Abstract
Introduction
Prior activation of the kappa opioid system by repeated stress or agonist administration has been previously shown to potentiate the rewarding properties of subsequently administered cocaine. In the present study, intermittent and uncontrollable footshock, a single session of forced swim, or acute administration of the kappa agonist U50,488 (5 mg/kg) were found to reinstate place preference in mice previously conditioned with cocaine (15 mg/kg) and subsequently extinguished by repeated training sessions without drug.
Results and discussion
Stress-induced reinstatement did not occur for mice pretreated with the kappa opioid receptor antagonist norbinaltorphimine (10 mg/kg) and did not occur in mice lacking either kappa opioid receptors (KOR −/−) or prodynorphin (Dyn −/−). In contrast, the initial cocaine conditioning and extinction rates were not significantly affected by disruption of the kappa opioid system. Cocaine-injection also reinstated conditioned place preference in extinguished mice; however, cocaine-primed reinstatement was not blocked by kappa opioid system disruption.
Conclusion
The results suggest that stress-induced drug craving in mice may require activation of the dynorphin/kappa opioid system.
Keywords: Cocaine, Reinstatement, Stress, Dynorphin, Kappa receptor antagonist
Introduction
A central problem in treating drug addiction is the vulnerability to relapse during abstinence (Leshner 1997), and a better understanding of the underlying mechanisms of relapse may identify important therapeutic targets and suggest novel treatments. Towards this goal, animal models of relapse were developed that identified triggers for reinstatement of drug self-administration (de Wit and Stewart 1981, 1983; Gerber and Stretch 1975; Stretch and Gerber 1973). Drug reinstatement studies have shown that presentation of drug-associated cues, drug priming, and acute footshock stress each increased drug self-administration (see Bossert et al. 2005; Epstein et al. 2006; Shaham et al. 2003). While the direct extension from animal models of reinstatement to human addiction experience may be problematic (Katz and Higgins 2003), a relationship between stress and drug addiction has been repeatedly noted (see Koob 2006), and treatments that enhance stress resilience might reduce the frequency of relapse.
Previously, we found that the dynorphin/kappa opioid system was one of the key mediators of the stress response affecting the reinforcing properties of cocaine (McLaughlin et al. 2003). Repeated swim stress exposure or repeated social defeat stress, before cocaine conditioning, significantly enhanced subsequent place preference (CPP) to the cocaine-paired compartment in wild-type C57Bl/6 mice, but stress did not affect cocaine CPP in mice pretreated with the kappa receptor antagonist norbinaltorphimine (norBNI) or lacking prodynorphin-derived opioid peptides (McLaughlin et al. 2003, 2006b). Although co-administration of kappa agonists with cocaine inhibited CPP (Shippenberg et al. 1996), pharmacological activation of the kappa opioid receptor by U50,488 60-min before cocaine conditioning mimicked stress exposure and potentiated cocaine CPP (McLaughlin et al. 2006a). These results suggest that repeated stress exposure induced the release of endogenous dynorphins that activate kappa opioid receptors and subsequently enhanced the rewarding properties of cocaine. As kappa receptor activation is aversive in rats (Shippenberg and Herz 1986) and produces dysphoria in humans (Pfeiffer et al. 1986), we rationalize these results by hypothesizing that the dysphoria produced by stress-induced dynorphin release enhances the rewarding properties by increasing the euphorigenic valence of cocaine.
Release of endogenous dynorphins may also mediate a component of stress-induced drug craving in reinstatement models. Consistent with this prediction, rodents given either an intermittent footshock or a forced swim stressor failed to reinstate drug-seeking behaviors after administration of kappa receptor antagonists (Beardsley et al. 2005; Carey et al. 2007). In this study, we extend these findings by determining the effects of the kappa antagonist norbinaltorphimine and disruption of the kappa system by gene deletion of either the kappa opioid receptor or the endogenous ligand dynorphin on forced-swim-stress- and foot-shock-stress-induced reinstatement of cocaine-conditioned place preference. Extension of these findings by using different forms of stress and using place preference conditioning significantly expands our understanding of the role of the dynorphin/kappa opioid system in stress-induced reinstatement.
Materials and methods
Animals and housing
Male C57Bl/6 mice (Charles River Laboratories, Wilmington, MA), KOR gene knockout (−/−) mice, and Dyn (−/−) mice weighed 20–30 g at the beginning of the study. Breeding and genotyping of KOR (−/−) and Dyn (−/−) (backcrossed onto on C57Bl/6 backgrounds) and their corresponding wild-type littermate (+/+) controls were generated by crossing heterozygotic parents as previously described (McLaughlin et al. 2003, 2006a, b). Mice were housed three to four per cage in self-standing plastic cages (28 cm L × 16 cm W × 13 cm H) lined with “Bed-o’Cobs” and habituated to isolated decentralized housing 1 week before the start of the study. The colony room was maintained on a 12-h light/dark cycle (lights on at 07:00) with food pellets and water available ad libitum. Procedures were approved by the UW IACUC.
Drugs
Cocaine–HCl (NIDA Drug Supply, Bethesda, MD, USA), norbinaltorphimine (norBNI, Sigma, St Louis, MO, USA) and U50,488 (TOCRIS, Ellisville, MO, USA) were dissolved in sterile physiological saline. Drugs and vehicle were administered in volumes of 1 ml/100 g body weight.
Conditioned place preference and reinstatement
The CPP apparatus consisted of two large Plexiglas outer chambers separated by a smaller inner chamber as previously described (McLaughlin et al. 2003). The two outer chambers were made visually distinct with 2.5-cm-wide alternating black and white stripes orientated either vertically or horizontally, while the smaller inner chamber was completely white. The floors of both outer chambers were covered with a depth of approximately 1–2 cm of shredded wood chip bedding (Beta chip, NEPCO, Warrensburg, NY, USA), while the center chamber remained uncovered Plexiglas.
Footshock stress was administered in one side of a shuttle box apparatus (16.5 cm L × 19 cm W × 10 29.2 cm H) equipped with a stainless steel grid floor and stand alone shock generator (Coulbourn Instruments, Allentown, PA, USA). Forced swim stress was conducted in opaque plastic 5-l beakers (40 cm H × 25 cm diameter) filled with 3.5l of 30°C (±1°C) water, as previously described (McLaughlin et al. 2003). The conditioning, extinction, and reinstatement protocols are summarized (Fig. 1a).
