Abstract
Striatal spine loss is a key pathological feature of human Parkinson’s disease (PD) that can be induced after complete degeneration of the nigrostriatal dopaminergic system in rodent models of parkinsonism. In line with these observations, our findings reveal a significant (30–50%) reduction in spine density in both the caudate nucleus and putamen of severely DA-depleted striata of MPTP-treated monkeys; the sensorimotor post-commissural putamen being the most severely affected region for both dopamine depletion and spine loss. Using MPTP-treated monkeys with complete or partial striatal dopamine (DA) denervation, we also demonstrate that striatal spine loss is an early pathological feature of parkinsonism, which progresses along a positive rostrocaudal and mediolateral gradient in parallel with the extent of striatal dopamine denervation. Quantitative electron microscopy immunocytochemistry for D1 dopamine receptor (D1) in the striatum of control and severely DA-depleted animals revealed that both D1-immunoreactive and immunonegative spines are lost in the putamen of MPTP-treated monkeys.
These data demonstrate that striatal spine loss in MPTP-treated monkeys is an early pathological event of parkinsonism, tightly correlated with the degree of nigrostriatal dopamine denervation that likely affects both direct and indirect striatofugal pathways.
INTRODUCTION
Dopamine (DA) plays a critical role in regulating spine density on striatal medium-sized spiny neurons (MSNs) (Arbuthnott et al., 2000; Robinson and Kolb, 1999). A significant reduction in spine density has been demonstrated in postmortem tissue from human parkinsonians (Stephens et al., 2005; Zaja-Milatovic et al., 2005) and in 6-hydroxydopamine (6-OHDA) rodent model of Parkinson’s disease (PD) (Ingham et al., 1989, 1998). The role of DA in modulating striatal spine morphogenesis is also despicted by the significant increase in the number of spines on MSNs in animals treated with psychostimulants (Robinson and Kolb, 1999; Norrholm et al., 2003). Rodent models of PD, characterized by a severe loss of striatal dopamine, show a significant reduction in overall spine density accompanied by a corresponding decrease in the total number of putative glutamatergic terminals forming asymmetric synapses (Ingham et al., 1993; 1998). Therefore, dopamine is a key transmitter that plays an important role in regulating the growth, maintenance and plasticity of dendritic spines in the striatum. However, because rodent and human data gathered so far have been collected from patients or animal models at the end stage of parkinsonism, it is not clear if striatal spine loss is an early pathological event that progresses with the degeneration of the nigrostriatal system or a late phenomenon that occurs only in severely dopamine-depleted striata.
Although the etiology of the degenerative process that underlies clinical deterioration in PD remains unknown, it is well established that the dopaminergic innervation of specific striatal regions, like the postcommissural sensorimotor putamen, is more sensitive to degeneration than that of other striatal areas in both PD patients and animal models of Kish et al., 1988; Brooks et al., 1990; Iravani et al., 2005). parkinsonism ( There is also clear evidence that striatal dopaminergic denervation precedes nigral neuronal loss (Bernheimer et al., 1973; Herkenham et al., 1991; Wu et al., 2003) suggesting that striatal dysfunction in dopamine transmission and, likely, MSNs spine pathology, are early steps towards nigrostriatal dopaminergic degeneration and death of nigral neurons in PD.
In this study, we took advantage of the progressive degenerative process induced by low doses of MPTP to assess the effects of partial or severe dopaminergic depletion on spine loss in MSNs of specific functional striatal sub-regions in monkeys. Furthermore, based on evidence that the striatum comprises two segregated populations of MSNs (Gerfen et al., 1990) that display a different degree of spine loss in rodent models of parkinsonism (Day et al., 2006), we performed a quantitative electron microscopic analysis of the density of striatal D1-immunoreactive and non-immunoreactive spines between normal and MPTP-treated monkeys.
Findings of these studies have been presented in abstract forms (Villalba et al., 2006, 2007).
MATERIALS AND METHODS
1.- Animals and Tissue Preparation
In total, 6 control (one male and five females) and 6 MPTP-treated (one male and five females) juvenile (3–6 years old) Rhesus monkeys (Macaca mulatta) (Yerkes National Primate Research Center colony) were used in this study. The housing, feeding and experimental conditions used in these studies were in line with those of the National Institutes of Health guidelines and approved by Emory University Institutional Animal Care and use Committee.
1.1.- MPTP Injections and Parkinsonism
In four monkeys, MPTP was injected unilaterally through the right carotid (total dose 2–3 mg/kg; Sigma-Aldrich, St-Louis, MO) under general isoflurane anesthesia (1–3%), while the other two animals received intramuscular injections of MPTP (total dose 4.3 and 8 mg/kg; Sigma-Aldrich, St-Louis, MO). Following these injections, behavioral changes and parkinsonian motor signs were measured with quantitative methods routinely used in our laboratory to assess parkinsonian behaviors in MPTP-treated monkeys (Bergman et al., 1990; Wichmann et al., 2001; 2006; Soares et al., 2004; Bogenpohl et al., 2007). In brief, the monkey’s behavior was documented through observations of spontaneous cage activity or using a quantitative computer-assisted behavioral system to score the occurrence of spontaneous limb movements and a general activity monitoring system using observation cages equipped with infrared beams. The four monkeys that received intracarotid MPTP injections developed obvious signs of parkinsonisms on the side of the body contralateral to the injected carotid, while the ipsilateral side was not significantly affected. One of the systemically injected monkeys that received a total dose of 8 mg/kg MPTP over a 5 months period developed stable bilateral parkinsonian motor signs (bradikynesia, rigidity, flexed limb and body posture). The other animal, that was systemically administered 4.3 mg/kg MPTP over 2 months, did not develop any significant motor impairment. The survival time following the last MPTP injection was 6 months for the two systemically injected monkeys and 6 to 18 months for the intracarotid treated animals.
