Abstract
More than 25% of children survive to hospital discharge after in-hospital cardiac arrests, and 5% to 10% survive after out-of-hospital cardiac arrests. This review of pediatric cardiopulmonary resuscitation addresses the epidemiology of pediatric cardiac arrests, mechanisms of coronary blood flow during cardiopulmonary resuscitation, the 4 phases of cardiac arrest resuscitation, appropriate interventions during each phase, special resuscitation circumstances, extracorporeal membrane oxygenation cardiopulmonary resuscitation, and quality of cardiopulmonary resuscitation. The key elements of pathophysiology that impact and match the timing, intensity, duration, and variability of the hypoxic-ischemic insult to evidence-based interventions are reviewed. Exciting discoveries in basic and applied-science laboratories are now relevant for specific subpopulations of pediatric cardiac arrest victims and circumstances (eg, ventricular fibrillation, neonates, congenital heart disease, extracorporeal cardiopulmonary resuscitation). Improving the quality of interventions is increasingly recognized as a key factor for improving outcomes. Evolving training strategies include simulation training, just-in-time and just-in-place training, and crisis-team training. The difficult issue of when to discontinue resuscitative efforts is addressed. Outcomes from pediatric cardiac arrests are improving. Advances in resuscitation science and state-of-the-art implementation techniques provide the opportunity for further improvement in outcomes among children after cardiac arrest.
Keywords: cardiopulmonary resuscitation, cardiac arrest, ECMO, extracorporeal membrane oxygenation, pediatric, pediatric advanced life support, PALS, therapies
Pediatric cardiac arrests are among the most stressful clinical experiences for pediatricians and families. When the Pediatric Advanced Life Support (PALS) course was first developed in 1988, outcomes from pediatric cardiac arrests were dismal. Therefore, the original course focused on prevention of cardiac arrest through early recognition and treatment of respiratory failure and shock rather than treatment of cardiac arrest. Over the last 25 years, survival to hospital discharge has improved to 27% of children after in-hospital cardiac arrest, and most survive with favorable neurologic outcomes.1
In this article we review the state-of-the-art in pediatric cardiac arrest: epidemiology, the 4 phases of cardiac arrest and appropriate interventions, mechanisms of blood flow during cardiopulmonary resuscitation (CPR), postarrest supportive care, special resuscitation circumstances (pediatric ventricular fibrillation [VF], post–congenital heart surgery, extracorporeal membrane oxygenation [ECMO] CPR), prevention of cardiac arrest with rapid-response teams, and implementation of innovative simulation training programs.
EPIDEMIOLOGY OF PEDIATRIC CARDIAC ARREST
Cardiovascular disease is the most common cause of disease-related death, resulting in ~1 million deaths per year in North America. Approximately 16 000 American children (8–20 in 100 000 children per year) suffer a cardiac arrest each year. Critical factors that influence survival outcomes include the environment in which the arrest occurs, the preexisting condition of the child, the duration of “no flow” (period of time when there is no pulsatile flow and the patient is not undergoing CPR) before resuscitation, the initial electrocardiographic rhythm detected, and the quality of the basic and advanced life support interventions provided. Almost two thirds of in-hospital pediatric patients with cardiac arrest achieve return of spontaneous circulation (ROSC), and approximately one fourth survive to hospital discharge, of which almost three quarters have a good neurologic outcome (Table 1).1–17 Outcomes after pediatric out-of-hospital arrests are much worse than those after in-hospital arrests (Table 2).4,12,13,18–26 Survival to hospital discharge typically occurs for <10% of these children, and many have severe neurologic sequelae. These poor outcomes are in part caused by prolonged periods of no flow, because many out-of-hospital cardiac arrests are not witnessed and only 30% of children are provided with bystander CPR.13 This low bystander CPR rate is especially unfortunate, because bystander CPR more than doubles patient survival rates.27
TABLE 1.
Summary of Representative Studies of Outcome After In-Hospital Pediatric Cardiac Arrest
| Author (Year) | Settinga | No. of Patients | ROSC, n (%) | Survival to Discharge, n (%) | Favorable Neurologic Survival, n (%) |
|---|---|---|---|---|---|
| Thiagarajan et al16 (2007) | IH, ECMO | 682 | NA | 261 (38) | NR |
| de Moss15 (2006) | All ICUs | 91 | 75 (82) | 23 (25) | 10 of 14 assessed |
| Meaney et al5 (2006) | All ICU patients <21 y old | 464 | 233 (50) | 105 (22) | 67 (14) |
| Nadkarni et al1 (2006) | IH | 880 | 459 (52) | 236 (27) | 154 (18) |
| Samson et al8 (2006) | IH (all CAs) | 855 | 430 (50) | 216 (25) | 190 (22) |
| Initial VF/VT rhythm only | 104 | 73 (70) | 36 (35) | 34 (33) | |
| Tibballs and Kinney17 (2006) | IH | 111 | 81 (73) | 40 (36) | NR |
| Lopez-Herce et al4 (2005) | Mixed IH and OOH CA | 213 | 110 (52) | 45 (21) | 34 (16) |
| Extracorporeal Life Support Organization130 (2005) | IH, ECMO | 232 | NA; all needed ECMO | 88 (38) | NR |
| Reis et al7 (2002) | IH | 129 | 83 (64) | 21 (16) | 19 (15) |
| Chamnanvanakij and Perlman2 (2000) | IH, NICU patients with chest compressions for bradycardia | CPR: 14; CA: 25 | CPR: 14 (100); CA: not documented | CPR: 14 (100); CA: 19 (76) | CPR: 5 (36), 36% LTF; CA: 2 (8) |
| Parra et al6 (2000) | Pediatric CICU | 32 | 24 (63) | 14 (44) | 8 (25) |
| Suominen et al10 (2000) | IH | 118 | 74 (63) | 1-y survival: 21 (18) | NR |
| Young and Seidel13 (1999) | Meta-analysis IH | 544 | NR | 129 (24) | NR |
| Slonim et al9 (1997) | IH PICU | 205 | NR | 28 (14) | NR |
| Torres et al11 (1997) | IH | 92 | NR | 1-y survival: 9 (10) | 7 (8) |
| Zaritsky et al14 (1987) | IH | 53 | NR | 5 (9) | NR |
NA, indicates not applicable; NR, not reported; IH, in hospital; OOH, out of hospital; CPR, patients who received <2 minutes of CPR; CA, patients who received >2 minutes of CPR; LTF, lost to follow-up.
Patient population.
TABLE 2.
Summary of Representative Studies of Outcome After Out-of-Hospital Pediatric Cardiac Arrest
| Author (Year) | Settinga | No. of Patients | ROSC, n (%) | Survival to Discharge, n (%) | Favorable Neurologic Survival, n (%) |
|---|---|---|---|---|---|
| Osmond et al (2006) | OOH | 503 | NR | 10 (2) | NR |
| Berg et al19 (2005) | OOH, shockable rhythm | 13 | 13 (100) | 0 (0) | 0 (0) |
| Donoghue et al131 (2005) | OOH, review | 5693 | NR | 689 (12) | 228 (4) |
| Lopez-Herce et al4 (2005) | Mixed IH and OOH | 213 | 110 (52) | 45 (21) | 34 (16) |
| Sirbaugh et al24 (1999) | OOH CA | 300 | 33 (11) | 6 (2) | 1(<1) |
| Young and Seidel13 (1999) | Meta-analysis OOH | 1568 | NR | 132 (8) | NR |
| Suominen et al26 (1998) | OOH, traumatic arrests | 41 | 10 (24) | 3 (7) | 2 (5) |
| Suominen et al25 (1997) | OOH | 50 | 13 (26) | 8 (16) | 6 (12) |
| Schindler et al23 (1996) | OOH | 80 | 43 (54) | 6 (8) | 0 (0) |
| Dieckmann and Vardis20 (1995) | OOH | 65 | 3 (5) | 2 (3) | 1 (1.5) |
| Kuisma et al22 (1995) | OOH | 34 | 10 (29) | 5 (15) | 4 (12) |
OOH indicates out of hospital; IH, in hospital; NR, not reported.
Patient population.
Survival outcomes after pediatric in-hospital cardiac arrests are higher than those after adult in-hospital cardiac arrests. Although pediatric arrests are less commonly arrhythmogenic arrests caused by ventricular tachycardia (VT)/VF (10% of pediatric arrests vs 25% of adult arrests), 27% of the children survive to hospital discharge compared with 17% of the adults (adjusted odds ratio: 2.3 [95% confidence interval: 2.0–2.7]).1 The superior overall pediatric survival rate reflects a substantially higher survival rate among children with asystole or pulseless electrical activity (PEA) compared with adults with asystole or PEA (24% vs 11%). Further investigations have shown that the superior survival rate among children is predominantly a result of much better survival among infants and preschool-aged children compared with older children or adults.5 It may be that the superior outcomes in the younger children are related in part to CPR resulting in better myocardial and cerebral blood flows in these small children with compliant chest walls.28,29 In addition, survival from pediatric in-hospital CPR is more likely in hospitals staffed with pediatric physicians.18
PHASES OF RESUSCITATION: PREARREST, NO FLOW, CPR LOW FLOW, AND POST-ROSC
Interventions to improve outcome from pediatric cardiac arrest should be targeted to optimize therapies according to the etiology, timing, duration, intensity, and “phase” of resuscitation as suggested in Table 3. There are at least 4 phases of cardiac arrest: (1) prearrest; (2) no flow (untreated cardiac arrest); (3) low flow (CPR); and (4) postresuscitation. The prearrest phase represents the greatest opportunity to impact patient survival by preventing pulseless cardiopulmonary arrest. Because early recognition, prevention, and anticipation of cardiac arrest is better than treatment, the Pediatric Advanced Life Support course has traditionally focused on early recognition and treatment of respiratory failure and shock to prevent cardiac arrests. With the same intent, medical emergency teams (METs) (rapid-response teams) are being trained to recognize and intervene to prevent cardiac arrests.30,31 Preventing common childhood accidents by enforcing the use of car restraint devices and pool safety can decrease the incidence of cardiac arrest. Child safety seats are recommended to prevent traumatic arrests, and self-closing fences around swimming pools are recommended to prevent drowning-associated cardiac arrests.
TABLE 3.
Phases of Cardiac Arrest and Resuscitation
| Phase | Interventions |
|---|---|
| Prearrest (protect) | Optimize community education regarding child safety |
| Optimize patient monitoring and rapid emergency response | |
| Recognize and treat respiratory failure and/or shock to prevent cardiac arrest | |
| Arrest (no flow) (preserve) | Minimize interval to BLS and ACLS (organized response) |
| Minimize interval to defibrillation, when indicated | |
| Low flow (CPR) (resuscitate) | Push hard, push fast |
| Allow full chest recoil | |
| Minimize interruptions in compressions | |
| Avoid overventilation | |
| Titrate CPR to optimize myocardial blood flow (coronary perfusion pressures and exhaled CO2) | |
| Consider adjuncts to improve vital organ perfusion during CPR | |
| Consider ECMO if standard CPR/ALS not promptly successful | |
| Postresuscitation Short-term | Optimize cardiac output and cerebral perfusion |
| Treat arrhythmias, if indicated | |
| Avoid hyperglycemia, hyperthermia, hyperventilation | |
| Consider mild postresuscitation systemic hypothermia | |
| Debrief to improve future responses to emergencies | |
| Longer-term rehabilitation (regenerate) | Early intervention with occupational and physical therapy |
| Bioengineering and technology interface | |
| Possible future role for stem cell transplantation |
BLS indicates basic life support; ACLS, advanced cardiac life support; ALS, advanced life support.
