Abstract
It is well established that calcitonin is a potent inhibitor of bone resorption; however, a physiological role for calcitonin acting through its cognate receptor, the calcitonin receptor (CTR), has not been identified. Data from previous genetically modified animal models have recognized a possible role for calcitonin and the CTR in controlling bone formation; however, interpretation of these data are complicated, in part because of their mixed genetic background. Therefore, to elucidate the physiological role of the CTR in calcium and bone metabolism, we generated a viable global CTR knockout (KO) mouse model using the Cre/loxP system, in which the CTR is globally deleted by >94% but <100%. Global CTRKOs displayed normal serum ultrafiltrable calcium levels and a mild increase in bone formation in males, showing that the CTR plays a modest physiological role in the regulation of bone and calcium homeostasis in the basal state in mice. Furthermore, the peak in serum total calcium after calcitriol [1,25(OH)2D3]-induced hypercalcemia was substantially greater in global CTRKOs compared with controls. These data provide strong evidence for a biological role of the CTR in regulating calcium homeostasis in states of calcium stress.
Key words: calcitonin, knock-out/in, bone histomorphometry
INTRODUCTION
The calcitonin receptor (CTR) is a G protein–coupled cell surface receptor that signals through intracellular activation of adenylate cyclase and phospholipase C.(1) CTRs are expressed in osteoclasts, renal, and neural cells.(1) Calcitonin, a 32 amino acid peptide, acting through interaction with the CTR, is the most potent inhibitor of osteoclastic bone resorption identified to date, both in vitro and in vivo. Calcitonin is secreted primarily by thyroid C cells in response to elevated serum calcium levels but is also secreted by other tissues in the neck and thorax. The main recognized actions of calcitonin in calcium homeostasis are to inhibit bone resorption,(2) to decrease calcium tubular reabsorption in the kidney, thereby increasing urinary calcium excretion,(3) and to regulate 1α,25(OH)2D3 production in the kidney.(4) The use of calcitonin as a therapeutic agent in the treatment of hypercalcemia and bone disease has been severely limited by calcitonin's short duration of action.(5,6)
Whereas it is well established that calcitonin potently inhibits bone resorption through the interaction with the CTR on osteoclasts in vitro, the physiological role of calcitonin remains an area of debate. In an attempt to further elucidate the physiological role of calcitonin, two genetically modified mouse models have been previously generated: a global calcitonin/calcitonin gene–related peptide knockout (CT/CGRP KO)(7) and a model haploinsufficient for the CTR,(8) the phenotypes of which suggest an additional role of the CTR in regulating bone formation. Surprisingly, CT/CGRP KOs were found to have increased trabecular bone volume compared with controls because of an increase in bone formation.(7) However, the interpretation of the physiological actions of calcitonin from the phenotype of the CT/CGRP KO is complicated by the fact that CGRP also has effects on osteoclasts and osteoblasts, as well as potent effects on vascular elements.(9) Furthermore, the initial characterization of the CT/CGRP KO mice was carried out on mice of mixed genetic background. Gagel et al.(10) recently reported that the bone phenotype of increased trabecular bone formation is no longer evident after the backcrossing of the global-CT/CGRP KO mice to a homogeneous C57BL/6 background; however, they instead show an age-related phenotype, which includes increased cortical porosity. We have shown that deletion of one copy of the CTR in mice results in CTR haploinsufficiency with CTR expression decreased by 50%.(8) Despite the potent inhibitory action of calcitonin on osteoclasts, haploinsufficient CTR mice have increased bone formation, with no effect on bone resorption. It has been proposed that the CTR may affect bone formation by influencing coupling between osteoclasts and osteoblasts, secondary to modulating osteoclast activity.(11)
Evidence exists to suggest that calcitonin also plays a role in regulating calcium and conserving bone during times of calcium stress, such as hypercalcemia, pregnancy, and lactation. Serum calcium levels remain elevated for a prolonged period in thyroidectomized patients challenged with a calcium load.(12) This ability of calcitonin to reduce serum calcium levels seems to be dependent on the rate of bone turnover in both humans and rodents. In states of high bone turnover, such as in childhood or patients with malignancy induced hypercalcemia, calcitonin rapidly lowers serum calcium, with little effect observed in adults.(13,14) Calcitonin may also play a physiological role in pregnancy and lactation, because expression of calcitonin in the uterus is required for implantation of the blastocyst,(15) and homozygous deletion of the CTR during embryogenesis is lethal.(8)
It has been suggested that the physiological function of calcitonin may be to protect the bone against bone-resorbing stimuli through its acute inhibition of bone resorption.(16) Despite the many studies investigating the actions of calcitonin on calcium homeostasis and bone metabolism, the physiological role of calcitonin acting through the CTR still remains unresolved. The data from the previously described animal models identifying a possible role for calcitonin and the CTR in controlling bone formation are intriguing but complex. As noted above, studies in the CT/CGRP KOs initially reported increased bone formation; however, these data were confounded by potential effects arising from the deletion of CGRP.(7) Subsequent reports in CT/CGRP KO mice on a homogenous genetic background failed to confirm the previously described anabolic effects of calcitonin/CGRP deletion on bone formation.(10) We have previously shown that mice haploinsufficient for the CTR have increased bone formation with no effect on bone resorption in adult male mice, whereas homozygous deletion of exons 6 and 7 of the CTR is embryonic lethal.(8) Therefore, to clarify the effects of calcitonin through the CTR on bone resorption and to extend the data on its potential role in bone formation, we generated a new, viable genetically modified mouse model using the Cre-loxP system, in which the CTR is globally deleted by >94% but <100% (global CTRKOs). We used our global CTRKOs as a model to further elucidate the physiological role of calcitonin in bone and calcium metabolism, basally and in calcium stress.