1.2.- Animal Perfusion
Animals were deeply anesthetized with an overdose of pentobarbital (100 mg/kg, iv) and perfused transcardially with cold oxygenated Ringer’s solution, followed by 2 liters of fixative containing 4% paraformaldehyde and 0.1% glutaraldehyde in phosphate buffer (PB; 0.1 M, pH 7.4). After perfusion, the brains were taken out from the skull and cut into 10 mm-thick blocks in the frontal plane. Tissue sections (60 μm-thick), were obtained with a Vibratome collected in cold phosphate-buffered saline (PBS; 0.01 M, pH 7.4), and treated with sodium borohydride (1% in PBS, 20 min).
2.- Golgi Impregnation
The Golgi impregnation technique was used on 60 μm-thick vibratome striatal coronal sections from normal and MPTP-treated monkeys. Sections were immersed in 1% osmium tetroxide (30 min) followed by subsequent overnight incubations in 3.5% potassium dichromate and 2% silver nitrate. Golgi-impregnated sections were then dehydrated in graded ethyl alcohol, embedded in resin (Durcupan ACM; Fluka, Buchs, Switzerland), mounted, and coverslipped. The resin was polymerized at 60°C (48 hours).
3.- Immunocytochemistry
3.1.- Primary Antibodies
All commercially available antibodies used in this study have been well characterized using immunoblots on brain tissue or transfected cells, peptide pre-adsorption and omission of primary antibodies. The source and dilution of these antibodies were: 1) Mouse monoclonal anti-TH antibody (dilution 1:1,000; Chemicon, Temecula, CA; catalog #: MAB 318), 2) Rat monoclonal anti-D1 dopamine receptor (D1) antibody (dilution: 1:75; Sigma, St Louis, MO; catalog #: D-187; Levey et al., 1993).
3.2.- Immunoperoxidase Labeling for Light and Electron Microscopy
The avidin-biotin complex method (ABC, Vectastain Standard kit) and the chromogen diaminobenzidine (DAB) were used for the peroxidase reaction (Galvan et al., 2006; Raju et al., 2006; Mitrano et al., 2007). For light microscopy (LM), the sections were mounted onto gelatin-coated slides, dehydrated, and then coverslipped with Permount. The tissue was examined with a Leica DMRB microscope (Leica Microsystems, Inc., Bannockburn, IL) and images were taken with a CCD camera (Leica DC 500; Leica IM50 software). For electron microscopy (EM), immunostained sections were post-fixed in osmium tetroxide and dehydrated in a graded series of alcohol and propylene oxide. The sections were then embedded in resin (Durcupan, ACM, Fluka) on microscope slides and put in the oven (60°C) for 48 hours. After examination in the light microscope, the region of interest, ie the dorsal part of the postcommissural putamen, was cut out from the slides and glued on the top of the resin blocks with cyanocrylate glue. The ultrathin sections (60 nm) were then cut using an ultramicrotome (Leica Ultracut T2), collected on Pioloform-coated single slot grids, stained with lead citrate and examined with a Zeiss EM 10C electron microscope. Ultrathin sections were obtained from 5 different blocks. From each block, 3–5 grids (4–5 sections/grid) were analyzed in the EM. Electron micrographs were taken at 25,000X magnification with a CCD camera (DualView 300W; DigitalMicrograph software, version 3.10.1; Gatan, Inc., Pleasanton, CA), saved and printed for quantitative analysis.
4.- Data Analysis
4.1.- Quantitative Analysis of Dendritic Spine Density in Golgi-impregnated Neurons
In sections from control and DA-depleted animals, only neurons with a long dendritic process that emerged from a visible soma were selected. The number of spines along the longest dendrites of these well-impregnated MSNs was quantified from the start to the most distal visible end (usually secondary or tertiary dendrites) using the 100X immersion oil objective and a computer-assisted tracing system (Neurolucida v7; MicrobrightFiled Inc., USA). In partially DA-depleted animals, the longest dendrites were quantitatively analyzed from impregnated MSNs selected from specific sriatal areas characterized by different levels of TH immunoreactivity in adjacent sections.
4.2.- D1-immunolabeled Spine Analysis
Blocks of tissue were prepared from commissural putamen sections in control and MPTP-treated parkinsonian monkeys. The observer was blind to the source of these blocks to avoid any bias in the analysis of the tissue. To minimize false negatives, and taking in consideration the limited access of antibodies, only ultrathin sections from the most superficial sections of blocks were scanned in the electron microscope. A total of 100 electron micrographs per monkey were taken at a magnification of 25,000X with each electron micrograph representing an area of approximately 12.56 μm2. All labeled and unlabeled dendritic spines with identifiable asymmetric synapses were quantified.
4.3.- Statistical Analysis
Statistics were performed using the SigmaStat 2.03 software. All data were expressed as means ± S.E.M and compared by T-test to determine statistical differences between control and MPTP-treated monkeys. To make sure there was no significant inter-individual difference in spine density between animals of the same group, the density of spines from Golgi-impregnated neurons in control (n=3) and MPTP-treated (n=4) monkeys were initially compared statistically using one-way ANOVAs. Based on this analysis, the average spine density values for each group (pre-commissural, commissural and post-commissural caudate and putamen) of control and MPTP-treated animals were compared with T-test.
