Abstract
Adoptive immunotherapy for treatment of cancers and infectious diseases is often hampered by a high degree of variability in the final T cell product and in the limited in vivo function and survival of ex vivo expanded antigen-specific cytotoxic T cells (CTL). This has stimulated interest in development of standardized artificial antigen presenting cells (aAPC) to reliably expand antigen specific CTL. However, for successful immunotherapy the aAPC ex vivo generated CTL must have anti-tumor activity in vivo. Here, we demonstrate that HLA-Ig based aAPC stimulated tumor-specific CTL from human peripheral blood T lymphocytes showed robust expansion and functional activity in a human/SCID mouse melanoma model. HLA-Ig based aAPC expanded CTL were detected in the peripheral blood up to 15 days after transfer. Non-invasive bioluminescence imaging of tumor bearing mice demonstrated antigen dependent localization of transferred CTL to the tumor site. Moreover, adoptive transfer of HLA-Ig based aAPC generated CTL inhibited the tumor growth both in prevention and treatment modes of therapy and was comparable to that achieved by dendritic cell expanded CTL. Thus, our data demonstrate potential therapeutic in vivo activity of HLA-Ig based aAPC expanded CTL to control tumor growth.
Keywords: Human/SCID, Antigen-specific CD8+ T cells, aAPC, Melanoma, Adoptive immunotherapy
Introduction
Adoptive immunotherapy using antigen-specific CD8+ cytotoxic T lymphocytes (CTL) provides a promising approach for the treatment of cancers and infectious diseases [1–3]. One major obstacle to its broad application has been the lack of reproducible and cost effective methods to generate clinically relevant numbers of antigen-specific CTL. Currently, adoptive immunotherapeutic approaches in patients with cytomegalovirus (CMV), human immunodeficiency virus (HIV), Epstein-Barr virus (EBV), and melanoma involve the infusion of T cells that have been expanded ex vivo using autologous dendritic cells (DC), virally infected B cells and/or allogenic feeder cells [4–13]. In addition to expensive and time consuming generation process for antigen presenting cells, these methods require up to several months to produce therapeutic numbers of antigen-specific T cells. Thus, great efforts are being made to develop alternative culture protocols for adoptive immunotherapy [14]. Recently, we and others have developed artificial antigen presenting cells (aAPC) for generating large numbers of antigen-specific T lymphocytes. These aAPC include both cell-based technologies and acellular technologies such as genetically engineered insect cells, mouse fibroblasts, human leukemia cell lines, exosomes, magnetic beads, and artificial liposomes [15, 16]. One of the important issues with all these methods is to optimize the generation of antigen-specific CTL with maximal therapeutic potential, which largely depends on the signals provided to T cells during ex vivo expansion.
Malignant melanoma is a tumor type in which adoptive immunotherapy holds significant promise. In melanoma, several tumor-associated antigens have been recently identified as targets for immunotherapy [17]. The identification of tumor-associated antigens has led to novel approaches to augment T cell mediated anti-tumor immune responses [12, 18–22]. Mart-1 (melanoma antigen recognized by T cells-1), belongs to the group of melanocyte differentiation antigens that has been used as a target antigen for several T cell based immunotherapeutic treatments because of its high immunogenicity and preferential expression on tumor cells [23]. Regression of metastatic melanoma has been reported in patients undergoing adoptive transfer therapy with highly specific tumor-reactive, Mart-1 specific T cells [10, 22, 24] showing the clinical potential of immunotherapies targeting this antigen. We have shown that aAPC, made by coupling HLA-Ig and anti-CD28 antibody to magnetic beads, compare favorably with autologous DC for generation of antigen-specific CTL from multiple donors [25]. However, the in vivo functional efficacy of aAPC expanded CTL was not known. Therefore, we used HLA-Ig based aAPC to expand tumor specific CTL from peripheral blood T lymphocytes and tested their efficacy in vivo.
Here, we demonstrate that aAPC-induced T cell cultures showed rapid expansion of tumor specific CTL with potent functional activity. aAPC expanded Mart-1 specific CTL could survive intact in vivo up to 2 weeks after transfer. We also show that, using non-invasive bioluminescence imaging of tumor bearing mice aAPC generated CTL traffic to the tumor site as early as 3 days after transfer in an antigen-dependent fashion. Simultaneous injections of aAPC generated Mart-1 specific CTL and HLA-A2+ Mart-1 expressing melanoma cells resulted in the inhibition of tumor growth in vivo, similar to that seen with DC expanded CTL. More importantly, adoptive transfer of aAPC expanded CTL into tumor bearing mice decreased the growth of established melanoma. Our data for the first time demonstrate in real time the localization of aAPC-induced CTL at the tumor site and their ability to decrease the growth of established solid tumor in vivo. Together, these results suggest that tumor-specific CTL generated using HLA-Ig based aAPC are biologically competent and further highlight the potential clinical use of aAPC expanded CTL for the treatment of cancer.
Materials and methods
Mice and tumor cells
SCID-beige (C·B-17) mice (female, 6–8 week old, hereafter referred to as SCID mice) which lack T, B and NK cells were procured from Taconic (Rockville, MD) and maintained in the animal facility of Johns Hopkins University (JHU), Baltimore, MD. All experiments performed on these animals were approved by the JHU Animal Care and Use Committee. Human melanoma cell lines MeI493 (HLA-A2+ Mart-1+ [26], hereafter referred as HLA-A2+) and MeE384 (HLA-A2− Mart-1+ [26], hereafter referred as HLA-A2−) were cultured in complete RPMI 1640 [RPMI 1640 medium supplemented with non-essential amino acids (100×, Sigma-Aldrich, St Louis, MO), sodium pyruvate (100×, Gibco, Invitrogen Corporation, Carlsbad, CA), vitamin solution (100×, Gibco), 2-mercaptoethanol (1,000×, Gibco) and 10 μM ciprofloxacin (Serologicals Proteins Inc., Kankakee, IL)] with 10% fetal calf serum (HyClone, Logan, UT). Both HLA-A2+ and HLA-A2− melanoma cell lines (5–10 × 106) grew as a subcutaneous tumor in SCID mice, 8–10 days post injection and no significant difference was observed in their tumor growth potential (data not shown).
Peptides
Peptides used in this study (Mart-126–35: ELAGIGILTV, and CMV-pp65495–503: NLVPMVATV) were synthesized by Commonwealth Biotechnologies, Inc. (Richmond, VA). The modified Mart-1 peptide (alanine to leucine at position 2) has been previously shown to have increased binding affinity for HLA-A*0201 [27]. The purity (>98%) of each peptide was confirmed by mass-spectral analysis and high-pressure liquid chromatography.
Preparation of artificial antigen presenting cells
aAPC were prepared by coupling HLA-A2 Ig and anti-CD28 human monoclonal antibody onto Dynabeads M-450 epoxy (Catalog # 140.11, Dynal Biotech Inc., Lake Success, NY) as per the established procedure in our laboratory [25]. For peptide loading, aAPC were incubated in a final peptide concentration of 30 μg/ml at 4°C for 24 h. aAPC were stored in the peptide solution at 4°C until use.
HLA-A2.1+ lymphocytes
The institutional ethics committee approved this study. All donors gave written informed consent before enrolling in the study. The blood was drawn from HLA-A2.1+ healthy individuals and peripheral blood mononuclear cells (PBMC) were separated by BD VacutainerCPT cell preparation tube (Becton-Dickinson, Franklin Lakes, NJ).
In vitro generation of dendritic cells
Monocytes were isolated from PBMC using CD14+ microbeads (Miltenyi Biotec, Auburn, CA). The CD14+ cells were cultured in complete RPMI 1640 medium supplemented with 3% autologous serum, 100 ng/ml human granulocyte-macrophage colony-stimulating factor (R&D Systems, Inc., Minneapolis, MN), 50 ng/ml IL-4 (R&D Systems), and 5 ng/ml transforming growth factor-β1 (R&D Systems). After 6 days of culture, 100 ng/ml lipopolysaccharide (Sigma) was added for 24 h to mature the DC [28]. The mature DC displayed typical cell surface markers of DC such as CD1a+, CD14− and CD86+. For peptide loading, DC were harvested, washed thrice and incubated with 30 μg/ml peptide in complete RPMI 1640 medium.
