Abstract
To study putative brain circuits involved in habituation to stress, rats were exposed daily (30 min for 15 days) to an environment in the presence (Chronic) or absence (Acute) of loud noise (105 dB sound pressure level—SPL A Scale). Behavioral and endocrine measures of stress were taken throughout this habituation period, and both measures displayed strong habituation in the Chronic group. All rats were killed immediately after the day 16 exposure, constituting an acute stressor for the Acute group, and regional brain activity was assessed using c-fos mRNA induction with in situ hybridization. Hearing damage could not easily explain these results because additional rats exposed to a similar stress protocol exhibited no changes in auditory brainstem evoked potentials. c-fos mRNA induction in the central auditory system was similar between the Acute and Chronic groups, particularly at lower auditory processing levels, also arguing against a simple reduction in auditory processing in the chronically stressed rats. However, c-fos mRNA expression was reduced in chronically, as compared to acutely, stressed rats in several regions previously implicated in audiogenic stress (lateral septum, bed nucleus of the stria terminalis, some preoptic areas, and the paraventricular hypothalamic nucleus). Interestingly, the orbitofrontal cortex was the only region displaying higher c-fos mRNA induction in the chronically as compared to acutely stressed rats. This region has connections to several stress-responsive areas and may thus be a critical region actively inhibiting stress.
Keywords: Adaptation, Behavior, BNST, Corticosterone, Immediate-early genes, Noise
INTRODUCTION
Acute stress induces a number of endocrine, physiologic, and behavioral responses that are generally aimed at coping with the specific challenges encountered (Levine and Ursin, 1991; Ursin and Olff, 1993; Akil and Morano, 1995). However, when stressors are repeatedly experienced, these responses can lead to several pathological changes (physical and psychological). Under certain conditions, the impact of repeated challenges is reduced through adaptive mechanisms, generally termed habituation (Christoffersen, 1997), that act to minimize further physical and psychological insult. In general, habituation to glucocorticoid and catecholamine release, some of the most widely studied stress responses, often develop following the relatively predictable exposures of experimental animals to the same (homotypic) stressor (McCarty et al., 1992; Marti and Armario, 1998). Importantly, these responses are either normal or facilitated in habituated animals exposed to a different (heterotypic), not previously experienced stressor (Marti and Armario, 1998). These findings have led to the suggestion that habituation occurs in the central limb of stress-responsive circuits (Akana and Dallman, 1997), thus allowing the hypothalamo–pituitary–adrenocortical (HPA) axis to respond normally, or even become sensitized, to new challenges.
In an attempt to define the brain centers associated with habituation to intermittent chronic stress, a number of investigators have measured brain activation using immediate–early gene induction (Melia et al., 1994; Umemoto et al., 1994; Watanabe et al., 1994; Chen and Herbert, 1995; Stamp and Herbert, 1999). These studies have shown that c-fos induction was most reliably reduced in the paraventricular nucleus of the hypothalamus (PVN), which controls the activity of the HPA axis, in chronically stressed rats. Because the neural afferents that control PVN activity, associated with the stressors in the above studies are poorly understood, these studies did not consistently single out activity changes in specific afferents linked with the PVN.
The aim of the present study was thus to determine how intermittent chronic stress would alter the brain activity, as measured by c-fos mRNA induction, in response to a stressor with a characterized set of PVN afferents. Recently, audiogenic stress was associated with activity in restricted forebrain areas (Campeau and Watson, 1997) that project directly to the PVN. In turn, many of these forebrain regions receive auditory information from the thalamus (Campeau and Watson, 2000), which is necessary for audiogenic stress-induced corticosterone release (Campeau et al., 1997). This circuit thus provides a background against which, activity changes in afferents to the PVN can more directly be tested in relation to chronic stress. One concern that needed to be addressed was whether repeated exposure to loud noise that produces habituation (Armario et al., 1984), results from progressive peripheral hearing damage. To test this possibility, the hearing thresholds of rats were tested prior to, and after repeated audiogenic stress, by measuring auditory-evoked brainstem potentials (Jewett et al., 1970; Yamasoba and Dolan, 1997). These potentials globally measure the activity of the auditory nerve and lower auditory brainstem structures, thereby reflecting possible peripheral damage to cochlear hair cells. The generalization of habituation to intermittent chronic audiogenic stress was also tested across different response systems by measuring corticosterone release and behavioral activity (Irwin et al., 1989; Segal et al., 1989; Britton et al., 1992; Campeau and Watson, 1997).
MATERIALS AND METHODS
Subjects
Twenty-six naive male albino Sprague–Dawley rats weighing 250–300 g (Charles River, Kingston, NY) were used. They were housed in plastic cages (20 × 25 × 50 cm) in pairs with water and laboratory chow continuously available. All rats were maintained on a 12:12 h light–dark cycle (lights on at 7:00 a.m.) and acclimatized to the colony room for a week before the experiments began. The background noise level in the quiet animal colony averaged approximately 50 dB (decibel—A scale) SPL (sound pressure level). All procedures were carried out between 7:00 a.m. and 1:00 p.m. All experiments and procedures performed were approved by the Animal Care and Use Committee of the University of Michigan and conformed to the United States of America National Institute of Health guidelines involving vertebrate animals in research.
Apparatus
The acoustic chamber was located in an adjacent room to the colony, and consisted of a ventilated and acoustically insulated wooden black enclosure (1.2 × 1.0 × 1.0 m) vertically divided by a shelf in the middle so that, up to six rats could be individually housed in clear plastic cages (20 × 25 × 50 cm). The enclosure was illuminated by four 15 W incandescent light bulbs, two in the upper corner of each shelf. The ceiling of each shelf was fitted with two 16 cm Kenwood three-way speakers, providing the auditory stimuli. White noise (0.2–20 kHz) was produced by a General Radio Random-Noise Generator (Type 1390-A), and amplified (SONY STR-D315 Receiver) to an intensity of 105 dB (A) SPL. Noise intensity was measured by placing a Radio Shack Realistic Sound Level Meter (A scale; no 33-2050) in the cages at several locations and taking an average of the different readings. The noise level provided by the ventilating fans and the white noise was approximately 60 dB (A) SPL, which will be referred to here as the experimental background noise level.
Behavioral Procedures
Rats were handled for approximately 2 min and placed in the experimental enclosure for 10 min daily for eight consecutive days. Twenty-four hours later, and for a total of 15 successive days, rats were placed in the experimental enclosure for 30 min. One group of rats (acute, n = 6) simply remained in the enclosure under experimental background noise level. Another group (chronic, n = 6) received white noise at an intensity of 105 dBA SPL. During the last 20 min of enclosure placement, the behavior of all rats was scored every 10 s in one of the following two categories: immobile (freezing posture or sitting posture with head up and no detectable movement except for breathing), or active (grooming, rearing, locomoting). On days 1, 4, and 8, a blood sample (150 μl) obtained from a small nick in a tail vein was collected immediately upon removing the rats from the experimental enclosure. On the afternoon of day 15, all rats were weighed. On the last day (16), rats from the acute and chronic groups were exposed to white noise (105 dBA SPL) for 30 min and were then immediately decapitated following noise exposure. Trunk blood was collected, the brains and pituitaries were rapidly removed and frozen in chilled isopentane (−40°C), and the adrenal glands and thymus glands were harvested for wet weight measurement. Rats that were not handled or exposed to the experimental enclosure served as naive controls (n = 6) and were treated as described above, immediately upon removal from the colony room on day 16.
