Abstract
OTK18 is a C2H2 type zinc finger protein involved in the regulation of HIV-1 replication in human mononuclear phagocytes. Previously, we reported that OTK18 expression in brain perivascular macrophages but not in microglia in HIV encephalitis brain. We have cloned the OTK18 promoter region proximal to the transcriptional start site, and determined the region responsible (−884/+1) for the basal transcriptional activity in a microglia cell line. Sequential deletion mutation analyses reveal three important response elements: YY1 (−805/−777), an HIV-1 response element for promoter activation; FoxD3 (−743/−725), a negative regulatory element; and Ets response element (−725/−707), a basal transcriptional activity response element. HIV-1 infection-induced upregulation of YY1 and c-Ets-1 protein, binding to the promoter region as determined by immunoblotting and chromatin immunoprecipitation and PCR assays, and induction of YY1 was also observed in virus-infected monocyte-derived macrophages. Silencing of FoxD3 and YY1 in the cell line by siRNA duplexes specific to these molecules significantly up- and down-regulated basal OTK18 promoter activity in FoxD3 and YY1 response element-dependent manners, respectively. On the other hand, infection of primary cultured human microglia significantly reduced YY1 expression and induced FoxD3 as determined by immunoblotting and reverse transcription real-time PCR. These data suggest that HIV-1 induces OTK18 expression through a YY1-mediated manner in human macrophages, although its gene expression is suppressed by FoxD3 upregulation and YY1 down-regulation in human microglia. This mechanism may explain the perivascular macrophage-specific expression of OTK18 in HIV encephalitis brains.
Keywords: Human, Macrophages, AIDS, Transcription Factors, Gene Regulation
Introduction
The human genome contains 564 members of Kruppel-associated box (KRAB) zinc finger proteins (Looman et al., 2002). The zinc finger genes are often clustered, such as KRAB A+B zinc finger gene family on chromosome 19q13.2 (ZNF45 family), (Dehal et al., 2001; Tang et al., 2002), and ZNF91 subfamily on chromosome 19p12-13.1 and 7 (Hamilton et al., 2006). These regions are known for frequent retroviral integration (Schroder et al., 2002), which suggests a functional correlation between retroviral gene expression and zinc finger protein expression. Interestingly, the “zinc-finger like” protein sequence of nucleocapsid protein is important for specific viral RNA recognition and packaging (Gorelick et al., 1988), suggesting a role for zinc finger domains as potential factors interfering in viral packaging.
Our previous studies demonstrated that OTK18/ZNF175, a C2H2 type zinc finger protein on chromosome 13q19.4, potently suppresses HIV-1 replication partly due to its direct inhibition of HIV-1 Tat mediated HIV-1 long terminal repeat (LTR) activation (Carlson et al., 2004a), which is mediated through two distinct regions of the LTR (Horiba et al., 2007). These studies implicate a general regulation of integrated retroviral life cycle by zinc finger proteins, although its gene regulation in the context of viral infection and integration is poorly understood. We have originally found that OTK18 is specifically expressed in brain perivascular macrophages but not in microglia of the HIV encephalitis brain (Carlson et al., 2004b). The HIV-1-induced OTK18 gene upregulation generally peaks around 7-day post infection in vitro. We hypothesize that OTK18 promoter is actively regulated by viral infection, and its gene regulation mechanism is different in macrophages and microglia. To understand the molecular mechanism of OTK18 promoter regulation and its potential therapeutic application for enhancing its antiretroviral activity, we have isolated and characterized the proximal 5’-flanking region of OTK18 promoter sequence. Here, we report that another GLI-Krüppel class of zinc finger proteins: Yingyang-1 (YY1) (Kang et al., 2004; Alvarez-Salas et al., 2005), cellular homologue to the viral E26 transformation-specific sequence-1 (c-Ets-1) (Dittmer, 2003); and forkhead box D3 (FoxD3) transcriptional repressor (Sutton et al., 1996; Katoh, 2004) are responsible for the OTK18 gene regulation. Viral infection upregulates their expression and binding to the OTK18 promoter sequence, although FoxD3 upregulation and YY1 down-regulation is prominent in microglia primary cultures. These data suggest that viral infection activates OTK18 promoter activity through differential regulation of YY1 and FoxD3 in macrophages and microglia.
Materials and Methods
Cell line and tissue culture
The human microglia cell line (a gift from M. Tardieu) (Janabi et al., 1995), which expresses the CD68 macrophage marker, was cultured at 37°C in a humidified 5% (v/v) CO2-air environment in Dulbecco’s modified Eagle’s medium (DMEM) (Sigma, St. Louis, MO) supplemented with 10% fetal bovine serum (FBS), 10 U/ml penicillin, and 10 µg/ml streptomycin (all reagents from Invitrogen).
Human monocytes were recovered from peripheral blood mononuclear cells of donors after leukopheresis and purified by counter-current centrifugal elutriation (Gendelman et al., 1988). Monocytes were cultured in DMEM, supplemented with 10% heat-inactivated human serum, 2 mM L-glutamine, gentamicin (50 µg/ml), ciprofloxacin (10 µg/ml), and macrophage colony-stimulating factor (MCSF, 1000 U/ml, Wyeth Pharmaceutical, Cambridge, MA). Monocytes were cultivated for 7 days and then referred to as monocyte-derived macrophages (MDM).
Human microglia were isolated as described (Chao et al., 1994; Borgmann et al., 2005). Fetal brain tissue (gestational age, 14 to 16 weeks) was obtained from the Birth Defects Laboratory, University of Washington, Seattle, in full compliance with the ethical guidelines of the NIH and the Universities of Washington and Nebraska Medical Center. The tissue was washed with cold Hanks balanced salt solution (Invitrogen, Carlsbad, CA) supplemented with Ca2+ and Mg2+ and then digested with 0.25% trypsin (Sigma) for 30 min at 37°C. Trypsin was neutralized with fetal bovine serum (FBS), and the tissue was further dissociated to obtain single-cell suspensions. The cells were resuspended in DMEM supplemented with a mixture containing 10% heat-inactivated FBS, 1,000 U of purified recombinant human macrophage colony stimulating factor (MCSF) per ml, penicillin and streptomycin (50 µg/ml), and 100 µg of neomycin per ml. The mixed culture was maintained under 5% CO2 for 7 days, and the medium was fully replaced to remove any cell debris. The microglia cells released with further incubation were collected and purified by preferential adhesion. The purity of microglia was confirmed by immunocytochemistry using anti-CD68 (>98%) and anti-glial fibrillar acidic protein (astrocyte marker, <2%). Microglia were cultured as adherent monolayers at a density of 2 × 106 cells/well in a 6-well plate and floating cells removed after 4 hours. All tissue culture reagents were screened before use and found negative for endotoxin (<10 pg/ml; Associates of Cape Cod, Inc., Woods Hole, MA) and mycoplasma contamination (Gen-probe II; Gen-probe Inc., San Diego, CA).
OTK18 promoter luciferase reporter gene construction
Matrix search of the 5’-flank proximal promoter region by the Transfac/Match program (http://www.gene-regulation.com/cgi-bin/pub/programs/match/bin/match.cgi) identified the cluster of response elements in −884/−565 region of the promoter. The −884/+1 and −565/+1 OTK18 promoter regions were PCR amplified and subcloned from a BAC clone BC330783 (CIT-HSPC_470E3), which contains OTK18 open-reading frame and 5’-flanking region, into pGL3-basic vector (promoter-less luciferase reporter plasmid, Promega, Madison, WI), and designated as pGL3-(−884/+1) and pGL3-(−565/+1) reporter constructs. The pGL3-(−884/+1) reporter construct was used as a template for the construction of the OTK promoter truncation and deletion clones developed by PCR and QuickChange II site-directed mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer’s protocol. All constructs were made using the same 3′ primer and the respective 5′ primer listed in Table 1: Primer 38 for pGL3-(−805/+1), 38A for pGL3-(−777/+1), 38B for pGL3-(−743/+1), 48 for clone pGL3-(−725/+1), 48A for pGL3-(−707/+1), 48B for clone pGL3-(−690/+1), 48C for clone pGL3-(−674/+1), 58 for pGL3-(−645/+1), 68 for pGL3-(−565/+1), 78 for pGL3-(−479/+1), DYY1 for pGL3-[−805/+1(ΔYY1) (containing null mutation in YY1 response element), FoxD3 for pGL3-[−746/+1(FoxD3*) (containing null mutation in FoxD3 response element), and ETS for pGL3-(−725/+1) (containing null mutation in ETS element). PCR was done using 100 ng of template, 200 nM of each primer and Herculase polymerase (Stratagene, La Jolla, CA) at 3 min at 94°C followed by 31 cycles of 1 min at 94°C, 1 min at 55°C and 1 min at 72°C. Amplicons were gel purified from 1% low melting agarose gel using Qiaquick gel extraction kit (Qiagen, Valencia, CA). Amplicons and pGL3-basic vector were digested overnight at 37°C with KpnI and HindIII and gel purified. Cut pGL3basic was dephosphorylated with calf intestinal alkaline phosphatase for 45 min at 37°C. Amplicons were ligated with cut pGL3basic for 20 min at room temperature with T4 ligase and transformed into DH5α E. coli competent cells (Invitrogen). All the clones were screened by KpnI and HindIII double digestion and the sequences were confirmed by the DNA Sequencing Core Facility.