Interventions during the no-flow phase of pulseless cardiac arrest focus on early recognition of cardiac arrest and prompt initiation of basic life support. The goal of effective CPR is to optimize coronary and cerebral perfusion and blood flow to critical organs during the low-flow phase. Basic life support with near-continuous effective chest compressions (eg, push hard, push fast, allow full chest recoil, minimize interruptions, and do not overventilate) is the emphasis in this phase. For VF and pulseless VT, rapid determination of electrocardiographic rhythm and prompt defibrillation when appropriate are critical. For cardiac arrests resulting from asphyxia and/or ischemia, provision of adequate myocardial perfusion and myocardial oxygen delivery with ventilation titrated to blood flow is important.
The postresuscitation phase is a high-risk period for brain injury, ventricular arrhythmias, and extension of reperfusion injuries. Injured cells can die or partially or fully recover function. Myocardial dysfunction and severe hypotension are common during the postresuscitation phase.32 Reperfusion injury occurs after the reestablishment of circulation after a period of ischemia. The reintroduction of blood flow can lead to inflammation and oxidative stress and can cause secondary injury. Interventions such as systemic hypothermia during the immediate postresuscitation phase strive to minimize reperfusion injury and support cellular recovery. The postarrest phase may have the most potential for innovative advances in the understanding of cell injury and death, inflammation, and apoptosis ultimately leading to novel interventions. Thoughtful attention to management of temperature (avoid hyperthermia), glucose level (normoglycemia), blood pressures (normotension), coagulation, and optimal ventilation (avoid hyperventilation) may be particularly important in this phase.33
The specific phase of cardiac arrest and resuscitation should dictate the timing, intensity, duration, and focus of interventions. Current understanding of the physiology of cardiac arrest and recovery only enables the titration of blood pressure, global oxygen delivery and consumption, body temperature, coagulation, and other physiological parameters to attempt to optimize outcome. Future strategies will likely take advantage of emerging discoveries and knowledge of cellular inflammation, thrombosis, reperfusion, mediator cascades, cellular markers of injury and recovery, and transplantation technology.
INTERVENTIONS DURING THE PREARREST PHASE: RAPID-RESPONSE TEAMS
The prearrest phase is the ideal phase to decrease mortality and morbidity from cardiac arrest by decreasing the incidence of cardiac arrest events. Patients suffering from in-hospital cardiac arrest often have abnormal physiological parameters in the hours before their event.34–36 Moreover, the precipitating causes of most pediatric cardiac arrests are acute respiratory insufficiency and circulatory shock.1 Rapid-response teams or METs are in-hospital emergency teams designed to respond to patients in imminent danger of decompensation and thereby prevent progression to cardiac arrest. Several “nonrandomized” adult studies have shown improvement in outcomes with the presence of METs,37,38 but a cluster-randomized trial in adults did not show a decrease in cardiac arrests or mortality.39 To date, there have been no prospective randomized trials establishing that either adult or pediatric METs prevent cardiac arrests. Nevertheless, 3 pediatric studies suggested that implementation of METs decreases the frequency of cardiac arrests compared with retrospective control periods before MET initiation.30,40,41 The first 2 studies revealed trends toward decreases of in-hospital cardiac arrests.30,40 Most recently, Sharek et al41 demonstrated that implementation of a pediatric MET decreased the mean monthly mortality rate by 18% (from 1.01 to 0.83 deaths per 100 discharges; P =.007) and the mean monthly code rate by >70% at a large children’s hospital. METs cannot prevent all cardiac arrests, but they should generally be able to transfer critically ill children to an ICU for better monitoring and more aggressive interventions to prevent cardiac arrest or to provide prompt advanced life support if the arrest occurs. The implication of this new paradigm is that any cardiac arrest that does not occur in a monitored unit should be reviewed as a sentinel event (ie, potentially avoidable).
MECHANISM OF CORONARY BLOOD FLOW DURING CPR
The coronary arteries provide blood flow from the aortic root to the myocardium. The normally beating heart is mainly perfused by coronary blood flow during diastole. When the heart arrests and no blood flows to the aorta, coronary blood flow ceases. During chest compressions, aortic pressure rises at the same time as right atrial pressure. During the decompression phase of chest compressions, the right atrial pressure falls faster and lower than the aortic pressure, which generates a pressure gradient that perfuses the heart with oxygenated blood during “diastole.” Coronary perfusion pressure below 15 mm Hg during CPR is a poor prognostic factor for ROSC.42
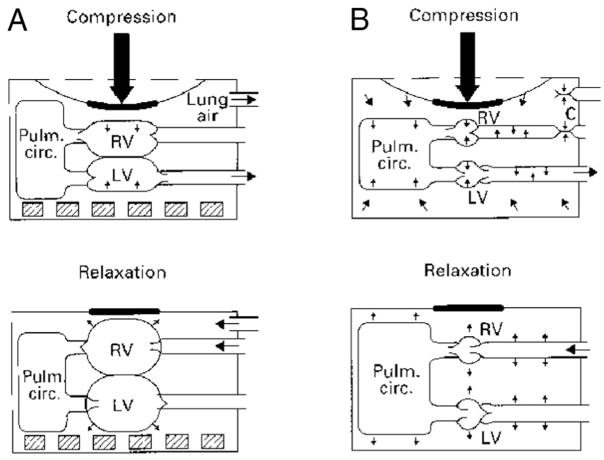
The importance of negative intrathoracic pressure on venous return during CPR, and thereby on coronary perfusion pressure and myocardial blood flow during CPR, was recently rediscovered (Fig 1). During the decompression phase, negative intrathoracic pressure can be enhanced by briefly impeding air flow to the lungs (ie, with an inspiratory impedance threshold device [ITD]). The increased negative pressure promotes venous return, cardiac output, and mean aortic pressure. The ITD is a small, disposable valve that can be connected directly to the tracheal tube or face mask to augment negative intrathoracic pressure during the inspiratory phase of spontaneous breathing and the decompression phase of CPR in animals or adult humans. It will selectively impede inspiratory airflow into the patient when the intrathoracic pressure is <0 atm, therefore increasing the amount of negative intrathoracic pressure with each chest decompression.43 The application of this concept has been shown in animal and adult human trials of CPR to improve vital organ perfusion pressures and myocardial blood flow.44–47 Despite encouraging outcomes in animal studies, 1 adult randomized, double-blinded study showed no difference in mortality rates between patients treated with the ITD versus a sham ITD for VF and asystolic arrests. The survival rate was higher with the ITD among a subgroup of patients with PEA.48
FIGURE 1.
A, Direct cardiac compressions; B, thoracic pump compressions. RV indicates right ventricle; LV, left ventricle; Pulm. circ., pulmonary circulation. (Reproduced with permission from Paradis NA, Halperin HR, Kern KB, Wenzel V, Chamberlain DA. Cardiac Arrest: The Science and Practice of Resuscitation Medicine. 2nd ed. Cambridge: Cambridge University Press; 2007)
Additional evidence that intrathoracic pressure manipulation during CPR can improve hemodynamics and outcomes is seen with studies of the active compression-decompression (ACD) device. ACD CPR consists of a handheld device that is fixed by suction to the anterior chest wall and, during the compression phase, forces blood out of the heart to perfuse vital organs. When actively pulling on the device, a vacuum is created within the thorax, drawing more blood back to the heart.49 The combination of ACD CPR with the ITD has been shown to further improve coronary artery perfusion pressures and systemic blood pressures significantly in animals and patients in cardiac arrest when compared with ACD CPR alone. The combination ACD ITD causes a more rapid decrease in negative intrathoracic pressure than any of the devices alone45 and is associated with a greater influx of venous blood back to the heart with each decompression phase.
INTERVENTIONS DURING CARDIAC ARREST (NO FLOW) AND CPR (LOW FLOW)
Airway and Breathing
During CPR, cardiac output and pulmonary blood flow are ~10% to 25% of that during normal sinus rhythm. Consequently, much less ventilation is necessary for adequate gas exchange from the blood traversing the pulmonary circulation during CPR. Animal and adult data indicate that overventilation during CPR is common and can substantially compromise venous return and cardiac output. Most concerning, these adverse hemodynamic effects during CPR combined with the interruptions in chest compressions to provide airway management and rescue breathing can contribute to worse survival outcomes.46,50–56
In animal models of sudden VF cardiac arrest, acceptable PaO2 and PaCO2 persist for 4 to 8 minutes during chest compressions without rescue breathing. Aortic oxygen and carbon dioxide concentrations do not vary from the prearrest state because there is no blood flow and aortic oxygen consumption is minimal. Adequate oxygenation and ventilation can continue without rescue breathing, because the lungs serve as a reservoir for oxygen during the low-flow state of CPR. Several retrospective studies of witnessed VF cardiac arrest in adults have also suggested that outcomes are similar after bystander-initiated CPR with either chest compressions alone or chest compressions plus rescue breathing. In contrast, animal studies of asphyxia-precipitated cardiac arrests have established that rescue breathing is a critical component of successful CPR.57,58 During asphyxia, blood continues to flow to tissues; therefore, arterial and venous oxygen saturations decrease while carbon dioxide and lactate increase. In addition, continued pulmonary blood flow before the cardiac arrest depletes the pulmonary oxygen reservoir. Therefore, asphyxia results in significant arterial hypoxemia and acidemia before resuscitation in contrast to VF. In this circumstance, rescue breathing can be life-saving.
Circulation
Basic life support with continuous effective chest compressions is generally the best way to provide circulation during cardiac arrest. Basic life support is often provided poorly or not at all. In 1 recent study, bystanders reported that they did not perform CPR because they panicked or were afraid they would do CPR incorrectly.59 The most critical elements are to push hard and push fast. Because there is no flow without chest compressions, it is important to minimize interruptions in chest compressions. To allow good venous return in the decompression phase of external cardiac massage, it is important to allow full chest recoil and to avoid overventilation. The latter can prevent venous return because of increased intrathoracic pressure.
Open-Chest CPR
Excellent standard closed-chest CPR generates ~10% to 25% of baseline myocardial blood flow and a cerebral blood flow that is ~50% of normal. By contrast, open-chest CPR can generate a cerebral blood flow that approaches normal. Although open-chest massage improves coronary perfusion pressure and increases the chance of successful defibrillation in animals and humans, surgical thoracotomy is impractical in many situations. Open-chest CPR is often provided to children after open-heart cardiac surgery and sternotomy. Earlier institution of open-chest CPR may warrant reconsideration in selected special resuscitation circumstances.