To test for potential hearing loss produced by this chronic intermittent loud noise exposure, two additional groups of rats (n = 4/group) were subsequently used. They were handled and placed in the experimental enclosure for 10 min daily for eight consecutive days. On the last handling day, hearing acuity of the rats was tested using auditory brainstem evoked potentials (ABR; see procedure below). Twenty-four hours later, and for a total of eight successive days, rats were placed in the experimental enclosure for 30 min. One group of rats (no noise, n = 4) simply remained in the enclosure under experimental background noise level. Another group (noise, n = 4) received white noise at an intensity of 105 dBA SPL. All rats were returned to the colony immediately after the 30 min exposure. On days 1, 3, and 8, hearing acuity was tested using ABR 60 min following removal from the experimental enclosure.
Corticosterone Radioimmunoassay
Blood was collected into ice-chilled tubes containing EDTA. Blood samples were centrifuged at 1500 g for 10 min, the plasma was pipetted into 0.5 ml Eppendorf microcentrifuge tubes, and stored at −20°C until assayed.
Corticosterone was measured by radioimmunoassay using a specific rabbit antibody raised in our laboratory, with less than 3% crossreactivity with other steroids (Dr Dana Helmreich, personal communication). Plasma samples were diluted 1:100 in 0.05 M sodium phosphate buffer containing 0.25% bovine serum albumin (BSA) pH 7.4, and corticosterone separated from binding protein by heat (70°C, 30 min). Duplicate samples of 200 μl to which 50 μl of trace (3H-corticosterone; Amersham 50 Ci/mmol, 10,000 cpm/tube) and 50 μl of antibody (final concentration 1:12,800) were incubated at 4°C overnight. Separation of bound from free corticosterone was achieved by adding 0.5 ml of chilled 1% charcoal –0.1% dextran mixture in buffer for 10 min at 4°C and centrifuged for 10 min at 3000 rpm (Sorvall RC-5B). The supernatant was poured into 4 ml scintillation fluid and bound 3H-corticosterone counted in a Packard CA2000 liquid scintillation analyzer and compared to a standard curve (range: 0–80 μg/dl). All samples were measured simultaneously to reduce interassay variability; within assay variability was less than 5%.
Auditory Brainstem Evoked (ABR) Potentials Measurements
Hearing threshold was evaluated with ABR (Jewett et al., 1970; Yamasoba and Dolan, 1997), before and after the first, third and eighth noise exposure to establish baseline thresholds and any subsequent changes. All ABR measurements were performed by a person who was not familiar with the treatments. Rats were anesthetized with a cocktail of 83% ketamine, 8% xylazine, and 8% acepromazine (1.0 ml/kg, i.m.). When unconscious, each rat was placed in an electrically shielded, sound-attenuating chamber (Acoustic Systems, Austin, TX). Needle electrodes were placed subcutaneously below the tested ear (neg), at the vertex (pos) and below the contralateral ear (ground). The electrodes were connected to a differential amplifier (P15 Grass amplifier, Grass Instruments, Boston, MA) with filter settings of 0.3–3.0 kHz and a gain of 100. The output of the amplifier was led to a custom designed band pass filter amplifier (0.3–3.0 kHz × 100 gain) for a total amplification of 10 k. The output of the amplification system was led to the A/D converter. The ABR waveforms and acoustic stimuli were analyzed and controlled by Tucker–Davis Technologies Data Acquisition System (TDT, Gainesville, FL). The ABR waveforms were generated from 1024 averaged responses. The sound stimulus consisted of 15 ms tone bursts, with a rise–fall time of 1 ms at 4, 12, and 24 kHz delivered from a shielded Beyer earphone via a tube into the ear canal. Calibration of sound stimuli was performed in a volume approximating the external ear canal and expressed as dB SPL. The sound intensity was initially varied in 10 dB steps from 85 dB SPL and in 5 dB steps near the threshold. Hearing threshold was estimated between the last two levels in which a response was present and then absent.
In Situ Hybridization Histochemistry
After rapid decapitation, brains were removed and frozen in isopentane chilled to −40 to −50°C, and stored at −80°C. Ten micron coronal sections were then cut on a Bright cryostat, thaw mounted onto polylysine coated slides, and stored at −80°C until further processed. Slides were fixed in a buffered 4% paraformaldehyde solution for 1 h. Tissue sections were then deproteinated with Proteinase K (1.0 μg/ml) for 10 min at 37°C. The slides were then rinsed in H2O for 5 min, acetylated in 0.1 M triethanolamine containing 0.25% acetic anhydride for 10 min, rinsed for an additional 5 min in H2O, and dehydrated in a progressive series of alcohols.
35S-labeled cRNA probes were generated for c-fos from cDNA subclones in transcription vectors using standard in vitro transcription methodology. The rat c-fos cDNA clone (courtesy of Dr T. Curran, St Jude Children’s Research Hospital) was subcloned in pGem3Z (courtesy of Dr C.A. Fox, University of Michigan) and cut with HindIII to yield a 680 nt cRNA probe. Riboprobes were labeled in a reaction mixture consisting of 1 μg linearized plasmid, 1X T7 or SP6 transcription buffer (BRL), 125 μCi 35S-UTP, 150 μM NTPs–UTP, 12.5 mM dithiothreitol, 20 U RNase inhibitor, and 6 U polymerase. The reaction was allowed to proceed for 120 min at 37°C, and probe was separated from free nucleotides over a Sephadex G50-50 column. Riboprobes were diluted in hybridization buffer to yield approximately 1.5 × 106 dpm/30 μl buffer. The hybridization buffer consisted of 50% formamide, 10% dextran sulfate, 2X SSC, 50 mM sodium phosphate buffer (pH = 7.4), 1X Denhardt’s solution, and 0.1 mg/ml yeast tRNA. Diluted probe (30 μl) was applied to each slide and sections were coverslipped. Slides were placed in sealed plastic boxes lined with filter paper moistened with 50% formamide in 50 mM sodium phosphate buffer, and were subsequently incubated for 12 h at 55°C. Coverslips were then removed, and slides were rinsed several times in 2X SSC (sodium saline citrate buffer). Slides were then incubated in RNase A (200 μg/ml) for 60 min at 37°C, washed successively in 2X, 1X, 0.5X and 0.1X SSC for 5–10 min each, and washed in 0.1X SSC for 60 min at 65°C. Slides were subsequently rinsed in fresh 0.1X SSC, dehydrated in a graded series of alcohols, and exposed to Kodak XAR X-ray film.
