Abstract
The mononuclear fraction from human umbilical cord blood (HUCB) contains a significant number of stem/progenitor cells that in theory could become any cell in the body, including neurons. Taking into consideration that transdifferentiation would be a very rare event and also knowing that overlapping genetic programs for hematopoiesis and neuropoiesis exist, we undertook a characterization of the HUCB mononuclear fraction, including analysis of cellular subpopulations and their morphology, cell viability, proliferation, and expression of neural and hematopoietic antigens. Two cell populations were apparent—adherent and floating fractions. The adherent fraction was mainly lymphocytes (~53%) expressing hematopoietic antigens. Upon replate, the floating population had many cells that expressed stem cell antigens. More of the cells in this subfraction expressed neural proteins. Neurotrophin receptors trkB and trkC were present in both cell fractions, although expression was higher in the floating fraction. Our initial characterization suggests that a subpopulation of cells exists within the HUCB mononuclear fraction that seems to have the potential to become neural cells, which could then be used in the development of cell-based therapies for brain injuries and diseases.
Keywords: Human, Umbilical cord blood, Stem/progenitor cell, Multipotential differentiation Hematopoietic and neural antigens, Neuropoiesis
INTRODUCTION
Human umbilical cord blood (HUCB) cells have been used as a powerful tool for the treatment of several blood-related diseases such as Fanconi anemia [1], leukemia [2, 3], thalassemia, sickle cell disease [4], and Wiscott-Aldrich syndrome [5]. Recently it was observed that cells derived from this source have the ability to express markers and morphologies of other cell types within the same mesodermal germ layer such as bone [6], fat [7], smooth muscle [8], and skeletal muscle [9]. More surprisingly, exposing HUCB cells to various experimental conditions showed that their progeny could also reveal properties typical of neuroectoderm-derived cells [10–13]. This multilineage differentiation capacity and the expression of neural properties suggests that HUCB cells may have the ability to transdifferentiate or become nonhematopoietic cells of various tissue lineages, including neural cells. If this is the case, they may therefore be useful for numerous cell-based therapies requiring either the replacement of individual cell types or substitution/replenishment of missing substances.
It has been reported that these cells can be used as a source of therapeutically effective substances with the ability to improve functional outcomes after stroke [14–16] and delay both onset of symptoms and death of animals with amyotrophic lateral sclerosis [17, 18]. Furthermore, these cells were shown to be useful in models of traumatic brain injury [19, 20] and spinal cord injuty [21, 22]. In the Sanfilippo animal model of mucopolysacharidosis IIIB, histopathological improvements were found after HUCB administration [23]. In the clinic, treatment of a young Krabbe’s leukodystrophy patient with HUCB infusions resulted in attenuated progression of the disease [24]. These studies rely more on the trophic effect of the mononuclear HUCB cells than on actual cellular replacement. Only a few transplantation studies have addressed the possibility that the mononuclear fraction may contain a small number of nondifferentiated cells that may, under specific circumstances, such as stroke or placement into a favorable neurogenic environment [12, 15], give rise to neural-like cells.
Knowing that overlapping genetic programs for hematopoiesis and neuropoiesis exist [25], in this study we explored the possibility that stem/progenitor cells in the mononuclear HUCB fraction can produce progeny that express neural antigens when grown as adherent or nonadherent cultures. We used culture conditions commonly used for neural tissue and examined through available immunocytochemical stainings and Western blot analysis whether, and to what extent, the antigens typical for neural lineages would overlap with hematopoietic surface antigens characteristic for hematopoietic stem/progenitor cells or their fully differentiated progeny. In addition, we investigated whether the hematopoietic cells, expressing neural antigens without previous exposure to neuralizing epigenetic factors, would attain the morphology of neural-derived cells. These observations will help us clarify the real contribution of the mononuclear HUCB fraction to the overall pool of hematopoietic cells that seem to be able to transdifferentiate toward neural lineages or determine whether this transdifferetiation is just a temporary coexpression of hematopoietic and neural markers triggered by unusual or artificial environmental cues.
MATERIALS AND METHODS
Wright-Giemsa Smears Before Culturing
To identify the cellular composition and morphology of the mononuclear HUCB fraction after thawing, Wright-Giemsa stain (Sigma-Aldrich, St. Louis, http://www.sigmaaldrich.com) was used. Using the wedge technique, smears of HUCB cells were prepared from a thawed sample, air dried, and stained for 30 seconds. After several washes in deionized water (pH 7.2), the slides were allowed to dry and coverslipped with water. The stained smears were analyzed under a light microscope, and representative images of various cell types from the smears were photographed at × 100. The sizes of individual hematopoietic cells at different maturational stages were measured using Image-pro Plus 4.1 software (Media Cybernetics, Inc., Silver Spring, MD, http://www.mediacy.com) (Table 1), and the cells were categorized based on their size and morphology [26].
Table 1.
Classification of the mononuclear HUCB cells before culture
| Cell type | Individual cells | Number of total cells | Percentage | Size (µm) |
|---|---|---|---|---|
| Myelocyte | 71 | 3,611 | 1.95 | 9.00–13.00 |
| Monocyte | 112 | 3,611 | 3.10 | 13.00–14.00 |
| Lymphocyte | 1,921 | 3,611 | 53.20 | 7.00–13.00 |
| Erythrocyte | 4 | 3,611 | 1.00 | 8.00–11.00 |
| Thrombocyte | 23 | 3,611 | 0.64 | 3.00–9.00 |
| Uncertain | 1,325 | 3,611 | 36.70 | 7.00–13.00 |
The table shows the occurrence (%) and size (µm) of HUCB cells from thawed samples prepared as smears and stained by Giemsa. Different affinities to eosin and methylene, the two main components of the dye, as well as the shape of the nucleus, cell size, nucleus/cytoplasm ratio, distribution of chromatin, color of cytoplasm, and cytoplasmic granules, allowed for recognition of individual blood lineages [26].
Abbreviation: HUCB, human umbilical cord blood.
Preparation of Mononuclear HUCB Cell Cultures
Adherent Culture
Cryopreserved samples of HUCB mononuclear fraction (supplied by Cambrex Corporation, East Rutherford, NJ, http://www.cambrex.com, or Saneron CCEL Therapeutics, Inc., Tampa, FL, http://www.saneron-ccel.com/pages/893814/) were thawed rapidly in a 37°C water bath, suspended in 10 ml of Dulbecco’s modified Eagle’s medium (DMEM) (Invitrogen, Carlsbad, CA, http://www.invitrogen.com) with 10% fetal bovine serum (FBS) (Invitrogen) and 0.1% gentamicin (Sigma-Aldrich) in a 15-ml conical tube (Falcon, BD Biosciences, San Diego, http://www.bdbiosciences.com), centrifuged (400g/7 minutes), and resuspended in 1 ml of the above-mentioned fresh medium. Cell viability (62%–90%) was assessed by a rapid staining procedure with 0.4% try-pan blue (Invitrogen). Cells were plated in the described medium on poly-l-lysine-coated (PLL) (10 µg/ml) eight-well chamber slides (Lab-Tek, Campbell, CA, http://www.labtek.net/) at a seeding density of 100,000 cells per cm2. All cultures were incubated at 37°C in 5% CO2 for 1–20 days in vitro (DIV), with fresh medium added every 5 days. Four donor samples were used in this experiment. Experiments were performed in duplicate for each time point.
Floating HUCB Fraction
Several cultures (20–34 DIV) with whole mononuclear HUCB cells were used to generate the floating population. The supernatants were collected, cells were spun down (400g/7 minutes), and the viability of the aliquot was determined (95%) using 0.4% trypan blue. The cells were replated into fresh DMEM (containing 10% FBS and 0.1% gentamicin) on PLL-coated (10 µg/ml) 24-well plates (Nunc, Rochester, NY, http://www.nuncbrand.com) at a seeding density of 100,000 cells per cm2. Fresh medium was added on day 5. The cultures were fixed and analyzed on day 10.