MATERIALS AND METHODS
Genetically modified mouse lines
Because the phenotype of genetically modified mice can vary depending on their genetic strain,(17,18) floxed CTR and Cre transgenic mouse lines were backcrossed onto a C57BL/6J background for at least six generations (>98% C57BL/6J) before use in experiments. All C57BL/6J mice were obtained from the Animal Resources Center (Canning Vale, West Australia, Australia). Mice were supplied with tap water and standard chow ad libitum and were housed at 22°C on a 12-h light/dark cycle in standard cages. All procedures involving animals were approved by the Austin Health Animal Ethics Committee.
Generation of floxed CTR mice
Exons 13 and 14 of the CTR gene, which encode the seventh transmembrane domain and the C terminus of the receptor, respectively, together with the 3′ untranslated region (UTR), were targeted for Cre-mediated deletion by insertion of loxP sites (Fig. 1). The targeting construct was generated from 129SvJ mouse genomic DNA using standard restriction enzyme digestion and ligation methods and contained a floxed neomycin resistance (Neo R) cassette and a thymidine kinase cassette to allow for positive and negative selection of homologous recombinants, respectively (Fig. 1). The Neo R cassette was a kind gift from Dr Shelley Ross (Monash Medical Center, Clayton, Victoria, Australia). The targeting construct was introduced into 129SvJ mouse embryonic stem (ES) cells by electroporation. ES cells were subjected to positive and negative selection with 175 μg/ml Geneticin (Gibco Invitrogen, Grand Island, NY, USA) and 200 nM gancyclovir (Roche Diagnostics, Mannheim, Germany), respectively. ES colonies were screened for homologous recombination events and correct integration of the targeting construct at both the 5′ and 3′ ends by PCR and sequencing. PCR conditions are available on request.
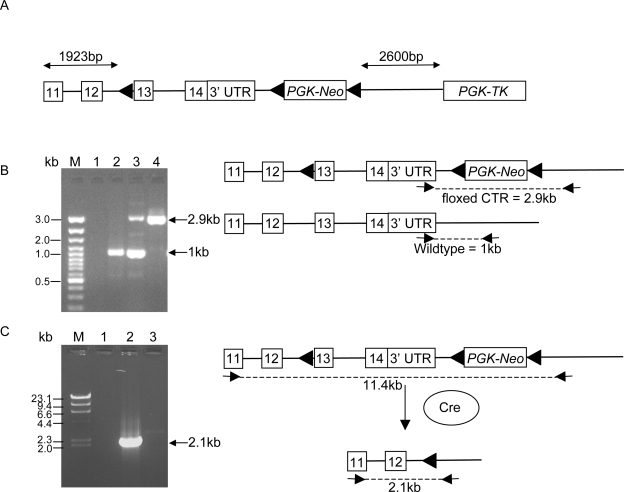
FIG. 1.
(A) Schematic diagram of floxed CTR targeting construct. loxP sites and their orientation are shown as black triangles. PGK-Neo, neomycin-positive selection cassette; PGK-TK, thymidine kinase–negative selection cassette. (B) Location of primers used for PCR analysis to distinguish mice homozygous for the floxed CTR allele from mice heterozygous for the floxed CTR allele. M, molecular weight marker. Lane 1, negative control; lane 2, wildtype control; lane 3, heterozygous floxed CTR, homozygous floxed CTR. (C) Location of primers used for PCR analysis to confirm Cre-mediated deletion of exons 13 and 14 and the 3′UTR of the CTR in global CTRKOs. M, λ-HindIII molecular weight marker. Lane 1, homozygous floxed CTR; lane 2, global CTRKO; lane 3, negative control.
One floxed CTR ES cell clone was expanded and injected into C57BL/6J-derived blastocysts by IngenKO (Monash Medical Center, Clayton, Victoria, Australia). Male chimeras were backcrossed to C57BL/6J female mice resulting in floxed CTR mice.
Global CTRKO mice
To generate global CTRKO mice, floxed CTR mice were bred with CMV-Cre transgenic mice obtained with permission from Dr Ursula Lichtenberg (Institute for Genetics, University of Cologne, Cologne, Germany). These mice express Cre recombinase ubiquitously under the control of the CMV promoter.(19) Het-floxed CTR littermates were used as controls in all analyses.
TRACP-CTRKO mice
To generate KO mice in which the CTR is deleted in osteoclasts, floxed CTR mice were bred with TRACP-Cre transgenic mice, which express Cre under the control of the TRACP promoter.(20)
Routine genotyping
Approximately 100 ng of genomic DNA isolated from tail biopsy was used as a template for PCR genotyping. The primer pair sequences used for genotyping mice were as follows: Cre coding sequence, forward 5′-GCGGCATGGTGCAAGTTGAAT-3′, reverse 5′-ACCCCCAGGCTAAGTGCCTT-3′; primers flanking CTR floxed region to distinguish het- and hom- floxed CTR from wildtype CTR, forward 5′-CTGACTTACCATAACATTGCATGT-3′, reverse, 5′-CGGTGAGTAATGAATGAAGTGAA-3′ (Fig. 1B); Cre-mediated recombined CTR fragment, forward 5′-GCTGGCTGAGTGCAGAAA-3′, reverse 5′-CGGTGAGTAATGAATGAAGTGAA-3′. PCR conditions are available on request.