Table 1.
Oligonucleotide sequences for gene constructions
| 3' primer: | 5'GGAATGCCAAGCTTACTTAGATCGC |
| 5’ primer 38: | 5'AAGGTACCCTCGAGTTTCCCATAATCAGCCATTTT3' |
| 5’ primer 38A: | 5'AAGGTACCCTCGAGTTATGATTGCTATTACTCACC3' |
| 5’ primer 38B: | 5'AAGGTACCCTCGAGTTTTTGTTTATTTGATTGAAC3' |
| 5’ primer 48: | 5'AAGGTACCCTCGAGACAACCTCTTCCTGAAAATT3' |
| 5’ primer 48A: | 5'AAGGTACCCTCGAGTTCTGCCCAGTTGGGCTGGC3' |
| 5’ primer 48B: | 5'AAGGTACCCTCGAGGGCTGACATTGATCTCAGTAA3' |
| 5’ primer 48C: | 5'AAGGTACCCTCGAGAGTAATTGTGTTTTTTTCCTT3' |
| 5’ primer 58: | 5'AAGGTACCCTCGAGTTATATTTTTTTCATTCGCC3' |
| 5’ primer 68: | 5'AAGGTACCCTCGAGGTTACTTCATCAATAGGTTC3' |
| 5’ primer 78 | 5'AAGGTACCCTCGAGCATTTTCAAAAGTGCTATTA3' |
| 5’ primer DYY1: | 5'AAGGTACCTTTCCCATAATCAGGGATTTTTTTAATTTTATGATTGC3' |
| 5’ primer FoxD3: | 5'AAGGTACCTTTTACTTTATTTGATTGAACAACCTCTTCCTG3' |
| 5’ primer ETS: | 5'AAGGTACCACAACCTCAACTGAAAATTCTGCCCAGTTG3' |
Pseudotyped HIV-1 generation and viral infection
Vesicular stomatitis virus-glycoprotein (VSV-G) pseudotyped dual-tropic HIV-1 subtype B YU-2 strain (Li et al., 1991) was generated by co-transfection of human embryonic kidney 293T cells with pYU-2 plasmid with pHIT/G plasmid (encoding VSV-G) (Fouchier et al., 1997) using Fugene-6 DNA transfection reagent (Roche Diagnostics, Indianapolis, IN). 48 hours after transfection, media were collected, and the virus titer was quantified by HIV-1 reverse transcriptase activity as described (Carlson et al., 2004a). Human microglial cells line or primary culture microglia were infected with 2,500 cpm/ml of non-pseudotyped or pseudotyped YU2 or 10,000 cpm/ml of non-pseudotyped HIV-1ADA for 4 hrs, followed by incubation with fresh media for the study.
Luciferase assays
Human microglial cells (105 cells/well of 24-well plates, Fisher Scientific, Pittsburg, PA) were transfected with luciferase vector (300 ng, pGL3 series or pHIV-LTR Luciferase vector), pTK-RL (50 ng, thymidine kinase-promoter driven Renilla luciferase expression vector as internal control) using CellPhect reagent (Promega) as described (Carlson et al., 2004a; Horiba et al., 2007). Twenty-four hours after transfection, cells were infected with different doses of HIV-1 strains (ADA, pseudotyped YU2, or YU2) for 4 hrs, and the cell extract was subjected to dual-luciferase assay at 48 hrs after the infection as measured by luminometer (Berthold Systems Inc., Aliquippa, PA) using the Dual-Luciferase kit (Promega). The data were presented as luciferase activity/TK-RL activity ratio.
PCR Detection of HIV-1 infection
We detected the HIV DNA in infected cells by PCR. Twenty-four hours after virus infection, genomic DNA was extracted from human microglial cells and subjected to PCR analysis of DNA preintegration complex formation as described previously using PCR primer sets amplifying the region between nucleotides 685 and 789 of the gag sequence: h-FR (5′-ACATCAAGCAGCCATGCAAAT-3′) and h-REV (5′-ATCTGGCCTGGTGCATAGG-3′) (Alfano et al., 2005). The thermal cycling conditions were 50°C for 2 min, 95°C for 12 min, and 30 cycles of 95°C for 15 s and 65°C for 1 min. One μl of the first PCR reaction was subjected to second round of 25 or 30 cycles of PCR, and the samples were subjected to 1.5% DNA agarose gel electrophoresis and ethidium bromide staining.
Immunoblotting
Cell protein lysates were obtained by incubating 10 min on ice with lysis buffer (0.3 M KCl, 20 mM Tris, 1% Triton-X-100, 2 mM EDTA, 500 µM NaOVa, 2 mM NaF). Lysates were centrifuged at 15,000 rpm, 10 min, 4°C with the supernatants collected. BCA assay (Pierce, Rockford, IL) was used to quantify the supernatant’s protein concentration. Equal levels of protein were run on 10% SDS-PAGE gels and transferred to 0.45 µM low-fluorescence PVDF membranes (Millipore, Billerica, CA). Membranes were blocked in blottoA [5% dried milk in TBST (150 mM NaCl, 50 mM Tris-Cl, pH 7.5, 0.05% Tween-20], and incubated with antibodies to YY1 (H-414, 1 µg/ml, Santa Cruz Biotechnology, Santa Cruz, CA), FoxD3 (MAB2819, 2 µg/ml, R & D Systems, Minneapolis, MN), β-actin (100ng/ml, Sigma), or c-ETS-1 (N-276, 1 µg/ml, Santa Cruz Biotechnology). After stringent rinsing in TBST, blots were incubated with horseradish peroxidase (HRP)-conjugated anti-rabbit or HRP-conjugated anti-mouse antibody. Blots were visualized after washing with TBST using ECL Plus western blotting detection system (GE Healthcare, Piscataway, NJ).
Chromatin Immunoprecipitation and PCR (ChIP) Assay
ChIP assays were performed using the ChIP-IT™ Assay Kit (Active Motif, Carlsbad, CA) according to the manufacturer’s protocol. Briefly, 4.5 × 106 cells were plated on 15-cm-diameter dishes. 16 hours after plating, selected cells were incubated with pYU2 virus (27,000 cpm/plate) for four hours and subsequently removed. At 48 hours post infection, media was removed and cells were crosslinked with formaldehyde (final concentration 1%) for 10 min at room temperature. After crosslinking, cells were collected then nuclei collected per kit instructions. Genomic DNA was enzymatically digested to reduce the DNA length to between 200 and 1,000 bp for 10 min 37°C. To normalize the amount of genomic DNA, the concentration was determined through averaging 3 aliquots of chromatin from which the genomic DNA was isolated per kit instructions. Chromatin, after being normalized for the amount of genomic DNA, was precleared by incubation with protein-G beads for 1.5 h at 4°C. The supernatant was incubated with anti-RNA polymerase II complex (positive control in ChIP-IT™ Assay Kit, Active Motif), YY1 (H-414, 2 µg, Santa Cruz Biotechnology), FoxD3 (MAB2819, 2 µg, R&D Systems), c-ETS-1 (N-276, 2 µg, Santa Cruz Biotechnology), or preimmune mouse IgG (negative control, 2 µg, Sigma) at 4°C overnight. Immunocomplexes were collected with protein-G sepharose FF (Amersham/GE Healthcare) at 4°C for 1.5 h. After extensive washing and reversal of crosslinks by heating 65°C for 1.5h, followed by proteinase K treatment 42°C for 1.5 h, DNA was recovered by DNA column per kit instructions and used as a template for PCR to amplify the region including the transcription factor binding sites of interest in the OTK promoter. The primer pairs were 5′TTCAGATCCTCACTCAGCCCTGC3′ (−834) (ChSense) and 5′GATCAATGTCAGCCAGCCCAACT3′ (−677) (CHAS) for amplification. Each PCR used 94°C for 3 min followed by 33 cycles of 20 s at 94°C, 30 s at 59°C and 30 s at 72°C. The PCR products were electrophoresed on 1.5% agarose gels and stained with ethidium bromide (Sigma).
siRNA duplex transfection
Pre-designed and validated siRNA duplexes against human FoxD3 (SI00420616), YY1 (SI00051912), and c-Ets-1 (SI00074249) were purchased from Qiagen. For the luciferase reporter study, cells (105 cells/well in 24-well plate) were co-transfectd with 0.8 µg of siRNA duplex or pJ6 empty vector, 100 ng of pGL3-serires luciferase reporter plasmid, and pTK-RLusing CellPhect reagent (Promega) according to the manufacturer’s instructions. Control Cy3-labeled siRNA (Ambion, Austin, TX) was used for monitoring the transfection efficiency, and >90% transfection efficiency was normally achieved in this system.