Dissection and Culture of Embryonic Tissue
Sprague-Dawley timed-pregnant rats (Harlan, Indianapolis, http://www.harlan.com) were anesthetized with Equithesin (3 ml/kg, i.p.), and the embryonic (E14-18) brains (15 embryos per dam) were removed from their skulls. The cortical and olfactory bulb [27, 28] tissues were dissected and mechanically dissociated in Hanks’ balanced salt solution/HEPES solution with 0.05% DNase. The cells were resuspended in DMEM, 10% FBS, and 0.1% gentamicin and plated on PLL-coated 24-well plates at a seeding density of 75,000 cells per cm2. Preplating viability ranged from 90%–95%. A day after plating, the medium was changed to Neurobasal (Gibco, Grand Island, NY, http://www.invitrogen.com) with 0.01% of B27 supplement (Gibco) and gentamicin. The cells were maintained at 37°C in 5% CO2 with 95% humidity for up to 10 DIV.
Viability of Cultured HUCB Cells
Before fixation, fluorescein diacetate/propidium iodide (FDA/PI) (Sigma-Aldrich) staining was used to assess the viability of the adherent mononuclear fraction at 1, 5, 10, 15, and 20 DIV. The numbers of living (green) and dead (red) cells were counted in 16 randomly selected fields per chamber slide per time point under an inverted fluorescent microscope. Cell viability was calculated as the number of living cells divided by the total number of cells multiplied by 100.
Isolation of CD133+ Cells from HUCB Mononuclear Fraction
Cryopreserved HUCB mononuclear fractions purchased from Cambrex Corporation were thawed rapidly in a 37°C water bath and then washed with phosphate-buffered saline (PBS) containing 2 mM EDTA (Sigma-Aldrich). The cell pellet was resuspended in a final volume of 300 µl of buffer (PBS, pH 7.2, supplemented with 0.5% bovine serum albumin and 2 mM EDTA) per 107 total cells, and then the CD133+ cells were separated out using a CD133 cell isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany, http://www.miltenyibiotec.com) according to manufacturer’s instructions. Briefly, after adding 100 µl FcR blocking reagent, cells were incubated with CD133 MicroBeads for 30 minutes at 4°C to 8°C. CD133+ cells then were isolated from the HUCB mononuclear fraction by a Mini MACs (a magnetic cell separator; Miltenyi Biotec). After separation, the total number of positive cells and the viability were determined using a hemacytometer. A total of 3.1 × 104 CD133+ cells was isolated from the HUCB mononuclear fraction, and viability was 98%. Immunocytochemical staining confirmed all isolated cells were positive for CD133.
Immunocytochemistry
Immunocytochemistry was used for identification of hematopoietic, embryonic, and neural-specific antigens in HUCB cells and primary embryonic cell cultures. The HUCB cultures (adherent and floating) were fixed with 4% paraformaldehyde in 0.1 M PBS (pH 7.4) at 1, 5, 10, 15, and 20 DIV, whereas primary cell cultures were fixed at day 10. After rinsing three times in PBS, the cells were blocked with 10% goat serum and 0.1% Triton X-100 (omitted for immunolabeling of surface markers) in PBS for 1 hour at room temperature (RT). They were then incubated with the primary antibody (Table 2) at 4°C for 24 hours, washed in PBS three times, and incubated with a species-appropriate secondary antibody (Alexa Fluor 594, 488; Molecular Probes Inc., Eugene, OR, http://probes.invitrogen.com; 1:800 and 1:500, respectively) for 1 hour at RT, washed again with cold PBS, and coverslipped with 95% glycerol. Cell nuclei were visualized with 4,6-diamidino-2-phenylindole hydrochloride (100 ng/ml; Sigma-Aldrich). All culture slides were examined under BX-60 or IX-71 microscopes (Olympus, Tokyo, http://www.olympus-global.com).
Table 2.
Antibodies used in the study
| Antigen | Specie | Cross reactivit | Dilution | Source |
|---|---|---|---|---|
| Hematopoietic | ||||
| CD133 | Mouse | Human | 1:50 | Miltenyi Biotec |
| CD117 | Mouse | Human | 1:50 | BD Pharmingen |
| CD15 | Mouse | Human | 1:50 | BD Pharmingen |
| CD45 | Mouse | Human | 1:50 | BD Pharmingen |
| Early neural | ||||
| Nestin | Mouse | Human | 1:100 | BD Transduction |
| Vimentin | Mouse | Human | 1:200 | Novocastra |
| A2B5 | Mouse | Human, rat, chicken, mouse | 1:200 | Chemicon |
| Neuronal | ||||
| TuJ1 | Rabbit | Mammals | 1:1,000 | Covance |
| NF68KD | Rabbit | Rat, mouse, bovine, monkey, human | 1:500 | Chemicon |
| MAP2 | Mouse | Human, rat, mouse | 1:1,500 | Chemicon |
| NSE | Rabbit | Human | 1:100 | Oncogene |
| Glia | ||||
| GFAP | Rabbit | Human, cow | 1:800 | DAKO |
| S100 | Rabbit | Human | 1:167 | Sigma-Aldrich |
| GalC | Rabbit | Human, animal | 1:150 | Sigma-Aldrich |
| Receptors | ||||
| trk A | Rabbit | Human | 1:500 | Chemicon |
| trk B | Rabbit | Human | 1:2,000 | Chemicon |
| trk C | Rabbit | Human | 1:2,000 | Chemicon |
| p75 NTR | Mouse | Human | 1:400 | Novus |
| CXCR4 | Rabbit | Human | 1:1,000 | Chemicon |
The table summarizes all primary antibodies used and their species specificity, dilutions, and sources.
Abbreviations: CD133, stem/progenitor cells; CD117 (c-kit), hematopoietic progenitor cell; CD15, neutrophils, eosinophils, monocytes; CD45, leukocytes; CXCR4, chemokine receptor 4 (SDF1 receptor); GalC, galactocerebroside C; GFAP, glial fibrillary acidic protein; MAP2, microtubule-associated protein 2; NF 68KD, neurofilament 68KD; NSE, neuron-specific enolase; p75NTR, low-affinity neurotrophin receptor (NGF, BDNF, NT4/5, NT3 receptor); trkA, tyrosine kinase receptor A (NGF, NT3 receptor); trkB, tyrosine kinase receptor B (BDNF, NT4/5, NT3 receptor); trkC, tyrosine kinase receptor C (NT3, BDNF, NT4/5 receptor); TuJ1, β-tubulin III.
Quantitative Analysis
To assess the initial composition of the mononuclear HUCB fraction, we counted the number of morphologically distinct cell types from three samples of HUCB cells (three to four smears per sample). The prevalence of each individual cell type was determined from 24 visual fields taken at × 40. The number of cells with similar morphology was divided by the total number of cells per field and multiplied by 100 (Table 1).
To estimate the number of cells positive for hematopoietic, embryonic, and neural markers, the number of immunopositive cells and the total number of cells were counted using fluorescent microscopy by a single individual. The percentage was calculated as the number of cells positive for the specific antigen divided by the total number of cells and then multiplied by 100. For each antigenic marker, counts were taken from five randomly selected fields in the well at × 20 magnification and expressed as mean ± SEM. The total number of counted cells for each antigen ranged from 1,500 to 2,500. Analysis of variance was used to determine the differences in the number of cells expressing the specific antigen at each of the different culturing periods (1, 5, 10, 15, and 20 DIV). The criterion of significance was set at p < .05.
The staining intensity of each antigen was also assessed quantitatively using a 0–4 ordinal scale, in which 0 = absent, 1 = minimal, 2 = moderate, 3 = high, and 4 = maximal. The average degree of immunostaining was then calculated for each antigen in five fields.
To compare the two subpopulations (adhered and floating) of HUCB cells, statistical analysis of the data was performed using the program GB-STAT version 7.0 (DynamicMicrosystems, Inc., Silver Spring, MD, http://www.gbstat.com) by Student’s t-test with a significance level of p < .05 (two-tailed).
