Abstract
Background/Aims
Not all alcoholics develop liver disease (ALD). Thus, excessive ethanol consumption is necessary, but not sufficient, to induce alcoholic steatohepatitis (ASH) & ALD. Since endotoxemia is present in patients with ALD, it has been proposed that gut-derived, circulating endotoxin is the necessary co-factor for ASH. But, it is not known whether endotoxemia is the consequence or the trigger for ALD. Accordingly, the aim of the current study was to determine whether endotoxemia occurs prior to development of ASH and whether gut leakiness is the primary cause of the endotoxemia in an animal model of ASH.
Methods
Time courses for development of gut hyperpermeability, nitric oxide production, oxidative injury to the gut, endotoxemia, and liver injury were assessed in rats during 10 weeks of daily alcohol gavage.
Results
Liver fat and serum transaminase increased after 2 weeks, but evidence of liver cell injury and inflammation (ASH) occurred after 8 weeks. Gut leakiness, intestinal oxidative injury, and endotoxemia occurred in weeks 2–4 and progressed thereafter.
Conclusions
That alcohol-induced gut leakiness and endotoxemia preceded steatohepatitis indicates they are not the consequence of ALD. Our data support the hypothesis that gut leakiness resulting in endotoxemia is a key co-factor (trigger) for ASH.
Keywords: intestinal hyperpermeability, alcoholic steatohepatitis, nitric oxide, oxidative stress, endotoxemia, alcoholic liver disease
Introduction
Among the most serious complications of alcoholism is alcoholic liver disease (ALD) that includes alcoholic steatohepatitis (ASH) and cirrhosis (1–5). It is now accepted that steatohepatitis is required for development of cirrhosis. But, the mechanisms linking EtOH consumption to steatohepatitis are not fully established. Since only about 30% of alcoholics develop ALD (4), additional factors besides heavy EtOH consumption must be involved.
A most likely candidate is gut-derived endotoxin. Indeed, several recent clinical observations and experimental studies strongly suggest that endotoxin is the key cofactor (6–10). However, these studies have not: (a) ruled out the possibility that endotoxemia is a consequence of and not a cause of ASH, (b) identified the underlying mechanism causing the endotoxemia.
To address the first issue, the cause and effect question, we did a time course study to test the hypothesis that endotoxemia caused by chronic EtOH feeding precedes the development of ASH. To address the question of the mechanism of the endotoxemia, we tested the hypothesis that the endotoxemia is caused by alcohol-induced gut leakiness. We explored this mechanism because we recently showed that only alcoholics with ALD, and not those without ALD, had intestinal hyperpermeability (10). We also reported that oats supplementation prevented both gut leakiness and liver injury in alcohol fed rats (11). These findings suggest that gut leakiness is the mechanism causing the endotoxemia associated with ALD (11).
Equally unclear is the mechanism mediating EtOH-induced gut leakiness. Several lines of evidence suggest that the culprit is EtOH-induced tissue oxidative stress. First, oxidative stress has been postulated to be a key mechanism of alcohol-induced organ injury such as occurs in the CNS (12), pancreas (13), heart (14) and liver (15). Second, in vitro studies have shown that alcohol activates oxidative pathways including upregulation of iNOS (16–18). Third, our recent in vitro study showed that alcohol disrupts the barrier integrity of monolayers of intestinal cells and that alcohol-induced disruption is due to oxidative injury to the cytoskeleton (17). This mechanism needed to be investigated in vivo.
The goal of this study was to fill the above mentioned gaps in our knowledge by conducting a study to determine the time course for development of gut hyperpermeability, oxidative injury to the gut, endotoxemia, and liver injury during 10 weeks of daily alcohol gavage in rats.
Materials and Methods
Animal Subjects
Male Sprague-Dawley rats (250–300 g at intake) were obtained from Harlan (Indianapolis, IN). During experiments, each rat was given either alcohol or an isocaloric amount of dextrose in liquid rat chow intragastrically (by gavage; 2 cc) 2X daily. The dose was gradually increased every 2 to 3 days up to a maximum of 6 g/kg/day by 2 weeks. Study Day 1 was defined as the first day rats received 6 g/kg/day alcohol. Rats received chow ad libitum and were weighed daily. Intestinal permeability was measured just before sacrifice. Rats were sacrificed after 2, 4, 8 and 10 weeks of administration of 6 g/kg alcohol (see Table 1 for N in each group). Sacrifice was done by CO2 inhalation, followed immediately by cardiac puncture (for blood draw) and harvesting of intestine and liver.
Table 1.
Indices of hepatic inflammation & liver cell injury
| Group | Week 2 | Week 4 | Week 8 | Week 10 | Specific Liver Disease Criteria |
|---|---|---|---|---|---|
| Dextrose fed rats | 0 (7) | 0 (6) | 0 (7) | 0 (6) | Necroinflammatory Histology score |
| Alcohol fed rats | 0 (11) | 0 (12) | 2*(±0.2) (10) | 4*(±0.5 ) (6) | Necroinflammatory Histology score |
| Dextrose fed rats | 0.16 (±0.2) | 0.13 (±0.2) | 0.16 (±0.2) | 0.13 (±0.02) | Liver MPO (U/mg tissue) |
| Alcohol fed rats | 0.15 (± 0.2) | 0.26 (± 0.05) | 0.58* (± .07) | 2.37* (± 0.3) | Liver MPO (U/mg tissue) |
| Dextrose fed rats | 65.27 (± 7) | 65.27 (± 7) | 65.27 (± 7) | 65.27 (± 7) | Serum ALT (U/dl) |
| Alcohol fed rats | 90.74*(± 10) | 79.29* (± 8) | 100.84* (± 11) | 90.37* (± 10) | Serum ALT (U/dl) |
| Dextrose fed rats | 82.38 (± 9) | 82.38 (± 9) | 82.38 (± 9) | 82.38 (± 9) | Serum AP (U/dl) |
| Alcohol fed rats | 90.75 (±10) | 110.83 (±12) | 264.39* (± 27) | 220.47* (± 21) | Serum AP (U/dl) |
mean ± S.E.;
p ≤ 0.05 Alcohol fed rats vs. Dextrose fed rats; number in () represents number of rats in each group
All animal protocols and practices were reviewed and approved in advance by the Rush University Institutional Animal Care and Use Committee (IACUC) in accordance with guidelines set forth by the Office of Laboratory Animal Welfare, NIH, and the publications: U.S. Government Principles for the Utilization and Care of Vertebrate Animals used in Testing, Research, and Training, and the Guide for the Care and Use of Laboratory Animals. Every effort was made to minimize animal pain or discomfort.
Intestinal permeability
We used an oral sugar test to assess intestinal permeability as we described (11, 19, 20). After an 8 h fast, rats were given, intragastrically, 2.0 ml of a solution containing 107 mg/kg lactulose, 30 mg/kg mannitol, 15 mg/kg sucralose, and 570 mg/kg sucrose. Rats were housed individually in metabolic cages and urine samples were collected for 5h. To promote urine output, each rat was subcutaneously injected with 10 ml of lactated Ringer’s solution, just prior to sugar administration. Urinary sugar levels were measured by gas chromatography as we reported (19, 20).