Real-time reverse-transcription polymerase chain reaction (RT2-PCR)
RT2-PCR was performed as described previously (Carlson et al., 2004b). Briefly, total RNA from virus-infected or uninfected primary cultured microglia (2×106 cells per prep) at 7 day post-infection was isolated using Trizol (Invitrogen, Carlsbad, CA) and Mini RNA isolation kit (Qiagen). Poly-A RNA was reverse transcribed by Moloney murine leukemia virus (M-MuLV) reverse transcriptase and oligo-dT12–18 (Stratagene). After quantification of transcribed cDNA, 100ng of cDNA was subjected to real-time PCR per reaction using commercially available certified primer sets and probe for human FoxD3, YY1, c-Ets-1, and β-actin cDNA (Applied Biosystems, Foster City, CA) using ABI 7700. The cycle-threshold (Ct) cycle of each sample was determined by the real-time PCR, and the results were presented as ΔCt (average Ct cycle of sample group - average Ct cycle of β-actin group) (N = 8). Lower ΔCt corresponds to higher copies of original mRNA in the total RNA.
Statistics
All data were normally distributed and presented as mean values ± standard errors of mean (SEM). In case of multiple mean comparisons, the data were analyzed by analysis of variances (ANOVA), followed by Newman-Keuls (for one-way ANOVA) or Bonferroni (for two-way ANOVA) multiple comparison tests using statistics software (Prism 4.0, Graphpad Software, Inc., San Diego, CA). In case of single mean comparison, data were analyzed by Student’s t-test. A p value of less than 0.05 was regarded as significant difference.
Results
Deletion mutation analysis of OTK18 5’-flank proximal promoter region
The eukaryotic promoter region is commonly divided into three parts: 1) the core promoter responsible for the actual binding of the transcription apparatus, typically situated ~35 bp upstream of the transcription start site (TSS); 2) the proximal promoter, a region containing several regulatory elements ranging up to a few hundred base pairs upstream of the TSS; and 3) the distal promoter, which can range up to several thousands of base pairs upstream of the TSS and contains additional regulatory elements called enhancers and silencers (Abeel et al., 2008). To understand the induction of OTK18 by HIV-1, we focused on the −1000/+1 range of the OTK18 5’-flank proximal promoter region, and using Transfac/Match program we identified a cluster of response element in −884/−565 range of the promoter region. We subcloned the −884/+1 and −565/+1 regions from a corresponding human genome PAC clone into the pGL3-basic luciferase reporter plasmid and examined their promoter activities in a microglia cell line by a transient expression system in the context of viral infection.
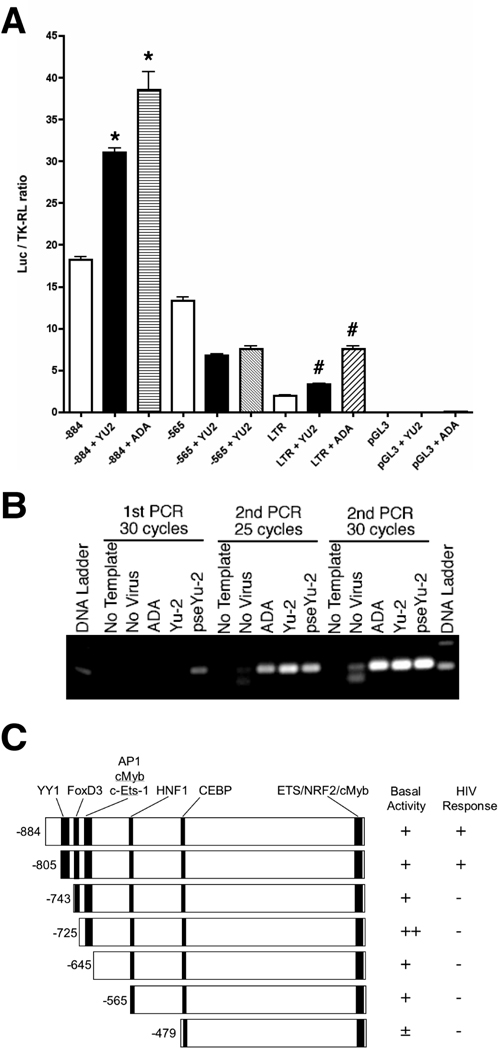
The microglia cell line was originally developed by the transformation of human primary microglia with SV40 large-T antigen (Janabi et al., 1995; Janabi et al., 1998); however, after several passages this cell line expresses CD68, a macrophage maker (Holness and Simmons, 1993) but CD45−CD163−CD14low as determined by FACS (data not shown). In contrast, primary cultured MDM and human microglia are CD45+CD163+CD14+ and CD45+CD163−CD14+, respectively, suggesting that the original microglia-like phenotype of the cell line is lost. However, this cell line can be easily transfected with DNA by lipofection and infected by HIV-1ADA and HIV-1YU2 in low efficiency. We transfected OTK18 promoter-luciferase reporter gene (−884/+1 or −565/+1) and infected with HIV-1ADA and HIV-1YU2 (Fig. 1A). Viral infection significantly enhanced the promoter activity of −884/+1 region but not −565/+1. As a positive control, we also transfected HIV long-terminal repeat-luciferase reporter plasmid (LTR-Luc) as described (Carlson et al., 2004a), and confirmed the viral infection-induced LTR activation in this system (Fig. 1A). To confirm the viral infection of the cell line, genomic DNA was isolated from infected cells at 24 hr post-infection and subjected to 2 rounds of PCR against proviral gag sequence (Fig. 1B). Infection of VSV-G pseudotyped YU2 shows viral DNA synthesis from the first round of PCR, although 2 rounds of PCR were necessary after infection of non-pseudotyped ADA or YU2, suggesting that viral infection is inefficient in the transformed cell line (Fig. 1B). Thus, we infected this cell line with pseudotyped YU2 for the rest of the study.
Figure 1. OTK18 promoter luciferase reporter gene induction and viral infection of a human cell line.
A. Cells were transfected with luciferase reporter plasmid pGL3-(−884/+1), pGL3-(−565/+1), pHIV-LTR-Luc, or control pGL3 with pTK-RL (thymidine kinase promoter-driven Renilla luciferase expression vector), and infected with VSV-G pseudotyped HIV-1YU2 (YU2, 2,500 cpm/ml) or HIV-1ADA (ADA, 2,500 cpm/ml). Dualluciferase assays were performed at 48 hrs after the infection. The results were shown as luciferase activity/TK-RL activity ratio. * denotes p < 0.01 as determined by ANOVA and Newman Keuls post-hoc, respectively. B, Detection of HIV DNA after viral infection. Cells were infected with non-pseudotyped ADA (10,000 cpm/ml), Yu-2 (2,500 cpm/ml), and pseudotyped Yu-2 (pseYu-2), and genomic DNA was isolated at 24 hr after infection. The samples were subjected to 2 rounds of PCR against proviral gag sequence as described (30 cycles in 1st round and 25 or 30 cycles in 2nd round). C, Schematic presentation of OTK18 promoter response elements (YY1, FOXD3, cMyb/AP1, C/EBP, c-Ets-1, NRF, cMyb), design of deletion mutants (−884/+1, −805/+1, −743/+1, −725/+1, −645/+1, −565/+1, and −479/+1), and results of basal luciferase activity and induction by pseudotyped YU2 infection.