Control experiments were performed on tissue sections pre-treated with RNase A (200 μg/ml at 37°C for 60 min) prior to hybridization; this treatment prevented labeling. Alternatively, some control sections were hybridized with the sense cDNA strands, which in all cases did not lead to significant hybridization to tissue sections.
Importantly, three to five slides for a given brain region from each rat included in the study were processed simultaneously to allow direct comparisons in the same regions. Multiple in situ hybridizations were thus performed at different levels of the brain with all animals represented to reduce the effects of technical variations within regions. Sections from all rats in the same region were all exposed on the same X-ray film to further minimize variations. Semi-quantitative analyses were performed on digitized images from X-ray films in the linear range of the gray values obtained from our acquisition system (Northern Light lightbox model B 95, a Pulnix TV camera model TM-745 fitted with a Nikkor 55 mm lens, connected to an A/D converter onboard a Macintosh Quadra 840AV, captured with NIH Image v1.62). Signal pixels in a region of interest were defined as being 3.5 standard deviations above the mean gray value of a cell poor area close to the region of interest. The number of pixels and the average pixel values above the set background were then computed for each regions of interest, and multiplied, giving an integrated mean gray value measure. An average of four–eight measurements were made on different sections (which included bilateral counts made in all cases), for each region of interest, and these values were further averaged to obtain a single integrated mean gray value per region for each rat. Sections undergoing in situ hybridization were stained with cresyl violet and used extensively in the determination of regional boundaries on the digitized images.
Statistics
A repeated-measures (days) analysis of variance (ANOVA) with Treatments (Acute vs. Chronic) as a between-subjects variable was performed on the group means of active behaviors over the last 20 min periods of each session. This analysis was followed by independent t-tests on each day to determine the source of the significant interaction obtained with the initial ANOVA. One-way ANOVA was performed on body, adrenal and thymus weights. A repeated-measures (days) ANOVA with treatments (Acute vs. Chronic) as a between-subjects variable was performed on corticosterone values, followed by independent t-tests on each sampling day to determine the source of the significant interaction obtained with the initial ANOVA. The auditory brainstem evoked potentials were assessed using a repeated-measures ANOVA with frequency (4, 12, and 24 kHz) and days (Baseline, 1, 4, and 8) as within-subjects variables and treatments (background noise and loud noise) as between-subjects variable. Statistical significance for these tests were set to p = 0.05.
One-way ANOVAs were performed on the mean integrated densities obtained from each region where c-fos mRNA induction was measured. This was followed by Tukey’s HSD post-hoc multiple means comparison to determine more exactly the source of the differences obtained with the initial ANOVA. Given the number of tests performed, the statistical significance criterion was raised to p = 0.01 for the initial ANOVAs, but the ensuing conservative Tukey’s HSD post-hoc multiple means comparisons criterion was left at p = 0.05 for regions initially found to show significant c-fos mRNA differences between the treatment groups.
RESULTS
Behavior
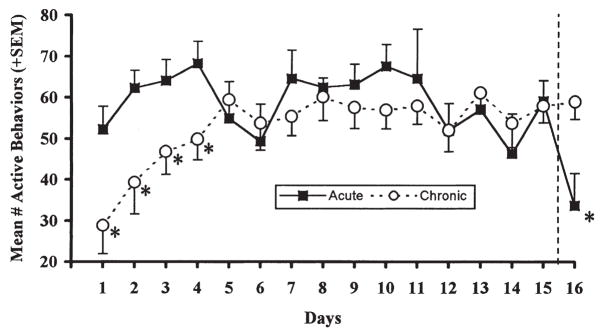
Figure 1 shows that the mean number of active behaviors in the chronically exposed rats was significantly reduced during the initial days of loud noise exposure, compared to the rats in the background noise condition (Acute group). This impression was statistically verified by a significant days × treatments interaction, [F(15, 150) = 1.86, p = 0.032], indicating that the initially reduced activity of the chronically exposed rats disappeared with repeated loud noise exposure. Additional independent t-tests on each day revealed that the Chronic group displayed significantly lower activity during the last 20 min presentation of the loud noise compared to the Acute group on the first four days (all ps < 0.05), but not thereafter. On the last exposure day (day 16), upon the first loud noise exposure for the Acute group, their activity level was significantly reduced compared to the chronic group [t(10) = 2.83, p = 0.018].
FIGURE 1.
Mean number of active behaviors (± SEM) observed during the last 20 min of daily loud noise (105 dBA SPL) exposure (Chronic) or experimental cage placement without loud noise (Acute) for 15 consecutive days. On day 16, the same measure is represented when both groups were exposed to loud noise. *p < 0.05 compared to Acute or Chronic group, as indicated.
Body, Adrenal and Thymus Weights
Body weight was measured 24 h prior to killing, and the several organs were weighed immediately after killing in the Acute, Chronic and Naïve groups, respectively. These results are presented in Table I. One-way analysis of variance on body [F(2,15) = 1.33, p = 0.30], adrenal [F(2,15) = 0.56, p = 0.58], and thymus weights [F(2, 15) = 0.79, p = 0.47], provided no significant group differences among any of these measures. The results were similar, when the adrenal and thymus weights were converted to mg/body weight.
TABLE I.
Body, adrenal and thymus weights (mean ± SEM)
| Body (g) | Adrenals (mg) | Thymus (mg) | |
|---|---|---|---|
| Naive | 383.3 (±6.42) | 50.6 (±2.85) | 677.9 (±31.49) |
| Acute | 374.7 (±7.33) | 47.4 (±2.34) | 721.3 (±24.69) |
| Chronic | 366.3 (±8.28) | 46.9 (±2.84) | 654.2 (±52.70) |
No significant differences were observed for any of the body or organ wet weights among the Naive (n = 6), Acute (n = 6) and Chronic (n = 6) groups.
Corticosterone
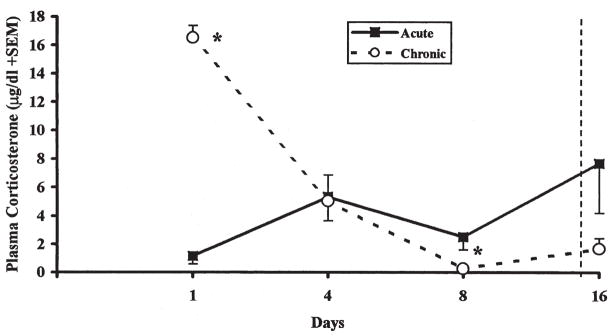
Figure 2 depicts the plasma corticosterone levels, immediately after removal from the experimental enclosure on days 1, 4, 8 and 16. Similar to the activity measure, a significant days × treatment interaction [F(3, 30) = 20.41, p < 0.001], indicated that the initially elevated plasma corticosterone levels during the initial day of loud noise exposure in the Chronic group significantly decreased with additional loud noise exposure. Indeed, these levels were reduced to the levels of the Acute group on day 4 [t(10) = 0.14, p > 0.05], and reliably lower than the Acute group on day 8 [t(10) = 2.69, p = 0.023]. On day 16, the first and only exposure of the Acute rats to loud noise, the high variability among rats in this group precluded the demonstration of significantly higher plasma corticosterone levels compared to the repeatedly exposed rats [t(10) = 1.84, p > 0.05].
