Summary
Aim
This study was designed to test the hypothesis that immediate post-shock chest compressions improve outcome from prolonged ventricular fibrillation (VF) compared with typical “hands off” period (i.e., delayed post-shock compressions) associated with AED use.
Materials and methods
After 7.5 min of untreated VF, 36 domestic swine (26 ± 1 kg) were treated with 200 J biphasic shocks and randomly assigned to: (1) 1 min of immediate post-shock chest compressions or (2) simulated pre-hospital automated external defibrillator (AED) care with delays in post-shock chest compressions. Return of spontaneous circulation (ROSC) occurred in 7/18 immediate chest compressions animals within 2 min of the first shock versus 0/18 AED animals (P < 0.01). Ten of 18 immediate chest compressions animals attained ROSC compared with 3/18 AED animals (P < 0.05). Nine of 18 immediate chest compressions swine were alive at 24 and 48 h compared with 3/18 AED swine (P < 0.05). All 48-h survivors had good neurologic outcomes. Among the 21 animals that defibrillated with the first shock, ROSC was attained in 7/10 immediate chest compressions animals within 2 min of the first shock compared with 0/11 AED animals (P = 0.001), and 48-h survival was attained in 8/10 versus 3/11, respectively (P < 0.05).
Conclusions
Immediate post-shock chest compressions can substantially improve outcome from prolonged VF compared with simulated pre-hospital AED care.
Keywords: Chest compressions, Cardiac arrests, Ventricular fibrillation, Defibrillation, Cardiopulmonary resuscitation, Heart arrest, Automated external defibrillator (AED), Outcome
Introduction
More than 300,000 Americans suffer a cardiac arrest each year, mostly in the pre-hospital setting.1 Patients with ventricular fibrillation (VF) are those most likely to survive an arrest, and successful resuscitation from VF is time-dependent.1–5 Unfortunately, emergency medical service providers are generally not available until 7–12 min after collapse from VF, and defibrillation from such prolonged VF typically results in a non-perfusing rhythm (pulseless electrical activity or asystole).2,6–8 Successful resuscitation from these non-perfusing rhythms depends on prompt, effective cardiopulmonary resuscitation (CPR).1–5
The first defibrillators available for most pre-hospital VF are automated external defibrillators (AEDs).4 These lightweight, simple-to-operate devices can be quickly and easily carried to the side of a cardiac arrest victim. They have automated electrocardiographic rhythm analysis systems for differentiation of “shockable” versus “non-shockable” rhythms, and shock advisory systems. These automated systems allow earlier defibrillation by first responders with limited diagnostic skills, but require mandatory “hands off” periods with no provision of potentially life-saving CPR.
Recent studies have established that interruptions in chest compressions adversely affect hemodynamics during CPR for VF.9–12 During these interruptions, cardiac output, coronary perfusion and cerebral perfusion approach zero. In addition, the aortic diastolic pressure decreases substantially during the interruptions, and slowly increases after re-institution of chest compressions.9–11 These hemodynamic phenomena can result in both worse myocardial blood flow and a lower survival rate.9–13
We have previously established that delays in chest compressions associated with AED rhythm analysis and shock advisory before and after defibrillation attempts for prolonged VF result in worse survival compared with prompt manual defibrillation and CPR (i.e., minimal interruptions and delays).13 Numerous animal and clinical studies have demonstrated that pre-shock chest compressions can improve outcome for VF in the circulatory phase.14–19 However, laboratory and clinical investigations have not specifically addressed the value of immediate post-shock compressions. In the present study, we evaluated whether immediate post-shock chest compressions (without waiting for a re-analysis of the rhythm) can improve resuscitation outcomes. We hypothesized that immediate post-shock chest compressions would improve survival from prolonged VF compared with the typical ‘hands off’ period of no compressions associated with AED use. The primary outcome measure was good neurologic outcome at 48-h post-arrest.
Materials and methods
Animal preparation
Experimental protocols were approved by The University of Arizona Institutional Animal Care and Use Committee and followed the guidelines of the American Physiological Society. Thirty-six healthy female domestic swine (26 ± 1 kg) underwent masked anesthesia with 3–5% isoflurane, followed by oral endotracheal intubation. They were mechanically ventilated with a volume-limited, time-cycled ventilator (Narkomed 2A, North American Drager, Telford, PA) on a mixture of room air and titrated isoflurane (1–3%). The tidal volume was initially set at 15 ml/kg and ventilator rate at 14 breaths/min; ventilator settings were adjusted to maintain end-tidal carbon dioxide at 40 ± 5 mmHg by an inline capnometer (Hewlett-Packard model 47210A, Palo Alto, CA).