Plasma Endotoxin
Blood was collected from rats at the time of sacrifice. Serum samples were then analyzed for endotoxin by kit (Kinetic-QLC; Whittaker Bioproducts).
Liver Injury
a. Liver Histology
Formalin fixed liver tissues were stained with H&E. Blinded assessment was done by a GI pathologist (SJ). Histological slides were assessed for liver disease by grading steatosis, necrosis, inflammation & fibrosis (11). Our scoring system is based on one widely used for the scoring of liver disease (21). Severity of steatosis (% of liver cells containing fat) was scored 1+ to 4+, corresponding to the fraction of liver cells with fat. These were, respectively, <25%, 26–50%, 51–75%, and >75%. Necrosis was quantified as the # of necrotic foci/mm2 and # of Councilman Bodies per HPF and severity of necrosis graded on a scale of 0–4. Severity of inflammation was graded on a scale of 0–4 based on the extent [# of inflammatory foci per HPF and # of HPF with inflammatory foci] and density of inflammatory foci (from a few inflammatory cells to dense inflammatory infiltrate). Severity of fibrosis was scored on a scale of 0 to 4 using trichrom staining. However, the fibrosis score in all rats was zero and thus fibrosis grade was not included in the histology score. A total histological score (including fatty liver grade) and necroinflammatory score (inflammation and necrosis score representing presence and severity of steatohepatitis) was then calculated. The maximum histology score and necroinflammatory score were 12 and 8 respectively. When assessing slides for pathology studies, at least 3 different sections were studied for each rat. Alcoholic steatohepatitis (ASH) was defined as the presence, in the liver, of inflammatory cell infiltration, spotty necrosis, and liver cell necrosis.
b. Liver Fat Content
Total liver fat was measured gravimetrically as previously described (22) and used by us (20).
c. Liver Myeloperoxidase Level
MPO is present in myeloid lineage cells such as neutrophils and has been reliably used to assess the presence and severity of inflammation (23). Liver MPO was measured using the Myeloperoxidase Assay Kit (Cytostore, Calgary, Alberta, Canada) and final values expressed as MPO units/mg tissue.
Serum alanine aminotransferase (ALT) and alkaline phosphatase (AP)
ALT & AP were measured in blinded serum samples by the Rush University Medical Center clinical laboratories and the data provided as Units means/dl ± S.E.
NO Levels in Intestinal Mucosa and Urine
Nitrite and nitrate in urine and tissue samples were measured (μM/ml or μM/mg) using the Nitrate/Nitrite Colorimetric Assay Kit (Cayman Chemical, Ann Arbor, MI).
Analysis of Inducible-Nitric Oxide Synthase (iNOS) Levels
Tissues were homogenized and samples (20 μg protein/lane) were separated by 7.5% SDS-PAGE and quantitated as described (17).
Quantitative Slot-Immunoblotting for oxidation (carbonylation) and nitration (nitrotyrosination)
Oxidation and nitration of mucosal proteins was assessed by measuring protein carbonyl and protein nitrotyrosine formation using a slot-blotting method we previously described (24, 25).
Data and Statistical Analysis
Data are presented as mean ± S.E. For parametric analyses of two groups, we used Student’s t-test; ANOVA was used when we compared multiple groups. Least Standard Deviation (LSD) was used for post-hoc analysis and a paired t-test for comparison of paired data such as intestinal permeability. p ≤ 0.05 was regarded as significant.
Results
Alcohol-fed rats gained significantly less weight than dextrose control fed rats (week 10 dextrose fed rats 395 ± 10g vs. alcohol-fed 365 ± 10g; p ≤ .05). Below we present the results as they relate to seven key questions this study addressed.
(i) Can chronic alcohol consumption disrupt intestinal permeability in rats?
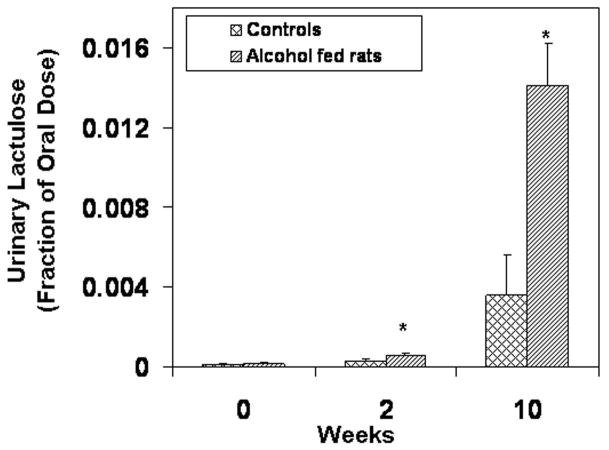
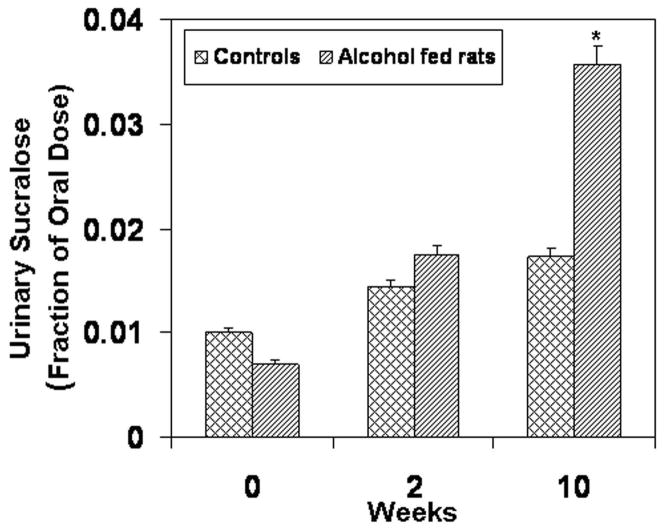
Daily feeding of alcohol to rats disrupted intestinal barrier function as early as 2 weeks after the start of 6 g/kg alcohol administration. Five hour urinary lactulose (small bowel permeability index) was significantly higher in alcohol-fed rats than in control rats as early as week 2 (Fig. 1a, p= 0.04). Urinary lactulose was also significantly higher in alcohol-fed rats than in dextrose fed rats at 10 weeks (p<0.015). Urinary sucralose (index of whole gut [small bowel + large bowel] permeability) was also increased in alcohol-fed rats and the difference was significant by week 10 (p≤0.05, Fig. 1b).
Figure 1.
Figure 1a. Intestinal Permeability Measured by Urinary Lactulose. Intestinal permeability was measured by urinary lactulose (Methods). Five-hour urinary lactulose (a marker of small bowel permeability) increased throughout the study in both groups. Lactulose levels in the alcohol-fed group were significantly greater (p<.05) than in the control group at weeks 2,4,6,8, and 10. Only weeks 2 and 10 are shown. Data are means of fraction of oral dose recovered (e.g. .016= 1.6% recovered) ± S.E. for N= 6 or more rats for each time point.
Figure 1b. Intestinal Permeability Measured by Urinary Sucralose. Five-hour urinary sucralose (a marker of whole gut permeability) increased throughout the study although sucralose levels in the alcohol-fed group were only significantly greater than in the control group at week 10. Data are means of fraction of oral dose recovered ± S.E. for N= 6 or more rats for each time point.
