Abstract
While the presence of abnormal late gadolinium enhancement (LGE) in cardiac amyloidosis has been well established, its prognostic implication and utility to identify cardiac involvement in patients with systemic amyloidosis is unknown. We sought to assess the diagnostic and prognostic significance of cardiovascular magnetic resonance (CMR) imaging in patients with light chain (AL) amyloidosis but unknown cardiac involvement. CMR with LGE was performed in 28 patients with systemic amyloidosis. The presence of cardiac amyloidosis was determined by a separate clinical evaluation. The performance of LGE for the prediction of cardiac amyloidosis and prognostic implications of LGE were determined. LGE was observed in 19 (68%) patients. The sensitivity, specificity, positive predictive value and negative predictive value of LGE for the identification of clinical cardiac involvement was 86%, 86%, 95%, and 67% respectively. During a median follow-up of 29 months, there were 5 deaths (82% survival). LGE itself did not predict survival (p=0.62). LGE volume positively correlated to serum level of B-type natriuretic peptide (BNP) (R=0.64, p≤0.001) and in multivariable analysis, LGE volume proved the strongest independent predictor of BNP. BNP was correlated to New York Heart Association class (p=0.03). Reduced right ventricular end-diastolic volume (p < 0.01) and stroke volume (p = 0.02) were associated with mortality. In conclusion, in patients with systemic amyloidosis, LGE is highly sensitive and specific for the identification of cardiac involvement, but does not predict survival. LGE does correlate strongly to heart failure severity as assessed by BNP.
Keywords: Amyloidosis, Cardiovascular magnetic resonance imaging, Cardiomyopathy, Congestive Heart Failure
Introduction
Cardiovascular magnetic resonance (CMR) imaging is a powerful non-invasive tool to identify cardiac amyloidosis because it is a volumetric technique that accurately measures chamber dimensions, volumes, function, and mass while affording the ability to characterize intrinsic interstitial abnormalities through the means of late gadolinium enhancement (LGE) CMR.1 Initial applications of CMR to cardiac amyloidosis described intrinsic abnormalities in myocardial tissue contrast.2,3 More recently, abnormalities in LGE have been reported in patients with cardiac amyloidosis that were associated with features of advanced disease such as increased left ventricular (LV) mass.4–6 Prior published studies have only recruited subjects with known cardiac amyloidosis, leaving undefined the role of LGE in the evaluation of patients with systemic amyloidosis but unknown cardiac involvement. Furthermore, the association of abnormal LGE with clinical outcomes is unknown. We sought to assess the diagnostic and prognostic role of LGE in patients with systemic light chain (AL) amyloidosis.
Methods
We recruited 28 patients (20 male; age 62 ± 11 years) with systemic light-chain amyloidosis referred to the Boston University Amyloid Treatment and Research Program. Clinical data and CMR were collected following written informed consent as approved by the Institutional Review Board of Boston University Medical Center. All patients had biopsy-proven amyloid disease and a plasma cell dyscrasia as determined by bone marrow biopsy and hematologic consultation. All patients underwent a comprehensive clinical evaluation including echocardiography and electrocardiography. Subjects were followed for a median of 29 months (range 5 months to 3 years) following initial evaluation and CMR.
The ultimate determination of cardiac amyloid involvement was made by endomyocardial biopsy (n = 6, 29%) or by clinical criteria including all of the following consistent with previously published studies7: echocardiographic findings of increased wall thickness > 12 mm, symptoms of congestive heart failure (New York Heart Association class 2 or greater), and low electrocardiographic voltage (≤0.5 mV in the limb leads). None of the patients had a history of hypertension (with the exception of one who was included by virtue of a positive cardiac biopsy).
Transthoracic two-dimensional and Doppler echocardiograms (Sonos 5500 and/or iE 33, Philips Medical Systems, Andover, MA) were analyzed on a commercial workstation (XCelera, Philips Medical Systems, Andover, MA). Mean left ventricular (LV) wall thickness was measured from the anteroseptal and posterior wall measurements taken from the parasternal long-axis view. Electrocardiographic voltage was determined by the mean of the measurements of the absolute magnitude of the QRS complex in limb leads I, II, and III. All echocardiograms were interpreted by readers blinded to clinical and biopsy information.
