Abstract
The goal of this critical review is to provide a critical analysis of the chain dynamics responsible for the action of micro- and nanoparticles of mucoadhesive biomaterials. The objective of using bioadhesive controlled drug delivery devices is to prolong their residence at a specific site of delivery, thus enhancing the drug absorption process. These mucoadhesive devices can protect the drug during the absorption process in addition to protecting it on its route to the delivery site. The major emphasis of recent research on mucoadhesive biomaterials has been on the use of adhesion promoters, which would enhance the adhesion between synthetic polymers and mucus. The use of adhesion promoters such as linear or tethered polymer chains is a natural result of the diffusional characteristics of adhesion. Mucoadhesion depends largely on the structure of the synthetic polymer gels used in controlled release applications.
Keywords: bioadhesion, mucoadhesion, molecular dynamics, hydrogels, tethered chains
1. Introduction
1.1 General Concepts of Mucoadhesion
Mucus is a viscous and heterogeneous biological product that coats many epithelial surfaces [1]. Mucus-secreting cells are widely spread in different locations in the body, including the nasal, ocular, buccal area, and the gastrointestinal, reproductive and respiratory tracts.
Mainly, the mucus serves as a lubricant to minimize shear stresses and as a protection barrier against harmful substances. However, mucus can perform other important functions [2–5]. Goblet cells located in the epithelium are unicellular mucus-secreting glands.
Mucus is stored in large granules in the goblet cell and can be released by exocytosis or exfoliation of the whole cell [6]. Mucus granules are mainly store in the apical side of the goblet cell, which results in the characteristic balloon shape of these cells. Although the secretion of mucus can vary depending on age, sex, body location and health condition, the average mucus turnover is approximately 6 hours [2]. Goblet cells experience two types of granules exocytosis: basal secretion, which is characterized by a low level, continuous and unregulated secretion, and stimulated secretion, which is a regulated exocytosis of granules in response to extracellular stimuli.
The stimulated pathway can dramatically increase the mucus secretion [7]. In pathological conditions, secretion of mucus can considerably vary. For example, in ulceration or inflammation, the intestinal mucous layer is thinner [8]. In physiological conditions, it has been observed that the mucous layer on the gastric and duodenal epithelial surfaces has a thickness between 5 and 200 μm in the rat, to twice this variation in the human [9].
Mucus consists mainly of water (up to 95% weight), inorganic salts (about 1% weight), carbohydrates and lipids (less than 1%) and glycoproteins (no more than 5% weight). Mucus glycoproteins are also called mucins and consist of a protein core with branched oligosaccharide chains attached over 63% of its length [2]. Approximately 80% by weight of the glycoprotein consists of oligosaccharides, which make the mucin more hydrosoluble and also protects the protein core from proteolytic degradation.
Mucins are responsible of the gel-like properties of the mucus [2]. Glycoprotein concentrations determine the cohesion of the mucus. When a critical mucin concentration is achieved, the hydrodynamic volumes of the molecules start overlapping and a gel is formed.
The main amino acids in the branched protein blocks are serine and threonine, which are linked to the oligosaccharide chains by O-glycosidic bonds. The sugar residues composing the oligosaccharide side chains are galactose, fucose, N-acetylglusocamine, N-acetylgalactosamine and sialic acid. Generally the oligosaccharide chain is linked to the protein core through an ether bond between the −1 position hydroxyl group from the N-acetylgalactosamine and the hydroxyl group from the serine or threonine amino acids [10]. Oligosaccharide chains are normally 2 to 19 residues long and often fucose, sialic acid, sulfate esters of galactose and N-acetylglusocamine are the terminal groups [11]. Opposite to the rich serine and threonine branched blocks, the unbranched blocks of the protein core have a normal amino acids composition [10].
Mucin glycoproteins exhibit molecular weights between 0.5 to 40 × 106 Da, although the average MW is 1.8 × 106 Da [2]. They consist of 4 to 6 subunits linked together [12]. Mucin subunits are joined together through disulfide bonds between the cysteine residues present in the non-glycosylated areas of the protein core [4]. Intermolecular interactions have also been detected between mucin molecules. They are believed to be non-covalent, being the hydrogen-bonding, hydrophobic interactions and physical entanglement the main intermolecular interactions. Figure 1 shows a scheme of the mucus layer covering the epithelial surface. Goblet cells and mucin glycoproteins are also shown.
Figure 1.
Mucus layer on epithelial surface. Goblet cells produce, store and secrete mucus. Mucin glycoproteins consist of a protein core with oligosaccharide side chains over their length.
1.2 Mechanisms involved in mucoadhesion
Mucoadhesion can be defined as the phenomenon of the attachment of natural or synthetic polymers to a mucosal surface. In order to develop and/or improve mucoadhesive materials, it is essential to understand the forces and mechanisms that lead to an effective bond between a polymer and a mucous layer. Yet, the mechanisms of mucoadhesion are not completely clear.
In general, it is agreed that the process involved in the mucoadhesion phenomenon can be described in three steps: First of all, the wetting and swelling of the polymer should allow an intimate contact with the tissue, secondly interpenetration of the polymer chains and entanglement between the polymer and the mucin chains should be attained and finally, the formation of weak chemical bonds should be possible [13, 14]. Certain polymeric hydrogels can exhibit mucoadhesive properties. Some of the characteristics that have shown to increase hydrogel mucoadhesive properties include: the presence of a high amount of hydrogen bonding chemical groups, such as hydroxyls and carboxyls, anionic surface charges, high polymer molecular weight, high polymer chain flexibility and surface tensions that will induce spreading into the mucus layer [15].
There exist three main types of interactions between a polymer and the mucous layer: physical or mechanical bonds, secondary chemical bonds and covalent chemical bonds. [16–19].
Physical bonds imply the entanglement of mucin glycoproteins with the polymer chains, and the interpenetration of the mucin chains in the polymer matrix. This interpenetration of macromolecules will depend on the respective chain flexibility and diffusion coefficients. [15].
Secondary chemical interactions include ionic bonds, van der Waals interactions and hydrogen bonding. Hydrogen bonding is probably the most important secondary chemical interaction in mucoadhesion. Some of the functional groups that form hydrogen bonds are hydroxyls, carboxyls, sulfate and amino groups. For this reason, polymers such as poly(vinyl alcohol), poly(acrylic acid), poly(hydroxyalkyl methacrylate) have shown good mucoadhesive properties in the past. Even these types of forces are weak; numerous interaction sites lead to strong mucoadhesion [15].
Covalent bonds are attained by the chemical reaction of the polymer and the substrate. Even this type of bond is permanent, the necessity of such type of system should be evaluated since both the mucus turnover and the epithelial desquamation would result in the detachment and loose of the polymer from the tissue anyway.
1.3 Theories on mucoadhesion
To date, no individual theory has been accepted to explain mucoadhesion as a phenomenon occurring via one singular mechanism. However, several theories have been developed and are used to describe the complex phenomenon of mucoadhesion. Some of these theories are founded on physical interactions while some others are based on chemical interactions. Here, a brief description of the predominant theories on mucoadhesion is given.
The electronic theory assumes that the mucoadhesive polymer and the mucin glycoproteins have different electronic structures, resulting in the formation of a double layer of electrical charge at the interface. Attraction across the electrical double layer leads to adhesion of the two surfaces [20].
According to the adsorption theory, the formation of a successful mucoadhesive bond is the result of van der Waals forces and hydrogen bonds [21].
The wetting theory is mainly applied in semisolid and liquid mucoadhesive systems and relates the ability of a mucoadhesive polymer to spread over a tissue. This theory uses surface tensions at the interfaces to calculate the spreading coefficient, which is an indicative parameter of the mucoadhesion properties of the polymer [22].
The fracture theory examines the force involved in the separation of the polymer surface and the biological surface after adhesion. This force is related to the mucoadhesive capabilities of the polymer evaluated [16].