(ii) Can chronic alcohol consumption cause endotoxemia in rats?
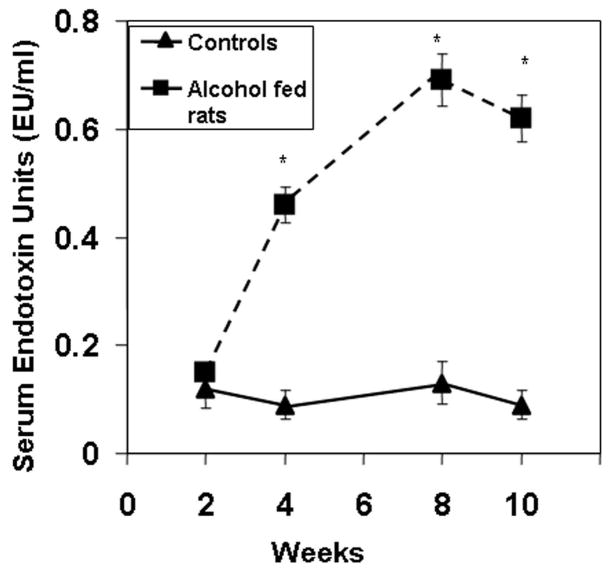
Endotoxin was assessed in serum obtained from blood at sacrifice. Serum endotoxin values remained low and unchanged throughout the study in dextrose fed rats. In contrast, daily alcohol feeding led to significant endotoxemia as early as 4 weeks. Serum endotoxin levels were even greater (~6 fold rise) at 8 and 10 weeks (Fig. 2, p≤.05).
Figure 2.
Serum Endotoxin Levels. Serum endotoxin was determined for control and alcohol fed rats at sacrifice at the indicated weeks as described in Methods. Serum endotoxin levels increased throughout the study. Levels were significantly higher in alcohol-fed rats as early as week 4. Data are expressed as mean endotoxin Units (EU) per ml serum ± S.E. for N=6 or more rats for each time point.
(iii) Can chronic alcohol consumption cause steatohepatitis (ASH) in rats?
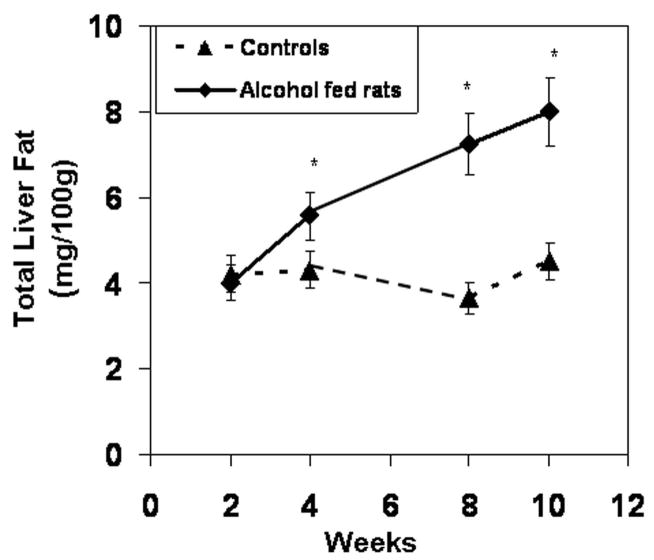
Daily feeding of alcohol to rats caused a progressive increase in liver fat content over the 10 weeks (Fig. 3). Steatosis was detectable histologically as early as 2 weeks (Fig. 4). In contrast, alcoholic steatohepatitis (ASH), defined by the presence of inflammatory cell infiltration, spotty necrosis and liver cell necrosis, was not seen histologically until week 8 (Table 1). The severity of ASH (defined by necroinflammatory score and liver MPO levels) was greatest at week 10 (Table 1) with the appearance of liver cell necrosis and Councilman Bodies (Fig. 4). The distribution of the inflammatory cell infiltrate and liver cell necrosis was always in the pericentral zone of the liver acini. Alcohol fed rats also had elevated serum transaminase (ALT) levels (Table 1) as early as 2 weeks. Daily alcohol feeding also caused elevation of serum alkaline phophatase after 4 weeks (Table 1) with markedly elevated serum levels after 8 weeks of alcohol feeding when the first signs of liver inflammation were noted histologically and remained elevated at 10 weeks.
Figure 3.
Total Liver Fat. Liver fat content was determined for both treatment groups after sacrifice (Methods). Liver fat content increased in alcohol-fed rats. It was significantly higher in alcohol-fed rats as early as week 4 and remained higher than controls thereafter. Data are means of total liver fat (mg fat/100gm liver tissue) ± S.E. for N=6 or more rats for each time point.
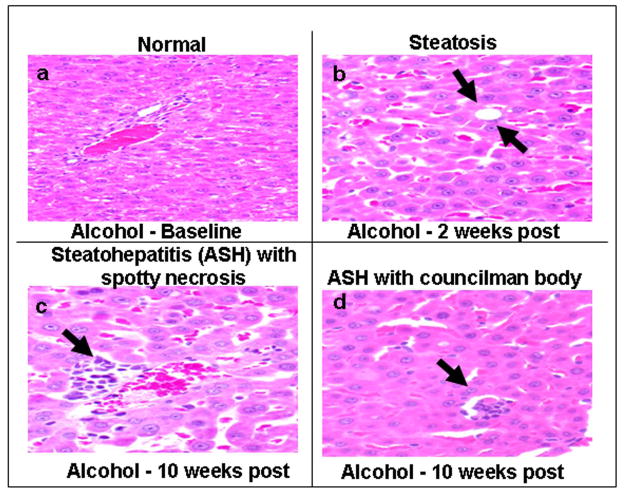
Figure 4.
Histological Analysis of Liver Tissue. Liver tissue obtained at sacrifice was fixed and stained with H&E (Methods) and representative samples are shown. Panel a shows a normal liver section from a control rat. Panel b shows that macro and micro vesicular steatosis (arrows) in the liver is observed as early as week 2 in alcohol-fed rats although not significantly different until week 4 (Fig. 4). Panel c shows inflammatory cells and spotty necrosis (arrow) in the liver in alcohol-fed rats at week 10. Panel d shows a necrotic hepatocyte ‘Councilman’s Body’ (arrow) characteristic of alcoholic cirrhosis (ALD) in alcohol fed rats at 10 weeks.
(iv) Is endotoxemia involved in development of alcoholic steatohepatitis?