Generation of antigen specific cytotoxic T lymphocytes
CD8+ T lymphocytes were enriched from PBMC of HLA-A2+ donor by negative selection using a CD8+ T cell isolation kit (Miltenyi Biotec, Auburn, CA). The enriched CD8+ T cells (>90%) were used for generation of antigen-specific CTL, according to the established method in our laboratory [25]. Briefly, purified CD8+ T cells (2 × 104 cells/well) were cultured with aAPC (2 × 104 beads/well) or DC (1 × 104 cells/well) loaded with either Mart-1 or CMV-pp65 peptide in complete RPMI 1640 medium supplemented with 5% autologous serum and 3% T cell growth factor (TCGF) in a 96-well plate at 37°C in a 5% CO2 incubator. TCGF was prepared as described earlier [29]. The T cells were restimulated with peptide pulsed aAPC or DC once a week for 4 weeks, as mentioned above.
CD8+ T cells having 50–70% specificity for Mart-1 antigen (referred to hereafter as Mart-1 specific CTL) as determined by tetramer staining were used in all the experiments in this study. CMV-pp65 specific CTL (CD8+ T cells with >90% specificity for CMV-pp65 antigen) were used as a negative control.
Flow cytometric analysis
The antigen specificity of the CTL was tested by staining with anti-human CD8 monoclonal antibody (clone UCHT-4, Sigma-Aldrich) and HLA-A2 tetramer loaded with either Mart-1 peptide (hereafter referred as Mart-1 tetramer) or CMV-pp65 peptide (hereafter referred as CMV-pp65 tetramer) (Beckman Coulter Inc., San Diego, CA). For phenotypic analysis, the cells were stained with anti-human CD8 antibody and the antibodies against CD45RA (clone HI100), CD45RO (clone UCHL1), CD62 ligand (clone Dreg-56) and CCR7 (clone 3D12). All antibodies against surface markers were purchased from BD Biosciences, San Diego, CA. Samples were analyzed using a FACScalibur flow cytometer and CELLquest software.
Analysis of in vitro effector functions
51Cr-release assay
The cytotoxic activity of antigen specific CTL was measured as follows. HLA-A2+ and HLA-A2− melanoma cell lines were pulsed with 51Cr (Catalog # CJS4, Amersham Biosciences, Piscataway, NJ) for 1 h at 37°C in a 5% CO2 incubator and used as targets. The Mart-1 specific CTL were incubated with 51Cr-pulsed melanoma cells (HLA-A2+ or HLA-A2−) for 4 h at 37°C. Different effector to target (E/T) ratios were used with 2,000 target cells/well. The targets plated without T cells and targets with 0.2% Triton X-100 were used as spontaneous release and maximum release, respectively. After the incubation, the supernatants were collected and dried in a scintillator containing Lumaplate-96 (Catalog # 6006633, PerkinElmer Life Sciences, Shelton, CT) overnight. The radioactivity was measured using a TopcountNXT Microplate Scintillation & Luminescence Counter (Packard Instrument Company, Downers Grove, IL). Triplicate wells were averaged and the percentage of antigen specific cytotoxicity was calculated as follows: percent specific killing = (sample release − spontaneous release)/(maximum release − spontaneous release) × 100.
Cytometric bead array
To analyze the effector cytokines production upon activation by endogenous antigen on the tumor cells, the Mart-1 specific CTL (2 × 105/well) were incubated alone or were stimulated with either HLA-A2+ or HLA-A2− melanoma cells (2 × 105/well) for 6 h at 37°C. After the incubation, the culture supernatants were collected and tested for IL-2, IL-4, IL-5, IL-10, TNF-α and IFN-γ using cytometric bead array (Human Th1/Th2 cytokine kit, BD Biosciences) according to the manufacturer’s recommendations.
Analysis of survival of Mart-1 specific CTL in vivo
SCID mice were injected subcutaneously (s.c.) with HLA-A2+ melanoma cells (1 × 107 cells/mouse) for tumor induction. After 2 weeks (tumor size about 25–50 mm2), the mice were transferred intravenously (i.v.) in the tail vein with Mart-1 specific CTL (5 × 106 cells/mouse). CMV-pp65-specific CTL was used as a negative control. (The number of antigen-specific CTL used in this and other in vivo experiments was based on our preliminary experiments.) All mice received two injections of recombinant human IL-2 (rhIL-2) (2 × 105 IU/mouse) (Chiron Corporation, Emeryville, CA) intraperitoneally (i.p.) on days 0 and 2. Blood was drawn serially before and on 7, 15 and 21 days after transfer and flow cytometric analysis was done by staining the peripheral blood lymphocytes with anti-human CD8 antibody.
Production of lentivirus with luciferase gene and transduction of Mart-1 specific CTL
Lentivirus pseudotyped with vesicular stomatitis virus G (VSV-G) envelope was generated by transfection of 293T cells using lipofectamine 2000 (Invitrogen, Carlsbad, CA) as previously described [30]. The viral supernatants were collected and filtered through 0.45-μm filters to remove cell debris and frozen in aliquots at −80°C. If necessary, viral supernatants were concentrated using centrifugal filter devices [Centricon Plus-20 (MW CO 100, 000), Millipore Corporation, Bedford, MA]. The viral titers were determined by transduction of 293T cells (1 × 105 cells per well in 6-well plate) with serially diluted viral supernatants in the presence of 8 μg/ml of polybrene (Sigma-Aldrich). Transducing units (TU) were calculated based on numbers of transduced GFP+ cells analyzed by flow cytometry. The viral titer was typically in between 2 and 3 × 106 TU/ml.
For transduction of antigen specific CTL, the purified CD8+ T cells were cultured with Mart-1 peptide loaded aAPC for 4 days. Then the cells (2.5 × 105) were collected, mixed with 1 ml of viral supernatant in the presence of polybrene (8 μg/ml) and centrifuged at 3000 rpm for 3 h at room temperature. After centrifugation, the supernatant was aspirated; the cells were resuspended in a fresh complete RPMI 1640 medium and cultured with peptide loaded aAPC as described above to expand Mart-1 specific CTL transduced with luciferase gene. Approximately, 15–30% of total CD8+ T cells were positive for GFP after 48 h of transduction, as analyzed by flow cytometry (data not shown). The luciferase activity of transduced cells was determined using Bright-Glo system (Promega Corporation, Madison, WI). Briefly, the cells (1 × 106) were lysed with Glo lysis buffer, d-Luciferin substrate was added and luciferase activity was measured using a TopCount luminescence counter (Packard).
In vivo bioluminescence imaging
SCID mice were injected s.c. with HLA-A2+ (1 × 107 cells/mouse) for the induction of tumor growth. HLA-A2− melanoma was used as a negative control. After 2 weeks (tumor size about 25–50 mm2), the mice were adoptively transferred i.v. in the tail vein with luciferase transduced Mart-1 specific CTL (1 × 107 cells/mouse). In addition, these mice received two injections of rhIL-2 (2 × 105 IU/mouse) i.p. on days 0 and 2. All the mice were injected i.p. with d-Luciferin substrate (300 mg/Kg body weight) (Catalog # XR-1001, Xenogen Corporation, Alameda, CA) and in vivo imaging was done after 15 min using a Xenogen IVIS200 equipped with XGI-8 gas anesthesia system and Living Image Software (Xenogen Corporation).
Adoptive transfer of Mart-1 specific CTL and its effects on the tumor growth in vivo
SCID mice (n = 4–5) were transferred i.v. with aAPC generated Mart-1 specific CTL (3 × 106 cells/mouse) and challenged s.c. with HLA-A2+ melanoma cells (1 × 107 cells/mouse) on the same day. CMV-pp65 specific CTL was used as a negative control.