Western Blot
HUCB cells were cultured for either 5 or 10 DIV. Positive controls were prepared from male Sprague-Dawley rat brains (5 months old) euthanatized by decapitation. Longer-term cultures of the floating replated HUCB population (190 days), which highly expressed nestin and TuJ1, were also used as a positive control. The cell pellets or harvested brain tissue samples were suspended in the homogenization buffer (pH 8.0). The samples were homogenized by ultrasound and centrifuged (800g/20 minutes) to remove the nuclear fraction, and the resulting supernatants were collected. Protein concentration (for each sample) was assayed by bicinchoninic acid (Pierce, Rockford, IL, http://www.piercenet.com). The defined amount of protein (50 µg/40 µl) was separated by SDSPAGE on a 7%–10% polyacrylamide gel, transferred on nitrocellulose membranes, and incubated for 1 hour at RT with 5% nonfat powdered milk in Tris-buffered saline containing 0.1% Tween 20 (TTBS) (pH 8.3). Immunodetection was performed using a monoclonal antibody against Nestin (1:100; BD Biosciences), TuJ1 (1:1,000; Covance, Princeton, NJ, http://www.covance.com), A2B5 (1:200; Chemicon, Temecula, CA, http://www.chemicon.com), or GFAP (1:500; Harlan) overnight at 4°C. After washing in TTBS, a secondary antibody conjugated to alkaline phosphatase (1:3,000; Bio-Rad, Hercules, CA, http://www.bio-rad.com) was applied for 1 hour at RT, and then the sample was washed again with TTBS. The reaction product was detected using a chemiluminescent protein detection system (Bio-Rad).
RESULTS
Cellular Composition of the Mononuclear HUCB Fraction Before Culturing
Upon thaw of the HUCB mononuclear cells, Wright-Giemsa smears were prepared to determine the ratio of differentiated and undifferentiated leukocytes (Fig. 1A). Two samples from four donors each were used. The stained cells were categorized into lymphoid (54%) and myeloid (7%) lineages based on the appearance of their nuclei and cytoplasm. The cells from the lymphoid lineage consisted of lymphoblasts (Fig. 1A, part A' [H]) and small lymphocytes (Fig. 1A, part A' [I]). Cells of the myeloid lineage had a characteristic granular cytoplasm (granulocytes) and consisted of monocytes (Fig. 1A, part A' [F, G]) and myeloid (Fig. 1A, part A' [B–E]) cells. Thirty-nine percent of the cells were not as easily defined. The morphology of these cells was round and similar to lymphocytes. Additionally, 1%–2% of cells resembled immature thrombocytes and erythrocytes. The number of mature erythrocytes was negligible (0.78%, Table 1).
Figure 1.
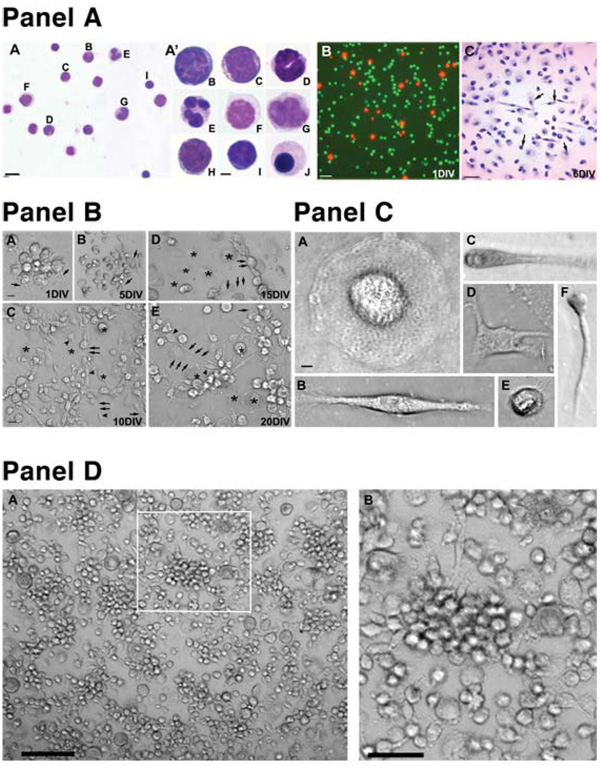
The Wright-Giemsa staining and morphology of a mononuclear HUCB fraction before and after culturing. (Panel A): Part A, Giemsa stained smear of HUCB cells. Scale bar = 10 µm. Part A' shows high magnification of individual cell types depicted on the previous image: (B) myeloblast, (C) promyelocyte, (D) band, (E) neutrophil, (F) promonocyte, (G) monocyte, (H) lymphoblast, (I) small lymphocyte, and (J) orthochromic normoblast. Scale bar = 2 μm. Part B shows viability of cultured mononuclear HUCB cells; living cultures were stained with fluorescein diacetate/ propidium iodide to detect healthy (green) and dead (red) cells. Part C shows bright-field photomicrograph of Giemsastained cultured HUCB cells (6 DIV) demonstrating heterogeneous morphologies (arrows). Scale bar = 20 µm. (Panel B): Morphology of the adherent population of mononuclear HUCB cells cultured for 20 days. (A): One day after plating, cells (arrowheads) formed small clusters from which a few cells sent out tiny processes (arrows). During the next culture period (5–10 days), the clusters became loosely packed and many cells extended longer processes interconnecting cell groups. (B, C): Besides small round cells either with or without processes (arrows), numerous larger round cells (asterisks) were found. These cells were always tightly adhered to the bottom of the culture well. (D, E): In the next two time periods (15 and 20 DIV), small cells (arrowheads) with longer processes were interspersed either with individual or groups of round flat cells (asterisks). Scale bar = (A) 10 µm and (B–E) 20 µm. (Panel C): Different cell types were observed in adherent cultures: (A) large egg-shaped cells; (B) bipolar and (D) multipolar cells; (E) small round cells with multiple hair-like spines; and small cells with (C) thicker or (F) thinner processes. Scale bar = 2 µm. (Panel D): The morphology of cultured HUCB cells prepared from floating fractions. Morphologically, the cells were similar to the adherent population, with a striking absence of big round egg-like cells. (A): Numerous clusters (arrow) of uniform round cells with occasional processes were observed. (B): Higher magnification of the boxed area from (A). Scale bar = (A) 40 µm and (B) 20 µm. Abbreviations: DIV, days in vitro; HUCB, human umbilical cord blood.
Viability and Morphology of Cultured Mononuclear HUCB Cells
The viability of the HUCB cells at thaw ranged from 65%–85%. After 5 DIV, FDA/PI fluorescent staining revealed that viability remained high (85%), whereas at later time points (10, 15, and 20 DIV; Fig. 1A [B]), viability was maintained at 50%–60%. The morphology of plated cells changed rapidly over time (Fig. 1A [C]). These initially small, round cells attained a new morphological appearance within 24–48 hours after plating. Numerous cells within clusters started to send out tiny processes. Later on (5–20 DIV), clearly distinct cell types appeared (Fig. 1B). The large egg-shaped cells without processes were common. They tightly adhered to the culture plate and frequently had numerous small cells on their surface or in close vicinity to the cell. The most frequently observed cell type was the small, round cell, either with one or two processes of variable length or with a ciliated surface.
HUCB Cells Reveal Immunoreactivity for Hematopoietic and Neural Antigens
We cultured HUCB mononuclear cells with serum-containing medium because these cells grow better in serum. The same lot of serum was used throughout the study, which allowed us to compare the morphology and cell type–specific antigens across several platings and time points (1, 5, 10, 15, and 20 DIV). Under these conditions, we found that there were two subpopulations of cells in the HUCB mononuclear fraction, an adherent and a floating population. Cellular composition, morphology, viability, and developmental potential of these two subpopulations are different.
Adherent Monolayer Cultures
Expression of Hematopoietic Antigens
We found that most adherent HUCB cells (90.85% ± 3.33%) expressed common leukocyte antigen (CD45) at all examined time points (Fig. 2A [A]). The morphology of these CD45+ cells was generally round without differentiation. Only a few cells expressed CD133 (2.72% ± 1.88%; Fig. 2A [C]) and CD117 (6.67% ± 4.23%) on day 1 (Fig. 3A). Both of these antigens were used for identifying stem/progenitor cells. These cells were small and round and were scattered throughout the culture. In addition, we noted that only a small number of CD15+ cells were present in this subfraction over all time points, although expression peaked at 10 DIV (18.13% ± 5.95%; Fig. 2A [B]). CD15 is normally expressed on 90% of human circulating granular cells and 30%–60% of circulating monocytes. It is also known as stage-specific embryonic antigen (SSEA-1) or Lewis X because it recognizes the carbohydrate epitope 3-fucosyl-N-acetyl-lactosamine on embryonic stem cells.