4.4.- Photomicrographs Production
Pictures were digitally acquired, imported in TIFF format into Adobe Photoshop software (version 9.0; Adobe System, Inc. San Jose, CA) and adjusted only for brightness and contrast, to optimize the quality of the images for analysis. Micrographs were then compiled into figures in Adobe Illustrator 12.0. Some images were pseudo-colored using the NIH Image program ImageJ.
RESULTS
Striatal Spine Loss in Severely Dopamine-depleted Striatum
In a first series of experiments, striatal spine loss was examined in the dorsal striatum of four severely dopamine-depleted monkeys that displayed significant parkinsonian motor signs. The whole extent of the caudate nucleus and putamen of these animals were almost completely devoid of TH innervation (Fig. 1A, A′, B, B′). Only the nucleus accumbens showed significant immunoreactivity (Fig. 1A′). At the midbrain level, the ventral tier SNc was severely damaged, whereas a significant number of TH-immunoreactive neurons and processes remained in the ventral tegmental area and dorsal tier SNc (Fig. 1C, C′).
Figure 1.
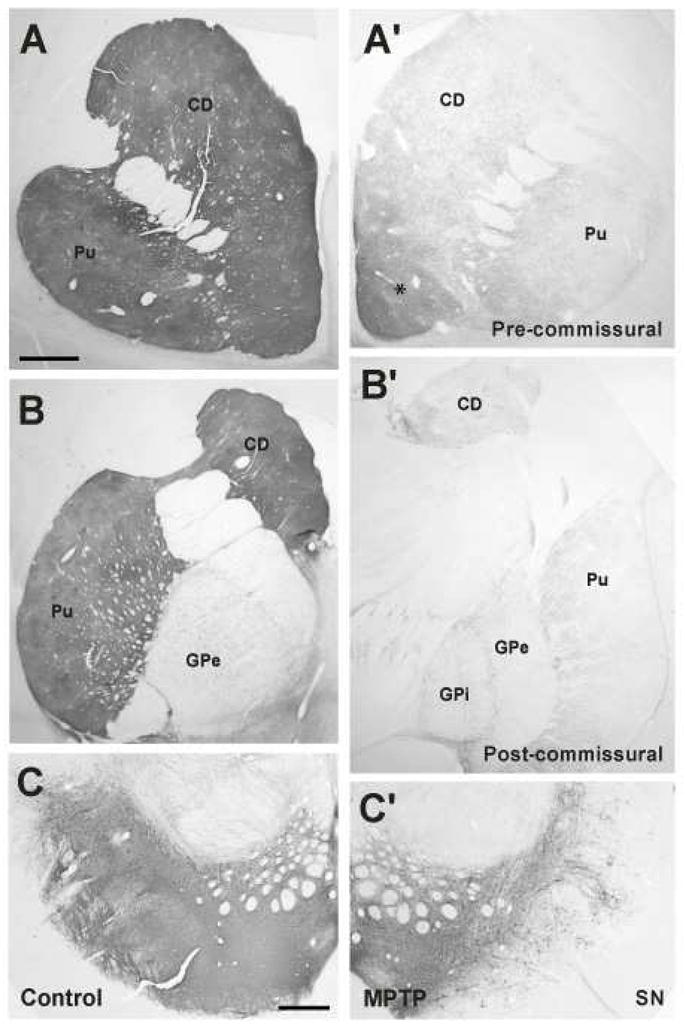
Tyrosine hydroxylase immunoreactivity (TH-IR) of coronal striatal sections from control (A – C) and severely dopamine (DA)-depleted monkeys (A′–C′). Strong TH-IR was found in the pre- (A) and post-commissural (B) striatum as well as in the substantia nigra (SN) of all control animals. An almost complete and uniform depletion of TH-IR was observed in the dorsal striatum and the ventral tier of the SNc of MPTP-treated monkeys (A′–C′). Only the ventral striatum (accumbens nucleus, *), ventral tegmental area and dorsal tier SNc showed remaining TH-IR in these animals. CD: caudate; Pu: putamen; SN: substantia nigra; GPi: Globus pallidus, internal segment; GPe: Globus pallidus, external segment. Scale bar in A (applies to A′, B and B′) and in C (applies to C′) = 1 mm.
