Abstract
Purpose
Expression of various members of the ErbB family (EGFR/ErbB-1, ErbB-2, ErbB-3, and ErbB-4) has been associated with stage of disease and survival in patients with urothelial carcinoma (UC). We examined the correlation of expression of ErbB family receptors with progression of UC, and survival.
Methods
A UC tissue array was constructed from 248 archival paraffin blocks, and quality control studies were ascertained. The tissue microarray was stained for EGFR, ErbB-2, ErbB-3, and ErbB-4, and was analyzed using an automated reader. Patient data included grade, stage, growth pattern, recurrence, and survival.
Results
Kaplan-Meier estimates of the 5-year overall and recurrence-free survival were 58% and 27%, respectively. Patients with high-grade, invasive, or non-papillary disease had a worse prognosis than patients with low-grade, superficial, or papillary disease (p <0.0001). High EGFR or low ErbB-4 expression was associated with non-papillary, high-grade, and invasive tumors (p <0.002), as well as a significantly shorter recurrence-free survival (p = 0.028) and overall survival (p = 0.047), respectively. Levels of ErbB-2 and ErbB-3 expression were not associated with overall or recurrence-free survival.
Conclusion
The expression profiles of ErbB-4 and EGFR are prognostic in UC and may help in selecting high-risk patients with bladder cancer for more aggressive therapy.
Keywords: urothelial carcinoma, cystectomy, ErbB receptors, tissue microarray, prognosis
INTRODUCTION
The initial presentation of bladder cancer can portray a wide spectrum of biologic behavior that may range from less aggressive, low-grade, superficial disease to high-grade, muscle-invasive cancer with high risk of disease progression and metastasis. Although conventional clinico-pathologic parameters such as high grade and stage of the tumor are known to be predictors of recurrence, progression, and survival, an improved understanding of some biologic aspects of bladder cancer has provided novel prognostic and therapeutic approaches. Profiling of molecular pathways has gained increasing use to improve prognostication in patients with bladder cancer in an effort to provide optimal therapy prior to the development of progression and/or distant metastasis.
Among important mediators of oncogenesis, the protein tyrosine kinases have been shown to be involved in regulatory processes, including proliferation, migration, and cellular transformation. Members of the ErbB family receptors of protein tyrosine kinases share structural homologies and include the epidermal growth factor receptor (EGFR), ErbB-2, ErbB-3, and ErbB-4. Overexpression of ErbB receptors have both prognostic and therapeutic significance in various malignancies including breast cancer.1 However, the prognostic significance of ErbB expression for patients with urothelial carcinoma (UC) remains controversial, since several contradictory reports have been published.1–7
One purpose for quantifying ErbB status in patients with UC of the bladder is to identify potential targets for combined therapeutic interventions. Another is to identify high-risk features that might direct therapy. Since receptor dimerization and cross-phosphorylation of the ErbB receptors are evident in vitro, the implications of coexpression patterns in bladder cancer in the clinical setting need further clarification. We evaluated the expression of all four members of the ErbB family on a tissue microarray to determine the correlations between tumor expression and patient overall and recurrence-free survival.
MATERIALS AND METHODS
Patient population
The study population, based on patients treated from 1986–2002, was 80% male and 20% female, and the median age was 67 years (range, 43–86 years). Of the patients included, 15% were African-American and 85% were Caucasian. Information on pathologic characteristics of their tumors, the date of diagnosis, the date of the last follow-up visit, recurrence of disease, and the status of the patient (alive or dead) was collected.
Tumor samples and follow-up data were collected from transurethral resection or cystectomy and archived according to the laboratory protocol approved by the Institutional Review Board of The University of Texas M. D. Anderson Cancer Center. All patients had primary UC of the bladder, and none of the patients whose tumor samples were used in this study received prior chemotherapy or radiotherapy. Since some of the sections were detached from the glass slide during tissue processing for immunohistochemistry, not all proteins could be measured from all cores. Consequently, the numbers of samples used in the various analyses are slightly different, as shown in the tabled results. Further, for a very small number of cases, survival and/or recurrence-free survival information was not available. In assessing all contrasts, however, all potentially informative samples were used.