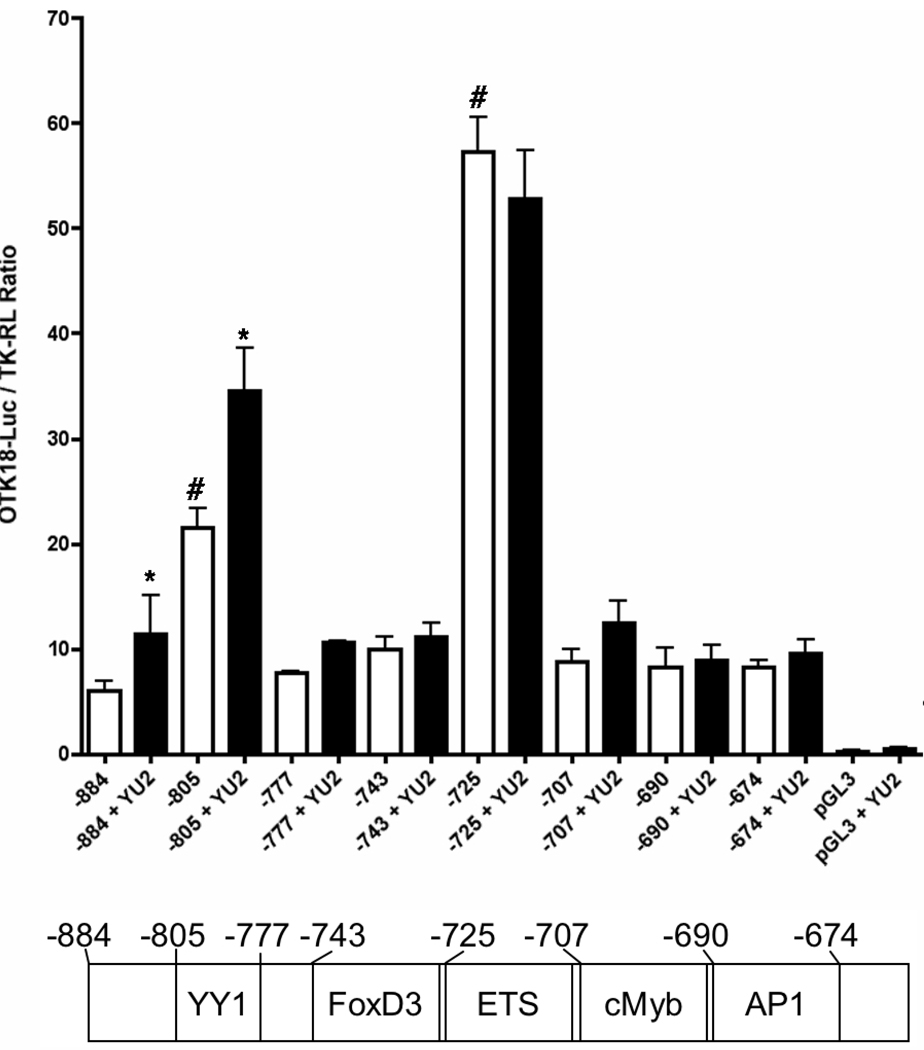
Transfac/Match program identified several transcriptional elements in the −884/+1 region (Fig. 1C). Thus, we introduced site-directed truncation mutants of the 5’-proximal region to dissect the transcriptional elements using −805/+1, −743/+1, −725/+1, −645/+1, −565/+1, and −479/+1 and examined their basal and HIV-inducible promoter activities. Viral-induced promoter activation was present in −884/+1 and −805/+1 but not in the other constructs, whereas basal activity was present in all the constructs except −479/+1 (Fig. 1C). Interestingly, basal activity was highly increased in −725/+1. Based on these data, we focused on several elements in the −805/−674 region, which contains YY1, FoxD3, c-Ets-1, cMyb, and AP1 sites (Fig. 2, lower panel), and constructed −777/+1, −707/+1, −690/+1, and −674/+1 truncation mutants. As shown in Fig. 2, we found that the deletion of YY1 element (−777/+1 and after) results in loss of HIV-1-induced promoter activation. Deletion of FoxD3 element (−725/+1) significantly increased basal activity but lacks in HIV-1 induction. This increased basal activity is diminished by deletion of c-Ets-1 element (−707/+1). There was no searchable element in the −743/−725 region other than FoxD3, suggesting that it is responsible for the suppression. These data suggest that the YY1 element (−805/−777) corresponds to the viral induced promoter activation, the FoxD3 element (−743/−725) suppresses the basal promoter activity, and the c-Ets-1 element (−725/−707) mediatesthe high basal promoter activity in the absence of FoxD3 element.
Figure 2. Deletion mutation analysis of the −848/−674 region of OTK18 5’-proximal promoter region.
The cell line was transfected with a series of pGL3 constructs with pTK-RL, infected with pseudotyped YU2 (2,500 cpm/ml, black columns), and subjected to dual-luciferase assays. * and # denote p<0.05 vs. uninfected control of the same pGL3 construct-transfected group or −884/+1 group (white column) as determined by ANOVA and Newman Keuls post-hoc, respectively.
YY1, c-Ets-1, and FoxD3 regulate basal and virus-induced OTK18 promoter activity
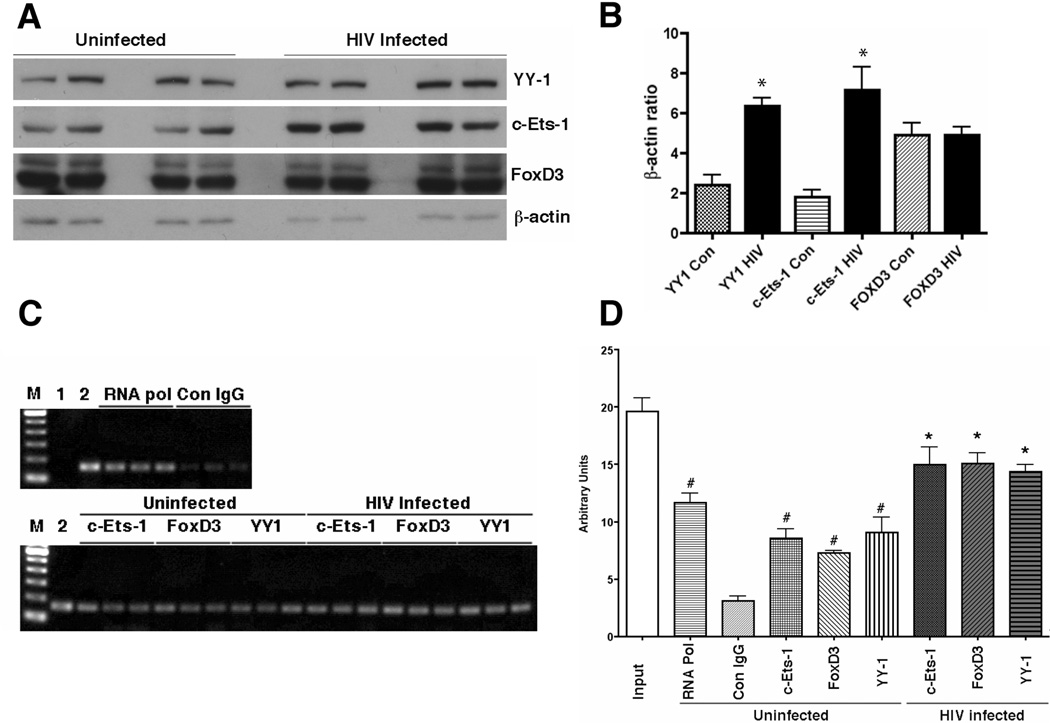
YY1 is a ubiquitously expressed transcriptional factor involved in inflammation and toll-like receptor (TLR)-induced cyclooxygenase-2 gene expression in macrophages (Bjorkbacka et al., 2004; Joo et al., 2007). FoxD3, however, is documented as a transcriptional repressor restricted in human embryonic stem cells (known as genesis) (Sutton et al., 1996) or in the Spemann organizer and later in premigratory neural crest cells in Xenopus laevis embryo (known as XFD-6) (Pohl and Knochel, 2001). C-Ets-1 is involved in a variety of developmental and cellular process, expressed in monocytic lineage cells, including macrophages, and involved in gene expression of key growth factor receptors and inflammatory factors, including the receptor for MCSF (c-fms) (Reddy et al., 1994) and heme oxygenase-1 (Chung et al., 2005). We examined and confirmed the expression of these transcriptional factors in the cell line by immunoblotting (Fig. 3A), and found that viral infection significantly increased the expression of YY1 and c-Ets-1 but no FoxD3 (Fig. 3B). Since FoxD3 expression has not been documented in human macrophages, we have also confirmed the FoxD3 mRNA expression by RT-PCR using two different PCR primer sets in the cell line and primary human MDM (data not shown), establishing the expression of FoxD3 in both mRNA and protein in human macrophages. We examined if these molecules bind to the corresponding region of OTK18 promoter, and the effect of viral infection by ChIP assay using a primer set which amplifies −834/−677 region of OTK18 promoter. As shown in Fig 3C and D, c-Ets-1, FoxD3, and YY1 bind to the promoter region, which is significantly higher than the control group (Con IgG), and its binding activity is significantly increased by viral infection. RNA polymerase II serves as a positive control group, which binds to any transcriptional active site of the genome. These data suggest that c-Ets-1, FoxD3, and YY1 actually exist in the cell line, and bind to the identified specific OTK18 promoter region, which is enhanced by viral infection.
Figure 3. Immunoblotting and ChIP assay of transcriptional factors after viral infection.