After obtaining a surgical plane of anesthesia, introducer sheaths were placed in the right internal and external jugular veins and right carotid artery by cut-down technique. High-fidelity, solid-state, micromanometer-tipped catheters (MPC-500, Millar Instruments, Houston, TX) were advanced through the carotid artery and external jugular vein into thoracic locations under fluoroscopic guidance. The internal jugular catheter was advanced into the pulmonary artery.
Measurements
Electrocardiographic lead II, right atrial pressure, aortic pressure, pulmonary artery pressure, and end-tidal carbon dioxide were measured continuously throughout the experiment from the time of the insertion of the catheters until the end of the post-resuscitation intensive care period, and recorded on a Fujitsu Lifebook 530T laptop computer using data-acquisition system DI-220-PGH and Windaq software (Dataq Instruments, Inc., Akron, OH). Oxygen saturation, PCO2, PO2, pH, and hemoglobin were measured with a blood gas analyzer (IL-1306 with model 482 co-oximeter, Instrumentation Laboratories, Lexington, MA).
The initial post-shock rhythm was defined at 5 s after defibrillation attempt.4 Pulseless electrical activity was defined as any organized electrical activity with a systolic blood pressure less than 50 mmHg (consistent with a non-palpable pulse in a pre-hospital setting). Survival and neurologic status were evaluated 48 h after the initial cardiac arrest by an individual blinded to the animal’s experimental group. Neurologic status was evaluated with swine cerebral performance categories, as previously described.10,11,13 Swine cerebral performance category is a global assessment of neurologic function. Category 1 was assigned to pigs that appeared normal, based on level of consciousness, gait, feeding behavior, response to an approaching human, and response to human restraint. Category 2, mildly abnormal, was assigned when the pigs had subtle dysfunction with regard to these characteristics. Category 3 referred to more severe dysfunction: inability to stand, walk, or eat. Category 4, vegetative state or deep coma, referred to pigs with minimal response to noxious stimuli. Category 5 referred to animals with no response to their environment. Categories 1 and 2 were considered good neurologic outcome.10,11,13
Experimental protocol
After baseline hemodynamic and blood gas data were collected, a pacing electrode was positioned in the right ventricle. Isoflurane was discontinued, and VF was induced with a 60-cycle alternating current to the endocardium, confirmed by the ECG waveform and precipitous decline in aortic pressure. Ventilation was discontinued. After 7.5-min of untreated VF (simulating the time for first responding emergency medical personnel to arrive with a defibrillator), the animals received a single 200 J biphasic shock (Medtronic Emergency Response Systems, Redmond, WA). The animals were then randomly provided with either immediate post-shock CPR or delayed post-shock CPR to simulate typical pre-hospital AED defibrillation (Figure 1).
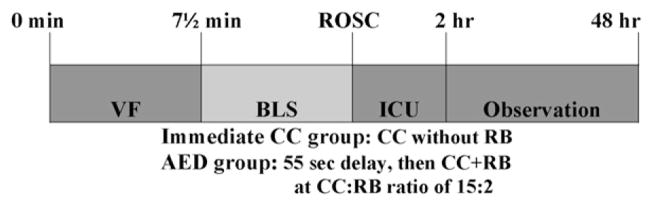
Figure 1.
Graphic representation of the experimental time-line. After 7.5 min of untreated VF, animals were randomized in the BLS (basic life support) period to: (1) 1 min of immediate post-shock continuous chest compressions without rescue breaths or (2) simulated AED care with no compressions for 55 s, followed by delayed post-shock chest compressions and rescue breathing with a compression:ventilation ratio of 15:2. Animals that attained ROSC (return of spontaneous circulation) were provided with ICU (intensive care unit) care (i.e., mechanical ventilation and anesthesia).