Tumor identification, preparation, and tissue microarray (TMA) construction was performed as previously described.8 In brief, histologic slides from 248 bladder cancers were reviewed and the most representative, well-preserved areas of the tumor tissue were selected and marked. The tumors were classified according to the three-tier World Health Organization (WHO) histological grading system and growth pattern (papillary vs. nonpapillary).9 The depth of invasion was recorded according to the tumor-node-metastasis (TNM) staging system.10 Stage T1 (lamina propria invasion) was divided into T1a (no muscularis mucosae invasion) and T1b (muscularis mucosae invasion). As in our previous publications, the tumors were dichotomized into superficial (Ta–T1a) and invasive (T1b and higher) groups.11
The donor paraffin blocks were punched in areas of interest using a microarray instrument (Beecher Instruments, Inc., Sun Prairie, WI) and 0.6 mm cores of the tumor tissue were transferred to a recipient block. The recipient microarray block contained 85 noninvasive low-grade (Grade 1–2) papillary carcinomas, 25 noninvasive high-grade (Grade 3) papillary carcinomas, 18 invasive high-grade (Grade 3) papillary carcinomas, and 120 invasive high-grade (Grade 3) nonpapillary carcinomas. Overall, the microarray contained 110 superficial (Ta–T1a) and 138 invasive (T1b and higher) urothelial carcinomas of the bladder. The reproducibility of staining in multiple tissue cores from the same tumor was assessed on a separate microarray containing five different cores each from four randomly selected low-grade superficial and four high-grade invasive urothelial carcinomas of the bladder.
Immunohistochemical staining of UC specimens
Immunohistochemical studies were performed on formalin-fixed paraffin-embedded tissue samples from the TMA using the avidin-biotin peroxidase complex method, and the results related to the corresponding clinico-pathologic data. Tumor tissue was fixed in formalin and embedded in paraffin according to standard procedures. Tissue sections (2 μm thick) of formalin-fixed, paraffin-embedded specimens were deparaffinized in xylene followed by treatment with a graded series of alcohol [100%, 95%, and 80% ethanol/double-distilled H2O (v/v)], rehydrated in PBS (pH 7.5), and pretreated with Tris buffer (pH 8) at 99°C for 45 minutes. Endogenous peroxidase was blocked by the use of 0.3% hydrogen peroxide in 90% methanol for 5 minutes and stained with the following antibodies against: extracellular domain of the EGFR, Clone 31G7 (Zymed Laboratories, Inc., San Francisco, CA), ErbB-2 cytoplasmic domain (Lab Vision Corp., Fremont, CA), ErbB-3 C-terminus (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and ErbB-4 aa 1249–1264 (Lab Vision Corp., Fremont, CA) overnight at 4°C. The bound primary antibodies were visualized by avidin-biotin complex assay (DAKO Corp., Carpenteria, CA) with 3,3′-diaminobenzidine as a chromagen (DAKO) and hematoxylin as a counterstain. Sections of three normal ureters obtained from patients undergoing nephrectomy for renal cell carcinoma with no evidence of urothelial neoplasia involving the pelvis and ureter were included in the microarray and served as internal positive controls. As a negative control for the secondary antibodies and the color detection system, slides of bladder tumor microarrays including normal ureters were processed without the primary antibodies. The specificity of the staining was additionally assessed on microarray sections exposed to primary antibody blocked with the corresponding immunizing peptide at 1:10 molar ratio. For this purpose recombinant full-length EGFR was purchased from Invitrogen Corp. (Carlsbad, CA), a specific ErbB-3 peptide was acquired from Santa Cruz Biotechnology (sc-7390P) and the ErbB-4 immunizing peptide was custom synthesized by GenScript Corp. (Piscataway, NJ). No immunizing peptide was available for ErbB-2.