CMR was performed on a 1.5 Tesla CMR system (Philips Gyroscan ACS NT). Ventricular volumes and function were assessed using an electrocardiographic-gated, breath-hold, steady-state free precession technique with contiguous short-axis slices obtained from the base to the apex in serial 8–10 second breath-holds. The following scan parameters were applied: slice thickness 10 mm, gap of 0 mm, field-of-view 320 mm, repetition time 3.2 ms, echo time 1.6 ms, temporal resolution 30 ms, spatial resolution 2 mm × 2 mm in-plane, flip angle 50 degrees. LGE-CMR was acquired using a 2-dimensional, spoiled, segmented inversion recovery, gradient-echo sequence approximately 15 minutes after an intravenous injection of 0.2 mmol/kg gadopentatate dimeglumine (Magnevist, Schering AG, Berlin, Germany). Inversion time was selected using a standardized algorithm based myocardial and blood T1 values and dependent upon subject heart-rate, time of imaging from contrast injection, and dose of contrast administered8. LGE was performed with serial breath-holds in the contiguous short-axis plane matched spatially to the cine images. Scan parameters include: slice thickness 10 mm, trigger delay mid-diastole, field of view 320 mm, flip angle 15 degrees, repetition time 3.6 ms, echo time 1.2 ms, spatial resolution 2 mm × 2 mm, turbo field-echo factor 32, number of averages 2. In addition, a variable inversion time sequence (Look-Locker9) was obtained in selected amyloid patients (n = 4) to demonstrate the efficacy of myocardial signal suppression.
Measurement of creatinine clearance identified 3 patients with creatinine clearance < 30 ml/min/1.73 m2 and 3 patients with creatinine clearance between 30 – 60 ml/min/1.73 m2. Four of the six were scanned prior to 2005, and two of the six scanned in 2006.
All CMR analyses were performed and verified by two observers blinded to clinical or echocardiographic data. Biventricular volumes, mass, and function were analyzed with a commercial workstation (QMass MR, Medis Software, Leesburg, VA, USA). Endo- and epicardial contours were manually traced. The presence and quantification (in grams of myocardium) of LGE was determined by a semi-automated technique using a signal intensity threshold of 6 standard deviations above that of myocardium that demonstrated the lowest signal intensity (effectively nulled). Numerous lower signal intensity thresholds were tested (data not shown), but 6-standard deviations provided the highest reproducibility and least variability. The presence or absence of LGE was treated as a categorical variable as well as a continuous variable (LGE volume in grams, and LGE in grams/total myocardial mass in grams termed % LGE) in different statistical analyses.
For statistical analysis, all data were analyzed using the R statistical package version 2.2 (R Foundation statistical software, Vienna, 2007). All hypothesis tests were two-tailed with significance level 0.05. Unless otherwise noted, data are presented as mean ± standard deviation. Inter- and intra-observer variability testing of LGE volume was determined by Bland-Altman analysis. Unpaired t-tests were used to compare means between groups. Bivariate associations between categorical variables were assessed using Fisher’s exact test, and between continuous variables using Pearson or Spearman correlation coefficients as appropriate. Finally, multiple linear regression models were used to predict B-type natriuretic peptide (BNP) with different subsets of independent variables.
Results
Of the 28 patients with systemic amyloidosis, cardiac involvement was identified in 21 (75%) by cardiac biopsy (n=6) and by clinical criteria (n=15). Following observation for 25 ± 10 months (median 29 months), there were 5 deaths (18%, 3 male, 2 female), all of whom were in the group with cardiac involvement. Heart failure was the primary or secondary cause of death in all patients.
In respect to treatment received, 18 patients (64%) received high dose melphalan followed by stem cell transplantation, and the remainder received an oral melphalan-based regimen. Two were referred for cardiac transplantation. The decision to proceed with stem cell transplantation was made apart from the CMR findings. There was no relationship between treatment received (high-dose vs. oral melphalan) and survival.