All mice received two injections of rhIL-2 (2 × 105 IU/mouse) i.p. on days 0 and 2. These mice were followed for induction of tumor growth and tumor size was measured using a caliper and the products of perpendicular diameters were determined. In addition, upon termination of the experiment, tumor was removed from all groups of mice and tumor weight was taken. In another set of experiments, DC-expanded Mart-1 specific CTL was used as a positive control to compare the efficacy of aAPC generated Mart-1 specific CTL in controlling tumor growth.
For treatment experiments, SCID mice were injected s.c. with HLA-A2+ melanoma cells (1 × 107 cells/mouse) for tumor induction. After 2 weeks (tumor size about 25–50 mm2), the mice were infused i.v. in the tail vein with aAPC generated Mart-1 specific CTL (3 × 106 cells/mouse) and were injected with two doses of rhIL-2 (2 × 105 IU/mouse) i.p. on days 0 and 2. CMV-pp65 specific CTL was used as a negative control. Thereafter tumor size was recorded as mentioned above.
Statistical analysis
The data were analyzed using two tailed Student’s t test and Wilcoxon rank sum test.
Results
Generation, phenotypic and functional characterization of Mart-1-specific CTL
To test the in vivo functional activity of HLA-Ig based aAPC expanded tumor specific CTL, the HLA-A2 restricted peptide derived from melanoma-associated antigen, Mart-1 was chosen as a model antigen. CD8+ T cells isolated from peripheral blood mononuclear cells (PBMC) of healthy individuals were stimulated with Mart-1 peptide loaded aAPC and tested for their antigen specificity and level of expansion. After four rounds of in vitro stimulation, 55.5% of total CD8+ T cells were Mart-1 specific (Fig. 1a). Starting with a population of 10 × 106 total CD8+ T cells, that were less than 0.1% specific, approximately 50 × 106 Mart-1 specific CTL could be generated within a month. This rapid expansion of Mart-1 specific CTL was seen consistently in several donors tested (Table 1).
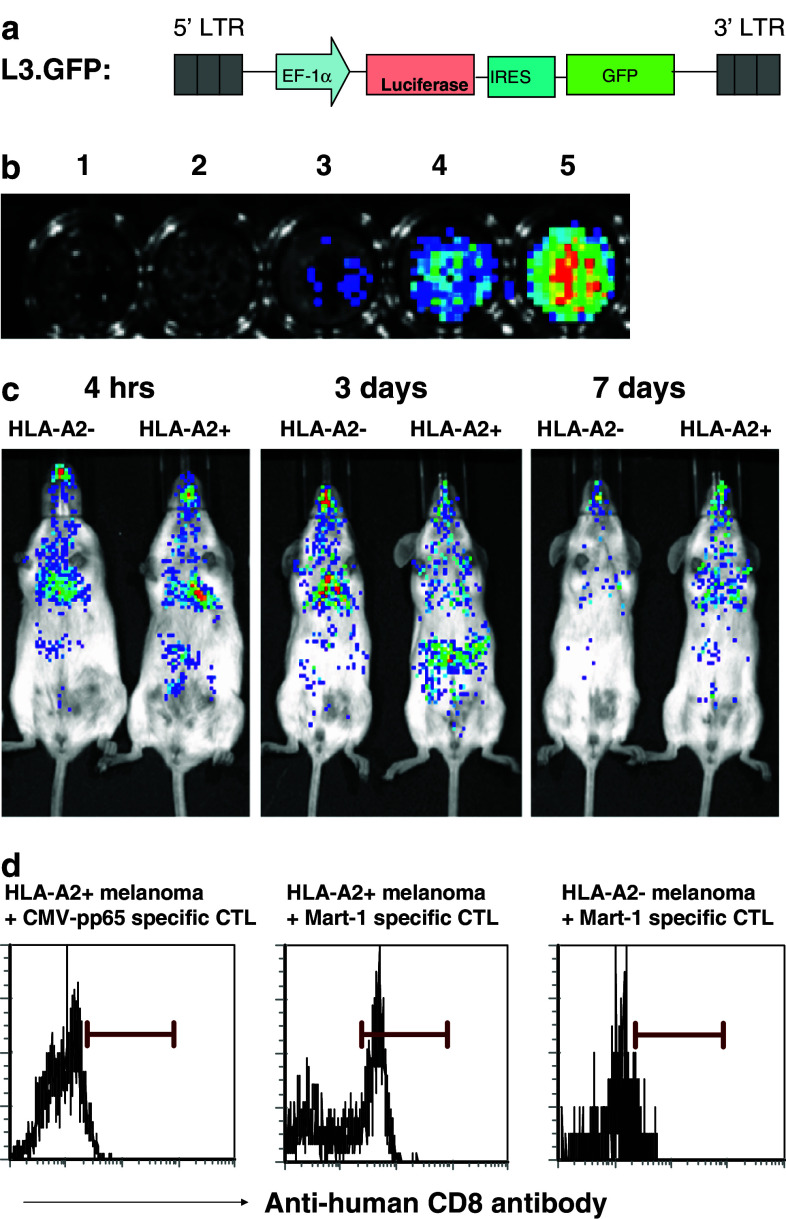
Fig. 1.
In vitro characterization of aAPC generated Mart-1 specific CTL. a Human peripheral blood CD8+ T cells were cultured with Mart-1 peptide loaded aAPC for 4 weeks to induce and expand antigen specific CTL as detailed in “Materials and methods”. After 4 weeks, the CTL were stained with anti-human CD8 antibody and with either Mart-1 tetramer (upper panel) or control CMV-pp65 tetramer (lower panel) and analyzed by flow cytometry. The percentage of peptide specific CTL within total CD8+ T lymphocytes is shown in the upper right corner. b Cytotoxic activity of Mart-1-specific CTL was tested against melanoma cells expressing endogenous antigen as targets using 51Cr-release assay. % specific lysis by Mart-1 specific CTL is shown for HLA-A2+ and HLA-A2− melanoma targets. Values represent triplicates at varying effector-target ratios. The effector cell numbers represent total CD8+ T cells. c Mart-1 specific CTL were incubated alone or stimulated with either HLA-A2+ or HLA-A2− melanoma cells for 6 h and the supernatants were analyzed for IL-2, IL-4, IL-5, IL-10, TNF-α and IFN-γ using cytometric bead array. Values are shown as mean ± SD of duplicate wells in the assay. The Mart-1 specific CTL for both assays were obtained after four stimulations with peptide loaded aAPC. Data is representative of three independent experiments
Table 1.
Expansion and yield of Mart-1 specific T cells from different donors using HLA based aAPC
| Donors | Experiments | % of Mart-1 tetramer specific cells | Total number of cells yielded (starting from 10 × 106 CD8+ T cells) |
|---|---|---|---|
| Donor 1 | 1 | 61.3 | 64.0 × 106 |
| 2 | 67.0 | 92.0 × 106 | |
| 3 | 58.5 | 74.0 × 106 | |
| Donor 2 | 1 | 38.2 | 71.0 × 106 |
| 2 | 32.0 | 54.0 × 106 | |
| Donor 3 | 1 | 34.2 | 46.0 × 106 |
| 2 | 26.6 | 42.0 × 106 |
A summary of the frequency and total cell number of Mart-1-specific CTL induced by aAPC. The Mart-1 specific CTL were cultured for 4 weeks before analyzing. The frequency of Mart-1-specific CTL was analyzed by tetramer staining
We then characterized the phenotype of aAPC expanded CTL. After 4 weeks, the aAPC generated T cells were largely CD3+CD8+ cells with more than 93% of the cells expressing the memory cell marker CD45RO, but were negative for CD45RA. Further analysis showed that 22% of the cells within total CD8+ T lymphocytes expressed CD62 ligand, and only 3% of the cells expressed CCR7. Thus, the aAPC expanded CTL were CD45RA−, CD45RO+, CCR7− and CD62L+/−; characteristics of effector memory T cells. In contrast, the CD8+ T cells before culture were mostly CD45RA+, CD45RO−, CCR7− and CD62L+. No staining by the cells with isotype control antibodies was seen either before or after 4 weeks of culture. Additional T cell receptor repertoire analysis revealed that the resulting CTL population contained 4–5 clonal T cell populations. In a representative example we found T cells that clonally expressed TCR Vβ 8, 9, 14, 17 and 22.