Ratio of Compressions to Ventilation
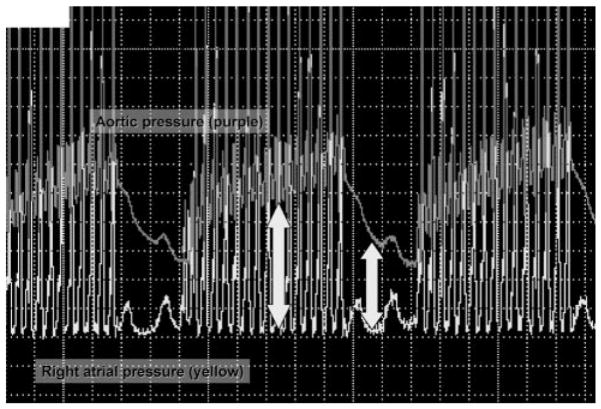
Ideal compression/ventilation ratios for pediatric patients are unknown. Recent physiological estimates60 suggest the amount of ventilation needed during CPR is much less than the amount needed during a normal perfusing rhythm, because the cardiac output during CPR is only 10% to 25% of that during normal sinus rhythm. The best ratio of compressions to ventilations depends on many factors including the compression rate, the tidal volume, the blood flow generated by compressions, and the time that compressions are interrupted to perform ventilations. A chest-compression/ventilation ratio of 15:2 delivers the same minute ventilation as CPR with a chest-compression/ventilation ratio of 5:1 in a mannequin model of pediatric CPR, but the number of chest compressions delivered was 48% higher with the 15:2 ratio.61,62 Chest compressions maintain increased aortic pressure and coronary perfusion pressure. When chest compressions cease, the aortic pressure rapidly decreases, thus decreasing coronary perfusion pressure (Fig 2). Increasing the ratio of compressions to ventilations minimizes these interruptions, thus increasing coronary blood flow. The benefits of positive pressure ventilation (increased arterial content of oxygen and carbon dioxide elimination) must be balanced against the adverse consequence of impeding circulation.
FIGURE 2.
Coronary perfusion pressure (the difference between aortic pressure and right atrial pressure).
Medications Used During Cardiac Arrest
Although animal studies indicate that epinephrine can improve initial resuscitation success after both asphyxial and VF cardiac arrests, no single medication has been shown to improve survival outcome from pediatric cardiac arrest. Medications commonly used for CPR in children are vasopressors (epinephrine or vasopressin), antiarrhythmics (amiodarone or lidocaine), calcium chloride, and sodium bicarbonate. Calcium and bicarbonate are most likely beneficial during special resuscitation circumstances (eg, prolonged cardiac arrest, post-bypass, hyperkalemia), but nonselective administration of these medications may be associated with worse survival outcomes.1,63
During CPR, epinephrine’s α-adrenergic effect increases systemic vascular resistance, increasing diastolic blood pressure, which in turn increases coronary perfusion pressure and blood flow and increases the likelihood of the ROSC. Epinephrine also increases total cerebral blood flow during good-quality CPR, because peripheral vasoconstriction directs a greater proportion of flow to the cerebral circulation.64–66 Recent animal data indicate that epinephrine can decrease local cerebral microcirculatory blood flow.67 The β-adrenergic effect increases myocardial contractility and heart rate and relaxes smooth muscle in the skeletal muscle vascular bed and bronchi, although this effect is of less importance. Epinephrine also increases the vigor and intensity of VF, increasing the likelihood of successful defibrillation. High-dose epinephrine (0.05–0.2 mg/kg) improves myocardial and cerebral blood flow during CPR more than standard-dose epinephrine (0.01–0.02 mg/kg) and may increase the incidence of initial ROSC.68,69 However, prospective and retrospective studies have indicated that use of high-dose epinephrine in adults or children does not improve survival and may be associated with a worse neurologic outcome.70,71 A randomized, blinded, controlled trial of rescue high-dose epinephrine versus standard-dose epinephrine after failed initial standard-dose epinephrine for pediatric in-hospital cardiac arrest demonstrated a worse 24-hour survival rate in the high-dose epinephrine group (1 of 27 vs 6 of 23; P <.05).72
Vasopressin is a long-acting endogenous hormone that acts at specific receptors to mediate systemic vasoconstriction (V1 receptor) and reabsorption of water in the renal tubule (V2 receptor). In randomized, controlled trials of in-hospital and out-of-hospital arrests in adults, vasopressin had comparable efficacy to epinephrine.73,74 Vasopressin during prolonged pediatric cardiac arrest may result in ROSC when standard medications have failed.75 Future research is necessary to further assess the efficacy of vasopressin use in conjunction with epinephrine in pediatric cardiac.
POSTRESUSCITATION INTERVENTIONS
Temperature Management
Mild induced hypothermia is the most clinically promising recent goal-directed postresuscitation therapy for adults. Two seminal articles have established76,77 that induced hypothermia (32°C–34°C) could improve outcome for comatose adults after resuscitation from VF cardiac arrest. In both randomized, controlled trials, the inclusion criteria were patients older than 18 years who were persistently comatose after successful resuscitation from nontraumatic VF. Interpretation and extrapolation of these studies to children is difficult. Fever after cardiac arrest, brain trauma, stroke, and other ischemic conditions are associated with poor neurologic outcome. Hyperthermia after cardiac arrest is common in children.78 It is reasonable to believe that mild induced systemic hypothermia may benefit children resuscitated from cardiac arrest. However, benefit from this treatment has not been rigorously studied and reported in children or in any patients with non-VF arrests. Emerging neonatal trials of selective brain cooling and systemic cooling show promise in neonatal hypoxic-ischemic encephalopathy (HIE), suggesting that induced hypothermia may improve outcomes.79,80
Postresuscitation Myocardial Support
Postarrest myocardial stunning and hypotensive shock occur commonly after successful resuscitation in animals, adults, and children and are generally reversible among long-term survivors. Postarrest myocardial stunning is pathophysiologically similar to sepsis-related post–cardiopulmonary bypass myocardial dysfunction, including increases in inflammatory mediator and nitricoxide production. Although the optimal management of post–cardiac arrest hypotension and myocardial dysfunction has not been established, data suggest that aggressive hemodynamic support may improve outcomes. Controlled trials in animal models have shown that dobutamine, milrinone, or levosimendan can effectively ameliorate post–cardiac arrest myocardial dysfunction.81–85 In clinical observational studies, fluid resuscitation has been provided for patients with hypotension and concomitant low central venous pressure, and various vasoactive infusions, including epinephrine, dobutamine, and dopamine, have been provided for the myocardial dysfunction.33,76,86–90 Furthermore, mechanical circulatory support with ECMO when compared with non-ECMO use was associated with improved survival in 1 retrospective study.15 General critical care principles suggest that appropriate therapeutic goals are adequate blood pressures and adequate myocardial, cerebral, and systemic blood flows and oxygen delivery. However, the definition of “adequate” is elusive. Reasonable interventions for vasodilatory shock with low central venous pressure include fluid resuscitation and vasoactive infusions. Appropriate considerations for left ventricular myocardial dysfunction include euvolemia, inotropic infusions, and afterload reduction.
Glucose Control
Hyperglycemia after adult cardiac arrest is associated with worse neurologic outcome after controlling for duration of arrest and presence of cardiogenic shock.91 In animal models of asphyxial and ischemic cardiac arrest, administration of insulin and glucose, but not administration of glucose alone, improved neurologic outcome compared with administration of normal saline.92 Data for evidence-based titration of specific end points in children are not available.
POSTRESUSCITATION OUTCOMES AND QUALITY OF LIFE
Information about predictors of neurologic outcome after both adult and pediatric cardiac arrests is limited. Barriers to assessment of neurologic outcomes of children after cardiac arrests include the constantly changing developmental context that occurs with brain maturation. Prediction or prognosis for future neuropsychological status is a complex task, particularly after an acute neurologic insult. There is little information about the predictive value of clinical neurologic examinations, neurophysiological diagnostic studies (eg, electroencephalography or somatosensory evoked potentials), biomarkers, or imaging (computed tomography, MRI, or positron emission tomography) on eventual outcome after cardiac arrest or other global hypoxic-ischemic insults in children. Computed tomography scans are not sensitive in detecting early neurologic injury. The value of MRI studies after pediatric cardiac arrest is not yet clear. However, MRI with diffusion weighting should provide valuable information about hypoxic-ischemic injury in the subacute and recovery phases. Emerging data suggest that burst-suppression pattern on postarrest electroencephalograms is sensitive and specific for poor neurologic outcome.93 One study showed that somatosensory evoked potential was highly sensitive and specific in pediatric patients after cardiac arrest.94 However, somatosensory evoked potential is not standardized in the pediatric population and is difficult to interpret. Many children who suffer a cardiac arrest have substantial preexisting neurologic problems.1 Thus, comparison to prearrest neurologic function of a child is difficult and adds another dimension/barrier to the assessment and prediction of postarrest neurologic status.
Biomarkers are emerging tools for predicting neurologic outcome. In an adult study, serum levels of neuron-specific enolase (NSE) and S100-B protein showed prognostic value. An NSE level of >33μg/L and S100-B level of >0.7 μg/L were highly sensitive and specific for poor neurologic outcome (death or persisting unconsciousness).95 One adult study investigated the patterns of release in patients after cardiac arrest who underwent induced therapeutic hypothermia. A decrease in NSE levels in the hypothermic group was significantly associated with good outcome 6 months after arrest.96 One investigation that examined 9 pediatric patients after cardiac arrest concluded that elevated S100-B levels may be associated with poor outcomes.97 Recently, preliminary data from a study that examined NSE and S100-B levels after cardiac arrest demonstrated that elevated NSE levels are associated with poor neurologic outcomes when measured 48 hours after ROSC.98 Additional efforts are ongoing to determine if biomarker levels after pediatric cardiac arrest will help predict prognosis for outcomes in the future.
SPECIAL RESUSCITATION CIRCUMSTANCES
Pediatric VF
VF is an uncommon, but not rare, electrocardiographic rhythm during out-of-hospital pediatric cardiac arrests. Two studies reported VF as the initial rhythm in 19% to 24% of out-of-hospital pediatric cardiac arrests after excluding sudden infant death syndrome (SIDS) deaths. In studies that included SIDS victims, the frequency decreased to the range of 6% to 10%; however, SIDS patients typically are asystolic, because they have been dead long before emergency medical services arrival.99 The incidence of VF varies according to setting and age.100 In special circumstances, such as tricyclic antidepressant overdose, cardiomyopathy, post–cardiac surgery, and prolonged QT syndromes, VF is a more likely rhythm during cardiac arrest. Commotio cordis, or mechanically initiated VF caused by relatively low-energy chest-wall impact during a narrow window of repolarization (10–30 milliseconds before the T wave peak in swine models), is reported predominantly in children aged 4 to 16 years. Out-of-hospital VF cardiac arrest is uncommon in infants but occurs more frequently in children and adolescents. Although VF is often associated with underlying heart disease and generally considered the “immediate cause” of cardiac arrest (ie, an arrhythmogenic arrest), “subsequent” VF can also occur during resuscitation efforts (ie, after an initial rhythm of asystole or PEA). Asphyxia-associated VF is also well documented among pediatric near-drowning patients.101 Traditionally, VF and VT have been considered “good” cardiac arrest rhythms that result in better outcomes than asystole and PEA. Survival to discharge is much more common among children with an initial shockable rhythm than among children with shockable rhythms occurring later during the resuscitation.8 Data suggest that outcomes after initial VF/VT are “good,” but outcomes after subsequent VF/VT are substantially worse, even compared with initial asystole/PEA without subsequent VF/VT.