RNA isolation, cDNA synthesis, and quantitative real-time PCR
Total RNA was isolated from kidney and bone of control and global CTRKO male and female mice at 6 wk of age, as previously described.(21) Total RNA (2 μg) was treated with 2 units of DNase I (DNA–Free Kit; Ambion), and cDNA was synthesized from 1 μg of DNase-treated RNA using random hexamers (Promega, Madison, WI, USA) and M-MLV reverse transcriptase, according to the manufacturer's instructions. Quantitative real-time PCR (Q-PCR) was performed in duplicate using 10 ng cDNA per 25-μl reaction on an Applied Biosystems 7500 Real Time PCR system, using Applied Biosystems Taqman gene expression assays (60 cycles of Q-PCR). A no template control was included in all Q-PCR reactions.
To quantitate CTR mRNA levels in the bone and kidney of female and male control and global CTRKOs, three to eight samples were analyzed per group using a custom designed CTR gene expression assay, which amplifies from exon 13–14 with a probe directed to the exon13/exon14 boundary (forward primer: 5′-GTGGCGACTATCTACTGCTTCTG-'3; reverse primer: 5′-TGAAGCGCCAGTGGACG-3′; probe: 5′-CAACCATGAGGTGCAAGT-3′). Absolute expression was determined using 60 cycles of Q-PCR and the ΔCT method with CTR CT values normalized to eukaryotic 18S rRNA (Assay ID: 4310893E). To quantitate the degree of mRNA deletion of exons 13 and 14 of the CTR in global CTRKOs, 10 ng of cDNA from a global CTRKO was amplified in the presence of decreasing concentrations of control cDNA (10, 5, 1, 0.1, and 0.01 ng). This was performed in cDNA isolated from bone and kidney from two independent control and global CTRKO mice.
Assessment of osteoclast function in vitro
Osteoclasts were derived from bone marrow of control and global CTRKOs, as described.(22) CTR activity was tested on resulting osteoclasts. Culture media was replaced with media containing RANKL alone (50 ng/ml), or RANKL plus 0.01 nM salmon calcitonin (sCT). Cells were incubated for 30 min, fixed with 4% paraformaldehyde for 10 min, and stained with Phalloidin-Texas red (Sigma; 10 μg/ml) for actin ring assembly. Nuclei were stained for 2 min with 4', 6-diamidino-2-phenylindole (DAPI) solution (1 μg/ml) in PBS. Slides were mounted with ProLong Gold, antifade reagent with DAPI (Invitrogen), sealed, and examined by confocal microscopy.
Serum and bone collection
For dynamic histomorphometry experiments, mice received two intraperitoneal injections of 20 mg/kg body weight calcein (Sigma), with a 7-day interval between injections. Three days after the second injection, mice were anesthetized by isoflurane, and blood was collected through the tail vein for serum PTH and serum calcium analyses and by cardiac puncture for remaining biochemical analyses. After death, the femora and the spine were dissected, and the surrounding soft tissue was removed.
Bone histomorphometry
Femur length was measured using a digital caliper (Etalon). Distal femora from 6-, 12-, and 24-wk-old mice were prepared for quantitative histomorphometry, using established resin embedding techniques as described previously.(23) Five-micrometer-thick longitudinal sections were cut using a Polycut motorized microtome (Reichert). Sections were stained using a modified von Kossa silver technique and counterstained with H&E for osteoclast and osteoid surface calculations.(23) Trabecular bone volume, thickness, and number were calculated in the metaphyseal region below the growth plate excluding the primary spongiosa, using a Leica Quantimet Image Analysis System using QWin software (Cambridge Instruments).(23) Dynamic markers of bone turnover (osteoclast surface, osteoid surface, mineralizing surface, mineral apposition rate, bone formation rate) were estimated in the same area of the secondary spongiosa of the distal femoral metaphysis as described previously.(24)
Quantitative μCT
Femora and vertebrae (L5) from 6-, 12-, and 24-wk-old mice were evaluated using desktop microtomography (μCT40; Scanco Medical), as described previously.(24) For trabecular morphology, the following parameters were assessed: trabecular bone volume/tissue volume, number, thickness, separation, connectivity density, and structural model index (SMI). Cortical thickness was assessed at the midfemoral diaphysis.
Baseline biochemical analyses
Serum intact PTH was measured in serum collected through the tail vein(18) by two-site ELISA (Immutopics, San Clemente, CA, USA), and serum calcitonin was measured by a two-site immunoradiometric assay (IRMA; Immutopics), according to the manufacturer's instructions. The C-terminal telopeptide α1 chain of type I collagen (X-laps) was determined in serum by ELISA (RatLaps ELISA; Nordic Bioscience Diagnostics). Serum calcium and total protein were measured on an automated chemical analyser, using manufacturer recommended methods (Austin Pathology; Austin Health).
Induction of hypercalcemia in control and global CTRKOs
At 6 wk of age, control and global CTRKOs were fed a modified AIN-76 diet(25) containing low (0.02%) calcium for 2 wk to limit the contribution of serum calcium arising from calcitriol mediated intestinal absorption and to maximize the release of calcium from the bone through calcitriol's action to increase bone resorption.(26) A baseline blood sample was collected through the tail vein, and mice were treated with 0.5 μg calcitriol [1,25(OH)2D3; Wako Chemicals], administered subcutaneously in the nape of the neck daily each morning for 2 days. Blood samples were collected at 45 and 50.5 h after first calcitriol treatment. Food was removed after the blood collection at 45 h, and mice were killed by cervical dislocation after blood collection at 50.5 h after first calcitriol treatment.