Figure 2.
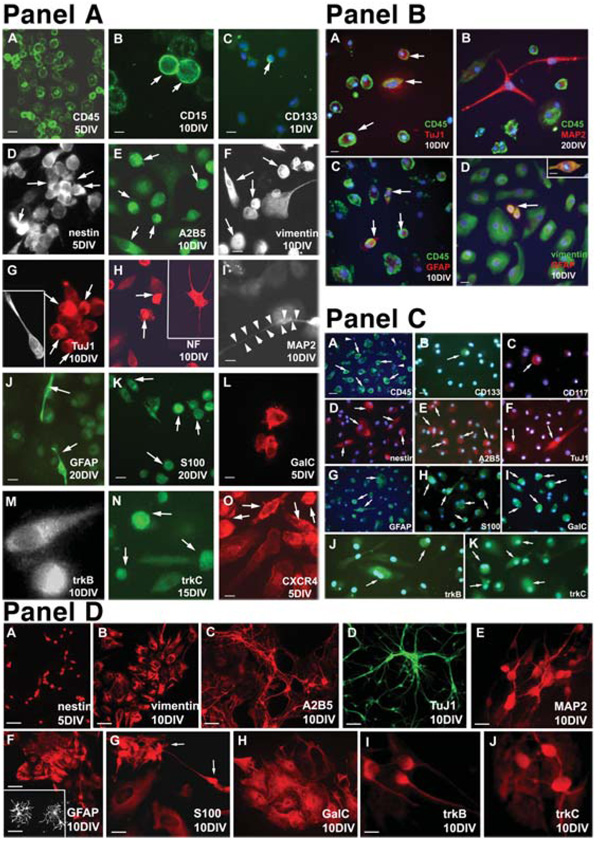
Fluorescent images. (Panel A): Immunocytochemical characterization of (A–C) adherent mononuclear HUCB cells showing the expression of hematopoietic CD antigens. (A): Almost every cell revealed surface CD45 (green) antigen at 1, 5, 10, 15, and 20 days. (B): Only a few cells were positive for the antibody against CD15 (green, arrows) throughout the entire course of the study. (C): The presence of immature progenitors expressing CD133 (green, arrow) was recorded only in short-term (1 DIV) cultures. DAPI counterstaining was used for visualization of the entire cell population. Scale bar = (A, B) 20 µm and (C) 10 µm. (D–F): Several early neural markers were expressed in HUCB cells. Cells positive for (D) nestin, (E) A2B5, and (F) vimentin were observed throughout the entire culturing period. Arrows in (D–F) point to immunoreactive cells. (G–I): HUCB cells expressed early and mature neuronal antigens. Ten days after culturing, only a few cells expressed TuJ1 (G, arrows) and MAP2 (I). The arrow points to the MAP2-positive soma, whereas arrow-heads delineate the long process. (H): At the same time, many cells were immunoreactive for NF68KD (arrows). Scale bar = (D–I) 20 µm. (J–L): Cultured HUCB cells express glial antigens. (J): Numerous cells were positive for GFAP. The arrows point to two cells with bipolar morphology. In sister cultures, the positivity for other glial markers such as S100 (K, arrows) and GalC (L) was detected. Scale bar = (J, K) 20 µm and (L) 10 µm. (M–O): Expression of neurotrophin and chemokine receptors. (M): Positivity for trkB was found on day 5 only, whereas trkC (N, arrows) was present throughout the whole culture period. (O): CXCR4 was present in numerous cells in all studied intervals (arrows). Scale bar = (M–O) 10 µm. (Panel B): Coexpression of hematopoietic and neural antigens in cultured HUCB cells. (A): Several CD45-positive (green) cells coexpressing TuJ1 (red). Hematopoietic CD markers are localized on the cell surface (arrows) of many HUCB cells, whereas weak cytoplasmic TuJ1 expression was found occasionally. Nuclear DAPI labeling (blue) confirms that not every HUCB cell expresses CD antigens. (B): For longer cultures, a cell with distinct processes positive for mature neuronal marker MAP2 (red) and negative for CD45. On the other hand, cells with round morphology were CD45 immunoreactive (green). (C): Coexpression of GFAP (red) and CD45 (green) was also found (arrows). (D): HUCB cells were immunopositive for vimentin (green), and some also expressed GFAP (red). The arrow indicates vimentin/GFAP-postive cells. Inset shows a double-labeled vimentin/GFAP-positive (orange) cell at a higher magnification. Scale bar = (A–C, inset in D) 10 µm and (D) 20 µm. (Panel C): Fluorescent images of replated floating HUCB cells. (A–C): Hematopoietic markers, including (A) CD45, (B) CD133, and (C) CD117. Arrowheads point to cells negative for the specific marker, whereas arrows indicate cells expressing one of the CD antigens. (D, E): Expression of (D) nestin and (E) A2B5 (arrows) was higher in the replated floating fraction than in the adhered HUCB cells. (F–I): Cells positive for (F) TuJ1, (G) GFAP, (H) S100, and (I) GalC. Morphologically, this population of cells rarely acquired the classical appearance of neural derivatives. (J, K): Expression of neurotrophin receptors (J) trkB and (K) trkC was also detected. Arrows in (F–K) point to positively labeled cells. Blue DAPI counterstaining was used for clear identification of all cultured cells. Scale bar = (A, D) 20 µm and (B, C, E–K) 40 µm. (Panel D): Expression of neural antigens in E14–E18 primary cultures. (A–C): Cells isolated from the embryonic striatum were immunopositive for (A) nestin and (B) vimentin, and cultures prepared from the embryonic olfactory bulb revealed positivity for (C) A2B5. Scale bar = (A) 10 µm and (B, C) 40 µm. TuJ1 (D) and MAP2 (E) were found in cortical cultures after 10 days. Cells expressing these two neuronal antigens revealed classical neuronal morphology. (F): Small neuronal bodies and long, branching processes differed from flat, larger cells expressing GFAP. Inset shows the detailed morphology of GFAP-positive cells taken from E18 striatal culture. Scale bar = (D) 40 µm, (E) 10 µm, and 20 µm. Scale bar in inset = 10 µm. (G, H): Cortex-derived cells also expressed two other glial antigens. Arrows in (G) point to S100-positive cells. Scale bar = 20 µm. (I, J): Cortical cells also reveal positivity for neurotrophin receptors. Scale bar = 10 µm. Abbreviations: DAPI, 4,6-diamidino-2-phenylindole; DIV, days in vitro; HUCB, human umbilical cord blood.
Figure 3.
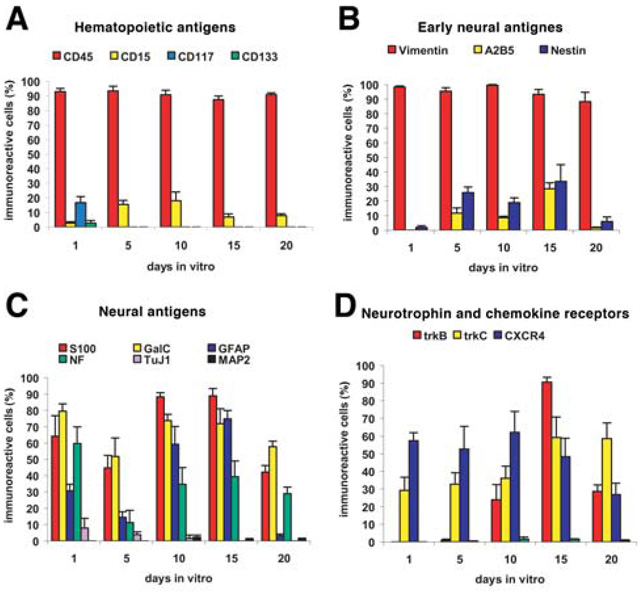
Antigen expression in adherent HUCB cells cultured in serum from 1–20 days in vitro. (A): Expression of early hematopoietic and stem cell antigens. (B): Expression of early neural/stem cell antigens. (C): Expression of antigens indicative of immature and mature neurons, astrocytes, and oligodendrocytes. (D): The adherent HUCB cells also expressed both neurotrophin receptors and the receptor for the chemokine stromal-derived factor 1, CXCR4. Abbreviation: HUCB, human umbilical cord blood.