Defibrillation (defined as termination of VF) is necessary for successful resuscitation from VF cardiac arrest. The termination of fibrillation can result in asystole, PEA, or a perfusing rhythm. The goal of defibrillation is return of an organized electrical rhythm with pulse. When prompt defibrillation is provided soon after the induction of VF in a cardiac catheterization laboratory, the rates of successful defibrillation and survival approach 100%, and when automated external defibrillators (AEDs) are used within 3 minutes of adult witnessed VF, long-term survival can occur in >70%.102,103 In general, the mortality rate increases by 7% to 10% per minute of delay to defibrillation. Early and effective near-continuous chest compressions can attenuate the incremental increase in mortality rate with delayed defibrillation. Because pediatric cardiac arrests are commonly a result of progressive asphyxia and/or shock, the initial treatment of choice is prompt CPR. Therefore, rhythm recognition is relatively less emphasized than with adult cardiac arrests. The earlier that VF can be diagnosed, the more successfully we can treat it.
Recently, AEDs were recommended for children <8 years old104,105; however, there is insufficient evidence to support a recommendation for or against the use of AEDs in children <1 year of age.
Postoperative Congenital Heart Disease Considerations
The postoperative cardiac patient may require resuscitation because of a persistent low cardiac output state but also may experience an acute decompensation such as a respiratory event, an arrhythmia, a feed-associated vagal episode, aortopulmonary shunt occlusion, pulmonary hypertensive crisis, or a coronary event. Over the first 6 to 18 hours after aortic cross-clamp, a low cardiac output state occurs and can progress to hypotension, inadequate coronary artery perfusion pressure, and cardiac arrest. Milrinone has been shown to reduce the incidence of low cardiac output syndrome in infants and children.106 Persistent hypoxemia in the shunted single-ventricle patient is concerning for evolving shunt thrombosis. If suspected, systemic heparinization to prevent ongoing thrombosis, increasing systemic vascular resistance (fluid boluses and phenylephrine) to promote increased antegrade shunt flow, and an emergent cardiac catheterization for definitive diagnosis and intervention are warranted.
Patients with persistent pulmonary hypertension and associated right ventricular failure are at high risk for cardiac arrest. The presence of an intra-atrial communication may help maintain systemic cardiac output at the expense of hypoxemia. Increases in intra-thoracic pressure caused by positive pressure ventilation can result in unacceptable afterload on the right ventricle, inadequate cardiac output, and ultimately cardiac arrest. Phosphodiesterase inhibitors, fluid volume, oxygen, and sodium bicarbonate remain the mainstays of resuscitation, with isoproterenol and inspired nitric oxide used specifically for patients with pulmonary hypertension.
Premature and Newly Born Infants
Worldwide, ~4 million newborns suffer from birth asphyxia, resulting in ~1 million deaths and 1 million with neurologic sequelae.107 Most recently, resuscitation research after perinatal birth asphyxia has focused on how to attenuate neurologic injury and mortality by using room-air resuscitation or systemic and/or head cooling. Room-air resuscitation has been studied extensively and may improve the overall mortality rate, but it has no effect on the rates of HIE as compared with patients who are resuscitated with 100% oxygen.107–110 In 2005, the International Liaison Committee on Resuscitation stated that there was “insufficient evidence to specify the concentration of oxygen to be used at initiation of resuscitation.”105 Hyperoxia is discouraged because of the risk of oxidant injury.111 Additional randomized and blinded studies are needed to gain more conclusive evidence for future recommendations.
Recent studies that assessed the use of selective head cooling after perinatal birth asphyxia to reduce HIE have shown no benefit in a severely encephalopathic group, but a significant benefit has been seen in the children with moderate encephalopathy.79 A randomized, controlled trial that examined whole-body cooling after perinatal asphyxia showed a reduction in mortality and disability in patients with moderate and severe encephalopathy.112 The 2005 American Heart Association guidelines for emergency cardiovascular care and CPR, written before the publication of the previous studies, did not recommend cooling because of insufficient data. Recently, a systematic review of 8 studies concluded that systemic and head cooling in the perinatally asphyxiated population when compared with the normothermic population does improve mortality rates and neurodevelopmental outcomes.113 Additional studies are underway, and cooling is being used clinically.105
ECMO CPR
ECMO CPR is the use of venoarterial ECMO to reestablish circulation and provide controlled reperfusion after cardiac arrest. Outcome studies have been published, but prospective, controlled studies are lacking. Nevertheless, these series have reported extraordinary results with the use of ECMO as a rescue therapy for pediatric cardiac arrests, especially from potentially reversible acute postoperative myocardial dysfunction or arrhythmias.6,114–118 In 1 study, 11 children who suffered cardiac arrest in the PICU after cardiac surgery were placed on ECMO during CPR after 20 to 110 minutes of CPR. Six of these 11 children were long-term survivors without apparent neurologic sequelae. Most remarkably, Morris et al119 reported on 66 children placed on ECMO during CPR. The median duration of CPR before establishment of ECMO was 50 minutes, yet 35% (23 of 66) of these children survived to hospital discharge. Most recently, these data have been corroborated by a retrospective study from the Extracorporeal Life Support Organization database, characterizing 682 patients over a 13-year period who received ECMO CPR. More than one third of the patients survived to discharge.16 Specific to the cardiac surgery population, patients with shunted single-ventricle circulation supported with ECMO showed survival-to-hospital-discharge rates of ~39%.118
Although there are more patients receiving ECMO CPR, the proportion of those treated with ECMO CPR who survive has not improved, potentially implicating the importance of selecting patients with reversible disease etiologies. It is important to emphasize that the children who survived with ECMO CPR despite prolonged CPR had brief periods of no flow, excellent CPR during the low-flow period, and a well-controlled postresuscitation phase. CPR and ECMO are not curative treatments. They are simply cardiopulmonary supportive measures that may allow tissue perfusion, optimization of microcirculatory flow, and viability until recovery from the precipitating disease process.
QUALITY OF CPR AND RESUSCITATION INTERVENTIONS
Despite evidence-based guidelines, extensive provider training, and provider credentialing in resuscitation medicine, the quality of CPR is typically poor. CPR guidelines recommend target values for selected CPR parameters related to rate and depth of chest compressions and ventilations, avoidance of CPR-free intervals, and complete release of sternal pressure between compressions.120 Unfortunately, in clinical practice, slow compression rates, inadequate depth of compression, and substantial pauses are the norm. An approach of “push hard, push fast, minimize interruptions, allow full chest recoil, and do not overventilate” can markedly improve myocardial, cerebral, and systemic perfusion and will likely improve outcomes.50 The quality of postresuscitative management has also been demonstrated to be critically important for improving resuscitation survival outcomes.33
Measuring the quality of CPR and avoiding overventilation during cardiac arrest resuscitation were reemphasized recently by consensus of the International Liaison Committee on Resuscitation and the American Heart Association.121 Although the correct amount, timing, intensity, and duration of ventilation that are required during CPR is controversial, there is no controversy that measurement and titration of the amount of ventilation to the amount of blood perfusion are desirable. Thus, additional technology that is safe, accurate, and practical would improve detection and feedback for the “quality of CPR.”
Recent technology has been developed that monitors the quality of CPR by force sensors and accelerometers and can provide verbal feedback to the CPR administrator regarding the frequency and depth of chest compressions and the volume of ventilations. Recent pediatric data illustrated that intensive training and real-time corrective feedback can help chest-compression quality approach age-specific American Heart Association CPR guideline targets.122 Improving the quality of postresuscitative management is another fertile area for improved cardiac arrest care. Improvements in postresuscitation care can improve resuscitation survival outcomes.33
WHEN SHOULD CPR BE DISCONTINUED?
Several factors determine the likelihood of survival after cardiac arrest, including the mechanism of the arrest (eg, traumatic, asphyxial, progression from circulatory shock), location (eg, in hospital or out of hospital), response (ie, witnessed or unwitnessed, with or without bystander CPR), underlying pathophysiology (ie, cardio-myopathy, congenital defect, drug toxicity, or metabolic derangement), and the potential reversibility of underlying diseases. These factors should all be considered before deciding to terminate resuscitative efforts. Continuation of CPR has been traditionally considered futile beyond 15 minutes of CPR or when >2 doses of epinephrine are needed.123 Presumably, in part because of improvements in CPR quality and postresuscitation care, improved outcomes from in-hospital CPR efforts beyond 15 minutes or 2 doses of epinephrine are increasingly the norm.1,7 The potential for excellent outcomes despite prolonged CPR has been highlighted by the ECMO-CPR data noted above.16,114,115,119,124 Conversely, the decision to discontinue CPR prematurely is final and cannot be rescinded. In the first decade of the 21st century, there is no simple answer to the important clinical question: when should CPR be discontinued?
SPECIAL ISSUES IN SIMULATION, ADVANCED EDUCATION, AND IMPLEMENTATION OF PROGRAMS
Just-in-time and just-in-place training concepts were developed on the basis of studies and recent reviews by experts for resuscitation training. Just-in-time training is the teaching of specific psychomotor skills such as resuscitation and airway management just before the skills are likely to be used (eg, before elective endotracheal intubation or while awaiting arrival of a child in cardiac arrest). Just-in-place training teaches providers specific skills at the location where the skills would be used. Psychomotor skills and team function are the primary skills necessary during resuscitation; however, these skills decay within 6 weeks after resuscitation training.125 Just-in-time and just-in-place refresher training seems reasonable for enhancing operational performance and improving patient safety. This training can incorporate some of the advantages of simulation (eg, abilities to plan and shape training opportunities), provide a safe environment for both patients and students, provide an opportunity to prepare for rare but complicated and important clinical events, and provide opportunities for repeated performances (practice).126 DeVita et al127 evaluated the efficiency of the code team (crisis-management team) training with adult high-fidelity simulation manikins. They measured the survival of the manikin in a simulated scenario and the task-completion rate as outcomes in 3 simulated training sessions. The team performance showed improvement in overall simulation survival and task-completion rates from 0% to 90% and 31% to 89%, respectively. Hunt et al128,129 used simulated cardiac arrest and trauma stabilization “mock codes” successfully to identify deficiencies in treatment of children with cardiac arrests and trauma. Such simulations are likely to drive resuscitation implementation in the future.