Quantification of staining in UC specimens
The staining intensity of each antigen in the tissue microarray was measured using an automated scanner GenoMx (Automated Digital Image System, Bio Genex, San Ramon, CA) following the manufacturer’s recommendations. The area of tumor was outlined in each tissue core, and its staining intensity was compared to the intensity of staining in normal urothelium for each protein. For each tumor sample, the relative expression level (REL) of each protein was calculated as follows: REL = P × (M + H), where P was the area of the tumor with positive staining (%), M was the area of the tumor with medium staining intensity, and H was the area of the tumor with high staining intensity. The tumors were divided into two groups, that is, those with REL scores above and below the median value for each protein.
Statistical analysis
Differences in the relative expression levels of markers between clinical groups were compared using Van der Waerden Score tests. The Kaplan-Meier product-limit method was used to determine the overall survival probability for subgroups on the basis of the staining score. The median receptor staining intensity was used as the cutpoint to dichotomize patients into high- and low-score groups. Survival curves were compared using log-rank tests. A two-sided P value of 0.05 was considered significant for all tests. Univariate and multivariate Cox proportional hazards models were fit to assess the relationship between the immunostaining scores and survival in the absence and presence of other clinical covariates. Statistical analyses were performed using the R (version 1.9.0, http://www.r-project.org/) and S-plus (Insightful Corp.) statistical packages.
RESULTS
Superficial bladder cancer was diagnosed in 34% of patients (18% Ta and 16% T1), while 66% of patients had more advanced stage tumors (42% T2-4N0, 24% TxN+) on final pathologic analysis. The majority of tumors were high grade (73%), and 50% were characterized by non-papillary growth patterns. Patients with high-grade disease had a worse prognosis than patients with low-grade disease (median overall survival, 25 months versus 84.6 months, p <0.0001); patients with invasive disease had a worse prognosis than those without invasion (median overall survival, 20.9 months versus 75.2 months, p <0.0001); and, similarly, patients with non-papillary tumors had a worse prognosis than those without (median overall survival, 23.5 months versus 67.4 months, p <0.0001). Kaplan-Meier estimates of the 5-year overall and recurrence-free survival for all patients were 58% and 27%, respectively.
Staining of tumors by all the ErbB family members was predominantly membranous with occasional detectable immunoreactivity within the cytoplasm. Normal urothelium stained positively for all the ErbB family members and the staining was predominantly membranous with minimal diffuse cytoplasmic reaction. An example of EGFR (fig. 1) and ErbB-4 (fig. 2) is included to demonstrate this pattern of predominant membrane staining. Normal urothelium and tumor samples that were not incubated with primary antibody did not show any positive reaction. The specificity of staining for EGFR, ErbB-3 and ErbB-4 was verified by pre-incubation of the primary antibody with its respective immunizing peptide which showed >80% reduction in the staining intensity. The reproducibility of staining patterns was tested on a separate microarray containing four low-grade superficial (Ta, T1a) and four high-grade invasive (T1b and higher) tumors. An example of these control studies is shown for EGFR in figure 3.
Figure 1.