Expression of Early and Mature Neural Antigens
To explore the potential of the adherent HUCB cells to produce neuron-like cells, we investigated the expression of neural proteins in these cells. Cells were immunofluorescently labeled with specific antigens, and the percentage of cells expressing each antigen was determined at all examined time points during the study (Fig. 2, Fig 3). Many antigens typically found in the brain were expressed in the adherent fraction of HUCB cells. The highest frequency of expression was observed at 10–15 DIV. Nestin expression increased from 1.9% ± 1.3% after 1 DIV to 33.44% ± 11.51% after 15 DIV (Fig. 2A [D], Fig. 3B). Similarly, A2B5 (Fig. 2A [E]) expression peaked (28% ± 4%) at 15 DIV and decreased to 1.59% ± 0.46% by 20 DIV. In addition, regardless of their morphology and the length of the culturing period, almost all cells (average, 95.55% ± 2.3%) expressed vimentin, which is not only a marker of early neural cells but also a marker of mesenchymal origin. Besides these three antigens which are indicative of immature cells, we also detected mature glial antigens. GFAP was highly expressed in round cells at all times except 20 DIV, when expression was negligible (Fig. 3C) and strictly confined to small cells with two processes (Fig. 2A [J]). At the same time, S100 was highly expressed by many medium-sized cells. Further, the oligodendrocyte marker galactocerebroside was detected on the surface of round and irregularly shaped cells (Fig. 2A [K]) throughout the entire culture period.
HUCB cells within the adherent fraction in our study conditions were less likely to express either immature or mature neuronal markers. Immature neuronal antigen TuJ1 was present in a small population of round-shaped cells or cells with single processes at 1, 5, and 10 DIV (Fig. 2A [G]), although expression was highest at 1 DIV (7.9% ± 5.9%). We did not find TuJ1+ cells at 15 or 20 DIV. The mature neuronal antigen, MAP2, was almost absent in adherent HUCB cells (Fig. 3C). Those rare MAP2+ cells, however, had a neuron-like morphology with small somas and long, thin processes (Fig. 2A [I]). Interestingly, NF68KD expression was found in 34.86% ± 10.14% of the cells at all studied intervals (Fig. 3C). NF68KD predominantly stains neurons of the central and peripheral nervous system. This antigen was mostly expressed in cells with multiple processes (Fig. 2A [H]).
To elucidate whether there is coexpression of hematopoietic and neural antigens within the same cells, we first labeled adherent cultures with the antibody recognizing CD45 and afterward labeled cells with TuJ1, MAP2, or GFAP (Fig. 2B). Some HUCB cells coexpressed CD45 antigen and TuJ1 (Fig. 2B [A]; CD45+/TuJ1+) or GFAP (Fig. 2B [C]; CD45+/GFAP+). Interestingly, CD45 was not coexpressed with MAP2 (Fig. 2B [B]), and the morphologies of these CD45−/MAP2+ cells were different from the CD45-positive cells. MAP2 immunoreactive cells (CD45−/MAP2+) were well-differentiated, extending long processes. We also observed positive cells with coexpression of vimentin and GFAP antigens (vimentin+/GFAP+).
Presence of Neurotrophin and Chemokine Receptors
In this study, we also investigated the expression of neurotrophic receptors in the adherent HUCB subfraction, including the high-affinity receptors (trkA, trkB, and trkC) and low-affinity receptor p75NTR. The presence of neurotrophin receptors suggests that these cells could be induced into becoming neural cells. We noted that most of the HUCB cells in the adherent cultures expressed trkB and trkC. The trkA and p75NTR receptors, however, were rare in the adherent fraction (Fig. 3D). From 5 DIV, trkB was detected and reached a peak at 15 DIV (90.44% ± 2.86%; Fig. 2A [M]; Fig. 3D), whereas trkC was expressed on round cells at all time points (Fig. 2A [N]; Fig. 3D). In this experiment, we also examined the expression of CXCR4, the stromal derived factor-1 (SDF-1) receptor; SDF-1 is a chemokine important in migration of hematopoietic cells as well as in the development of the central nervous system (CNS). Half of the cultured HUCB cells expressed this antigen, although the morphology of these CXCR4+ cells was varied (Fig. 2A [O]).
Replated Floating Mononuclear HUCB Fraction
Morphology and Phenotype
We collected the floating population from 20- to 34-DIV HUCB cultures. Viability of these cells was high (90%–95%). After replating, these cells attached and practically all cells began to differentiate within 24–48 hours (Fig. 1D). The major difference in morphology between the replated floating fraction and the adherent fraction was that there were no large, egg-shaped cells in the replated floating fraction. In addition, there were numerous cells with long, thin processes in this fraction.
When we compared the incidence of hematopoietic antigens presented in both the adherent and floating-replate fractions, we found substantially fewer CD45-labeled cells and a large number of immature cells expressing CD133 and CD117 surface markers (Table 3) in the replated floating fraction; further, there was a significant increase in the number of cells expressing A2B5 (p < .001) and both trkB and trkC (p < .05) and a significant decrease in all mature glial markers (p < .05 to .001). This finding suggests that there may be more stem/progenitor cells present in the replated floating fraction that could be induced into a neuronal lineage. Even so, when we separated the CD133+ cell population from the whole mononuclear fraction, these cells failed to proliferate under the same culture conditions used with both the adherent and floating fractions. Although they maintained their cellular morphology throughout the study period, all of the cells died within 3 weeks of culturing.
Table 3.
Incidence of hematopoietic and neural antigens in both populations of cultured HUCB cells
| Adherent | Floating | |||||
|---|---|---|---|---|---|---|
| Antigen | Positive | Total | Percentage | Positive | Total | Percentage |
| CD133 | 0 | 525 | 0 | 14 | 428 | 3.31 ± 2.27a |
| CD117 | 0 | 340 | 0 | 21 | 523 | 4.10 ± 0.53a |
| CD45 | 501 | 551 | 90.85 ± 3.13 | 287 | 467 | 61.43 ± 4.87a |
| Nestin | 119 | 626 | 18.97 ± 3.3 | 146 | 603 | 24.29 ± 1.06 |
| A2B5 | 67 | 773 | 8.68 ± 0.91 | 218 | 671 | 32.43 ± 0.88a |
| TuJ1 | 6 | 369 | 1.76 ± 1.97 | 12 | 520 | 2.40 ± 1.38 |
| MAP2 | 11 | 441 | 2.41 ± 1.17 | 0 | 580 | 0 |
| GFAP | 315 | 531 | 59.35 ± 10.76 | 148 | 432 | 34.38 ± 3.47b |
| S100 | 315 | 357 | 88.36 ± 2.53 | 326 | 497 | 65.63 ± 7.17a |
| GalC | 219 | 297 | 73.77 ± 3.75 | 229 | 508 | 45.02 ± 8.61b |
| trkB | 98 | 410 | 23.81 ± 8.86 | 131 | 355 | 36.79 ± 4.90b |
| trkC | 234 | 647 | 36.16 ± 6.79 | 342 | 505 | 67.77 ± 6.33b |
All data presented as means; percentage presented as mean ± SEM. The comparison is made between the adherent and replated floating HUCB fraction on day 10. Significant differences between the two groups were assessed by Student’s t-test
p < .001
p < .05.
Abbreviation: HUCB, human umbilical cord blood.
We also used adult rat brain (5 months old) as a positive control for CNS antigen expression, degree of immunofluorescent labeling, and true neural morphologies (Fig. 2D; Table 4).
Table 4.