The mean BNP level in the patients that survived was lower than those that died (423 ± 622 pg/ml vs. 1090 ± 1956 pg/ml, p < 0.01). In addition, patients with cardiac amyloidosis had higher BNP (688 ± 1087 pg/ml) as compared to those without cardiac involvement (109 ± 211 pg/ml, p=0.03). Overall mean New York Heart Association heart failure class was 2.0 ± 1.0 with a difference observed between those with (2.3 ± 0.9) and without (1.3 ± 0.5) cardiac involvement (p= 0.01). Heart failure class was also higher for those that died as compared to those that survived (2.8 ± 0.4 vs. 1.9 ± 0.9, p < 0.01). BNP-level and heart failure class were positively correlated (R=0.42, p= 0.03).
CMR data are summarized in Table 1. Patients with cardiac amyloidosis had greater LV mass (p < 0.01), right ventricular (RV) mass (p < 0.01), LV mass-to-volume ratio (p = 0.01), and RV mass-to-volume ratio (p < 0.01) as compared to those without cardiac amyloidosis.
Table 1.
Cardiac magnetic resonance imaging measured parameters in patients with systemic amyloidosis with and without clinical cardiac amyloid involvement
| Variable | Cardiac Amyloid | P value | |
|---|---|---|---|
| Yes (n=21) | No (n=7) | ||
| Left ventricular ejection faction (%) | 57 ± 13 | 65 ± 8 | 0.06 |
| Left ventricular stroke volume (ml) | 88 ± 32 | 108 ± 22 | 0.07 |
| Left ventricular end-diastolic volume (ml) | 155 ± 45 | 169 ± 44 | 0.47 |
| Left ventricular mass (g) | 186 ± 61 | 135 ± 31 | 0.009 |
| Left ventricular mass/Left ventricular volume (g/ml) | 1.23 ± 0.4 | 0.85 ± 0.3 | 0.01 |
| Right ventricular ejection faction (%) | 53 ± 13 | 60 ± 6 | 0.09 |
| Right ventricular stroke volume (ml) | 74 ± 31 | 92 ± 27 | 0.16 |
| Right ventricular end-diastolic volume (ml) | 138 ± 48 | 156 ± 45 | 0.38 |
| Right ventricular wall thickness (mm) | 6 ± 2.5 | 4 ± 2.0 | 0.06 |
| Right ventricular mass (g) | 54 ± 19.6 | 38 ± 7.5 | 0.006 |
| Right ventricular mass/Right ventricular volume (g/ml) | 0.40 ± .013 | 0.26 ± 0.09 | 0.004 |
CMR parameters were also assessed for prognosis and are presented in Table 2. RV volumetric parameters were associated with survival, while LV volumetric parameters were not. Patients that died during follow-up had lower RV end-diastolic volume (p < 0.01) and RV stroke volume (p = 0.02) while RV ejection fraction was similar (p = 0.74) as compared to those that survived. This observation persisted when normalizing RV end-diastolic volume and stroke volume to body surface area.
Table 2.
Cardiac magnetic resonance imaging measured parameters in survivors and non-survivors with systemic amyloidosis
| Variable | Survivor | P value | |
|---|---|---|---|
| Yes (n=23) | No (n=5) | ||
| Left ventricular ejection fraction (%) | 60 ± 13 | 57 ± 13 | 0.68 |
| Left ventricular mass (g) | 179 ± 61 | 147 ± 49 | 0.25 |
| Right ventricular ejection fraction (%) | 55 ± 10 | 52 ± 21 | 0.74 |
| Right ventricular end-diastolic volume (ml) | 151 ± 46 | 102 ± 25 | 0.007 |
| Right ventricular stroke volume (ml) | 84 ± 30 | 52 ± 22 | 0.02 |
| Right ventricular wall thickness (mm) | 6 ± 2 | 5 ± 3 | 0.68 |
| Right ventricular mass (g) | 52 ± 18.6 | 39 ± 15.8 | 0.15 |
| Right ventricular mass/Right ventricular volume (g/ml) | 0.36 ± 0.13 | 0.40 ± .015 | 0.66 |
| Late Gadolinium enhancement (g) myocardium) | 27 ± 35 | 35 ± 49 | 0.74 |
| % Late Gadolinium enhancement of total myocardial mass | 13 ± 15 | 24 ± 34 | 0.51 |
LGE was observed in 19 patients (68%) overall. Inter-observer and intra-observer agreement for the presence of LGE was 100%. For LGE quantification, inter-observer agreement was −0.5 ± 6 g (R=0.99, p<0.001) and intra-observer agreement was 1.4 ± 9 g (R=0.90, p<0.0001).