Another important parameter for adoptive immunotherapeutic use is the ability of aAPC generated CTL to recognize tumor cells that express low amounts of endogenous antigen on their surface. We therefore stimulated aAPC-induced Mart-1 specific CTL with tumor targets expressing endogenous Mart-1 antigen and tested their cytotoxic activity and cytokines secretion profile using 51Cr release assay and cytometric bead array, respectively. Mart-1 specific CTL showed dose dependent lysis of HLA-A2+ melanoma targets, 27% of specific lysis was seen at an effector to target ratio of 33:1; whereas control HLA-A2− melanoma cells were not lysed (Fig. 1b). Furthermore, Mart-1 specific CTL produced both Tc1 and Tc2 type cytokines including IFN-γ, IL-2, IL-4 and IL-5 upon stimulation with HLA-A2+ melanoma targets, whereas control HLA-A2− melanoma targets did not. Lower amounts of TNF-α and IL-10 were elicited by the HLA-A2+ tumor cells (Fig. 1c). None of the cytokines were produced either by T cells alone or by HLA-A2+ or HLA-A2− melanoma cells alone (data not shown). Overall, the results demonstrate that aAPC can efficiently induce and expand antigen specific CTL against melanoma associated antigen, Mart-1 and these aAPC-induced CTL have the characteristics of effector memory CD8+ T cells with the ability to show potent anti-tumor effector functions in vitro.
Survival of aAPC generated Mart-1 specific CTL in vivo
In order to exert their anti-tumor functions in vivo, adoptively transferred antigen specific CTL must have the ability to survive without undergoing apoptosis. We therefore monitored the fate of adoptively transferred Mart-1 specific CTL in the peripheral blood at different time points after transfer into tumor bearing mice. PBMC obtained immediately before and 7, 15 and 21 days after infusion were analyzed for the presence of transferred CTL. Our results (Fig. 2) show that 7% of transferred CTL could be detected in the blood, 7 days after the infusion. Further increase in the number of CD8+ T cells (10–12%) was observed on day 15 which decreased to 2% by day 21; suggesting the disappearance of transferred CTL in the blood after 2 weeks of transfer. In contrast, non-cognate CMV-pp65-specific CTL could not be detected in the blood after the transfer into tumor bearing mice and Mart-1 specific CTL could not be detected in mice bearing A2-negative tumors (data not shown). Thus, our data show that aAPC expanded Mart-1 specific CTL could survive in vivo and furthermore the survival and persistence of transferred Mart-1 specific CTL were antigen-dependent.
Fig. 2.
Survival of aAPC expanded Mart-1 specific CTL in vivo. SCID mice were injected s.c. with HLA-A2+ melanoma cells for tumor induction. After 2 weeks, Mart-1 specific CTL (5 × 106 cells/mouse) were transferred i.v. and the animals were also injected i.p. with recombinant human IL-2 (rhIL-2) (2 × 105 IU/mouse) on days 0 and 2. CMV-pp65 specific CTL served as a negative control. Blood was drawn serially on day 0, 7, 15 and 21 after T cell transfer. The peripheral blood lymphocytes were stained with anti-human CD8 antibody and analyzed by flow cytometry. Data is shown for two representative mice. The percentage of human CD8+ T cells within total peripheral blood lymphocytes is given in the lower right corner. A representative of two independent experiments is shown
Localization of aAPC expanded Mart-1 specific CTL at the tumor site
To visualize the kinetics of CTL trafficking in living tumor bearing animals, bioluminescence imaging was performed using aAPC-induced Mart-1 specific CTL, transduced with luciferase gene (Mart-1-Luc CTL). The schematic diagram of lentiviral vector used in this study is shown in Fig. 3a. The Mart-1-Luc CTL was tested for their in vitro luciferase activity before their adoptive transfer into tumor bearing mice. The results (Fig. 3b) demonstrate the dose dependent activity of luciferase in the cell lysates.
Fig. 3.
In vivo tracking and localization of aAPC expanded Mart-1 specific CTL. a The lentiviral vector used in this study was HIV-1 based self-inactivating vector containing internal human elongation factor 1α (EF1α) promoter. L3.GFP was designed to express luciferase gene and GFP gene in the single construct where the expression of GFP is directed by IRES. b The generation of luciferase gene transduced Mart-1 specific CTL is described in detail in “Materials and methods”. The luciferase transduced Mart-1 specific CTL (1 × 106 cells) were lysed using lysis buffer, d-Luciferin substrate was added and the luminescence was measured using Xenogen IVIS200. The luciferase activity in the total cell lysate is shown. Samples: 1 buffer, 2 untransduced cell lysate, 3 10 μl, 4 25 μl, and 5 100 μl of transduced cell lysates. c SCID mice were injected s.c. with HLA-A2+ melanoma to induce tumor growth and after 2 weeks, luciferase transduced Mart-1 specific CTL (1 × 107 cells/mouse) were transferred i.v. into tumor bearing mice. These mice also received rhIL-2 (2 × 105 IU/mouse) on days 0 and 2. HLA-A2− melanoma bearing mice and HLA-A2+ tumor bearing mice without any T cell transfer served as an additional control. All the mice were injected i.p. with d-Luciferin substrate (300 mg/Kg body weight) and in vivo images were taken after 15 min using Xenogen IVIS 200. Similar results were seen with repeat experiments. d Tumor bearing (HLA-A2+ or HLA-A2−) SCID mice were adoptively transferred with Mart-1 or CMV-pp65 specific CTL as mentioned above. On day 3, tumor samples were harvested, single cell suspension were made and stained with anti-human CD8 antibody. Flow cytometric analysis was performed
SCID mice were injected s.c. with HLA-A2+ or HLA-A2− melanoma cells and after 2 weeks, Mart-1-Luc CTL were transferred i.v. into tumor bearing mice. The mice were then injected i.p. with d-Luciferin substrate and images were taken. The results show that independent of the melanoma type (HLA-A2+ or HLA-A2−), Mart-1-Luc CTL could be detected in the lungs, the predominant site of harboring T cells after intravenous injection at 4 h post-transfer but at that time did not localize to the tumor (Fig. 3c). The initial localization of adoptively transferred T cells to the lungs has been observed in different animal models as well as in humans [31–33]. Interestingly, imaging after 3 days of transfer demonstrated that the Mart-1-Luc CTL preferentially accumulated at the tumor site in the HLA-A2+ melanoma tumor bearing mice, whereas at that time CTL did not localize to the HLA-A2− melanoma (Fig. 3c). Mart-1-Luc CTL could no longer be detected on day 7 (Fig. 3c) and day 15 (data not shown) after infusion. No luminescence signals were detected in untreated tumor bearing mice in any of the days tested (data not shown). Together, these results show that adoptively transferred aAPC generated tumor specific CTL were able to track to the tumor site in an antigen-dependent manner.
We also tested the intratumoral localization of aAPC generated Mart-1 CTL. Mart-1 or CMV specific CTL were infused i.v. into tumor bearing mice and on day 3, the tumor samples were harvested and tested for the presence of human CD8+ T cells. The results (Fig. 3d) show the presence of transferred human CD8+ T cells (Mart-1 specific CTL) only in the HLA-A2+ tumor tissue but not in the HLA-A2− tumor. In contrast, the transferred CMV specific CTL did not localize to the HLA-A2+ tumor site (Fig. 3d). Our data demonstrate that localization to the tumor was dependent on both the tumor expressing the appropriate HLA-A2 complex, and CTL being antigen-specific and HLA-A2 restricted. Thus intratumoral localization of aAPC generated Mart-1 specific CTL is antigen specific.
aAPC generated Mart-1 specific CTL inhibit the growth of melanoma in vivo
To analyze the effects of adoptively transferred Mart-1 specific CTL on the onset and growth of melanoma in vivo, SCID mice were transferred i.v with Mart-1 specific CTL and injected s.c. with HLA-A2+ melanoma cells on the same day for prevention studies. Mice injected with Mart-1 specific CTL showed a marked reduction in HLA-A2+ melanoma tumor growth (P < 0.025) when compared to untreated or CMV specific CTL treated groups (Fig. 4a). Mart-1 specific CTL-treated mice had a 2–3-fold decrease in tumor growth on day 36 as compared to controls, while there was no significant difference in the tumor growth between the control groups. Moreover, transfer of Mart-1 specific CTL delayed tumor progression for about 15 days when compared to controls. As expected, adoptive transfer of Mart-1 specific CTL did not have any significant effect on the HLA-A2− melanoma tumor growth in vivo (data not shown).