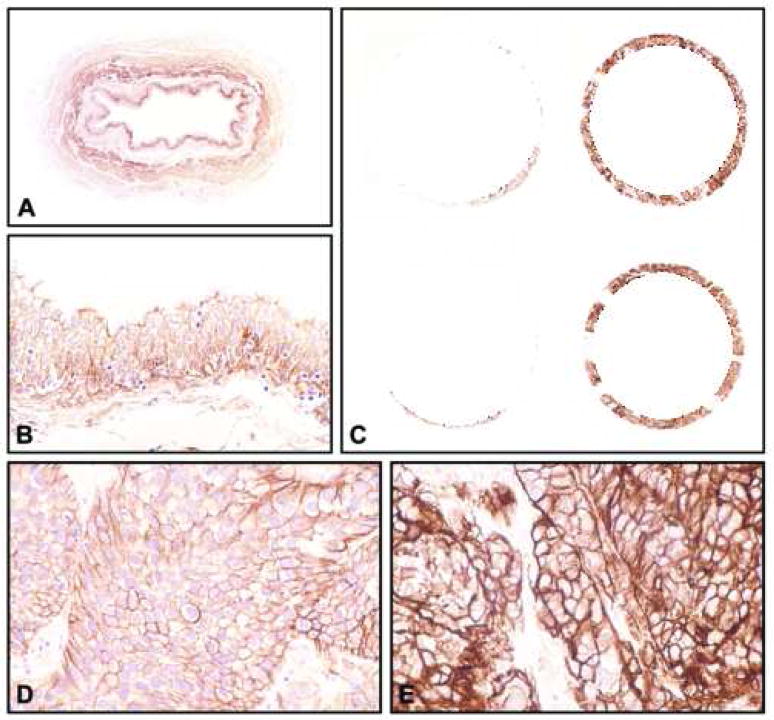
Expression of EGFR tested in a tissue microarray containing samples of 248 bladder tumors. (A and B) Baseline expression levels in normal urothelium (A, x8; B, x200). (C) Low power view of representative tissue cores corresponding to high-grade (Grade 3) invasive (T1b-T4) TCC with low (left column) and high (right column) levels of EGFR (x18). (D) Higher magnification of a tissue core shown in C with low levels of EGFR (x400). (E) Higher magnification of a tissue core shown in C with high levels of EGFR (x400). Note that low expression levels of EGFR are similar to its baseline expression in normal urothelium.
Figure 2.
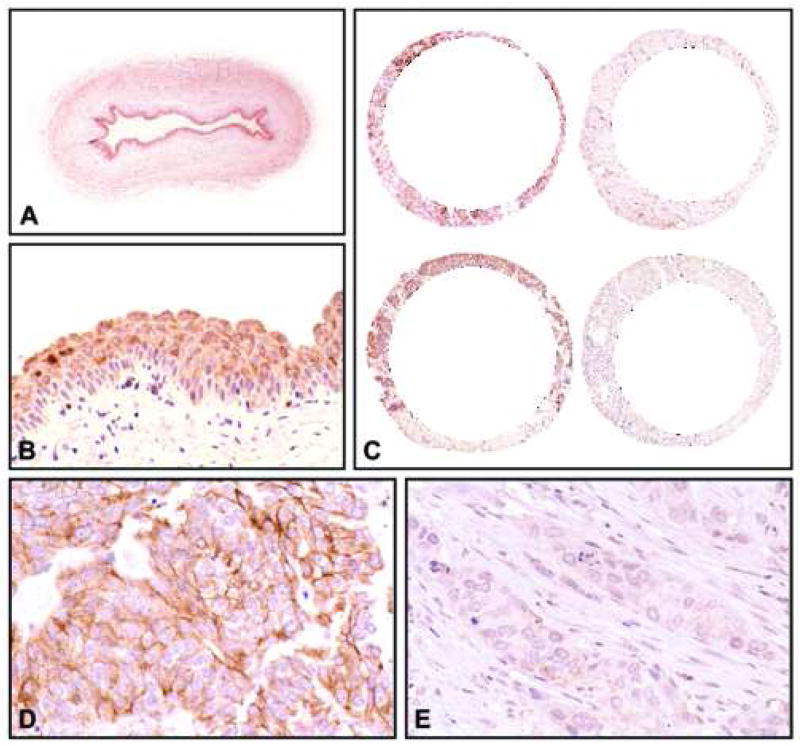
Expression of ErbB-4 tested in a tissue microarray containing samples of 248 bladder tumors. (A and B) Baseline expression levels in normal urothelium (A, x8; B, x200). (C) Low power view of representative tissue cores corresponding to high-grade (Grade 3) invasive (T1b-T4) TCC with retention (left column) and loss (right column) of ErbB-4 expression (x18). (D) Higher magnification of a tissue core shown in C with retention of ErbB-4 expression (x400). (E) Higher magnification of a tissue core shown in C with loss of ErbB-4 expression (x400). Note that tumors with retention of expression show levels of ErbB-4 similar to its baseline expression in normal urothelium.
Figure 3.