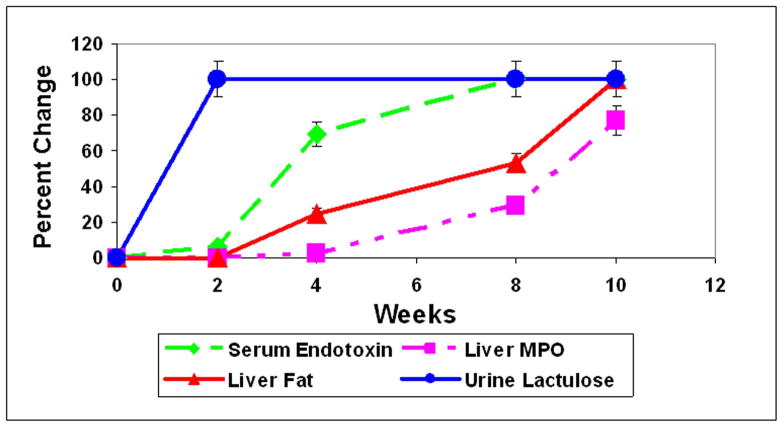
Our summarized time course data (Fig. 5) show that endotoxemia (4 weeks) occurred prior to development of steatohepatitis (MPO, 8 weeks) and thus it is highly unlikely that endotoxemia is the consequence of liver inflammation. Instead, our data support the model in which endotoxemia is involved in initiation of the hepatic necroinflammatory cascade and development of alcoholic steatohepatitis (ASH).
Figure 5.
Time course summary for permeability (lactulose), endotoxemia, liver fat, and liver MPO in alcohol fed rats. Values for urinary lactulose, serum endotoxin, liver fat, and liver MPO were determined as described (Methods) and significance has been shown for specific data in previous figures. Data are presented as percent change ± S.E. for weeks 2, 4, 8, and 10.
(v) Is gut leakiness contributing to alcohol-induced endotoxemia?
Statistically significant small bowel leakiness (increased urinary lactulose; Fig. 1) occurred 2 weeks after daily alcohol gavage, preceding endotoxemia that occurred after 4 weeks of alcohol exposure. Colonic leakiness occurred after additional weeks of alcohol exposure (increased urinary sucralose representing both small bowel and colon leakiness), corresponding to more severe endotoxemia (Fig. 2). These findings suggest that gut leakiness may be the mechanism, at least in part, of endotoxemia in alcohol fed rats.
(vi) What is the mechanism of alcohol-induced gut leakiness?
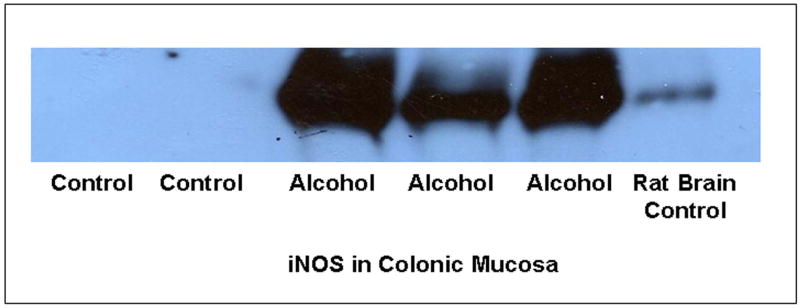
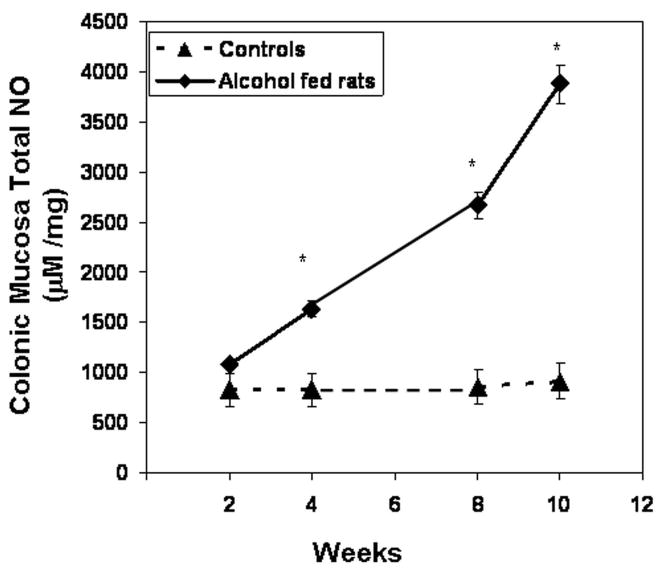
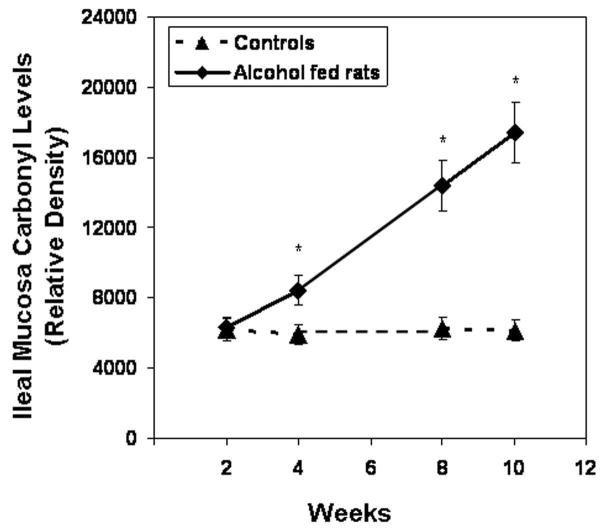
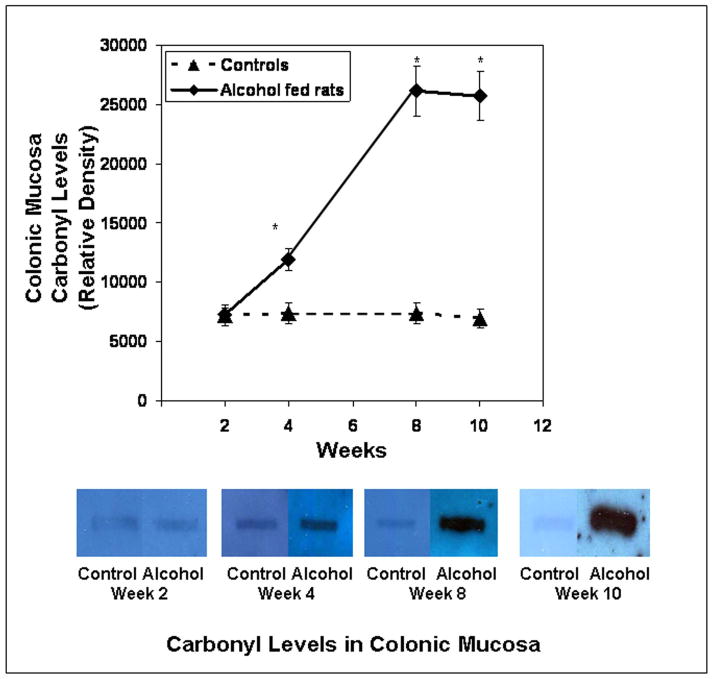
We found that daily alcohol feeding causes oxidative stress as indicated by 9-fold levels of total urinary NO (NO2+NO3)(week 10 control 92 ± 9μM vs. alcohol-fed 857 ± 92μM; p ≤ .05). To determine the source of alcohol-induced tissue oxidative stress, we measured iNOS protein in the colonic mucosa. Daily alcohol feeding upregulated mucosal iNOS (Fig. 6, p < 0.05) in alcohol fed rats. Mucosal iNOS increased to 400% of control values. This increase was associated with increases in intestinal mucosal total NO, the reaction product of this enzyme (Fig. 7), More importantly, chronic alcohol consumption caused intestinal mucosal oxidative stress with markedly elevated tissue protein nitration (nitrotyrosine levels; Fig. 8) and protein oxidation (protein carbonyl levels; Fig. 9) in jejunal, ileal and colonic mucosa of alcohol-fed rats.