FIGURE 2.
Plasma corticosterone concentration (μg/dl ± SEM) measured immediately following 30 min loud noise exposure (Chronic) or experimental cage placement (Acute) on days 1, 4, and 8. Plasma corticosterone concentrations were measured again on day 16, when both groups were exposed to loud noise (right of dashed line). *p < 0.05 compared to Acute group.
Auditory Brainstem Evoked (ABR) Potentials
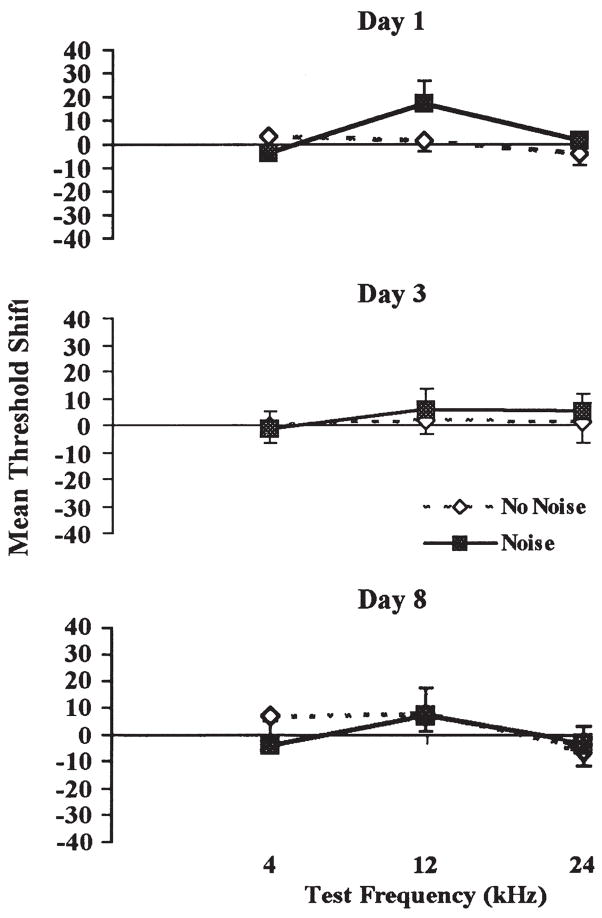
Auditory brainstem evoked potentials that were measured in a group of rats, repeatedly exposed to the experimental background (no noise) and another group, repeatedly exposed to loud noise (noise), did not differ initially in auditory thresholds at any of the three frequencies (4, 12, and 24 kHz) tested [F(1, 6) = 0.30, p > 0.05], prior to any exposure (data not shown). Likewise, no evidence of threshold shifts emerged in response to repeated loud noise exposure, as determined by a repeated-measures ANOVA with days, frequencies and treatments, [F(6, 36) = 1.50, p > 0.05]. Mean group changes (shifts) in auditory thresholds, computed by subtracting each animal’s baseline auditory threshold measurement from each subsequent test day measurement at each frequency, are illustrated in Fig. 3.
FIGURE 3.
Mean threshold shifts (dB) of auditory brainstem potentials in response to pure tones of 4, 12, and 24 kHz, 1 h after the end of 30 min loud noise (105 dBA SPL) exposure (Chronic Group) or experimental cage placement (Acute Group) on days 1 (top), 3 (middle), and 8 (bottom). No significant threshold shifts were observed at any time or frequency.
c-fos mRNA Induction
The levels and statistical results of the c-fos mRNA induction measured in several brain areas of naïve, acutely or chronically treated rats following the last 30 min of loud noise exposure on day 16 are presented in Table II. The regionally measured c-fos mRNA induction generally fell within three distinct patterns. First, a number of brain areas failed to show significant c-fos mRNA induction in acutely and chronically exposed rats compared to naïve animals (see Table II). Second, additional brain regions measured displayed significant c-fos mRNA induction in both the acutely and chronically treated rats compared to naïve controls. These regions included most of the lower auditory brain areas quantified (the cochlear nuclei, nucleus of the trapezoid bodies, superior olivary complex, nuclei of the lateral lemniscus, and the external and central nuclei of the inferior colliculus), many hypothalamic areas (the lateral and posterior aspects, the anterior area, and the ventromedial and dorsomedial nuclei), suprachiasmatic nucleus, the cingulate cortex, anteroventral thalamic nucleus, the lateral geniculate nucleus, the dentate gyrus, and the basomedial nucleus of the amygdala. Illustrations of these results in some brain areas are provided in Fig. 4.
TABLE II.