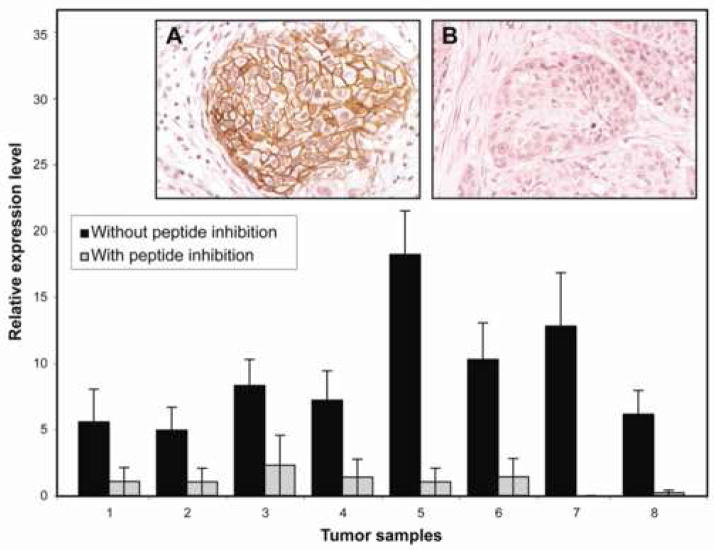
An example of the control studies showing the reproducibility of EGFR expression measurement (calculation of REL) in 5 different core samples each from four randomly selected low-grade superficial (1–4) and four high-grade invasive (5–8) urothelial carcinomas of the bladder. The results are presented for staining with (gray bars) and without (black bars) pre-incubation with the immunizing peptide. The insert shows expression patterns of EGFR with the primary antibody (A) and after inhibition with its immunizing peptide (B) (x200).
Patients whose tumors expressed higher EGFR and lower ErbB-4 levels had a significantly shorter recurrence-free survival (p = 0.028) and overall survival (p = 0.0037), respectively (table 1). Based on published literature, we predicted that the levels of EGFR expression would be correlated with outcome, but were intrigued that levels of ErbB-4 expression were prognostic; in patients whose tumors retained ErbB-4 expression, the 5-year overall survival rate was 65% compared with 35% for those whose tumors expressed lower levels of ErbB-4 (p = 0.0037), and the median overall survival was 10.1 years and 2.8 years, respectively (fig. 4A). In the multivariate analysis that adjusted for grade, invasiveness, and growth pattern, higher EGFR level remained significantly associated with recurrence-free survival (p <0.04), but ErbB-4 level was no longer significantly associated with overall survival. In subset analyses looking at patients with invasive and non-invasive disease separately, the only statistically significant correlation was between high EGFR expression and decreased recurrence-free survival in non-invasive disease (p = 0.026). There was no such correlation to overall survival or for patients with invasive disease. Of note, ErbB-2 and ErbB-3 levels were not associated with overall or recurrence-free survival. High EGFR and low ErbB-4 levels were significantly associated with non-papillary, high-grade, and invasive tumors (p <0.002) (table 2). Although ErbB-2 level was associated with growth pattern and grade, it was not significantly associated with invasive disease. ErbB-3 level was not associated with growth pattern, grade, or stage.
Table 1.
Summary of Cox proportional hazards analysis of variables that predict overall and recurrence-free survival
| Patients (#) | OS | RFS | |
|---|---|---|---|
| EGFR | 182 | NS | 0.028 |
| HER-2 | 184 | NS | NS |
| HER-3 | 128 | NS | NS |
| HER-4 | 124 | 0.0037 | NS |
| Combined EGFR and HER-4 | 0.075 | NS | |
| High EGFR/low HER-4 | 32 | ||
| Low EGFR/high HER-4 | 35 | ||
| Growth pattern | <0.0001 | NS | |
| Papillary | 128 | ||
| Non-papillary | 120 | ||
| Grade | <0.0001 | NS | |
| Low | 84 | ||
| High | 164 | ||
| Invasiveness | <0.0001 | NS | |
| Superficial | 110 | ||
| Invasive | 138 |
Figure 4.