For the immediate post-shock chest compression group, 1 min of metronome-guided continuous chest compressions was provided to all animals without regard to rhythm or pulse pressures, followed by a 10-s interval simulating assessment for rhythm. Because the experimental group strategy was to minimize post-shock chest compression delays and interruptions, a simulated “quick look at the AED screen” was used rather than the longer automated rhythm analysis. For animals remaining in VF, a 300 J biphasic shock was provided, followed promptly by another minute of chest compressions. For animals with a systolic pressure >50 mmHg (i.e., presumed signs of circulation), no further interventions were provided. For animals in PEA (i.e., systolic pressure < 50 mmHg), another minute of chest compressions was provided. Each set of chest compressions was followed by another 10-s interval without compressions, simulating assessment for rhythm.
For the “AED” group, when PEA occurred after the initial 200 J shock, 1 min of standard AHA-recommended CPR with chest compressions and rescue breathing was provided after a delay of 55 s prior to first chest compressions (based on delays documented in the pre-hospital literature and our previous laboratory studies with an AED).4,5,13,20,21 If the second rhythm analysis indicated a shockable rhythm (VT/VF), then the animal received a second shock of 300J, followed by another 55-s rhythm analysis and shock advisory period.
For both groups chest compressions were provided manually by a single experienced research technician who was blinded to arterial blood pressures and end-tidal carbon dioxide values during the experiment. The compressions were approximately 1/3 of the antero-posterior diameter of the chest at a metronome-guided rate of 100 compressions/min. For the “AED” group, rescue breaths were provided with 100% oxygen, and the ratio of compressions-to-ventilations was 15:2.4 Neither group received medications during the cardiac arrest BLS period, because this protocol was intended to model the care provided by the first EMS responder in the USA who typically does not have intravenous access available and cannot administer intravenous medications.
Restoration of spontaneous circulation was defined as an unassisted pulse with a systolic arterial pressure >50 mmHg, and a pulse pressure >20 mmHg lasting >1 min. If animals did not attain restoration of spontaneous circulation within 20 min of the initial shock, resuscitative efforts were terminated. All successfully resuscitated animals were supported with 100% oxygen and mechanical ventilation for 2 h. All pigs received 20 ml/kg of normal saline intravenously during the intensive care period to replenish “third space” losses due to surgical procedures and capillary leak. After 2 h of supportive care, all animals were weaned from pharmacological and ventilatory support. Animals surviving the intensive care period were transferred to observation cages for the next 48 h. After the 48-h neurological assessment, surviving animals were sacrificed by infusion of barbiturate-based euthanasia solution.
Data analysis
Comparisons of continuous variables were evaluated by unpaired Student’s t-test, and described as mean ± S.E.M. Comparisons of discrete variables were evaluated by Chi-square analysis or Fisher’s exact test, as appropriate. All statistical analyses were performed with Statview 5.0 software.
Results
At pre-arrest baseline, the two groups did not differ in weight, hemodynamic status, arterial and mixed venous blood gases, and hemoglobin concentration (Table 1). Rates of successful return of spontaneous circulation and 48-h survival were substantially superior in the immediate post-shock chest compressions group (Figure 2, Table 2). Return of spontaneous circulation occurred in 7 of 18 immediate chest compression animals within 2 min of the first shock versus none of the 18 AED animals (P < 0.01); all seven attaining ROSC within 2 min were 48-h survivors with good neurologic outcomes.
Table 1.
Pre-arrest baseline data
| AED care | Immediate post-shock CPR | |
|---|---|---|
| Weight (kg) | 26 ± 1 | 25 ± 1 |
| HR (bpm) | 108 ± 5 | 127 ± 9 |
| AoS (mmHg) | 81 ± 3 | 73 ± 3 |
| AoD (mmHg) | 54 ± 3 | 49 ± 2 |
| RA (mmHg) | 6 ± 1 | 6 ± 1 |
| C.O. (l/min) | 2.7 ± 0.2 | 2.5 ± 0.1 |
| SaO2% | 93 ± 1 | 91 ± 1 |
| PCO2 (mmHg) | 41 ± 1 | 42 ± 1 |
| pHa | 7.45 ± 0.01 | 7.43 ± 0.01 |
| Hgb (gm/dl) | 8.6 ± 0.2 | 8.9 ± 0.2 |
AED care, control group with typical pre-hospital AED defibrillation; immediate post-shock CPR, experimental group; data described as mean ± S.E.M.