Three patients had clinical cardiac amyloidosis but no evidence of LGE (all diagnosed by cardiac biopsy), and one patient had LGE but did not meet the criteria for clinical cardiac amyloidosis. The presence of LGE had a sensitivity of 86%, specificity 86%, positive predictive value 95%, and negative predictive value 67%, for the identification of clinical cardiac involvement. The pattern of LGE was diffuse and predominantly sub-endocardial as has been previously described4 (Figures 1 and 2), with absence of LGE in patients without cardiac involvement (Figure 3).
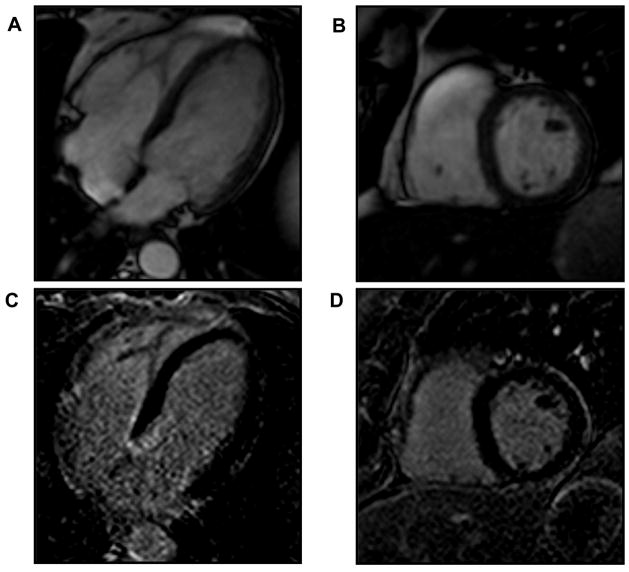
Figure 1.
Quantification of late gadolinium enhancement (LGE) in cardiac amyloidosis was performed using a semi-automated signal intensity thesholding technique. Panels A and B illustrate steady state free precession images of a patient with cardiac amyloidosis in the 4-chamber and short axis orientations respectively. Panel C is an LGE image from the short axis plane shown in B with clearly evident LGE (bright areas within the myocardium) in a diffuse, sub-endocardial pattern. Panel D illustrates a color overlay map of LGE based upon a signal intensity threshold of 6 standard deviation units above the nulled (dark appearing) myocardium.
Figure 2.
Diffuse sub-endocardial late gadolinium enhancement (LGE) is evident in this 2-chamber orientation from a patient with cardiac amyloidosis. Panel A illustrates an un-enhanced steady state free precession image, and panel B, the corresponding late gadolinium enhanced image.
Figure 3.
The absence of late gadolinium enhancement (LGE) is evident in this patient with systemic amyloidosis but no cardiac involvement. Panels A and B illustrate steady state free precession images in the 4-chamber and short axis orientations respectively. Panels C and D are corresponding LGE images showing normal myocardial signal characteristics (nulling) without evidence of LGE.
The presence of LGE did not predict mortality. Four deaths did occur in those with LGE, but 17 of 21 patients with LGE survived (p=0.62). However, in the absence of LGE, 6 of 7 (86%) patients survived suggesting that the absence of LGE may better predict survival then the presence of LGE predicts mortality. When treated as a continuous variable, the magnitude of LGE also did not differ between the patients that survived (27 ± 35 g) vs. those that died (35 ± 49 g, p=0.74, Table 2).
