Abstract
The mononuclear fraction of human umbilical cord blood (HUCBmnf) is a mixed cell population that multiple research groups have shown contains cells that can express neural proteins. In these studies, we have examined the ability of the HUCBmnf to express neural antigens after in vitro exposure to defined media supplemented with a cocktail of growth and neurotrophic factors. It is our hypothesis that by treating the HUCBmnf with these developmentally-relevant factors, we can expand the population, enhance the expression of neural antigens and increase cell survival upon transplantation. Prior to growth factor treatment in culture, expression of stem cell antigens is greater in the non-adherent HUCBmnf cells compared to the adherent cells (p < 0.05). Furthermore, treatment of the non-adherent cells with growth factors, increases BrdU incorporation, especially after 14 days in vitro (DIV). In HUCBmnf-embryonic mouse striata co-culture, a small number of growth factor treated HUCBmnf cells were able to integrate into the growing neural network and express immature (nestin and TuJ1) and mature (GFAP and MAP2) neural markers. Treated HUCBmnf cells implanted in the subventricular zone predominantly expressed GFAP although some grafted HUCBmnf cells were MAP2 positive. While short-term treatment of HUCBmnf cells with growth and neurotrophic factors enhanced proliferative capacity in vitro and survival of the cells in vivo, the treatment regimen employed was not enough to ensure long-term survival of HUCBmnf-derived neurons necessary for cell replacement therapies for neurodegenerative diseases.
Introduction
HUCBmnf cells are rich in stem/progenitor cells; like bone marrow, HUCBmnf cells are capable of self-renewal [1], proliferation, subsequent lineage commitment for multiple differentiated cell types [2] and can be used to reconstitute the blood and immune systems [3]. Some of these pluripotent mesodermal cells have recently been shown to differentiate into cells derived from other germ layers both in vitro [4] and in vivo [5]. Therefore, it is not surprising that HUCBmnf cells express numerous markers of either stemness or neural fate in vitro such as nestin, Musashi1, Oct-4, TuJ1, NCAM, A2B5, vimentin, GFAP, S100, GalC and MAP2 [6, 7]. Further, these cells express neurotrophic receptors trkB, trkC and p75NTR and cytokine receptor CXCR4 [7, 8]. Upon transplantation of HUCBmnf cells into the subventricular zone (SVZ), a neurogenic area in the adult brain, they continued to express the neural markers nestin and TuJ1 [9]. Since the genetic program for hematopoiesis and neuropoiesis overlap [10], HUCBmnf cells may have the potential to transdifferentiate into neural cells, especially with exposure to inducing factors.
Previous studies by our group have shown that there are at least two fractions in HUCBmnf cells—the adherent and non-adherent fractions; this latter fraction can be further subdivided into cells that freely float within the medium and cells that lightly sit on the top of the adherent fraction, but do not firmly adhere to it [7]. The adherent fraction is considered to function as a feeder layer to support the survival of the floating fraction; this feeder layer is analogous to the mesenchymal cells obtained from bone marrow that give rise to mesenchymal stem cells. It is, however, the non-adherent fraction that contains most of the proliferating cells and that we therefore believe contains most of the stem/progenitor cells. HUCBmnf stem cells are similar to bone marrow-derived adult stem cells within the blood. Unlike embryonic stem cells with their high proliferative potential and resistance to rejection, which therefore may contribute to fibrosis and malignancies, these adult stem/progenitor cells are relatively quiescent under normal circumstances [11]. The intrinsic replication potential of these quiescent stem cells needs to be stimulated by factors within the environment [12], without which the cells do not proliferate unrestrained.
In this study, we hypothesized that treatment of the HUCBmnf cells with growth, and neurotrophic factors could not only increase the number of cells through increased proliferation, but it could also enhance the ability of these cells to survive and differentiate into neural cells either in vitro or in vivo. Therefore, we cultured the non-adherent fraction of HUCBmnf under various culture conditions supplemented with growth and neurotrophic factors. We also transplanted these stimulated cells into the subventricular zone (SVZ) of 9 month old rats to explore whether the HUCBmnf progenitor cells are able to read specific cues within the environment to produce appropriate cell types.
Materials and methods
We performed a series of experiments to characterize the effects of growth and neurotrophic factor treatment on the HUCBmnf.
Experiment 1: cell proliferation of HUCBmnf after growth factor treatment
Preparation of HUCBmnf cultures
Cryopreserved HUCBmnf (supplied by Saneron CCEL Therapeutics, Inc.) was thawed in a 37 °C water bath as previously described [8]. Cells were initially suspended in a Dulbecco's modified Eagle's medium (DMEM, Gibco) with 10% fetal bovine serum (FBS, Gibco) and 0.1% Gentamicin (Sigma) and plated on either four-well plates (Nunc) or 100 mm dishes (Nunc) at a density of 100 000 cells cm-2. The media were supplemented as described below. The cells were incubated in 5% CO2 at 37 °C, and a fresh medium was added every 4-5 days. Cell viability on plating was determined by trypan-blue exclusion. Three independent replicates were performed.
Proliferative potential
To explore the proliferative potential, HUCBmnf from one donor was cultured with a growth medium (GM, table 1) containing DMEM with 10% FBS and 0.1% Gentamicin, and supplements of growth factors shown to increase proliferation of neural stem cells (human epidermal growth factor (hEGF, 10 ng ml-1; Sigma), human basic fibroblast growth factor (hbFGF, 10 ng ml-1; Sigma)), human leukemia inhibitory factor (hLIF, 10 ng ml-1; Chemicon) and human stem cell factor (hSCF, 10 ng ml-1; Sigma) every 4 days. Under the control condition, growth factors were excluded from the media. Bromodeoxyuridine (BrdU, 10 μM; Sigma) was added to the cultures 2 h before fixation. These cultures were then fixed with 4% paraformaldyhyde (PFA) diluted in a 0.1 M phosphate buffer (PB; pH 7.4) for 10 min at day 7, 14 and 21. The definition of factors used is listed in table 2.
Table 1.
Media composition and experimental protocol.
| Media | Abbreviation | Composition |
|---|---|---|
| Growth media | GM | DMEM, FBS (10%), Gentamacin (0.1%) hbFGF (10 ng ml-1), hEGF (10 ng ml-1), hLIF (10 ng ml-1), hSCF (10 ng ml-1) |
| Differentiation media | DM1 | DMEM/F12, FBS (10%), N2 (0.1%), ITS (1%) all-trans RA (1 μM), hβ-NGF (100 ng ml-1), hGDNF (10 ng ml-1) |
| Differentiation media | DM2 | DMEM/F12, FBS (10%), N2 (0.1%), ITS (1%), hbFGF (10 ng ml-1), hEGF (10 ng ml-1), hSHH (300 ng ml-1), all-trans RA (1 βM), dbcAMP (1 mM), hBDNF (10 ng ml-1), hFGF 8 (10 ng ml-1), hβ-NGF (100 ng ml-1), hGDNF (10 ng ml-1), |
| Procedure | ||
| Experiment 1 | Cell proliferation | |
| Protocol | Culture cells in GM for 7, 14, 21 days | |
| Experiment 2 | Cell differentiation | |
| Protocol 1 | Culture cells in GM → for 1, 14, 30 or 40 days | Change media to DMEM/F12, FBS (10%), N2 (0.1%), ITS (1%), all-trans RA (1 μM), hβ-NGF (100 ng ml-1), hGDNF (10 ng ml-1) for 14 days |
| Protocol 2 | Culture cells in GM → for 40 days | Change media to DMEM/F12, FBS (10%), N2 (0.1%), ITS (1%), hbFGF (10 ng ml-1), hEGF (10 ng ml-1), hSHH (300 ng ml-1) ↓ 3 days |
| Change media to DMEM/F12, FBS (10%), N2 (0.1%), ITS (1%), all-trans RA (1 μM), dbcAMP (1 mM), hSHH (300 ng ml-1) ↓ 3 days |
||
| Addition of hBDNF (10 ng ml-1), hNGF 8 (10 ng ml-1), ↓ 2 days |
||
| Addition of hβ-NGF (100 ng ml-1), hGDNF (10 ng ml-1) ↓ 6 days |
||
| Fixation |
Table 2.
Factors used in the media.
| Factors | Definition | Effect | Reference |
|---|---|---|---|
| hEGF | Human epidermal growth factor | Regulation of cell growth, proliferation and differentiation | [15] |
| hbFGF | Human basic fibroblast growth factor | Potent inducer of DNA synthesis in mesoderm and neuroectoderm lineages | [19] |
| hLIF | Human leukemia inhibitory factor | Inhibits differentiation of cells, maintaining multipotency of stem cells longer and selectively supports the survival of a population(s) of neural stem cells | [20] |
| hSCF | Human stem cell factor | Hematopoietic growth factor active early in hematopoiesis; also growth factor for multiple cell types | [21] |
| all-trans RA | All-trans retinoic acid | Induces cell differentiation and inhibits cell proliferation | [17] |
| hSHH | Human sonic hedgehog | Induces proliferation of primitive human hematopoietic cells | [16] |
| hFGF8 | Human fibroblast growth factor-8 | Stimulates the proliferation and activation of cells that express FGF receptors | [22] |
| hβ-NGF | Human nerve growth factor | Enhances survival, growth, and neurotransmitter biosynthesis of sympathetic and sensory neurons | [23] |
| hBDNF | Human brain-derived neurotrophic factor | Support growth and survival of neuronal and glial cells | [24] |
| hGDNF | Human glial-derived neurotrophic factor | Stimulates growth of dopaminergic neurons and autonomic motor neurons | [25] |
| dbcAMP | N6,2′-O-dibutyryladenosine 3′:5′ cyclic monophosphate | Promotes stem cell differentiation and increases expression of specific subtypes of Na+-dependent glutamate transporters | [26] |
BrdU immunochemistry
Cells were pretreated with 2N HCl at room temperature for 2 h, washed five times in 0.1 M phosphate buffered saline (PBS), incubated with rat anti-BrdU (Accurate; 1:400) followed by secondary antibodies conjugated to Alexa Fluor 594 (Molecular Probes; 1:1000). All cultures were examined using Olympus BX-60 or IX-71 microscopes.
Quantitative analysis
To assay the response of HUCBmnf to growth factor stimulation, the number of BrdU positive (+) cells and the total number of cells were counted (ten fields per well, two wells/sample) at each time point. The percentage of positively labeled cells was subsequently calculated and expressed as mean ± standard error of the mean (SEM). Student's t-test was used to determine the differences between experimental conditions with the criterion of significance set at p <0.05.