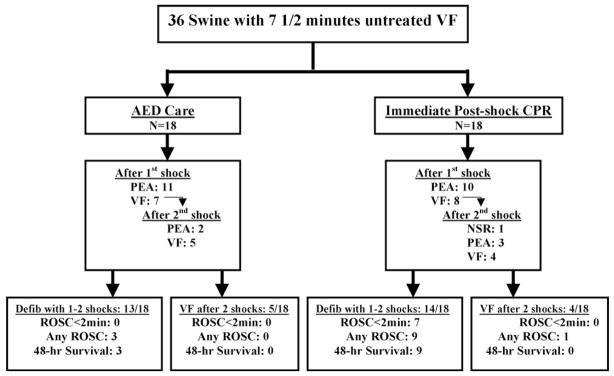
Figure 2.
Graphic representation of outcomes of the animals in the two experimental groups after 7.5 min of VF (ventricular fibrillation), including electrocardiographic response to the first and second shocks, as well as the resuscitation outcomes among animals defibrillated with the first two shocks (Defib with 1 to 2 shocks) and those not defibrillated after 2 shocks (VF after 2 shocks). AED refers to automated external defibrillator; CC, chest compressions; PEA, pulseless electrical activity; NSR, normal sinus rhythm; ROSC, return of spontaneous circulation.
Table 2.
Resuscitation outcomes
| AED care | Immediate post-shock CPR | |
|---|---|---|
| ROSC <2 min | 0/18 | 7/18** |
| ROSC | 3/18 | 10/18* |
| 24-h survival | 3/18 | 9/18* |
| 48-h survival | 3/18 | 9/18* |
| 48-h good neuro | 3/18 | 9/18* |
AED care, control group with typical pre-hospital AED defibrillation and post-shock delays in chest compressions for rhythm analysis and shock advisory; immediate post-shock CPR, experimental group; ROSC, return of spontaneous circulation; ROSC <2 min, return of spontaneous circulation within 2 min of first shock; 48-h good neuro, 48-h survival with good neurological outcome;
P < 0.05;
P < 0.01.
Ten of 18 immediate chest compression animals attained ROSC compared with three of 18 AED animals (P < 0.05). Nine of 18 immediate chest compression swine were alive at 24 and 48 h compared with three AED swine (P < 0.05). The only animal with ROSC that did not survive to 24 h was also the only animal that needed a dopamine infusion to maintain adequate blood pressure during the 2-h ICU period. All 48-h survivors had good neurologic outcomes. Seven of 9 immediate chest compression 48-h survivors and 2/3 AED 48-h survivors appeared normal (cerebral performance category 1), and the other three had mild abnormalities (cerebral performance category 2).
Five seconds after the first shock, 10/18 immediate chest compression animals and 11/18 AED animals defibrillated into PEA, and the rest remained in VF (Figure 2). None defibrillated into a perfusing rhythm after the first shock. All but one of the 48-h survivors defibrillated into PEA with the first (n = 9) or second (n = 2) shock. The other 48-h survivor was defibrillated into a perfusing rhythm after the second shock (i.e., after a 1-min interval of post-shock CPR prior to the second shock). Therefore, all 12 animals with 48-h survival needed post-shock chest compressions for successful resuscitation.
Among the 21 animals that defibrillated into a non-perfusing rhythm with the first shock, 7/10 immediate chest compression animals attained sustained return of spontaneous circulation within 2 min of the first shock compared with 0/11 AED animals (P = 0.001). Similarly, 8/10 immediate chest compression animals attained ROSC within 5 min of the first shock compared with none of the AED animals (P < 0.001). Moreover, 8/10 of these immediate chest compression animals survived to 48-h with good neurologic outcomes (i.e., all eight with ROSC < 5 min) compared with 3/11 AED animals (P < 0.05).
During the first minute of CPR, right atrial compression pressure (a surrogate of compression force) did not differ in the two groups (83 ± 6 mmHg with immediate CPR vs. 94 ± 6 mmHg with AED). The aortic relaxation pressure was higher in the immediate CPR group (23 ± 2 mmHg vs. 15 ± 2 mmHg, P < 0.05), as was the resultant coronary perfusion pressure (14 ± 2 mmHg vs. 9 ± 2 mmHg, P < 0.05), because the aortic pressure decreased in the AED group during the long delay prior to provision of chest compressions.
