevance of the primary findings of the MTA: Success rates based on severity of ADHD and ODD symptoms at the end of treatment. J Am Acad Child Adolesc Psychiatry. 2001;40:168–179. doi: 10.1097/00004583-200102000-00011. [DOI] [PubMed] [Google Scholar]
- Tillman R. Geller B. Frazier J. Zimerman B. Klages T. Bolhofner K. Children with a prebubertal and early adolescent phenotype from pediatric versus psychiatric facilities. J Am Acad Child Adolesc Psychiatry. 2005;44:776–781. doi: 10.1097/01.chi.0000164588.10200.ed. [DOI] [PubMed] [Google Scholar]
- Tohen M. Kryzhanovskaya L. Carlson G. Delbello M. Wozniak J. Kowatch R. Wagner K. Findling R. Lin D. Roberston-Plouch C. Xu W. Dittman R. Biederman J. Olanzapine versus placebo in the treatment of adolescents with bipolar mania. Am J Pysch. 2007;164:1547–1556. doi: 10.1176/appi.ajp.2007.06111932. [DOI] [PubMed] [Google Scholar]
- Vitiello B. Long term effects of stimulant medications on the brain: possible relevance to the treatment of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2001;11:25–34. doi: 10.1089/104454601750143384. [DOI] [PubMed] [Google Scholar]
- Wagner KD. Kowatch RA. Emslie GJ. Findling RL. Wilens TE. McCague K. D'Souza J. Wamil A. Lehman RB. Berv D. Linden D. A double-blind, randomized, placebo-controlled trial of oxcarbazepine in the treatment of bipolar disorder in children and adolescents. Am J Psychiatry. 2006;163:1179–1186. doi: 10.1176/ajp.2006.163.7.1179. [DOI] [PubMed] [Google Scholar]
- Waxmonsky J. Gnagy E. O'Connor B. Straub L. Molina B. Bukstein O. Pelham W. Prevalence, predictive factors of bipolar disorder (BP) in young adults with pediatric attention deficit hyperactivity disorder (ADHD). Scientific Proceedings of 160th Annual Meeting of the American Psychiatric Association; Washington (DC): American Psychiatric Association Press; 2007. [Google Scholar]
- Young RC. Biggs JT. Ziegler E. Meyer DA. A rating scale for mania: Reliability, validity and sensitivity. Br J Psychiatry. 1978;133:429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Youngstrom EA. Danielson CK. Findling RL. Gracious BL. Calabrese JR. Factor structure of the Young Mania Rating Scale for use with youths ages 5 to 17 years. J Clin Child Adolesc Psychol. 2002;31:567–572. doi: 10.1207/S15374424JCCP3104_15. [DOI] [PubMed] [Google Scholar]
- Youngstrom EA. Findling RL. Calabrese JR. Gracious BL. Demeter C. Bedoya DD. Price M. Comparing the diagnostic accuracy of six potential screening instruments for bipolar disorder in youths aged 5 to 17 years. J Am Acad Child Adolesc. 2004;43:847–858. doi: 10.1097/01.chi.0000125091.35109.1e. [DOI] [PubMed] [Google Scholar]
- Youngstrom EA. Meyers O. Kogos Youngstrom J. Calabrese J. Findling RL. Diagnostic and measurement issues in the assessment of pediatric bipolar disorder: Implications for understanding mood disorder across the life cycle. Dev Psychopathol. 2006;18:989–1021. doi: 10.1017/S0954579406060494. [DOI] [PubMed] [Google Scholar]