Fig. 1.
a Timeline for the sequence of behavioral manipulations. Preconditioning test: animals tested for initial chamber bias. Conditioning phase: animals were injected with saline and confined to the initial chamber for 30 min; 4 h later, mice were injected with cocaine (15 mg/kg) and confined to the drug-chamber for 30 min. Mice were conditioned in this manner for 4 days. Conditioning test day: Animals were tested (30 min) for cocaine place preference. Extinction phase 1 and 2: Daily 30 min sessions were used to extinguish cocaine place preference. Criterion to move on to the next test phase required that the time spent in the drug-paired chamber during an extinction trial be within 10% of the time spent in the drug-paired chamber during the pre-conditioning test day for three of four consecutive days. After extinction phase 1, animals are given a single session of footshock stress (0.8 mA, 40-s variable interval, 15 min), forced swim stress (15 min, 30°C), or an injection of the kappa receptor agonist U50,488 (5 mg/kg). After extinction phase 2, saline and cocaine priming (10 mg/kg) injections were given on consecutive days. b Number of C57Bl/6 mice used for the saline control and norBNI groups, KOR (−/−) mice and wild-type littermates KOR(+/+), and Dyn(−/−) and wild-type Dyn (+/+) littermates that started and completed the present study. c Mean number (±SEM) of trials needed to extinguish cocaine place preference before footshock stress, forced swim stress, U50,488 and saline/cocaine priming sessions for each group
On day 1 of the conditioning procedure, mice were placed into the small central chamber and had 30 min access to all chambers of the CPP apparatus to assess baseline preference. Animal movement was recorded by an overhead video camera and the time spent in each chamber later analyzed with Ethovision software (Noldus, The Netherlands). A biased place preference design was used in which animals were assigned to receive cocaine in their least preferred chamber while saline was to be administered in their preferred chamber. Biased place preference protocols have previously been used and produce comparable results as other designs (see Bardo et al. 1995; McLaughlin et al. 2003, 2006a). Saline and cocaine conditioning sessions were conducted daily over the next 4 days. Each day, animals were injected with saline and placed in their preferred chamber for 30 min. Four hours later, animals were injected with cocaine (15 mg/kg, s.c.) and placed in the opposite chamber for 30 min. A 30-min post-conditioning test session was performed on the fifth day to assess cocaine place preference.
During the extinction phase, animals were given daily 30 min sessions, with access to all chambers of the CPP apparatus, while in a drug-free state. Cocaine place preference was considered extinguished when the time in the drug-paired box returned to within 10% of their preconditioning preference on three out of four consecutive days. Two extinction phases were used: the first after cocaine conditioning and the second after the footshock or forced swim stress sessions. C57Bl/6 mice meeting extinction criteria were randomly assigned to the saline or norBNI groups and were injected 1 h before the footshock stress. Mice were habituated to the apparatus for 5 min before the start of the footshock stressor. Mice then received 15 min of intermittent and uncontrollable footshock (0.8 mA, 0.5 s “on”, 40 s variable interval, range 10–70 s) in a Plexiglas chamber located in a room different from the CPP testing room. After footshock, mice were returned (within 1–2 min) to the CPP room and place preference assessed. The footshock intensity (0.8 mA) was chosen because it is typically used in rat reinstatement studies. Although a 0.8-mA intensity of footshock may have produced greater stress in mice than in rats, it is comparable to studies in which footshock has been used as a stressor in mice (Song et al. 2007).
The effect of kappa antagonism on forced-swim-stress-induced reinstatement was assessed in a separate group of C57Bl/6 mice. After extinction of cocaine CPP, mice were pretreated with either saline or norBNI and then exposed to forced swim (15 min at 30°C) 1h after injection. Immediately after the forced swim session, mice were towel dried thoroughly, placed in a transport box, and taken to the CPP apparatus room for place preference testing. The time between the end of the forced swim session and placement of the mice in the CPP chamber was approximately 1 to 2 min. To mitigate the hypothermic effects of forced swim on body temperature, we used water maintained at 30°C. A previous study from this laboratory has shown that the body temperature in animals forced to swim in 30°C water slightly decreased during the forced swim test but had returned to baseline levels 10 min after the end of the forced swim session (McLaughlin et al. 2003).
The effects of norBNI on a U50,488-induced reinstatement were examined in another group of C57Bl/6 mice. In a previous study, we have shown that the kappa receptor agonist U50,488 potentiated a cocaine CPP and induced analgesia in a manner similar to that produced by a forced swim stressor (McLaughlin et al. 2006a). After successful extinction of cocaine CPP, mice were injected (i.p.) with either saline or norBNI 1 h before a subsequent injection of the kappa agonist U50,488 (5 mg/kg). Thirty minutes later, chamber preference was assessed in the CPP apparatus for 30 min. The dose of U50,488 (5 mg/kg) and the timing of testing after injection (30 min) were the lowest dose to use that had produced antinociception and the earliest time after administration that did not produce aversive effects, respectively (McLaughlin et al. 2006a).
After the footshock stress and forced swim stress test days, mice were given daily 30-min sessions, with access to all chambers of the CPP apparatus, while in a drug-free state to extinguish any preference for the drug-paired box that may have carried over from the previous stress test sessions. Once mice met extinction criterion, all animals received a saline injection (s.c.) immediately before CPP testing (saline test day) to assess the effects of s.c injections. On the following day, mice were injected with saline or norBNI (10 mg/kg, i.p.), respectively, 1h before a cocaine priming injection (10 mg/kg, s.c.). Mice were immediately placed in the CPP apparatus after cocaine administration and then tested for 30 min.
Data analysis
Raw untransformed data showing the absolute time spent in the drug-paired chamber during all phases of the reinstatement protocol are tabled in panel a of Figs. 2, 3, 4, 5, and 6 to outline place preference behavior throughout the study. Panel b of Figs. 2, 3, 4, 5, and 6 graphs the transformed data used in statistical analyses. One-way and two-way repeated measures analysis of variance (ANOVA) tests were conducted where appropriate. The difference in the time spent in the drug-paired box during the test day (post-conditioning, footshock/forced swim/U50,488 and cocaine primed injections) was subtracted from the preceding pretest day(s) (pre-conditioning, the mean of the 3 days that met extinction criterion during extinction phase 1, and saline primed injection, respectively) and analyzed with a two-way repeated measures ANOVA. Five separate ANOVAs were performed to analyze differences between norBNI-pretreated and saline controls (one ANOVA for each for the footshock, forced swim, and U50,488 stressors), KOR (−/−) and KOR (+/+), and Dyn (−/−) and Dyn (+/+) groups across the reinstatement phases (Statistica software, StatSoft Inc, Tulsa, OK, USA). Significant group × phase interactions were further analyzed with Tukey honestly significant difference (HSD) post hoc tests. All analyses used an alpha level of p < 0.05 to determine significance.
Fig. 2.