Telomerase activity
Adherent and non-adherent fractions of HUCBmnf cells were cultured in GM for 14 and 30 days. The adherent and non-adherent cells were harvested, and the telomerase activity was assessed using the TRAPEZE® XL Telomerase Detection Kit (Chemicon), according to the manufacturer's protocol. Briefly, the pelleted cells were washed with 0.1 M PBS, resuspended in a CHAPS XL Lysis buffer (200 μl/105 cells) and incubated on ice for 30 min. In addition to the samples (SPLs), negative control (heat-treated sample; SPLC) and telomerase positive control (CTL1), minus telomerase control (CTL2) and PCR/ELISA positive control (CTL3) were performed for each assay. PCR amplification was performed as follows. The tubes (samples and controls) were placed in a thermocycler for 30 min at 30 °C. Thirty-six cycles (94 °C/30 s, 59 °C/30 s, 72 °C for 1 min) were performed, followed by incubations at 72 °C/3 min, 55 °C/24 min and 4 °C. The PCR reactions were measured using a microtiter plate reader at absorbance 450 nm (fluorescein) and 690 nm (sulforhodamine). The relative fluorescence units were calculated as absorbance at 450 nm minus absorbance at 690 nm. When assessed against controls, net increase in the absorbance for the samples should be greater than 0.15.
Experiment 2: neural induction of HUCBmnf with differentiating factors
To determine the neural potential of HUCBmnf cells, we used two different protocols that exposed the HUCBmnf cells to factors that would be present during neural development. HUCBmnf was first cultured in proliferation media, GM, as defined in experiment 1 for 1, 14, 30 or 40 days, and then the non-adherent fraction was collected from these cultures and replated with a differentiation medium (DM). At all media changes, half the media were removed from the culture and replaced with fresh media. We used neural induction media which are conventionally [13] used in primary neural cultures. Two different protocols were adopted in this study: in the first protocol, we added a combination of factors at once [14]; in the second protocol, we modified the first protocol and design to sequentially add factors on the basis of the physiological role of the individual factors during development (table 2).
First protocol (table 1)
The differentiating media (DM1) were DMEM/F12 (Gibco) with 10% FBS, 0.1% N2, 1% insulin-transferrin-selenium (ITS, Gibco) and all-trans retinoic acid (RA, 1 μM; Sigma), human nerve growth factor (hNGF, 100 ng ml-1), human glial-derived neurotrophic factor (GDNF, 10 ng ml-1; Invitrogen). After either 1, 14 or 30 days in GM, the floating fraction was collected and replated in DM1 for 14 days. These cultures were then fixed with 4% PFA diluted in 0.1 M PB for 10 min.
Second protocol (table 1)
After 40 days in the GM, the non-adherent fraction, including floating cells in the media and those cells sitting lightly on the top of the adherent layer, was lifted by 0.05% trypsin and 0.53 mM EDTA (Sigma), and replated in DM2 for another 14 days. The base of the differentiating media (DM2) was DMEM/F12 with 10% FBS, 1% N2 and 1% ITS. Factors and reagents were sequentially added starting with hEGF (10 ng ml-1), hbFGF (10 ng ml-1), human sonic hedgehog (hSHH, 300 ng ml-1; R&D systems) for the first 3 days of differentiation. These factors are important for stimulating proliferation [15, 16]. These remaining proliferation promoting factors are gradually also removed to be replaced by factors that inhibit proliferation and promote differentiation such as all-trans RA (1 μM) [17], N6,2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate (dbcAMP, 1 mM; Sigma) [18] and hSHH (300 ng ml-1) for a period of 3 days. Beginning on day 6, neuralizing factors were introduced into the media. On day 6, BDNF and human fibroblast growth factor-8 (hFGF-8, 10 ng ml-1; Chemicon) were added to the cultures while hGDNF (10 ng ml-1) and hNGF-β (100 ng ml-1) were supplied on days 8 to 10. After 14 days, the cultures were fixed with 4% PFA diluted in 0.1 M PB for 10 min. The definitions of factors used in this section are listed in table 2.
Immunocytochemistry
The cultured HUCBmnf cells were immunocytochemically labeled to detect cell-type-specific antigen expression. After washing three times in 0.1 M PBS, cells were blocked with 10% goat serum with 1% Triton X100 at room temperature (RT) for 1 h. Cells were then incubated with primary antibodies (table 3): rabbit anti-trkB (1:2000; Chemicon), mouse anti-trkA (1:2000; Chemicon), mouse anti-trkC (1:2000; Chemicon), mouse anti-p75NR (1:300; Chemicon), mouse anti-human nucleoli (1:30; MAB 1277, Chemicon), mouse anti-Oct-4 (1:100; R&D), mouse anti-human mitochondria (1:30; Chemicon), rabbit anti-neuronal class III β-tubulin (TuJ1, 1:1000; Covance), rabbit anti-microtubule-associated protein 2 (MAP2, 1:1000; Chemicon), mouse anti-synaptophysin (1:1000, Chemicon), rabbit anti-glial fibrillary acidic protein (GFAP, 1:500; Dako), anti-CNPase (1:100 Chemicon), mouse anti-human CD34 (hematopoietic stem cell antigen), CD4 (T-cell), CD44 (hematopoietic cellular adhesion molecule) and CD45 (common leukocyte antigen) (all at 1:50; BD PharMingen), mouse anti-human CD56 (neural cellular adhesion molecule) (1:50; Chemicon), mouse anti-nestin (1:100; BD Transduction), rabbit anti-Ki67 (proliferation marker) (1:1000; Chemicon). After 24 h incubation with primary antibodies, appropriate secondary antibodies (Alexa Fluor 594 (1:600) and 488 (1:1000); Molecular Probes) were used. Vectashield with DAPI (Vector Laboratories) was added to the fixed cultures to prevent fading during photomicroscopy as well as to aid in visualization of cell nuclei. All cultures were examined using Olympus BX-60 or IX-71 microscopes.
Table 3.
Antibodies for immunocytochemistry.
| Antigen | Tissue specificity | Reference |
|---|---|---|
| CD4 | Present on a subset of T lymphocytes (`helper/inducer' T cells) and is also expressed at a lower level on monocytes, tissue macrophages and granulocytes | [27] |
| CD34 | Hematopoietic progenitor cells and the most primitive pluripotent stem cells and the small vessel endothelium of a variety of tissues | [28] |
| CD44 | T Cells, B cells, monocytes, granulocytes, erythrocytes, medullary thymocytes, endothelial cells and a high proportion of epithelial cells, fibroblasts and a wide variety of tumors | [29] |
| CD45 | Human hematopoietic lineage (including hematopoietic stem cells) with the exception of mature red cells | [30] |
| CD56 | Most neuroectodermal-derived cell lines, tissues and neoplasm such as retinoblastoma, medulloblastoma, astrocytomas and neuroblastoma. Also known as neural cell adhesion molecule (NCAM) | [31] |
| Oct-4 | Expressed by undifferentiated embryonic stem cells and embryonic germ cells | [32] |
| Nestin | Stem/progenitor cells, glioma cells, and tumor endothelial cells in the mammalian CNS | [33] |
| Class III β-tubulin | Also referred to as TuJ1. Expressed in central and peripheral nervous systems (CNS and PNS) where it is prominently expressed during fetal and postnatal development. | [34] |
| MAP2 | Microtubule-associated protein 2. Essential for the development and maintenance of neuronal morphology | [35] |
| GFAP | Glial fibrillary acidic protein. Astrocytes and certain other astroglia in the central nervous system, in satellite cells in peripheral ganglia, and in nonmyelinating Schwann cells in peripheral nerves. In addition, neural stem cells frequently strongly express GFAP, consistent with the current hypothesis that neural stem cells are a sub-population of astrocytes | [36, 37] |
| Vimentin | It is detected in neuroepithelium and early progenitors in brain | [38] |
| CNPase | Myelin-associated protein expressed on oligodendrocytes and Schwann cells | [39] |
| Ki 67 | Expressed by proliferating cells in all phases of the active cell cycle (G1, S, G2 and M phase). It is absent in resting (G0) cells | [40] |
Quantitative analysis
The number of cells positively labeled for specific antigens and the total number of cells were counted and statistics performed as described in experiment 1. The total number of counted cells for each antigen ranged from 2500 to 3500.
Experiment 3: co-culture of HUCBmnf with mouse embryonic striatal cells
The goal of this study was to determine in a simple model system how HUCBmnf cells interact with neural cells. Therefore, we co-cultured non-adherent cells from HUCBmnf with embryonic mouse striatal cells.
HUCBmnf cells were grown in a NeuroCult® medium with human proliferation supplement (StemCell Technologies) with hEGF (10 ng ml-1), hFGF (20 ng ml-1) and heparin (20 ng ml-1; StemCell Tech) for 40 DIV and then harvested in preparation for co-culturing with mouse embryonic striatal cells (E14, catalogue number 00330, StemCell Technologies). The mouse cells were thawed 24 h prior to co-culturing in a 37 °C water bath and then suspended in a mouse NeuroCult® medium (StemCell Technologies) with mouse proliferation supplement (StemCell Technologies) and hEGF (20 ng ml-1) for 1 day. The mouse and human cells were then co-cultured in mouse NeuroCult® media supplemented with differentiation supplement (StemCell Technologies) and hNGF (100 ng ml-1), hFGF8 (10 ng ml-1) and RA (1 μM) for 7 days. In some instances, the HUCBmnf cells were pre-labeled by a CM-DiI cell tracer (Molecular Probes, 5 μM in culture media). Initial viability and quantification was determined by trypan-blue dye exclusion. Upon conclusion of the study, the cells were fixed with 4% PFA diluted in 0.1 M PB for 10 min. Immunocytochemistry and quantification of cells positively labeled for nestin, TuJ1, MAP2 and GFAP were performed as described in experiment 2.
Experiment 4: transplantation of HUCBmnf treated with growth factors
In previous studies, we have found that only a small percentage of untreated HUCBmnf cells transplanted into the neurogenic SVZ of normal neonate and young adult rats survive and few of these are differentiated into neural-like cells [41]. In this study, we hypothesized that pretreating these cells with growth factors would enhance survival of these cells within the SVZ of adult rats and increase the number of cells that differentiated into neurons.