Mean integrated gray values/100 presented as arbitrary units
| Experimental group means (± SEM) |
||||||
|---|---|---|---|---|---|---|
| Brain region | Naive | Acute | Chronic | |||
| Forebrain | ||||||
| Nucleus accumbens* | 15 | (7.3) | 133† | (29) | 44 | (9.0) |
| Anterior BNST* | 1.3 | (0.3) | 32† | (11) | 4.4 | (0.6) |
| Caudate nucleus dorsal | 21 | (3.9) | 69 | (18) | 67 | (12) |
| Caudate nucleus ventral | 9.3 | (3.2) | 13 | (2.9) | 12 | (1.6) |
| Medial septum | 3.8 | (1.3) | 7.4 | (1.1) | 4.3 | (0.6) |
| Lateral septum* | 2.6 | (0.4) | 203† | (66) | 55 | (12) |
| Septohypothalamic nucleus | 2.0 | (0.5) | 107 | (52) | 13 | (2.7) |
| Amygdala | ||||||
| Anterior cortical nuclei | 96 | (8.8) | 109 | (9.4) | 112 | (15) |
| Basolateral nucleus | 43 | (6.7) | 80 | (11) | 48 | (8.4) |
| Basomedial nucleus* | 14 | (2.0) | 37‡ | (3.6) | 28‡ | (5.1) |
| Central nucleus | 28 | (3.9) | 40 | (4.9) | 28 | (5.0) |
| Lateral nucleus* | 22 | (4.6) | 114† | (13) | 32 | (2.9) |
| Medial nucleus* | 25 | (3.8) | 64‡ | (7.9) | 43 | (5.9) |
| Hippocampus | ||||||
| Dentate gyrus* | 45 | (4.1) | 80‡ | (8.4) | 90‡ | (9.5) |
| CA1* | 31 | (4.5) | 89‡ | (11) | 57 | (9.0) |
| CA3 | 71 | (12) | 105 | (9.1) | 97 | (12) |
| Cortex | ||||||
| Cingulate* | 77 | (15) | 560‡ | (60) | 421‡ | (121) |
| Infralimbic* | 22 | (3.8) | 106† | (10) | 59 | (14) |
| Insular | 112 | (24) | 174 | (49) | 133 | (34) |
| Orbitofrontal* | 102 | (20) | 329† | (39) | 617¶ | (64) |
| Parietal | 487 | (150) | 711 | (256) | 591 | (219) |
| Piriform | 399 | (65) | 575 | (85) | 565 | (46) |
| Sensory | 1455 | (377) | 2071 | (612) | 1943 | (582) |
| Temporal (Auditory)* | 1530 | (273) | 4011† | (252) | 2140 | (412) |
| Hypothalamus | ||||||
| Anterior area* | 18 | (4.3) | 147‡ | (23) | 115‡ | (14) |
| Arcuate nucleus | 96 | (11) | 86 | (15) | 124 | (15) |
| Dorsomedial nucleus* | 60 | (13) | 312‡ | (65) | 207 | (29) |
| Lateral nucleus* | 21 | (4.6) | 65‡ | (14) | 60‡ | (9.3) |
| Lateral preoptic area* | 1.7 | (0.3) | 14‡ | (3.4) | 9.3 | (2.4) |
| Medial preoptic area* | 1.8 | (0.3) | 79‡ | (24) | 28 | (5.9) |
| Medial preoptic nucleus* | 1.3 | (0.4) | 12† | (2.4) | 5.3 | (0.7) |
| Paraventricular nucleus* | 3.2 | (0.7) | 61† | (24) | 11 | (1.9) |
| Posterior area* | 33 | (6.8) | 151‡ | (10) | 106‡ | (21) |
| Suprachiasmatic nucleus* | 74 | (9.0) | 37‡ | (7.2) | 45 | (8.6) |
| Supramammillary nucleus* | 8.2 | (1.0) | 104† | (18) | 57¶ | (10) |
| Ventromedial nucleus* | 12 | (2.1) | 71‡; | (16) | 49‡ | (7.8) |
| Thalamus | ||||||
| Anterodorsal nucleus | 87 | (15) | 75 | (13) | 68 | (12) |
| Anteroventral nucleus* | 62 | (9.7) | 137‡ | (12) | 141‡ | (18) |
| Lateral geniculate nucleus* | 258 | (35) | 445‡ | (68) | 456‡ | (61) |
| Medial geniculate body* | 123 | (17) | 713† | (40) | 373¶ | (52) |
| Posterior intralaminar nucleus* | 19 | (2.1) | 90† | (6.3) | 33 | (4.9) |
| Subparafascicular nucleus* | 17 | (3.3) | 215† | (19) | 111¶ | (16) |
| Midbrain, Pons, Modulla | ||||||
| Dorsolateral central gray* | 21 | (4.7) | 280† | (18) | 114¶ | (9.1) |
| Lateroventral central gray* | 37 | (9.2) | 132† | (16) | 61 | (12) |
| Cochlear nuclei* | 194 | (38) | 943‡ | (135) | 914‡ | (95) |
| Cuneiform nucleus* | 20 | (3.6) | 136† | (11) | 45 | (6.1) |
| Inferior colliculus cen/dor* | 965 | (109) | 2001‡ | (167) | 1977‡ | (198) |
| Inferior colliculus external* | 1140 | (111) | 2772‡ | (217) | 2311‡ | (131) |
| Nuc lateral lemniscus* | 108 | (16) | 667‡ | (34) | 644‡ | (51) |
| Nuc trapezoid bodies* | 46 | (4.8) | 445‡ | (31) | 383‡ | (43) |
| Superior olivary complex* | 54 | (5.9) | 408‡ | (39) | 372‡ | (20) |
| Locus coeruleus* | 3.6 | (0.6) | 12‡ | (2.3) | 7.5 | (0.7) |
| Raphe dorsal | 29 | (10) | 67 | (15) | 35 | (6.3) |
| Dorsal tegmental nucleus | 26 | (6.6) | 40 | (6.1) | 49 | (12) |
| Ventral tegmental nucleus | 21 | (4.3) | 28 | (0.7) | 28 | (2.8) |
| Cerebellum | ||||||
| Hemispheres* | 2149 | (547) | 4563 | (547) | 5342‡ | (997) |
| Vermis* | 3211 | (667) | 9081‡ | (1146) | 10,756‡ | (1283) |
Mean integrated gray values (divided by 100) measured in the Naïve (n6), Acute (n6), and Chronic (n6) groups (SEM).
One-way ANOVA, p ≤ 0.02 (all significant areas are presented in bold characters to facilitate the visualization of areas activated by loud noise).
Tukey’s HSD multiple means comparisons: p0.05 vs. Naive and Chronic groups.
Tukey’s HSD multiple means comparisons: p0.05 vs. Naive group.
Tukey’s HSD multiple means comparisons: p0.05 vs. Naïve and Acute groups.
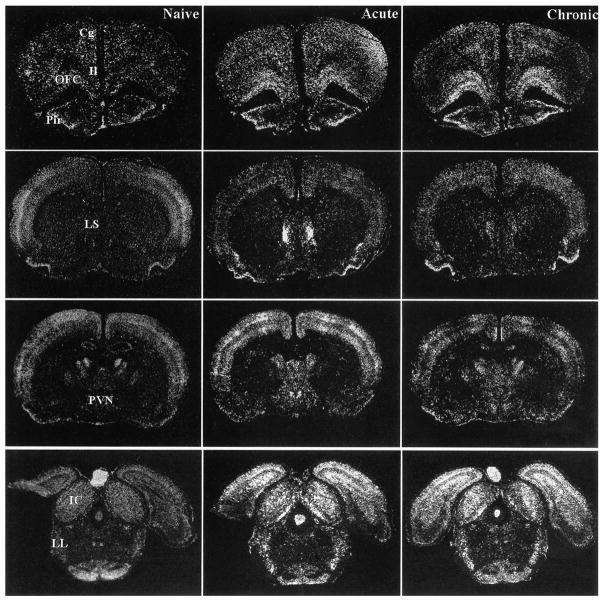
FIGURE 4.
Representative autoradiograms of coronal brain sections displaying c-fos mRNA expression in Naïve (left), Acute (middle), and Chronic (right) rats at different levels of the neuraxis. The orbitofrontal cortex (OFC) showed significantly higher c-fos mRNA induction in Chronic animals than in Acute or Naïve rats. Many regions, particularly at lower auditory levels, showed similar c-fos mRNA induction in Acute and Chronic rats, as compared to Naïve animals, including the inferior colliculus (IC) and the nuclei of the lateral lemniscus (LL). Additional regions, particularly those found to be specifically responsive to stress, showed lower c-fos mRNA induction in Chronic, as compared to Acute rats, with the Chronic values not being different from Naïve values in many instances. Some examples included the lateral septum (LS) and paraventricular nucleus of the hypothalamus (PVN). Other abbreviations: Cg: cingulate cortex; Il: infralimbic cortex; Pir: piriform cortex.