Finally, the diffusion theory is based on the diffusion and interpenetration of macromolecular chains. When the mucoadhesive polymer is brought in contact with the mucous layer, the concentration gradient at the interface provokes the spontaneous diffusion of the polymer chains into the mucus and the glycoprotein chains of the mucus into the polymeric matrix. Depending on the chain concentration gradient and the diffusion coefficient of the macromolecules through the polymeric matrix, the diffusion rate will vary. [23].
1.4 Methods of analysis of mucoadhesion
Since the early 1980’s, a vast variety of methods to evaluate the potential mucoadhesive properties of new polymeric materials has been developed. The diversity in physical forms of the mucoadhesive devices invented led to the generation of a wide variety of techniques for mucoadhesion evaluation.
A large number of methods found in the literature are based on the measurement of the force necessary to separate a mucoadhesive material from a biological membrane. Peel, shear and tensile forces can be determined depending on the direction in which the mucoadhesive material is detached from the biological surface.
Peel forces are measured when evaluating mucoadhesive devices for buccal or transdermal applications. Within the shear strength tests, the Wilhelmy plate method developed by Smart al. [24, 25], is one of the most remarkable methods. In this method, a glass plate coated with the mucoadhesive material to be tested is submerged in a mucin solution. A microbalance connected to the plate measures the forces due to surface tension on the plate as the system containing the mucin solution is pulled away from the mucoadhesive material. This force measured is related to the wettability of the mucin on the polymer surface and corresponds to the adhesive force between the mucoadhesive polymer and the mucin glycoprotein.
Tensile tests have been widely used for the evaluation of a large diversity of mucoadhesive devices. For example, Ponchel et al. [26] analyzed the tensile force required to separate a mucoadhesive tablet from animal mucosa. This force is then used to calculate the work of adhesion. This parameter has been extensively used as a good indicator of the mucoadhesive properties of a material and is calculated by the integration of the force vs. displacement curve obtained in the tensile experiments.
Other notable mucoadhesion techniques include the method developed by Robinson et al. [27, 28] where human epithelial cells are labeled with fluorescent probes and placed in contact with a mucoadhesive polymer. The interaction between the epithelial cell membrane and the polymer is investigated. More recently, other methods used to examine the molecular interactions at cell surfaces include the force microscopy techniques [29, 30].
Mikos and Peppas [31] invented the flow channel method in which a mucoadhesive polymer particle is placed on a mucus surface in a Plexiglas ® channel. A laminar flow of air is directed over the microparticle and photographs are taken to analyze the static and dynamic behavior of the polymer particle. Other techniques used for the evaluation of mucoadhesive particles include the electrobalance method [32] and contact angle measurements [33].
The falling film technique developed by Ho et al. [34] is also a remarkably simple method for the evaluation of mucoadhesive particles. In this method, spherical latex particles are coated with a mucoadhesive material and are suspended in a buffer solution of a known concentration. The particle solution is then pumped over a rat small intestine cut lengthwise and placed in a cylindrical channel. The eluted solution is collected and remaining particles in the solution are counted. The portion of particles that remained adhered in the mucosal tissue is an indication of the mucoadhesive properties of the material tested.
Staining methods have also been developed for the evaluation of mucoadhesive polymers. A colloidal gold staining technique was developed by Park [35], where mucin-gold conjugates interacted with a hydrogel surface resulting in a red coloration. More recently, a direct staining method to evaluate the attachment of a polymer to human buccal cells has been proposed [36].
Hassan and Gallo [37] reported the rheological method for mucoadhesion evaluation. This method is based on the idea that when a mucoadhesive polymer is mixed with mucin, there is a synergistic increase in viscosity. However the contradictory results obtained in some experiments suggest that this method should not be used as a single technique to evaluate mucoadhesion [38, 39].
Other techniques used to study the interaction between mucoadhesive polymers and mucin glycoproteins has been done by Huang et al. [40] with the use of the surface force apparatus (SFA). The SFA measures the magnitude and distance dependence of the molecular force acting between two surfaces, with resolutions of the measured force up to 10 nN and distances up to 1 •.
Some in vivo methods to assess mucoadhesion properties of polymers include the gamma scintigraphy [41–43] and the use of radioisotopes to measure the gastrointestinal transit of the mucoadhesive device [44, 45].
2 Mucoadhesive Hydrogels As Drug Delivery Devices
2.1 Design of new mucoadhesive biomaterials
The interest in developing mucoadhesive drug delivery systems has its origins in the early 1980s when Nagai et al. [46] developed an innovative adhesive tablet for the local treatment of aphthae. Also, they showed that the use of a mucoadhesive polymer increased peptide’s bioavailability when administrated nasally [47].
Some of the pioneering work also includes the development of mucoadhesive ointments based on polyacrylic acid [48] and poly(methyl methacrylate) [49]. Most of this initial work on mucoadhesion was done by use of conventional polymers and in the form of tablets [26], powders [50, 43] or films [25]. The satisfactory results obtained during the development of these early mucoadhesive formulations suggested that the field of mucoadhesion should be furthered studied. The advantageous properties that mucoadhesive devices brought in contrast to conventional drug release systems should be undoubtedly exploited.
To date, a wide variety of mucoadhesive biomaterials have been used for the development of new pharmaceutical devices, including synthetic and natural polymers. The majority of the current synthetic mucoadhesive polymers are polyacrylic acid and cellulose derivatives. Some of the polyacrylic acid derivative polymers are Carbopol, polycarbophil, polyacrylic acid, polyacrylates, poly(methyl vinylether-co-methacrylic) acid, poly(2-hydroxyethyl methacrylate), polymethacrylate, poly(alkyl cyanoacrylate), poly(isohexyl cyanoacrylate) and poly(isobutyl cyanoacrylate). Some cellulose derivative polymers are carboxymethyl cellulose, hydroxyethyl cellulose, hydroxypropyl cellulose, sodium carboxymethyl cellulose, methyl cellulose and methyl hydroxyl ethyl cellulose. Additionally, semi-natural mucoadhesive polymers include chitosan, and some gums such as guar, xanthan, gellan carrageenan, pectin and alginate. Some other synthetic mucoadhesive polymers include poly(N-vinyl pyrrolidone) and poly(vinyl alcohol) [51].
Current mucoadhesive polymers can be improved by molecularly modifying their chemical structures. One of these examples is the work of Bernkop-Schürch et al. [52]. They propose the use of thiol groups in polymers to increase mucoadhesion. They showed that polycarbophil-cysteine conjugates exhibited high mucoadhesion due to the formation of disulfide bonds with the cysteine-rich domains in mucins.
Other novel modifications have been the use of lectins. Lectins are proteins with the property of binding to carbohydrates with significant specificity. Lectin-conjugated polymers are known as the second generation of mucoadhesive since they are designed to specifically bind to receptors on the epithelial cells. This is the reason why these types of polymers are also called cytoadhesives. Although this property brings an advantage over mucoadhesive polymers, we cannot forget that the polymer will have to diffuse through the whole mucus layer before reaching the epithelial cells membrane. As mention before, one of the roles of this mucus layer is to act as a shield to protect the cells underneath. Also, in some body locations the mucus coat can have a thickness up to 400 μm. Then, cytoadhesive polymers should not only have the ability to specifically bind the cell membrane but also should be capable of diffusing across the entire mucus layer to find the epithelial surface.
Currently, some of the other molecular modifications that are being introduced in mucoadhesive polymers are based on the diffusion theory. Voyutskii proposed the diffusion theory of adhesion between rubbery polymers in 1963 [23]. According to this theory, when two rubbery polymers are brought to intimate contact, the polymer chains have enough mobility to diffuse across the initial interface due to a chemical potential gradient. In the case of a polymer-mucus system, the polymer chains diffuse and interpenetrate into the mucus and the mucin glycoproteins into the mucoadhesive polymer.