FUTURE DIRECTIONS AND POTENTIAL OBSTACLES
Exciting new epidemiologic studies, such as the National Registry of CardioPulmonary Resuscitation for in-hospital cardiac arrests and the large-scale, multicenter out-of-hospital Resuscitation Outcome Consortium funded by the National Heart, Lung, and Blood Institute, are providing new data to guide our resuscitation practices and generate hypotheses for new approaches to improving outcomes. The Resuscitation Outcomes Consortium epidemiologic data are collected on patients of all ages, but the initial interventional studies only enrolled patients >15 years old. It is increasingly clear that excellent basic life support is often not provided in either out-of-hospital or in-hospital settings. Innovative technical advances, such as directive and corrective real-time feedback, can increase the likelihood of effective basic life support. In addition, team dynamic training and performance feedback can substantially improve self-efficacy and functional implementation. Mechanical interventions such as ECMO or other cardiopulmonary bypass systems are already commonplace interventions during prolonged in-hospital cardiac arrests. Technical advances are likely to further improve our ability to provide such mechanical support.
In the past, the concept of evidence-based pediatric cardiac arrest recommendations seemed fanciful. Recommendations were based on extrapolated animal and adult data. Pediatric cardiac arrest clinical trials have started with the randomized, controlled trial of high-dose epinephrine versus standard-dose epinephrine as rescue therapy for in-hospital pediatric cardiac arrests. Clinical trials are necessary for appropriate evidence-based recommendations for treatment of pediatric cardiac arrests. It is likely that the evolution of systems such as “cardiac arrest centers,” similar to trauma, stroke, and myocardial infarction centers, is likely to facilitate the appropriate intensive care for children who require specialized postresuscitation care.
CONCLUSIONS
Outcomes from pediatric cardiac arrest and CPR seem to be improving. The evolution of clinical practice to integrate the pathophysiology and timing, intensity, duration, and variability of the hypoxic-ischemic insult may lead to more patient-specific and time-specific goal-directed therapy and better outcomes. Exciting discoveries in basic and applied-science laboratories are on the immediate horizon for specific subpopulations of cardiac arrest victims. By strategically focusing therapies to specific phases of cardiac arrest and resuscitation and to evolving pathophysiology, there is great promise that critical care interventions will lead the way to more successful cardiopulmonary and cerebral resuscitation in children.
Acknowledgments
Drs Berg and Nadkarni receive research grant support from the National Institutes of Health and unrestricted research grant support from Laerdal Foundation for Acute Care Medicine; and Dr Topjian has indicated she has no financial relationships relevant to this article to disclose.
Abbreviations
- CPR
cardiopulmonary resuscitation
- ECMO
extracorporeal membrane oxygenation
- ROSC
return of spontaneous circulation
- VT
ventricular tachycardia
- VF
ventricular fibrillation
- PEA
pulseless electrical activity
- MET
medical emergency team
- ITD
impedance threshold device
- ACD
active compression-decompression
- HIE
hypoxic-ischemic encephalopathy
- NSE
neuron-specific enolase
- SIDS
sudden infant death syndrome
- AED
automated external defibrillator
References
- 1.Nadkarni VM, Larkin GL, Peberdy MA, et al. First documented rhythm and clinical outcome from in-hospital cardiac arrest among children and adults. JAMA. 2006;295(1):50–57. doi: 10.1001/jama.295.1.50. [DOI] [PubMed] [Google Scholar]
- 2.Chamnanvanakij S, Perlman JM. Outcome following cardiopulmonary resuscitation in the neonate requiring ventilatory assistance. Resuscitation. 2000;45(3):173–180. doi: 10.1016/s0300-9572(00)00184-2. [DOI] [PubMed] [Google Scholar]
- 3.Hintz SR, Benitz WE, Colby CE, Sheehan AM, Rycus P, Van Meurs KP. Utilization and outcomes of neonatal cardiac extracorporeal life support: 1996–2000. Pediatr Crit Care Med. 2005;6(1):33–38. doi: 10.1097/01.PCC.0000149135.95884.65. [DOI] [PubMed] [Google Scholar]
- 4.López-Herce J, Garcia C, Dominguez P, et al. Outcome of out-of-hospital cardiorespiratory arrest in children. Pediatr Emerg Care. 2005;21(12):807–815. doi: 10.1097/01.pec.0000190230.43104.a8. [DOI] [PubMed] [Google Scholar]
- 5.Meaney PA, Nadkarni VM, Cook EF, et al. Higher survival rates among younger patients after pediatric intensive care unit cardiac arrests. Pediatrics. 2006;118(6):2424–2433. doi: 10.1542/peds.2006-1724. [DOI] [PubMed] [Google Scholar]
- 6.Parra DA, Totapally BR, Zahn E, et al. Outcome of cardiopulmonary resuscitation in a pediatric cardiac intensive care unit. Crit Care Med. 2000;28(9):3296–3300. doi: 10.1097/00003246-200009000-00030. [DOI] [PubMed] [Google Scholar]
- 7.Reis AG, Nadkarni V, Perondi MB, Grisi S, Berg RA. A prospective investigation into the epidemiology of in-hospital pediatric cardiopulmonary resuscitation using the international Utstein reporting style. Pediatrics. 2002;109(2):200–209. doi: 10.1542/peds.109.2.200. [DOI] [PubMed] [Google Scholar]
- 8.Samson RA, Nadkarni VM, Meaney PA, Carey SM, Berg MD, Berg RA. Outcomes of in-hospital ventricular fibrillation in children. N Engl J Med. 2006;354(22):2328–2339. doi: 10.1056/NEJMoa052917. [DOI] [PubMed] [Google Scholar]
- 9.Slonim AD, Patel KM, Ruttimann UE, Pollack MM. Cardiopulmonary resuscitation in pediatric intensive care units. Crit Care Med. 1997;25(12):1951–1955. doi: 10.1097/00003246-199712000-00008. [DOI] [PubMed] [Google Scholar]
- 10.Suominen P, Olkkola KT, Voipio V, Korpela R, Palo R, Räsänen J. Utstein style reporting of in-hospital paediatric cardiopulmonary resuscitation. Resuscitation. 2000;45(1):17–25. doi: 10.1016/s0300-9572(00)00167-2. [DOI] [PubMed] [Google Scholar]
- 11.Torres A, Jr, Pickert CB, Firestone J, Walker WM, Fiser DH. Long-term functional outcome of inpatient pediatric cardiopulmonary resuscitation. Pediatr Emerg Care. 1997;13(6):369–373. doi: 10.1097/00006565-199712000-00002. [DOI] [PubMed] [Google Scholar]
- 12.Tunstall-Pedoe H, Bailey L, Chamberlain DA, Marsden AK, Ward ME, Zideman DA. Survey of 3765 cardiopulmonary resuscitations in British hospitals (the BRESUS Study): methods and overall results. BMJ. 1992;304(6838):1347–1351. doi: 10.1136/bmj.304.6838.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Young KD, Seidel JS. Pediatric cardiopulmonary resuscitation: a collective review. Ann Emerg Med. 1999;33(2):195–205. doi: 10.1016/s0196-0644(99)70394-x. [DOI] [PubMed] [Google Scholar]
- 14.Zaritsky A, Nadkarni V, Getson P, Kuehl K. CPR in children. Ann Emerg Med. 1987;16(10):1107–1111. doi: 10.1016/s0196-0644(87)80465-1. [DOI] [PubMed] [Google Scholar]
- 15.de Mos N, van Litsenburg RR, McCrindle B, Bohn DJ, Parshuram CS. Pediatric in-intensive-care-unit cardiac arrest: incidence, survival, and predictive factors. Crit Care Med. 2006;34(4):1209–1215. doi: 10.1097/01.CCM.0000208440.66756.C2. [DOI] [PubMed] [Google Scholar]
- 16.Thiagarajan RR, Laussen PC, Rycus PT, Bartlett RH, Bratton SL. Extracorporeal membrane oxygenation to aid cardiopulmonary resuscitation in infants and children. Circulation. 2007;116(15):1693–1700. doi: 10.1161/CIRCULATIONAHA.106.680678. [DOI] [PubMed] [Google Scholar]
- 17.Tibballs J, Kinney S. A prospective study of outcome of in-patient paediatric cardiopulmonary arrest. Resuscitation. 2006;71(3):310–318. doi: 10.1016/j.resuscitation.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 18.Donoghue AJ, Nadkarni VM, Elliott M, Durbin D. Effect of hospital characteristics on outcomes from pediatric cardiopulmonary resuscitation: a report from the national registry of cardiopulmonary resuscitation. Pediatrics. 2006;118(3):995–1001. doi: 10.1542/peds.2006-0453. [DOI] [PubMed] [Google Scholar]
- 19.Berg MD, Samson RA, Meyer RJ, Clark LL, Valenzuela TD, Berg RA. Pediatric defibrillation doses often fail to terminate prolonged out-of-hospital ventricular fibrillation in children. Resuscitation. 2005;67(1):63–67. doi: 10.1016/j.resuscitation.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 20.Dieckmann RA, Vardis R. High-dose epinephrine in pediatric out-of-hospital cardiopulmonary arrest. Pediatrics. 1995;95(6):901–913. [PubMed] [Google Scholar]
- 21.Gerein RB, Osmond MH, Stiell IG, Nesbitt LP, Burns S OPALS Study Group. What are the etiology and epidemiology of out-of-hospital pediatric cardiopulmonary arrest in Ontario, Canada? Acad Emerg Med. 2006;13:653–658. doi: 10.1197/j.aem.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 22.Kuisma M, Suominen P, Korpela R. Paediatric out-of-hospital cardiac arrests: epidemiology and outcome. Resuscitation. 1995;30(2):141–150. doi: 10.1016/0300-9572(95)00888-z. [DOI] [PubMed] [Google Scholar]
- 23.Schindler MB, Bohn D, Cox PN, et al. Outcome of out-of-hospital cardiac or respiratory arrest in children. N Engl J Med. 1996;335(20):1473–1479. doi: 10.1056/NEJM199611143352001. [DOI] [PubMed] [Google Scholar]