Animals
Transplantation experiments were performed on 9 month old adult Fisher 344 rats (NIA, Bethesda, MD) weighing 400-510 g (n = 8). The animals had ad libitum access to food and water and were housed in pairs in a temperature-controlled room (22 °C) on a 12 h light/dark cycle. This study was conducted under the oversight of the University of South Florida's Institutional Animal Care and Use Committee and adhered to the federal guidelines set forth in the Guide for the Care and Use of Laboratory Animals.
Cell preparation
Cryopreserved HUCBmnf were cultured in DMEM supplemented with 10% FBS supplied with hEGF (10 ng ml-1), hFGF (10 ng ml-1), hLIF (10 ng ml-1) and hSCF (10 ng ml-1) for 40 days. A fresh complete medium was added every 5 days. Non-adherent cells including the floating and sitting fractions were then harvested and suspended in a Hank's balanced salt medium (Gibco) at a concentration of 50 000 cells μl-1. Cell viability ranged between 90% and 95%.
Cell transplantation
After anesthetization with isoflurane (Halocarbon Lab) at 2-5% in O2 at 2 l min-1, the animals received a unilateral stereotaxic injection of cells (100 000 cells in 2 μl). Cells were implanted unilaterally into the right SVZ (anteroposterior (AP): 1.6, mediolateral (ML): 1.5, dorsoventral (DV): -4.2, with toothbar set at -2.3 [41] at a rate of 0.5 μl min-1. The needle then was withdrawn after being left in place for 3 min. The incision was closed with surgical suture (3.0, Dexon). Cyclosporin A (10 mg kg-1, i.p.) was administered beginning on the day of surgery and continuing for the duration of the study. In addition, an antibiotic (Baytril, 0.15 ml, i.m.) and analgesic (Ketofen, 0.05 ml, i.m.) were also administered on the day of surgery. Animals were euthanatized 3, 7, 14 or 21 days after transplantation.
Tissue processing
Animals were transcardially perfused with 0.1 M PB followed by 4% PFA in PB. The brains were post-fixed for 24 h and cryopreserved in 30% sucrose for 48 h, embedded in tissue freezing compound (TBS), and cryosectioned in the sagittal plane at 30 μm thicknesses. To verify graft localization in the brain, every sixth section was Nissl stained with 0.1% cresyl violet 30 s, dehydrated and coverslipped with Permount (Fisher Scientific). For immunohistochemistry, sections containing grafts were selected and processed to identify human-specific antigen (human mitochondria or nucleoli) and double stained with neural-specific markers (TuJ1, GFAP, MAP2).
Results
Experiment 1: cell proliferation of HUCBmnf after growth factor treatments
Characteristics of the HUCBmnf cells
Several important immature hematopoietic and neural markers that play a role in hematopoiesis, stemness and neurogenesis were detected in non-adherent cultures, including CD117 (4 ± 1%), CD34 (29 ± 3%) nestin (23 ± 1%) and TuJ1 (2% ± 1%), these immature antigens were observed more often in the non-adherent fraction than in the adherent fraction. Conversely, the expression of the common leukocyte marker CD45 (figure 1(c) in panel A) declined over time in culture in the non-adherent fraction (figure 1 panel B). We also observed that a large number of cells expressed CD56 (NCAM; figure 1(b) in panel A). NCAM plays an important role in cell migration and process innervation during embryogenesis in addition to contact-mediated interactions between neural cells [42].
Figure 1.
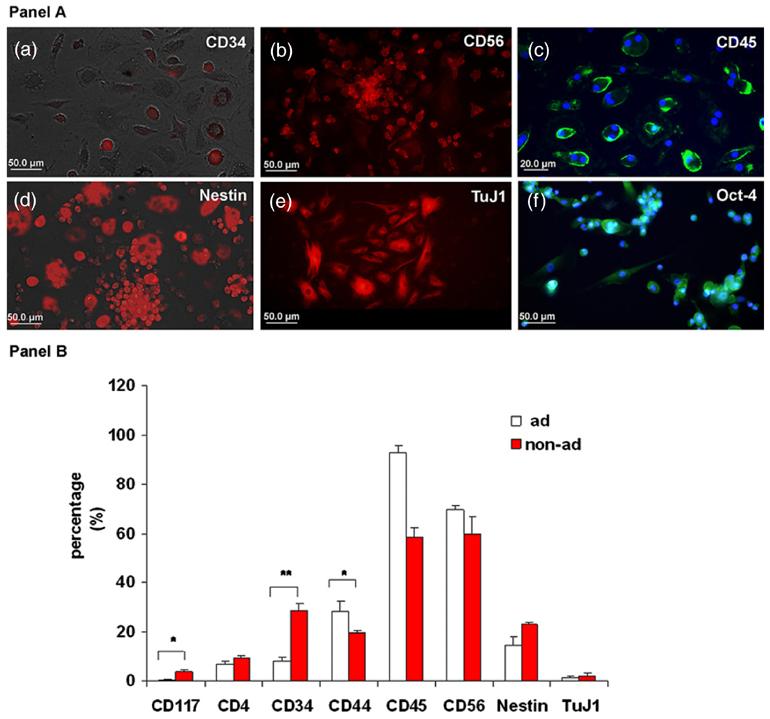
Expression of immature hematopoietic and neural antigens in the HUCBmnf non-adherent fraction. Panel A: representative photomicrographs of replated non-adherent HUCBmnf cells that express immature hematopoietic and non-hematopoietic markers after 14 DIV. (a) 29 ± 3% of CD34 positive (+) small round cells (red) were found. In addition, many CD56 (NCAM) immunoreactive cells were also found (b). (c) While 58 ± 5% of the non-adherent cells still expressed common leukocyte antigen CD45 (green), 93 3% of the adherent HUCBmnf cells were positive for CD45, suggesting that they maintained their hematopoietic heritage. A number of cells in the non-adherent HUCBmnf were negative for this antigen. (d) The immature neural marker, nestin, (red) as well as early neuronal marker TuJ1 ((e), red) were expressed. (f) The majority of the replated non-adherent HUCBmnf cells (93 ± 6%) were immunopositive for the stem cell marker Oct-4 (green). DAPI (blue) counterstaining was used to visualize all nucleated cells in the culture. Scale bar = 50 μm (a), (b), (d), (e) and (f), and 20 μm in (c). Panel B: after 14 DIV, the expression of immature hematopoietic markers (CD117 and CD34) and the neural marker, nestin, was greater in the non-adherent HUCBmnf cells compared to the adherent cells while the hematopoietic markers (CD45 and CD56) were predominantly found in adherent cells. Significant differences between the two groups were assessed by Student's t-test (*p < 0.05, **p < 0.001).
In addition to these early markers of embryonic, hematopoietic and neural stem cells, the HUCBmnf cells have a defined morphology when cultured. HUCBmnf cells were usually observed as three layers after culturing in a serum containing medium (DMEM with 10% FBS). These layers were (1) an adherent layer, which was usually formed by days 5-10 during culture, that mainly consisted of bigger flat cells that attached tightly to the culture dish and did not proliferate. This layer was previously found to mainly consist of fibroblasts, macrophages, endothelial cells and several extracellular matrix proteins [43]. (2) The cells in the layer that was loosely resting upon the adherent layer were small and round while the adherent cells were big and flat (adherent cells; figure 2(a); see arrows). Viability of these cells which formed clusters and usually appeared on days 7-40 in culture was high. Compared to the adherent cells, these cells exhibited greater proliferative capacity and potential to differentiate into multiple phenotypes. (3) The floating layer consisted of a large number of small cells that were mainly CD34+ cells and that floated in the supernatant of the HUCBmnf cultures, indicating that these were hematopoietic stem cells [7].
Figure 2.
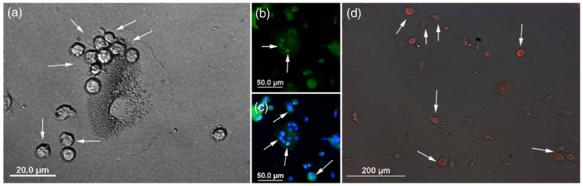
HUCBmnf cells in the non-adherent fraction express an early stem cell antigen. (a) A brightfield photomicrograph showing the small, round non-adherent HUCBmnf cells (arrows) sitting on the larger, adherent egg-like cells after 8 DIV. (b) and (c) Fluorescent photomicrographs indicated that these small, round cells expressed Oct-4 (green, arrows), while the bigger egg-like adherent cell was negative for Oct-4. DAPI (blue) was used to visualize cell nuclei. (d) The floating fraction from the identical HUCBmnf culture contained mostly Oct-4 positive, undifferentiated cells (red, arrows) shortly after replating for 1 DIV in DMEM with serum. Scale bar = 20 μm in (a); 50 μm in (b) and (c); 200 μm in (d).
Proliferative potential measured with Oct-4 and BrdU histochemistry
We used two assays to examine the proliferative potential of the HUCBmnf cells—immunohistochemical labeling for Oct-4 and BrdU incorporation into proliferating cells. In both sitting and floating subfractions of the non-adherent layer, the majority of cells (93 ± 6%) were Oct-4+ (figures 2(b), (c) (see arrows) and (d) (red), and figure 1(f) (green) in panel A). BrdU+ cells were observed in both adherent and non-adherent fractions after 14 days in culture (figure 3 panel B) under standard culture conditions. When the non-adherent cells were treated with GM (EGF, bFGF and LIF), we detected 5% BrdU+ cells on day 7, 17% BrdU+ cells on day 14, and 10% on day 21 (figure 3(c) in panel A). There was a peak five fold increase in proliferation in the growth factor treated group on day 7. These non-adherent HUCBmnf cells retained morphologic heterogeneity when cultured in DMEM without growth factors (figure 3(a) in panel A) as did GM-treated cultures (figure 3(b) in panel A). After replating, these non-adherent cells rapidly adhered and differentiated, especially the cells in the GM-treated cultures.
Figure 3.