Effect of footshock stress and cocaine priming condition on reinstatement of cocaine-seeking behavior in mice pretreated with either saline or norBNI. a Time (s) spent in the drug-paired chamber during each phase of the conditioned place preference reinstatement protocol. b Difference in time (s) spent in the drug-paired chamber during the post-conditioning, footshock, and forced swim test days. The difference in time spent in the drug paired chamber was calculated by subtracting the time spent in the drug-paired chamber during the post-conditioning (post cond), footshock stress, and cocaine primed test days from the time spent in the drug-paired chamber during the pre-conditioning (pre cond), the mean of the 3 days that met criteria during extinction phase 1 (Ext 1), and saline primed tests, respectively. c Total distance traveled (m) during each phase of the reinstatement protocol. Error bars represent SEM. Asterisk represents a significant difference between groups, p<0.05. Number symbol denotes significant difference between the cocaine-primed reinstatement phase and all other phases, p<0.05
Fig. 3.
Effect of forced swim stress exposure on cocaine-seeking behavior in C57Bl/6 mice pretreated with saline or norBNI. a Time (s) spent in the drug-paired chamber during each phase of the conditioned place preference reinstatement protocol. b Difference in time (s) spent in the drug-paired chamber during the post-conditioning (post cond), forced swim stress, and cocaine primed test days (see Fig. 2 for more details). c Total distance traveled (m) during each phase of the reinstatement protocol. Error bars represent SEM. Asterisk represents a significant difference between groups, p<0.05. Number symbol denotes significant difference between the cocaine-primed reinstatement phase and all other phases, p<0.05
Fig. 4.
Effect of the kappa receptor agonist U50,488 on reinstatement of cocaine-seeking behavior in saline- or norBNI-pretreated groups. a Time (s) spent in the drug-paired chamber during each phase of the conditioned place preference reinstatement protocol. b Difference in time (s) spent in the drug-paired chamber after the post-conditioning and U50,488 test days. Saline or norBNI (10 mg/kg) were administered 1 h before U50,488 (5 mg/kg) injections. Place preference was performed 30 min after U50,488 injections. Error bars represent SEM. Asterisk represents a significant difference between groups, p<0.05
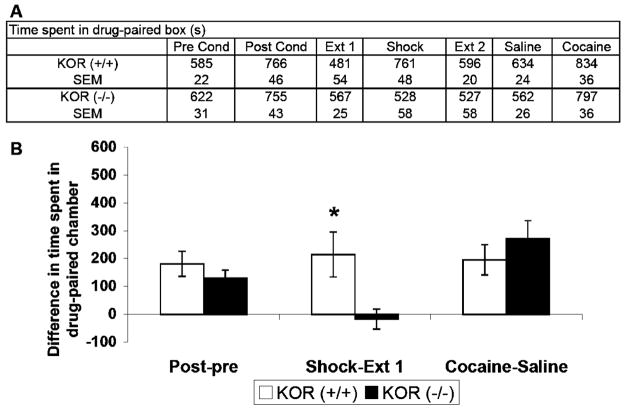
Fig. 5.
Effect of footshock stress and cocaine-primed reinstatement of cocaine-seeking behavior in KOR(−/−) and KOR(+/+) groups. a Time (s) spent in the drug-paired chamber during each phase of the conditioned place preference reinstatement protocol. b Difference in time (s) spent in the drug-paired chamber during the post-conditioning, footshock stress, and cocaine primed test days for the KOR(−/−) and KOR(+/+) groups (see Fig. 2 for more details). Error bars represent SEM. Asterisk represents a significant difference between groups, p<0.05
Fig. 6.
Effect of footshock stress and cocaine-primed reinstatement of cocaine-seeking behavior in Dyn (−/−) and Dyn (+/+) groups. a Time (s) spent in the drug-paired chamber during each phase of the conditioned place preference reinstatement protocol. b Difference in time (s) spent in the drug-paired chamber during the post-conditioning, footshock stress and cocaine prime test days for the Dyn (−/−) and Dyn (+/+) groups (see Fig. 2 for more details). Error bars represent SEM. Asterisk represents a significant difference between groups, p<0.05
Results
The within-subjects conditioned place preference reinstatement protocol produces stable extinction rates
Previous studies have used a conditioned place preference to study relapse of drug seeking (Kreibich and Blendy 2004; Mueller and Stewart 2000). The present study, however, differs in two aspects. First, a criterion was used to establish extinction of place preference to ensure that animals showed similar levels of extinguished behavior before reinstatement testing. Secondly, a within-subjects design was used to increase sensitivity and power and decrease the number of groups needed. The same animals were tested after both stress and drug priming sessions. An outline of the CPP reinstatement protocol used in the present study is depicted in Fig. 1a.
It should be noted that not all mice conditioned to cocaine successfully extinguished their place preference even after 21 extinction sessions (the upper limit for inclusion in the study) after either the cocaine conditioning phase or after reinstatement by the footshock or forced swim stressors. The data from mice that did not extinguish their place preference after either the conditioning or stress (footshock or forced swim) phase were excluded from statistical analysis. An independent group t test between the extinguished animals (mean = 198.95, SD = 97.83) and those that did not extinguish (mean = 237.96, SD = 154.37) was performed on cocaine place preference (post-conditioning test). Results revealed no significant differences between extinguished and non-extinguished groups (t = −1.64, p < 0.104), suggesting that the initial cocaine preference was not a factor in whether an animal extinguished place preference or not. In addition, data from animals that displayed an initial preference for a particular chamber that was greater than 66% of the total time on the pre-conditioning test session were also excluded. As summarized in Fig. 1b, approximately 50% of animals met extinction criteria for both extinction phases. A one-way ANOVA conducted on the differences between the groups for the number of trials to meet criteria before the footshock stress session was non-significant [F(7, 58) = 0.74, p < 0.64] (Fig. 1c). There were no significant differences between the groups in the number of extinction trials needed to meet extinction criteria for the second extinction phase [F(7, 58) = 2.00, p < 0.07]. Moreover, these extinction rates did not differ across groups of mice tested, suggesting that the mechanism of extinction learning was not different between the commercially bred (C57BL/6) and knockout, KOR and Dyn knockout and wild-type, mice strains. While intriguing, the basis for the difference in extinction success was not further explored in this study.