Expression of various antigens by both (adherent and floating-replated) HUCBs and primary cultures (E14-18 embryonic rat brain)
| Presence and staining intensity | |||
|---|---|---|---|
| Antigen | Adherent HUCB culture 10 DIV |
Floating-replated HUCB culture 10 DIV |
Primary neural culture 10 DIV |
| Nestin | +/4 | +/4 | +/4 |
| Vimentin | +/4 | +/4 | +/4 |
| A2B5 | +/1 | +/2 | +/3 |
| TuJ1 | +/3 | +/3 | +/4 |
| MAP2 | +/4 | −/0 | +/4 |
| NSE | −/0 | −/0 | +/4 |
| GFAP | +/3 | +/4 | +/4 |
| S100 | +/4 | +/4 | +/4 |
| GalC | +/3 | +/3 | +/4 |
| trk A | −/0 | −/0 | +/4 |
| trk B | +/3 | +/3 | +/4 |
| trk C | +/4 | +/4 | +/4 |
| p75NTR | +/1 | +/1 | +/4 |
The table illustrates the expression of numerous neural-related antigens between both (adherent and floating) HUCB cells and primary culture. All antigens used in this study were presented in primary culture. Staining intensity and extent assessed using a 0–4 ordinal scale, in which 0 = absent, 1 = minimal, 2 = moderate, 3 = high, and 4 = maximal.
Abbreviation: DIV, days in vitro; HUCB, human umbilical cord blood.
Western Blot
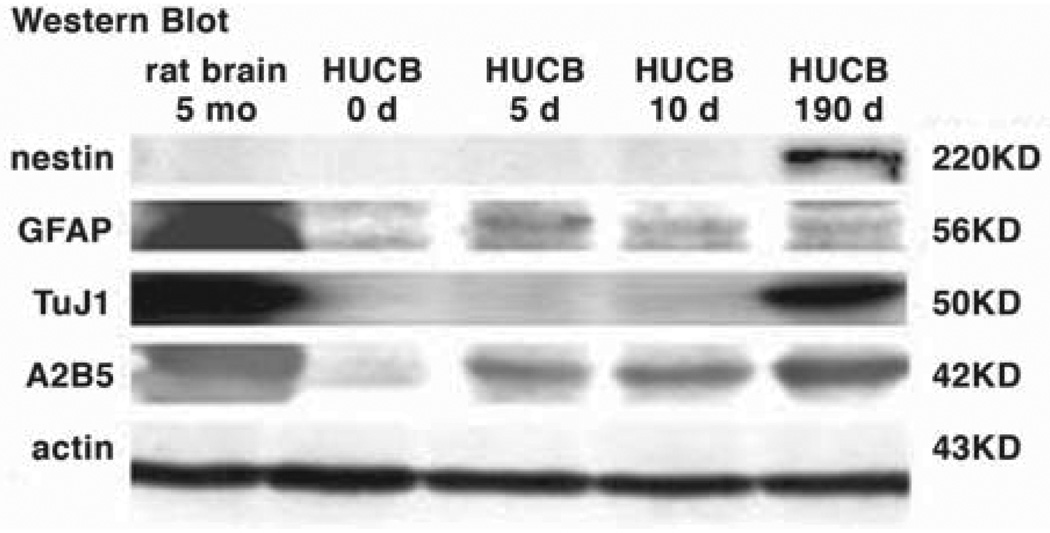
For Western blots, the following cell preparations were examined: HUCB mononuclear cells on thaw (HUCB day 0), the adherent HUCB cells cultured for 5 or 10 DIV; primary cultures from 5-month-old rat brain (positive control); and floating-replate HUCB cells (cultured for 190 DIV and having extensive TuJ1 expression). Western blot analysis confirmed immunocytochemical detection of the neural markers nestin, TuJ1, A2B5, and GFAP. Adherent HUCB cells showed a weak intensity band for nestin at 5 and 10 DIV (220 KD), whereas the floating-replate HUCB cells were strongly positive for nestin; we did not find nestin in the 5-month-old rat brain preparation, most likely because nestin is commonly present only during early development of brain. TuJ1 (50 KD) was weakly expressed in HUCB cells at thaw and at 5 and 10 DIV, whereas the floating-replate subpopulation and the rat brain had strong TuJ1 immunolabeling. Weak bands were seen for A2B5 (42 KD) and GFAP (56 KD) in all samples. The β-actin loading control demonstrated that similar amounts of protein were added in each lane (Fig. 4).
Figure 4.
Western blot analysis. Protein extracts from 5-month-old rat brain culture (lane 1) and from day 0 (lane 2), day 5 (lane 3), day 10 (lane 4), and day 190 (lane 5) of HUCB culture. Actin was used as a loading control. Abbreviation: HUCB, human umbilical cord blood.
DISCUSSION
In this study, we characterized the HUCB mononuclear fraction before and after culturing with serum-containing media without addition of induction factors such as neurotrophins, growth factors, or cytokines. Specifically, we examined the expression of embryonic (stem), hematopoietic, and neural antigens over time in culture. We categorized the raw HUCB mononuclear fraction into two different subpopulations—adherent and floating-replate. We found that there were a significant number of stem/progenitor cell antigens expressed on cells in the floating population as well as neural antigens.
Basic Characteristics of the HUCB Cells In Vitro
The freshly thawed HUCB mononuclear fraction was comprised of 53% mature lymphoid cells. Only 1.95% and 3.1% of HUCB mononuclear cells were myeloid cells or monocytes, respectively, and most of these were more immature than those reported for bone marrow [29]. These immature cells in HUCB may actively proliferate and have multipotential differentiation capabilities when cultured in the absence of extrinsic promoting factors [30]. Mayani et al. showed that survival and proliferation of these cultured cells depended on high seeding density (1 × 107 to 1 × 108/cm2). This may be because HUCB cells cultured at higher seeding densities contain a larger number of stromal precursors, which are able to develop and form a feeder layer (our putative adherent subpopulation); this layer in turn generates sufficient intrinsic promoting factors such as stem cell factor and interleukin 6 [31] to support long-term survival of the nonadherent HUCB population (our floating-replate subpopulation).
The cultured HUCB mononuclear fraction was heterogeneous, with six common morphological cell types (Fig. 1C). The two most common in our observations were the large egg-shaped cells and the small round cells. The large egg-shaped cells that attached tightly to culture vessels and preferred serum-containing medium were part of the adherent subfraction. This feeder layer has been described by Ye et al. [31], who demonstrated that the central components of this layer were fibroblasts, macrophages, endothelial cells, and several extracellular matrix proteins. The small round cells sit on the top of large egg-shaped cells or were floating in the medium and hence were part of the floating-replate population; these cells were highly proliferative and multipotent. The morphology of these cells changed over time in culture.
Expression of Hematopoietic and Neural Antigens
Fully 90% of the adherent HUCB cells expressed the common leukocyte antigen CD45. In addition, there were few cells in this fraction that transiently expressed stem/precursor antigens (CD133 and CD117). More cells that expressed early stem cell antigens were found in the floating-replated population of HUCB cells and not the adherent cell subpopulation. In our culture, these CD133+ cells seem to be quiescent because we did not see any changes in morphology or proliferation in purified cultures of CD133+ cells maintained under the same conditions as we used for the floating or adherent cultures. This is consistent with the hypothesis that adult stem cells exist in a quiescent state in the blood until an injury or disease state triggers the stem cells to proliferate or differentiate through the production of specific factors [32].
Another stem cell antigen, CD15+, or SSEA-1, was found in the HUCB cell cultures. This antigen is expressed on embryonic stem cells, primitive hematopoietic stem cells, and also within the developing human brain [33, 34]. The expression of CD15 antigen in both hematopoietic and neural lineages suggests that there may be a set of overlapping genes controlling genesis in both lineages.
The expression of neurotrophin receptors in HUCB cells certainly suggests that the HUCB cells could be driven to a neural fate. By binding to their appropriate receptors, neurotrophins play important roles in differentiation and survival of neural cells during CNS development. The activity of trk receptors themselves also affects cell survival by adenosine [35]. Although the large number of trkB+ and trkC+ cells observed in cultured HUCB cells indicated that cells are responsive to brain-derived neurotrophic factor, neurotrophin 3, and neurotrophin 4/5 and may be instrumental in transdifferentiation of the HUCB cells into neural cells, there is evidence that the neurotrophins play a functional role in the peripheral immune response. Neurotrophins are expressed by platelets [36] and B cells [37] and mediate activation and survival of eosinophils in allergic bronchial asthma [38]. They also enhance cytokine mRNA expression [39] in blood cells.