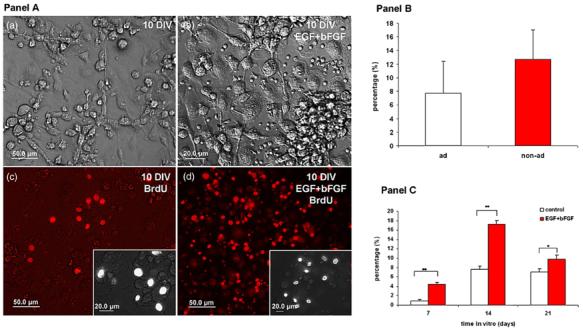
Proliferative capacity of non-adherent HUCBmnf after 10 DIV with or without growth factors. Panel A: (a) brightfield photomicrograph of HUCBmnf cultured for 10 DIV without growth factors. The culture in (b) was treated with hEGF and hbFGF. BrdU+ cells were found in both the non-treated (c) and growth factor treated (d) cultures. The insets in (c) and (d) show BrdU labeled cells at higher magnification. Scale bar = 50 μm in (a)-(d), and 20 μm in inset of (c) and (d). Panel B: the proliferative capacity of the non-adherent cells tended to be greater than in the adherent HUCBmnf cells at 14 DIV (p = 0.06). Panel C: within the nonadherent fraction, treatment of the cultures with growth factors increased BrdU incorporation into the cells. Significant differences between the two groups were assessed by Student's t-test (*p < 0.05, **p < 0.001).
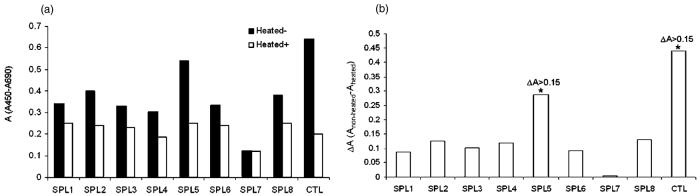
Telomerase activity
When we examined the telomerase activity in both fractions (adherent and non-adherent) of HUCBmnf before and after treatment of growth factors, we only observed a significant change in activity in SPL5, the non-adherent HUCBmnf cells, exposed to growth factors (figures 4(a) and (b); ΔA = 0.3).
Figure 4.
Telomerase activity in adherent and non-adherent HUCBmnf cells before and after treatment of growth factors. (a) Telomerase activity in relative absorbance units of all samples (black column) and their negative controls (heat-treated samples; white column). Sample 1 (SPL1) and sample 2 (SPL2)-adherent HUCBmnf cells (14 DIV) with or without growth factors treatment; sample 3 (SPL3) and sample 4 (SPL4)-adherent HUCBmnf cells (30 DIV) with or without growth factor treatment; sample 5 (SPL5) and sample 6 (SPL6)-non-adherent HUCBmnf cells (14 DIV) with or without growth factors treatment; sample 7 (SPL7) and sample 8 (SPL8)-nonadherent HUCBmnf cells (30 DIV) with or without growth factors treatment. CTL—telomerase positive control. (b) Telomerase activity standardized for activity in the heat-treated samples. Only ΔA values of SPL5 and CTL are more than 0.15.
Experiment 2: neural induction of HUCBmnf with differentiating factors
We used two experimental protocols in this experiment to examine the ability of growth/trophic factors to induce HUCBmnf cells' neuralization.
First protocol
In this experiment, cells were grown in growth media up to 40 days and then exposed to DM1 differentiation media in which we completely withdrew proliferation-promoting agents and simply added all neurotrophic factors at once. Under this protocol, the cellular morphology of the HUCBmnf cells changed with time and culture condition. Cells from the non-adherent fraction of HUCBmnf cells after 1 DIV were as heterogeneous as the full (untreated) HUCB population in culture (figure 5(a) in panel A); as time in culture increased to 14 DIV (figures 5(c) and (d) in panel A), differentiated cells with multiple cellular processes were clearly visible compared to small round cells present in culture after 1 DIV (figure 5(a) in panel A). After 30 DIV with DM1, differentiated cells were bipolar with long processes (figure 5(b) in panel A). The morphology of these cells was reminiscent of neurons and astrocytes.
Figure 5.
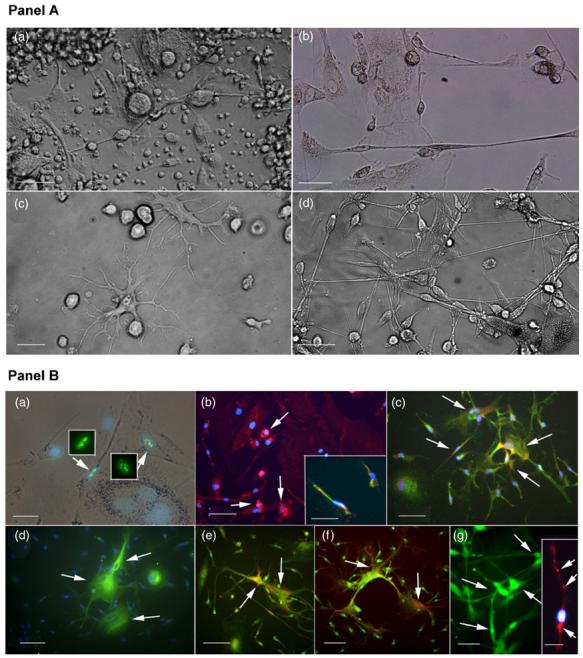
Morphology and immunophenotypes of HUCBmnf non-adherent fraction exposed to growth factors and re-cultured with defined differentiation media. Panel A: (a) Brightfield photomicrograph of non-adherent HUCBmnf cells cultured for 1 DIV and then re-cultured in DM1 for up to14 days. These cells retained the heterogeneous morphologies seen in cultures of full HUCBmnf. (b) Cells that were cultured for 30 DIV prior to replate in DM1 for another 14 DIV, were bipolar with small cell bodies and had long thin processes that formed a network. The occasional large egg-shaped cell, usually found in the adherent fraction, was also noted. (c) When cells were cultured in GM for 14 DIV prior to culturing with DM1 for 14 DIV, the cells became larger with multiple dendrite-like processes. (d) When the HUCBmnf were pre-cultured for 40 DIV and then replated in DM2 for 14 DIV, the cell bodies had one or two long processes, interconnecting to form a network similar to (b). Scale bar = 50 μm in (a) and (b), 20 μm in (c) and (d). Panel B: immunophenotypes of cultured non-adherent HUCB mnf. When these cells were pre-cultured for 14 DIV in GM and then replated in DM1 for 14 DIV, (a) Ki67 labeled nuclei (green, arrows) were visible within well differentiated cells. The inset shows the same Ki67 labeled cells under fluorescent illumination. (b) CD45+ expression was still present in these cultures (arrows). Some of these cells co-expressed vimentin (inset; CD45: red and vimentin: green). (c) Mostofthe cells (85 ± 4%) expressed the glial marker, GFAP (green). A small number of cells (12 ± 6% in this culture co-expressed GFAP and immature neural marker, nestin (GFAP: green and nestin: red; arrows). (d) few TuJ1+ cells (1 ± 0.7%, green, arrows) were detected in the culture and some of these co-expressed GFAP (e) (TuJ1: red and GFAP: green, arrows). (f) MAP2+ cells (green, arrows) were found in the HUCBmnf cultures maintained in GM for 40 DIV and then re-cultured in DM2 for up to 14 DIV. Some cells cultured under this condition were positive for synaptophysin (inset, red, arrows). Blue counterstaining was done with nuclear DAPI (blue). Scale bar = 50 μm in (a), (g) and inset in (a) and (g); 100 μm in (b)-(f).
When we examined the immunophenotypes of these cells, we found that most of the cells (90%) were differentiated with only a few cells (10%) still expressing the proliferative marker, Ki67 (figure 5(a) (green) in panel B). CD45 was still expressed in 58 ± 4% of the untreated cells (CD45; figure 5(b) (red) in panel B). Some of these CD45+ cells were also positive for the early neural marker, vimentin (figure 5(b) inset, CD45 (red) and vimentin (green) in panel B). While all cultures in DM1 did have cells positive for the immature neural markers nestin (figure 5(c) (red) in panel B) and TuJ1 (figure 5(d) (green) in panel B), they were few and far between; cells that contained either of these markers generally co-expressed the glial marker, GFAP (figures 5(c) (green) and (e) (green) in panel B). GFAP was observed in most of the cells from the replated non-adherent fraction in DM1.
Second protocol
In this protocol, the non-adherent cells were first cultured in GM for 40 days and then cultured in DM2 differentiation media, in which specific factors were sequentially added over time. After 14 DIV in DM2, clusters of bipolar cells with long processes that formed an interconnecting network covered the cell culture dish (figure 5(d) in panel A). Interestingly, when we examined these cells for the expression of neural markers, 30% of the cells were positive for the mature neuronal marker MAP2 (figure 5(g) (green) in panel B), some of them (5 ± 0.6%) expressed synaptophysin (figure 5 inset (red) in (g) in panel B). This is in contrast to the observations with DM1 cultured cells, very few of which expressed early neuronal markers let alone mature markers (table 4). Table 4 depicts the antigen profile when HUCBmnf cells were cultured in DM1 or DM2.
Table 4.
Antigen expression of HUCBmnf cells in DM1 and DM2.
| Antigen | DM1 (%) | DM2 (%) |
|---|---|---|
| Ki67 | 10 ± 3 | 5 ± 3 |
| CD45 | 58 ± 4 | 40 ± 2 |
| GFAP* | 85 ± 4 | 36 ± 10 |
| TuJ1 | 1 ± 0.7 | 12 ± 3 |
| MAP2* | 0 | 30 ± 8 |
| Synaptophysin* | 0 | 5 ± 0.6 |
Significant differences between the two groups were assessed by Student's t-test
p < 0.05
p < 0.001
Experiment 3: co-culture of HUCBmnf with mouse embryonic striatal cells
In order to learn more about how the HUCBmnf cells would interact with primary neural cells, we co-cultured non-adherent cells from HUCBmnf with embryonic mouse striatal cells in a NeuroCult® medium with human proliferation supplement (StemCell Technologies) and hEGF, hFGF and heparin for 40 DIV. The embryonic striatal cells (E14) were cultured for 1 day within a defined culture medium before the 40 DIV cultured HUCBmnf cells were added to the embryonic striatal cell culture. The CM-DiI labeled 95% of HUCBmnf cells (figure 6(a) in panel A). However, the CM-DiI at the concentration used adversely affected the cells, so we also used immunocytochemistry for human nucleoli and human mitochondria to distinguish the human cells from the mouse cells. We found that a small number of HUCBmnf progenitor cells (1%) integrated with mouse striatal cells and developed into cells expressing neural antigens. These cells expressed the neuronal markers TuJ1 (figure 6(c) in panel A) and MAP2 (figure 6(d) in panel A).
Figure 6.