Inactivation of the kappa opioid receptor does not affect cocaine conditioned place preference learning
Before extinction, C57Bl/6 mice, KOR (−/−) and KOR (+/+) as well as Dyn (−/−) and Dyn (+/+) animals were conditioned to associate the effects of cocaine with one chamber of the CPP apparatus and the effects of saline with the other chamber. For all groups tested, the time spent in the cocaine-paired chamber during the post-conditioning test day increased relative to the time spent in the drug-paired chamber during the preconditioning test day. Statistical analysis of the two-way repeated ANOVAs for each comparison yielded significant group × phase interactions: saline-footshock/norBNI-footshock, F(2, 34) = 3.92, p < 0.029; saline-forced swim/norBNI-forced swim, F(2, 24) = 3.59, p < 0.043, saline-U50,488/norBNI-U50,488, F(1, 11) = 5.32, p < 0.041, KOR (−/−)/(+/+), F(2, 30) = 5.09, p < 0.012; Dyn (−/−)/(+/+), F(2, 28) = 3.16, p < 0.04. Post hoc Tukey HSD tests showed non-significant differences (p > 0.05) between the two groups (saline pretreated compared to the norBNI pretreated and knockout compared to wild-type littermates) within each comparison. The results confirm that neither KOR gene nor prodynorphin gene deletion affected cocaine CPP in the absence of stress, as previously shown (McLaughlin et al. 2003, 2006a).
norBNI blocks footshock-stress- and forced-swim- stress-induced reinstatement
After extinction of cocaine preference, mice were subjected to a 15-min session of uncontrollable footshock and then immediately placed in the CPP apparatus. For each animal, the time spent in the drug-paired chamber after footshock was subtracted from the mean of the time spent in the drug-paired chamber during the 3 days that met the extinction criteria (extinction phase 1). Reinstatement of cocaine preference after footshock stress was readily seen in C57Bl/6 mice pretreated with saline, but was not evident in animals pretreated with norBNI (Fig. 2b). The significant group × phase interaction for the saline-footshock/norBNI-footshock comparison, [F(2, 34) = 3.92, p < 0.029] was followed by post hoc Tukey HSD tests that confirmed that norBNI significantly blocked footshock-induced reinstatement (p < 0.05) when compared to saline pretreated animals.
A separate group of C57Bl/6 mice were exposed to a single session of forced swim stress after extinction of cocaine place preference. Mice were injected with either saline or norBNI 1-hr before forced swim exposure. A single session of forced swim significantly reinstated preference for the cocaine-paired chamber, while norBNI-pretreated animals failed to show an increase in preference for the cocaine-paired chamber (Fig. 3b). The two-way repeated ANOVA yielded a significant group × phase interaction [F(2, 24) = 3.59, p < 0.043]. Post hoc tests confirmed that the saline-pretreated group spent a significantly greater amount of time in the drug-paired chamber than norBNI-pretreated animals (p < 0.05). Thus, as with footshock stress-induced reinstatement, KOR antagonism blocked reinstatement of cocaine seeking after a stressful forced swim session.
norBNI blocks U50,488 induced reinstatement of cocaine-seeking behavior
A separate group of C57Bl/6 mice were administered the kappa agonist U50,488 after extinction of cocaine place preference. Mice were injected with either saline or norBNI 1 h before U50,488 injections. U50,488 administration significantly reinstated preference for the cocaine-paired chamber, while norBNI-pretreated animals failed to show reinstatement of cocaine-seeking behavior (Fig. 4b). Analysis of the two-way repeated ANOVA resulted in a significant group × phase interaction [F(1, 11) = 5.32, p < 0.041], and the post hoc Tukey HSD test confirmed that the saline-U50,488 group spent a significantly greater amount of time in the drug-paired chamber than norBNI-U50,488-injected animals (p < 0.05).
Disruption of the kappa opioid receptor attenuates footshock-stress- and forced-swim-stress-induced cocaine-seeking behavior
Disruption of kappa receptor activity via KOR (−/−) and Dyn (−/−) blocked footshock-induced cocaine reinstatement (Figs. 5 and 6, respectively). The group × phase interactions for the KOR (−/−)/(+/+) [F(2, 30) = 5.09, p < 0.012] and Dyn (−/−)/(+/+) [F(2, 28) = 3.16, p < 0.04] analyses were significant. Post hoc Tukey HSD tests confirmed that inactivation of the KOR, via either deletion of the KOR itself or depletion of the endogenous KOR ligand, dynorphin, completely blocked cocaine-seeking behavior induced by footshock stress compared to their wild-type littermates (p < 0.05). The results showed that footshock stress significantly induced reinstatement of cocaine-seeking behavior in the saline control, KOR (+/+), and Dyn (+/+) groups but failed to do so in the norBNI-pretreated, KOR (−/−), and Dyn (−/−) groups. These results indicate that the activation of the kappa opioid receptor was required for the reinstatement of cocaine-seeking behavior after footshock stress.
Cocaine-priming injections induces reinstatement of cocaine-seeking behavior in norBNI-pretreated, KOR (−/−), Dyn (−/−), and their respective control groups
The role of KOR in mediating cocaine-induced reinstatement of cocaine-seeking behavior was assessed in the same animals that were tested for footshock-stress- and forced-swim-stress-induced reinstatement. After the footshock stress or forced swim stress testing, a second extinction phase was used to eliminate carryover effects of place preference that may have occurred. Only ten animals in the study that had met criteria for the first extinction phase failed to meet extinction criteria during the second extinction phase. All data from these animals were excluded from statistical analyses. To assess whether an i.p. injection was itself stressful and could elicit reinstatement of cocaine-seeking behavior, animals were injected with saline (i.p.).and then tested for place preference. For each group tested, the time spent in the drug-paired box after saline injection was compared to mean of the time spent in the drug-paired box during the 3 days used for meeting extinction criteria for the second extinction phase. Data were analyzed by two-way ANOVAs and followed up with post hoc Tukey HSD tests. Results showed that saline injections failed to reinstate cocaine-seeking behavior in any of the groups tested (data not shown). The day after the saline injection day, animals were injected with cocaine (10 mg/kg) and then tested for reinstatement of cocaine-seeking behavior. The difference in time spent in the drug-paired box after the injection of the cocaine prime was subtracted from the time spent in the drug-paired box after the saline injection test day. Significant group × phase interactions for each of the each comparison were further analyzed with Tukey HSD tests to determine the effects of the KOR manipulation on cocaine-prime induced reinstatement. Analysis of both the footshock-stressed and forced-swim-stressed groups ANOVAs yielded significant group × phase interactions [F(2, 34) = 3.92, p < 0.029 and F(2, 24) = 3.59, p < 0.043, respectively]. Post hoc analyses revealed non-significant differences in time spent in the drug-paired box between the saline-pretreated and norBNI-pretreated groups after the cocaine prime injection (p > 0.05). Omnibus ANOVAs for the KOR (−/−)/(+/+) and Dyn (−/−)/(+/+) comparisons were F(2, 30) = 5.09, p < 0.012 and F(2, 28) = 3.16, p < 0.04, respectively. Similarly, both the KOR (−/−) and KOR (+/+), as well as the Dyn (−/−) and Dyn (+/+), groups demonstrated increased preference for the drug-paired chamber with no significant difference between knockout and wild-type littermate groups (p > 0.05) after cocaine primed injections. The fact that all groups demonstrated increased cocaine-seeking behavior after a cocaine-primed injection suggests that the kappa opioid system does not mediate drug-primed reinstatement.