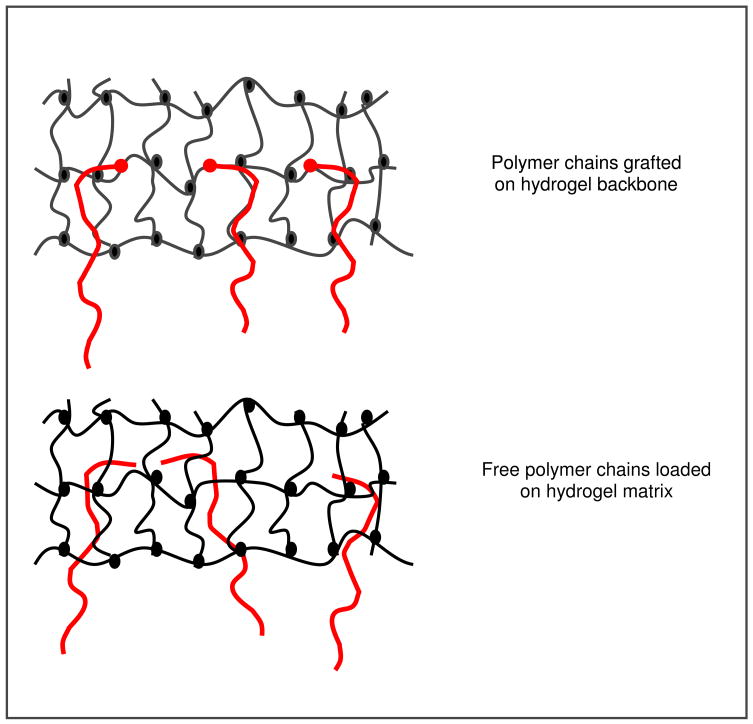
Peppas and collaborators [53] were the first ones to introduce the interdiffusion theory in the mucoadhesion field. They suggested that an increase in chain interpenetration could lead to an increase of mucoadhesion. Jabbari et al. [54] studied the mucin interpenetration at the poly[acrylic acid]/mucin interface using ATR/FTIR spectroscopy. The results demonstrated that the concentration of mucin inside the poly(acrylic acid) polymer increased over the time. Based on the chain interpenetration as a mechanism of mucoadhesion enhancement, Peppas and coworkers [55–57] proposed the use of adhesive promoters to enhance chain interpenetration and consequently increase polymer mucoadhesion. There are two ways of introducing adhesion promoters in a polymer: to load free polymer chains in the matrix or to graft the chains on the polymer surface.
Figure 2 shows a scheme of free polymer chains loaded in a hydrogel matrix and polymer chains grafted on the backbone of a hydrogel, as adhesion promoters. After intimate contact between the mucus layer and a mucoadhesive device loaded with free adhesion promoters, the concentration gradient across the interface provokes the diffusion of the chains. Then, formation of effective interactions at the interface occurs, such as physical entanglements and hydrogen bonds [5].
Figure 2.
Schematic representation of polymer chains incorporated in hydrogel matrixes as adhesion promoters. Polymer chains can be grafted onto the hydrogel backbone or freely added in the hydrogel network.
Peppas et al. [55–57] used a theoretical analysis to study the interpenetration of free chains in mucoadhesion. They concluded that the mobility of the diffused chains depended on the chain length and gel volume fraction.
Experimental observations were carried out by DeAscentis et al [58] when the addition of free poly(ethylene glycol) (PEG) chains as adhesion promoters, in crosslinked poly(2-hydroxethyl methacrylate) particles resulted in an increase in the mucoadhesive properties of the polymer. They explained this observation as a consequence of the free PEG chains penetration across the interface.
Sahlin and Peppas [59] used the near-field FTIR microscopy to study the diffusion of free PEG chains through a poly(acrylic acid) hydrogel. In 1997 [60], they confirmed the mucoadhesion enhancement properties of linear PEG chains by interpenetration in hydrogels.
Tethered polymer chains can also be used as adhesion promoters. Tethers have one of their ends chemically attached to the hydrogel surface and leave the other free, available for diffusion and interpenetration. When the hydrogel system gets into intimate contact with the mucus surface, the concentration gradient provokes the diffusion of the grafted chains across the interface. The covalent bond between the adhesion promoter chains and the backbone hydrogel structure prevents the lost of these chains. The adhesion promoter polymer chains may penetrate into the mucus and act as a bridge between the tissue and the mucoadhesive device. In other fields, the idea of surface-anchored polymers has been also used [61, 62].
Huang et al. [63] studied the gel-gel adhesion by tethered polymers using the single-chain mean-field (SCMF) theory. The theoretical results obtained provided guidelines for the design of new biomaterials with tethered polymer chains. Later, direct measurements of the interactions between PEG tether chains and mucin glycoproteins were evaluated using the surface force apparatus. [45]. Their results showed that, the use of PEG tethered structures could be very beneficial for the future design of new mucoadhesive drug delivery systems.
2.2 Hydrogels as mucoadhesive drug delivery systems
Mucoadhesive polymers can be classified as water soluble or water insoluble systems. Water soluble mucoadhesive polymers are normally linear or random polymeric materials which dissolve in water. Their residence time depends on their water dissolution rate. Water insoluble polymer networks are generally swellable networks that present a crosslinked chemical structure. The residence time of water insoluble mucoadhesive biomaterials depends on the mucus turnover or cell desquamation.
Hydrogels are three-dimensional, insoluble polymer network that swell in water and physiological fluids. They are composed of hydrophilic homopolymers or copolymers [64]. The crosslinks present in the chemical structure prevents their dissolution and confers them a characteristic physical integrity. Hydrogels have been extensively used in the medical and pharmaceutical fields since they resemble natural tissue more than any other type of synthetic biomaterial. They have a high water content and rubbery nature, which makes them biocompatible [65, 66]. Hydrogels have been used as contact lenses, membranes for biosensors, materials for artificial skin, linings for artificial hearts and drug delivery systems [67–72].
The network structure of a hydrogel will determine its properties as a drug delivery device. There are three main parameters that characterize the hydrogels network structure:
The volume fraction in the swollen state, ν2,S
The molecular weight of the polymer chain between two neighboring crosslinking points.
The corresponding mesh size, ξ
The polymer volume fraction in the swollen state is a measure of the amount of fluid absorbed and retained by the hydrogel. The molecular weight between crosslinks is a measure of the degree of crosslinking of the polymer. Since the polymerization is a random process, only average values of can be calculated. The mesh size provides a measure of the distance between consecutive junctions or crosslinks. It offers a measure of the space available between the macromolecular chains for drug diffusion.
Hydrogels can be classified as neutral or ionic depending on the type of charges in their pendant groups. Hydrogels are also capable of exhibiting a swelling behavior depending on the surrounding environment.
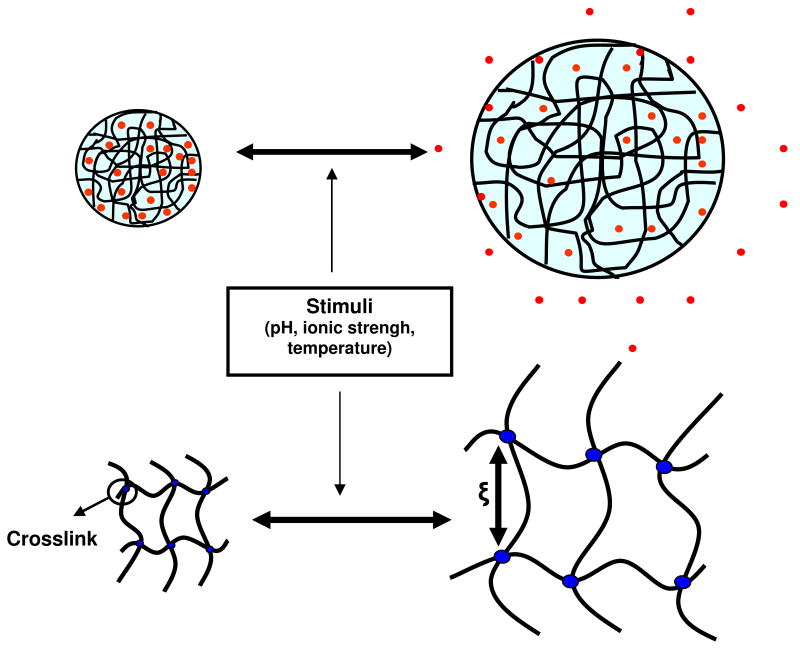
Physiologically responsive hydrogels have gained enormous attention in the last years [73, 74]. Some of the factors that affect the swelling behavior of this type of hydrogels are the pH, ionic strength, temperature, and electromagnetic radiation (Figure 3). pH-Sensitive hydrogels, with swelling behavior and tri-dimensional configuration dependent on the pH of the external environment, contain acidic or basic pendant groups. Changes in surrounding pH and ionic strength result in specific chemical groups’ ionization and consequent changes in hydrogels structure. These properties are very advantageous when designing drug delivery devices since the drug can be released at specific body locations and protected from certain hostile body environments.