Discussion
This study establishes that immediate post-shock chest compressions can improve outcome from prolonged VF compared with simulated pre-hospital AED defibrillation and its inherent ‘hands off’ period. As in the pre-hospital clinical setting, defibrillation from prolonged VF in this experiment routinely resulted in a non-perfusing rhythm.2–8 The treatment of choice for a post-shock non-perfusing rhythm, either pulse-less electrical activity or asystole, is myocardial perfusion. Therefore, it should not be surprising that provision of immediate continuous chest compressions after defibrillation for prolonged VF was so important for post-shock myocardial perfusion and successful resuscitation. Nevertheless, this is the first study to specifically address this issue, and immediate continuous post-shock chest compressions were rarely provided after defibrillation for out-of-hospital prolonged VF before the 2005 Guidelines of Cardiopulmonary Resuscitation and Emergency Cardiovascular Care.5,20–22
As expected, most of the benefit from immediate post-shock chest compressions was accrued in the first few minutes after the first shock, and was focused on the animals that defibrillated to a non-perfusing rhythm with the first shock. Seven of the nine immediate chest compression animals with 48-h survival attained sustained return of spontaneous circulation within 2 min of the first shock. In addition, among animals that defibrillated into a non-perfusing rhythm with the first shock, 7/10 immediate chest compression animals attained sustained return of spontaneous circulation within 2 min of the first shock compared with 0/11 AED animals (P = 0.001). Similarly, 8/10 of these immediate chest compression animals with first shock defibrillation survived to 48-h with good neurologic outcomes compared with 3/11 AED animals with first shock defibrillation (P < 0.05). That is, immediate post-shock chest compressions were remarkably effective for the group it was intended to help: animals initially defibrillated into a non-perfusing rhythm.
Prolonged VF is different from short duration VF in regard to myocardial bioenergetics, cellular electrophysiology, whole-organ myocardial electrophysiology, and response to therapy.13–16,23–25 Substantial, progressive depletion of myocardial high-energy phosphates occur during prolonged VF.23,25 Moreover, characteristic changes occur in the VF waveform during prolonged VF from a coarse waveform initially to a fine waveform over time. As the duration of VF increases and the waveform becomes fine, defibrillation into a perfusing rhythm is less likely. Experimental and clinical studies indicate that pre-shock chest compressions for prolonged VF can “coarsen” the VF waveform, and improve the rate of successful resuscitation.1,14–19 As VF becomes very prolonged, even circulatory support is not adequate for successful resuscitation. Furthermore, a recent clinical investigation demonstrated that even 10–20 s pauses in pre-shock compressions decrease defibrillation success.26
Therefore, Weisfeldt and Becker proposed a three-phase, time-sensitive model of VF.1 The first 4–5 min of untreated VF was coined the electrical phase, which can generally be successfully treated with shocks alone. The next 5–10 min of VF was termed the circulatory phase of prolonged VF, which generally requires pre-shock CPR for successful resuscitation. This is followed by the metabolic phase of prolonged VF, which requires metabolic therapies (e.g., post-resuscitation hypothermia) as well as circulatory support for successful resuscitation. The present investigation indicates that prompt post-shock CPR is another circulatory support strategy to improve outcomes during the circulatory phase of VF.
Previous animal studies with a variety of models have demonstrated that delays and interruptions in chest compressions of similar duration during CPR can adversely affect hemodynamics and outcomes.10–13 In addition, pre-hospital clinical studies substantiate that delays in post-shock chest compressions like those in our “AED” group are common, and that outcomes after such delays are quite poor. 5,20,21 Furthermore, in-hospital studies indicate lower chest compression rates result in worse outcomes, presumably because of the resultant decrease in perfusion.27 Based on these data and the high likelihood of termination of fibrillation after the first shock with modern biphasic defibrillators, the American Heart Association’s 2005 Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care recommends immediate post-shock CPR rather than post-shock rhythm checks and pulse checks.22 Recent clinical and animal studies provide general support for this approach.28–30 However, none of these previous animal or clinical studies specifically evaluated the strategy of immediate post-shock chest compressions without regard to rhythm analysis (i.e., minimizing delays and interruptions in post-shock myocardial perfusion) to improve outcome.