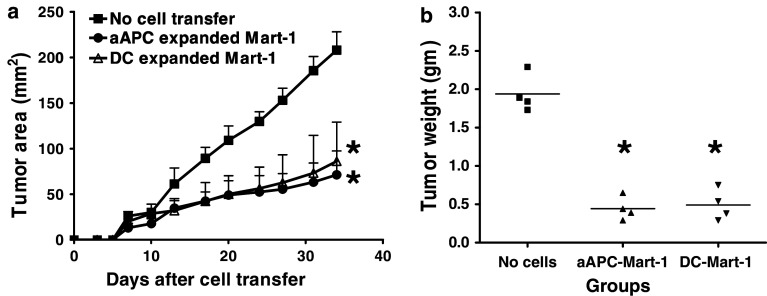
Fig. 4.
Pre-treatment of aAPC generated Mart-1 specific CTL inhibits the growth of melanoma in SCID mice. a SCID mice (n = 4) were infused i.v. with aAPC expanded Mart-1 specific CTL (3 × 106 cells/mouse) and injected s.c. with HLA-A2+ melanoma cells on the same day. All mice received rhIL-2 (2 × 105 IU/mouse) on days 0 and 2. Untreated mice and CMV-pp65 specific CTL treated mice were used as negative controls. [In vitro characterization of aAPC-induced CMV-pp65 specific CTL showed dose dependent lysis of target cells expressing endogenous antigen, documenting their antigen dependent effector functions (data not shown)]. After the transfer, all mice were monitored for the subcutaneous growth of melanoma as described. The products of perpendicular diameters are shown as mean ± SD. The difference in tumor growth between mice transferred with Mart-1 specific CTL and the control groups was statistically significant (* P < 0.025) as determined by Wilcoxon rank sum test. b After checking the tumor growth in different groups of mice as mentioned above (Fig. 4a), tumor was dissected out from all the mice and weight was determined. The difference in the mean tumor weight between the experimental and the control groups was statistically significant by Student’s t test (* P < 0.05). Both experiments were repeated at least three times
To further test the effect of Mart-1 specific CTL on the tumor burden, tumor mass was removed and the weight was determined. Figure 4b shows that the mice treated with Mart-1 specific CTL had a significantly decreased tumor weight, 4–5-fold in the mice injected with Mart-1 specific CTL, when compared to controls. However, the difference in the tumor weight between the control groups of mice was not significant. These results demonstrate that aAPC generated Mart-1 specific CTL were able to inhibit the tumor growth in vivo.
Anti-tumor efficacy of aAPC expanded CTL was comparable to DC generated CTL
Current adoptive immunotherapy approaches often use autologous peptide-pulsed DC to induce and expand antigen specific CTL. We therefore compared the efficacy of aAPC expanded Mart-1 specific CTL with that of the DC-expanded CTL in the tumor prevention model. Adoptive transfer of equal numbers of either aAPC or DC expanded Mart-1 specific CTL decreased the tumor growth significantly (P < 0.025) when compared to untreated group (Fig. 5a). The effect of aAPC expanded Mart-1 specific CTL on the tumor growth was similar to the effect mediated by DC expanded CTL. In addition, Fig. 5b shows that mice transferred with aAPC or DC expanded Mart-1 specific CTL had a reduced tumor weight that was statistically significant when compared to untreated mice. The tumor weight was 4–5-fold less in CTL treated groups when compared to controls. These results demonstrate that aAPC generated Mart-1 specific CTL were able to inhibit the tumor growth in vivo, similar to DC expanded CTL. It further suggests that aAPC provide a technical advantage for the generation of antigen specific T cells without a loss of functional ability.
Fig. 5.
Anti-tumor efficacy of aAPC generated Mart-1 specific CTL was comparable with DC based expansion. a SCID mice (n = 4–5) were transferred i.v. with equal number of Mart-1 specific CTL (3 × 106 cells/mouse) expanded using either aAPC or DC. Mice were also injected s.c. with HLA-A2+ melanoma cells on the same day. These mice received rhIL-2 (2 × 105 IU/mouse) on days 0 and 2. Untreated mice were used as a control. All mice were monitored for tumor growth and the tumor size was recorded. The difference obtained in tumor growth between mice transferred with aAPC or DC expanded Mart-1 specific CTL and control groups was statistically significant (* P < 0.025) as determined by Wilcoxon rank sum test. b After measuring the tumor growth, tumors were harvested and weight was determined at the end of the experiment. The difference in the tumor weight between the aAPC or DC expanded Mart-1 specific CTL treated and control groups was statistically significant by Student’s t test (* P < 0.05)
Treatment of established melanoma by transfer of aAPC-induced CTL
The treatment of established solid tumor is more difficult to achieve but is essential in most tumor immunotherapy settings. As a proof of principle, we evaluated the efficacy of aAPC-induced Mart-1 specific CTL in controlling the growth of an established subcutaneous tumor. SCID mice were injected with HLA-A2+ melanoma and after 2 weeks these mice were infused i.v. with antigen specific CTL. Adoptive transfer of Mart-1 specific CTL into the tumor-bearing mice significantly (P < 0.025) suppressed the growth of melanoma as compared to untreated or CMV-pp65 specific CTL treated groups (Fig. 6). The tumor growth was delayed about 10–15 days, with a twofold decrease in the tumor size in the mice treated with Mart-1 specific CTL. In contrast, no difference was seen in tumor growth between the untreated and control CMV-specific CTL treated groups. Thus, our data show that adoptive transfer of aAPC-induced Mart-1 specific CTL can significantly reduce established solid tumor growth.
Fig. 6.
Adoptive transfer of Mart-1 specific CTL decreases the growth of established melanoma. SCID mice (n = 4) were injected s.c. with HLA-A2+ melanoma cells for induction of tumor growth. After 2 weeks, these mice were infused i.v. with Mart-1 specific CTL (3 × 106 cells/mouse) and were also injected with rhIL-2 (2 × 105 IU/mouse) on days 0 and 2. Untreated mice and CMV-pp65 specific CTL treated mice served as controls. Thereafter, all mice were assessed for tumor growth. The products of perpendicular diameters were determined and expressed as mean ± SD. The difference in the tumor growth between Mart-1 specific CTL treated group and the control groups was statistically significant (* P < 0.025) as analyzed by Wilcoxon rank sum test. Data shown is representative of two independent experiments
Discussion
We studied the ability of HLA-Ig based aAPC to generate tumor specific CTL with in vivo anti-tumor activity for determining the potential clinical uses of aAPC-induced CTL in adoptive immunotherapy. We demonstrated here that HLA-Ig based aAPC could induce and expand therapeutic numbers of tumor specific CTL against Mart-1 with in vivo functional activity. aAPC expanded CTL could survive in vivo for several days. Furthermore, our study provides evidence that aAPC expanded CTL can localize preferentially to the tumor site in an antigen-specific fashion. We also show that adoptive transfer of Mart-1 specific CTL into mice resulted in the inhibition of tumor growth, both in prevention and treatment modes of therapy. Furthermore, the in vivo efficacy of aAPC expanded CTL was similar to the in vivo efficacy of DC expanded CTL. In addition, while the current aAPC preferentially induce effector memory T cells, the flexibility of the system enables one to exchange or add other costimulatory molecules, which may allow for generation of different T cells subsets, such as central memory T cells. Thus, HLA-Ig based aAPC represent a versatile technology useful for expanding antigen specific cells without a loss in functional efficacy. These findings highlight the potential clinical uses of aAPC-induced CTL in adoptive immunotherapy.