Figure 3.
Physiologically responsive hydrogel. Swelling and release of the drug occurs under certain external stimuli.
Complexation hydrogels have been previously studied as very promising controlled release devices for drugs and proteins [75–77]. This type of hydrogels is characterized by the association of chemical groups belonging to different polymer chains. Hydrogen bonding, among others, is one of the interactions responsible of these chemical associations between macromolecular chains [78–80].
Numerous monomers have been used for the synthesis of hydrogel biomaterials for pharmaceutical applications. Researchers are able to design many hydrogel structures by the use and combination of different monomers. Some of the monomers that have been commonly used for the synthesis of hydrogels for medical and pharmaceutical applications are detailed in table 2.1.
Table 1.
Monomers commonly used in the synthesis of hydrogels for medical and pharmaceutical applications [66]
| Monomer abbreviation | Monomer |
|---|---|
| HEMA | Hydroxyethyl methacrylate |
| HEEMA | Hydroxyethoxyethyl methacrylate |
| HDEEMA | Hydroxydiethoxyethyl methacrylate |
| MEMA | Methoxyethyl methacrylate |
| MEEMA | Methoxyethoxyethyl methacrylate |
| MDEEMA | Methoxydiethoxyethyl methacrylate |
| EGDMA | Ethylene glycol dimethacrylate |
| NVP | N-vinyl-2-pyrrolidone |
| NIPAAm | N-isopropyl AAm |
| VAc | Vinyl acetate |
| AA | Acrylic acid |
| MAA | Methacrylic acid |
| HPMA | N-(2-hydroxypropyl) methacrylamide |
| EG | Ethylene glycol |
| PEGA | PEG acrylate |
| PEGMA | PEG methacrylate |
| PEGDA | PEG diacrylate |
| PEGDMA | PEG dimethacrylate |
It is appreciable that many different hydrogel structures exhibiting a wide range of different physical and chemical characteristics could be possibly synthesized. Over the years, the practice of using “off-the-shelf” polymeric materials designed for use in consumer applications and adapted for medical purposes has been substitute by the molecular design of specific biomaterials for concrete drug delivery purposes. Some of the hydrogel polymers that can be used in controlled drug delivery are shown in table 2.2.
Table 2.
Common hydrogels used in the pharmaceutical field for the preparation of controlled drug delivery systems [65]
| Hydrogel polymer | Type of hydrogel |
|---|---|
| Poly(glycolic acid) (PGA) | Biodegradable Hydrogels |
| Poly(lactic acid) (PLA) | |
| PLA-PGA | |
| PLA-PEG | |
| Chitosan | |
| Dextran | |
| Dextran-PEG | |
| Polycyanoacrylates | |
| Fumaric acid-PEG | |
|
| |
| Non-Biodegradable Hydrogels | |
| Poly(hydroxyethyl methacrylate) (PHEMA) | Neutral |
| Poly(vinyl alcohol) (PVA) | |
| Poly(N-vinyl pyrrolidone) (PNVP) | |
| Poly(ethylene-co-vinyl acetate) (PEVAc) | |
|
| |
| Poly(acrylamide) (PAAm) | pH-Responsive |
| Poly(acrylic acid) (PAA) | |
| Poly(methacrylic acid) (PMAA) | |
| Poly(diethylaminoetyl methacrylate) (PDEAEMA) | |
| Poly(dimethylaminoethyl methacrylate) (PDMAEMA) | |
|
| |
| Poly(methacrylic acid-grafted-poly(ethylene glycol) (P(MAA-g-EG)) | Complexing hydrogels |
| Poly(acrylic acid-grafted-poly(ethylene glycol) (P(AA-g-EG)) | |
|
| |
| Poly(N-isopropyl acrylamide) (PNIPAAm) | Temperature-sensitive |
|
| |
| PNIPAAm/PAA | pH/Temperature-sensitive |
| PNIPAAm/PMAA | |
Mucoadhesive hydrogels are able to interact with the mucus and attach to mucosal surfaces, resulting in a prolonged residence time of the mucoadhesive drug release device in the body. As discussed in the previous sections, specific hydrogel chemical compositions and structures will determine the mucoadhesive properties of the biomaterial.
2.3 Drug release mechanisms from mucoadhesive hydrogels
Mucoadhesive hydrogels can be successfully used for the delivery of therapeutic agents since they are able to provide desirable drug release rates and localize and maintain the pharmaceutical device to a specific location in the body for a prolonged period of time. These are significant advantages over traditional pharmaceutical devices.
The study of the release of a drug over the time is crucial information in the drug delivery field. The usage of mathematical models when designing new pharmaceutical formulations as well as for the analysis of the experimental results is essential [81]. Most of the theoretical models to study the drug delivery phenomenon are based on basic diffusion equations. Different categories of controlled drug release systems exist since drug release can occur following different types of mechanisms [82]:
-Diffusion-controlled drug delivery systems
-Chemically-controlled drug delivery systems
-Swelling-controlled drug delivery systems
In this section, each of these systems will be discussed. Also, since diffusion plays an important role in all of them, a brief review regarding the fundamentals on diffusion is given.
Fundamentals of diffusion
The release of a drug from a mucoadhesive hydrogel involves the movement of the drug molecules through the bulk of the polymer. This phenomenon is known as diffusion and can be explained by mass transport fundamentals. From a macroscopic point of view, Fick’s laws of diffusion can explain the movement of drug molecules from the hydrogel matrix to the external environment. Equations (1) and (2) present Fick’s first and second law of diffusion, respectively. These are one-dimensional forms of Fick’s laws [83].
| (1) |
| (2) |
In these equations, the concentration and mass flux are designated as C and J, respectively. D is the diffusion coefficient. The independent variables of position and time are designated as x and t, respectively.
First Fick’s law of diffusion, Eq. (1), is used when we are at steady state or when the drug concentration within the diffusion volume does not change with respect to time. That would be the case of a zero order drug release. When steady state has not been reached, or when the concentration within the diffusion volume changes with respect to time, Fick’s second law of diffusion, Eq. (2) needs to be used to describe the diffusional process.
Diffusion-controlled drug delivery systems
There exist two different types of diffusion-controlled systems: the monolithic devices and the reservoir devices. In monolithic devices the drug is intimately mixed with the polymer which has rate-controlling properties. The drug can then be dissolved or dispersed in the polymer, resulting in two different types of monolithic systems. Therapeutic agents released from monolithic delivery systems do not follow zero order kinetics, although they provide a prolonged drug release.
In reservoir devices the drug is contained in a core which is surrounded by a rate-controlling polymeric membrane. Drug transport from the core through the external polymer membrane occurs by dissolution at one interface of the membrane and diffusion driven by a gradient in thermodynamic activity. Drug transport can be described by Fick’s first law (Eq. 1). If the thermodynamic activity of the drug in the reservoir remains constant and infinite sink conditions are maintained, the drug release rate will be constant and can be predicted since it depends on the membrane permeability and device configuration. Then, drug release will be independent of time, and zero order kinetics can be achieved.
Fick’s first law can be restated to calculate drug release rates from planar devices, as shown in Eq. 3
| (3) |
In this equation, Mt is the amount of drug released and dMt/dt is the release rate at time t. A is the total surface area of the planar device, K is the distribution coefficient, ΔC is the difference in concentration between the solutions on each side of the membrane, and ℓ is the diffusion distance.