- 24.Sirbaugh PE, Pepe PE, Shook JE, et al. A prospective, population-based study of the demographics, epidemiology, management, and outcome of out-of-hospital pediatric cardiopulmonary arrest. Ann Emerg Med. 1999;33(2):174–184. doi: 10.1016/s0196-0644(99)70391-4. [DOI] [PubMed] [Google Scholar]
- 25.Suominen P, Korpela R, Kuisma M, Silfvast T, Olkkola KT. Paediatric cardiac arrest and resuscitation provided by physician-staffed emergency care units. Acta Anaesthesiol Scand. 1997;41(2):260–265. doi: 10.1111/j.1399-6576.1997.tb04677.x. [DOI] [PubMed] [Google Scholar]
- 26.Suominen P, Rasanen J, Kivioja A. Efficacy of cardiopulmonary resuscitation in pulseless paediatric trauma patients. Resuscitation. 1998;36(1):9–13. doi: 10.1016/s0300-9572(97)00088-9. [DOI] [PubMed] [Google Scholar]
- 27.Holmberg M, Holmberg S, Herlitz J. Effect of bystander cardiopulmonary resuscitation in out-of-hospital cardiac arrest patients in Sweden. Resuscitation. 2000;47(1):59–70. doi: 10.1016/s0300-9572(00)00199-4. [DOI] [PubMed] [Google Scholar]
- 28.Dean JM, Koehler RC, Schleien CL, Michael JR, Chantarojanasiri T, Rogers MC, et al. Age-related changes in chest geometry during cardiopulmonary resuscitation. J Appl Physiol. 1987;62(6):2212–2219. doi: 10.1152/jappl.1987.62.6.2212. [DOI] [PubMed] [Google Scholar]
- 29.Kouwenhoven WB, Jude JR, Knickerbocker GG. Closed-chest cardiac massage. JAMA. 1960;173:1064–1067. doi: 10.1001/jama.1960.03020280004002. [DOI] [PubMed] [Google Scholar]
- 30.Brilli RJ, Gibson R, Luria JW, et al. Implementation of a medical emergency team in a large pediatric teaching hospital prevents respiratory and cardiopulmonary arrests outside the intensive care unit. Pediatr Crit Care Med. 2007;8(3):236–246. doi: 10.1097/01.PCC.0000262947.72442.EA. quiz 247. [DOI] [PubMed] [Google Scholar]
- 31.Tibballs J, Kinney S. A prospective before-and-after trial of a medical emergency team. Med J Aust. 2004;180(6):308, 310. doi: 10.5694/j.1326-5377.2004.tb05936.x. author reply 310. [DOI] [PubMed] [Google Scholar]
- 32.Laurent I, Monchi M, Chiche JD, et al. Reversible myocardial dysfunction in survivors of out-of-hospital cardiac arrest. J Am Coll Cardiol. 2002;40(12):2110–2116. doi: 10.1016/s0735-1097(02)02594-9. [DOI] [PubMed] [Google Scholar]
- 33.Sunde K, Pytte M, Jacobsen D, et al. Implementation of a standardised treatment protocol for post resuscitation care after out-of-hospital cardiac arrest. Resuscitation. 2007;73(1):29–39. doi: 10.1016/j.resuscitation.2006.08.016. [DOI] [PubMed] [Google Scholar]
- 34.Chaplik S, Neafsey PJ. Pre-existing variables and outcome of cardiac arrest resuscitation in hospitalized patients. Dimens Crit Care Nurs. 1998;17(4):200–207. doi: 10.1097/00003465-199807000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Smith AF, Wood J. Can some in-hospital cardio-respiratory arrests be prevented? A prospective survey. Resuscitation. 1998;37(3):133–137. doi: 10.1016/s0300-9572(98)00056-2. [DOI] [PubMed] [Google Scholar]
- 36.Buist MD, Jarmolowski E, Burton PR, Bernard SA, Waxman BP, Anderson J. Recognising clinical instability in hospital patients before cardiac arrest or unplanned admission to intensive care: a pilot study in a tertiary-care hospital. Med J Aust. 1999;171(1):22–25. doi: 10.5694/j.1326-5377.1999.tb123492.x. [DOI] [PubMed] [Google Scholar]
- 37.Bellomo R, Goldsmith D, Uchino S, et al. Prospective controlled trial of effect of medical emergency team on postoperative morbidity and mortality rates. Crit Care Med. 2004;32(4):916–921. doi: 10.1097/01.ccm.0000119428.02968.9e. [DOI] [PubMed] [Google Scholar]
- 38.Jones D, Bellomo R, Bates S, et al. Long term effect of a medical emergency team on cardiac arrests in a teaching hospital. Crit Care. 2005;9(6):R808–R815. doi: 10.1186/cc3906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hillman K, Chen J, Cretikos M, et al. Introduction of the medical emergency team (MET) system: a cluster-randomised controlled trial [published correction appears in Lancet. 2005; 366(9492):1164] Lancet. 2005;365(9477):2091–2097. doi: 10.1016/S0140-6736(05)66733-5. [DOI] [PubMed] [Google Scholar]
- 40.Tibballs J, Kinney S, Duke T, Oakley E, Hennessy M. Reduction of paediatric in-patient cardiac arrest and death with a medical emergency team: preliminary results. Arch Dis Child. 2005;90(11):1148–1152. doi: 10.1136/adc.2004.069401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sharek PJ, Parast LM, Leong K, et al. Effect of a rapid response team on hospital-wide mortality and code rates outside the ICU in a children’s hospital. JAMA. 2007;298(19):2267–2274. doi: 10.1001/jama.298.19.2267. [DOI] [PubMed] [Google Scholar]
- 42.Paradis NA, Martin GB, Rivers EP, et al. Coronary perfusion pressure and the return of spontaneous circulation in human cardiopulmonary resuscitation. JAMA. 1990;263(8):1106–1113. [PubMed] [Google Scholar]
- 43.Plaisance P, Lurie KG, Vicaut E, et al. Evaluation of an impedance threshold device in patients receiving active compression-decompression cardiopulmonary resuscitation for out of hospital cardiac arrest. Resuscitation. 2004;61(3):265–271. doi: 10.1016/j.resuscitation.2004.01.032. [DOI] [PubMed] [Google Scholar]
- 44.Lurie K, Zielinski T, McKnite S, Sukhum P. Improving the efficiency of cardiopulmonary resuscitation with an inspiratory impedance threshold valve. Crit Care Med. 2000;28(11 suppl):N207–N209. doi: 10.1097/00003246-200011001-00009. [DOI] [PubMed] [Google Scholar]
- 45.Lurie KG, Coffeen P, Shultz J, McKnite S, Detloff B, Mulligan K. Improving active compression-decompression cardiopulmonary resuscitation with an inspiratory impedance valve. Circulation. 1995;91(6):1629–1632. doi: 10.1161/01.cir.91.6.1629. [DOI] [PubMed] [Google Scholar]
- 46.Yannopoulos D, Aufderheide TP, Gabrielli A, et al. Clinical and hemodynamic comparison of 15:2 and 30:2 compression-to-ventilation ratios for cardiopulmonary resuscitation. Crit Care Med. 2006;34(5):1444–1449. doi: 10.1097/01.CCM.0000216705.83305.99. [DOI] [PubMed] [Google Scholar]
- 47.Yannopoulos D, Metzger A, McKnite S, et al. Intrathoracic pressure regulation improves vital organ perfusion pressures in normovolemic and hypovolemic pigs. Resuscitation. 2006;70(3):445–453. doi: 10.1016/j.resuscitation.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 48.Aufderheide TP, Pirrallo RG, Provo TA, Lurie KG. Clinical evaluation of an inspiratory impedance threshold device during standard cardiopulmonary resuscitation in patients with out-of-hospital cardiac arrest. Crit Care Med. 2005;33(4):734–740. doi: 10.1097/01.ccm.0000155909.09061.12. [DOI] [PubMed] [Google Scholar]
- 49.Wolcke BB, Mauer DK, Schoefmann MF, et al. Comparison of standard cardiopulmonary resuscitation versus the combination of active compression-decompression cardiopulmonary resuscitation and an inspiratory impedance threshold device for out-of-hospital cardiac arrest. Circulation. 2003;108(18):2201–2205. doi: 10.1161/01.CIR.0000095787.99180.B5. [DOI] [PubMed] [Google Scholar]
- 50.Edelson DP, Abella BS, Kramer-Johansen J, et al. Effects of compression depth and pre-shock pauses predict defibrillation failure during cardiac arrest. Resuscitation. 2006;71(2):137–145. doi: 10.1016/j.resuscitation.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 51.Abella BS, Sandbo N, Vassilatos P, et al. Chest compression rates during cardiopulmonary resuscitation are suboptimal: a prospective study during in-hospital cardiac arrest. Circulation. 2005;111(4):428–434. doi: 10.1161/01.CIR.0000153811.84257.59. [DOI] [PubMed] [Google Scholar]
- 52.Valenzuela TD, Kern KB, Clark LL, et al. Interruptions of chest compressions during emergency medical systems resuscitation. Circulation. 2005;112(9):1259–1265. doi: 10.1161/CIRCULATIONAHA.105.537282. [DOI] [PubMed] [Google Scholar]
- 53.Aufderheide TP, Sigurdsson G, Pirrallo RG, et al. Hyperventilation-induced hypotension during cardiopulmonary resuscitation. Circulation. 2004;109(16):1960–1965. doi: 10.1161/01.CIR.0000126594.79136.61. [DOI] [PubMed] [Google Scholar]
- 54.Aufderheide TP, Lurie KG. Death by hyperventilation: a common and life-threatening problem during cardiopulmonary resuscitation. Crit Care Med. 2004;32(9 suppl):S345–S351. doi: 10.1097/01.ccm.0000134335.46859.09. [DOI] [PubMed] [Google Scholar]
- 55.Ewy GA. Continuous-chest-compression cardiopulmonary resuscitation for cardiac arrest. Circulation. 2007;116(25):2894–2896. doi: 10.1161/CIRCULATIONAHA.107.751065. [DOI] [PubMed] [Google Scholar]
- 56.Ewy GA, Zuercher M, Hilwig RW, et al. Improved neurological outcome with continuous chest compressions compared with 30:2 compressions-to-ventilations cardiopulmonary resuscitation in a realistic swine model of out-of-hospital cardiac arrest. Circulation. 2007;116(22):2525–2530. doi: 10.1161/CIRCULATIONAHA.107.711820. [DOI] [PubMed] [Google Scholar]
- 57.Berg RA, Hilwig RW, Kern KB, Ewy GA. Bystander chest compressions and assisted ventilation independently improve outcome from piglet asphyxial pulseless “cardiac arrest”. Circulation. 2000;101(14):1743–1748. doi: 10.1161/01.cir.101.14.1743. [DOI] [PubMed] [Google Scholar]
- 58.Berg RA, Hilwig RW, Kern KB, Babar I, Ewy GA. Simulated mouth-to-mouth ventilation and chest compressions (bystander cardiopulmonary resuscitation) improves outcome in a swine model of prehospital pediatric asphyxial cardiac arrest. Crit Care Med. 1999;27(9):1893–1899. doi: 10.1097/00003246-199909000-00030. [DOI] [PubMed] [Google Scholar]