The phenotype of ex vivo expanded CTL is largely dependent on the signals delivered during CTL activation. In vitro characterization showed that aAPC generated Mart-1 specific CTL had the characteristics of effector memory T cells (CD45RA−, CD45RO+, CCR7− and CD62L+/−). Furthermore, in addition to killing of HLA-A2+ melanoma targets in vitro, they produced significant amounts of both Tc1 and Tc2 effector cytokines such as IFN-γ, IL-2, IL-4, and IL-5 upon activation by endogenously processed antigen on tumor cells. The effector memory cells have the capacity to migrate to nonlymphoid tissues [34–36] and selective homing of memory cells to human tumors has also been demonstrated [37, 38]. The absence of appropriate homing receptors and the inefficient trafficking of antigen specific CTL to the site of pathology has been reported in patients with CMV, EBV and HIV infections [39]. The effector memory T cells were shown to produce IFN-γ, IL-4 and IL-5 within hours following antigenic stimulation [40]. The finding that effector memory CD8+ cells can make both Tc1 and Tc2 cytokines, IFN-γ, IL-4 and IL-5, has also been reported for DC-based ex vivo expansion [41]. Thus, aAPC-induced CTL in addition to their rapid and robust expansion, have the ability to show rapid effector functions upon their encounter with tumor cells.
The survival of transferred antigen specific CTL in the recipients is crucial for the success of adoptive T cell therapy. By serial analysis of peripheral blood, we obtained a clear kinetics pattern of the frequency of transferred CTL in the treated tumor bearing mice. Our data show that the infused CTL were detectable in the blood for up to 15 days in the tumor bearing mice. Results from previous studies on the fate of transferred CTL are quite variable, ranging from no detectable CTL immediately after transfer [42, 43] to survival of CTL for several days [9, 44]. Two recent clinical studies where transferred Mart-1 CTL [13, 22] with low dose IL-2 were seen in the blood up to 14 days in melanoma patients also supports persistence of CTL. These variable results might be attributed to difference in the dose of IL-2 used or lack of CD4+ T cell help. The importance of IL-2 supplementation and CD4+ T cell help for the survival and persistence of adoptively transferred T cells has been documented [4, 7, 13]. Considering the fact that aAPC-induced CTL had relatively long term survival ability in xenogenic recipients (SCID mice), in the presence of CD4+ T cells help and supplementation of several doses of IL-2 in patients, these CTL might be able to survive even longer, upon infusion. Thus, our data suggest that the aAPC-induced CTL do not undergo apoptosis immediately after transfer and can survive intact for at least 2 weeks in the tumor bearing mice.
The homing of antigen-specific CTL to the tumor site is an important requirement for in vivo CTL anti-tumor function. Noninvasive, bioluminescence imaging was used to characterize the trafficking kinetics and tumor localization of transferred Mart-1 specific CTL. We found that the CTL distributed initially to the lungs as reported in several studies [22, 31–33], however the CTL were able to localize at the site of HLA-A2+ melanoma tumor as early as 3 days after transfer. It was further confirmed by FACS analysis that the intratumoral localization of aAPC expanded CTL was antigen dependent. Similar observations were made in human studies when DC expanded Mart-1 specific CTL were infused into melanoma patients [45]. Very few studies have attempted to analyze the trafficking and localization of transferred T cells at the tumor site [22, 45, 46]. These reports have used different methods for detection of infused T cells at the tumor site, such as radioactive labeling of T cells before transfer, flow cytometric analysis of transferred cells by staining the surface markers, etc. These methods utilize endpoint analysis, requiring tumor biopsies from animal/patient and do not reveal the kinetics of transferred cells within the same animal/patient. However, our data demonstrate the kinetics and localization of aAPC-induced tumor specific CTL at the tumor site within same animal and that this accumulation was antigen dependent. In our study, the transferred CTL could not be detected 7 days after transfer in the tumor bearing mice using bioluminescence imaging. While, our flow cytometric analysis showed the presence of these cells in the peripheral blood up to 2 weeks after transfer (Fig. 2), the absence of luciferase transduced CTL after 7 days of infusion could be due to the decreased expression of the luciferase gene over time in vivo as suggested by Morgan et al. [47]. Overall, our data highlight the ability of HLA-Ig based aAPC to expand therapeutically active T cells that track to and therefore control the tumor growth.
In terms of therapeutic efficacy, we observed that adoptive transfer of aAPC-induced Mart-1 specific CTL decreased the tumor growth significantly in both prevention and treatment experiments. Various groups have reported the importance of IL-2 for survival and function of transferred CTL in vivo in both animal models [48–50] and in patients [45]. The infusion of ex vivo expanded tumor specific T cells have been found to mediate tumor regression successfully in patients with metastatic melanoma in different settings [10, 11, 22, 45, 47]. In all these studies, tumor specific CTL were infused several times at weekly intervals followed by multiple doses of IL-2 to achieve tumor regression. In our study, a single transfer of CTL followed by two injections of IL-2 was still able to reduce the tumor growth significantly. Thus, the effect of aAPC-induced CTL on the tumor growth could be enhanced in a clinical setting by increasing the frequency of T cell transfer along with injection of multiple doses of IL-2. Moreover, concurrent transfer of tumor specific CD4+ T cells might further improve CTL survival and function. Although several models of artificial antigen presenting cells (aAPC) have been reported for expansion of antigen specific T cells [15], this is the first study to show the in vivo efficacy of aAPC expanded CTL in control of solid tumor growth.
The fact that either aAPC or DC generated Mart-1 specific CTL could not eliminate the tumor completely in our study may be due to the lack of CD4 help in SCID mice. While the human/SCID models are by their nature only partially reconstituted immune responses; they are proven to be useful in evaluating the therapeutic efficacy of adoptively transferred human tumor specific T cells. These models have been used extensively in studying adoptive immunotherapeutic treatment approaches for diseases including, but not limited to, EBV-associated lymphoma, B cell lymphoma, adenocarcinoma and melanoma [31, 48, 49, 51–53]. Anti-tumor efficacy of adoptively transferred CTL has been demonstrated in other immunocompetent animal models such as pmel-1 CD8+ antigen specific cells in C57BL/6 host [50, 54]. The functional efficacy of aAPC activated pmel-1 T cells is currently under investigation in our laboratory.
Our data show that HLA-Ig based aAPC, in addition to their capacity to support large scale production, can generate tumor specific CTL with in vivo biological functions; thereby suggesting the potential clinical applications of aAPC-induced CTL in adoptive immunotherapy. While some of the components for aAPC generation such as the anti-CD28 mAb and the magnetic bead are already available as a GMP product, for clinical grade aAPC it will be necessary to develop GMP grade HLA-A2-Ig as well. Nevertheless these findings help to overcome logistical limitations associated with generating clinically relevant numbers of antigen specific CTL with therapeutic potential for adoptive immunotherapy. Therefore, this new aAPC-based approach has the potential to replace currently used protocols to generate tumor specific CTL,which will help make adoptive immunotherapy a more viable and reliable approach for cancer treatment.
Acknowledgments
We thank Carl H June for providing anti-human CD28 antibody (clone 9.3), Drew Bennett for making HLA-A2 Ig dimer, and Richard Senatore for help with cell culture. This work has been supported by grants from National Institutes of Health, Bethesda, Maryland to JPS (AI 44129, CA108835 and AI 29575).
Conflict of interest statement
The authors declare that they have no commercial and financial interests.
Abbreviations
- aAPC
artificial Antigen presenting cell
- Mart-1
Melanoma antigen recognized by T cells-1
Contributor Information
Malarvizhi Durai, Phone: +1-410-6140642, FAX: +1-410-6143548, Email: mdurai1@jhmi.edu.