As with all animal cardiac arrest experiments, there are multiple potential experimental problems, especially lack of blinding and relevance to human pre-hospital VF cardiac arrest. Although this experiment could not be fully blinded by its very nature, each animal was randomly assigned to one of the two treatment groups, and the resuscitation and post-resuscitation protocols were standardized and strictly followed. Moreover, outcomes and other end-points were clearly defined a priori to minimize investigator bias. Finally, the resuscitator was blinded to the hemodynamic monitoring, and the research team member performing the neurologic examination was blinded to the animal’s treatment group. The two experimental groups received different compression-to-ventilation ratios. The immediate chest compression group received 1 min of continuous post-shock chest compressions, whereas the AED group received the typical 15:2 compression:ventilation ratio with 2 s/rescue breath extant at the time this study was performed (i.e., before 2005 Guideline changes). It is possible that the continuous chest compressions contributed to the better outcomes; however, many previous animal studies by our group have demonstrated no difference in outcome with continuous chest compressions versus 15:2 breaths with only 2 s/breath.31–34
Conclusions
This study establishes that immediate post-shock chest compressions can improve outcome from prolonged VF compared with simulated pre-hospital AED defibrillation and the typical delays in post-shock chest compressions. This finding does not negate the important benefits of an AED: easy availability and potential use by individuals untrained to diagnose and treat VF. To the contrary, this experiment supports the most recent recommendations for AED defibrillation of prolonged VF in the circulatory phase: immediate post-shock chest compressions without regard to rhythm instead of commencing post-shock chest compressions after substantial delays for automated rhythm analyses and shock advisories.
Acknowledgments
Dr. Robert Berg has been funded by NIH to study this issue. This investigation was supported by NIH RO1 HL71694-01.
Footnotes
A Spanish translated version of the summary of this article appears as Appendix in the final online version at doi:10.1016/j.resuscitation.2008.02.014.
Conflict of interest
The authors have no other potential conflicts in regard to this study.
References
- 1.Weisfeldt M, Becker LB. Resuscitation after cardiac arrest. A 3-phase time-sensitive model. JAMA. 2002;288:3035–8. doi: 10.1001/jama.288.23.3035. [DOI] [PubMed] [Google Scholar]
- 2.Kern KB, Valenzuela TD, Clark LL, et al. An alternative approach to advancing resuscitation science. Resuscitation. 2005;64:261–8. doi: 10.1016/j.resuscitation.2004.08.009. [DOI] [PubMed] [Google Scholar]
- 3.Ewy GA. Cardiocerebral resuscitation: the new cardiopulmonary resuscitation. Circulation. 2005;111(16):2134–42. doi: 10.1161/01.CIR.0000162503.57657.FA. [DOI] [PubMed] [Google Scholar]
- 4.American Heart Association in collaboration with International Liaison Committee on Resuscitation Guidelines 2000 for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. International Consensus on Science, Part 4: the automated external defibrillator: key link in the chain of survival. Circulation. 2000;102(Suppl 8):I60–76. [PubMed] [Google Scholar]
- 5.Valenzuela TD, Kern KB, Clark LL, et al. Interruptions of chest compressions during emergency medical system resuscitation. Circulation. 2005;112(9):1259–65. doi: 10.1161/CIRCULATIONAHA.105.537282. [DOI] [PubMed] [Google Scholar]
- 6.Niemann JT, Stratton SJ, Cruz B, Lewis RJ. Outcome of out-of-hospital postcountershock asystole and pulseless electrical activity versus primary asystole and pulseless electrical activity. Crit Care Med. 2001;29(12):2366–70. doi: 10.1097/00003246-200112000-00020. [DOI] [PubMed] [Google Scholar]