In a same manner, drug release rates from cylindrical delivery devices, with internal and external radii r0 and r1 and height h, can be calculated from the following restated Fick’s first law, as shown in Eq. 4
| (4) |
Drug release rates from spherical delivery devices can be also studied using the restated Fick’s first law shown in Eq. 5
| (5) |
From these equations, it is observable that drug release can be controlled by the geometry of the delivery system. Also, depending on the thickness of the membrane, the concentration difference of the drug across the membrane, the thermodynamic characteristics of the systems through the partition coefficient and the structure of the polymer via the drug diffusion coefficient, drug release can be modeled.
Chemically-controlled drug delivery systems
There are two different types of chemically-controlled drug delivery systems depending on the mechanisms that control the release of the therapeutic agent.
In pendant chain systems, the drug is covalently attached to a polymer backbone. The bond between the drug and the polymer is labile and can be broken by hydrolysis or enzymatic degradation.
In erodible drug delivery systems the release of the drug is controlled by the dissolution or degradation of the polymer. Contrary to pendant chain systems, the drug diffuses from erodible systems. Depending on whether diffusion or polymer degradation controls the release rate, the drug is released following different mechanisms. If erosion of the polymer is much slower than the diffusion of the drug through the polymer, then drug release can be treated as a diffusion-controlled drug delivery system. If the diffusion of the drug from the polymer matrix is very slow, then polymer degradation or erosion is the rate-controlling step. Two different types of erodible polymers can be found: hydrophilic erodible polymers and hydrophilic erodible polymers.
Hydrophilic erodible polymers are completely permeated by water and they undergo a bulk erosion process. Erosion throughout the polymer matrix takes place.
Hydrophobic erodible polymers can experience bulk erosion or surface erosion. In bulk erosion, degradation occurs throughout the bulk of the polymer and generally the analysis of the drug release kinetics is complex since it comprises erosion and diffusion.
Swelling-controlled drug delivery systems
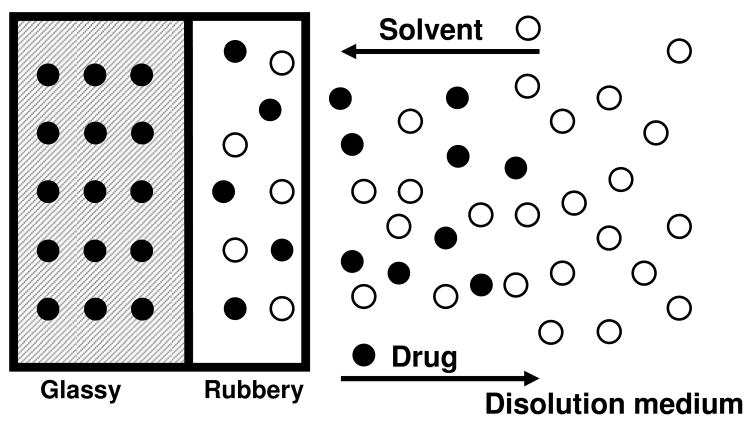
In this type of drug delivery systems, the influx of water in the hydrophilic polymer matrix and consequent swelling of the system controls the release of the drug. The drug is initially homogeneously dispersed in the glassy polymer matrix. Glassy polymers are essentially impermeable and the drug is then immobilized in the matrix. No possible drug diffusion from the glassy polymer can take place. When the polymer gets in contact with water or biological fluids the polymer matrix begin to swell and the swollen rubbery phase appears. The two phases are observed: the inner glassy phase and the external rubbery phase. Drug molecules are able to diffuse out through the rubbery phase (See Figure 4). Then, the velocity and position of the rubbery front controls the drug release rate. In swelling-controlled devices, the drug can be released following two different mechanisms: Diffusion and polymer chain relaxation, which occurs at the glassy-rubbery interface. Ritger and Peppas [84, 85] proposed a simple equation to determine the relative importance of diffusion and macromolecular relaxation on the overall drug delivery process. Eq. 6
Figure 4.
Swelling-controlled drug delivery system. When the glassy hydrogel gets in contact with water, the polymer matrix begin to swell and the rubbery phase appears. Drug molecules are able to diffuse out through the rubbery phase.
| (6) |
In this equation, Mt and M are the amounts of drug released at time t and at equilibrium, respectively. k is a proportionality constant and n is the diffusional exponent.
Ritger and Peppas [84, 85] introduced this exponential equation to describe the drug release behavior from polymeric matrixes and analysis of the Fickian and non-Fickian diffusional behavior in relation to the value of the exponent n was performed. Diffusional exponent values for planar, cylindrical and spherical drug release systems were related to the mechanism of release. Their results are displayed in Table 3
Table 3.
Values of diffusional exponent, n, and related drug release mechanism from various controlled released devices [84]
| Diffusional exponent, n | Drug release mechanism | ||
|---|---|---|---|
| Thin film | Cylindrical sample | Spherical sample | |
| 0.5 | 0.45 | 0.43 | Fickian diffusion |
| 0.5<n<1.0 | 0.45<n<1.0 | 0.43<n<1.0 | Anomalous transport |
| 1.0 | 1.0 | 1.0 | Case II transport (Zero-order release) |
3. P(AA-g-EG) Hydrogels as Promising Mucoadhesive Drug Carriers
Poly(acrylic acid) grafted with poly(ethylene glycol) copolymers, designated as P(AA-g-EG), are pH-responsive hydrogels, composed of an ionic network that swells in response to changes in pH of the environment. These types of hydrogels, and analogous copolymers synthesized with poly(methacrylic acid), have been studied in our group as advantageous carriers for the delivery of a wide range of drugs and proteins [75–77]. They are cataloged as complexation hydrogels [78, 80].
At pH values under the pKa of the acrylic acid, the ether group in the poly(ethylene glycol) (PEG) chain is able to form a hydrogen bond with the carboxylic group of the acrylic acid molecule. In this situation, the hydrogel presents a “collapsed” conformation, and drug diffusion through the network is difficult. As the pH gets more basic, ionization of the carboxylic groups occurs, and consequently the hydrogen bond between the PEG chains and the PAA backbone breaks up. Decomplexation and swelling of the network occurs in these conditions due to interchain electrostatic repulsion. In this situation, drugs or proteins contained in the hydrogel network can easily diffuse out of the network. Figure 5 shows the pH-responsive behavior of the P(AA-g-EG) hydrogels. Owing to these characteristics, drugs or proteins contained in the hydrogel network can be delivered at specific sites in the body, depending on the pH.
Figure 5.
Graphic illustration of the pH-responsive behavior of P(AA-g-EG) hydrogels.
In addition, such hydrogel systems exhibited the ability of inhibiting enzymatic activity, given their capability to chelate calcium [86]. This is a very beneficial feature overall for the delivery of peptides and proteins, which undergo complete enzymatic degradation when orally administrated.
Along with these properties, it has also been shown that such hydrogel systems have the capability of increasing the intestinal absorption of macromolecules by the reversible opening of the tight junctions between the intestinal cells [77, 87–89]. This property was found to be very valuable for the oral administration and absorption of proteins.
Adhesion to mucous tissues has been also a very important characteristic attributed to these copolymeric hydrogels, allowing the maintenance of the drug carrier to a specific location in the body for an extended period of time [90, 91].
Therefore, the indubitable benefits that the P(AA-g-EG) hydrogels can bring to the controlled drug delivery field are the motivation for the ongoing research based on these systems.
4. Conclusions
In this paper, we analyzed the idea that polymer chains tethered on hydrogel surfaces may have important applications in drug delivery fields, such as mucoadhesion promotion and site-specific drug targeting. The advantage of tethered structures is the ability to modify the surface properties of hydrogel systems, which is important for bioadhesion and site-specific targeting, while its bulk properties remain unaffected which can be optimized separately for controlled release.
The idea of using bioadhesive materials in contact with mucosal surfaces, as a strategy to improve the efficacy of therapeutic treatments, has been of great interest in the pharmaceutical field since the early developments in mucoadhesion. The main advantages that mucoadhesive drug release devices bring to the controlled release field are the maintenance of the delivery device to a specific location in the body for an extended period of time. These features are able to provide higher efficiency of local and systemic therapies. In topical treatments, the mucoadhesive pharmaceutical formulation is able to remain in contact with the biophase for a prolonged time.