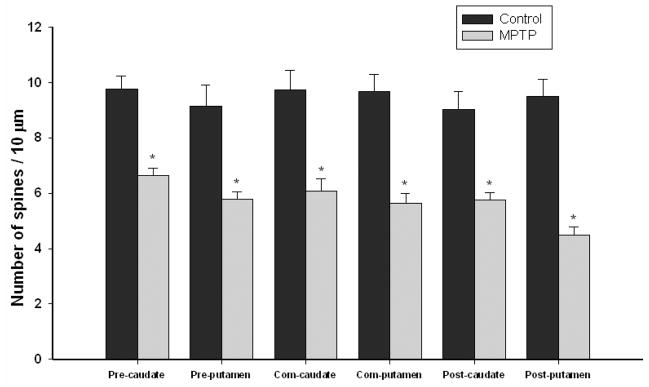
To determine the effects of dopamine loss on striatal spine density, sections of the caudate nucleus and putamen at the pre-commissural, commissural and post-commissural levels were Golgi-impregnated and used for quantitative measurements of total spine density on MSNs from both controls (Fig. 2A, C) and MPTP-treated (Fig. 2B, D) monkeys. Dendritic spines were only observed after the first ramification of the primary dendrites (Fig. 2A–D). Quantitative spine density was measured from the longest dendrites of randomly encountered Golgi-impregnated MSNs in all striatal regions from both control (Fig. 2A, A′, C, C′) and MPTP-treated monkeys (Fig. 2B, B′, D, D′). In controls, there was no significant difference in the mean densities of spines for the different striatal areas examined (one way ANOVAs analysis, P=0.86). In addition, there was no significant inter-individual difference in spine density between each of the 3 animals in the control group (one way ANOVAs, P=0.155) or the 4 animals in the MPTP group (one way ANOVAs, P=0.340), suggesting that the differences in spine density between normal and MPTP-treated animals are consistent across individuals and largely depend on drug treatment (Fig. 3). The spine density values collected from each of these animals were, therefore, pooled with others in their respective groups, and these averages were used for statistical comparisons using T-test between normal and MPTP-treated animals. As depicted in figure 3, a significant difference (*) in the mean density of spines between control and MPTP-treated groups was found in all striatal areas (P<0.001) (Fig. 3). In control animals, an average density of about 9 spines per 10 μm of dendritic length was measured in all striatal territories (Fig. 3). The analysis of 23–32 dendrites in each striatal region revealed a significant reduction (32–47%) in spine density measurement at all rostrocaudal levels of the caudate nucleus and putamen compared with controls (Fig. 3). The post-commisural putamen was the most severely affected striatal region (9.49 ±0.64 spines/10 μm in control vs. 4.48 ± 0.30 in MPTP) with almost 50% spine loss, while the pre-commissural caudate was the least affected striatal region (9.75 ± 0.47 spines/10 μm in control vs. 6.65 ± 0.27 in MPTP) with 32% reduction in spine density in MPTP-treated monkeys.
Figure 2.
Golgi-impregnated medium-sized spiny neurons (MSNs) in the caudate nucleus (A, A′, B, B′) and putamen (C, C′, D, D′) of control (A, A′, C, C′) and MPTP-treated monkeys (B, B′, D,D′). A′, B′, C′ and D′: High magnification images of dendrites labeled with an arrow in A, B, C and D, respectively. These dendrites correspond to the longest dendritic processes that were analyzed for quantitative studies. Note the dramatic reduction of spines on the dendrites of MSNs from MPTP-treated monkeys. Scale bar in A and C = 25 μm (valid for B and D). Scale bar in B′ = 5 μm (valid for A′, C′ and D′).
Figure 3.
Histograms showing the quantitative analysis of spine density on medium-sized spiny neurons (MSNs) in the pre-commissural, commissural and post-commissural caudate nucleus and putamen of controls and MPTP-treated monkeys. Spine density (spine number per 10 μm) was measured along the length of the longest dendrite of Golgi-impregnated (MSNs) in control and MPTP-treated monkeys. Statistical analysis (T-test) showed a significant difference (*) in the mean density of spines between control and MPTP-treated groups for all striatal areas (P<0.001). The differences in the mean densities of spines for the different striatal areas in control animals were not statistically different (ANOVA analysis, P=0.86). There was no significant inter-individual difference in spine density between animals in control (one way ANOVAs, P=0.155) or the MPTP group (one way ANOVAs, P=0.340). The average values for the number of spines per 10 μm in the different striatal areas are indicated inside the bars (mean ± SEM). Three control and five MPTP-treated monkeys were used in this analysis.
Striatal Spine Loss in Partially Dopamine-depleted Striatum
In a third group of monkeys, we tested the effects of partial dopamine depletion on striatal spine loss. The striatal tissue used for this part of the study was collected from either the contralateral side of the four intracarotid MPTP-treated monkeys or from the monkey that received systemic MPTP, but did not develop parkinsonian motor signs. We took advantage of the heterogeneous and progressive rostrocaudal and mediolateral gradient in dopamine denervation of the striatum to assess the degree of spine loss in subregions of the caudate nucleus and putamen showing differential degrees of dopamine denervation (Fig. 4). Pseudocolored images (ImageJ) of TH-immunostained striatal sections from partially dopamine-depleted MPTP-treated monkeys are shown in Fig. 4A′, B′. Overall, the density of spines was tightly correlated with the relative abundance of TH-immunoreactive fibers in these animals, ie. striatal areas with low TH innervation harbored MSNs with a lower density of dendritic spines. The commissural putamen level clearly illustrates this relationship showing that spine density in the severely dopamine-depleted lateral sector (regions 1 and 2 in Fig. 4B′) was significantly lower than in the less dopamine-denervated medial regions (region 4 in Fig. 4B′).
Figure 4.