- 7.Herlitz J, Bang A, Holmberg M, Axelsson A, Lindkvist J, Holmberg S. Rhythm changes during resuscitation from ventricular fibrillation in relation to delay until defibrillation, number of shocks delivered and survival. Resuscitation. 1997;34(1):17–22. doi: 10.1016/s0300-9572(96)01064-7. [DOI] [PubMed] [Google Scholar]
- 8.White RD, Russell JK. Refibrillation, resuscitation and survival in out-of-hospital sudden cardiac arrest victims treated with biphasic automated external defibrillators. Resuscitation. 2002;55(1):17–23. doi: 10.1016/s0300-9572(02)00194-6. [DOI] [PubMed] [Google Scholar]
- 9.Kern KB, Hilwig RW, Berg RA, Ewy GA. Efficacy of chest compression-only BLS CPR in the presence of an occluded airway. Resuscitation. 1998;39(3):179–88. doi: 10.1016/s0300-9572(98)00141-5. [DOI] [PubMed] [Google Scholar]
- 10.Berg RA, Sanders AB, Kern KB, et al. Adverse hemodynamic effects of interrupting chest compressions for rescue breathing during cardiopulmonary resuscitation for ventricular fibrillation cardiac arrest. Circulation. 2001;104(20):2465–70. doi: 10.1161/hc4501.098926. [DOI] [PubMed] [Google Scholar]
- 11.Kern K, Hilwig R, Berg RA, Sanders A, Ewy G. Importance of continuous chest compressions during CPR. Circulation. 2002;105:645–9. doi: 10.1161/hc0502.102963. [DOI] [PubMed] [Google Scholar]
- 12.Yu T, Weil MH, Tang W, et al. Adverse outcomes of interrupted precordial compression during automated defibrillation. Circulation. 2002;106(3):368–72. doi: 10.1161/01.cir.0000021429.22005.2e. [DOI] [PubMed] [Google Scholar]
- 13.Berg RA, Hilwig RW, Kern KB, Sanders AB, Xavier LC, Ewy GA. Automated external defibrillation versus manual defibrillation for prolonged ventricular fibrillation: lethal delays of chest compressions before and after countershocks. Ann Emerg Med. 2003;42(4):458–67. doi: 10.1067/s0196-0644(03)00525-0. [DOI] [PubMed] [Google Scholar]
- 14.Berg RA, Hilwig RW, Kern KB, Ewy GA. Precountershock cardiopulmonary resuscitation improves ventricular fibrillation median frequency and myocardial readiness for successful defibrillation from prolonged ventricular fibrillation: a randomized, controlled swine study. Ann Emerg Med. 2002;40(6):563–70. doi: 10.1067/mem.2002.129866. [DOI] [PubMed] [Google Scholar]
- 15.Berg RA, Hilwig RW, Ewy GA, Kern KB. Precountershock cardiopulmonary resuscitation improves initial response to defibrillation from prolonged ventricular fibrillation: a randomized, controlled swine study. Crit Care Med. 2004;32(6):1352–7. doi: 10.1097/01.ccm.0000127780.01362.e5. [DOI] [PubMed] [Google Scholar]
- 16.Yakaitis RW, Ewy GA, Otto CW, Taren DL, Moon TE. Influence of time and therapy on ventricular defibrillation in dogs. Crit Care Med. 1980;8(3):157–63. doi: 10.1097/00003246-198003000-00014. [DOI] [PubMed] [Google Scholar]
- 17.Cobb LA, Fahrenbruch CE, Walsh TR, et al. Influence of cardiopulmonary resuscitation prior to defibrillation in patients with out-of-hospital ventricular fibrillation. JAMA. 1999;281(13):1182–8. doi: 10.1001/jama.281.13.1182. [DOI] [PubMed] [Google Scholar]
- 18.Wik L, Hansen TB, Fylling F, et al. Delaying defibrillation to give basic cardiopulmonary resuscitation to patients with out-of-hospital ventricular fibrillation: a randomized trial. JAMA. 2003;289:1389–95. doi: 10.1001/jama.289.11.1389. [DOI] [PubMed] [Google Scholar]
- 19.Niemann JT, Cairns CB, Sharma J, Lewis RJ. Treatment of prolonged ventricular fibrillation: immediate countershock versus high-dose epinephrine and CPR preceding countershock. Circulation. 1992;85:281–7. doi: 10.1161/01.cir.85.1.281. [DOI] [PubMed] [Google Scholar]