- 59.Swor R, Khan I, Domeier R, Honeycutt L, Chu K, Compton S. CPR training and CPR performance: do CPR-trained bystanders perform CPR? Acad Emerg Med. 2006;13(6):596–601. doi: 10.1197/j.aem.2005.12.021. [DOI] [PubMed] [Google Scholar]
- 60.Idris AH, Staples ED, O’Brien DJ, et al. Effect of ventilation on acid-base balance and oxygenation in low blood-flow states. Crit Care Med. 1994;22(11):1827–1834. [PubMed] [Google Scholar]
- 61.Kinney SB, Tibballs J. An analysis of the efficacy of bag-valve-mask ventilation and chest compression during different compression-ventilation ratios in manikin-simulated paediatric resuscitation. Resuscitation. 2000;43(2):115–120. doi: 10.1016/s0300-9572(99)00139-2. [DOI] [PubMed] [Google Scholar]
- 62.Srikantan SK, Berg RA, Cox T, Tice L, Nadkarni VM. Effect of one-rescuer compression/ventilation ratios on cardiopulmonary resuscitation in infant, pediatric, and adult manikins. Pediatr Crit Care Med. 2005;6(3):293–297. doi: 10.1097/01.PCC.0000161621.74554.15. [DOI] [PubMed] [Google Scholar]
- 63.Srinivasan V, Morris MC, Helfaer MA, Berg RA, Nadkarni VM American Heart Association National Registry of CPR Investigators. Calcium use during in-hospital pediatric cardiopulmonary resuscitation: a report from the National Registry of Cardiopulmonary Resuscitation. Pediatrics. 2008;121(5) doi: 10.1542/peds.2007-1555. Available at: www.pediatrics.org/cgi/content/full/121/5/e1144. [DOI] [PubMed]
- 64.Berkowitz ID, Gervais H, Schleien CL, Koehler RC, Dean JM, Traystman RJ. Epinephrine dosage effects on cerebral and myocardial blood flow in an infant swine model of cardiopulmonary resuscitation. Anesthesiology. 1991;75(6):1041–1050. doi: 10.1097/00000542-199112000-00017. [DOI] [PubMed] [Google Scholar]
- 65.Koehler RC, Michael JR, Guerci AD, et al. Beneficial effect of epinephrine infusion on cerebral and myocardial blood flows during CPR. Ann Emerg Med. 1985;14(8):744–749. doi: 10.1016/s0196-0644(85)80050-0. [DOI] [PubMed] [Google Scholar]
- 66.Lindner KH, Ahnefeld FW, Bowdler IM, Prengel AW. Influence of epinephrine on systemic, myocardial, and cerebral acid-base status during cardiopulmonary resuscitation. Anesthesiology. 1991;74(2):333–339. doi: 10.1097/00000542-199102000-00021. [DOI] [PubMed] [Google Scholar]
- 67.Ristagno G, Sun S, Tang W, Castillo C, Weil MH. Effects of epinephrine and vasopressin on cerebral microcirculatory flows during and after cardiopulmonary resuscitation. Crit Care Med. 2007;35(9):2145–2149. doi: 10.1097/01.ccm.0000280427.76175.d2. [DOI] [PubMed] [Google Scholar]
- 68.Brown CG, Martin DR, Pepe PE, et al. A comparison of standard-dose and high-dose epinephrine in cardiac arrest outside the hospital. The Multicenter High-Dose Epinephrine Study Group. N Engl J Med. 1992;327(15):1051–1055. doi: 10.1056/NEJM199210083271503. [DOI] [PubMed] [Google Scholar]
- 69.Lindner KH, Ahnefeld FW, Bowdler IM. Comparison of different doses of epinephrine on myocardial perfusion and resuscitation success during cardiopulmonary resuscitation in a pig model. Am J Emerg Med. 1991;9(1):27–31. doi: 10.1016/0735-6757(91)90008-8. [DOI] [PubMed] [Google Scholar]
- 70.Callaham M, Madsen C, Barton C, Saunders C, Daley M, Pointer J. A randomized clinical trial of high-dose epinephrine and norepinephrine versus standard-dose epinephrine in pre-hospital cardiac arrest. JAMA. 1992;268(19):2667–2672. [PubMed] [Google Scholar]
- 71.Behringer W, Kittler H, Sterz F, et al. Cumulative epinephrine dose during cardiopulmonary resuscitation and neurologic outcome. Ann Intern Med. 1998;129(6):450–456. doi: 10.7326/0003-4819-129-6-199809150-00004. [DOI] [PubMed] [Google Scholar]
- 72.Perondi MB, Reis AG, Paiva EF, Nadkarni VM, Berg RA. A comparison of high-dose and standard-dose epinephrine in children with cardiac arrest. N Engl J Med. 2004;350(17):1722–1730. doi: 10.1056/NEJMoa032440. [DOI] [PubMed] [Google Scholar]
- 73.Stiell IG, Hébert PC, Wells GA, et al. Vasopressin versus epinephrine for inhospital cardiac arrest: a randomised controlled trial. Lancet. 2001;358(9276):105–109. doi: 10.1016/S0140-6736(01)05328-4. [DOI] [PubMed] [Google Scholar]
- 74.Wenzel V, Krismer AC, Arntz HR, Sitter H, Stadlbauer KH, Lindner KH. A comparison of vasopressin and epinephrine for out-of-hospital cardiopulmonary resuscitation. N Engl J Med. 2004;350(2):105–113. doi: 10.1056/NEJMoa025431. [DOI] [PubMed] [Google Scholar]
- 75.Mann K, Berg RA, Nadkarni V. Beneficial effects of vasopressin in prolonged pediatric cardiac arrest: a case series. Resuscitation. 2002;52(2):149–156. doi: 10.1016/s0300-9572(01)00470-1. [DOI] [PubMed] [Google Scholar]
- 76.Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–556. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 77.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–563. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 78.Hickey RW, Kochanek PM, Ferimer H, Graham SH, Safar P. Hypothermia and hyperthermia in children after resuscitation from cardiac arrest. Pediatrics. 2000;106(1 pt 1):118–122. doi: 10.1542/peds.106.1.118. [DOI] [PubMed] [Google Scholar]
- 79.Gluckman PD, Wyatt JS, Azzopardi D, et al. Selective head cooling with mild systemic hypothermia after neonatal encephalopathy: multicentre randomised trial. Lancet. 2005;365(9460):663–670. doi: 10.1016/S0140-6736(05)17946-X. [DOI] [PubMed] [Google Scholar]
- 80.Shankaran S, Laptook A, Wright LL, et al. Whole-body hypothermia for neonatal encephalopathy: animal observations as a basis for a randomized, controlled pilot study in term infants. Pediatrics. 2002;110(2 pt 1):377–385. doi: 10.1542/peds.110.2.377. [DOI] [PubMed] [Google Scholar]
- 81.Kern KB, Hilwig RW, Berg RA, et al. Postresuscitation left ventricular systolic and diastolic dysfunction: treatment with dobutamine. Circulation. 1997;95(12):2610–2613. doi: 10.1161/01.cir.95.12.2610. [DOI] [PubMed] [Google Scholar]
- 82.Meyer RJ, Kern KB, Berg RA, Hilwig RW, Ewy GA. Post-resuscitation right ventricular dysfunction: delineation and treatment with dobutamine. Resuscitation. 2002;55(2):187–191. doi: 10.1016/s0300-9572(02)00204-6. [DOI] [PubMed] [Google Scholar]
- 83.Niemann JT, Garner D, Khaleeli E, Lewis RJ. Milrinone facilitates resuscitation from cardiac arrest and attenuates postresuscitation myocardial dysfunction. Circulation. 2003;108(24):3031–3035. doi: 10.1161/01.CIR.0000101925.37174.85. [DOI] [PubMed] [Google Scholar]
- 84.Vasquez A, Kern KB, Hilwig RW, Heidenreich J, Berg RA, Ewy GA. Optimal dosing of dobutamine for treating postresuscitation left ventricular dysfunction. Resuscitation. 2004;61(2):199–207. doi: 10.1016/j.resuscitation.2004.01.002. [DOI] [PubMed] [Google Scholar]
- 85.Huang L, Weil MH, Tang W, Sun S, Wang J. Comparison between dobutamine and levosimendan for management of postresuscitation myocardial dysfunction. Crit Care Med. 2005;33(3):487–491. doi: 10.1097/01.ccm.0000156241.55872.15. [DOI] [PubMed] [Google Scholar]
- 86.Bernard S, Buist M, Monteiro O, Smith K. Induced hypothermia using large volume, ice-cold intravenous fluid in comatose survivors of out-of-hospital cardiac arrest: a preliminary report. Resuscitation. 2003;56(1):9–13. doi: 10.1016/s0300-9572(02)00276-9. [DOI] [PubMed] [Google Scholar]
- 87.Müllner M, Domanovits H, Sterz F, et al. Measurement of myocardial contractility following successful resuscitation: quantitated left ventricular systolic function utilising non-invasive wall stress analysis. Resuscitation. 1998;39(1–2):51–59. doi: 10.1016/s0300-9572(98)00122-1. [DOI] [PubMed] [Google Scholar]
- 88.Adrie C, Adib-Conquy M, Laurent I, et al. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation. 2002;106(5):562–568. doi: 10.1161/01.cir.0000023891.80661.ad. [DOI] [PubMed] [Google Scholar]
- 89.Laurent I, Adrie C, Vinsonneau C, et al. High-volume hemofiltration after out-of-hospital cardiac arrest: a randomized study. J Am Coll Cardiol. 2005;46(3):432–437. doi: 10.1016/j.jacc.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 90.Ruiz-Bailén M, Aguayo de Hoyos E, Ruiz-Navarro S, et al. Reversible myocardial dysfunction after cardiopulmonary resuscitation. Resuscitation. 2005;66(2):175–181. doi: 10.1016/j.resuscitation.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 91.Langhelle A, Tyvold SS, Lexow K, Hapnes SA, Sunde K, Steen PA. In-hospital factors associated with improved outcome after out-of-hospital cardiac arrest: a comparison between four regions in Norway. Resuscitation. 2003;56(3):247–263. doi: 10.1016/s0300-9572(02)00409-4. [DOI] [PubMed] [Google Scholar]
- 92.Berger PB. A glucose-insulin-potassium infusion did not reduce mortality, cardiac arrest, or cardiogenic shock after acute MI. ACP J Club. 2005;143(1):4–5. [PubMed] [Google Scholar]
- 93.Nishisaki A, Sullivan J, 3rd, Steger B, et al. Retrospective analysis of the prognostic value of electroencephalography patterns obtained in pediatric in-hospital cardiac arrest survivors during three years. Pediatr Crit Care Med. 2007;8(1):10–17. doi: 10.1097/01.pcc.0000256621.63135.4b. [DOI] [PubMed] [Google Scholar]
- 94.Schellhammer F, Heindel W, Haupt WF, Landwehr P, Lackner K. Somatosensory evoked potentials: a simple neurophysiological monitoring technique in supra-aortal balloon test occlusions. Eur Radiol. 1998;8(9):1586–1589. doi: 10.1007/s003300050591. [DOI] [PubMed] [Google Scholar]