norBNI does not affect locomotor activity after footshock, forced swim, or cocaine prime injections
The possibility that the KOR antagonist norBNI may have had an effect on locomotor ability during the footshock stress and forced swim stress sessions were examined by measuring the total distance traveled (m) during all CPP sessions. All groups showed similar levels of locomotor activity at each phase of the reinstatement protocol. The two-way repeated measures ANOVAs performed between the saline-footshock/norBNI-footshock [F(6, 106) = 1.29, p < 0.267] and saline-forced swim/norBNI-forced swim groups [F(6, 72) = 0.466, p < 0.831] on the total distance traveled were shown to be non-significant. The total distance traveled during the footshock stress test day was the same for both the norBNI- and saline-pretreated groups (Fig. 2c). Similarly, the total distance traveled by the norBNI- and saline-pretreated groups after the forced swim stress session were not different (Fig. 3c). The non-significant group × phase interactions suggest that pretreatment with norBNI does not adversely affect locomotor activity. Analysis of the main effect of phase for the saline-footshock/norBNI-footshock comparison [F(6, 102) = 71.76, p < 0.001] and the saline-forced swim/norBNI-forced swim comparison [F(6, 72) = 17.09, p < .0.001] were significant. Post hoc Tukey HSD tests revealed that the total distance traveled during the cocaine-prime test sessions were significantly greater than all other phases of the reinstatement protocol (p < 0.05). That is, after cocaine-prime injections, all animals demonstrated locomotor sensitization regardless of whether they were saline- or norBNI-pretreated.
Discussion
The role of the kappa opioid receptor in stress- and cocaine-priming-induced reinstatement
Stress exposure has been found to potentiate addictive drug related behaviors (for example Covington and Miczek 2005; McLaughlin et al. 2003, 2006a), and stress is a major precipitating factor for relapse of drug seeking (Epstein et al. 2006; Stewart 2003). The principal findings of the present study were that reinstatement of cocaine seeking by stress exposure, but not by cocaine priming, was kappa-opioid-receptor-mediated. We found that inescapable foot-shock, repeated forced swim, and the kappa agonist U50,488 all effectively reinstated cocaine-seeking behavior in C57Bl/6 mice previously conditioned to cocaine. Stress-induced reinstatement was completely blocked by norBNI and was not evident in KOR(−/−) or Dyn(−/−) mice. However, disruption of the dynorphin/KOR system did not affect reinstatement after a priming injection of cocaine. Thus, dynorphin and KOR activation seems to have a specific role in stress-induced reinstatement.
The findings of the present study are consistent with previous reports (Beardsley et al. 2005; Carey et al. 2007; Valdez et al. 2007). Beardsley et al. (2005) showed that intermittent and uncontrollable footshock was able to reinstate active lever responding on a lever previously associated with cocaine self-administration and that pre-treatment with the kappa antagonist JDTic attenuated footshock-induced cocaine-seeking behavior. Carey et al. (2007) also demonstrated that the kappa receptor antagonist arodyn blocked forced-swim-stress-induced reinstatement of cocaine-seeking behavior using a conditioned place preference protocol. Beardsley et al. (2005) and Carey et al. (2007) also demonstrated that antagonism of the kappa receptor failed to attenuate cocaine-induced reinstatement.
Opioid mechanisms have been broadly implicated in mediating stress-, cue-, and drug-priming-induced reinstatement of drug seeking. However, activation of mu, delta, or opioid receptor-like1 (ORL1) receptor seems to induce drug reinstatement in a manner distinct from the kappa opioid receptor. The preferential mu receptor antagonist naltrexone has been shown to attenuate alcohol- and heroin-induced reinstatement of alcohol and heroin-seeking behaviors, respectively, but failed to block footshock-induced reinstatement (Le et al. 1999; Shaham et al. 1996). Both naltrexone and the delta opioid antagonist naltrindole have been shown to block cue-induced reinstatement of alcohol in a self-administration protocol (Cioccocioppo et al. 2002). In contrast, the ORL1 receptor antagonist nociceptin reduced footshock-induced reinstatement of ethanol seeking, but failed to block footshock-induced reinstatement of cocaine seeking (Martin-Fardon et al. 2000). Although the present study demonstrated that the dynorphin/kappa opioid system mediates stress-induced reinstatement of cocaine-seeking behavior, further studies are needed to assess its role in reinstatement to other drugs of abuse.
In the present study, the kappa agonist U50,488 was also capable of inducing drug-seeking behavior, and this effect was blocked by norBNI pretreatment. This is consistent with the role of endogenous dynorphin suggested by the block of reinstatement in stress-exposed dyn(−/−) mice. Our findings are also generally consistent with those of Valdez et al. (2007) who recently showed that the kappa agonists enadoline and spiradoline were capable of reinstating extinguished operant lever pressing in squirrel monkeys; however, Valdez and colleagues (2007) found that naltrexone, but not norBNI, was able to block the agonist-induced reinstatement. The authors suggest that the failure of norBNI to attenuate cocaine-seeking behavior after administration of the κ1 receptor agonists enadoline and spiradoline may be due to norBNI’s greater effective- ness at antagonizing the κ2 receptor subtype. Differences between rodents and primates are intriguing, but a suggested role of KOR subtypes will require additional validation. The different results may also stem from methodological differences, particularly the chosen routes of administration. In addition, the findings that the selective kappa opioid antagonists JDTic and arodyn blocked reinstatement support the conclusion that it is the kappa opioid receptor, as opposed to the mu receptor, that plays an important role in stress-induced reinstatement.
Other pharmacological stressors, including corticotropin-releasing factor (CRF) and yohimbine administration, have also been found to reinstate cocaine-seeking behaviors, indicating a role for the CRF and adrenergic neurotransmitter systems in mediating stress-induced relapse (Erb et al. 2001; Erb and Stewart 1999; Lee et al. 2004; Leri et al. 2002). The relationship between the CRF, adrenergic, and dynorphin systems in stress-induced reinstatement has not been resolved; however, we recently reported that CRF-induced place aversion was mediated by dynorphin-dependent KOR activation (Land et al. 2008). Several additional lines of evidence support a possible dynorphin–CRF interaction in mediating stress-induced reinstatement. For instance, studies have shown that CRF and dynorphin are co-expressed in the same brain regions (Roth et al. 1983); CRF induces dynorphin release (Nikolarakis et al. 1987; Sirinathsinghji et al. 1989), and dynorphin mediates several CRF effects (Overton and Fisher 1989). More specifically, CRF has been shown to elicit cocaine-seeking behavior (Erb and Stewart 1999; Erb et al. 2001), and CRF antagonists have been shown to block both footshock- (Erb and Stewart 1999) and kappa-agonist-induced reinstatement (Valdez et al. 2007). Similar to the present findings, manipulations of either the CRF or adrenergic neurotransmitter systems blocked stress-induced reinstatement and failed to alter drug-priming-induced reinstatement. The CRF antagonist D-Phe-CRF (Erb et al. 1998), the alpha-2 adrenoreceptor agonists clonidine, lofexidine, and guanabenz (Erb et al. 2000), as well as a mixture beta-1 and beta-2 adrenoreceptor antagonists (Leri et al. 2002) were able to attenuate footshock-induced but not cocaine-induced reinstatement. It seems reasonable then that a mechanism for stress-induced reinstatement of drug-seeking behavior may involve not only the dynorphin/kappa system but also an interaction between the kappa opioid, CRF, and/or adrenergic neurotransmitter systems.