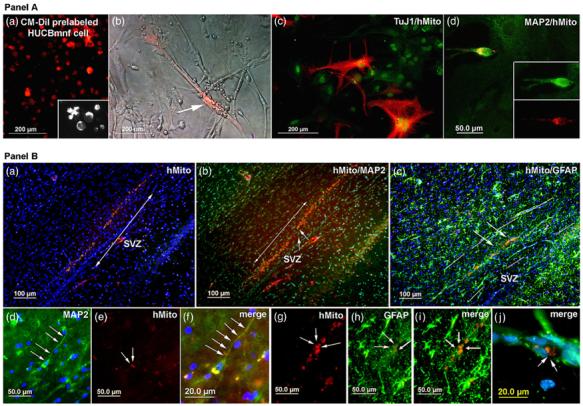
HUCBmnf non-adherent cells co-cultured with mouse embryonic striatal cells in vitro or grafted into rat brain. The HUCBmnf cells were cultured with growth factors for 40 DIV and then co-cultured with E14 mouse striata (ST). Panel A: (a) most cells (95%) were labeled by a CM-DiI cell tracer (red) before co-culture. The inset shows in greater detail CM-DiI pre-labeled cells at higher magnification (100 μm); (b) phase contrast/fluorescent photomicrograph showing a CM-DiI pre-labeled HUCBmnf cell surrounded by unlabeled mouse striatal cells. (c) Some cells (2%) co-expressed TuJ1 (red) and human mitochondrial antigen (green). (d) A few cells (1%) were positive for both MAP2 and human mitochondrial antigen (yellow). Panel B: photomicrograph of non-adherent HUCBmnf cells exposed to growth factors prior to transplantation into the SVZ of adult rats. (a) Fluorescent photomicrograph of sagittal brain section of a 9 month old rat showing surviving HUCBmnf cells within the SVZ to RMS. These cells were distributed around the injection site in a rat that survived 21 days post-transplantation. (b) The same photomicrograph as in panel A, but showing that some of the human mitochondrial labeled cells (red) within the RMS express MAP2 (green). (c) Some of these cells express both human mitochondrial antigen (red) and GFAP (green). (d) This is a higher magnification view of the area highlighted by small arrows in (b) in panel B. A MAP2+ cell with a long thin process (green; arrows) is clearly visible. (e) The same cell as in (d) is also labeled with human mitochondrial antibody (red). (f) A merged image of (d) and (e) with DAPI (blue) used to visualize the cell nuclei (g)-(j). Similarly, these images show GFAP+ /hMito+ (human mitochondria) cells within the needle tract in image (c) (arrows). Scale bar = 200 μm in (a)-(c) and 50 μm in panel A; 100 μm in (a)-(c) and 50 μmin (d), (e) and (g)-(i) and 20 μm in (f) and (j) in panel B.
Experiment 4: transplantation of HUCBmnf treated with growth factor
In this study, we wished to determine whether treatment of the HUCBmnf cells with growth factors would increase survival and neural differentiation when these cells were transplanted into the adult brain of a rat. After transplantation, rats survived for either 3, 7, 14 or 21 days. Only half the animals had surviving grafts at each time point. In those animals that had surviving grafts, the cells were visible within SVZ and RMS (figure 6(a) in panel B). Immunohistochemical staining with specific markers for human mitochondria confirmed these cells as human. Grafted cells were found around the injection site of those animals with both shorter survival times (3 days) and longer times (figure 6; 14 and 21 days). By 21 days post-transplant, few cells were observed within the injection tract, but the cells were migrating along the RMS (figure 6(a) in panel B). When we counted the number of cells within these grafts, only 0.3%, 1.2%, 0.5% and 0.3% of the 10 000 cells transplanted were observed after 3, 7, 14 and 21 days. Of the surviving human cells in the animals that survived for 14 and 21 days, approximately 10% were MAP2+ with one or two long processes (figures 6(b) (arrows) and (d)-(f) (arrows) in panel B) were found while 50% were GFAP+ (figures 6(c) (green, arrows) and (g)-(j) (green, arrows) in panel B).
Discussion
Compared to the adherent fraction, the cultured non-adherent HUCBmnf cells contain more cells expressing stem/progenitor antigens such as CD133+, and CD117+ cells [44] as well as cells that express immature neural markers [7]. The adherent cells in our cultures usually developed and formed a feeder layer [45] which may play an important role in supporting the long-term survival of the non-adherent HUCBmnf cells. Adherent cells isolated from bone marrow have been shown to give rise to mesenchymal stem cells [46], they can be expanded in culture and are able to differentiate into cells from all three germ layers. However, mesenchymal stem cells derived from adherent HUCBmnf have more mesengenic than neurogenic potential [47], even when the cells were transplanted into developing rat brain [48]. In our previous study in vivo, we transplanted non-pretreated HUCBmnf cells into young and ageing rodent brain, found that the survival of grafted HUCBmnf cells was restricted after 1 week although immuosuppression treatment was performed [41]. We therefore selected the non-adherent HUCBmnf cells to study further. When these cells were exposed to a neurogenic environment through treatment with growth factors and neurotrophins, they had increased proliferation, expression of neural antigens and survived longer in vivo.
HUCBmnf cells' neuralization potential
Besides traditional hematopoietic stem/progenitor cells such as CD34+, CD133+ and CD117+ cells which have a demonstrated pluripotency and can express antigens normally expressed by cells derived from other lineages [1, 49], solid evidence has also revealed the existence of non-hematopoietic stem/progenitor cells in HUCBmnf [50]. These non-hematopoietic stem/progenitor cells can spontaneously express the immature neural markers nestin, TuJ1, A2B5 and GFAP at a low frequency in the original culture without any specific induction factors. This may imply that this cell subpopulation has the potential to develop toward neural-like cell lineages. In support of this is the observation that some differentiated cells derived from HUCBmnf cells exhibited the mature neuronal marker, MAP2, with typical neuronal morphology, even though they were cultured with a regular serum-containing medium [7]. While the MAP2 expression is suggestive that neuronal antigens are present on the cord blood cells [51] and further that these cells are developing a structural polarity and functional compartmentalization similar to that of neurons, this has not been definitively demonstrated in vivo. Current evidence of function is limited to demonstration of the presence of voltage and ligand gated channels [50] on HUCBmnf-derived cells, and expression of a multitude of neurotransmitters [52]. Even so, these studies do not address structural polarity or functionality.
Effect of culture conditions on HUCBmnf progenitor cells
Effect of growth factors
Oct-4 antigen was expressed in 92% of the non-adherent HUCBmnf cells in contrast to only 14% in the adherent HUCBmnf cells. Oct-4 antigen is generally associated with totipotent embryonic stem and germ cells [53] and plays an important role in stem cell self-renewal and pluripotency that controls lineage commitment [54]. Whether the Oct-4 positive HUCBmnf cells are capable of differentiating across the three germ layers with or without specific inducing factors, needs to be investigated. It is therefore not surprising that there were more BrdU+, proliferating cells in the non-adherent HUCBmnf fraction. Further, it is encouraging that these cells were responsive to growth factor treatment. The number of BrdU incorporating cells in non-adherent HUCBmnf cells after 7 days of treatment with growth factors EGF, bFGF, LIF increased five fold.
In humans, telomerase activity, especially in the core catalytic subunit of telomerase, hTERT, is almost undetectable in normal somatic cells. The exception to this is proliferative cells of tissues that can undergo self-renewal [55]. For example, telomerase activity is high in hematopoietic stem cells. In this study, we only detected positive telomerase activity in day 14 non-adherent HUCBmnf cells treated with growth factors (hbFGF. hEGF, hLIF and hSCF). Meanwhile, the adherent fraction was negative for telomerase activity as were the day 30 non-adherent fraction samples regardless of growth factor treatment. Therefore, telomerase activity appears to be positively correlated with proliferation since telomerase activity was highest at 14 DIV, the time when we observed the most BrdU positive cells, and was non-existent at 30 DIV when proliferation was occurring at a slower rate. Similar findings have been reported by Stojko et al [56].
Effect of neurotrophic factors
We have previously shown that some progenitor cells from HUCBmnf may be able to differentiate into neural cell types [57]. The effect of the growth and differentiation media on the non-adherent HUCBmnf cells depended on the length of time in culture and the exposure to NGF, GDNF, RA and dbcAMP. When the HUCBmnf were grown for only 1 DIV after thaw in GM, the cells did not thrive when replated in either DM1 or DM2. For that reason, we extended the length of time the cells were cultured in GM up to 40 days. Further, the neural media (DM1 and DM2) were not optimal for the growth of hematopoietic cells. Those cells that did survive, however, exhibited a more neural morphology and immunophenotype. Fifty-eight ± 4% of these cells were CD45 negative, suggesting that they had lost their hematopoietic phenotype. When we examined whether there is co-localization of neural and hematopoietic markers in some of the cord-blood-derived cells, we do observe co-localization but this usually occurs with early neural markers [7] as opposed to more mature markers. For example, cells from shorter term cultures (14 DIV with growth factors treatment) mainly expressed GFAP and some of those cells co-expressed nestin or TuJ1 with the GFAP. One explanation is that these double labeled cells were caught at a transition stage in their development where antigen expression for GFAP was decreasing while neuronal antigens were increasing [58]. Cells from cultures grown longer in GM (30-40 DIV), however, developed longer processes and formed networks resembling primary neuronal cultures. Some of these cells (5 ± 0.6%) were also positive for synaptophysin. While cells in culture with both DM1 and DM2 for 30 DIV both developed networks of interconnecting cells, it was only the cells in DM2 that expressed MAP2, a marker of more mature neurons. The cells exposed to DM1 for 30 DIV expressed GFAP and early neural markers such as nestin and TuJ1. Therefore, the fate of the non-adherent HUCBmnf cells is influenced by both extrinsic cues (neurotrophic factors) and intrinsic cues (genetic predisposition) and these cues may change with time [59].
The nature of the stem cell in cord blood capable of producing a `neuron' is not clear. In a recent review of cord blood biology, Broxmeyer [3] presents evidence that there are hematopoieitic stem cells, mesenchymal stem cells, hematopoietic progenitors, endothelial progenitors and immune cells present within the cord blood. While HSCs reside within the CD34+ cell population and have been extensively characterized, a profile of specific antigenic expression of mesenchymal cells is not yet well-established, leading to isolation of these `MSCs' based on lineage negativity or adhesive properties. This has led to contradictory reports as to whether these stem cells are [60] or are not present in cord blood [61]. Within bone marrow, the MSCs are isolated based on their adherence to plastic and it is these cells that have been shown to `transdifferentiate'. Similarly, neural-like cells have been derived from CD34- cells [62] in cord blood. However, the CD34+ population of HSCs is itself a heterogeneous population. Another primitive stem cell marker is CD133. CD34+/CD133+ cells have been isolated from cord blood, and these cells have higher efficacy in repopulating the immune system of NOD/SCID mice [63] than CD34+/CD133- cells. These cells also can `transdifferentiate' into neural cells [64]. Further, neural stem cells also express a form of CD133 [65].