A, The cell line was infected with pseudotyped YU2 (2,500 cpm/ml) and total cell extract was prepared at 2 days post-infection. The samples were subjected to SDS-PAGE and immunoblotting of YY1, c-Ets-1, FoxD3, and β-actin. B, Densitometric quantification of protein levels and normalization by β-actin protein level (n=4). * denotes p<0.05 vs. uninfected control as determined by ANOVA and Newman Keuls post-hoc, respectively. C, ChIP assay of transcriptional factors and OTK18 promoter region in the cell line. Cells were infected with pseudotyped YU2 (2,500 cpm/ml), fixed with 4% paraformaldehyde, and subjected to ChIP assay according to the manufacturer’s instructions. After immunoprecipitation of chromatin complex with specific antibodies against RNA polymerase II complex (RNA pol), c-Ets-1, FoxD3, YY1, or control IgG (Con IgG), decrosslinked and released genomic DNA form precipitated chromatin complex was subjected to PCR against −834/−677 of OTK18 promoter sequence. Lame M, DNA standard markers from 100 to 600 bp; Lane 1, PCR without template genomic DNA; Lane 2, decrosslinked chromatin complex without immunoprecipitation (input value). D, Quantification of DNA agarose gel electrophoresis image. # and * denote p<0.05 vs. Con IgG or uninfected group of the same transcriptional factor immunoprecipitation as determined by ANOVA and Newman Keuls post-hoc, respectively.
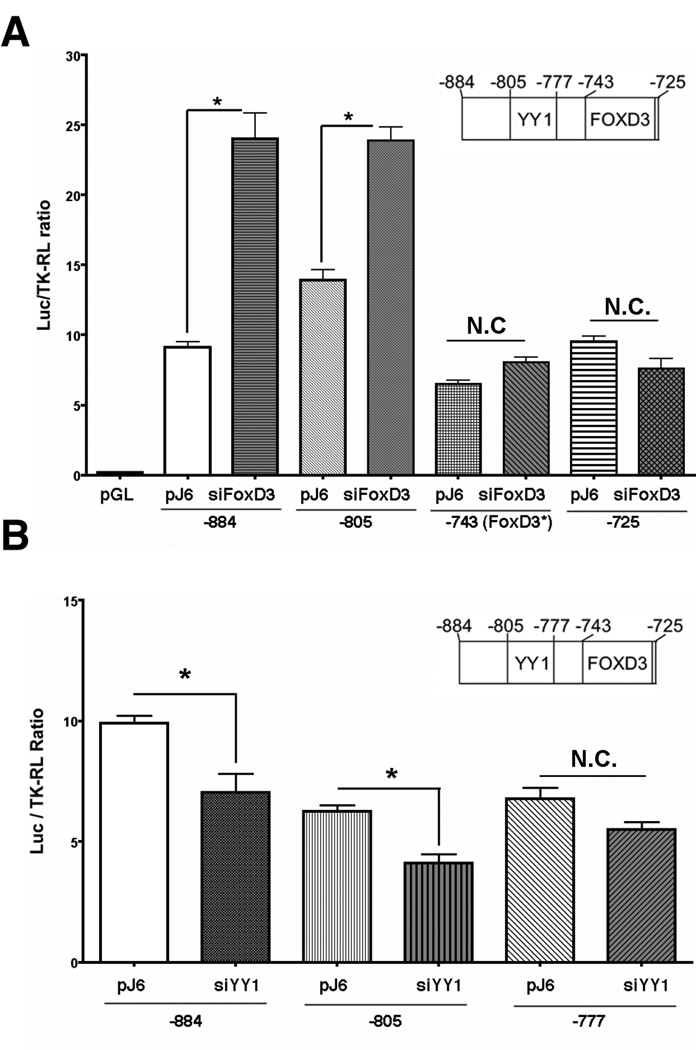
We further examined the effect of silencing the endogenous FoxD3 and YY1 on OTK18 promoter activity. For that purpose, pre-designed and verified siRNA duplex for human FoxD3 or YY1 was co-transfected with various pGL3-series luciferase and pTK-RL plasmids. The transfection efficiency of siRNA was >90% as determined by the transfection of Cy3-labeled siRNA control (data not shown). Three days after the siRNA transfection, the cells were co-transfected with pGL-series of OTK18 promoter luciferase reporters and TK-RL for the luciferase assays. Silencing of FoxD3 significantly increased the basal promoter activity of −884/+1 and −805/+1. This effect was diminished in −743/+1(FoxD3*), in which the consensus sequence of FoxD3 response element was mutated, and in −725/+1, which lacks FoxD3 response element (Fig. 4A). Silencing of YY1 significantly reduced the basal promoter activity of +884/+1 and −805/+1 but not −777/+1 lacking the YY1 response element as compared to the negative control (pJ6 empty vector-transfected group) (Fig. 4B). The basal activity of pGL3-[−805/+1(ΔYY1)], containing null mutation in the YY1 response element, was also reduced by YY1 silencing (data not shown), suggesting that YY1 may activate OTK18 promoter through its binding to another region. Indeed, YY1 can form a heterodimeric AP1 complex to bind to the AP1 site, which is also located in the −690/−674 region. These data suggest that endogenous FoxD3 suppresses basal promoter activity of OTK18 through its interaction with the FoxD3 response element at −743/−725, and YY1 enhances its basal activity through its interaction with the YY1 response element at −805/−777. Our attempts to silence endogenous c-Ets-1 were unsuccessful due to significant cell death after transfection of siRNA for human c-Ets-1, suggesting its role in cell survival. Over-expression of human YY1 or FoxD3 recombinant molecules by co-transfection of these expression vectors did not alter the promoter activity of OTK18, suggesting that some cellular signaling in addition to the upregulation of exogenous molecules may be necessary for their transcriptional activity in this system.
Figure 4. Effect of FoxD3 and YY1 silencing on OTK18 promoter regulation.
The cell line was co-transfected with pJ6 empty plasmid (negative control) or pre-verified siRNA against human FoxD3 (A) or YY1 (B), pGL3 series [−884/+1, −805/+1, −777/+1, −743/+1(FoxD3*), or −725/+1], and pTK-RL. PGL3-[−743/+1(FoxD3*)] reporter plasmid has null mutation in FoxD3 response element. The cells were subjected to dual-luciferase assay 2 days after the reporter gene transfection. * denotes p<0.05 vs. pJ6 transfected group of the same pGL3 series transfection as determined by ANOVA and Newman Keuls post-hoc, respectively. N.C. represents no statistical changes between groups.
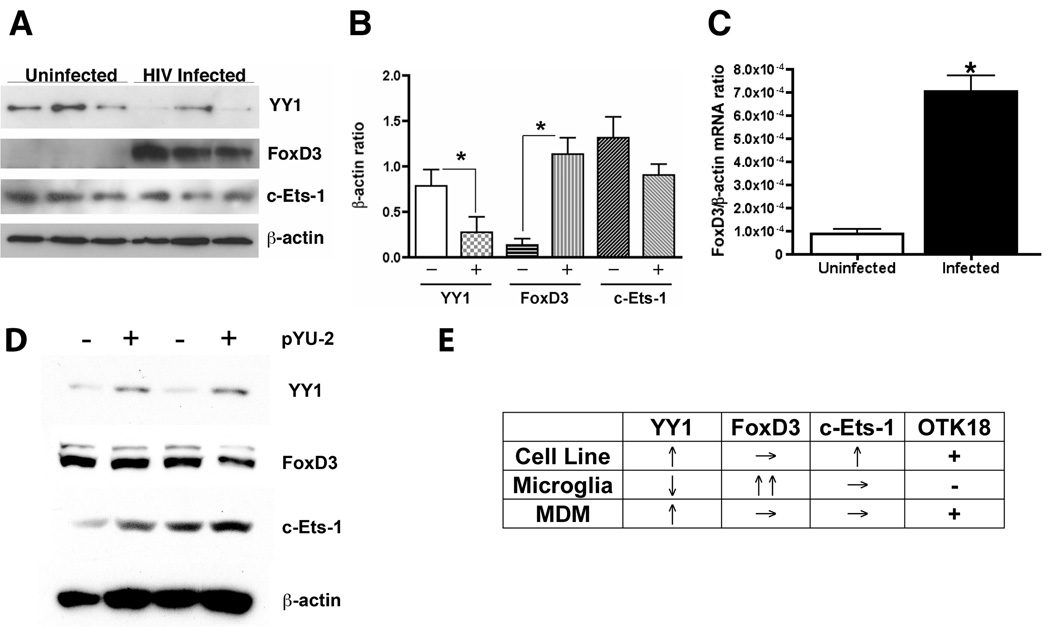
Differential regulation of YY1 and FoxD3 expression in virus-infected human primary microglia and MDM
Since our previous study identified the differential expression of OTK18 between brain perivascular macrophages and microglia of HIVE encephalitis brain, we examined if these transcriptional factors are differently regulated in human microglia. For that purpose, we cultured human primary microglia, infected with HIV-1, and subjected to immunoblotting. As shown in Fig 5A and B, YY1 was significantly down-regulated by viral infection. In contrast, FoxD3 expression was significantly upregulated by viral infection. The expression of c-Ets-1 was, however, unchanged by viral infection. We also confirmed the upregulation of FoxD3 in mRNA level as determined by RT2-PCR (Fig. 5C), demonstrating significantly higher FoxD3 mRNA level in infected group. Lastly, we tested how viral infection of MDM alter the expression of the three target transcription factors to determine if the data obtained from the cell line mimics the OTK18 gene regulation in MDM under the same experimental design. We confirmed upregulation of YY1 expression in virus-infected human MDM, whereas the expression of FoxD3 and c-Ets-1 was unchanged (Fig. 5D). These data suggest that OTK18 gene expression is suppressed by down-regulation of YY1 and upregulation of FoxD3 in human microglia, whereas viral infection of the cell line and MDM enhances YY1 expression (Fig. 5E).