Many of the brain regions analyzed for c-fos mRNA induction fell into the third category where only rats from the acutely exposed group demonstrated reliable induction above, both the chronically exposed and naïve rats. In most of these instances, the naïve and chronically exposed rats did not differ from each other (but see Table II for exceptions). These regions included the infralimbic cortex, the temporal (auditory) cortex, lateral septum, nucleus accumbens (core and shell analyzed together), anterior bed nucleus of the stria terminalis, the medial and lateral nuclei of the amygdala, several preoptic regions (medial nucleus, medial and lateral area), paraventricular nucleus of the hypothalamus (parvocellular region), supramammillary nucleus, posterior intralaminar nucleus, subparafascicular nucleus, medial geniculate body, the hippocampal CA1 region, cuneiform nucleus, ventral and dorsal periaqueductal gray, and the locus coeruleus. Some brain areas with these characteristics are illustrated in Fig. 4. Finally, a single brain area encompassing the region of the claustrum and orbitofrontal cortex displayed significantly higher c-fos mRNA induction in the chronically exposed group compared to the acutely treated and naïve rats, which is illustrated in Fig. 4. A similar, although non-significant trend was observed for the cerebellar vermis and its hemispheres.
DISCUSSION
The results of the present study indicated that the behavioral and endocrine responses associated with loud noise stress (Henkin and Knigge, 1963; Collu and Jequier, 1976; Borrell et al., 1980; Armario et al., 1984; Irwin et al., 1989; Segal et al., 1989; Britton et al., 1992; Campeau and Watson, 1997), were clearly attenuated following repeated intermittent daily presentation, following a classic pattern of habituation (Christoffersen, 1997). The rate of behavioral and endocrine habituation appeared relatively similar, even if the paucity of blood sampling prevented accurate rate delineation for corticosterone compared to the behavioral activity index. Both responses reached levels that were similar to those of non-stressed control animals by approximately the eighth exposure. However, the rate and final level of response habituation are likely to vary with stimulus characteristics and response systems (Marti and Armario, 1998), even within the auditory modality (Campeau, unpublished observations).
Although habituation to loud noise has been demonstrated previously, gradual hearing impairments might have led to the gradual response decrements observed (Armario et al., 1984). Measurement of ABR potentials in the present study ruled out the possibility that the observed habituation resulted from a gross and gradual peripheral hearing impairment, as shown by the similarity of auditory thresholds prior to, and up to 8 days after daily loud auditory stimulation. This is a time at which, with the parameters used in the present study, both the behavioral and endocrine stress responses were nearly indistinguishable from those of the background noise controls. Since possible hearing damage was not assessed for the entire 16 days, however, it is conceivable that the c-fos mRNA results could reflect such damage. This appears unlikely, given the fact that c-fos mRNA did not differ between the acute and chronic animals in most auditory structures analyzed. Also, because additional studies indicated that audiogenic stress-habituated animals respond with a normal rise in corticosterone to restraint or ether stress (Campeau, unpublished observations), fatigue or neurotransmitter depletion cannot readily explain the reduced behavioral and endocrine responses to repeated audiogenic stress.
The effect of prior, repeated audiogenic stress experience was assessed on the last experimental day by comparing the responses of the Chronic group to that of an otherwise identically-treated group receiving the loud noise for the first time (Acute group). The Acute rats responded to loud noise with a significant reduction in activity level, compared to the Chronic rats, in a fashion similar to the Chronic rats during their first exposure. However, they did not show a significant rise in corticosterone levels compared to the habituated rats on the last experimental day, and the levels did not reach the high levels observed during the first loud noise exposure of the chronically treated rats. A high degree of variation was obtained on this measure in the Acute rats, which partially accounts for this failure. Nevertheless, the behavioral data, combined with the pattern of c-fos mRNA induction in the acutely treated rats (discussed below), suggest that the loud noise was stressful to these animals.
Acute Audiogenic Stress and c-fos mRNA Induction
As reported previously, c-fos mRNA was expressed at very low levels in the brains of naïve rats (Campeau and Watson, 1997; Campeau et al., 1997; Campeau and Watson, 2000). However, significant c-fos mRNA induction was observed in several forebrain areas, including frontal, septal, amygdaloid, and medial basal areas, and several hypothalamic nuclei, of the acutely treated rats. Most auditory regions analyzed displayed reliable c-fos mRNA expression. A few key brainstem regions associated with defensive behavior (periaqueductal gray) also showed reliable c-fos mRNA induction. About a third of the regions investigated did not display reliable c-fos mRNA induction. This was the case in many cortical areas, several amygdaloid nuclei, the basal ganglia, and some brainstem nuclei. Overall, the brain activation pattern of the acutely exposed rats suggests that, as a group, these animals were stressed, in agreement with their behavioral activity data.
Habituation to Audiogenic Stress and c-fos mRNA Induction
One of the main goals of the present study was to determine the regional pattern of c-fos mRNA induction in the brains of animals repeatedly exposed to audiogenic stress. In the present study, several quantified brain areas of the chronically treated rats displayed a significant reduction in c-fos mRNA induction, when compared to the acutely treated animals. In many instances, this reduction was such that the c-fos mRNA levels from chronic rats were not different from that of the control naïve rats. These regions included forebrain (infralimbic cortex, lateral septum, nucleus accumbens, anterior bed nucleus of the stria terminalis, medial and lateral amygdaloid nuclei) hypothalamic (many preoptic nuclei, paraventricular nucleus of the hypothalamus, and supramammillary nucleus), and brainstem nuclei (posterior intralaminar, subparafascicular, and cuneiform nuclei, medial geniculate body, and periaqueductal gray). Some of these regions have specifically been reported to show a reduction of c-fos mRNA or Fos protein expression after repeated aggressive encounters (Martinez et al., 1998; Kollack-Walker et al., 1999). Additional studies employing repeated restraint/immobilization, have reported consistent regional reduction in immediate-early genes induction in many of the same regions found in the present study (Melia et al., 1994; Umemoto et al., 1994; Watanabe et al., 1994; Chen and Herbert, 1995; Stamp and Herbert, 1999). Importantly, many of the regions displaying reduction in c-fos mRNA induction in the present study were shown to project directly to the hypophysiotropic paraventricular nucleus, and were active during audiogenic stress (Campeau and Watson, 2000), providing evidence that the central limb of the HPA axis is significantly regulated by repeated stress.