Effect of neural cells on HUCBmnf cell development
Previous studies from our group have demonstrated that HUCBmnf cells expressed neural features when transplanted into the brains of both neonatal [8] and senescent rats [41]. However, in the adult brain, upon thaw, the full population of HUCBmnf only survived for 3 to 7 days. This is problematic if we hypothesize that HUCBmnf must exert their effects by developing into neural cells and integrating into the existing neural circuitry of the brain. We first pretreated non-adherent HUCBmnf cells with growth factors and began this set of studies to examine the interaction of the non-adherent HUCBmnf cells with embryonic mouse striatal cells (E14) in vitro. The contact co-culture system has been used for observing and verifying the interaction of varied cell types [66], although not specifically for hematopoietic and neural cell interactions. In our study, we used a contact co-culture system to observe in vitro the morphological changes and the expression of immunophenotypes of HUCBmnf cells in such culture condition and interconnection between HUCBmnf cells and primary neural cells. It is supposed to further confirm that HUCBmnf cells are able to survive with primary neural cells and to integrate into neural networks. Few of these cells were observed to differentiate into neural phenotypes in this co-culture system and form connections with the mouse neural cells. Some of them expressed neuronal-specific markers TuJ1 and MAP2 with typical neuronal morphology.
It has been suggested that cell fusion is a more likely explanation of the expression of neural antigens by the HUCBmnf cells. The original cell fusion events were described in relation to bone marrow-derived cells fusing with embryonic stem cells [67] but these events have also been described for hepatocytes, cardiomyocytes [68] and neurons [69]. There are also reports that cord blood cells can fuse with hepatocytes [70] and cardiomyocytes [71], although there is evidence that the HUCBmnf cells also appear to transdifferentiate in these models as well. However, fusion appears to be model-specific since fusion was not a major component of cord blood integration in the gastrointestinal tract [72]. Unfortunately, while the method we chose to identify the HUCBmnf cells in the brain after transplantation labeled the entire cell body and helped us to define the cellular morphology as well as cell survival, it could not address the issue of whether these cells contain genetic material from both the human and rat.
While pre-labeling the cells with a neuronal tracer has the advantage of putting an easily identifiable cell into the brain, there is also the disadvantage that the dye may be taken up into adjacent cells in contact with the labeled cell, particularly if the transplanted cell dies [41]. We have tried multiple tracers to identify the cord blood cells, with the common result that the cells do not retain the label for more than a few days (unpublished observation). An alternative would be to transfect the cells with GFP, but the efficiency is not necessarily very high [73].
In vivo, the transplanted HUCBmnf cells were recognized by expression of human mitochondrial proteins. Within the SVZ, we found that grafted cells migrated along the SVZ to rostral migratory stream, survived for 14 and 21 days, and mostly expressed the glial marker, GFAP. Occasionally, MAP2 positive cells were observed with processes. MAP2 is a marker for mature neurons with anatomical and functional polarity. These grafted MAP2 positive cells, although they are few in number, may imply that these cells are trying to make appropriate functional connections with the host cells. Compared to similar studies from our group [41] and others [48], in which those cells derived from either non-pretreated or adherently growing HUCBmnf cells did not express the neuronal marker MAP2 in vivo, the results of this study suggested that growth factor pretreatment can enhance not only the survivability but also the potential for neuralization in vivo. The SVZ is one of the two principal neurogenic regions present in the brain across the lifespan [74]. Recent studies suggest that GFAP positive astrocytes within these areas may be primary neuronal precursors and develop into interneurons during migration, although some do retain their glial phenotypes [75]. While the grafted HUCBmnf cells did migrate, they did not migrate long distances. We do not know the ultimate fate of these grafted, GFAP+ cells, whether they survived in the host brain, whether they remained GFAP positive or developed further. If they behaved like neural progenitors, then they may have remained quiescent, out of the cell cycle, until stimulated by the local environment [76]. Compared to those HUCBmnf cells without pretreatment which failed to survive longer than a week in vivo, the ability of pretreated HUCBmnf cells to survive in the host brain has been enhanced. Even so, the similar cell survival rate at all time points after grafting suggests that the cell loss is the result of a slow rejection of the xenograft, but may be something more akin to hyperacute rejection or anoikisis.
In summary, stem/progenitor-like cells derived from non-adherent HUCBmnf can rapidly respond to extrinsic growth or trophic factors with increased proliferation. At the same time, these growth factor pretreated cells were more prone to neuralization, suggesting that they can read cues from the neurogenic environment; this is further supported by the observation that they survived in vivo and subsequently attained features of neurons and glia. Recent reports further revealed that the non-hematopoietic fraction of HUCBmnf cells can functionally differentiate into neural-like cells with voltage- and ligand-gated channels similar to those found in primary neural cells [50], and CD34 deprived fraction of HUCBmnf cells was able to be driven toward neural lineage [51]. Further, HUCBmnf-derived stem cells have been used to establish a neural-like cell line [77].
HUCBmnf cells are being examined in experimental models of brain injury and disease such as stroke [78, 79], injured spinal cord [80], ALS and Sanfilippo syndrome B [81] where they have been shown to migrate to damaged brain and improve functional outcomes. Their effectiveness, however, does not solely rely on their ability to replace lost neurons, but is also a function of how the cells modify the local brain environment, decreasing inflammation, providing trophic support and inducing angiogenesis and neurogenesis. The therapeutic potential of these cells remains largely untapped and will be the focus of investigation in the future.
Acknowledgments
This paper is dedicated to Dr Tanja Zigova who was my former mentor, a scientist with a vision. She died in February 2004 before the completion of this project and manuscript. The work was supported by grants from National Institutes of Health/National Institutes on Aging (grant R01 AG20927) (AW and TZ). PS is co-founder of Saneron CCEL Therapeutics, Inc. (SCT). SGD, PB and AEW are consultants to SCT, and PS, SGD and AEW are inventors of HUCB patents.
References
- [1].Kim SY, Park SY, Kim JM, Kim JW, Kim MY, Yang JH, Kim JO, Choi KH, Kim SB, Ryu HM. Differentiation of endothelial cells from human umbilical cord blood AC133-CD14+ cells. Ann. Hematol. 2005;84:417–22. doi: 10.1007/s00277-004-0988-y. [DOI] [PubMed] [Google Scholar]
- [2].Goodwin HS, Bicknese AR, Chien SN, Bogucki BD, Quinn CO, Wall DA. Multilineage differentiation activity by cells isolated from umbilical cord blood: expression of bone, fat, and neural markers. Biol. Blood Marrow Transplant. 2001;7:581–8. doi: 10.1053/bbmt.2001.v7.pm11760145. [DOI] [PubMed] [Google Scholar]
- [3].Broxmeyer HE. Biology of cord blood cells and future prospects for enhanced clinical benefit. Cytotherapy. 2005;7:209–18. doi: 10.1080/14653240510027190. [DOI] [PubMed] [Google Scholar]
- [4].Rosada C, Justesen J, Melsvik D, Ebbesen P, Kassem M. The human umbilical cord blood: a potential source for osteoblast progenitor cells. Calcif. Tissue Int. 2003;72:135–42. doi: 10.1007/s00223-002-2002-9. [DOI] [PubMed] [Google Scholar]
- [5].Kong KY, Ren J, Kraus M, Finklestein SP, Brown RH., Jr Human umbilical cord blood cells differentiate into muscle in sjl muscular dystrophy mice. Stem Cells. 2004;22:981–93. doi: 10.1634/stemcells.22-6-981. [DOI] [PubMed] [Google Scholar]
- [6].McGuckin CP, Forraz N, Baradez MO, Navran S, Zhao J, Urban R, Tilton R, Denner L. Production of stem cells with embryonic characteristics from human umbilical cord blood. Cell Prolif. 2005;38:245–55. doi: 10.1111/j.1365-2184.2005.00346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Chen N, Hudson JE, Walczak P, Misiuta I, Garbuzova-Davis S, Jiang L, Sanchez-Ramos J, Sanberg PR, Zigova T, Willing AE. Human umbilical cord blood progenitors: the potential of these hematopoietic cells to become neural. Stem Cells. 2005;23:1560–70. doi: 10.1634/stemcells.2004-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Sanchez-Ramos JR, Song S, Kamath SG, Zigova T, Willing A, Cardozo-Pelaez F, Stedeford T, Chopp M, Sanberg PR. Expression of neural markers in human umbilical cord blood. Exp. Neurol. 2001;171:109–15. doi: 10.1006/exnr.2001.7748. [DOI] [PubMed] [Google Scholar]