Figure 5. Different induction of YY1 and FoxD3 in human primary microglia.
A, Immunoblotting of transcriptional factors in primary human microglia at 2 days after viral infection (pseudotyped YU2, 2,500 cpm/ml). B, Densitometric analysis of immunoreactive bands and normalization by β-actin band intensity in A (n=3). Uninfected (−) and infected (+) groups were statistically compared by ANOVA and Newman Keuls post-hoc (* denotes p<0.05). C, RT2-PCR analysis of human FoxD3 mRNA levels in uninfected or viral infected human microglia using pre-certified primer sets and probe. Lower ΔCt value is indicative of higher FoxD3 mRNA level in infected microglia. * denotes p<0.01 as determined by Student’s t-test. D, Immunoblotting of transcriptional factors in human monocyte-derived macrophages at 2 days after viral infection (pseudotyped YU2, 2,500 cpm/ml). E, summary of viral gene regulation of transcriptional factors in cell line, primary microglia, and MDM.
Discussion
In this paper, we have performed detailed mutational characterization of 884-bp fragment of the 5’-flank proximal region of OTK18 promoter and identified its unexpected regulation of three transcriptional factors, YY1, FoxD3, and c-Ets-1. We have originally reported the viral induction of OTK18 gene in human monocyte-derived macrophages (Carlson et al., 2004a), and this study identified that it is mediated through YY1 interaction with the YY1 response element in −805/−777 of the promoter sequence. Viral infection can non-specifically upregulate a number of transcriptional factors, thus OTK18 promoter activation may not be specific to HIV-1 infection. Indeed, we observed OTK18 promoter upregulation by recombinant adenovirus infection (unpublished observation), suggesting that both viral infection and inflammation can contribute to promoter regulation. However, we did not observe OTK18 expression in other neurodegenerative disorders or cytomegalovirus encephalitis patients (Carlson et al., 2004b), suggesting that there is some specificity of OTK18 gene expression in certain viral species.
YY1 protein was originally affinity purified as a DNA binding protein of the P5 promoter of adeno-associated virus, which is normally a silent promoter (Shi et al., 1991). It is known as a multifunctional zinc finger protein that can act as a transcriptional repressor, an activator, or an initiator element binding protein that directs and initiates transcription (Shi et al., 1997). Recent studies support the close relationship between viral infection, replication, and YY1 activation in T-cells and other HIV-1 co-receptor expressing cell lines. YY1 represses HIV-1 LTR activity (Margolis et al., 1994; Romerio et al., 1997; Coull et al., 2000; He and Margolis, 2002; Ylisastigui et al., 2005) and down-regulates HIV-1 chemokine co-receptors CXCR4 (Moriuchi et al., 1999; Cristillo and Bierer, 2003) and CCR5 (Moriuchi and Moriuchi, 2003). In addition, YY1 enhances osteoclast differentiation through activation of tartrate-resistant acid phosphatase gene expression (Shi et al., 2004) and TLR-induced cyclooxygenase-2 gene induction (Joo et al., 2007). Since HIV-1 endocytosis activates TLR in multiple monocytic lineage cells (Beignon et al., 2005; Alter et al., 2007; Meier et al., 2007), this could be the mechanism of viral-induced OTK18 gene expression. However, our attempt to stimulate several OTK18 deletion mutants with known dsRNA, ssRNA, and ssDNA TLR ligands [poly(I:C) for TLR3, imiquimod for TLR7/8, and CpG for TLR9) was unsuccessful (data not shown). Thus, this pathway is unlikely to be involved in the OTK18 promoter activation. However, over-expression of YY1 did not readily enhance OTK18 promoter activity in our system. This could be due to the fact that YY1 requires interaction with p300 and histone deacetylase (HDAC1/2) complexes for its deacetylation [Gordon, 2006 #984], since hyper-acetylation of YY1 is associated with its repression activity (Yao et al., 2001). Over-expression of YY1 is thus insufficient to alter its acetylation status to convert its function from a repressor to an activator. Thus, it is possible that viral infection and release of ssRNA activate ssRNA-sensing TLRs, which leads to the YY1-mediated transcriptional activation with its interaction with HDAC1/2 and p300.
FoxD3 belongs to the winged helix (formally HNF-3/forkhead) transcriptional regulatory family and is implicated in the regulation of a pluripotent gene Nanog, a downstream molecule of leukemia inhibitory factor and bone morphologic protein 4 signaling for self-renewal of embryonic stem cells (Pan and Thomson, 2007). It regulates neuronal migration and differentiation in the neural crest (Dottori et al., 2001; Pohl and Knochel, 2001). To the best of our knowledge, its expression in monocytic lineage and induction by viral infection is undocumented. Interestingly, FoxD3 gene expression is significantly enhanced in the prefrontal cortex of major depression disorders (Kang et al., 2007). Although its expression seems to be restricted to NeuN-positive neurons in the adult brain, microglial markers were unused for the co-localization study and it is possible that FoxD3 is also expressed in microglia in diseased brain. This is especially interesting since severe depression is a common complication of chronic AIDS patients including the elderly, women, adolescents, and children (Starace et al., 2002; Cook et al., 2004; Gaughan et al., 2004; Misdrahi et al., 2004), and FoxD3 expression might be a common mechanism in these two disorders. Further study will be necessary to correlate FoxD3 expression and depression in AIDS patients, and its role in cellular function and viral replication in microglia.
Ets-1 plays an important role for the promoter activity of HIV-1 LTR in T-cells (Seth et al., 1993; Freed and Martin, 2001). Disruption of the LTR region containing the Ets response element markedly impaired HIV-1 replication in activated peripheral blood mononuclear cells (Kim et al., 1993). Ets-1 has been shown to cooperatively interact with USF-1 in LTR activation via their adjacent DNA binding domains (E-box and Ets response element, respectively) (Sieweke et al., 1998). We also found that OTK18 suppresses HIV-1 Tat-induced LTR activation through its Ets response element (Horiba et al., 2007). This study suggests that Ets element (−725/−707) is responsible for the basal promoter activity of OTK18. This prompted us to test if OTK18 suppresses its own promoter activity via binding to the Ets response element. Indeed, co-transfection of OTK18 expression vector suppressed −884/+1 promoter activity (data not shown) suggesting a negative feedback system of OTK18 gene expression in monocytic lineage. In summary, our data suggest that viral infection of the cell line and potentially human macrophages activates OTK18 gene transcription by binding of YY1 to the YY1 response element on the OTK18 promoter. FoxD3 play a role in suppressing the promoter activity but its expression level was unchanged by viral infection. Induced OTK18 suppresses HIV-1 LTR promoter activity via its binding to the negative regulatory element and c-Ets-1 response element, and it may also suppress its own promoter activity as a negative feedback system. In case of human primary microglia, viral infection down-regulates YY1 and upregulates FoxD3 expression, which leads to suppression of OTK18 promoter activity, resulting in poor OTK18 expression in HIV-1 infected microglia. Thus, differential regulation of FoxD3 and YY1 may account for the alteration in OTK18 expression in microglia in HIV brains. Further study will be necessary to clarify the suppression of YY1 expression in human microglia. These data also suggest that upregulation of OTK18 in microglia may suppress viral infection and can ameliorate the CNS disease progression. Indeed, our recent clinical study on the OTK18 levels in plasma and cerebrospinal fluid in HIV patients revealed that there is a significant negative correlation between viral infection and OTK18 levels in both samples (Buescher et al., 2008), suggesting that OTK18 suppress viral replication in not only brain mononuclear phagocytes but also in periphery. Thus, identification of compounds which upregulates OTK18 may have therapeutic potential.
Acknowledgements
We would like to thank NIH AIDS Research and Reference Reagent Program for pHIV-LTR-Luc and YU2 plasmids, Byran Cullen for pHIT/G plasmid, Edward Sato for human YY1 expression plasmid, and Robert Hromas for human FoxD3 (Genesis) expression plasmid, CNND Tissue and Cell Core Facility (Li Wu and Anuja Ghorpade) for primary culture of human microglia and monocyte elutriation, Michael Jacobsen and Meg Marquardt for editorial assistance, Santi Gorantla for consultation in viral pseudotyping, and Myhanh Che for viral titration. This work is funded by National Institute of Health R01 MH072539 (TI), P01 NS043985 (TI), and NCRR P20RR15635 (TI). The authors declare no conflict of interest or financial interests in this work.