In agreement with the above studies using different stressors, many regions examined in the present study displayed significant c-fos mRNA induction that was not reduced by repeated audiogenic stress. Many of these regions were associated with auditory structures, especially at the lower levels of the neuraxis, beginning with the cochlear nuclei. This suggests that habituation to audiogenic stress is not a simple reduction in sensory activation, but might involve active inhibition at more central levels, where c-fos mRNA induction was reduced. Other regions displaying this non-habituated pattern also included many hypothalamic areas. These regions are not likely to be responsible for the response attenuation produced by chronic stress, at least for the behavioral and endocrine responses measured.
The pattern of c-fos mRNA induction observed in the present study is in many respects similar to results obtained in acutely stressed rats after lesions of the auditory thalamus (Campeau et al., 1997). These lesions abolished corticosterone release in response to acute audiogenic stress, in a manner similar to the effects of repeated audiogenic stress. Following auditory thalamic lesions, many of the same regions observed in the present study displayed reduced c-fos mRNA induction, whereas others showed similar induction levels as obtained in control animals. It was hypothesized that auditory thalamic lesions “disconnect” the forebrain from auditory inputs, thereby depriving stress-sensitive brain regions from the “stressful” character of loud noise. The present results might suggest that repeated audiogenic stress functionally achieves a similar brain state, somehow filtering the information and attenuating the “stressful” properties of loud noise.
Finally, a distinctive finding of the present study consisted in the observation of increased c-fos mRNA induction in the orbitofrontal cortical region of chronically, as compared to acutely treated, and naïve, rats. The deduced increase in activity in this region is interesting because it has several reciprocal connections with cortical, limbic, thalamic, hypothalamic, and brainstem areas (van der Kooy et al., 1982; Saper, 1985; Terreberry and Neafsey, 1987; Hurley et al., 1991; Cavada et al., 2000), many of which showed a reduction in c-fos mRNA induction in the present study. In addition to an important role ascribed to the orbitofrontal cortex in learning and memory (Bachevalier and Mishkin, 1986; Frey and Petrides, 2000; Schoenbaum et al., 2000), this region appears critical for appropriate response selection and inhibition based on discriminative signal contingencies (Jones and Mishkin, 1972; Eichenbaum et al., 1983). It is conceivable that an increase in activity of the orbitofrontal area helps to inhibit a range of responses in the endocrine, autonomic, and behavioral domains, based on prior experience with a repeatedly encountered stressful stimulus. Of additional interest, this region has been reported to be abnormal in the brain of clinically depressed patients (Drevets et al., 1997; Rajkowska et al., 1999). Since mood disorders such as major depression often develop after extended periods of stressful life events, a dysfunction in this region may be partly responsible for maladaptive responses that may lead to the elaboration of depressive symptoms.
A trend for an increase in c-fos mRNA induction in the cerebellar vermis and hemispheres of the chronically exposed rats was also observed. The cerebellar vermis, not its hemispheres, has previously been implicated in long-term habituation of the acoustic startle reflex (Leaton and Supple, 1986). Interestingly, the vermis is particularly important during the development of habituation, as opposed to the expression of habituation once it has been acquired (Lopiano et al., 1989). It is thus conceivable that the unreliable increase in c-fos mRNA induction in the present study might be related to the lesser function of the cerebellar vermis, once the chronically exposed rats had likely completely acquired the habituated responses by the end of the experiment. Alternatively, an increase in c-fos mRNA induction in the cerebellum of chronic rats might reflect the simple fact that rats presented with loud noise for the first time display reduced locomotor activation, as compared with the chronically treated rats. Thus, the roles of the cerebellum and orbitofrontal cortex deserve further attention with regard to the development and expression of habituation to chronic stressful stimuli.
Acknowledgments
S.J.W. was the recipient of NIMH grant MH-42251, and S.C. received a Post-doctoral Fellowship from the Medical Research Council of Canada.
References
- Akana SF, Dallman MF. Chronic cold in adrenalectomized, corticosterone (B)-treated rats: facilitated corticotropin responses to acute restraint emerge as B increases. Endocrinology. 1997;138:3249–3258. doi: 10.1210/endo.138.8.5291. [DOI] [PubMed] [Google Scholar]
- Akil H, Morano M. Stress. In: Bloom F, Kupfer D, editors. Psychopharmacology: The Fourth Generation of Progress. Raven Press; New York: 1995. pp. 773–785. [Google Scholar]
- Armario A, Castellanos JM, Balasch J. Adaptation of anterior pituitary hormones to chronic noise stress in male rats. Behav Neural Biol. 1984;41:71–76. doi: 10.1016/s0163-1047(84)90745-3. [DOI] [PubMed] [Google Scholar]
- Bachevalier J, Mishkin M. Visual recognition impairment follows ventromedial but not dorsolateral prefrontal lesions in monkeys. Behav Brain Res. 1986;20:249–261. doi: 10.1016/0166-4328(86)90225-1. [DOI] [PubMed] [Google Scholar]
- Borrell J, Torrellas A, Guaza C, Borrell S. Sound stimulation and its effects on the pituitary-adrenocortical function and brain catecholamines in rats. Neuroendocrinology. 1980;31:53–59. doi: 10.1159/000123050. [DOI] [PubMed] [Google Scholar]
- Britton KT, Segal DS, Kuczenski R, Hauger R. Dissociation between in vivo hippocampal norepinephrine response and behavioral/neuroendocrine responses to noise stress in rats. Brain Res. 1992;574:125–130. doi: 10.1016/0006-8993(92)90808-m. [DOI] [PubMed] [Google Scholar]
- Campeau S, Watson SJ. Neuroendocrine and behavioral responses and brain pattern of c-fos induction associated with audiogenic stress. J Neuroendocrinol. 1997;9:577–588. doi: 10.1046/j.1365-2826.1997.00593.x. [DOI] [PubMed] [Google Scholar]
- Campeau S, Watson SJ. Connections of some auditory-responsive posterior thalamic nuclei putatively involved in activation of the hypothalamo–pituitary–adrenocortical axis in response to audiogenic stress in rats: an anterograde and retrograde tract tracing study combined with Fos expression. J Comp Neurol. 2000;423:474–491. [PubMed] [Google Scholar]