Serum calcium and total protein analyses
Serum samples from the induced hypercalcemia studies were analyzed in duplicate for total calcium and protein content using the cresolphthalein-complexone (CPC) and Biuret methods, respectively (ThermoScientific, Victoria, Australia).
Calculations and statistical analysis
Ultrafiltrable calcium (UFCa) was calculated using the following formula described by Morris et al.(27): UFCa = total serum calcium – (0.02 × total protein). For calculating basal UFCa levels in control and global CTRKOs, this equation was derived by regression of total serum calcium on serum total protein pooled from control mice at 6, 12, and 24 wk of age.
Repeated-measures ANOVA was used to determine the effect of genotype, time, and the interaction of genotype and time on serum calcium after calcitriol treatment. To further analyze the effect of genotype on serum calcium levels at 45 and 50 h after calcitriol treatment, an unpaired Student's t-test was used. Because the relationship between bone histomorphometric parameters and age is not linear, the effects of CTR deletion on bone histomorphometry, μCT and serum biochemistry analyses were determined using an unpaired Student's t-test at each age group. All Student's t-tests were performed assuming equal variances; however, if the Levene's test of equality of error variances was <0.05, indicating that the variance was unequal, an unpaired Student's t-test assuming unequal variances was used. All statistical analyses were performed using SPSS 11. A value of p < 0.05 was considered significant.
RESULTS
TRACP-CTRKOs
We previously showed that global deletion of exons 6 and 7 of the CTR is embryonic lethal.(8) In these experiments, five breeding pairs, consisting of a het-floxed CTR, het-TRACP-Cre female and a hom-floxed CTR male, generated 46 female and 43 male offspring. No hom-TRACP-CTRKOs were generated, whereas the other expected genotypes were represented (data not shown) indicating that Cre-mediated deletion of exons 13 and 14 and the 3′UTR of the CTR driven by the TRACP promoter is embryonic lethal.
Characterization of Cre-mediated deletion of the CTR in global CTRKOs
To confirm that Cre-mediated deletion of exons 13 and 14 and the 3′ UTR of the CTR had occurred in global CTRKOs, PCR was performed on genomic DNA using a forward primer in exon 11 and a reverse primer downstream of the mouse CTR (Fig. 1C). A PCR product, representing the recombined CTR fragment of 2.1 kb, was amplified from the genomic DNA of global CTRKOs (Fig. 1C), confirming that Cre-mediated deletion of exons 13 and 14 and the 3′UTR of the CTR had occurred. In contrast to the hom-TRACP-CTR KOs, global CTRKO mice generated by breeding floxed CTR and CMV-Cre mice were viable, born at the expected mendelian ratios, and survived to adulthood, suggesting that deletion of the CTR in these mice was not complete. This was confirmed by performing PCR on genomic DNA from global CTRKOs using a forward primer in the endogenous CTR (located in exon 10) and a reverse primer unique to the floxed CTR construct (located 5′ of the first loxP site), showing the presence, albeit at very low levels, of the floxed CTR (data not shown), indicating that deletion of the CTR in global CTRKOs was <100%.
Q-PCR was performed to quantify the degree of deletion of the CTR in global CTRKOs at the mRNA level. After 60 cycles of real-time amplification, CTR mRNA levels were undetectable in global CTRKOs, independent of sex (n = 3–7/group). CTR mRNA levels were detected in heterozygous controls, and they did not differ between males (ΔCT = 22.8 ± 1.0, n = 8) and females (ΔCT = 19.4 ± 0.6, n = 4).
Despite these data showing that no CTR mRNA was detectable after 60 cycles of Q-PCR, an observation that is generally considered to indicate complete deletion of the target gene, we wished to assign a value to the degree of deletion in the global CTRKO mice. Therefore, relative quantitative analyses were performed by amplifying 10 ng of global CTRKOs cDNA in the presence of decreasing amounts of control cDNA ranging from 10 to 0.01 ng, representing 90–99.99% deletion. Deletion of exons 13 and 14 and the 3′UTR in global CTRKO mice was >90% in bone and kidney compared with controls (data not shown). To further define the degree of CTR deletion, a second relative quantitative analysis was performed using control cDNA concentrations from the kidney, ranging from 1 to 0.1 ng and representing 90–99% deletion. These results indicate that the amount of CTR deletion in the global CTRKO mice was >94% compared with controls (data not shown).
Osteoclast function in global CTRKOs
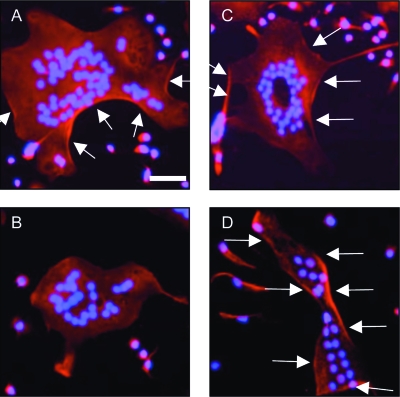
To assess the effect of CTR deletion on osteoclast function, the effect of calcitonin on actin ring formation was assessed in osteoclast-like cells derived from controls and global CTRKOs. In osteoclast-like cells derived from the bone marrow of controls, 30 min of treatment with 0.01 nM sCT resulted in complete disassembly of the actin ring (Figs. 2A and 2B). In contrast, sCT treatment of osteoclast-like cells derived from global CTRKOs had no effect on the actin ring (Figs. 2C and 2D).
FIG. 2.