Clinical implications of kappa antagonism on drug reinstatement
The dynorphin/kappa opioid system has been postulated to counteract the stimulating and rewarding properties produced by drugs of abuse (Carlezon et al. 2005), and this system has been found to be critical in the development and relapse to drug seeking (for review, see Shippenberg et al. 2007). Briefly, increased activation of the nucleus accumbens results in a cAMP-response-element-binding-protein-mediated increase in dynorphin release that acts on inhibitory kappa receptors on the terminals and cell body of dopaminergic ventral tegmental area neurons. Kappa agonists have been shown to decrease mesolimbic DA release and activity (Di Chiara and Imperato 1988; Chefer et al. 2005). Co-administration of kappa receptor agonists with cocaine has been shown to attenuate the rewarding and behavioral effects of cocaine. Pretreatment with kappa agonists has been shown to decrease self-administration of cocaine (Glick et al. 1995; Mello and Negus 1998; Negus et al. 1997; Schenk et al. 1999), cocaine place preference (Crawford et al. 1995; Schenk et al. 1999), locomotor sensitization (Schenk et al. 1999), and cocaine-induced reinstatement (Schenk et al. 1999, 2000). These findings have led to the hypothesis that cocaine self-administration could be attenuated by pretreatment with kappa agonists. However, the potential of kappa agonists as a pharmacotherapeutic tool to combat cocaine addiction may be tempered by their dysphoric effects (Mello and Negus 1998, Negus et al. 1997). Furthermore, using a protocol that distinguishes between the effects of choice and locomotor activity, Negus (2004) showed that the kappa agonist U50,488 decreased overall responses on both the food and cocaine levers, but actually increased the percentage of cocaine choice. This finding demonstrated that despite decreased dopamine activity by kappa agonists, the rewarding effects of cocaine were intact. A lack of beneficial effect of kappa agonists on cocaine self-administration was also found in cocaine-dependent people given either enadoline or butorphanol (Walsh et al. 2001). Taken together, these results suggest that kappa agonists may not produce the desired reduction in cocaine craving. In contrast, kappa opioid receptor antagonism blocked several types of stress-induced reinstatement in animal models, and they may have beneficial effects in stress-induced relapse in recovering addicts.
Footshock-stress-induced reinstatement has repeatedly been shown to increase cocaine-seeking behavior using both the self-administration and conditioned place preference paradigms (Erb et al. 1996; Shaham et al. 1996; Shalev et al. 2000; Wang et al. 2000). Most reinstatement models using operant lever protocols have delivered foot-shock in the same operant chambers in which self-administration had occurred. When footshock or restraint stress was administered in an environment other than the self-administration chambers, the stress failed to elicit reinstatement (Shalev et al. 2000). In contrast, both the footshock and forced swim were administered in a different apparatus and room in the reinstatement protocol described in the present study. This is consistent with recent reports from other research groups also showing that stress exposure outside the experimental chamber was able to reinstate drug seeking (Carey et al. 2007; Kreibich and Blendy 2004, Ribeiro Do Cotuo et al. 2006). Differences between reinstatement results obtained using operant self-administration protocols and place-conditioning protocols likely reflect the differences in the underlying learning mechanisms.
In our study, a single session of forced swim stress was able to reinstate cocaine-seeking behavior when animals were tested immediately after the stressor. The ability of forced swim stress to induce reinstatement cocaine-seeking behavior has been reported after a 1-h (Carey et al. 2007) and up to a 24-h delay (Kreibich and Blendy 2004). As with the present study, footshock stress has been shown to induce drug reinstatement when administered immediately (Erb et al. 1996; Shaham and Stewart 1996; Shaham et al. 1996). Recently, it was reported that externally administered social defeat, tail pinch, and restraint stressors acutely induced reinstatement of morphine-seeking behavior (Ribeiro Do Couto et al. 2006). Differences between acute and prolonged stress exposure and the duration of vulnerability after stress exposure will be important to fully understand.
In conclusion, we have shown that activation of the kappa opioid system by footshock forced swim or U50,488 administration reinstated cocaine-seeking behavior. The present findings extend previous work by demonstrating that disruption of a functioning dynorphin/kappa opioid system by pretreatment with the kappa antagonist norBNI or by KOR or prodynorphin gene disruption abolishes reinstatement. The results of the present study support the suggestion that kappa opioid antagonists might be effective therapeutic tools for reducing stress-induced drug relapse.
Acknowledgments
We thank Dr. Ute Hochgeschwender for the prodynorphin knockout mice (Dyn −/−) and Dr. John Pintar for the kappa opioid receptor (KOP-r) knockout mice (KOR−/−). William Giardino helped with the behavioral assays. We also thank Dan Messinger for performing the mouse genotyping and managing the mouse-breeding colony. This work was supported by USPHS grants RO1 DA16898, KO5 DA20570 (CC), and T32 DA07278 (VR) from the National Institute on Drug Abuse.
Abbreviations
- norBNI
norbinaltorphimine
- CPP
conditioned place preference
- HSD
Tukey honestly significant difference
- KOR
kappa opioid peptide receptor
- Dyn−/−
prodynorphin gene knockout mice
Footnotes
Disclosure/Conflict of interest statement The authors have no conflicts of interest to declare.