The CXCR4 chemokine receptor for SDF-1 is associated with normal development of the nervous system and is widely distributed throughout the body. SDF-1 induces leukocyte and hematopoietic progenitor migration through interactions with CXCR4; SDF-1 and a gradient of chemokine ligands also direct migration of the primordial germ cells [40, 41]. The deletion of CXCR4/SDF-1 genes in mice resulted in fetal mortality with abnormal cerebellar development [42]. Although this chemokine is expressed in response to injury or disease, which may be useful for the development of the HUCB cells as a therapeutic agent, SDF-1’s role in the neural development is not yet completely understood.
The presence of stem cell antigens within the HUCB population that we have examined suggests that there is a stem/progenitor population that may give rise to both hematopoietic cells and cells normally derived from other germ cell layers (e.g., neural cells) under the appropriate conditions. Certainly, when we examined the expression of neural proteins in HUCB cultures, many of the cells were positive. All of the neural antigens chosen (nestin, vimentin, A2B5, TuJ1, MAP2, GFAP, S100, NF68KD [NFL], and neurotrophin receptors) were observed within the HUCB subpopulations. We observed more glial antigens expressed in the adherent population and more neuronal antigens in the floating population. Our results are consistent with our earlier observations [13] and the observations of other laboratories [10, 11] and could support an argument for transdifferentiation. However, without a functional assay, alternative explanations are more likely. We observed coexpression of both hematopoietic and neural antigens in some cells. In vivo, the coexpression of two different lineage markers could be indicative of cell fusion with the host cells, but this could not apply to our culture dish, in which only HUCB cells were plated. An alternative explanation is that mononuclear cells do express some of these antigens normally. GFAP is found in some cells within the bone marrow compartment of the hematopoietic system [43]. A third possible explanation was elaborated by Lu et al. [44], who demonstrated that transdifferentiation of mesenchymal stem cells from bone marrow into neural cells is more likely a function of cellular toxicity with resulting changes to the cytoskeleton and not a change in the cellular differentiation pathways. Clearly the best demonstration that the cells have attained a neural phenotype will be through functional assays showing not only a neural morphology and protein expression but also synapse formation, neural electrophysiological activity, and neurotransmitter release. Even if we can demonstrate the true conversion of the HUCB cells into neural phenotypes, in vitro expansion of the cells may be problematic, as this may silence the cells’ capacity to differentiate and engraft [45]. Further studies are warranted to better understand how to best use these cells in the development of cell therapies to restore brain function.
ACKNOWLEDGMENTS
Tanja Zigova died in February 2004, during the preparation of this manuscript. This paper is dedicated to the memory of Dr. Zigova: an erudite scholar, a visionary scientist, and a gentlewoman. She was a beloved mentor, an inspiration, and a friend. We are grateful to Marci McCall for editorial assistance. This work was supported by NIH/NIA grant R01 AG20927-01 (to T.Z.).
Footnotes
DISCLOSURES
P.R.S. is cofounder and owner of Saneron CCEL Therapeutics, Inc. A.E.W., S.G.D., T.Z., and J.S.R. are consultants to Saneron CCEL Therapeutics, Inc. P.R.S., S.G.D., J.S.R., and A.E.W. are coinventors on HUCB-related patents.
REFERENCES
- 1.de Medeiros CR, Silva LM, Pasquini R. Unrelated cord blood transplantation in a Fanconi anemia patient using fludarabine-based conditioning. Bone Marrow Transplant. 2001;28:110–112. doi: 10.1038/sj.bmt.1703090. [DOI] [PubMed] [Google Scholar]
- 2.Ooi J, Iseki T, Nagayama H, et al. Unrelated cord blood transplantation for adult patients with myelodysplastic syndrome-related secondary acute myeloid leukaemia. Br J Haematol. 2001;114:834–836. doi: 10.1046/j.1365-2141.2001.03049.x. [DOI] [PubMed] [Google Scholar]
- 3.Tezuka K, Nakayama H, Honda K, et al. Treatment of a child with myeloid/NK cell precursor acute leukemia with L-asparaginase and unrelated cord blood transplantation. Int J Hematol. 2002;75:201–206. doi: 10.1007/BF02982029. [DOI] [PubMed] [Google Scholar]
- 4.Locatelli F, Rocha V, Reed W, et al. Related umbilical cord blood transplantation in patients with thalassemia and sickle cell disease. Blood. 2003;101:2137–2143. doi: 10.1182/blood-2002-07-2090. [DOI] [PubMed] [Google Scholar]
- 5.Krisiukeniene A, Sitkauskiene B, Sakalauskas R. Wiskott-Aldrich syndrome: the possibilities of diagnosis and treatment [in Lithuanian] Medicina (Kaunas) 2003;39:211–216. [PubMed] [Google Scholar]
- 6.Rosada C, Justesen J, Melsvik D, et al. The human umbilical cord blood: a potential source for osteoblast progenitor cells. Calcif Tissue Int. 2003;72:135–142. doi: 10.1007/s00223-002-2002-9. [DOI] [PubMed] [Google Scholar]
- 7.Goodwin HS, Bicknese AR, Chien SN, et al. Multilineage differentiation activity by cells isolated from umbilical cord blood: expression of bone, fat, and neural markers. Biol Blood Marrow Transplant. 2001;7:581–588. doi: 10.1053/bbmt.2001.v7.pm11760145. [DOI] [PubMed] [Google Scholar]
- 8.Simper D, Stalboerger PG, Panetta CJ, et al. Smooth muscle progenitor cells in human blood. Circulation. 2002;106:1199–1204. doi: 10.1161/01.cir.0000031525.61826.a8. [DOI] [PubMed] [Google Scholar]
- 9.Pesce M, Orlandi A, Iachininoto MG, et al. Myoendothelial differentiation of human umbilical cord blood-derived stem cells in ischemic limb tissues. Circ Res. 2003;93:51–62. doi: 10.1161/01.RES.0000090624.04507.45. [DOI] [PubMed] [Google Scholar]
- 10.Sanchez-Ramos JR. Neural cells derived from adult bone marrow and umbilical cord blood. J Neurosci Res. 2002;69:880–893. doi: 10.1002/jnr.10337. [DOI] [PubMed] [Google Scholar]
- 11.Bicknese AR, Goodwin HS, Quinn CO, et al. Human umbilical cord blood cells can be induced to express markers for neurons and glia. Cell Transplant. 2002;11:261–264. [PubMed] [Google Scholar]
- 12.Zigova T, Song S, Willing AE, et al. Human umbilical cord blood cells express neural antigens after transplantation into the developing rat brain. Cell Transplant. 2002;11:265–274. [PubMed] [Google Scholar]
- 13.Walczak P, Chen N, Hudson JE, et al. Do hematopoietic cells exposed to a neurogenic environment mimic properties of endogenous neural precursors? J Neurosci Res. 2004;76:244–254. doi: 10.1002/jnr.20042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen J, Sanberg PR, Li Y, et al. Intravenous administration of human umbilical cord blood reduces behavioral deficits after stroke in rats. Stroke. 2001;32:2682–2688. doi: 10.1161/hs1101.098367. [DOI] [PubMed] [Google Scholar]
- 15.Willing AE, Lixian J, Milliken M, et al. Intravenous versus intrastriatal cord blood administration in a rodent model of stroke. J Neurosci Res. 2003;73:296–307. doi: 10.1002/jnr.10659. [DOI] [PubMed] [Google Scholar]
- 16.Willing AE, Vendrame M, Mallery J, et al. Mobilized peripheral blood cells administered intravenously produce functional recovery in stroke. Cell Transplant. 2003;12:449–454. doi: 10.3727/000000003108746885. [DOI] [PubMed] [Google Scholar]