References
- Abeel T, Saeys Y, Bonnet E, Rouze P, Van de Peer Y. Generic eukaryotic core promoter prediction using structural features of DNA. Genome Res. 2008;18:310–323. doi: 10.1101/gr.6991408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfano M, Grivel JC, Ghezzi S, Corti D, Trimarchi M, Poli G, Margolis L. Pertussis toxin B-oligomer dissociates T cell activation and HIV replication in CD4 T cells released from infected lymphoid tissue. Aids. 2005;19:1007–1014. doi: 10.1097/01.aids.0000174446.40379.3b. [DOI] [PubMed] [Google Scholar]
- Alter G, Suscovich TJ, Teigen N, Meier A, Streeck H, Brander C, Altfeld M. Single-stranded RNA derived from HIV-1 serves as a potent activator of NK cells. J Immunol. 2007;178:7658–7666. doi: 10.4049/jimmunol.178.12.7658. [DOI] [PubMed] [Google Scholar]
- Alvarez-Salas LM, Benitez-Hess ML, Dipaolo JA. YY-1 and c-Jun transcription factors participate in the repression of the human involucrin promoter. Int J Oncol. 2005;26:259–266. doi: 10.3892/ijo.26.1.259. [DOI] [PubMed] [Google Scholar]
- Beignon AS, McKenna K, Skoberne M, Manches O, DaSilva I, Kavanagh DG, Larsson M, Gorelick RJ, Lifson JD, Bhardwaj N. Endocytosis of HIV-1 activates plasmacytoid dendritic cells via Toll-like receptor-viral RNA interactions. J Clin Invest. 2005;115:3265–3275. doi: 10.1172/JCI26032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorkbacka H, Fitzgerald KA, Huet F, Li X, Gregory JA, Lee MA, Ordija CM, Dowley NE, Golenbock DT, Freeman MW. The induction of macrophage gene expression by LPS predominantly utilizes Myd88-independent signaling cascades. Physiol Genomics. 2004;19:319–330. doi: 10.1152/physiolgenomics.00128.2004. [DOI] [PubMed] [Google Scholar]
- Borgmann K, Gendelman HE, Ghorpade A. Isolation and HIV-1 infection of primary human microglia from fetal and adult tissue. Methods Mol Biol. 2005;304:49–70. doi: 10.1385/1-59259-907-9:049. [DOI] [PubMed] [Google Scholar]
- Buescher JL, Duan F, Sun J, Price RW, Ikezu T. OTK18 Levels in Plasma and Cerebrospinal Fluid Correlate with Viral Load and CD8 T-cells in Normal and AIDS Patients. J Neuroimmune Pharmacol. 2008 doi: 10.1007/s11481-008-9125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson K, Leisman G, Limoges J, Pohlman G, Horiba M, Buescher J, Gendelman H, Ikezu T. Molecular Characterization of a Putative Anti-Retroviral Transcriptional Factor, OTK18. J Immunol. 2004a;172:381–391. doi: 10.4049/jimmunol.172.1.381. [DOI] [PubMed] [Google Scholar]
- Carlson KA, Limoges J, Pohlman GD, Poluektova LY, Langford D, Masliah E, Ikezu T, Gendelman HE. OTK18 expression in brain mononuclear phagocytes parallels the severity of HIV-1 encephalitis. J Neuroimmunol. 2004b;150:186–198. doi: 10.1016/j.jneuroim.2004.01.021. [DOI] [PubMed] [Google Scholar]
- Chao CC, Gekker G, Hu S, Peterson PK. Human microglial cell defense against Toxoplasma gondii. The role of cytokines. J Immunol. 1994;152:1246–1252. [PubMed] [Google Scholar]
- Chung SW, Chen YH, Perrella MA. Role of Ets-2 in the regulation of heme oxygenase-1 by endotoxin. J Biol Chem. 2005;280:4578–4584. doi: 10.1074/jbc.M409125200. [DOI] [PubMed] [Google Scholar]
- Cook JA, Grey D, Burke J, Cohen MH, Gurtman AC, Richardson JL, Wilson TE, Young MA, Hessol NA. Depressive symptoms and AIDS-related mortality among a multisite cohort of HIV-positive women. Am J Public Health. 2004;94:1133–1140. doi: 10.2105/ajph.94.7.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JJ, Romerio F, Sun JM, Volker JL, Galvin KM, Davie JR, Shi Y, Hansen U, Margolis DM. The human factors YY1 and LSF repress the human immunodeficiency virus type 1 long terminal repeat via recruitment of histone deacetylase 1. J Virol. 2000;74:6790–6799. doi: 10.1128/jvi.74.15.6790-6799.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristillo AD, Bierer BE. Regulation of CXCR4 expression in human T lymphocytes by calcium and calcineurin. Mol Immunol. 2003;40:539–553. doi: 10.1016/s0161-5890(03)00169-x. [DOI] [PubMed] [Google Scholar]
- Dehal P, Predki P, Olsen AS, Kobayashi A, Folta P, Lucas S, Land M, Terry A, Ecale Zhou CL, Rash S, Zhang Q, Gordon L, Kim J, Elkin C, Pollard MJ, Richardson P, Rokhsar D, Uberbacher E, Hawkins T, Branscomb E, Stubbs L. Human chromosome 19 and related regions in mouse: conservative and lineage-specific evolution. Science. 2001;293:104–111. doi: 10.1126/science.1060310. [DOI] [PubMed] [Google Scholar]
- Dittmer J. The biology of the Ets1 proto-oncogene. Mol Cancer. 2003;2:29. doi: 10.1186/1476-4598-2-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dottori M, Gross MK, Labosky P, Goulding M. The winged-helix transcription factor Foxd3 suppresses interneuron differentiation and promotes neural crest cell fate. Development. 2001;128:4127–4138. doi: 10.1242/dev.128.21.4127. [DOI] [PubMed] [Google Scholar]
- Fouchier RA, Meyer BE, Simon JH, Fischer U, Malim MH. HIV-1 infection of non-dividing cells: evidence that the amino-terminal basic region of the viral matrix protein is important for Gag processing but not for post-entry nuclear import. Embo J. 1997;16:4531–4539. doi: 10.1093/emboj/16.15.4531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freed E, Martin M. HIVs and Their Replication. In: Knipe D, Howley P, editors. Fields Virology. 4 Edition. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 1986–1991. [Google Scholar]
- Gaughan DM, Hughes MD, Oleske JM, Malee K, Gore CA, Nachman S. Psychiatric hospitalizations among children and youths with human immunodeficiency virus infection. Pediatrics. 2004;113:e544–551. doi: 10.1542/peds.113.6.e544. [DOI] [PubMed] [Google Scholar]
- Gendelman HE, Orenstein JM, Martin MA, Ferrua C, Mitra R, Phipps T, Wahl LA, Lane HC, Fauci AS, Burke DS, et al. Efficient isolation and propagation of human immunodeficiency virus on recombinant colony-stimulating factor 1-treated monocytes. J Exp Med. 1988;167:1428–1441. doi: 10.1084/jem.167.4.1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorelick RJ, Henderson LE, Hanser JP, Rein A. Point mutants of Moloney murine leukemia virus that fail to package viral RNA: evidence for specific RNA recognition by a "zinc finger-like" protein sequence. Proc Natl Acad Sci U S A. 1988;85:8420–8424. doi: 10.1073/pnas.85.22.8420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton AT, Huntley S, Tran-Gyamfi M, Baggott DM, Gordon L, Stubbs L. Evolutionary expansion and divergence in the ZNF91 subfamily of primate-specific zinc finger genes. Genome Res. 2006;16:584–594. doi: 10.1101/gr.4843906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He G, Margolis DM. Counterregulation of chromatin deacetylation and histone deacetylase occupancy at the integrated promoter of human immunodeficiency virus type 1 (HIV-1) by the HIV-1 repressor YY1 and HIV-1 activator Tat. Mol Cell Biol. 2002;22:2965–2973. doi: 10.1128/MCB.22.9.2965-2973.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holness CL, Simmons DL. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood. 1993;81:1607–1613. [PubMed] [Google Scholar]
- Horiba M, Martinez L, Buescher JL, Sato S, Limoges J, Jiang Y, Jones C, Ikezu T. OTK18, a zinc finger protein, regulates human immunodeficiency virus type I long terminal repeat through two distinct regulatory regions. J Gen Virol. 2007;88:236–241. doi: 10.1099/vir.0.82066-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janabi N, Peudenier S, Heron B, Ng KH, Tardieu M. Establishment of human microglial cell lines after transfection of primary cultures of embryonic microglial cells with the SV40 large T antigen. Neurosci Lett. 1995;195:105–108. doi: 10.1016/0304-3940(94)11792-h. [DOI] [PubMed] [Google Scholar]