The volume of LGE was correlated to RV mass and RV mass-to-volume ratio (both R=0.52, p < 0.01), LV mass (R=0.44, p = 0.03), and to the level of serum B-natriuretic peptide (R=0.64, p < 0.001). When LGE myocardium was expressed as a percentage of the total myocardial mass (% LGE), this correlation to BNP was even stronger (R=0.75, p <0.001). LV ejection fraction (R=−0.39, p=0.04), RV ejection fraction (R=−0.40, p=0.04), RV wall thickness (R=0.48, p=0.01), and RV mass-to-volume ratio (R=0.38, p=0.05) also correlated with BNP, while LV mass (R=0.17), LV end-diastolic volume (R=−0.11), and RV-end diastolic volume (R=−0.18) did not. A multiple regression model was developed including independent predictors that were significant from bivariate correlations (including LGE volume, % LGE, LV ejection fraction, RV ejection fraction, LV end-diastolic volume, RV wall thickness, and left atrial dimension) that demonstrated % LGE as the strongest independent predictor of BNP (Table 3). A forward stepwise procedure for model selection identified a smaller subset of independent predictors, but % LGE remained the strongest of those variables selected.
Table 3.
Multivariable analysis of late gadolinium enhancement to predict B-type natriuretic peptide level
| Variable | Estimate | 95% CI | P value |
|---|---|---|---|
| Model 1: | |||
| Late gadolinium enhancement | −18.7 | (−47.6, 10.1) | 0.22 |
| Late gadolinium enhancement (% of left ventricular mass) | 58.0 | (5.6, 110.4) | 0.046 |
| Left ventricular ejection fraction | −40.8 | (−91.8, 10.2) | 0.14 |
| Right ventricular ejection fraction | 12.6 | (−23.8, 49.0) | 0.51 |
| Left ventricular end-diastolic volume | −1.8 | (−9.9, 6.3) | 0.67 |
| Right ventricular wall thickness | 119.6 | (−29.3, 268.6) | 0.14 |
| Left atrial dimension | −6.5 | (−47.4, 34.3) | 0.76 |
| Model 2: | |||
| Late gadolinium enhancement (% of left ventricular mass) | 32.0 | (15.7, 48.2) | 0.001 |
| Left ventricular ejection fraction | −28.6 | (−62.3, 5.1) | 0.09 |
| Model 3: | |||
| Late gadolinium enhancement (% of left ventricular mass) | 38.4 | (23.4, 53.4) | <0.001 |
Discussion
The accurate identification of cardiac involvement in patients with systemic AL amyloidosis is an essential aspect of clinical evaluation. In our experience, amyloidosis is often first identified in a non-cardiac organ, hence the presence of cardiac involvement is unknown. This study is the first to examine whether CMR might assist in the determination of cardiac involvement in a cohort of patients with known light-chain amyloidosis but unknown cardiac involvement as an adjunct to a standard clinical evaluation. Using LGE imaging, we found that CMR conferred a very high positive predictive value (95%) for the detection of cardiac amyloid involvement, similar to that recently reported in a cohort of patients with restrictive cardiomyopathy by Vogelsberg et al.10 Furthermore, we found that the volume of enhanced myocardium directly varied with clinical heart failure symptoms as determined by serum BNP measurement but did not predict survival. Over a 2-year period, we also found that CMR-determined abnormalities of right ventricular end-diastolic volume and stroke volume were also associated with increased mortality.
With the exception of the Vogelsberg report, this study differs from those published previously that have examined the application of CMR to cardiac amyloidosis. These prior reports have demonstrated the ability of CMR to identify abnormalities in cardiac amyloidosis by means of LGE4–6,10 but recruited patients with known cardiac amyloidosis, while our study recruited patients with systemic amyloidosis but unknown cardiac involvement at the time of CMR. We are the first to report survival data and clinical correlates of CMR imaging in cardiac amyloidosis, however. Since the presence of cardiac amyloidosis was found in 75% of our cohort, we were able to determine the performance characteristics of LGE imaging.
LGE abnormalities in cardiac amyloidosis are thought to be related to the expansion of the interstitial compartment by the infiltrating amyloid protein4 with global subendocardial4 or transmural5 patterns. In the study by Maceira et al, selection of a short delay from gadolinium injection to LGE imaging (5 minutes at 0.1 mmol/kg contrast dose) was required to obtain optimal images due to the rapid clearance of gadolinium from the blood pool and the abnormal myocardial T1. While we likewise observed the rapid clearance of gadolinium from the blood pool, we utilized a higher dose of contrast (0.2 mmol/kg) and therefore chose to image at a later delay from injection with resultant preservation of blood pool signal. We observed a similar pattern of LGE as has been reported with the highest signal intensity in the sub-endocardium, but also with regions of diffuse gray (likely indicative of diffuse infiltration), and others of more complete nulling (perhaps indicative of relative sparing) also observed. We were able to generate images of comparable diagnostic quality to those reported previously with good contrast so as to permit quantification of LGE volume with a high degree of reproducibility. Despite the differing acquisition techniques, in our cohort we observed similar sensitivity (86% vs. 80%), specificity (86% vs. 94%), and positive predictive value (95% vs. 92%) as that reported by Vogelsberg et al 10 for the CMR detection of cardiac amyloidosis.