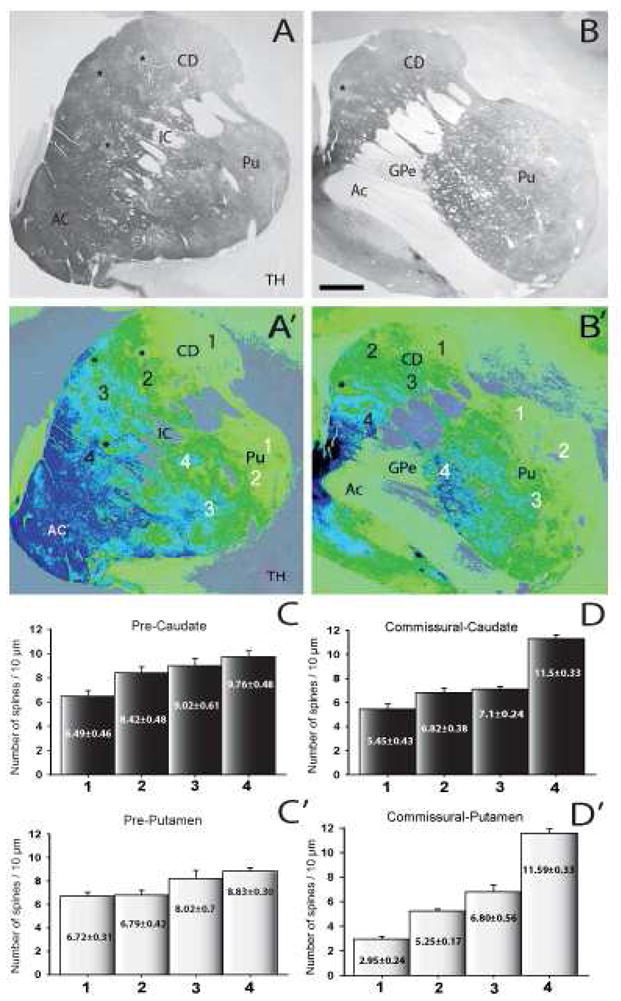
(A and B): Tyrosine hydroxylase immunoreactivity (TH-IR) of coronal sections from the pre-commissural (A), and commissural (B) striatum of partially dopamine-denervated MPTP-treated monkeys (N=3). (A′ and B′): Pseudo-colored images (NIH ImageJ program) of TH-immunostained sections showed in A and B. (C–D′): Histograms showing the quantitative analysis of the dendritic spine density of Golgi-impregnated neurons from striatal areas with different degrees of TH-IR depletion. Each bar represents the mean of spine density (mean ± SEM) per 10μm of dendritic length from at least 10 neurons in the striatal areas labelled with the corresponding number and color. Abbreviations: AC: accumbens nucleus; Ac: anterior commissure; CD: caudate; IC: internal capsule; Pu: putamen; GPe: Globus pallidus, external segment. Asterisks indicate striatal patch-like areas poor in TH. Scale bar in B = 1mm (valid for A).
Changes in the Density of D1-Immunoreactive Spines in the Striatum of MPTP-treated Monkeys
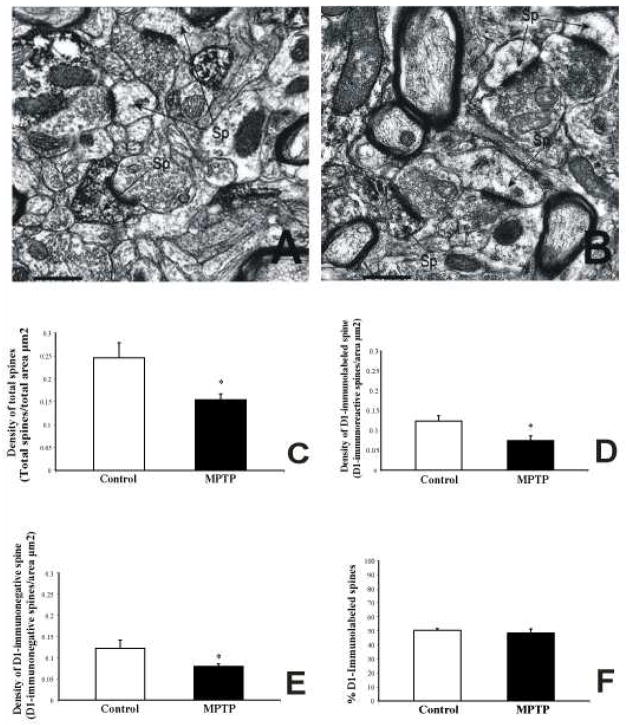
In order to determine if the loss of dendritic spines in DA-denervated striatum affects both direct and indirect pathway neurons, we measured the relative density of total spines and D1-containing spines in the commissural putamen of three control and three severely dopamine-depleted MPTP-treated monkeys. A total of 300 electron micrographs of labeled and unlabeled elements from the surface of D1-immunostained striatal tissue in 3 control and 3 MPTP-treated animals were analyzed (Fig. 5). As shown in previous rodent studies (Hersch et al., 1995; Lei et al., 2004), D1 immunoreactivity was strongly expressed in dendrites and spines of MSNs in control monkeys (Fig. 5A, B). On average, the density of total spines and D1-immunoreactive spines was decreased by 30% and 33%, respectively in the MPTP-treated striatum (total spine density: control=0.23±0.03, MPTP=0.16±0.01; D1-labeled spines: control=0.12±0.01; MPTP=0.08±0.01; T-test, P<0.05) (Fig. 5C, D). These differences were found to be highly significant using Student’s T -test (P<0.05). Similarly, the density of D1-immunonegative spines was decreased by 27% in the dopamine-depleted putamen (D1-unlabeled spines: control=0.11±0.02; MPTP=0.08±0.02; T-test, P<0.05) (Fig. 5E). These results suggest that both D1-immunoreactive and D1-immunonegative spines are lost following striatal dopamine denervation in MPTP-treated monkeys. These data are further supported by quantitative measurements showing no significant difference in the relative percentages of D1-immunoreactive versus non-immunoreactive spines between control and MPTP-treated monkeys (50.3±1.3% in control vs. 48.4±2.9% in MPTP-treated cases) (Fig. 5F).
Figure 5.