- Campeau S, Akil H, Watson SJ. Lesions of the medial geniculate nuclei specifically block corticosterone release and induction of c-fos mRNA in the forebrain associated with loud noise stress in rats. J Neurosci. 1997;17:5979–5992. doi: 10.1523/JNEUROSCI.17-15-05979.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10:220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Chen X, Herbert J. Regional changes in c-fos expression in the basal forebrain and brainstem during adaptation to repeated stress: correlations with cardiovascular, hypothermic and endocrine responses. Neuroscience. 1995;64:675–685. doi: 10.1016/0306-4522(94)00532-a. [DOI] [PubMed] [Google Scholar]
- Christoffersen GR. Habituation: events in the history of its characterization and linkage to synaptic depression. A new proposed kinetic criterion for its identification. Prog Neurobiol. 1997;53:45–66. doi: 10.1016/s0301-0082(97)00031-2. [DOI] [PubMed] [Google Scholar]
- Collu R, Jequier JC. Pituitary response to auditory stress: effects of treatment with a-methyl-p-tyrosine. Usefulness of a factorial mixed design for statistical analysis. Can J Physiol Pharmacol. 1976;54:596–602. doi: 10.1139/y76-082. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Clegg RA, Feeley A. Reexamination of functional subdivisions of the rodent prefrontal cortex. Exp Neurol. 1983;79:434–451. doi: 10.1016/0014-4886(83)90224-8. [DOI] [PubMed] [Google Scholar]
- Frey S, Petrides M. Orbitofrontal cortex: a key prefrontal region for encoding information. Proc Natl Acad Sci USA. 2000;97:8723–8727. doi: 10.1073/pnas.140543497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkin RI, Knigge KM. Effect of sound on the hypothalamic– pituitary –adrenal axis. Am J Physiol. 1963;204:910–914. doi: 10.1152/ajplegacy.1963.204.4.710. [DOI] [PubMed] [Google Scholar]
- Hurley KM, Herbert H, Moga MM, Saper CB. Efferent projections of the infralimbic cortex of the rat. J Comp Neurol. 1991;308:249–276. doi: 10.1002/cne.903080210. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Segal DS, Hauger RL, Smith TL. Individual behavioral and neuroendocrine differences in responsiveness to audiogenic stress. Pharmacol Biochem Behav. 1989;32:913–917. doi: 10.1016/0091-3057(89)90058-0. [DOI] [PubMed] [Google Scholar]
- Jewett DL, Romano MN, Williston JS. Human auditory-evoked potentials: possible brain stem components detected on the scalp. Science. 1970;167:1517–1518. doi: 10.1126/science.167.3924.1517. [DOI] [PubMed] [Google Scholar]
- Jones B, Mishkin M. Limbic lesions and the problem of stimulus-reinforcement associations. Exp Neurol. 1972;36:362–377. doi: 10.1016/0014-4886(72)90030-1. [DOI] [PubMed] [Google Scholar]
- Kollack-Walker S, Don C, Watson SJ, Akil H. Differential expression of c-fos mRNA within neurocircuits of male hamsters exposed to acute or chronic defeat. J Neuroendocrinol. 1999;11:547–559. doi: 10.1046/j.1365-2826.1999.00354.x. [DOI] [PubMed] [Google Scholar]
- van der Kooy D, McGinty JF, Koda LY, Gerfen CR, Bloom FE. Visceral cortex: a direct connection from prefrontal cortex to the solitary nucleus in rat. Neurosci Lett. 1982;33:123–127. doi: 10.1016/0304-3940(82)90238-5. [DOI] [PubMed] [Google Scholar]
- Leaton RN, Supple WF., Jr Cerebellar vermis: essential for long-term habituation of the acoustic startle response. Science. 1986;232:513–515. doi: 10.1126/science.3961494. [DOI] [PubMed] [Google Scholar]
- Levine S, Ursin H. What is stress? In: Brown MR, Koob GF, Rivier C, editors. Stress: Neurobiology and Neuroendocrinology. Marcel Dekker; New York: 1991. pp. 3–21. [Google Scholar]
- Lopiano L, de’Sperati C, Bergui M, Montarolo PG. Role of the cerebellar vermis in the long-term habituation of the acoustic startle response in the rat. Exp Brain Res. 1989;17:380–384. [Google Scholar]
- Marti O, Armario A. Anterior pituitary response to stress: time-related changes and adaptation. Int J Dev Neurosci. 1998;16:241–260. doi: 10.1016/s0736-5748(98)00030-6. [DOI] [PubMed] [Google Scholar]
- Martinez M, Phillips PJ, Herbert J. Adaptation in patterns of c-fos expression in the brain associated with exposure to either single or repeated social stress in male rats. Eur J Neurosci. 1998;10:20–33. doi: 10.1046/j.1460-9568.1998.00011.x. [DOI] [PubMed] [Google Scholar]
- McCarty R, Konarska M, Stewart RE. Adaptation to stress: a learned response? In: Kvetnansky R, McCarty R, Axelrod J, editors. Stress: Neuroendocrine and Molecular Approaches. Gordon and Breach Science; New York: 1992. pp. 521–535. [Google Scholar]
- Melia KR, Ryabinin AE, Schroeder R, Bloom FE, Wilson MC. Induction and habituation of immediate early gene expression in rat brain by acute and repeated restraint stress. J Neurosci. 1994;14:5929–5938. doi: 10.1523/JNEUROSCI.14-10-05929.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Saper CB. Organization of cerebral cortical afferent systems in the rat. II. Hypothalamocortical projections. J Comp Neurol. 1985;237:21–46. doi: 10.1002/cne.902370103. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Chiba AA, Gallagher M. Changes in functional connectivity in orbitofrontal cortex and basolateral amygdala during learning and reversal training. J Neurosci. 2000;20:5179–5189. doi: 10.1523/JNEUROSCI.20-13-05179.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R, Swick D. Audiogenic stress response: behavioral characteristics and underlying monoamine mechanisms. J Neural Transm. 1989;75:31–50. doi: 10.1007/BF01250642. [DOI] [PubMed] [Google Scholar]
- Stamp JA, Herbert J. Multiple immediate–early gene expression during physiological and endocrine adaptation to repeated stress. Neuroscience. 1999;94:1313–1322. doi: 10.1016/s0306-4522(99)00368-1. [DOI] [PubMed] [Google Scholar]
- Terreberry RR, Neafsey EJ. The rat medial frontal cortex projects directly to autonomic regions of the brainstem. Brain Res Bull. 1987;19:639–649. doi: 10.1016/0361-9230(87)90050-5. [DOI] [PubMed] [Google Scholar]
- Umemoto S, Noguchi K, Kawai Y, Senba E. Repeated stress reduces the subsequent stress-induced expression of Fos in rat brain. Neurosci Lett. 1994;167:101–104. doi: 10.1016/0304-3940(94)91037-5. [DOI] [PubMed] [Google Scholar]
- Ursin H, Olff M. The stress response. In: Stanford SC, Salmon P, editors. Stress: from Synapse to Syndrome. Academic Press; San Diego: 1993. pp. 3–22. [Google Scholar]
- Watanabe Y, Stone E, McEwen B. Induction and habituation of c-fos and zif/268 by acute and repeated stressors. NeuroReport. 1994;5:1321–1324. [PubMed] [Google Scholar]
- Yamasoba T, Dolan DF. Chronic strychnine administration into the cochlea potentiates permanent threshold shift following noise exposure. Hearing Res. 1997;112:13–20. doi: 10.1016/s0378-5955(97)00092-0. [DOI] [PubMed] [Google Scholar]