For example, mucoadhesion of formulations topically applied to the buccal or ocular areas where there exists a constant physical stress and wash out effects helps avoid the displacement of the pharmaceutical device, thus increasing drugs efficacy. When the mucoadhesive formulation is intended for systemic use, the extended period of time in which the device remains in contact with the mucosal absorptive membrane results, in most of the cases, to an increased drug bioavailability.
Among different mucoadhesive polymers, poly(acrylic acid) has been shown to exhibit very good mucoadhesive properties. Based on this polymer, chemical industries have developed excipients such us Carbopol® or Noveon®, widely used in the pharmaceutical, food and personal care industries. However, the evolution in the chemical and biomedical engineering areas has allowed the personalized synthesis of polymeric biomaterials with specific, desired properties. For example, we expect that modification of poly(acrylic acid) or poly(methacrylic acid)-based polymers will provide superior carriers for drug and protein delivery.
The use of adhesion promoters in order to increase interpenetration of macromolecules and enhance mucoadhesion is another promising approach.
Acknowledgments
Various aspects of the work reported here that comes from our laboratories have been supported by grants from the National Science Foundation (grant No. BES-97-06538) and the National Institutes of Health (grants No. GM-56231-01 and EB-000246-14).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Marriott C, Gregory NP. In: Mucus Physiology and Pathology, Bioadhesive Drug Delivery Systems. Lenaerts V, Gurny, editors. CRC; Boca Raton: 1990. pp. 1–24. [Google Scholar]
- 2.Marriot C, Hughes DRL. Mucus Physiology and Pathology, Bioadhesion-Possibilities and Future Trends. In: Gurny R, Junginger HE, editors. Wissenschaftliche Verlagsgesellschaft mbH. Stuttgart: 1990. pp. 29–43. [Google Scholar]
- 3.Peppas NA, Robinson JR. Bioadhesives for Optimization of Drug Delivery. J Drug Target. 1995;3:183–1842. doi: 10.3109/10611869509015943. [DOI] [PubMed] [Google Scholar]
- 4.Puchelle E. Rheology, Biochemistry and Functions of Mucus. Biorheology. 1987;24:411–423. [PubMed] [Google Scholar]
- 5.Helliway M. The use of bioadhesives in targeted delivery within the gastrointestinal tract. Adv Drug Deliv Rev. 1993;11:221–251. [Google Scholar]
- 6.Berne RM, Levey MN. Physiology. Mosby; St. Louis, MO: 1988. [Google Scholar]
- 7.Verdugo P. Goblet cells secretion and mucogenesis. Ann Rev Physiol. 1990;52:157–176. doi: 10.1146/annurev.ph.52.030190.001105. [DOI] [PubMed] [Google Scholar]
- 8.Gupta PK, Leung SS, Robinson JR. In: Bioadhesives/Mucoadhesives in Drug Delivery to the Gastrointestinal Tract, Bioadhesive Drug Delivery Systems. Lenaerts V, Gurny R, editors. CRC; Boca Raton, FL: 1990. pp. 65–92. [Google Scholar]
- 9.Allen A, Hutton DA, Pearson JP, Sellars LA. Mucus glycoprotein structure, gel formation and gastrointestinal mucus function, Mucus and Mucosa. Ciba Foundation Symposium. 1984;109:137–156. doi: 10.1002/9780470720905.ch10. [DOI] [PubMed] [Google Scholar]
- 10.Strous GJ, Dekker J. Mucin-type Glycoproteins. Crit Rev Biochem Mol Biology. 1992;27:57–92. doi: 10.3109/10409239209082559. [DOI] [PubMed] [Google Scholar]
- 11.MacAdam A. The Effects of Gasto-Intestinal Mucus on Drug Adsoption. Adv Drug Delivery Rev. 1993;11:201–220. [Google Scholar]
- 12.Fiebrig I, Harding SE, Rowe AJ, Hyman SC, Davis SD. Transmission Electron Microscopy Studies on Pig Gastric Mucin and its Interactions with Chitosan. Carbohydrate Polym. 1995;28:239–244. [Google Scholar]
- 13.Ponchel G, Duchêne D. In: Development of a bioadhesive tablet, High Performance Biomaterials. Szycher M, editor. Technomic Publishing; Lancaster, Pa: 1991. pp. 231–242. [Google Scholar]
- 14.Duchêne D, Touchard F, Peppas NA. Pharmaceutical and medical aspects of bioadhesive systems for drug administrations. Drug Dev Ind Pharm. 1988;14:283–318. [Google Scholar]
- 15.Mathiowitz E, Chickering D, Jacob J, Santos D. In: Bioadhesive Drug Delivery Systems, Encyclopedia of Controlled Drug Delivery. Mathiowitz E, editor. Wiley; New York, NY: 1999. pp. 9–45. [Google Scholar]
- 16.Mathiowitz E, Chickering D. In: Definitions, mechanisms and theories of bioadhesion, Bioadhesive Drug Delivery Systems: Fundamentals, Novel Approaches and Development. Mathiowitz E, Chickering D, Lehr CM, editors. Marcel Dekker; New York, NY: 1999. pp. 1–10. [Google Scholar]
- 17.Smart JD. The basics underlying mechanisms of mucoadhesion. Adv Drug Deliv Rev. 2005;57:1556–1568. doi: 10.1016/j.addr.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Tur KM, Ch’ng HS. Evaluation of possible mechanisms of mucoadhesion. Int J Pharm. 1998;160:61–74. [Google Scholar]
- 19.Dergim Z, Kellaway IW. An investigation of the interfacial attraction between poly(acrylic acid) and glycoprotein. Int J Pharm. 1998;175:6–16. [Google Scholar]
- 20.Derjaguin BV, Aleinikova IN, Toporov YP. On the role of electrostatic forces in the adhesion of polymer particles to solid surfaces. Prog Surf, Sci. 1994;45:119–123. [Google Scholar]
- 21.Kinloch AJ. The science of adhesion: I. Surface and interfacial aspects. J Mater Sci. 1980;15:2141–2166. [Google Scholar]
- 22.Lehr CM, Bouwstra JA, Bodde HE, Junginger HE. A surface energy analysis of mucoadhesion: contact angle measurements on polycarbophil and pig intestinal mucosa in physiologically relevant fluids. Pharm Res. 1992;970:75. doi: 10.1023/a:1018931811189. [DOI] [PubMed] [Google Scholar]
- 23.Voyutskii SS. Autohesion and Adhesion of High Polymers. Interscience; New York, NY: 1963. [Google Scholar]
- 24.Smart JD, Kellaway IW. In Vitro Techniques for Measuring Mucoadhesion. J Pharm Pharmacol. 1982;34:70P. [Google Scholar]
- 25.Smart JD, Kellaway IW, Worthington HEC. An In Vitro investigation of mucosa-adhesive materials for use in controlled drug delivery. J Pharm Phamacol. 1984;36:295–299. doi: 10.1111/j.2042-7158.1984.tb04377.x. [DOI] [PubMed] [Google Scholar]
- 26.Ponchel G, Touchard F, Duchêne D, Peppas NA. Bioadhesive analysis of controlled release systems. I. Fracture and interpenetration analysis in poly(acrylic acid)-containing systems. J Control Release. 1987;5:129–141. [Google Scholar]
- 27.Park K, Robinson JR. Bioadhesive polymers as platforms for oral-controlled drug delivery: Method to study bioadhesion. Int J Pharm. 1984;19:107–127. [Google Scholar]
- 28.Park K, Ch’ng HS, Robinson JR. In: Alternative approaches to oral controlled drug delivery: Bioadhesives and in situ systems, Recent Advances in Drug Delivery. Anderson JM, Kim SW, editors. Plenum Press; New York, NY: 1984. pp. 163–183. [Google Scholar]