- Janabi N, Di Stefano M, Wallon C, Hery C, Chiodi F, Tardieu M. Induction of human immunodeficiency virus type 1 replication in human glial cells after proinflammatory cytokines stimulation: effect of IFNgamma, IL1beta, and TNFalpha on differentiation and chemokine production in glial cells. Glia. 1998;23:304–315. [PubMed] [Google Scholar]
- Joo M, Wright JG, Hu NN, Sadikot RT, Park GY, Blackwell TS, Christman JW. Yin Yang 1 enhances cyclooxygenase-2 gene expression in macrophages. Am J Physiol Lung Cell Mol Physiol. 2007;292:L1219–L1226. doi: 10.1152/ajplung.00474.2006. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Adams DH, Simen A, Simen BB, Rajkowska G, Stockmeier CA, Overholser JC, Meltzer HY, Jurjus GJ, Konick LC, Newton SS, Duman RS. Gene expression profiling in postmortem prefrontal cortex of major depressive disorder. J Neurosci. 2007;27:13329–13340. doi: 10.1523/JNEUROSCI.4083-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JH, Chang SY, Yeom DH, Kim SA, Um SJ, Hong KJ. Weakening of the repressive YY-1 site on the thrombospondin-1 promoter via c-Jun/YY-1 interaction. Exp Mol Med. 2004;36:300–310. doi: 10.1038/emm.2004.41. [DOI] [PubMed] [Google Scholar]
- Katoh M. Human FOX gene family (Review) Int J Oncol. 2004;25:1495–1500. [PubMed] [Google Scholar]
- Kim JY, Gonzalez-Scarano F, Zeichner SL, Alwine JC. Replication of type 1 human immunodeficiency viruses containing linker substitution mutations in the −201 to −130 region of the long terminal repeat. J Virol. 1993;67:1658–1662. doi: 10.1128/jvi.67.3.1658-1662.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kappes JC, Conway JA, Price RW, Shaw GM, Hahn BH. Molecular characterization of human immunodeficiency virus type 1 cloned directly from uncultured human brain tissue: identification of replication-competent and - defective viral genomes. J Virol. 1991;65:3973–3985. doi: 10.1128/jvi.65.8.3973-3985.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looman C, Abrink M, Mark C, Hellman L. KRAB zinc finger proteins: an analysis of the molecular mechanisms governing their increase in numbers and complexity during evolution. Mol Biol Evol. 2002;19:2118–2130. doi: 10.1093/oxfordjournals.molbev.a004037. [DOI] [PubMed] [Google Scholar]
- Margolis DM, Somasundaran M, Green MR. Human transcription factor YY1 represses human immunodeficiency virus type 1 transcription and virion production. J Virol. 1994;68:905–910. doi: 10.1128/jvi.68.2.905-910.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier A, Alter G, Frahm N, Sidhu H, Li B, Bagchi A, Teigen N, Streeck H, Stellbrink HJ, Hellman J, van Lunzen J, Altfeld M. MyD88-dependent immune activation mediated by human immunodeficiency virus type 1-encoded Toll-like receptor ligands. J Virol. 2007;81:8180–8191. doi: 10.1128/JVI.00421-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misdrahi D, Vila G, Funk-Brentano I, Tardieu M, Blanche S, Mouren-Simeoni MC. DSM-IV mental disorders and neurological complications in children and adolescents with human immunodeficiency virus type 1 infection (HIV-1) Eur Psychiatry. 2004;19:182–184. doi: 10.1016/j.eurpsy.2003.06.009. [DOI] [PubMed] [Google Scholar]
- Moriuchi M, Moriuchi H. YY1 transcription factor down-regulates expression of CCR5, a major coreceptor for HIV-1. J Biol Chem. 2003;278:13003–13007. doi: 10.1074/jbc.M204980200. [DOI] [PubMed] [Google Scholar]
- Moriuchi M, Moriuchi H, Margolis DM, Fauci AS. USF/c-Myc enhances, while Yin-Yang 1 suppresses, the promoter activity of CXCR4, a coreceptor for HIV-1 entry. J Immunol. 1999;162:5986–5992. [PubMed] [Google Scholar]
- Pan G, Thomson JA. Nanog and transcriptional networks in embryonic stem cell Pluripotency. Cell Res. 2007;17:42–49. doi: 10.1038/sj.cr.7310125. [DOI] [PubMed] [Google Scholar]
- Pohl BS, Knochel W. Overexpression of the transcriptional repressor FoxD3 prevents neural crest formation in Xenopus embryos. Mech Dev. 2001;103:93–106. doi: 10.1016/s0925-4773(01)00334-3. [DOI] [PubMed] [Google Scholar]
- Reddy MA, Yang BS, Yue X, Barnett CJ, Ross IL, Sweet MJ, Hume DA, Ostrowski MC. Opposing actions of c-ets/PU.1 and c-myb protooncogene products in regulating the macrophage-specific promoters of the human and mouse colony-stimulating factor-1 receptor (c-fms) genes. J Exp Med. 1994;180:2309–2319. doi: 10.1084/jem.180.6.2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romerio F, Gabriel MN, Margolis DM. Repression of human immunodeficiency virus type 1 through the novel cooperation of human factors YY1 and LSF. J Virol. 1997;71:9375–9382. doi: 10.1128/jvi.71.12.9375-9382.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroder AR, Shinn P, Chen H, Berry C, Ecker JR, Bushman F. HIV-1 integration in the human genome favors active genes and local hotspots. Cell. 2002;110:521–529. doi: 10.1016/s0092-8674(02)00864-4. [DOI] [PubMed] [Google Scholar]
- Seth A, Hodge DR, Thompson DM, Robinson L, Panayiotakis A, Watson DK, Papas TS. ETS family proteins activate transcription from HIV-1 long terminal repeat. AIDS Res Hum Retroviruses. 1993;9:1017–1023. doi: 10.1089/aid.1993.9.1017. [DOI] [PubMed] [Google Scholar]
- Shi Y, Lee JS, Galvin KM. Everything you have ever wanted to know about Yin Yang 1. Biochim Biophys Acta. 1997;1332:F49–F66. doi: 10.1016/s0304-419x(96)00044-3. [DOI] [PubMed] [Google Scholar]
- Shi Y, Seto E, Chang LS, Shenk T. Transcriptional repression by YY1, a human GLI-Kruppel-related protein, and relief of repression by adenovirus E1A protein. Cell. 1991;67:377–388. doi: 10.1016/0092-8674(91)90189-6. [DOI] [PubMed] [Google Scholar]
- Shi Z, Silveira A, Patel P, Feng X. YY1 is involved in RANKL-induced transcription of the tartrate-resistant acid phosphatase gene in osteoclast differentiation. Gene. 2004;343:117–126. doi: 10.1016/j.gene.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Sieweke MH, Tekotte H, Jarosch U, Graf T. Cooperative interaction of ets-1 with USF-1 required for HIV-1 enhancer activity in T cells. Embo J. 1998;17:1728–1739. doi: 10.1093/emboj/17.6.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starace F, Bartoli L, Aloisi MS, Antinori A, Narciso P, Ippolito G, Ravasio L, Moioli MC, Vangi D, Gennero L, Coronado OV, Giacometti A, Nappa S, Perulli ML, Montesarchio V, La Gala A, Ricci F, Cristiano L, De Marco M, Izzo C, Pezzotti P, D'Arminio Monforte A. Cognitive and affective disorders associated to HIV infection in the HAART era: findings from the NeuroICONA study. Cognitive impairment and depression in HIV/AIDS. The NeuroICONA study. Acta Psychiatr Scand. 2002;106:20–26. doi: 10.1034/j.1600-0447.2002.02289.x. [DOI] [PubMed] [Google Scholar]
- Sutton J, Costa R, Klug M, Field L, Xu D, Largaespada DA, Fletcher CF, Jenkins NA, Copeland NG, Klemsz M, Hromas R. Genesis, a winged helix transcriptional repressor with expression restricted to embryonic stem cells. J Biol Chem. 1996;271:23126–23133. doi: 10.1074/jbc.271.38.23126. [DOI] [PubMed] [Google Scholar]
- Tang M, Waterman M, Yooseph S. Zinc finger gene clusters and tandem gene duplication. J Comput Biol. 2002;9:429–446. doi: 10.1089/10665270252935557. [DOI] [PubMed] [Google Scholar]
- Yao YL, Yang WM, Seto E. Regulation of transcription factor YY1 by acetylation and deacetylation. Mol Cell Biol. 2001;21:5979–5991. doi: 10.1128/MCB.21.17.5979-5991.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylisastigui L, Kaur R, Johnson H, Volker J, He G, Hansen U, Margolis D. Mitogen-activated protein kinases regulate LSF occupancy at the human immunodeficiency virus type 1 promoter. J Virol. 2005;79:5952–5962. doi: 10.1128/JVI.79.10.5952-5962.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]