Phalloidin staining of actin rings in osteoclast-like cells derived from the bone marrow of (A) control, (B) control treated for 30 min with 0.01 nM salmon calcitonin (sCT), (C) global CTRKO, and (D) global CTRKO treated for 30 min with 0.01 nM sCT. Arrows indicate actin ring. Nuclei of osteoclasts are counter-stained with DAPI. Scale bar represents 50 μm.
Bone phenotype of male and female CTRKOs
Body weight did not differ between male or female global CTRKOs compared with controls at any age group (data not shown). Femur length was unaffected by global deletion of the CTR in females at any age but was decreased by ∼4% in global CTRKO males compared with controls at 6, 12, and 24 wk of age (Table 1). Because the magnitude of this decrease in femur length in global CTRKO males was small, the size of the region for trabecular bone histomorphometry analyses was not modified.
Table 1.
Femoral Length and Static Bone Histomorphometry of the Distal Femoral Metaphysis in Male and Female Control and Global CTRKOs at 6, 12, and 24 wk of Age
| 6 wk | Male control (n = 10) | Male global CTRKO (n = 9) | Female control (n = 10) | Female global CTRKO (n = 22) |
| Femur length (mm) | 14.2 ± 0.2 | 13.6 ± 0.2* | 13.6 ± 0.2 | 13.5 ± 0.2 |
| BV/TV (%) | 23.3 ± 2.4 | 20.9 ± 2.5 | 14.9 ± 1.1 | 15.8 ± 0.5 |
| TbTh (μm) | 33.8 ± 1.9 | 35.3 ± 2.5 | 28.1 ± 1.0 | 29.3 ± 0.7 |
| TbN (/mm) |
6.8 ± 0.3 |
5.8 ± 0.5 |
5.3 ± 0.3 |
5.4 ± 0.2 |
|
12 wk |
Male control (n = 14) |
Male global CTRKO (n = 13) |
Female control (n = 16) |
Female global CTRKO (n = 17) |
| Femur length (mm) | 16.0 ± 0.1 | 15.5 ± 0.1* | 15.2 ± 0.1 | 15.0 ± 0.1 |
| BV/TV (%) | 18.1 ± 2.0 | 14.9 ± 1.1 | 8.1 ± 0.6 | 7.3 ± 0.4 |
| TbTh (μm) | 36.1 ± 2.4 | 31.9 ± 1.5 | 26.8 ± 0.9 | 24.0 ± 0.9* |
| TbN (/mm) |
4.8 ± 0.3 |
4.6 ± 0.2 |
3.0 ± 0.2 |
3.0 ± 0.1 |
|
24 wk |
Male control (n = 14) |
Male global CTRKO (n = 15) |
Female control (n = 15) |
Female global CTRKO (n = 11) |
| Femur length (mm) | 16.3 ± 0.1 | 15.8 ± 0.1† | 16.0 ± 0.1 | 16.1 ± 0.2 |
| BV/TV (%) | 10.2 ± 0.9 | 11.4 ± 1.2 | 3.6 ± 0.5 | 4.6 ± 0.6 |
| TbTh (μm) | 32.9 ± 1.7 | 32.5 ± 2.3 | 22.5 ± 1.5 | 23.1 ± 0.8 |
| TbN (/mm) | 3.0 ± 0.1 | 3.4 ± 0.2 | 1.5 ± 0.1 | 1.9 ± 0.2 |
Histomorphometric variables include trabecular bone volume/tissue volume (BV/TV), trabecular thickness (TbTh), and trabecular number (TbN). Values are mean ± SE.
* p < 0.05 vs. control within sex and age group.
† p < 0.005 vs. control within sex and age group.
Trabecular bone formation was increased by 20–40% in the distal femur of male global CTRKOs compared with controls at 12 and 24 wk of age as measured by mineralizing surface (p < 0.05) and bone formation rate (p < 0.05), whereas mineral apposition rate and osteoid surface were unchanged (Table 2). Osteoclast surface was unaffected in male global CTRKOs compared with controls (Table 2). Despite this increase in bone formation in male global CTRKOs, trabecular bone volume, number, and thickness did not differ from controls in the distal femur (Table 1) or vertebra (Table 3). Trabecular separation, however, was increased in vertebra of male global CTRKOs at 24 wk of age (p < 0.05; Table 3). Cortical thickness at the midfemoral diaphysis was unaffected in male global CTRKOs at any age (data not shown).
Table 2.