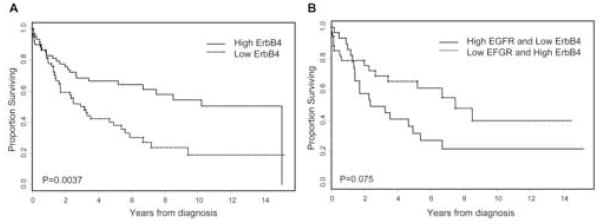
Kaplan-Meier estimate of overall survival stratified by ErbB-4 expression (A), or combined EGFR and ErbB-4 expression (B) in urothelial tumors.
Table 2.
Associations between receptors of the ErbB family and pathologic characteristics of the bladder tumors
| EGFR | HER-2 | HER-3 | HER-4 | |||||
|---|---|---|---|---|---|---|---|---|
| Rela | Pb | Rela | Pb | Rela | Pb | Rela | Pb | |
| Growth pattern | (0.0007) | (0.037) | (NS) | (<0.0001) | ||||
| Papillary | 33.5±24.4 | 34.3±30.1 | 30.6±28.0 | 19.1±20.8 | ||||
| Non-papillary | 46.2±30.6 | 26.4±26.7 | 25.7±22.5 | 4.6±7.1 | ||||
| Grade | (0.002) | (NS) | (NS) | (<0.0001) | ||||
| Low | 31.4±25.2 | 30.0±27.8 | 30.4±29.0 | 19.4±21.1 | ||||
| High | 44.0±28.9 | 31.8±29.7 | 26.8±23.5 | 7.5±12.6 | ||||
| Stage | (<0.0001) | (0.036) | (NS) | (<0.0001) | ||||
| Superficial | 31.1±23.8 | 35.3±25.6 | 31.7±28.0 | 20.6±21.0 | ||||
| Invasive | 46.6±29.7 | 26.8±27.8 | 25.8±23.3 | 5.6±9.6 | ||||
Rel - relative expression
P-value
We evaluated whether there was any correlation between the expression levels of receptors of the ErbB family in bladder cancer tumors. We found that the strongest statistically significant correlation was between EGFR and ErbB-4 (correlation coefficient −0.38, p <0.0001) compared with EGFR and ErbB-2 (correlation coefficient −0.27, p = 0.001), and with ErbB-3 and ErbB-4 (correlation coefficient 0.14, p <0.0001). Since EGFR and ErbB-4 levels were significantly associated with survival, we compared patients whose tumors expressed both high EGFR and low ErbB-4 with patients whose tumors expressed low EGFR and retained ErbB-4 expression. In order to evaluate the combination, we dichotomized the levels of expression into high versus low, with the median being the cutoff value, rather than using the level of receptor expression as a continuous variable. Kaplan-Meier curve demonstrated that patients whose tumors expressed both a high level of EGFR and a low level of ErbB-4 had a shorter overall survival than patients whose tumors expressed a low level of EGFR, but retained ErbB-4 expression; however, the difference attained only borderline statistical significance (p = 0.075) (fig. 4B).
DISCUSSION
We demonstrated that high EGFR and low ErbB-4 levels were associated with high-grade, invasive phenotype of UC. Similarly, high EGFR and low ErbB-4 levels were associated with shorter recurrence-free and overall survival for UC patients.
EGFR expression had previously been shown to predict for disease progression to muscle-invasive or metastatic UC and was found to be an independent prognostic factor for death in a multivariate analysis.12 Our study confirmed these findings; we found that patients with higher levels of EGFR had decreased rates of recurrence-free survival compared to patients with low levels of EGFR expression. In contrast to several reports, EGFR expression was also found to be prognostic in a multivariate analysis that adjusted for grade, invasiveness, and growth pattern. Interestingly, tumors that did not lose ErbB-4 expression were associated with a more favorable prognosis in our study. Patients with tumors that had not lost ErbB-4 expression had improved 5-year overall survival rates (65% versus 35%) and were more likely to have superficial, low-grade tumors.