- 20.Berg MD, Clark LL, Valenzuela TD, Kern KB, Berg RA. Post-shock chest compression delays with automated external defibrillator use. Resuscitation. 2005;64(3):287–91. doi: 10.1016/j.resuscitation.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 21.Van Alem AP, Chapman FW, Lank P, Hart AM, Koster RW. A prospective, randomized and blinded comparison of first shock success of monophasic and biphasic waveforms in out-of-hospital cardiac arrest. Resuscitation. 2003;58(1):17–24. doi: 10.1016/s0300-9572(03)00106-0. [DOI] [PubMed] [Google Scholar]
- 22.Emergency Cardiovascular Care Committee, Subcommittees of the American Heart Association. American Heart Association Guidelines for Cardiopulmonary Resuscitation and Emergency Cardiovascular Care. Circulation. 2005;112(Supplement IV):1–211. doi: 10.1161/CIRCULATIONAHA.105.166550. [DOI] [PubMed] [Google Scholar]
- 23.Angelos MD, Rath DP, Zhu H, Beckley PD, Robitaille PM. Flow requirements in ventricular fibrillation: an in vivo nuclear magnetic resonance analysis of the left ventricular high-energy phosphate pool. Ann Emerg Med. 1999;34:583–8. doi: 10.1016/s0196-0644(99)70159-9. [DOI] [PubMed] [Google Scholar]
- 24.Noc M, Weil MH, Gazmuri RJ, Sun S, Biscera J, Tang W. Ventricular fibrillation voltage as a monitor of the effectiveness of cardiopulmonary resuscitation. J Lab Clin Med. 1994;124(3):421–6. [PubMed] [Google Scholar]
- 25.Kern KB, Garewal HS, Sanders AB, et al. Depletion of myocardial adenosine triphosphate during prolonged untreated ventricular fibrillation: effect on defibrillation success. Resuscitation. 1990;20(3):221–9. doi: 10.1016/0300-9572(90)90005-y. [DOI] [PubMed] [Google Scholar]
- 26.Edelson DP, Abella BS, Kramer-Johansen J, et al. Effects of compression depth and pre-shock pauses predict defibrillation failure during cardiac arrest. Resuscitation. 2006;71(2):137–45. doi: 10.1016/j.resuscitation.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Abella BS, Sandbo N, Vassilatos P, et al. Chest compression rates during cardiopulmonary resuscitation are suboptimal: a prospective study during in-hospital cardiac arrest. Circulation. 2005;111:428–34. doi: 10.1161/01.CIR.0000153811.84257.59. [DOI] [PubMed] [Google Scholar]
- 28.Rea TD, Helbock M, Perry S, et al. Increasing use of cardiopulmonary resuscitation during out-of-hospital ventricular fibrillation arrest. Survival implications of guideline changes. Circulation. 2006;114:2760–5. doi: 10.1161/CIRCULATIONAHA.106.654715. [DOI] [PubMed] [Google Scholar]
- 29.Rea TD, Shah S, Kudenchuk PJ, Copass MK, Cobb LA. Automated external defibrillators: to what extent does the algorithm delay CPR? Ann Emerg Med. 2005;46:132–41. doi: 10.1016/j.annemergmed.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 30.Tang W, Snyder D, Wang J, et al. One-shock versus three-shock defibrillation protocol significantly improves outcome in a porcine model of prolonged ventricular fibrillation cardiac arrest. Circulation. 2006;113:2683–9. doi: 10.1161/CIRCULATIONAHA.105.592121. [DOI] [PubMed] [Google Scholar]
- 31.Berg RA, Kern KB, Sanders AB, Otto CW, Hilwig RW, Ewy GA. Bystander cardiopulmonary resuscitation. Is ventilation necessary? Circulation. 1993;88:1907–15. doi: 10.1161/01.cir.88.4.1907. [DOI] [PubMed] [Google Scholar]
- 32.Berg RA, Wilcoxson D, Hilwig RW, Kern KB, Sanders AB, Otto CW, Eklund DK, Ewy GA. The need for ventilatory support during bystander CPR. Ann Emerg Med. 1995;26:342–50. doi: 10.1016/s0196-0644(95)70084-6. [DOI] [PubMed] [Google Scholar]
- 33.Berg RA, Kern KB, Hilwig RW, Berg MD, Sanders AB, Otto CW, Ewy GA. Assisted ventilation does not improve outcome in a porcine model of single-rescuer bystander cardiopulmonary resuscitation. Circulation. 1997;95:1635–41. doi: 10.1161/01.cir.95.6.1635. [DOI] [PubMed] [Google Scholar]
- 34.Berg RA, Kern KB, Hilwig RW, Ewy GA. Assisted ventilation during ‘bystander’ CPR in a swine acute myocardial infarction model does not improve outcome. Circulation. 1997;96:4364–71. doi: 10.1161/01.cir.96.12.4364. [DOI] [PubMed] [Google Scholar]