Dynamic Histomorphometric Analyses of the Distal Femoral Metaphysis in Male and Female Control and Global CTRKOs at 6, 12, and 24 wk of Age
| Male control | Male global CTRKO | Female control | Female global CTRKO | |
| 6 wk | ||||
| MS (%) | 25.2 ± 2.3 (8) | 30.3 ± 2.3 (8) | 28.2 ± 1.0 (9) | 23.3 ± 1.3* (8) |
| MAR (μm/d) | 1.6 ± 0.2 (8) | 2.1 ± 0.3 (8) | 1.5 ± 0.1 (9) | 1.7 ± 0.3 (8) |
| BFR (μm2/μm/d) | 0.42 ± 0.09 (8) | 0.68 ± 0.14 (8) | 0.43 ± 0.03 (9) | 0.39 ± 0.07 (8) |
| Osteoclast surface (%BS) | 15.6 ± 2.4 (10) | 18.2 ± 1.7 (9) | 18.0 ± 1.7 (10) | 17.2 ± 1.6 (14) |
| Osteoid surface (%BS) | 23.2 ± 1.8 (10) | 24.4 ± 2.6 (9) | 25.9 ± 1.9 (10) | 29.1 ± 2.2 (14) |
| 12 wk | ||||
| MS (%) | 33.2 ± 2.3 (11) | 40.0 ± 1.6 (12)† | 35.6 ± 2.6 (10) | 35.2 ± 3.1 (10) |
| MAR (μm/d) | 1.3 ± 0.1 (11) | 1.4 ± 0.1 (12) | 1.9 ± 0.1 (10) | 2.0 ± 0.1 (10) |
| BFR (μm2/μm/d) | 0.42 ± 0.03 (11) | 0.55 ± 0.05 (12)† | 0.66 ± 0.05 (10) | 0.69 ± 0.06 (10) |
| Osteoclast surface (%BS) | 4.5 ± 0.8 (12) | 3.6 ± 0.9 (12) | 5.8 ± 1.1 (12) | 5.4 ± 1.1 (12) |
| Osteoid surface (%BS) | 21.4 ± 2.3 (12) | 27.8 ± 2.6 (12) | 39.1 ± 4.1 (12) | 38.9 ± 2.9 (12) |
| 24 wk | ||||
| MS (%) | 35.0 ± 1.7 (11) | 42.8 ± 2.1 (7)† | 43.2 ± 3.0 (9) | 41.7 ± 2.0 (10) |
| MAR (μm/d) | 1.1 ± 0.1 (11) | 1.3 ± 0.1 (6) | 1.4 ± 0.1 (9) | 1.6 ± 0.1 (10) |
| BFR (μm2/μm/d) | 0.38 ± 0.03 (11) | 0.53 ± 0.04 (6)† | 0.64 ± 0.04 (9) | 0.67 ± 0.04 (10) |
| Osteoclast surface (%BS) | 4.9 ± 1.3 (10) | 4.6 ± 1.3 (10) | 6.1 ± 2.0 (10) | 4.6 ± 1.4 (10) |
| Osteoid surface (%BS) | 23.0 ± 2.4 (10) | 29.7 ± 3.8 (10) | 43.6 ± 4.9 (10) | 44.6 ± 4.4 (10) |
Dynamic histomorphometric variables include mineralizing surface (MS), mineral apposition rate (MAR), bone formation rate (BFR), osteoclast surface/bone surface (BS), and osteoid surface/BS. Values are mean ± SE. Number per group shown in parentheses.
* p < 0.01 vs. control within age group.
† p < 0.05 vs. control within age group.
Table 3.
μCT Analyses of L5 in Male and Female Control and Global CTRKOs at 6, 12, and 24 wk of Age
| 6 wk | Male control (n = 9) | Male global CTRKO (n = 8) | Female control (n = 12) | Female global CTRKO (n = 14) |
| BV/TV (%) | 28.8 ± 0.6 | 27.2 ± 0.9 | 26.0 ± 0.3 | 24.6 ± 0.4* |
| TbTh (μm) | 53.2 ± 0.8 | 52.2 ± 0.8 | 49.0 ± 0.6 | 49.3 ±0.6 |
| TbN (/mm) | 5.9 ± 0.1 | 5.5 ± 0.1 | 5.6 ± 0.1 | 5.3 ± 0.06* |
| TbSp (μm) | 160.4 ± 3.5 | 169.3 ± 5.0 | 169.5 ± 4.8 | 179.7 ± 2.2 |
| Conn Dens (mm−3) | 281.5 ± 9.2 | 257.2 ± 11.6 | 288.9 ± 11.1 | 262.4 ± 8.2 |
| SMI |
0.88 ± 0.07 |
0.95 ± 0.08 |
0.94 ± 0.04 |
1.07 ± 0.05 |
|
12 wk |
Male control (n = 10) |
Male global CTRKO (n = 10) |
Female control (n = 10) |
Female global CTRKO (n = 10) |
| BV/TV (%) | 31.1 ± 1.1 | 30.2 ± 0.7 | 26.6 ± 0.9 | 25.9 ± 0.8 |
| TbTh (μm) | 57.3 ± 0.9 | 57.3 ± 1.2 | 55.9 ± 0.4 | 54.3 ± 0.5* |
| TbN (/mm) | 5.7 ± 0.1 | 5.4 ± 0.1 | 4.7 ± 0.2 | 4.4 ± 0.1 |
| TbSp (μm) | 165.5 ± 4.9 | 176.5 ± 4.0 | 209.8 ± 7.6 | 222.6 ± 6.0 |
| Conn Dens (mm−3) | 219.5 ± 13.2 | 220.2 ± 10.7 | 176.1 ± 8.8 | 191.1 ± 6.5 |
| SMI |
0.40 ± 0.10 |
0.41 ± 0.10 |
0.62 ± 0.08 |
0.57 ± 0.09 |
|
24 wk |
Male control |
Male global CTRKO (n = 10) |
Female control (n = 9) |
Female global CTRKO (n = 8) |
| BV/TV (%) | 27.8 ± 0.8 | 26.9 ± 0.6 | 18.7 ± 1.3 | 23.7 ± 2.0† |
| TbTh (μm) | 55.8 ± 1.3 | 57.0 ± 0.9 | 58.8 ± 0.5 | 56.5 ± 0.6* |
| TbN (/mm) | 5.1 ± 0.1 | 4.8 ± 0.1 | 3.3 ± 0.1 | 3.9 ± 0.3 |
| TbSp (μm) | 182.0 ± 2.2 | 196.2 ± 5.3* | 309.8 ± 13.6 | 270.5 ± 19.2 |
| Conn Dens (mm−3) | 176.7 ± 5.2 | 180.0 ± 6.1 | 82.1 ± 11.7 | 173.6 ± 37.8* |
| SMI | 0.64 ± 0.07 | 0.76 ± 0.07 | 1.27 ± 0.16 | 0.63 ± 0.17* |
μCT analyses include trabecular bone volume/tissue volume (BV/TV), trabecular thickness (TbTh), trabecular number (TbN), trabecular separation (TbSp), connectivity density (Conn Dens), and structural model index (SMI). Values are mean ± SE.