(A and B): Electron micrographs of D1-immunolabeled spines from control (A) and MPTP-treated monkeys (B). (C–F): Statistical analysis of D1-immunoreactive spine density. (C): Histograms showing a significant decrease in the density of total spines in MPTP-treated monkeys compared with control (total spine density: control=0.23±0.03; MPTP=0.16±0.01, T-test, P<0.05). (D and E): Histograms showing the density of D1-immunoreactive spines (D) and D1-immunonegative spines (E) in the putamen of control and MPTP-treated monkeys. A significant reduction in both D1-labeled (control=0.12±0.01; MPTP=0.08±0.01; T-test, P<0.05) and unlabeled (control=0.11±0.02; MPTP=0.08±0.002; T-test, P<0.05) spines was found in MPTP-treated monkeys. (F): There was no significant difference in the overall percentage of D1-immunoreactive spines in control animals compared with MPTP-treated monkeys. Three control and three MPTP-treated monkeys were used in this analysis. A total of 600 electron micrographs were analyzed (300 in control and 300 in MPTP-animals). Average area/picture=12.56μm2. Abbreviation: Sp: spine. Scale bar in A and B: 0.5 μm
DISCUSSION
The present study provides further evidence for significant striatal spine pathology in parkinsonism. In line with recent human parkinsonian data (Zaja-Milatovic et al., 2005), a regional pattern of spine loss was found in the striatum of MPTP-treated monkeys. Neurons in the postcommissural sensorimotor putamen were significantly more affected than cognitive- and limbic-related striatal cells in the anterior putamen and caudate nucleus. Our findings also demonstrate that striatal spine loss is an early pathogenic feature of parkinsonism, tightly linked with the severity of dopamine depletion, which occurs in both symptomatic parkinsonian monkeys and animals with partial striatal dopamine depletion that do not show any signs of motor abnormalities. Finally, another main conclusion of our study is that both D1-immunoreactive and D1-negative spines are lost following MPTP-induced dopamine depletion in the monkey striatum, suggesting that both direct and indirect pathway neurons are pathologically affected in this animal model.
Striatal spine loss has been reported in various rodent models of parkinsonism and human parkinsonians (Ingham et al., 1993; Cheng et al., 1997; Stephens et al., 2005; Zaja-Milatovic et al., 2005). Furthermore, in line with our data, previous studies have reported an increased sensitivity for spine pathology in the post-commissural sensorimotor putamen compared to other striatal regions in both human PD and MPTP-treated monkeys (Hornykiewicz et al., 2001; Stephens et al., 2005; Zaja-Milatovic et al., 2005). It is well established that the progressive degeneration of the dopaminergic nigrostriatal system either in PD or animal models of parkinsonism follows a specific pattern that first involves dopaminergic inputs to the postcommissural sensorimotor striatum (Damier et al., 1999; Dauer and Przedborski, 2003; Iravani et al., 2005). Cognitive areas of the anterior putamen/caudate nucleus and limbic-related ventral striatal regions are less sensitive, being dopamine-denervated later in the course of the disease (Hornykiewicz et al., 2001; Iravani et al., 2005). Our findings demonstrate that striatal spine loss also follows a specific pattern of degeneration that appears to be highly dependent on the degree of dopamine denervation, further suggesting the important role of dopamine in regulating striatal spine plasticity. This dependence was particularly well demonstrated in the partially dopamine-depleted monkeys, indicating that spine degeneration is an early pathogenic feature of PD. Interestingly, although considerable spine loss can be found in the sensorimotor striatum of partially dopamine-depleted monkeys, this pathology did not result in significant motor impairment. Knowing the important role of spine plasticity in mediating proper glutamatergic transmission at corticostriatal and thalamostriatal synapses, one may wonder about the compensatory mechanisms that are put in place to mediate normal motor behavior despite considerable striatal spine pathology in the sensorimotor striatum. Another possibility is that striatal spine loss underlies non-motor, cognitive deficits, not thoroughly assessed in these animals, but known to occur in early MPTP-treated monkeys and humans prior to the development of parkinsonian motor deficits (Stern et al., 1990; Schneider and Pope-Coleman, 1995).
Recent rodent data have provided convincing evidence that spine loss selectively affects striatal D2-containing neurons of the indirect pathway in reserpine-treated 17–25-day-old BAC D1 and BAC D2 EGFP mice (Day et al., 2006). These findings were supported by quantitative immunoelectron microscopy data from 6-OHDA-treated adult rats showing a significant decrease in the number of striatal D1-negative spines (Day et al., 2006). These observations are at odds with our data and findings of previous Golgi studies in both human parkinsonians and rodent models of parkinsonism showing a rather homogeneous loss of spines across large populations of Golgi-impregnated MSNs (Ingham et al., 1989; 1998; Stephens et al., 2005; Zaja-Milatovic et al., 2005). In each of these studies, there was a minimal degree of variability in the density of spines on randomly selected dendrites of Golgi-impregnated striatal neurons, suggesting a homogeneous spine loss across both populations of striatal MSNs. Our findings further support these data and also provide electron microscopic evidence for a reduced density of both D1-immunoreactive and D1-negative (presumably D2-containing) spines in the putamen of MPTP-treated monkeys. Whether these apparent discrepancies rely on species differences, chronic versus acute dopamine toxin exposure or other technical/sampling issues remain to be established.
Although the changes in relative densities of D1-immunolabeled and D1-immunonegative spines presented in our study provide strong evidence for the loss of both populations of spines in the putamen of MPTP-treated monkeys, our findings do not reveal any information on the exact number of spines in the striatum of normal and MPTP-treated monkeys. Therefore, because the tissue sections used in our study were not prepared for unbiased stereological measurements, our findings cannot be fairly compared with those obtained in rodents by Day et al. (2006). A rigorous stereological analysis of the total number of D1-immunoreactive and D1-immunonegative spines in MPTP-treated monkeys is needed to fully address this issue.