We tested CMR against our standard clinical assessment. In this cohort, nearly 1/3 of patients had cardiac biopsy proven amyloidosis, while the remainder had cardiac involvement determined clinically using echocardiography, electrocardiography, physical examination, and BNP measurement. This clinical algorithm was been well established and recently proposed as an alternative to cardiac biopsy to assess for cardiac involvement.11
Most CMR studies of cardiac amyloidosis were performed prior to the recognition of the potential deleterious effects of gadolinium administration (nephrogenic systemic fibrosis) to patients with severe renal impairment.12 In this cohort, using current guidelines for creatinine clearance, 3 patients would have not received contrast (creatinine clearance < 30 ml/min/1.73 m2) and 3 more would have fallen in an intermediate risk stratum (creatinine clearance between 30 and 60 ml/min/1.73 m2).12 Clearly, the benefit of LGE imaging in patients with systemic amyloidosis would have to be balanced against the potential risk of contrast administration. In our cohort, 79% had renal function that would fall within safety standards for contrast administration.
The presence or volume of enhanced myocardium did not predict mortality. This interpretation is limited by the relatively small numbers of patients who died relative to the entire cohort. However, nearly 90% of patients without LGE survived, suggesting that the absence of LGE not only correlates to the absence of cardiac involvement but also, in a consistent manner, predicts survival. Subjects who died had smaller RV end-diastolic volumes and stroke volume, increased wall mass, but similar RV ejection fractions. Previous work using echocardiography has paradoxically suggested that RV dilation (relative to the LV) could portend mortality in patients with cardiac amyloidosis.13 In this study, we present RV size relative to population norms, rather than the patient’s own LV, perhaps explaining the difference observed.
While the cohort of patients with amyloidosis in this study is consistent with previously published work4, overall numbers of patients were relatively small. Despite following patients for over 2 years, deaths were relatively infrequent owing to effective treatment. These small sample sizes limit generalization of this study’s conclusions without validation in a larger cohort. In our cohort of patients with systemic amyloidosis, we ultimately determined that approximately 75% had cardiac involvement. This is higher than is generally appreciated (approximately 50%)14,15 likely reflecting our limited sample size and naturally affected the test characteristics of LGE to assess cardiac amyloidosis.
Acknowledgments
The authors gratefully acknowledge the assistant of Lois Goepfort, RN in subject recruitment.
Funding Source: This research was supported by an NIH PO1 HL68705 to MS, NIH T32 HL07224-29 to FLR, and the Amyloid Research Fund at Boston University School of Medicine, Boston MA.