- 17.Chen R, Ende N. The potential for the use of mononuclear cells from human umbilical cord blood in the treatment of amyotrophic lateral sclerosis in SOD1 mice. J Med. 2000;31:21–30. [PubMed] [Google Scholar]
- 18.Garbuzova-Davis S, Willing AE, Zigova T, et al. Intravenous administration of human umbilical cord blood cells in a mouse model of amyotrophic lateral sclerosis: distribution, migration, and differentiation. J Hematother Stem Cell Res. 2003;12:255–270. doi: 10.1089/152581603322022990. [DOI] [PubMed] [Google Scholar]
- 19.Lu D, Sanberg PR, Mahmood A, et al. Intravenous administration of human umbilical cord blood reduces neurological deficit in the rat after traumatic brain injury. Cell Transplant. 2002;11:275–281. [PubMed] [Google Scholar]
- 20.Cairns K, Finklestein SP. Growth factors and stem cells as treatments for stroke recovery. Phys Med Rehabil Clin N Am. 2003;14:S135–S142. doi: 10.1016/s1047-9651(02)00059-1. [DOI] [PubMed] [Google Scholar]
- 21.Saporta S, Kim JJ, Willing AE, et al. Human umbilical cord blood stem cells infusion in spinal cord injury: engraftment and beneficial influence on behavior. J Hematother Stem Cell Res. 2003;12:271–278. doi: 10.1089/152581603322023007. [DOI] [PubMed] [Google Scholar]
- 22.Zhao ZM, Li HJ, Liu HY. Intraspinal transplantation of CD34+ human umbilical cord blood cells after spinal cord hemisection injury improves functional recovery in adult rats. Cell Transplant. 2004;13:113–122. doi: 10.3727/000000004773301780. [DOI] [PubMed] [Google Scholar]
- 23.Sanberg RR, Willing AE, Austin LA, et al. Cerebral intraventricular transplantation of human umbilical cord blood cells as a potential treatment of Sanfilippo syndrome type B. Exp Neurol. 2003;181:104. [Google Scholar]
- 24.Galvin-Parton PA. Screening for GALC to make neonatal diagnosis and initial neonatal stem cell treatment with umbilical cord blood. Pediatr Transplant. 2003;7:83–85. doi: 10.1034/j.1399-3046.2003.00032.x. [DOI] [PubMed] [Google Scholar]
- 25.Terskikh AV, Easterday MC, Li L, et al. From hematopoiesis to neuropoiesis: evidence of overlapping genetic programs. Proc Natl Acad Sci U S A. 2001;98:7934–7939. doi: 10.1073/pnas.131200898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carr JH, Rodak BF. Clinical Hematology Atlas. Philadelphia: W.B. Saunders; 1999. [Google Scholar]
- 27.Zigova T, Graziadei PP, Monti Graziadei AG. Olfactory bulb transplantation into the olfactory bulb of neonatal rats. Brain Res. 1990;513:315–319. doi: 10.1016/0006-8993(90)90473-o. [DOI] [PubMed] [Google Scholar]
- 28.Zigova T, Betarbet R, Soteres BJ, et al. A comparison of the patterns of migration and the destinations of homotopically transplanted neonatal subventricular zone cells and heterotopically transplanted telencephalic ventricular zone cells. Dev Biol. 1996;173:459–474. doi: 10.1006/dbio.1996.0040. [DOI] [PubMed] [Google Scholar]
- 29.Mikami T, Eguchi M, Kurosawa H, et al. Ultrastructural and cytochemical characterization of human cord blood cells. Med Electron Microsc. 2002;35:96–101. doi: 10.1007/s007950200012. [DOI] [PubMed] [Google Scholar]
- 30.Mayani H, Lansdorp PM. Biology of human umbilical cord blood-derived hematopoietic stem/progenitor cells. Stem Cells. 1998;16:153–165. doi: 10.1002/stem.160153. [DOI] [PubMed] [Google Scholar]
- 31.Ye ZQ, Burkholder JK, Qiu P, et al. Establishment of an adherent cell feeder layer from human umbilical cord blood for support of long-term hematopoietic progenitor cell growth. Proc Natl Acad Sci U S A. 1994;91:12140–12144. doi: 10.1073/pnas.91.25.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fortunel N, Batard P, Hatzfeld A, et al. High proliferative potential-quiescent cells: a working model to study primitive quiescent hematopoietic cells. J Cell Sci. 1998;111:1867–1875. doi: 10.1242/jcs.111.13.1867. [DOI] [PubMed] [Google Scholar]
- 33.Mai JK, Krajewski S, Reifenberger G, et al. Spatiotemporal expression gradients of the carbohydrate antigen (CD15) (Lewis X) during development of the human basal ganglia. Neuroscience. 1999;88:847–858. doi: 10.1016/s0306-4522(98)00266-8. [DOI] [PubMed] [Google Scholar]
- 34.Mai JK, Winking R, Ashwell KW. Transient CD15 expression reflects stages of differentiation and maturation in the human subcortical central auditory pathway. J Comp Neurol. 1999;404:197–211. [PubMed] [Google Scholar]
- 35.Lee FS, Rajagopal R, Chao MV. Distinctive features of Trk neurotrophin receptor transactivation by G protein-coupled receptors. Cytokine Growth Factor Rev. 2002;13:11–17. doi: 10.1016/s1359-6101(01)00024-7. [DOI] [PubMed] [Google Scholar]
- 36.Lommatzsch M, Zingler D, Schuhbaeck K, et al. The impact of age, weight and gender on BDNF levels in human platelets and plasma. Neurobiol Aging. 2005;26:115–123. doi: 10.1016/j.neurobiolaging.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 37.Edling AE, Nanavati T, Johnson JM, et al. Human and murine lymphocyte neurotrophin expression is confined to B cells. J Neurosci Res. 2004;77:709–717. doi: 10.1002/jnr.20176. [DOI] [PubMed] [Google Scholar]
- 38.Nassenstein C, Braun A, Erpenbeck VJ, et al. The neurotrophins nerve growth factor, brain-derived neurotrophic factor, neurotrophin-3, and neurotrophin-4 are survival and activation factors for eosinophils in patients with allergic bronchial asthma. J Exp Med. 2003;198:455–467. doi: 10.1084/jem.20010897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bayas A, Kruse N, Moriabadi NF, et al. Modulation of cytokine mRNA expression by brain-derived neurotrophic factor and nerve growth factor in human immune cells. Neurosci Lett. 2003;335:155–158. doi: 10.1016/s0304-3940(02)01152-7. [DOI] [PubMed] [Google Scholar]
- 40.Imitola J, Raddassi K, Park KI, et al. Directed migration of neural stem cells to sites of CNS injury by the stromal cell-derived factor 1alpha/CXC chemokine receptor 4 pathway. Proc Natl Acad Sci U S A. 2004;101:18117–18122. doi: 10.1073/pnas.0408258102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Peng H, Huang Y, Rose J, et al. Stromal cell-derived factor 1-mediated CXCR4 signaling in rat and human cortical neural progenitor cells. J Neurosci Res. 2004;76:35–50. doi: 10.1002/jnr.20045. [DOI] [PubMed] [Google Scholar]
- 42.Banisadr G, Fontanges P, Haour F, et al. Neuroanatomical distribution of CXCR4 in adult rat brain and its localization in cholinergic and dopaminergic neurons. Eur J Neurosci. 2002;16:1661–1671. doi: 10.1046/j.1460-9568.2002.02237.x. [DOI] [PubMed] [Google Scholar]
- 43.Wislet-Gendebien S, Leprince P, Moonen G, et al. Regulation of neural markers nestin and GFAP expression by cultivated bone marrow stromal cells. J Cell Sci. 2003;116:3295–3302. doi: 10.1242/jcs.00639. [DOI] [PubMed] [Google Scholar]
- 44.Lu P, Blesch A, Tuszynski MH. Induction of bone marrow stromal cells to neurons: differentiation, transdifferentiation, or artifact? J Neurosci Res. 2004;77:174–191. doi: 10.1002/jnr.20148. [DOI] [PubMed] [Google Scholar]
- 45.Glimm H, Oh IH, Eaves CJ. Human hematopoietic stem cells stimulated to proliferate in vitro lose engraftment potential during their S/G(2)/M transit and do not reenter G(0) Blood. 2002;96:4185–4193. [PubMed] [Google Scholar]