Figure 6.
Colonic mucosa iNOS protein. Expression of iNOS protein (130 kDa) was determined by western blotting in control and alcohol fed rat colonic mucosa (Methods). Equal amounts of protein (50μg) were loaded per lane except the lane 6 iNOS positive control (10μg) from rat brain (Cayman Chemical). Blot is representative of more than three experiments.
Figure 7.
Colonic mucosa total NO. Total NO (NO2 + NO3) was determined from colonic mucosa tissue from control and alcohol fed rats after sacrifice (Methods). Data are means of Total NO (μM/mg) ± S.E. for N=6 or more rats for each time point.
Figure 8.
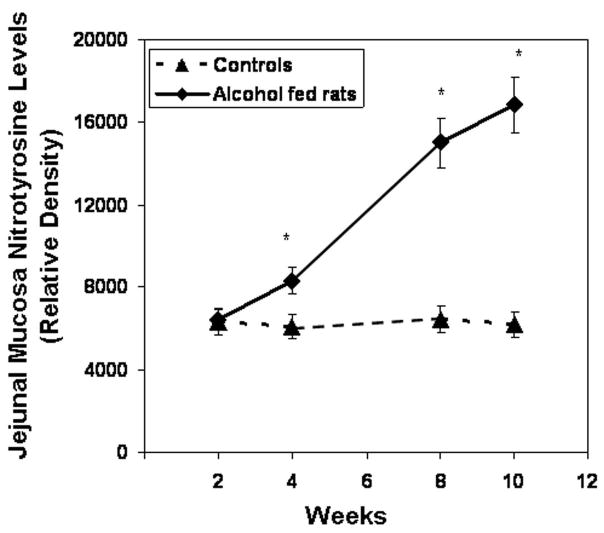
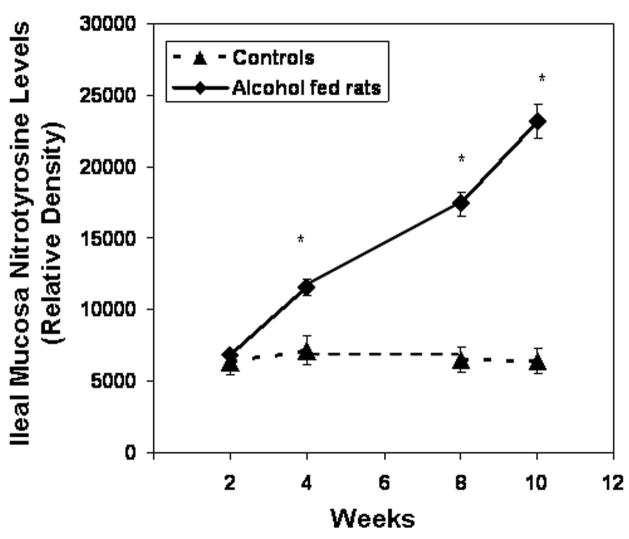
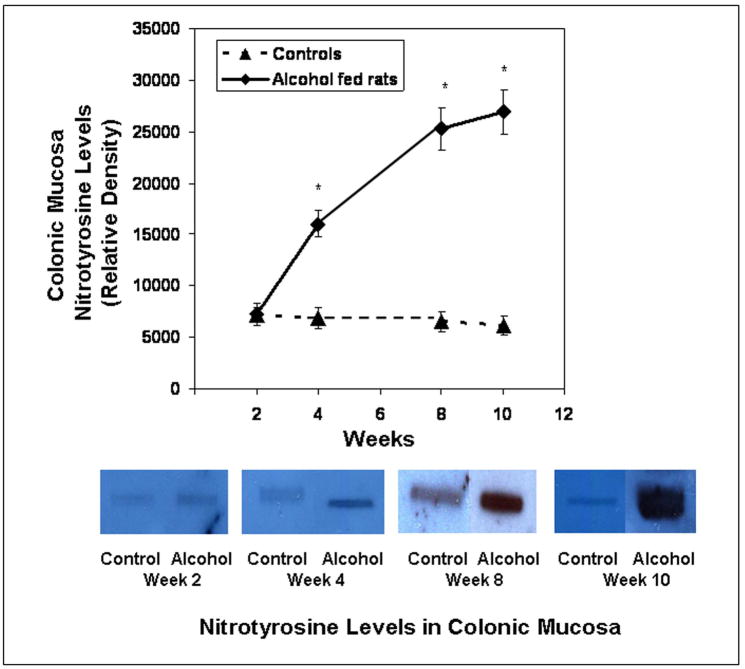
Slot blot and densitometry measurement of nitrotyrosine levels in intestinal tissue. Levels of the oxidative stress marker nitrotyrosine epitope were determined by slot blot and quantitative densitometry in tissue samples obtained after sacrifice from Ileum (Fig. 8a), jejunum (Fig. 8b), and colonic (Fig. 8c) mucosa (Methods). Data shown are means of relative densities ± S.E. for N=6 or more rats and N=3 blots for each time point. Additionally, for colonic mucosa representative slot blot data images are shown (Fig. 8c).
Figure 9.
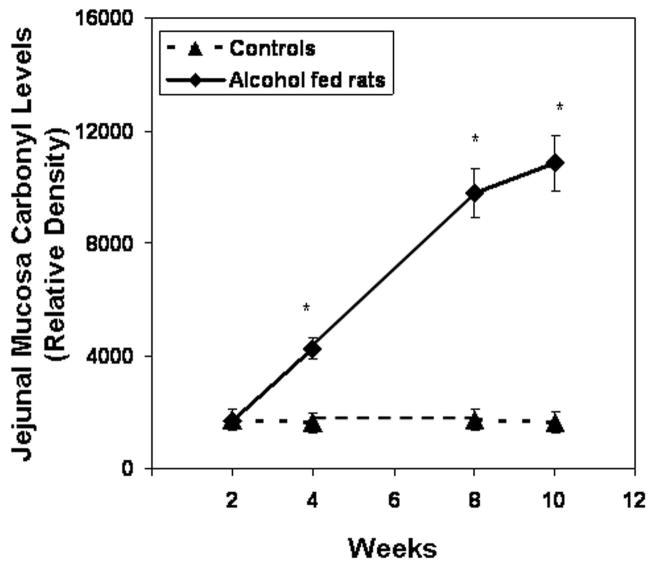
Slot blot and densitometry measurement of carbonyl levels in intestinal tissue. Levels of the oxidative stress marker carbonyl epitope were determined by slot blot and quantitative densitometry in tissue samples obtained after sacrifice from Ileum (Fig. 9a), jejunum (Fig. 9b), and colonic (Fig. 9c) mucosa (Methods). Data shown are means of relative densities ± S.E. for N=6 or more for each time point. Additionally, for colonic mucosa representative slot blot data images are shown (Fig. 9c).
(vii) Is oxidative stress involved in alcohol-induced gut leakiness?