References
- Beardsley PM, Howard JL, Shelton KL, Carroll FI. Differential effects of the novel kappa opioid receptor antagonist, JDTic on reinstatement of cocaine-seeking induced by footshock stressors vs. cocaine primes and its antidepressant-like effects in rats. Psychopharmacology. 2005;183:118–126. doi: 10.1007/s00213-005-0167-4. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Rowlett JK, Harris MJ. Conditioned place preference using opiate and stimulant drugs: a meta-analysis. Neurosci Biobehav Rev. 1995;19:39–51. doi: 10.1016/0149-7634(94)00021-r. [DOI] [PubMed] [Google Scholar]
- Bossert JM, Ghitza UE, Lu L, Epstein DH, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur J Pharmacol. 2005;526:36–50. doi: 10.1016/j.ejphar.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Carey AN, Borozny K, Aldrich JV, McLaughlin JP. Reinstatement of cocaine place-conditioning prevented by the peptide kappa-opioid receptor antagonist arodyn. Eur J Pharmacol. 2007;569:84–89. doi: 10.1016/j.ejphar.2007.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Duman RS, Nestler EJ. The many faces of CREB. Trends Neurosci. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Chefer VI, Czyzyk T, Bolan EA, Moron J, Pintar JE, Shippenberg TS. Endogenous kappa-opioid receptor systems regulate mesoaccumbal dopamine dynamics and vulnerability to cocaine. J Neurosci. 2005;25:5029–5037. doi: 10.1523/JNEUROSCI.0854-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cioccociopo R, Martin-Fardon R, Weiss F. Effect of selective blockade of m1 or d opioid receptors on reinstatement of alcohol-seeking behavior by drug-associated stimuli in rats. Neuropsychopharmacology. 2002;27:391–399. doi: 10.1016/S0893-133X(02)00302-0. [DOI] [PubMed] [Google Scholar]
- Covington HE, Miczek KA. Intense cocaine self-administration after episodic social defeat stress, but not after aggressive behavior: dissociation from corticosterone activation. Psychopharmacology. 2005;183:331–340. doi: 10.1007/s00213-005-0190-5. [DOI] [PubMed] [Google Scholar]
- Crawford CA, McDougall SA, Bolanos CA, Hall S, Berger SP. The effects of the kappa-opioid agonist, U50,488 on cocaine-induced conditioned and unconditioned behaviors and Fos immunoreactivity. Psychopharmacology. 1995;120:392–399. doi: 10.1007/BF02245810. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Wit H, Stewart J. Drug reinstatement of cocaine-reinforced responding in the rat. Psychopharmacology. 1981;75:134–143. doi: 10.1007/BF00432175. [DOI] [PubMed] [Google Scholar]
- De Wit H, Stewart J. Drug reinstatement of heroin-reinforced responding in the rat. Psychopharmacology. 1983;79:29–31. doi: 10.1007/BF00433012. [DOI] [PubMed] [Google Scholar]
- Epstein DH, Preston KL, Stewart J, Shaham Y. Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology. 2006;189:1–16. doi: 10.1007/s00213-006-0529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Stewart J. A role for the bed nucleus of the stria terminalis, but not the amygdala, in the effects of corticotrophin-releasing factor on stress-induced reinstatement of cocaine seeking. J Neurosci. 1999;19:RC35. doi: 10.1523/JNEUROSCI.19-20-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology. 1996;128:408–412. doi: 10.1007/s002130050150. [DOI] [PubMed] [Google Scholar]
- Erb S, Shaham Y, Stewart J. The role of corticotropin-releasing factor and corticosterone in stress- and cocaine-induced relapse to cocaine seeking in rats. J Neurosci. 1998;18:5529–5536. doi: 10.1523/JNEUROSCI.18-14-05529.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb S, Hitchcott PK, Rajabi H, Mueller D, Shaham Y, Stewart J. Alpha-2 adrenoreceptor agonists block stress-induced reinstatement of cocaine seeking. Neuropsychopharmacology. 2000;23:138–150. doi: 10.1016/S0893-133X(99)00158-X. [DOI] [PubMed] [Google Scholar]
- Erb S, Salamaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from the central nucleus of the amygdala to the bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- Gerber GJ, Stretch R. Drug-induced reinstatement of extinguished self-administration behavior in monkeys. Pharmacol Biochem Behav. 1975;3:1055–1061. doi: 10.1016/0091-3057(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Glick SD, Maisonnueve IM, Raucci J, Archer S. Kappa-opioid inhibition of morphine and cocaine self-administration in rats. Brain Res. 1995;681:147–152. doi: 10.1016/0006-8993(95)00306-b. [DOI] [PubMed] [Google Scholar]
- Katz JL, Higgins ST. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology. 2003;168:21–30. doi: 10.1007/s00213-003-1441-y. [DOI] [PubMed] [Google Scholar]
- Koob GF. The neurobiology of addiction: a neuroadaptational view relevant for diagnosis. Addiction. 2006;101(Suppl 1):23–30. doi: 10.1111/j.1360-0443.2006.01586.x. [DOI] [PubMed] [Google Scholar]
- Kreibich AS, Blendy JA. cAMP response element-binding protein is required for stress but not cocaine-induced reinstatement. J Neurosci. 2004;24:6686–6692. doi: 10.1523/JNEUROSCI.1706-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Land BB, Bruchas MR, Lemos JC, Xu M, Melief EJ, Chavkin C. The dysphoric component of stress is encoded by activation of the dynorphin kappa-opioid system. J Neurosci. 2008;28:407–414. doi: 10.1523/JNEUROSCI.4458-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le AD, Poulos CX, Harding S, Watchus J, Juzytsch W, Shaham Y. Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress. Neuropsychopharmacology. 1999;21:435–444. doi: 10.1016/S0893-133X(99)00024-X. [DOI] [PubMed] [Google Scholar]
- Lee B, Tiefenbacher S, Platt DM, Spealman RD. Pharmaco- logical blockade of a2-adrenoreceptors induces reinstatement of cocaine-seeking behavior in Squirrel monkeys. Neuropsychopharmacology. 2004;29:686–693. doi: 10.1038/sj.npp.1300391. [DOI] [PubMed] [Google Scholar]
- Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshner AI. Addiction is a brain disease, and it matters. Science. 1997;278:45–47. doi: 10.1126/science.278.5335.45. [DOI] [PubMed] [Google Scholar]
- Martin-Fardon R, Ciccocioppo R, Mass M, Weiss F. Nociception prevents stress-induced ethanol- but not cocaine seeking behavior in rats. Neuroreport. 2000;11:1939–1943. doi: 10.1097/00001756-200006260-00026. [DOI] [PubMed] [Google Scholar]