* p < 0.05 vs. control within age group.
† p < 0.01 vs. control within age group.
Modest changes in bone structure were observed in female global CTRKOs. Trabecular bone volume in the vertebrae of female global CTRKOs was decreased by 6% at 6 wk of age (p < 0.05) compared with controls, and this was caused by a reduction in trabecular number of 5% (p < 0.05), whereas trabecular separation was not affected (Table 3). Female global CTRKOs, however, were able to maintain this level of vertebral trabecular bone volume throughout adulthood until 24 wk of age, whereas control females, as expected, displayed age-related trabecular loss. This resulted in female global CTRKOs having higher trabecular bone volume by 27% (p < 0.01) and connectivity density by 100% compared with controls at 24 wk of age (p < 0.05; Table 3). Despite the ability to maintain trabecular number during aging, trabecular thickness was decreased at 12 and 24 wk of age compared with controls (p < 0.05; Table 3). SMI was decreased in female global CTRKOs in the vertebra at 24 wk of age compared with controls (p < 0.05; Table 3), indicating that the trabecular structures were more rod-like than plate-like in female global CTRKOs.
In contrast to the vertebra, femoral trabecular bone volume was unaffected by CTR deletion in females at any age despite a decrease in trabecular thickness of 11% at 12 wk of age compared with controls (p < 0.05; Table 1). Mineralizing surface in the distal femoral metaphysis was decreased by 18% in female global CTRKOs at 6 wk of age (p < 0.01); however, no further changes were observed in the dynamic histomorphometric variables analyzed in the distal femur at any age (Table 2).
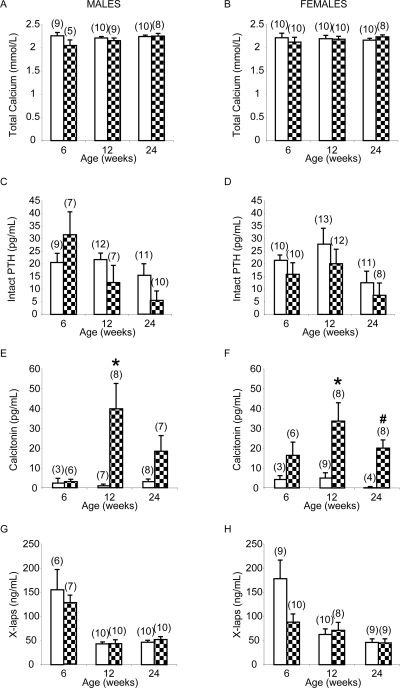
Increased serum calcitonin with no change in serum calcium in adult global CTRKOs
Serum calcitonin was strikingly increased in male and female global CTRKOs at each time studied except at 6 wk of age in the male global CTRKOs. For example, from basal levels <5 pg/ml, levels were increased to 40 and 34 pg/ml in male and female global CTRKOs, respectively, at 12 wk of age (p < 0.05) and to 20 pg/ml in females at 24 wk of age (p < 0.005; Figs. 3E and 3F). Total serum calcium, total protein, calculated ultrafiltrable calcium, intact PTH, and X-laps were unaffected by global deletion of the CTR in males and females at all ages on a normal calcium diet (Figs. 3A–3D, 3G, and 3H, and data not shown).
FIG. 3.
Serum biochemistry in control (white bars) and global CTRKOs (patterned bars): total serum calcium in (A) males and (B) females; intact PTH in (C) males and (D) females; calcitonin in (E) males and (F) females; and X-laps in (G) males and (H) females. Values are mean ± SE; numbers in each group are shown in parentheses. *p < 0.05, # p < 0.005 vs. control at the same time point.
Induced hypercalcemia in global CTRKOs
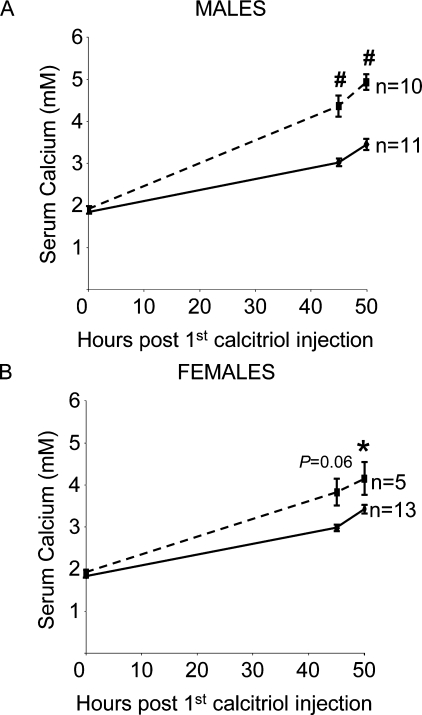
Baseline serum calcium levels did not differ between male and female global CTRKOs and controls fed a low Ca2+ diet for 2 wk (Fig. 4). Calcitriol treatment successfully induced hypercalcemia in male and female control and global CTRKOs, with the peak in serum calcium levels occurring ∼50 h after the first treatment with calcitriol (p < 0.0001; Fig. 4). Peak serum calcium levels after calcitriol-induced hypercalcemia were substantially higher in male and female global CTRKOs by 44% (1.5 mM; p < 0.0001) and 21% (0.7 mM; p < 0.05), respectively, compared with controls (Fig. 4). Serum total protein was unaffected in male and female global CTRKOs and controls at any time point after calcitriol treatment (data not shown). The small volumes of serum did not allow for measurement of total protein in all mice; therefore, ultrafiltrable calcium levels were not calculated.