- 29.Patel D, Smith JR, Smith AW, Grist N, Barnett P, Smart JD. An atomic force microscopy investigation of bioadhesive polymer adsorption onto human buccal cells. Int J Pharm. 2000;200:271–277. doi: 10.1016/s0378-5173(00)00396-3. [DOI] [PubMed] [Google Scholar]
- 30.Zur Mühlen E, Koschinski P, Gehring S, et al. In: Force microscopy of cells to measure bioadhesion, Bioadhesive drug delivery systems: Fundamentals, novel approaches, and development. Mathiowitz E, Chickering DE III, Lehr CM, editors. Marcel Dekker; New York, NY: 1999. pp. 197–221. [Google Scholar]
- 31.Mikos AG, Peppas NA. Bioadhesive analysis of controlled release systems. IV. An experimental method for testing the adhesion of microparticles with mucus. J Control Release. 1990;12:31–37. [Google Scholar]
- 32.Chickering DE, Mathiowitz E. Bioadhesive microspheres. 1. A Novel electrobalance-based method to study adhesive interactions between individual microspheres and intestinal-mucosa. J Control Release. 1995;34:251–262. [Google Scholar]
- 33.Mikos AG, Mathiowitz E, Langer R, Peppas NA. Interaction of polymer microspheres with mucin gels as a means of characterizing polymer retention on mucus. J Colloid Interface Sci. 1991;143:366–373. [Google Scholar]
- 34.Ho NFL, Teng CLC. Mechanistic studies in the simultaneous flow and adsorption of polymer-coated latex particles on intestinal mucus I: Methods and physical model development. J Control Release. 1987;6:133–149. [Google Scholar]
- 35.Park K. A new approach to study mucoadhesion-Colloidal gold staining. Int J Pharm. 1989;53:209–217. [Google Scholar]
- 36.Kockisch S, Rees GD, Young SA, Tsibouklis J, Smart JD. A direct-staining method to evaluate the mucoadhesion of polymers from aqueous dispersion. J Control Release. 2001;77:1–6. doi: 10.1016/s0168-3659(01)00444-8. [DOI] [PubMed] [Google Scholar]
- 37.Hassan EE, Gallo JM. A simple rheological method for the in vitro assessment of mucin-polymer bioadhesive bond strength. Pharm Res. 1990;7:491–495. doi: 10.1023/a:1015812615635. [DOI] [PubMed] [Google Scholar]
- 38.Hägerström H, Paulsson M, Edsman K. Evaluation of mucoadhesion for two polyelectrolyte gels in simulated physiological conditions using a rheological method. Eur J Pharm Sci. 2000;9:301–309. doi: 10.1016/s0928-0987(99)00070-6. [DOI] [PubMed] [Google Scholar]
- 39.Hägerström H, Edsman K. Limitations of the rheological mucoadhesion method: the effect of the choice of conditions and the rheological synergism parameter. Eur J Pharm Sci. 2003;18:349–357. doi: 10.1016/s0928-0987(03)00037-x. [DOI] [PubMed] [Google Scholar]
- 40.Huang Y, Efremova N, Peppas NA, Leckband DE. Direct Measurement of Interactions between Tethered PEG Chains and Adsorbed Mucin Layers. Langmuir. 2002;18:836–845. [Google Scholar]
- 41.Soane RJ, Frier M, Perkins AC, Jones NS, Davis SS, Illum L. Evaluation of the clearance characteristics of bioadhesive systems in humans. Int J Pharm. 1999;178:55–65. doi: 10.1016/s0378-5173(98)00367-6. [DOI] [PubMed] [Google Scholar]
- 42.Meseger G, Gurny R, Buri P. In vivo evaluation of dosage forms: application of gamma scintigraphy to non-enternal routes of administration. J Drug Target. 1994;2:269–288. doi: 10.3109/10611869409015908. [DOI] [PubMed] [Google Scholar]
- 43.Harris D, Fell JT, Sharma HL, Taylor DC. GI transit of potential bioadhesive formulations in man- A scintigraphic study. J Control Release. 1990;12:45–53. [Google Scholar]
- 44.Qaqish RB, AmijI M. Synthesis of a fluorescent chitosan derivative and its application for the study of chitosan-mucin interactions. Carbohydr Polym. 1999;38:99–107. [Google Scholar]
- 45.Riley RG, Green KL, Smart JD, et al. The gastrointestinal transit profile of C-14 labeled poly(acrylic acids): an in vivo study. Biomaterials. 2001;22:1861–1867. doi: 10.1016/s0142-9612(00)00369-0. [DOI] [PubMed] [Google Scholar]
- 46.Nagai T. Adhesive topical drug delivery system. J Control Release. 1985;2:121–134. [Google Scholar]
- 47.Nagai T, Machida Y. Mucosal adhesive dosage forms. Pharm Int. 1985;6:196–200. [Google Scholar]
- 48.Ishida M, Nambu N, Nagai T. Highly viscous gel ointment containing Carbopol for application to the oral mucosa. Chem Pharm Bull. 1983;31:4561–4564. doi: 10.1248/cpb.31.4561. [DOI] [PubMed] [Google Scholar]
- 49.Bremecker KD, Strempel H, Klein G. Novel concept for a mucosal adhesive ointment. J Pharm Sci. 1984;73:548–552. doi: 10.1002/jps.2600730429. [DOI] [PubMed] [Google Scholar]
- 50.Park H, Robinson JR. Physico-chemical properties of water insoluble polymers important to mucin-epithelial adhesion. J Control Release. 1985;2:47–57. [Google Scholar]
- 51.Lee JW, Park JH, Robinson JR. Bioadhesive-Based Dosage Forms: The Next Generation. J Pharm Sci. 2000;89:850–866. doi: 10.1002/1520-6017(200007)89:7<850::AID-JPS2>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 52.Bernkop-Schnurch A, Schwarz A, Steininger S. Polymers with thiol groups; A new generation of mucoadhesive polymers? Pharm Res. 1999;16:876–881. doi: 10.1023/a:1018830204170. [DOI] [PubMed] [Google Scholar]
- 53.Gurny R, Meyer JM, Peppas NA. Bioadhesive intraoral release systems: design testing and analysis. Biomaterials. 1984;5:336–340. doi: 10.1016/0142-9612(84)90031-0. [DOI] [PubMed] [Google Scholar]
- 54.Jabbari E, Wisniewski N, Peppas NA. Evidence of mucoadhesion by chain interpenetration at a poly(acrylic acid)/mucin interface using ATR-FTIR spectroscopy. J Control Release. 1993;26:99–108. [Google Scholar]
- 55.Mikos AG, Peppas NA. In: Scaling concepts and molecular theories of adhesion of synthetic polymers to glycoproteinic networks, Bioadhesive Drug Delivery Systems. Lenaerts V, Gurny G, editors. CRC Press; Boca Raton, FL: 1990. pp. 25–42. [Google Scholar]
- 56.Mikos AG, Peppas NA. In: Kinetics of mucus-polymer interaction, Bioadhesion-Possibilities and future trends. Gurny R, Junginger HE, editors. Wissenchaftliche; Stuttgart: 1990. pp. 65–85. [Google Scholar]
- 57.Peppas NA. Molecular calculations of poly(ethylene glycol) transport across a swollen poly(acrylic acid)/mucin interface. J Biomater Sci Polym Edn. 1998;9:535–542. doi: 10.1163/156856298x00028. [DOI] [PubMed] [Google Scholar]
- 58.DeAscentiis A, DeGrazia JL, Bowman CN, Colombo P, Peppas NA. Mucoadhesion of poly(2-hydroxyethyl-methacrylate) is improved when linear poly(ethylene oxide) chains are added to the polymer network. J Control Release. 1995;33:197–201. [Google Scholar]
- 59.Sahlin JJ, Peppas NA. Investigation of polymer diffusion in hydrogel laminates using near-field FTIR microscopy. Macromolecules. 1996;29:7124–7129. [Google Scholar]