Statistically significant small bowel leakiness (increased urinary lactulose) occurred 2 weeks after daily alcohol gavage, preceding both intestinal oxidative stress and endotoxemia that occurred after 4 weeks of alcohol exposure. Thus, our time course experiments are consistent with the idea that alcohol-induced oxidative stress might contribute, at least in part, to development of the gut leakiness in alcohol-fed rats.
Discussion
Several studies have provided evidence that gut-derived bacterial products are the necessary cofactors for development of ALD in a minority of alcoholics (6–10). For example, alcoholics with ALD have high serum endotoxin levels, and serum endotoxin levels correlate with ALD severity (26). Peripheral monocytes from ALD patients are primed for producing cytokines and oxidants (reactive oxygen and nitrogen species) and this priming seems to be due to endotoxin (27–29). Still others showed that alcohol fed rats with liver damage have high endotoxin levels in the portal vein, and there is a strong correlation between endotoxin levels and the severity of liver damage in these rats (30).
Indeed, it is well-established that alcohol exerts its damaging effect on the liver in synergy with endotoxin. For example, neither alcohol alone nor endotoxin alone (at least not low endotoxin levels) cause severe liver injury, but a combination of these two agents was sufficient to cause significant liver damage. Endotoxin can prime and activate macrophages (Kupffer cells) in rats chronically exposed to EtOH so that they overproduce cytokines such as TNF-α, IL-6, and IL-8 (8, 31). These cytokines not only injure hepatocytes directly, but also, they can initiate a hepatic necroinflammatory cascade, which includes migration of leukocytes, including neutrophils, into the liver (15, 31). These leukocytes produce injurious products, especially oxidants resulting from nitric oxide (16) such as peroxynitrite, which can cause liver cell necrosis. The EtOH-endotoxin synergy, along with other direct metabolic effects of EtOH on the liver (e.g., hypoxia, perturbation of NO-dependent pathways), not only can initiate liver injury, but also, can create a vicious circle that sustains a chronic necroinflammatory process and hastens the onset of liver failure.
Nevertheless, these studies have not ruled out the possibility that endotoxemia is a consequence of and not a prerequisite for ALD. The finding that lowering serum endotoxin level by oral administration of non-absorbable antibiotics (7) or lactobacillus (6) attenuates alcohol-induced liver damage in alcohol fed rats support the idea that endotoxin is key for promoting severe liver injury but does not provide direct evidence for endotoxin as a trigger for ASH. Our time course study, for the first time, demonstrates that endotoxemia precedes ASH and thus supports the view that endotoxin is not the consequence of liver disease and could act as a trigger for development of ASH. Our finding that fatty liver occurs early and before significant endotoxemia supports the hypothesis that simple steatosis is primarily due to the direct effects of alcohol on hepatic lipid metabolism and is not dependent on endotoxin (32). Unlike steatosis, steatohepatitis appears to be dependent on endotoxin. Although the mechanism of endotoxemia in alcoholics is not fully established, it is clear that the source of the endotoxin is the gut flora. Endotoxin, which is generated by bacteria in the gut lumen, permeates into the portal circulation and then travels to the liver where it is usually taken up and eliminated by Kupffer cells. Hence, abnormally high blood endotoxin levels (endotoxemia) could be due to: (i) increased production of endotoxin by small bowel bacterial overgrowth or abnormal gut flora (dysbiosis), (ii) increased permeation of endotoxin through the intestinal wall (gut leakiness), (iii) reduced liver clearance due to portal blood shunting that occurs in advanced liver disease and portal hypertension, and/or (iv) reduced endotoxin clearance due to defective liver Kupffer cell function. Gut leakiness may have a particularly large effect because it can potentiate the other three mechanisms. This could happen because integrity of the intestinal barrier is a gate keeper. Changes in the integrity of the intestinal barrier can blunt or accentuate the effect of the other possible mechanisms of endotoxemia.
Both small bowel and colonic bacteria can be the source of endotoxemia. Thus, it is important to assess both small bowel and colonic permeability to determine whether gut leakiness is the source of endotoxemia. Unfortunately, there is no universally accepted method to assess in vivo intestinal permeability. But, use of poorly absorbed sugars to assess small and large bowel permeability has now been validated in several studies in both healthy and disease states (33, 34). Mannitol (M) and lactulose (L) are poorly absorbed in the small bowel and their concentration in the urine (or L/M ratio) is a valid marker of small bowel leakiness. Since both lactulose and mannitol are extensively metabolized by colonic bacteria, they are not appropriate substrates to assess colonic permeability. In contrast, a poorly absorbed sugar, sucralose, is not metabolized by bacteria and thus is an appropriate marker for assessing total gut (small bowel and colon) permeability (35).
We used the sugar test to address the question whether gut leakiness is involved in alcohol-induced endotoxemia and if so, where is the site of leakiness in the gut. We now report that gut leakiness indeed occurs after daily alcohol feeding and the time course of gut leakiness and endotoxemia suggests that gut leakiness is, at least in part, responsible for endotoxemia in alcohol fed rats. Our current finding confirms our prior observation in alcoholic subjects (10) and alcohol-fed rats (11). Gut leakiness as the source of endotoxemia in alcoholics was also suggested by others (15). Indeed, other reports show that alcohol consumption substantially disturbs intestinal mucosal structure and function in both man and animals (18, 36–39). Our time course study provides yet another and more direct evidence for the importance of gut leakiness in alcohol-induced endotoxemia. We also report, for the first time, that alcohol disrupts barrier function throughout the gut. The deleterious effect of alcohol on small bowel barrier function (increased urinary lactulose) appears to be more rapid and occurs after only 2 weeks of exposure. In contrast, longer exposure to daily alcohol is required for disruption of colonic barrier (increased urinary sucralose). It is interesting that the magnitude of endotoxemia in the first 4 weeks of alcohol feeding, when the small bowel was leaky, was modest; while endotoxemia was more severe in the second month (4–8 weeks) of alcohol exposure when total gut leakiness was present. This finding is compatible with permeation of the high endotoxin content of the colon. But, the contribution of defective clearance of endotoxin by an injured liver should also be considered. Further studies are needed to determine the mechanism for the differences of time courses between disruption of small bowel and colonic barrier function.
How alcohol causes gut leakiness is not yet established. The integrity of intestinal barrier function depends on both healthy epithelial cells and an intact paracellular pathway, which appears to be the main route for permeation of macromolecules such as endotoxin (33). Oxidative injury to key proteins regulating this paracellular pathway is a plausible mechanism because oxidative stress is responsible for alcohol-induced tissue injury and organ failure (40–42). More specifically, alcohol increases oxidative stress and we (16, 43, 44) and others (45, 46) have shown that oxidative stress disrupts the monolayer barrier. Our data now support the key contribution of tissue oxidative injury in alcohol-induced gut leakiness. Our data also suggest that alcohol–induced oxidative injury to the gut is a result of upregulation of iNOS. Upregulation of iNOS causes overproduction of NO and then peroxynitrite and superoxide. NO can generate superoxide via a process called NOS uncoupling that refers to uncoupling of NADPH oxidation as well as by disruption of cytochrome C function (47, 48). Eventually peroxynitrite and superoxide anion cause nitration and carbonylation of key proteins responsible for integrity of barrier resulting in gut leakiness. Our in vitro studies (16, 17, 49) suggest the following cascade of events: alcohol activates NF-kB resulting in upregulation of iNOS. Increased iNOS increases NO production and high levels of peroxynitrite and superoxide resulting in nitration and carbonylation of tight junctional and cytoskeletal proteins. This oxidative damage to these key proteins disrupts integrity of intestinal barrier. Our rat model can not differentiate between luminal versus bloodstream effects of alcohol on the gut. But, since both apical and basolaterally applied alcohol promote monolayer hyperpermeability, one can conclude that both local and the systemic effects of alcohol could be involved in gut leakiness.