Conflicting reports on this topic had been published in breast cancer;13, 14 however, more recently Memon et al15 found that ErbB-4 levels were associated with better survival in patients with bladder cancer, which is consistent with the findings of our study. These findings may be a result of transcriptional regulation of the ErbB-4 gene in patients with UC.16 We also found that among members of the ErbB receptor family, the strongest significant correlation was between the levels of EGFR and ErbB-4 receptors. Interestingly, patients whose tumors expressed high levels of EGFR and low levels of ErbB-4 had shorter overall survival than patients whose tumors expressed low levels of EGFR and retained expression of ErbB-4. Although it was not confirmed in our study due to small sample size of the subset analysis, a combination of these two variables may lead to better prognostication for patients with bladder cancer than when variables are used alone.
The importance of ErbB-2 and ErbB-3 in bladder cancer progression and survival has yet to be clarified. Several studies performed on various tumors have shown that higher levels of ErbB-2 are associated with poor prognosis,7, 17, 18 whereas other studies found the contrary.6 Similarly, reports regarding the association between the levels of ErbB-3 and prognosis are also conflicting.6, 15, 19, 20 Differences in both the incidence and the prognostic significance of ErbB-2 and ErbB-3 expression are very likely attributable to the use of different methodologies, including assessment of receptor status (i.e., detection of amplification vs. detection of over-expression), method employed (i.e., polymerase chain reaction, fluorescent in situ hybridization, immunohistochemistry), and definition of receptor positivity.
CONCLUSION
The expression profile of high EGFR and loss of ErbB-4 may identify a distinct phenotype of urothelial carcinoma with an adverse prognosis and may help in selecting patients with bladder cancer for more aggressive therapy. Approaches to modification of the activity of EGFR and ErbB-4 may also have therapeutic potential for the treatment of selected patients with urothelial carcinoma of the bladder.
Acknowledgments
Supported by the Cancer Center Core Grant CA16672 from the National Cancer Institute, the GU Bladder SPORE CA91846 and the T32 training grant.
Key of Definitions
- UC
urothelial carcinoma
- TCC
transitional cell carcinoma
- OS
overall survival
- DSS
disease specific survival
- RFS
recurrence free survival
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rajkumar T, Stamp GW, Pandha HS, Waxman J, Gullick WJ. Expression of the type 1 tyrosine kinase growth factor receptors EGF receptor, c-erbB2 and c-erbB3 in bladder cancer. J Pathol. 1996;179:381. doi: 10.1002/(SICI)1096-9896(199608)179:4<381::AID-PATH603>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 2.Mellon K, Wright C, Kelly P, Horne CH, Neal DE. Long-term outcome related to epidermal growth factor receptor status in bladder cancer. J Urol. 1995;153:919. [PubMed] [Google Scholar]
- 3.Sriplakich S, Jahnson S, Karlsson MG. Epidermal growth factor receptor expression: predictive value for the outcome after cystectomy for bladder cancer? BJU Int. 1999;83:498. doi: 10.1046/j.1464-410x.1999.00914.x. [DOI] [PubMed] [Google Scholar]
- 4.Liukkonen T, Rajala P, Raitanen M, Rintala E, Kaasinen E, Lipponen P. Prognostic value of MIB-1 score, p53, EGFr, mitotic index and papillary status in primary superficial (Stage pTa/T1) bladder cancer: a prospective comparative study. The Finnbladder Group. Eur Urol. 1999;36:393. doi: 10.1159/000020039. [DOI] [PubMed] [Google Scholar]