Jonathan P. Schneck, Phone: +1-410-6144589, FAX: +1-410-6143548, Email: jschnec1@jhmi.edu
References
- 1.Dudley ME, Rosenberg SA. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nat Rev Cancer. 2003;3:666–675. doi: 10.1038/nrc1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melief CJ, Kast WM. T-cell immunotherapy of tumors by adoptive transfer of cytotoxic T lymphocytes and by vaccination with minimal essential epitopes. Immunol Rev. 1995;145:167–177. doi: 10.1111/j.1600-065X.1995.tb00081.x. [DOI] [PubMed] [Google Scholar]
- 3.Riddell SR, Greenberg PD. Principles for adoptive T cell therapy of human viral diseases. Annu Rev Immunol. 1995;13:545–586. doi: 10.1146/annurev.iy.13.040195.002553. [DOI] [PubMed] [Google Scholar]
- 4.Riddell SR, Watanabe KS, Goodrich JM, Li CR, Agha ME, Greenberg PD. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of T cell clones. Science. 1992;257:238–241. doi: 10.1126/science.1352912. [DOI] [PubMed] [Google Scholar]
- 5.Riddell SR, Walter BA, Gilbert MJ, Greenberg PD. Selective reconstitution of CD8+ cytotoxic T lymphocyte responses in immunodeficient bone marrow transplant recipients by the adoptive transfer of T cell clones. Bone Marrow Transplant. 1994;14(Suppl 4):S78–84. [PubMed] [Google Scholar]
- 6.Brodie SJ, Lewinsohn DA, Patterson BK, Jiyamapa D, Krieger J, Corey L, Greenberg PD, Riddell SR. In vivo migration and function of transferred HIV-1-specific cytotoxic T cells. Nat Med. 1999;5:34–41. doi: 10.1038/4716. [DOI] [PubMed] [Google Scholar]
- 7.Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED, Riddell SR. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–1044. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 8.Heslop HE, Brenner MK, Rooney CM. Donor T cells to treat EBV-associated lymphoma. N Engl J Med. 1994;331:679–680. doi: 10.1056/NEJM199409083311017. [DOI] [PubMed] [Google Scholar]
- 9.Heslop HE, Ng CY, Li C, Smith CA, Loftin SK, Krance RA, Brenner MK, Rooney CM. Long-term restoration of immunity against Epstein-Barr virus infection by adoptive transfer of gene-modified virus-specific T lymphocytes. Nat Med. 1996;2:551–555. doi: 10.1038/nm0596-551. [DOI] [PubMed] [Google Scholar]
- 10.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–854. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rosenberg SA, Packard BS, Aebersold PM, Solomon D, Topalian SL, Toy ST, Simon P, Lotze MT, Yang JC, Seipp CA, et al. Use of tumor-infiltrating lymphocytes and interleukin-2 in the immunotherapy of patients with metastatic melanoma. A preliminary report. N Engl J Med. 1988;319:1676–1680. doi: 10.1056/NEJM198812223192527. [DOI] [PubMed] [Google Scholar]
- 12.Mackensen A, Meidenbauer N, Vogl S, Laumer M, Berger J, Andreesen R. Phase I study of adoptive T-cell therapy using antigen-specific CD8+ T cells for the treatment of patients with metastatic melanoma. J Clin Oncol. 2006;24:5060–5069. doi: 10.1200/JCO.2006.07.1100. [DOI] [PubMed] [Google Scholar]
- 13.Yee C, Thompson JA, Roche P, Byrd DR, Lee PP, Piepkorn M, Kenyon K, Davis MM, Riddell SR, Greenberg PD. Melanocyte destruction after antigen-specific immunotherapy of melanoma: direct evidence of t cell-mediated vitiligo. J Exp Med. 2000;192:1637–1644. doi: 10.1084/jem.192.11.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Milone MC, June CH. Adoptive immunotherapy: new ways to skin the cat? Clin Immunol. 2005;117:101–103. doi: 10.1016/j.clim.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Kim JV, Latouche JB, Riviere I, Sadelain M. The ABCs of artificial antigen presentation. Nat Biotechnol. 2004;22:403–410. doi: 10.1038/nbt955. [DOI] [PubMed] [Google Scholar]
- 16.Oelke M, Krueger C, Giuntoli RL, 2nd, Schneck JP. Artificial antigen-presenting cells: artificial solutions for real diseases. Trends Mol Med. 2005;11:412–420. doi: 10.1016/j.molmed.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 17.Rosenberg SA. Progress in human tumour immunology and immunotherapy. Nature. 2001;411:380–384. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 18.Boon T, Cerottini JC, Van den Eynde B, van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–365. doi: 10.1146/annurev.iy.12.040194.002005. [DOI] [PubMed] [Google Scholar]
- 19.Maeurer MJ, Storkus WJ, Kirkwood JM, Lotze MT. New treatment options for patients with melanoma: review of melanoma-derived T-cell epitope-based peptide vaccines. Melanoma Res. 1996;6:11–24. doi: 10.1097/00008390-199602000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Rosenberg SA. The immunotherapy of solid cancers based on cloning the genes encoding tumor-rejection antigens. Annu Rev Med. 1996;47:481–491. doi: 10.1146/annurev.med.47.1.481. [DOI] [PubMed] [Google Scholar]
- 21.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meidenbauer N, Marienhagen J, Laumer M, Vogl S, Heymann J, Andreesen R, Mackensen A. Survival and tumor localization of adoptively transferred Melan-A-specific T cells in melanoma patients. J Immunol. 2003;170:2161–2169. doi: 10.4049/jimmunol.170.4.2161. [DOI] [PubMed] [Google Scholar]
- 23.Boon T, Coulie PG, Van den Eynde B. Tumor antigens recognized by T cells. Immunol Today. 1997;18:267–268. doi: 10.1016/S0167-5699(97)80020-5. [DOI] [PubMed] [Google Scholar]
- 24.Dudley ME, Wunderlich JR, Shelton TE, Even J, Rosenberg SA. Generation of tumor-infiltrating lymphocyte cultures for use in adoptive transfer therapy for melanoma patients. J Immunother. 2003;26:332–342. doi: 10.1097/00002371-200307000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Oelke M, Maus MV, Didiano D, June CH, Mackensen A, Schneck JP. Ex vivo induction and expansion of antigen-specific cytotoxic T cells by HLA-Ig-coated artificial antigen-presenting cells. Nat Med. 2003;9:619–624. doi: 10.1038/nm869. [DOI] [PubMed] [Google Scholar]
- 26.Mackensen A, Wittnebel S, Veelken H, Noppen C, Spagnoli GC, Lindermann A. Induction and large-scale expansion of CD8+ tumor specific cytotoxic T lymphocytes from peripheral blood lymphocytes by in vitro stimulation with CD80-transfected autologous melanoma cells. Eur Cytokine Netw. 1999;10:329–336. [PubMed] [Google Scholar]
- 27.Valmori D, Fonteneau JF, Lizana CM, Gervois N, Lienard D, Rimoldi D, Jongeneel V, Jotereau F, Cerottini JC, Romero P. Enhanced generation of specific tumor-reactive CTL in vitro by selected Melan-A/MART-1 immunodominant peptide analogues. J Immunol. 1998;160:1750–1758. [PubMed] [Google Scholar]
- 28.Kurokawa T, Oelke M, Mackensen A. Induction and clonal expansion of tumor-specific cytotoxic T lymphocytes from renal cell carcinoma patients after stimulation with autologous dendritic cells loaded with tumor cells. Int J Cancer. 2001;91:749–756. doi: 10.1002/1097-0215(200002)9999:9999<::AID-IJC1141>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 29.Oelke M, Moehrle U, Chen JL, Behringer D, Cerundolo V, Lindemann A, Mackensen A. Generation and purification of CD8+ melan-A-specific cytotoxic T lymphocytes for adoptive transfer in tumor immunotherapy. Clin Cancer Res. 2000;6:1997–2005. [PubMed] [Google Scholar]