Although the broad functional and pathophysiological implications of striatal spine loss in PD remain to be established, the fact that spines are the major targets of extrinsic glutamatergic inputs from the cerebral cortex and thalamus, and the main sites of long term synaptic plasticity (Smith and Bolam, 1990; Raju et al., 2006; Bourne and Harris, 2008) strongly suggest that proper axo-spinous transmission is a central component of normal basal ganglia functioning. Recent functional evidence for highly specific interactions between convergent axo-spinous glutamatergic and dopaminergic afferents in the striatum (Bamford et al., 2004), indeed, suggests that this change in synaptic connectivity may contribute to ineffectively timed and patterned striatofugal activity, thereby lead to pathophysiological basal ganglia discharges in PD (Wichmann et al., 2007).
Acknowledgments
The authors thank Dr. T. Wichmann for some of the MPTP-treated animals used in this study. This research was supported by NIH grant R01 NS 037948 to Y. Smith and the NIH base grant to the Yerkes National Primate Research Center (RR 00165).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arbuthnott GW, Ingham CA, Wickens JR. Dopamine and synaptic plasticity in the neostriatum. J Anat. 2000;196(Pt 4):587–596. doi: 10.1046/j.1469-7580.2000.19640587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamford NS, Robinson S, Palmiter RD, Joyce JA, Moore C, Meshul CK. Dopamine modulates release from corticostriatal terminals. J Neurosci. 2004;24:9541–9552. doi: 10.1523/JNEUROSCI.2891-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–1438. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- Bogenpohl J, Pare J-P, Smith Y. Subcellular localization of Adenosine A2A receptors in the striatum and globus pallidus of monkey and rat. IXth Triennial Meeting of the International Basal Ganglia Society.2007. [Google Scholar]
- Bourne JN, Harris KM. Balancing structure and function at hippocampal dendritic spines. Ann Rev Neurosci. 2008 doi: 10.1146/annurev.neuro.31.060407.125646. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks DJ, Ibanez V, Sawle GV, Quinn N, Lees AJ, Mathias CJ, Bannister R, Marsden CD, Frackowiak RS. Differing patterns of striatal 18F-dopa uptake in Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy. Ann Neurol. 1990;28:547–555. doi: 10.1002/ana.410280412. [DOI] [PubMed] [Google Scholar]
- Cheng HW, Rafols JA, Goshgarian HG, Anavi Y, Tong J, McNeill TH. Differential spine loss and regrowth of striatal neurons following multiple forms of deafferentation: a Golgi study. Exp Neurol. 1997;147:287–298. doi: 10.1006/exnr.1997.6618. [DOI] [PubMed] [Google Scholar]
- Damier P, Hirsch EC, Agid Y, Graybiel AM. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain. 1999;122(Pt 8):1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- Day M, Wang Z, Ding J, An X, Ingham CA, Shering AF, Wokosin D, Ilijic E, Sun Z, Sampson AR, Mugnaini E, Deutch AY, Sesack SR, Arbuthnott GW, Surmeier DJ. Selective elimination of glutamatergic synapses on striatopallidal neurons in Parkinson disease models. Nat Neurosci. 2006;9:251–259. doi: 10.1038/nn1632. [DOI] [PubMed] [Google Scholar]
- Galvan A, Kuwajima M, Smith Y. Glutamate and GABA receptors and transporters in the basal ganglia: what does their subsynaptic localization reveal about their function? Neuroscience. 2006;143:351–375. doi: 10.1016/j.neuroscience.2006.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y, Gundersen HJG, Van der Zee E, West MJ. Unbiased stereological estimation of the total number of synapses in a brain region. J Neurocytol. 1996;25:805–919. doi: 10.1007/BF02284843. [DOI] [PubMed] [Google Scholar]
- Gerfen CR, Engber TM, Maham LC, Susel Z, Chase TN, Monsma FJ, Jr, Sibley DR. D1 and D2 Dopamine Receptor-Regulated Gene Expression of Striatonigral and Striatopallidal Neurons. Science. 1990;250:1429–1432. doi: 10.1126/science.2147780. [DOI] [PubMed] [Google Scholar]
- Herkenham M, Little MD, Bankiewicz K, Yang SC, Markey SP, Johannessen JN. Selective retention of MPP+ within the monoaminergic systems of the primate brain following MPTP administration: an in vivo autoradiographic study. Neuroscience. 1991;40:133–158. doi: 10.1016/0306-4522(91)90180-v. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. Chemical neuroanatomy of the basal ganglia--normal and in Parkinson’s disease. J Chem Neuroanat. 2001;22:3–12. doi: 10.1016/s0891-0618(01)00100-4. [DOI] [PubMed] [Google Scholar]
- Ingham CA, Hood SH, Arbuthnott GW. Spine density on neostriatal neurones changes with 6-hydroxydopamine lesions and with age. Brain Res. 1989;503:334–338. doi: 10.1016/0006-8993(89)91686-7. [DOI] [PubMed] [Google Scholar]
- Ingham CA, Hood SH, Taggart P, Arbuthnott GW. Plasticity of synapses in the rat neostriatum after unilateral lesion of the nigrostriatal dopaminergic pathway. J Neurosci. 1998;18:4732–4743. doi: 10.1523/JNEUROSCI.18-12-04732.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingham CA, Hood SH, van Maldegem B, Weenink A, Arbuthnott GW. Morphological changes in the rat neostriatum after unilateral 6-hydroxydopamine injections into the nigrostriatal pathway. Exp Brain Res. 1993;93:17–27. doi: 10.1007/BF00227776. [DOI] [PubMed] [Google Scholar]