- 5.Miyamoto H, Kubota Y, Noguchi S, Takase K, Matsuzaki J, Moriyama M, et al. C-ERBB-2 gene amplification as a prognostic marker in human bladder cancer. Urology. 2000;55:679. doi: 10.1016/s0090-4295(99)00604-4. [DOI] [PubMed] [Google Scholar]
- 6.Chow NH, Chan SH, Tzai TS, Ho CL, Liu HS. Expression profiles of ErbB family receptors and prognosis in primary transitional cell carcinoma of the urinary bladder. Clin Cancer Res. 2001;7:1957. [PubMed] [Google Scholar]
- 7.Sato K, Moriyama M, Mori S, Saito M, Watanuki T, Terada K, et al. An immunohistologic evaluation of C-erbB-2 gene product in patients with urinary bladder carcinoma. Cancer. 1992;70:2493. doi: 10.1002/1097-0142(19921115)70:10<2493::aid-cncr2820701017>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 8.Kim JH, Tuziak T, Hu L, Wang Z, Bondaruk J, Kim M, et al. Alterations in transcription clusters underlie development of bladder cancer along papillary and nonpapillary pathways. Lab Invest. 2005;85:532. doi: 10.1038/labinvest.3700250. [DOI] [PubMed] [Google Scholar]
- 9.Mostofi FK. Histological typing of urinary bladder tumours. In: Mostofi FK, Davis CJ, Sesterhenn IA, editors. Histological typing of urinary bladder tumours. Berlin, New York: Springer; 1999. [Google Scholar]
- 10.Sobin LH, Wittekind C. TNM Classification of Malignant Tumors. New York: John Wiley; 1997. [Google Scholar]
- 11.Sen S, Zhou H, Zhang RD, Yoon DS, Vakar-Lopez F, Ito S, et al. Amplification/overexpression of a mitotic kinase gene in human bladder cancer. J Natl Cancer Inst. 2002;94:1320. doi: 10.1093/jnci/94.17.1320. [DOI] [PubMed] [Google Scholar]
- 12.Nguyen PL, Swanson PE, Jaszcz W, Aeppli DM, Zhang G, Singleton TP, et al. Expression of epidermal growth factor receptor in invasive transitional cell carcinoma of the urinary bladder. A multivariate survival analysis. Am J Clin Pathol. 1994;101:166. doi: 10.1093/ajcp/101.2.166. [DOI] [PubMed] [Google Scholar]
- 13.Bieche I, Onody P, Tozlu S, Driouch K, Vidaud M, Lidereau R. Prognostic value of ERBB family mRNA expression in breast carcinomas. Int J Cancer. 2003;106:758. doi: 10.1002/ijc.11273. [DOI] [PubMed] [Google Scholar]
- 14.Suo Z, Risberg B, Kalsson MG, Willman K, Tierens A, Skovlund E, et al. EGFR family expression in breast carcinomas. c-erbB-2 and c-erbB-4 receptors have different effects on survival. J Pathol. 2002;196:17. doi: 10.1002/path.1003. [DOI] [PubMed] [Google Scholar]
- 15.Memon AA, Sorensen BS, Meldgaard P, Fokdal L, Thykjaer T, Nexo E. The relation between survival and expression of HER1 and HER2 depends on the expression of HER3 and HER4: a study in bladder cancer patients. Br J Cancer. 2006;94:1703. doi: 10.1038/sj.bjc.6603154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amsellem-Ouazana D, Bieche I, Tozlu S, Botto H, Debre B, Lidereau R. Gene expression profiling of ERBB receptors and ligands in human transitional cell carcinoma of the bladder. J Urol. 2006;175:1127. doi: 10.1016/S0022-5347(05)00317-4. [DOI] [PubMed] [Google Scholar]
- 17.Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- 18.Gullick WJ. The role of the epidermal growth factor receptor and the c-erbB-2 protein in breast cancer. Int J Cancer Suppl. 1990;5:55. doi: 10.1002/ijc.2910460708. [DOI] [PubMed] [Google Scholar]
- 19.Witton CJ, Reeves JR, Going JJ, Cooke TG, Bartlett JM. Expression of the HER1–4 family of receptor tyrosine kinases in breast cancer. J Pathol. 2003;200:290. doi: 10.1002/path.1370. [DOI] [PubMed] [Google Scholar]
- 20.Pawlowski V, Revillion F, Hebbar M, Hornez L, Peyrat JP. Prognostic value of the type I growth factor receptors in a large series of human primary breast cancers quantified with a real-time reverse transcription-polymerase chain reaction assay. Clin Cancer Res. 2000;6:4217. [PubMed] [Google Scholar]