Footnotes
Disclosures: No conflicts of Interest are declared
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lima JAC, Desai MY. Cardiovascular magnetic resonance imaging: Current and emerging applications. J Am Coll Cardiol. 2004;44:1164–1171. doi: 10.1016/j.jacc.2004.06.033. [DOI] [PubMed] [Google Scholar]
- 2.Celletti F, Fattori R, Napoli G, Leone O, Rocchi G, Bacchi Reggiani L, Gavelli G. Assessment of restrictive cardiomyopathy of amyloid or idiopathic etiology by magnetic resonance imaging. Am J Cardiol. 1999;83:798–801. doi: 10.1016/s0002-9149(98)00998-9. [DOI] [PubMed] [Google Scholar]
- 3.Fattori R, Rocchi G, Celletti F, Bertaccini P, Rapezzi C, Gavelli G. Contribution of magnetic resonance imaging in the differential diagnosis of cardiac amyloidosis and symmetric hypertrophic cardiomyopathy. Am Heart J. 1998;136:824–830. doi: 10.1016/s0002-8703(98)70127-9. [DOI] [PubMed] [Google Scholar]
- 4.Maceira AM, Joshi J, Prasad SK, Moon JC, Perugini E, Harding I, Sheppard MN, Poole-Wilson PA, Hawkins PN, Pennell DJ. Cardiovascular magnetic resonance in cardiac amyloidosis. Circulation. 2005;111:186–193. doi: 10.1161/01.CIR.0000152819.97857.9D. [DOI] [PubMed] [Google Scholar]
- 5.Perugini E, Rapezzi C, Piva T, Leone O, Bacchi-Reggiani L, Riva L, Salvi F, Lovato L, Branzi A, Fattori R. Noninvasive evaluation of the myocardial substrate of cardiac amyloidosis by gadolinium cardiac magnetic resonance. Heart. 2006;92:343–349. doi: 10.1136/hrt.2005.061911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van den Driesen RI, Slaughter RE, Strugnell WE. MR findings in cardiac amyloidosis. Am J Roentgenol. 2006;186:1682–1685. doi: 10.2214/AJR.04.0871. [DOI] [PubMed] [Google Scholar]
- 7.Palladini G, Campana C, Klersy C, Balduini A, Vadacca G, Perfetti V, Perlini S, Obici L, Ascari E, d’Eril GM, Moratti R, Merlini G. Serum N-terminal pro-brain natriuretic peptide is a sensitive marker of myocardial dysfunction in AL amyloidosis. Circulation. 2003;107:2440–2445. doi: 10.1161/01.CIR.0000068314.02595.B2. [DOI] [PubMed] [Google Scholar]
- 8.Yeon SB, Sabir A, Clouse M, Martinezclark PO, Peters DC, Hauser TH, Gibson CM, Nezafat R, Maintz D, Manning WJ, Botnar RM. Delayed-enhancement cardiovascular magnetic resonance coronary artery wall imaging: comparison with multislice computed tomography and quantitative coronary angiography. J Am Coll Cardiol. 2007;50:441–447. doi: 10.1016/j.jacc.2007.03.052. [DOI] [PubMed] [Google Scholar]
- 9.Flacke S, Allen JS, Chia JM, Wible JH, Periasamy MP, Adams MD, Adzamli IK, Lorenz CH. Characterization of viable and nonviable myocardium at MR imaging: Comparison of gadolinium-based extracellular and blood pool contrast materials versus manganese-based contrast materials in a rat myocardial infarction model. Radiology. 2003;226:731–738. doi: 10.1148/radiol.2263020151. [DOI] [PubMed] [Google Scholar]
- 10.Vogelsberg H, Mahrholdt H, Deluigi CC, Yilmaz A, Kispert EM, Greulich S, Klingel K, Kandolf R, Sechtem U. Cardiovascular magnetic resonance in clinically suspected cardiac amyloidosis: noninvasive imaging compared to endomyocardial biopsy. J Am Coll Cardiol. 2008;51:1022–1030. doi: 10.1016/j.jacc.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 11.Selvanayagam JB, Hawkins PN, Paul B, Myerson SG, Neubauer S. Evaluation and management of cardiac amyloidosis. J Am Coll Cardiol. 2007;50:2101–2110. doi: 10.1016/j.jacc.2007.08.028. [DOI] [PubMed] [Google Scholar]
- 12.Grobner T, Prischl FC. Gadolinium and nephrogenic systemic fibrosis. Kidney Int. 2007;72:260–264. doi: 10.1038/sj.ki.5002338. [DOI] [PubMed] [Google Scholar]
- 13.Patel AR, Dubrey SW, Mendes LA, Skinner M, Cupples A, Falk RH, Davidoff R. Right ventricular dilation in primary amyloidosis: An independent predictor of survival. Am J Cardiol. 1997;80:486–492. doi: 10.1016/s0002-9149(97)00400-1. [DOI] [PubMed] [Google Scholar]
- 14.Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation. 2005;112:2047–2060. doi: 10.1161/CIRCULATIONAHA.104.489187. [DOI] [PubMed] [Google Scholar]
- 15.Merlini G, Bellotti V. Molecular mechanisms of amyloidosis. N Engl J Med. 2003;349:583–596. doi: 10.1056/NEJMra023144. [DOI] [PubMed] [Google Scholar]