- 30.Zhou X, Cui Y, Huang X, Yu Z, Thomas AM, Ye Z, Pardoll DM, Jaffee EM, Cheng L. Lentivirus-mediated gene transfer and expression in established human tumor antigen-specific cytotoxic T cells and primary unstimulated T cells. Hum Gene Ther. 2003;14:1089–1105. doi: 10.1089/104303403322124800. [DOI] [PubMed] [Google Scholar]
- 31.Brentjens RJ, Latouche JB, Santos E, Marti F, Gong MC, Lyddane C, King PD, Larson S, Weiss M, Riviere I, Sadelain M. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9:279–286. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 32.Costa GL, Sandora MR, Nakajima A, Nguyen EV, Taylor-Edwards C, Slavin AJ, Contag CH, Fathman CG, Benson JM. Adoptive immunotherapy of experimental autoimmune encephalomyelitis via T cell delivery of the IL-12 p40 subunit. J Immunol. 2001;167:2379–2387. doi: 10.4049/jimmunol.167.4.2379. [DOI] [PubMed] [Google Scholar]
- 33.Dudley ME, Wunderlich J, Nishimura MI, Yu D, Yang JC, Topalian SL, Schwartzentruber DJ, Hwu P, Marincola FM, Sherry R, Leitman SF, Rosenberg SA. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother. 2001;24:363–373. doi: 10.1097/00002371-200107000-00012. [DOI] [PubMed] [Google Scholar]
- 34.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291:2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 35.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 36.Tough DF. Deciphering the relationship between central and effector memory CD8+ T cells. Trends Immunol. 2003;24:404–407. doi: 10.1016/S1471-4906(03)00169-8. [DOI] [PubMed] [Google Scholar]
- 37.Beckhove P, Feuerer M, Dolenc M, Schuetz F, Choi C, Sommerfeldt N, Schwendemann J, Ehlert K, Altevogt P, Bastert G, Schirrmacher V, Umansky V. Specifically activated memory T cell subsets from cancer patients recognize and reject xenotransplanted autologous tumors. J Clin Invest. 2004;114:67–76. doi: 10.1172/JCI20278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Feuerer M, Beckhove P, Bai L, Solomayer EF, Bastert G, Diel IJ, Pedain C, Oberniedermayr M, Schirrmacher V, Umansky V. Therapy of human tumors in NOD/SCID mice with patient-derived reactivated memory T cells from bone marrow. Nat Med. 2001;7:452–458. doi: 10.1038/86523. [DOI] [PubMed] [Google Scholar]
- 39.Chen G, Shankar P, Lange C, Valdez H, Skolnik PR, Wu L, Manjunath N, Lieberman J. CD8 T cells specific for human immunodeficiency virus, Epstein-Barr virus, and cytomegalovirus lack molecules for homing to lymphoid sites of infection. Blood. 2001;98:156–164. doi: 10.1182/blood.V98.1.156. [DOI] [PubMed] [Google Scholar]
- 40.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 41.Oelke M, Kurokawa T, Hentrich I, Behringer D, Cerundolo V, Lindemann A, Mackensen A. Functional characterization of CD8(+) antigen-specific cytotoxic T lymphocytes after enrichment based on cytokine secretion: comparison with the MHC-tetramer technology. Scand J Immunol. 2000;52:544–549. doi: 10.1046/j.1365-3083.2000.00810.x. [DOI] [PubMed] [Google Scholar]
- 42.Mitchell MS, Darrah D, Yeung D, Halpern S, Wallace A, Voland J, Jones V, Kan-Mitchell J. Phase I trial of adoptive immunotherapy with cytolytic T lymphocytes immunized against a tyrosinase epitope. J Clin Oncol. 2002;20:1075–1086. doi: 10.1200/JCO.20.4.1075. [DOI] [PubMed] [Google Scholar]
- 43.Tan R, Xu X, Ogg GS, Hansasuta P, Dong T, Rostron T, Luzzi G, Conlon CP, Screaton GR, McMichael AJ, Rowland-Jones S. Rapid death of adoptively transferred T cells in acquired immunodeficiency syndrome. Blood. 1999;93:1506–1510. [PubMed] [Google Scholar]
- 44.Economou JS, Belldegrun AS, Glaspy J, Toloza EM, Figlin R, Hobbs J, Meldon N, Kaboo R, Tso CL, Miller A, Lau R, McBride W, Moen RC. In vivo trafficking of adoptively transferred interleukin-2 expanded tumor-infiltrating lymphocytes and peripheral blood lymphocytes. Results of a double gene marking trial. J Clin Invest. 1996;97:515–521. doi: 10.1172/JCI118443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci USA. 2002;99:16168–16173. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fisher B, Packard BS, Read EJ, Carrasquillo JA, Carter CS, Topalian SL, Yang JC, Yolles P, Larson SM, Rosenberg SA. Tumor localization of adoptively transferred indium-111 labeled tumor infiltrating lymphocytes in patients with metastatic melanoma. J Clin Oncol. 1989;7:250–261. doi: 10.1200/JCO.1989.7.2.250. [DOI] [PubMed] [Google Scholar]
- 47.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–129. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cochlovius B, Perschl A, Adema GJ, Zoller M. Human melanoma therapy in the SCID mouse: in vivo targeting and reactivation of melanoma-specific cytotoxic T cells by bi-specific antibody fragments. Int J Cancer. 1999;81:486–493. doi: 10.1002/(SICI)1097-0215(19990505)81:3<486::AID-IJC25>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 49.Lozupone F, Rivoltini L, Luciani F, Venditti M, Lugini L, Cova A, Squarcina P, Parmiani G, Belardelli F, Fais S. Adoptive transfer of an anti-MART–1(27–35)-specific CD8+ T cell clone leads to immunoselection of human melanoma antigen-loss variants in SCID mice. Eur J Immunol. 2003;33:556–566. doi: 10.1002/immu.200310032. [DOI] [PubMed] [Google Scholar]
- 50.Overwijk WW, Theoret MR, Finkelstein SE, Surman DR, de Jong LA, Vyth-Dreese FA, Dellemijn TA, Antony PA, Spiess PJ, Palmer DC, Heimann DM, Klebanoff CA, Yu Z, Hwang LN, Feigenbaum L, Kruisbeek AM, Rosenberg SA, Restifo NP. Tumor regression and autoimmunity after reversal of a functionally tolerant state of self-reactive CD8+ T cells. J Exp Med. 2003;198:569–580. doi: 10.1084/jem.20030590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Arditti FD, Greenberg R, Dekel B, Marcus H, Nagler A, Berrebi A, Skornick Y, Reisner Y. Human colon adenocarcinoma in the SCID/CB6 radiation chimera is susceptible to adoptive transfer of allogeneic human peripheral blood mononuclear cells. J Hematother Stem Cell Res. 2002;11:883–893. doi: 10.1089/152581602321080547. [DOI] [PubMed] [Google Scholar]
- 52.Cochlovius B, Stassar M, Christ O, Raddrizzani L, Hammer J, Mytilineos I, Zoller M. In vitro and in vivo induction of a Th cell response toward peptides of the melanoma-associated glycoprotein 100 protein selected by the TEPITOPE program. J Immunol. 2000;165:4731–4741. doi: 10.4049/jimmunol.165.8.4731. [DOI] [PubMed] [Google Scholar]
- 53.Wagar EJ, Cromwell MA, Shultz LD, Woda BA, Sullivan JL, Hesselton RM, Greiner DL. Regulation of human cell engraftment and development of EBV-related lymphoproliferative disorders in Hu-PBL-scid mice. J Immunol. 2000;165:518–527. doi: 10.4049/jimmunol.165.1.518. [DOI] [PubMed] [Google Scholar]
- 54.Overwijk WW, Tsung A, Irvine KR, Parkhurst MR, Goletz TJ, Tsung K, Carroll MW, Liu C, Moss B, Rosenberg SA, Restifo NP. gp100/pmel 17 is a murine tumor rejection antigen: induction of “self”-reactive, tumoricidal T cells using high-affinity, altered peptide ligand. J Exp Med. 1998;188:277–286. doi: 10.1084/jem.188.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]