- Iravani MM, Syed E, Jackson MJ, Johnston LC, Smith LA, Jenner P. A modified MPTP treatment regime produces reproducible partial nigrostriatal lesions in common marmosets. Eur J Neurosci. 2005;21:841–854. doi: 10.1111/j.1460-9568.2005.03915.x. [DOI] [PubMed] [Google Scholar]
- Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. N Engl J Med. 1988;318:876–880. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- Levey AI, Hersch SM, Rye DB, Sunahara RK, Niznik HB, Kitt CA, Price DL, Maggio R, Brann MR, Ciliax BJ, et al. Localization of D1 and D2 dopamine receptors in the brain with subtypespecific antibodies. Proc Natl Acad Sci U S A. 1993;90:8861–8865. doi: 10.1073/pnas.90.19.8861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitrano DA, Smith Y. Comparative analysis of the subcellular and subsynaptic localization of mGluR1a and mGluR5 metabotropic glutamate receptors in the shell and core of the nucleus accumbens in rat and monkey. J Comp Neurol. 2007;500:788–806. doi: 10.1002/cne.21214. [DOI] [PubMed] [Google Scholar]
- Norrholm SD, Bibb JA, Nestler EJ, Ouimet CC, Taylor JR, Greengard P. Cocaine-induced proliferation of dendritic spines in nucleus accumbens is dependent on the activity of cyclin-dependent kinase-5. Neuroscience. 2003;116:19–22. doi: 10.1016/s0306-4522(02)00560-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju DV, Shah DJ, Wright TM, Hall RA, Smith Y. Differential synaptology of vGluT2-containing thalamostriatal afferents between the patch and matrix compartments in rats. J Comp Neurol. 2006;499:231–243. doi: 10.1002/cne.21099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson TE, Kolb B. Alterations in the morphology of dendrites and dendritic spines in the nucleus accumbens and prefrontal cortex following repeated treatment with amphetamine or cocaine. Eur J Neurosci. 1999;11:1598–1604. doi: 10.1046/j.1460-9568.1999.00576.x. [DOI] [PubMed] [Google Scholar]
- Schneider JS, Pope-Coleman A. Cognitive deficits precede motor deficits in a slowly progressing model of parkinsonism in the monkey. Neurodegeneration. 1995;4:245–255. doi: 10.1016/1055-8330(95)90014-4. [DOI] [PubMed] [Google Scholar]
- Smith Y, Bolam JP. The output neurones and the dopaminergic neurones of the substantia nigra receive a GABA-containing input from the globus pallidus in the rat. J Comp Neurol. 1990;296:47–64. doi: 10.1002/cne.902960105. [DOI] [PubMed] [Google Scholar]
- Soares J, Kliem MA, Betarbet R, Greenamyre JT, Yamamoto B, Wichmann T. Role of external pallidal segment in primate parkinsonism: comparison of the effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonism and lesions of the external pallidal segment. J Neurosci. 2004;24:6417–6426. doi: 10.1523/JNEUROSCI.0836-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens B, Mueller AJ, Shering AF, Hood SH, Taggart P, Arbuthnott GW, Bell JE, Kilford L, Kingsbury AE, Daniel SE, Ingham CA. Evidence of a breakdown of corticostriatal connections in Parkinson’s disease. Neuroscience. 2005;132:741–754. doi: 10.1016/j.neuroscience.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Stern Y, Tetrud JW, Martin WR, Kutner SJ, Langston JW. Cognitive change following MPTP exposure. Neurology. 1990;40:261–264. doi: 10.1212/wnl.40.2.261. [DOI] [PubMed] [Google Scholar]
- Villalba R, Lee H, Raju D, Smith Y. Dopaminergic denervation and spine loss in the striatum of MPTP-treated monkeys. IXth Triennial Meeting of the International Basal Ganglia Society; 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba R, Verrault M, Smith Y. Spine Loss in the striatum of MPTP-treated monkeys: A correlation with the degree of striatal dopaminergic denervation. Society for Neuroscience 2006 [Google Scholar]
- West MJ. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. TINS. 1999;22:51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Kliem MA, DeLong MR. Antiparkinsonian and behavioral effects of inactivation of the substantia nigra pars reticulata in hemiparkinsonian primates. Exp Neurol. 2001;167:410–424. doi: 10.1006/exnr.2000.7572. [DOI] [PubMed] [Google Scholar]
- Wichmann T, Smith Y, Vitek J. Basal Ganglia: Anatomy and Physiology. In: Factor S, Weiner W, editors. Parkinson’s Disease: Diagnosis and Clinical Management. Demos; New York: 2007. [Google Scholar]
- Wichmann T, Soares J. Neuronal firing before and after burst discharges in the monkey basal ganglia is predictably patterned in the normal state and altered in parkinsonism. J Neurophysiol. 2006;95:2120–2133. doi: 10.1152/jn.01013.2005. [DOI] [PubMed] [Google Scholar]
- Wu DC, Teismann P, Tieu K, Vila M, Jackson-Lewis V, Ischiropoulos H, Przedborski S. NADPH oxidase mediates oxidative stress in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. Proc Natl Acad Sci U S A. 2003;100:6145–6150. doi: 10.1073/pnas.0937239100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaja-Milatovic S, Milatovic D, Schantz AM, Zhang J, Montine K, Sami A, Deutch AY, Montine TJ. Dendritic degeneration in neostriatal medium spiny neurons in Parkinson disease. Neurology. 2005;64:545–547. doi: 10.1212/01.WNL.0000150591.33787.A4. [DOI] [PubMed] [Google Scholar]