- [9].Zigova T, Song S, Willing AE, Hudson JE, Newman MB, Saporta S, Sanchez-Ramos J, Sanberg PR. Human umbilical cord blood cells express neural antigens after transplantation into the developing rat brain. Cell Transplant. 2002;11:265–74. [PubMed] [Google Scholar]
- [10].Terskikh AV, Easterday MC, Li L, Hood L, Kornblum HI, Geschwind DH, Weissman IL. From hematopoiesis to neuropoiesis: evidence of overlapping genetic programs. Proc. Natl Acad. Sci. USA. 2001;98:7934–9. doi: 10.1073/pnas.131200898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Traycoff CM, Abboud MR, Laver J, Clapp DW, Srour EF. Rapid exit from G0/G1 phases of cell cycle in response to stem cell factor confers on umbilical cord blood CD34+ cells an enhanced ex vivo expansion potential. Exp. Hematol. 1994;22:1264–72. [PubMed] [Google Scholar]
- [12].Chivu M, Diaconu CC, Bleotu C, Alexiu I, Brasoveanu L, Cernescu C. The comparison of different protocols for expansion of umbilical-cord blood hematopoietic stem cells. J. Cell. Mol. Med. 2004;8:223–31. doi: 10.1111/j.1582-4934.2004.tb00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Farso MC, Carroll FY, Beart PM. Establishment of primary cultures of rat olfactory bulb under serum-free conditions for studies of cellular injury. Cell Tissue Res. 2006;323:343–9. doi: 10.1007/s00441-005-0056-5. [DOI] [PubMed] [Google Scholar]
- [14].Long XX, Olszewsk M, Huang W, Kletzel M. Neural cell differentiation in vitro from adult human bone marrow mesenchymal stem cells. Stem Cells Dev. 2005;14:65–9. doi: 10.1089/scd.2005.14.65. [DOI] [PubMed] [Google Scholar]
- [15].Fallon JH, Seroogy KB, Loughlin SE, Morrison RS, Bradshaw RA, Knaver DJ, Cunningham DD. Epidermal growth factor immunoreactive material in the central nervous system: location and development. Science. 1984;224:1107–9. doi: 10.1126/science.6144184. [DOI] [PubMed] [Google Scholar]
- [16].Zhou JX, Li-wei Jia LW, Liu WM, Miao CL, Shuang Liu S, Cao YJ, Duan EK. Role of sonic hedgehog in maintaining a pool of proliferating stem cells in the human fetal epidermis. Human Reprod. 2006;21:1698–704. doi: 10.1093/humrep/del086. [DOI] [PubMed] [Google Scholar]
- [17].Scheibe RJ, Wagner JA. Retinoic acid regulates both expression of the nerve growth factor receptor and sensitivity to nerve growth factor. J. Biol. Chem. 1992;267:17611–6. [PubMed] [Google Scholar]
- [18].Gochenauer GE, Robinson BM. Dibutyryl-cAMP (dbcAMP) up-regulates astrocytic chloride-dependent L-[3H]glutamate transport and expression of both system xc- subunits. J. Neurochem. 2001;78:276. doi: 10.1046/j.1471-4159.2001.00385.x. [DOI] [PubMed] [Google Scholar]
- [19].Monfils MH, Driscoll I, Kamitakahara H, Wilson B, Flynn C, Teskey GC, Kleim JA, Kolb B. FGF-2-induced cell proliferation stimulates anatomical, europhysiological and functional recovery from neonatal motor cortex injury. Eur. J. Neurosci. 2006;24:739–49. doi: 10.1111/j.1460-9568.2006.04939.x. [DOI] [PubMed] [Google Scholar]
- [20].Carpenter MK, Cui X, Hu ZY, Jackson J, Sherman S, Seiger A, Wahlberg LU. In vitro expansion of a multipotent population of human neural progenitor cells. Exp. Neurol. 1999;158:265–78. doi: 10.1006/exnr.1999.7098. [DOI] [PubMed] [Google Scholar]
- [21].Jin K, Mao XO, Sun YJ, Xie L, Greenberg DA. Stem cell factor stimulates neurogenesis in vitro and in vivo. J. Clin. Invest. 2002;110:311–9. doi: 10.1172/JCI15251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fukuchi-Shimogori T, Grove EA. Neocortex patterning by the secreted signaling molecule FGF8. Science. 2001;294:1071–4. doi: 10.1126/science.1064252. [DOI] [PubMed] [Google Scholar]
- [23].Woszczycka-Korczynska I, Lewin-Kowalik J, Gorka D, Olakowska E. Neutrophins in biology and medicine. Pol. Merkuriusz. Lek. 2006;20:602–5. [PubMed] [Google Scholar]
- [24].Rossi C, et al. Brain-derived neurotrophic factor (BDNF) is required for the enhancement of hippocampal neurogenesis following environmental enrichment. Eur. J. Neurosci. 2006;24:1850–6. doi: 10.1111/j.1460-9568.2006.05059.x. [DOI] [PubMed] [Google Scholar]
- [25].Anitha M, Chandrasekharan B, Salgado JR, Grouzmann E, Mwangi S, Sitaraman SV, Srinivasan S. Glial-derived neurotrophic factor modulates enteric neuronal survival and proliferation through neuropeptide y. Gastroenterology. 2006;131:1164–78. doi: 10.1053/j.gastro.2006.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Faherty S, Kane MT, Quinlan LR. Self-renewal and differentiation of mouse embryonic stem cells as measured by Oct4 gene expression: effects of LIF, serum-free medium, serum-free medium, retinoic acid, and dbcAMP. In Vitro Cell. Dev. Biol.—Anim. 2005;41:356–63. doi: 10.1007/s11626-005-0008-0. [DOI] [PubMed] [Google Scholar]
- [27].Macon WR, Salhany KE. T-cell subset analysis of peripheral T-cell lymphomas by paraffin section immunohistology and correlation of CD4/CD8 results with flow cytometry. Am. J. Clin. Pathol. 1998;109:610–7. doi: 10.1093/ajcp/109.5.610. [DOI] [PubMed] [Google Scholar]
- [28].Osawa M, Hanada K, Hamada H, Nakauchi H. Long-term lymphohematopoietic reconstitution by a single CD34-low/negative hematopoietic stem cell. Science. 1996;273:242–5. doi: 10.1126/science.273.5272.242. [DOI] [PubMed] [Google Scholar]
- [29].Haynes BF, Telen MJ, Hale LP, Denning SM. CD44—a molecule involved in leukocyte adherence and T-cell activation. Immunol. Today. 1989;10:423–8. doi: 10.1016/0167-5699(89)90040-6. [DOI] [PubMed] [Google Scholar]
- [30].Zhang M, Moran M, Round J, Low TA, Patel VP, Tomassian T, Hernandez JD, Miceli MC. CD45 signals outside of lipid rafts to promote ERK activation, synaptic raft clustering, and IL-2 production. J. Immunol. 2005;174:1479–90. doi: 10.4049/jimmunol.174.3.1479. [DOI] [PubMed] [Google Scholar]
- [31].Hens J, Nuydens R, Geerts H, Senden NH, Van de Ven WJ, Roebroek AJ, van de Velde HJ, Ramaekers FC, Broers JL. Neuronal differentiation is accompanied by NSP-C expression. Cell Tissue Res. 1998;292:229–37. doi: 10.1007/s004410051054. [DOI] [PubMed] [Google Scholar]
- [32].Niwa H, Miyazaki J, Smith AG. Quantitative expression of Oct-3/4 defines differentiation, dedifferentiation or self-renewal of ES cells. Nature Genet. 2000;24:372–6. doi: 10.1038/74199. [DOI] [PubMed] [Google Scholar]
- [33].Dahlstrand J, Lardelli M, Lendahl U. Nestin mRNA expression correlates with the central nervous system progenitor cell state in many, but not all, regions of developing central nervous system. Dev. Brain Res. 1995;84:109–29. doi: 10.1016/0165-3806(94)00162-s. [DOI] [PubMed] [Google Scholar]
- [34].Nakamura Y, Yamamoto M, Oda E, Yamamoto A, Kanemura Y, Hara M, Suzuki A, Yamasaki M, Okano H. Expression of tubulin beta II in neural stem/progenitor cells and radial fibers during human fetal brain development. Lab. Invest. 2003;83:479–89. doi: 10.1097/01.lab.0000063930.75913.b3. [DOI] [PubMed] [Google Scholar]
- [35].Riederer BM, Draberova E, Viklicky V, Draber P. Changes of MAP2 phosphorylation during brain development. J. Histochem. Cytochem. 1995;43:1269–84. doi: 10.1177/43.12.8537643. [DOI] [PubMed] [Google Scholar]
- [36].Johansson S, Lee IH, Olson L, Spenger C. Olfactory ensheathing glial co-grafts improve functional recovery in rats with 6-OHDA lesions. Brain. 2005;28:2961–76. doi: 10.1093/brain/awh644. [DOI] [PubMed] [Google Scholar]
- [37].Takano T, Tian GF, Peng W, Lou N, Libionka W, Han X, Nedergaard M. Astrocyte-mediated control of cerebral blood flow. Nat. Neurosci. 2006;9:260–7. doi: 10.1038/nn1623. [DOI] [PubMed] [Google Scholar]
- [38].Hutchins JB, Casagrande VA. Vimentin: changes in distribution during brain development. Glia. 1989;2:55–66. doi: 10.1002/glia.440020107. [DOI] [PubMed] [Google Scholar]
- [39].Sheedlo HJ, Sprinkle TJ. The localization of 2′:3′ -cyclic nucleotide 3′ -phosphodiesterase in bovine cerebrum by immunofluorescence. Brain Res. 1983;288:330–3. doi: 10.1016/0006-8993(83)90112-9. [DOI] [PubMed] [Google Scholar]
- [40].Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 1984;133:1710–5. [PubMed] [Google Scholar]
- [41].Walczak P, Chen N, Hudson JE, Willing AE, Garbuzova-Davis SN, Song S, Sanberg PR, Sanchez-Ramos J, Bickford PC, Zigova T. Do hematopoietic cells exposed to a neurogenic environment mimic properties of endogenous neural precursors? J. Neurosci. Res. 2004;76:244–54. doi: 10.1002/jnr.20042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Rutishauser U, Acheson A, Hall A, Mann DM, Sunshine J. The neural cell adhesion molecule (NCAM) as a regulator of cell-cell interactions. Science. 1988;240:53–7. doi: 10.1126/science.3281256. [DOI] [PubMed] [Google Scholar]
- [43].Ye ZQ, Burkholder JK, Qiu P, Schultz JC, Shahidi NT, Yang NS. Establishment of an adherent cell feeder layer from human umbilical cord blood for support of long-term hematopoietic progenitor cell growth. Proc. Natl Acad. Sci. USA. 1994;91:12140–1. doi: 10.1073/pnas.91.25.12140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Baal N, Reisinger K, Jahr H, Bohle RM, Liang O, Munstedt K, Rao CV, Preissner KT, Zygmunt MT. Expression of transcription factor Oct-4 and other embryonic genes in CD133 positive cells from human umbilical cord blood. Thromb. Haemost. 2004;92:767–75. doi: 10.1160/TH04-02-0079. [DOI] [PubMed] [Google Scholar]