- McLaughlin JP, Marton-Popovici M, Chavkin C. κ Opioid receptor antagonism and prodynorphin gene disruption block stress-induced behavioral responses. J Neurosci. 2003;23:5674–5683. doi: 10.1523/JNEUROSCI.23-13-05674.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Land BB, Li S, Pintar JE, Chavkin C. Prior activation of kappa opioid receptors by U50,488 mimics repeated forced swim stress to potentiate cocaine place preference conditioning. Neuropharmacology. 2006a;31:787–794. doi: 10.1038/sj.npp.1300860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin JP, Li S, Valdez J, Chavkin TA, Chavkin C. Social defeat stress-induced behavioral responses are mediated by the endogenous kappa opioid system. Neuropsychopharmacology. 2006b;31:1241–1248. doi: 10.1038/sj.npp.1300872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK, Negus SS. Effects of kappa opioid agonists on cocaine- and food-maintained responding by Rhesus monkeys. J Pharmacol Exp Ther. 1998;286:812–824. [PubMed] [Google Scholar]
- Mueller D, Stewart J. Cocaine-induced conditioned place preference: reinstatement by priming injections of cocaine after extinction. Behav Brain Res. 2000;115:39–47. doi: 10.1016/s0166-4328(00)00239-4. [DOI] [PubMed] [Google Scholar]
- Negus SS. Effects of the kappa opioid agonist U50,488 and the kappa opioid antagonist nor-binaltorphimine on choice between cocaine and food in rhesus monkeys. Psychopharmacology. 2004;176:204–213. doi: 10.1007/s00213-004-1878-7. [DOI] [PubMed] [Google Scholar]
- Negus SS, Mello NK, Portoghese PS, Lin CE. Effects of kappa opioids on cocaine self-administration by Rhesus monkeys. J Pharmacol Exp Ther. 1997;282:44–55. [PubMed] [Google Scholar]
- Nikolarakis KE, Almeida OF, Herz A. Feedback inhibition of opioid peptide release in the hypothalamus of the rat. Neuroscience. 1987;23:143–148. doi: 10.1016/0306-4522(87)90278-8. [DOI] [PubMed] [Google Scholar]
- Overton JM, Fisher LA. Modulation of central nervous system actions of corticotrophin-releasing factor by dynorphin-related peptides. Brain Res. 1989;488:233–240. doi: 10.1016/0006-8993(89)90713-0. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A, Brantl V, Herz A, Emrich HM. Psychotomimesis mediated by kappa opiate receptors. Science. 1986;233:774–776. doi: 10.1126/science.3016896. [DOI] [PubMed] [Google Scholar]
- Ribeiro Do Couto B, Aguilar MA, Manzanedo C, Rodriguez-Arias M, Armario A, Miňarro J. Social stress is as effective as physical stress in reinstating morphine-induced place preference in mice. Psychopharmacology. 2006;185:459–470. doi: 10.1007/s00213-006-0345-z. [DOI] [PubMed] [Google Scholar]
- Roth KA, Weber E, Borchas JD, Chang D, Chang JK. Immunoreactive dynorphin-(1–8) and corticotrophin-releasing factor in subpopulation of hypothalamic neurons. Science. 1983;219:189–191. doi: 10.1126/science.6129700. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B, Shippenberg TS. U69593, a kappa-opioid agonist, decreases cocaine self-administration and decreases cocaine-produced drug-seeking. Psychopharmacology. 1999;144:339–346. doi: 10.1007/s002130051016. [DOI] [PubMed] [Google Scholar]
- Schenk S, Partridge B, Shippenberg TS. Reinstatement of extinguished drug-taking behavior in rats: effect of the kappa-opioid receptor agonist, U69593. Psychopharmacology. 2000;151:85–90. doi: 10.1007/s002130000476. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Stewart J. Effects of opioid and dopamine receptor antagonists on relapse induced by stress and reexposure to heroin in rats. Psychopharmacology. 1996;125:385–391. doi: 10.1007/BF02246022. [DOI] [PubMed] [Google Scholar]
- Shaham Y, Rajabi H, Stewart J. Relapse to heroin-seeking in rats under opioid maintenance: the effects of stress, heroin priming, and withdrawal. J Neurosci. 1996;16:1957–1963. doi: 10.1523/JNEUROSCI.16-05-01957.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology. 2003;168:3–20. doi: 10.1007/s00213-002-1224-x. [DOI] [PubMed] [Google Scholar]
- Shalev U, Highfield D, Yap J, Shaham Y. Stress and relapse to drug seeking in rats: studies on the generality of the effect. Psychopharmacology. 2000;150:337–346. doi: 10.1007/s002130000441. [DOI] [PubMed] [Google Scholar]
- Shippenberg TS, Herz A. Differential effects of mu and kappa opioid systems on motivational processes. NIDA Res Monogr. 1986;75:563–566. [PubMed] [Google Scholar]
- Shippenberg TS, LeFevour A, Heidbreder C. kappa-Opioid receptor agonists prevent sensitization to the conditioned rewarding effects of cocaine. J Pharmacol Exp Ther. 1996;276:545–554. [PubMed] [Google Scholar]
- Shippenberg TS, Zapata A, Chefer VI. Dynorphin and the pathophysiology of drug addiction. Pharmacol Ther. 2007;116:306–321. doi: 10.1016/j.pharmthera.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirinathsinghji DJS, Nikolarakis KE, Herz A. Corticotropin-releasing factor stimulates the release of methionine-enkephalin and dynorphin from the neostriatum and globus pallidus of the rat: in vitro and in vivo studies. Brain Res. 1989;490:276–291. doi: 10.1016/0006-8993(89)90245-x. [DOI] [PubMed] [Google Scholar]
- Song M, Wang XY, Zhao M, Wang XY, Zhai HF, Lu L. Role of stress in acquisition of alcohol-conditioned place preference in adolescent and adult mice. Alcohol Clin Exp Res. 2007;31:2001–2005. doi: 10.1111/j.1530-0277.2007.00522.x. [DOI] [PubMed] [Google Scholar]
- Stewart J. Stress and relapse to drug seeking: studies in laboratory animals shed light on mechanisms and sources of long-term vulnerability. Am J Addict. 2003;12:1–17. [PubMed] [Google Scholar]
- Stretch R, Gerber GJ. Drug-induced reinstatement of amphetamine self-administration behaviour in monkeys. Can J Psychol. 1973;27:168–177. doi: 10.1037/h0082466. [DOI] [PubMed] [Google Scholar]
- Valdez GR, Platt DM, Rowlett JK, Ruedi-Bettschen D, Spealman RD. agonist-induced reinstatement of cocaine seeking in squirrel monkeys: a role for opioid and stress-related mechanisms. J Pharmacol Exp Ther. 2007;323:525–533. doi: 10.1124/jpet.107.125484. [DOI] [PubMed] [Google Scholar]
- Walsh SL, Geter-Douglas B, Strain EC, Bigelow GE. Enadoline and butorphanol: evaluation of k-agonists on cocaine pharmacodynamics and cocaine self-administration in humans. J Pharmacol Exp Ther. 2001;299:147–158. [PubMed] [Google Scholar]
- Wang B, Luo F, Zhang WT, Han JS. Stress or drug priming induces reinstatement of extinguished conditioned place preference. Neuroreport. 2000;11:2781–2784. doi: 10.1097/00001756-200008210-00034. [DOI] [PubMed] [Google Scholar]