- 60.Sahlin JJ, Peppas NA. Enhanced hydrogel adhesion by polymer interdiffusion: Use of linear poly(ethylene glycol) as an adhesion promoter. J Biomater Sci Polym Edn. 1997;8:421–436. doi: 10.1163/156856297x00362. [DOI] [PubMed] [Google Scholar]
- 61.De Gennes PG. Soft interfaces. Cambridge University Press; Cambridge, UK: 1997. [Google Scholar]
- 62.Leger L, Raphael E, Hervet H. Surface-anchored polymer chains: their role in adhesion and friction. Adv Polym Sci. 1999;138:185–225. [Google Scholar]
- 63.Huang Y, Szleifer I, Peppas NA. Gel-gel adhesion by tethered polymers. J Chem Phys. 2001;114:3809–3816. [Google Scholar]
- 64.Lowman AM, Peppas NA. In: Hydrogels, Encyclopedia of controlled drug delivery. Mathiowitz E, editor. Wiley; New York, NY: 1999. pp. 397–418. [Google Scholar]
- 65.Peppas NA, Huang Y, Torres-Lugo M, Ward JH, Zhang J. Physicochemical foundations and structural design of hydrogels in medicine and biology. Annu Rev Biomed Eng. 2000;02:9–29. doi: 10.1146/annurev.bioeng.2.1.9. [DOI] [PubMed] [Google Scholar]
- 66.Peppas NA, Bures P, Leobandung W, Ichikawa H. Hydrogels in pharmaceutical formulations. Eur J Pharm Biopharm. 2000;50:27–46. doi: 10.1016/s0939-6411(00)00090-4. [DOI] [PubMed] [Google Scholar]
- 67.Dziubla TD, Lowman AM, Peppas NA. Evaluation of Poly(ethylene glycol)-Based Copolymers for Contact Lenses. Trans Soc Biomater. 2001;27:232. [Google Scholar]
- 68.Peppas NA. Hydrogels. In: Ratner BD, Hoffman AS, Schoen FJ, Lemons JE, editors. Biomaterials Science: An Introduction to Materials in Medicine. 2. Academic Press; New York, NY: 2004. pp. 100–107. [Google Scholar]
- 69.Peppas NA, Langer R. New challenges in biomaterials. Science. 1994;263:1715–1720. doi: 10.1126/science.8134835. [DOI] [PubMed] [Google Scholar]
- 70.Park K. Controlled Drug Delivery: Challenges and Strategies. American Chemical Society; Washington, DC: 1997. [Google Scholar]
- 71.Brannon-Peppas L, Peppas NA. In: Polymer Science of Controlled Release Systems, Controlled Release Systems. Gürsoy AZ, editor. KSSD; Istanbul: 2002. pp. 1–40. [Google Scholar]
- 72.Peppas NA, Wood KM, Blanchette JO. Hydrogels for Oral Delivery of Therapeutic Proteins. Expert Opin Biol Ther. 2004;4:881–887. doi: 10.1517/14712598.4.6.881. [DOI] [PubMed] [Google Scholar]
- 73.Peppas NA, Zhang J. In: Diffusional Behavior in pH- and Temperature Sensitive Interpenetrating Polymeric Networks Used in Drug Delivery, Biomaterials and Drug Delivery Systems towards the New Millennium. Park KD, Kwon IC, Yui N, Jeong SY, Park K, editors. Seoul, Korea: 2000. pp. 87–96. [Google Scholar]
- 74.Peppas NA. In: Kinetics of Smart Hydrogels, Reflexive Polymers and Hydrogels: Understanding and Designing Fast-responsive Polymeric Systems. Yui N, Mrsny R, Park K, editors. CRC Press; Boca Raton, FL: 2004. pp. 99–113. [Google Scholar]
- 75.Lowman AM, Morishita M, Kajita M, Nagai T, Peppas NA. Oral delivery of insulin using pH-responsive complexation gels. J Pharm Sci. 1999;88:933–937. doi: 10.1021/js980337n. [DOI] [PubMed] [Google Scholar]
- 76.Foss AC, Goto T, Morishita M, Peppas NA. Development of acrylic-based copolymer for oral insulin delivery. Eur J Pharm Biopharm. 2004;57:163–169. doi: 10.1016/S0939-6411(03)00145-0. [DOI] [PubMed] [Google Scholar]
- 77.Torres-Lugo M, Garcia M, Record R, Peppas NA. pH-Sensitive hydrogels as gastrointestinal tract absorption enhancers: Transport mechanisms of salmon calcitonin and other model molecules using the caco-2 cell model. Biotechnol Prog. 2002;18:612–616. doi: 10.1021/bp0101379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lowman AM, Peppas NA. Molecular analysis of interpolymer complexation in graft copolymer networks. Polymer. 2000;41:73–80. [Google Scholar]
- 79.Kim B, Peppas NA. Analysis of molecular interactions in poly(methacrylic acid-g-ethylene glycol) hydrogels. Polymer. 2003;44:3701–3707. [Google Scholar]
- 80.Lowman AM. In: Complexing polymers in drug delivery, Handbook of Pharmaceutical Controlled Release Technology. Wise DL, Brannon-Peppas L, Klibanov AM, Langer RS, Mikos AG, Peppas NA, Trantolo DJ, Wnek GR, Yaszemski MJ, editors. Marcel Dekker; New York, NY: 2000. pp. 89–98. [Google Scholar]
- 81.Narasimhan B, Peppas NA. In: The role of modeling studies in the development of future controlled release devices, Controlled Drug Delivery: Challenges and strategies. Park K, editor. American Chemical Society; Washington, DC: 1997. pp. 529–557. [Google Scholar]
- 82.Heller J. In: Use of polymers in controlled release of active agents, Controlled Drug Delivery, Fundamentals and Applications. Robinson JR, Lee VHL, editors. Marcel Dekker; New York, NY: 1987. pp. 179–212. [Google Scholar]
- 83.Burnette RR. In: Theory of mass transfer, Controlled Drug Delivery, Fundamentals and Applications. Robinson JR, Lee VHL, editors. Marcel Dekker; New York, NY: 1987. pp. 95–138. [Google Scholar]
- 84.Ritger PL, Peppas NA. A simple equation for description of solute release I. Fickian and non-Fickian release from non-swellable devices in form of slabs, sphere, cylinders or discs. J Control Release. 1987;5:23–36. [PubMed] [Google Scholar]
- 85.Peppas NA. Analysis of Fickian and non-Fickian drug release from polymers. Pharm Acta Helv. 1985;60:110 – 111. [PubMed] [Google Scholar]
- 86.Madsen F, Peppas NA. Complexation graft copolymers networks: swelling properties, calcium binding and proteolytic enzyme inhibition. Biomaterials. 1999;20:1701–1708. doi: 10.1016/s0142-9612(99)00071-x. [DOI] [PubMed] [Google Scholar]
- 87.Kavimandan NJ, Peppas NA, Morishita M, Goto T, Nagai T, Takayama K. Experimental Investigation of the Effect of Complexation Hydrogels on Insulin Transport across Model Intestinal Cell Monolayers. Drug Deliv Syst. 2003;18:283. [Google Scholar]
- 88.Blanchette JO, Kavimandan NJ, Peppas NA. Principles of Transmucosal Delivery of Therapeutic Agents. Biomed & Pharmacother. 2004;58:142–151. doi: 10.1016/j.biopha.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 89.López JE, Peppas NA. Cellular Evaluation of Insulin Transmucosal Delivery. J Biomater Sci Polym Edn. 2004;15:385–396. doi: 10.1163/156856204323005262. [DOI] [PubMed] [Google Scholar]
- 90.Peppas NA, Keys KB, Torres-Lugo M, Lowman AM. Poly(ethylene glycol)-containing Hydrogels in Drug Delivery. J Control Release. 1999;62:81–87. doi: 10.1016/s0168-3659(99)00027-9. [DOI] [PubMed] [Google Scholar]
- 91.Peppas NA. Devices Based on Intelligent Biopolymers for Oral Protein Delivery. Int J Pharm. 2004;277:11–17. doi: 10.1016/j.ijpharm.2003.03.001. [DOI] [PubMed] [Google Scholar]