In summary, our findings provide the first evidence that alcohol-mediated gut leakiness and endotoxemia precede the development of alcoholic liver disease and thus might be the necessary trigger for development of ASH. Further studies are needed to determine whether inhibition of iNOS upregulation in alcohol fed rats prevents gut leakiness, endotoxemia and steatohepatitis. Controlled clinical trials in alcoholics to see if interventions that prevent gut leakiness and endotoxemia prevent ALD must wait until an effective intervention to prevent gut leakiness is developed. We acknowledge that the best approach to prevent ALD is abstinence but unfortunately the long term success of maintaining sobriety is very poor. Thus, alternative approaches such as preventing gut leakiness would still have a positive impact in patients’ quality of life of sober alcoholics at risk for or suffering from ALD.
Acknowledgments
This study was supported by NIH grant AA13745 (AK).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Maher JJ. Alcoholic steatosis and steatohepatitis. Semin Gastrointest Dis. 2002;13:31–39. [PubMed] [Google Scholar]
- 2.Burbige EJ, Lewis DR, Jr, Halsted CH. Alcohol and the gastrointestinal tract. Med Clin North Am. 1984;68:77–89. doi: 10.1016/s0025-7125(16)31242-1. [DOI] [PubMed] [Google Scholar]
- 3.Galambos JT. Alcoholic hepatitis: its therapy and prognosis. Prog Liver Dis. 1972;4:567–588. [PubMed] [Google Scholar]
- 4.Grant BF, Dufour MC, Harford TC. Epidemiology of alcoholic liver disease. Semin Liver Dis. 1988;8:12–25. doi: 10.1055/s-2008-1040525. [DOI] [PubMed] [Google Scholar]
- 5.Maher JJ. Treatment of alcoholic hepatitis. J Gastroenterol Hepatol. 2002;17:448–455. doi: 10.1046/j.1440-1746.2002.02722.x. [DOI] [PubMed] [Google Scholar]
- 6.Nanji AA, Khettry U, Sadrzadeh SM. Lactobacillus feeding reduces endotoxemia and severity of experimental alcoholic liver (disease) Proc Soc Exp Biol Med. 1994;205:243–247. doi: 10.3181/00379727-205-43703. [DOI] [PubMed] [Google Scholar]
- 7.Adachi Y, Moore LE, Bradford BU, Gao W, Thurman RG. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995;108:218–224. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 8.Bhagwandeen BS, Apte M, Manwarring L, Dickeson J. Endotoxin induced hepatic necrosis in rats on an alcohol diet. J Pathol. 1987;152:47–53. doi: 10.1002/path.1711520107. [DOI] [PubMed] [Google Scholar]
- 9.Bode C, Kugler V, Bode JC. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J Hepatol. 1987;4:8–14. doi: 10.1016/s0168-8278(87)80003-x. [DOI] [PubMed] [Google Scholar]
- 10.Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol. 1999;94:200–207. doi: 10.1111/j.1572-0241.1999.00797.x. [DOI] [PubMed] [Google Scholar]
- 11.Keshavarzian A, Choudhary S, Holmes EW, Yong S, Banan A, Jakate S, et al. Preventing gut leakiness by oats supplementation ameliorates alcohol-induced liver damage in rats. J Pharmacol Exp Ther. 2001;299:442–448. [PubMed] [Google Scholar]
- 12.Davis RL, Dertien J, Syapin PJ. Ethanol-induced modulation of inducible nitric-oxide synthase activity in human A172 astrocytoma cells. Alcohol Clin Exp Res. 2002;26:1404–1411. doi: 10.1097/01.ALC.0000030841.92766.80. [DOI] [PubMed] [Google Scholar]
- 13.Chowdhury P, Gupta P. Pathophysiology of alcoholic pancreatitis: an overview. World J Gastroenterol. 2006;12:7421–7427. doi: 10.3748/wjg.v12.i46.7421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liedtke AJ, DeMuth WE. Effects of alcohol on cardiovascular performance after experimental nonpenetrating chest trauma. Am J Cardiol. 1975;35:243–250. doi: 10.1016/0002-9149(75)90008-9. [DOI] [PubMed] [Google Scholar]
- 15.Lumeng L, Crabb DW. Alcoholic liver disease. Curr Opin Gastroenterol. 2000;16:208–218. doi: 10.1097/00001574-200005000-00003. [DOI] [PubMed] [Google Scholar]
- 16.Banan A, Choudhary S, Zhang Y, Fields JZ, Keshavarzian A. Ethanol-induced barrier dysfunction and its prevention by growth factors in human intestinal monolayers: evidence for oxidative and cytoskeletal mechanisms. J Pharmacol Exp Ther. 1999;291:1075–1085. [PubMed] [Google Scholar]
- 17.Banan A, Fields JZ, Decker H, Zhang Y, Keshavarzian A. Nitric oxide and its metabolites mediate ethanol-induced microtubule disruption and intestinal barrier dysfunction. J Pharmacol Exp Ther. 2000;294:997–1008. [PubMed] [Google Scholar]
- 18.Draper LR, Gyure LA, Hall JG, Robertson D. Effect of alcohol on the integrity of the intestinal epithelium. Gut. 1983;24:399–404. doi: 10.1136/gut.24.5.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farhadi A, Keshavarzian A, Fields JZ, Sheikh M, Banan A. Resolution of common dietary sugars from probe sugars for test of intestinal permeability using capillary column gas chromatography. J Chromatogr B Analyt Technol Biomed Life Sci. 2006;836:63–68. doi: 10.1016/j.jchromb.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 20.Farhadi A, Keshavarzian A, Holmes EW, Fields J, Zhang L, Banan A. Gas chromatographic method for detection of urinary sucralose: application to the assessment of intestinal permeability. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;784:145–154. doi: 10.1016/s1570-0232(02)00787-0. [DOI] [PubMed] [Google Scholar]
- 21.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 22.Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 23.Malle E, Furtmuller PG, Sattler W, Obinger C. Myeloperoxidase: a target for new drug development? Br J Pharmacol. 2007;152:838–854. doi: 10.1038/sj.bjp.0707358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Banan A, Zhang LJ, Shaikh M, Fields JZ, Farhadi A, Keshavarzian A. Novel effect of NF-kappaB activation: carbonylation and nitration injury to cytoskeleton and disruption of monolayer barrier in intestinal epithelium. Am J Physiol Cell Physiol. 2004;287:C1139–C1151. doi: 10.1152/ajpcell.00146.2004. [DOI] [PubMed] [Google Scholar]