- 95.Piazza O, Cotena S, Esposito G, De Robertis E, Tufano R. S100B is a sensitive but not specific prognostic index in comatose patients after cardiac arrest. Minerva Chir. 2005;60(6):477–480. [PubMed] [Google Scholar]
- 96.Tiainen M, Roine RO, Pettila V, Takkunen O. Serum neuron-specific enolase and S-100B protein in cardiac arrest patients treated with hypothermia. Stroke. 2003;34(12):2881–2886. doi: 10.1161/01.STR.0000103320.90706.35. [DOI] [PubMed] [Google Scholar]
- 97.Berger RP, Kochanek PM. Urinary S100B concentrations are increased after brain injury in children: a preliminary study. Pediatr Crit Care Med. 2006;7(6):557–561. doi: 10.1097/01.PCC.0000244426.37793.23. [DOI] [PubMed] [Google Scholar]
- 98.Topjian AMM, Roth C, Zuppa A, Drott H, Ichord R, Helfaer MA, Nadkarni V. Neuron specific enolase and S100B predict neurologic outcome after pediatric cardiac arrest. Presented at: Pediatric Critical Care Colloquium; Snowbird, UT. February 21–24, 2006. [Google Scholar]
- 99.Smith BT, Rea TD, Eisenberg MS. Ventricular fibrillation in pediatric cardiac arrest. Acad Emerg Med. 2006;13(5):525–529. doi: 10.1197/j.aem.2005.12.019. [DOI] [PubMed] [Google Scholar]
- 100.Appleton GO, Cummins RO, Larson MP, Graves JR. CPR and the single rescuer: at what age should you “call first” rather than call fast? Ann Emerg Med. 1995;25(4):492–494. doi: 10.1016/s0196-0644(95)70264-4. [DOI] [PubMed] [Google Scholar]
- 101.Graf WD, Cummings P, Quan L, Brutocao D. Predicting outcome in pediatric submersion victims. Ann Emerg Med. 1995;26(3):312–319. doi: 10.1016/s0196-0644(95)70079-x. [DOI] [PubMed] [Google Scholar]
- 102.Caffrey SL, Willoughby PJ, Pepe PE, Becker LB. Public use of automated external defibrillators. N Engl J Med. 2002;347(16):1242–1247. doi: 10.1056/NEJMoa020932. [DOI] [PubMed] [Google Scholar]
- 103.Valenzuela TD, Roe DJ, Nichol G, Clark LL, Spaite DW, Hardman RG. Outcomes of rapid defibrillation by security officers after cardiac arrest in casinos. N Engl J Med. 2000;343(17):1206–1209. doi: 10.1056/NEJM200010263431701. [DOI] [PubMed] [Google Scholar]
- 104.Samson RA, Berg RA, Bingham R Pediatric Advanced Life Support Task Force, International Liaison Committee on Resuscitation for the American Heart Association; European Resuscitation Council. Use of automated external defibrillators for children: an update—an advisory statement from the Pediatric Advanced Life Support Task Force, International Liaison Committee on Resuscitation. Pediatrics. 2003;112(1 pt 1):163–168. doi: 10.1542/peds.112.1.163. [DOI] [PubMed] [Google Scholar]
- 105.American Heart Association. 2005 American Heart Association (AHA) guidelines for cardiopulmonary resuscitation (CPR) and emergency cardiovascular care (ECC) of pediatric and neonatal patients: pediatric advanced life support. Pediatrics. 2006;117(5) doi: 10.1542/peds.2006-0346. Available at: www.pediatrics.org/cgi/content/full/117/5/e1005. [DOI] [PubMed]
- 106.Hoffman TM, Wernovsky G, Atz AM, et al. Efficacy and safety of milrinone in preventing low cardiac output syndrome in infants and children after corrective surgery for congenital heart disease. Circulation. 2003;107(7):996–1002. doi: 10.1161/01.cir.0000051365.81920.28. [DOI] [PubMed] [Google Scholar]
- 107.Saugstad OD, Rootwelt T, Aalen O. Resuscitation of asphyxiated newborn infants with room air or oxygen: an international controlled trial: the Resair 2 study. Pediatrics. 1998;102(1) doi: 10.1542/peds.102.1.e1. Available at: www.pediatrics.org/cgi/content/full/102/1/e1. [DOI] [PubMed]
- 108.Davis PG, Tan A, O’Donnell CP, Schulze A. Resuscitation of newborn infants with 100% oxygen or air: a systematic review and meta-analysis. Lancet. 2004;364(9442):1329–1333. doi: 10.1016/S0140-6736(04)17189-4. [DOI] [PubMed] [Google Scholar]
- 109.Saugstad OD, Ramji S, Vento M. Resuscitation of depressed newborn infants with ambient air or pure oxygen: a meta-analysis. Biol Neonate. 2005;87(1):27–34. doi: 10.1159/000080950. [DOI] [PubMed] [Google Scholar]
- 110.Tan A, Schulze A, O’Donnell CP, Davis PG. Air versus oxygen for resuscitation of infants at birth. Cochrane Database Syst Rev. 2005;(2):CD002273. doi: 10.1002/14651858.CD002273.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Balan IS, Fiskum G, Hazelton J, Cotto-Cumba C, Rosenthal RE. Oximetry-guided reoxygenation improves neurological outcome after experimental cardiac arrest. Stroke. 2006;37(12):3008–3013. doi: 10.1161/01.STR.0000248455.73785.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Shankaran S, Laptook AR, Ehrenkranz RA, et al. Whole-body hypothermia for neonates with hypoxic-ischemic encephalopathy. N Engl J Med. 2005;353(15):1574–1584. doi: 10.1056/NEJMcps050929. [DOI] [PubMed] [Google Scholar]
- 113.Shah PS, Ohlsson A, Perlman M. Hypothermia to treat neonatal hypoxic ischemic encephalopathy: systematic review. Arch Pediatr Adolesc Med. 2007;161(10):951–958. doi: 10.1001/archpedi.161.10.951. [DOI] [PubMed] [Google Scholar]
- 114.Dalton HJ, Siewers RD, Fuhrman BP, et al. Extracorporeal membrane oxygenation for cardiac rescue in children with severe myocardial dysfunction. Crit Care Med. 1993;21(7):1020–1028. doi: 10.1097/00003246-199307000-00016. [DOI] [PubMed] [Google Scholar]
- 115.del Nido PJ, Dalton HJ, Thompson AE, Siewers RD. Extracorporeal membrane oxygenator rescue in children during cardiac arrest after cardiac surgery. Circulation. 1992;86(5 suppl):II300–II304. [PubMed] [Google Scholar]
- 116.Tecklenburg FW, Thomas NJ, Webb SA, Case C, Habib DM. Pediatric ECMO for severe quinidine cardiotoxicity. Pediatr Emerg Care. 1997;13(2):111–113. doi: 10.1097/00006565-199704000-00007. [DOI] [PubMed] [Google Scholar]
- 117.Thalmann M, Trampitsch E, Haberfellner N, Eisendle E, Kraschl R, Kobinia G. Resuscitation in near drowning with extracorporeal membrane oxygenation. Ann Thorac Surg. 2001;72(2):607–608. doi: 10.1016/s0003-4975(00)02307-9. [DOI] [PubMed] [Google Scholar]
- 118.Morris MC, Wernovsky G, Nadkarni VM. Survival outcomes after extracorporeal cardiopulmonary resuscitation instituted during active chest compressions following refractory inhospital pediatric cardiac arrest. Pediatr Crit Care Med. 2004;5(5):440–446. doi: 10.1097/01.pcc.0000137356.58150.2e. [DOI] [PubMed] [Google Scholar]
- 119.Ravishankar C, Dominguez TE, Kreutzer J, et al. Extracorporeal membrane oxygenation after stage I reconstruction for hypoplastic left heart syndrome. Pediatr Crit Care Med. 2006;7(4):319–323. doi: 10.1097/01.PCC.0000227109.82323.CE. [DOI] [PubMed] [Google Scholar]
- 120.Wik L, Kramer-Johansen J, Myklebust H, et al. Quality of cardiopulmonary resuscitation during out-of-hospital cardiac arrest. JAMA. 2005;293(3):299–304. doi: 10.1001/jama.293.3.299. [DOI] [PubMed] [Google Scholar]
- 121.ECC Committee, Subcommittees and Task Forces of the American Heart Association. 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2005;112(24 suppl):IV1–IV203. doi: 10.1161/CIRCULATIONAHA.105.166550. [DOI] [PubMed] [Google Scholar]
- 122.Sutton RMND, Donoghue D, Arbogast K, et al. The first quantitative report of pediatric in-hospital CPR. Presented at: 5th World Congress on Pediatric Critical Care; June 24–27, 2007; Geneva, Switzerland. [Google Scholar]
- 123.Zaritsky A. Cardiopulmonary resuscitation in children. Clin Chest Med. 1987;8(4):561–571. [PubMed] [Google Scholar]
- 124.Thiagarajan RR, Bratton SL. Extracorporeal membrane oxygenation for cardiac arrest: when to use it, and what are the outcomes? Crit Care Med. 2006;34(4):1285–1286. doi: 10.1097/01.CCM.0000202432.07027.C7. [DOI] [PubMed] [Google Scholar]
- 125.Stross JK. Maintaining competency in advanced cardiac life support skills. JAMA. 1983;249(24):3339–3341. [PubMed] [Google Scholar]
- 126.Grenvik A, Schaefer JJ, 3rd, DeVita MA, Rogers P. New aspects on critical care medicine training. Curr Opin Crit Care. 2004;10(4):233–237. doi: 10.1097/01.ccx.0000132654.52131.32. [DOI] [PubMed] [Google Scholar]
- 127.DeVita MA, Schaefer J, Lutz J, Dongilli T, Wang H. Improving medical crisis team performance. Crit Care Med. 2004;32:S61–S65. doi: 10.1097/01.ccm.0000110872.86812.1c. [DOI] [PubMed] [Google Scholar]
- 128.Hunt EA, Hohenhaus SM, Luo X, Frush KS. Simulation of pediatric trauma stabilization in 35 North Carolina emergency departments: identification of targets for performance improvement. Pediatrics. 2006;117(3):641–648. doi: 10.1542/peds.2004-2702. [DOI] [PubMed] [Google Scholar]
- 129.Hunt EA, Walker AR, Shaffner DH, Miller MR, Pronovost PJ. Simulation of in-hospital pediatric medical emergencies and cardiopulmonary arrests: highlighting the importance of the first 5 minutes. Pediatrics. 2008;121(1) doi: 10.1542/peds.2007-0029. Available at: www.pediatrics.org/cgi/content/full/121/1/e34. [DOI] [PubMed]
- 130.Conrad SA, Rycus PT, Dalton H. Extracorporeal Life Support Registry Report 2004. Asaio J. 2005;51(1):4–10. doi: 10.1097/01.mat.0000151922.67540.e9. [DOI] [PubMed] [Google Scholar]
- 131.Donoghue AJ, Nadkarni V, Berg RA, Osmond MH, Wells G, Nesbitt L, Stiell IG. Out-of-hospital pediatric cardiac arrest: an epidemiologic review and assessment of current knowledge. Ann Emerg Med. 2005;46(6):512–522. doi: 10.1016/j.annemergmed.2005.05.028. [DOI] [PubMed] [Google Scholar]