- [45].Gao L, Chen X, Zhang X, Liu Y, Kong P, Peng X, Liu L, Liu H, Zeng D. Human umbilical cord blood-derived stromal cell, a new resource of feeder layer to expand human umbilical cord blood CD34(+) cells in vitro. Blood Cells Mol. Dis. 2006;36:322–8. doi: 10.1016/j.bcmd.2005.12.036. [DOI] [PubMed] [Google Scholar]
- [46].Jiang Y, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–9. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- [47].Gang EJ, Hong SH, Jeong JA, Hwang SH, Kim SW, Yang IH, Ahn C, Han H, Kim H. In vitro mesengenic potential of human umbilical cord blood-derived mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2004;321:102–8. doi: 10.1016/j.bbrc.2004.06.111. [DOI] [PubMed] [Google Scholar]
- [48].Coenen M, Kogler G, Wernet P, Brustle O. Transplantation of human umbilical cord blood-derived adherent progenitors into the developing rodent brain. J. Neuropathol. Exp. Neurol. 2005;64:681–8. doi: 10.1097/01.jnen.0000173892.24800.03. [DOI] [PubMed] [Google Scholar]
- [49].D'Arena G, Musto P, Cascavilla N, Di Giorgio G, Fusilli S, Zendoli F, Carotenuto M. Human umbilical cord blood: immunophenotypic heterogeneity of CD34+ hematopoietic progenitor cells. Haematologica. 1998;81:404–9. [PubMed] [Google Scholar]
- [50].Sun W, Buzanska L, Domanska-Janik K, Salvi RJ, Stachowiak MK. Voltage-sensitive and ligand-gated channels in differentiating neural stem-like cells derived from the nonhematopoietic fraction of human umbilical cord blood. Stem Cells. 2005;23:931–45. doi: 10.1634/stemcells.2004-0316. [DOI] [PubMed] [Google Scholar]
- [51].Habich A, Jurga M, Markiewicz I, Lukomska B, Bany-Laszewicz U, Domanska-Janik K. Early appearance of stem/progenitor cells with neural-like characteristics in human cord blood mononuclear fraction cultured in vitro. Exp. Hematol. 2006;34:914–25. doi: 10.1016/j.exphem.2006.03.010. [DOI] [PubMed] [Google Scholar]
- [52].Fan CG, Zhang QJ, Tang FW, Han ZB, Wang GS, Han Z. Human umbilical cord blood cells express neurotrophic factors. Neurosci. Lett. 2005;380:322–5. doi: 10.1016/j.neulet.2005.01.070. [DOI] [PubMed] [Google Scholar]
- [53].Reubinoff BE, Pera MF, Fong CY, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat. Biotechnol. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- [54].Scholer HR, Hatzopoulos AK, Balling R, Suzuki N, Gruss P. A family of octamer-specific proteins present during mouse embryogenesis: evidence for germline-specific expression of an Oct factor. Embo. J. 1989;8:2543–50. doi: 10.1002/j.1460-2075.1989.tb08392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wu KD, Moore MA. Determination of telomerase activity and telomere length. Methods Mol. Med. 2005;113:207–23. doi: 10.1385/1-59259-916-8:207. [DOI] [PubMed] [Google Scholar]
- [56].Stojko R, Glogowska J, Wilczok A, Mazurek U, Witek A, Wilczok T. Estimation of telomerase transcriptive activity in the umbilical cord and the mother's venous blood cells. Ginekol. Pol. 2003;74:1376–8. [PubMed] [Google Scholar]
- [57].Sanberg PR, Willing AE, Garbuzova-Davis S, Saporta S, Liu G, Sanberg CD, Bickford PC, Klasko SK, El-Badri NS. Umbilical cord blood-derived stem cells and brain repair. Ann. N.Y. Acad. Sci. 2006;1049:67–83. doi: 10.1196/annals.1334.008. [DOI] [PubMed] [Google Scholar]
- [58].Wislet-Gendebien S, Bruyere F, Hans G, Leprince P, Moonen G, Rogister B. Nestin-positive mesenchymal stem cells favour the astroglial lineage in neural progenitors and stem cells by releasing active BMP4. BMC Neurosci. 2004;5:33. doi: 10.1186/1471-2202-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Harris AS, Hartenstein V. Cellular determination. In: Zigmond MJ, Bloom FE, Landis SC, Roberts JL, Squire LR, editors. Fundamental Neuroscience. Academic; San Diego, CA: 1999. pp. 507–9. [Google Scholar]
- [60].Erices A, Conget P, Minguell JJ. Mesenchymal progenitor cells in human umbilical cord blood. Br. J. Haematol. 2000;109:235–42. doi: 10.1046/j.1365-2141.2000.01986.x. [DOI] [PubMed] [Google Scholar]
- [61].Wexler S, Donaldson AC, Denning-Kendall P, Rice C, Bradley B, Hows JM. Adult bone marrow is a rich source of human mesenchymal `stem' cells but umbilical cord and mobilized adult blood are not. Br.J.Haematol. 2003;121:368–74. doi: 10.1046/j.1365-2141.2003.04284.x. [DOI] [PubMed] [Google Scholar]
- [62].Bicknese AR, Goodwin HS, Quinn CO, Henderson VC, Chien SN, Wall DA. Human umbilical cord blood cells can be induced to express markers for neurons and glia. Cell Transplant. 2002;11:261–4. [PubMed] [Google Scholar]
- [63].de Wynter EA, et al. CD34+AC133+ cells isolated from cord blood are highly enriched in long-term culture-initiating cells, NOD/SCID-repopulating cells and dendritic cell progenitors. Stem Cells. 1998;16:387–96. doi: 10.1002/stem.160387. [DOI] [PubMed] [Google Scholar]
- [64].Jang YK, Park JJ, Lee MC, Yoon BH, Yang YS, Yang SE, Kim SU. Retinoic acid-mediated induction of neurons and glial cells from human umbilical cord-derived hematopoietic stem cells. J. Neurosci. Res. 2004;75:573–84. doi: 10.1002/jnr.10789. [DOI] [PubMed] [Google Scholar]
- [65].Schwartz PH, Bryant PJ, Fuja TJ, Su H, O'Dowd DK, Klassen H. Isolation and characterization of neural progenitor cells from post-mortem human cortex. J. Neurosci. Res. 2003;74:838–51. doi: 10.1002/jnr.10854. [DOI] [PubMed] [Google Scholar]
- [66].Kitahara T, Hiromura K, Ikeuchi H, Yamashita S, Kobayashi S, Kuroiwa T, Kaneko Y, Ueki K, Nojima Y. Mesangial cells stimulate differentiation of endothelial cells to form capillary-like networks in a three-dimensional culture system. Nephrol. Dial. Transplant. 2005;20:42–9. doi: 10.1093/ndt/gfh572. [DOI] [PubMed] [Google Scholar]
- [67].Ying QL, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature. 2002;416:545–8. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- [68].Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–73. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- [69].Weimann JM, Johansson CB, Trejo A, Blau HM. Stable reprogrammed heterokaryons form spontaneously in Purkinje neurons after bone marrow transplant. Nat. Cell. Biol. 2003;5:959–66. doi: 10.1038/ncb1053. [DOI] [PubMed] [Google Scholar]
- [70].Tanabe Y, et al. Analyses to clarify rich fractions in hepatic progenitor cells from human umbilical cord blood and cell fusion. Biochem. Biophys. Res. Commun. 2004;324:711–8. doi: 10.1016/j.bbrc.2004.09.115. [DOI] [PubMed] [Google Scholar]
- [71].Ishikawa F, et al. Purified human hematopoietic stem cells contribute to the generation of cardiomyocytes through cell fusion. FASEB. J. 2006;20:950–2. doi: 10.1096/fj.05-4863fje. [DOI] [PubMed] [Google Scholar]
- [72].Ishikawa F, et al. Human cord blood- and bone marrow-derived CD34+ cells regenerate gastrointestinal epithelial cells. FASEB. J. 2004;18:1958–60. doi: 10.1096/fj.04-2396fje. [DOI] [PubMed] [Google Scholar]
- [73].Irons H, Lind JG, Wakade CG, Yu G, Hadman M, Carroll J, Hess DC, Borlongan CV. Intracerebral xenotransplantation of GFP mouse bone marrow stromal cells in intact and stroke rat brain: graft survival and immunologic response. Cell Transplant. 2004;13:283–94. doi: 10.3727/000000004783983990. [DOI] [PubMed] [Google Scholar]
- [74].Aguirre AA, Chittajallu R, Belachew S, Gallo V. NG2-expressing cells in the subventricular zone are type C-like cells and contribute to interneuron generation in the postnatal hippocampus. J. Cell Biol. 2004;165:575–89. doi: 10.1083/jcb.200311141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Peretto P, Giachino C, Aimar P, Fasolo A, Bonfanti L. Chain formation and glial tube assembly in the shift from neonatal to adult subventricular zone of the rodent forebrain. J. Comp. Neurol. 2005;487:407–27. doi: 10.1002/cne.20576. [DOI] [PubMed] [Google Scholar]
- [76].Morrison SJ, Shah NM, Anderson DJ. Regulatory mechanisms in stem cell biology. Cell. 1997;88:287–98. doi: 10.1016/s0092-8674(00)81867-x. [DOI] [PubMed] [Google Scholar]
- [77].Buzanska L, Jurga M, Stachowiak EK, Domanska-Janik K. Neural stem-like line derived from a nonhematopoietic population of human umbilical cord blood. Stem Cells Dev. 2006;15:391–406. doi: 10.1089/scd.2006.15.391. [DOI] [PubMed] [Google Scholar]
- [78].Willing AE, Jiang L, Milliken M, Poulos S, Zigova T, Song S, Hart C, Sanchez-Ramos J, Sanberg PR. Intravenous versus intrastriatal cord blood administration in a rodent model of stroke. J. Neurosci. Res. 2003;73:296–307. doi: 10.1002/jnr.10659. [DOI] [PubMed] [Google Scholar]
- [79].Newcomb JD, Ajmo CT, Sanberg CD, Sanberg PR, Pennypacker KR, Willing AE. Timing of cord blood treatment after experimental stroke determines therapeutic efficacy. Cell Transplant. 2006;15:213–25. doi: 10.3727/000000006783982043. [DOI] [PubMed] [Google Scholar]
- [80].Saporta S, Kim JJ, Willing AE, Fu ES, Davis CD, Sanberg PR. Human umbilical cord blood stem cells infusion in spinal cord injury: engraftment and beneficial influence on behavior. J. Hematother. Stem Cell Res. 2003;12:271–8. doi: 10.1089/152581603322023007. [DOI] [PubMed] [Google Scholar]
- [81].Garbuzova-Davis S, Willing AE, Zigova T, Saporta S, Justen EB, Lane JC, Hudson JE, Chen N, Davis CD, Sanberg PR. Intravenous administration of human umbilical cord blood cells in a mouse model of amyotrophic lateral sclerosis: distribution, migration, and differentiation. J. Hematother. Stem Cell Res. 2003;12:255–70. doi: 10.1089/152581603322022990. [DOI] [PubMed] [Google Scholar]