- 25.Keshavarzian A, Banan A, Farhadi A, Komanduri S, Mutlu E, Zhang Y, et al. Increases in free radicals and cytoskeletal protein oxidation and nitration in the colon of patients with inflammatory bowel disease. Gut. 2003;52:720–728. doi: 10.1136/gut.52.5.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bigatello LM, Broitman SA, Fattori L, Di Paoli M, Pontello M, Bevilacqua G, et al. Endotoxemia, encephalopathy, and mortality in cirrhotic patients. Am J Gastroenterol. 1987;82:11–15. [PubMed] [Google Scholar]
- 27.Criado-Jimenez M, Rivas-Cabanero L, Martin-Oterino JA, Lopez-Novoa JM, Sanchez-Rodriguez A. Nitric oxide production by mononuclear leukocytes in alcoholic cirrhosis. J Mol Med. 1995;73:31–33. doi: 10.1007/BF00203616. [DOI] [PubMed] [Google Scholar]
- 28.Hunt NC, Goldin RD. Nitric oxide production by monocytes in alcoholic liver disease. J Hepatol. 1992;14:146–150. doi: 10.1016/0168-8278(92)90150-n. [DOI] [PubMed] [Google Scholar]
- 29.McClain CJ, Cohen DA. Increased tumor necrosis factor production by monocytes in alcoholic hepatitis. Hepatology. 1989;9:349–351. doi: 10.1002/hep.1840090302. [DOI] [PubMed] [Google Scholar]
- 30.Nanji AA, Khettry U, Sadrzadeh SM, Yamanaka T. Severity of liver injury in experimental alcoholic liver disease. Correlation with plasma endotoxin, prostaglandin E2, leukotriene B4, and thromboxane B2. Am J Pathol. 1993;142:367–373. [PMC free article] [PubMed] [Google Scholar]
- 31.Hill DSS, McClain C, Diehl A, Tsukamoto H. Cytokines and Liver Disease. Marcel Dekker Ltd.; 1997. pp. 401–425. [Google Scholar]
- 32.Hall PD. Pathological spectrum of alcoholic liver disease. Alcohol Alcohol Suppl. 1994;2:303–313. [PubMed] [Google Scholar]
- 33.Hollander D. The intestinal permeability barrier. A hypothesis as to its regulation and involvement in Crohn’s disease. Scand J Gastroenterol. 1992;27:721–726. doi: 10.3109/00365529209011172. [DOI] [PubMed] [Google Scholar]
- 34.Menzies IS, Laker MF, Pounder R, Bull J, Heyer S, Wheeler PG, et al. Abnormal intestinal permeability to sugars in villous atrophy. Lancet. 1979;2:1107–1109. doi: 10.1016/s0140-6736(79)92507-8. [DOI] [PubMed] [Google Scholar]
- 35.Hansen J, Cherwitz DL, Allen JI. The role of tumor necrosis factor-alpha in acute endotoxin-induced hepatotoxicity in ethanol-fed rats. Hepatology. 1994;20:461–474. [PubMed] [Google Scholar]
- 36.Rubin E, Rybak BJ, Lindenbaum J, Gerson CD, Walker G, Lieber CS. Ultrastructural changes in the small intestine induced by ethanol. Gastroenterology. 1972;63:801–814. [PubMed] [Google Scholar]
- 37.Lindenbaum J, Lieber CS. Effects of chronic ethanol administration on intestinal absorption in man in the absence of nutritional deficiency. Ann N Y Acad Sci. 1975;252:228–234. doi: 10.1111/j.1749-6632.1975.tb19161.x. [DOI] [PubMed] [Google Scholar]
- 38.Keshavarzian A, Iber FL, Dangleis MD, Cornish R. Intestinal-transit and lactose intolerance in chronic alcoholics. Am J Clin Nutr. 1986;44:70–76. doi: 10.1093/ajcn/44.1.70. [DOI] [PubMed] [Google Scholar]
- 39.Bode JC, Knuppel H, Schwerk W, Lorenz-Meyer H, Durr HK. Quantitative histomorphometric study of the jejunal mucosa in chronic alcoholics. Digestion. 1982;23:265–270. doi: 10.1159/000198760. [DOI] [PubMed] [Google Scholar]
- 40.Ishii H. Oxidative stress in alcoholic liver injury. Alcohol Clin Exp Res. 1996;20:162A–167A. doi: 10.1111/j.1530-0277.1996.tb01768.x. [DOI] [PubMed] [Google Scholar]
- 41.Kurose I, Higuchi H, Kato S, Miura S, Ishii H. Ethanol-induced oxidative stress in the liver. Alcohol Clin Exp Res. 1996;20:77A–85A. doi: 10.1111/j.1530-0277.1996.tb01736.x. [DOI] [PubMed] [Google Scholar]
- 42.Reuben A. Alcohol and the liver. Curr Opin Gastroenterol. 2007;23:283–291. doi: 10.1097/MOG.0b013e3280f27582. [DOI] [PubMed] [Google Scholar]
- 43.Banan A, Choudhary S, Zhang Y, Fields JZ, Keshavarzian A. Oxidant-induced intestinal barrier disruption and its prevention by growth factors in a human colonic cell line: role of the microtubule cytoskeleton. Free Radic Biol Med. 2000;28:727–738. doi: 10.1016/s0891-5849(00)00160-x. [DOI] [PubMed] [Google Scholar]
- 44.Banan A, Fields JZ, Zhang Y, Keshavarzian A. iNOS upregulation mediates oxidant-induced disruption of F-actin and barrier of intestinal monolayers. Am J Physiol Gastrointest Liver Physiol. 2001;280:G1234–G1246. doi: 10.1152/ajpgi.2001.280.6.G1234. [DOI] [PubMed] [Google Scholar]
- 45.Rao RK, Baker RD, Baker SS, Gupta A, Holycross M. Oxidant-induced disruption of intestinal epithelial barrier function: role of protein tyrosine phosphorylation. Am J Physiol. 1997;273:G812–G823. doi: 10.1152/ajpgi.1997.273.4.G812. [DOI] [PubMed] [Google Scholar]
- 46.Rao RK, Basuroy S, Rao VU, Karnaky KJ, Jr, Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J. 2002;368:471–481. doi: 10.1042/BJ20011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moncada S, Erusalimsky JD. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat Rev Mol Cell Biol. 2002;3:214–220. doi: 10.1038/nrm762. [DOI] [PubMed] [Google Scholar]
- 48.Antunes F, Boveris A, Cadenas E. On the mechanism and biology of cytochrome oxidase inhibition by nitric oxide. Proc Natl Acad Sci U S A. 2004;101:16774–16779. doi: 10.1073/pnas.0405368101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Banan A, Keshavarzian A, Zhang L, Shaikh M, Forsyth CB, Tang Y, et al. NF-kappaB activation as a key mechanism in ethanol-induced disruption of the F-actin cytoskeleton and monolayer barrier integrity in intestinal epithelium. Alcohol. 2007;41:447–460. doi: 10.1016/j.alcohol.2007.07.003. [DOI] [PubMed] [Google Scholar]