FIG. 4.
Effect of global CTR deletion on total serum calcium after calcitriol-induced hypercalcemia in (A) males and (B) females. Values are mean ± SE. Solid lines, controls; dashed lines, global CTRKOs. *p < 0.05, # p < 0.001 vs. control at the same time point.
DISCUSSION
It is well established that calcitonin is a potent inhibitor of osteoclastic bone resorption(28) and, whereas it is effective in treating hypercalcemia of malignancy, a physiological role for calcitonin acting through the CTR has not been identified. This study provides strong evidence for a physiological role of the CTR in protecting against induced hypercalcemia. Whereas global deletion of the CTR had minimal effects on basal calcium homeostasis, these mice showed a significantly elevated hypercalcemic response to acute calcitriol treatment compared with controls.
Previous attempts to determine the physiological role of calcitonin in bone have been made through the use of genetically modified mouse models. Studies in CT/CGRP KO and haploinsufficient CTR mice have uncovered an unexpected role for calcitonin and the CTR in bone formation.(7,8) To further elucidate the physiological role of the CTR, we generated a global CTRKO mouse line. Using the Cre-loxP system to generate the global CTRKOs has overcome a number of limitations experienced by the earlier CT/CGRP KO and haploinsufficient CTR mouse models. In our global CTRKOs, the CTR was deleted by >94%, abolishing the inhibitory action of calcitonin in osteoclasts. Osteoclasts derived from the bone marrow of global CTRKOs did not respond to calcitonin in vitro. However, unlike the CTR-deficient (CTR−/−) mice,(8) these mice were viable and survive throughout adulthood.
The results from this study clearly show that the seventh transmembrane domain and C terminus of the CTR are essential for normal CTR function. Deletion of these domains of the CTR by >94% in the global CTRKOs inhibited the response of osteoclasts to calcitonin treatment in vitro as shown by the disassembly of the actin ring, consistent with previous observations of decreased internalization and activation of second messengers in vitro by CTR mutants lacking either the seventh transmembrane domain or seventh transmembrane domain and C terminus.(29,30) Further supportive evidence for these domains in CTR function is provided by the failure to generate viable TRACP-CTRKO mice. Presumably, it is the high expression of the TRACP promoter driving Cre-mediated recombination of the CTR in the placenta,(31) rather than the Cre-mediated deletion of the CTR in osteoclasts, that results in the embryonic lethality in this model.
The mild bone phenotype of the male and female global CTRKO mice indicates a modest physiological role for the CTR in regulating bone turnover in basal states. Consistent with our previous findings in male haploinsufficient CTR mice,(8) global deletion of the CTR in adult males resulted in increased trabecular bone formation rates, whereas markers of bone resorption, osteoclast surface and serum X-laps, remained unaffected, supporting the hypothesis that the CTR regulates bone formation. This has been proposed to occur through a putative coupling between osteoclasts and osteoblasts, with calcitonin modulating the expression of a factor produced by osteoclasts, but may also be mediated by calcitonin acting through the CTR in other tissues. These data, however, do not rule out the possibility that there may also be a mild increase in osteoclast activity and/or an increase in osteoclast survival in the absence of the CTR in global CTRKOs, which is not reflected by measurable increases in the histomorphometric marker, osteoclast surface, or in the circulating bone resorption marker, X-laps. The observation that trabecular separation was increased in the vertebra of male CTRKOs at 24 wk of age by 7% does, however, support a mild increase in bone resorption in the male CTRKOs. In contrast to the haploinsufficient CTR mice, the magnitude of bone formation observed in the global CTRKOs in this study did not result in an increase in trabecular bone volume, which is consistent with an increase in overall bone turnover with no net loss of trabecular bone. The differences in the magnitude of elevated bone formation in the absence of the CTR in the global CTRKOs and haploinsufficient CTR mouse models may be attributed to the differences in genetic background.(17,32) The haploinsufficient CTR mice were of mixed C57BL/6:SV129J background (∼87.5% C57BL/6, 12.5% SV129/J) and were maintained by cross-mating mice heterozygous for the deleted CTR (D Galson, personal communication), whereas our global CTRKOs were on a homogeneous C57BL/6 background. Further supportive evidence for strain differences and lack of discernible phenotype is the loss of increased bone formation phenotype in the CT/CGRP KOs(7) when backcrossed to a homogenous C57BL/6 background.(10)
In contrast to males, deletion of the CTR in females resulted in a 6% loss of trabecular bone in the vertebra at 6 wk of age compared with controls. This was as a result of decreased trabecular number, suggesting increased bone resorption, whereas no differences were observed in the femur. The failure to detect any accompanying differences in the serum or histomorphometric markers of bone resorption in female global CTRKO mice is perhaps not surprising given the small magnitude of bone loss observed in these mice. Of interest, however, was the ability of the female global CTRKOs to maintain this reduced level of vertebral trabecular bone volume throughout aging by preserving trabecular number despite the age-related thinning of trabeculae, whereas the control mice, as expected, displayed age-related trabecular loss. This resulted in female global CTRKO mice having increased vertebral trabecular bone volume compared with controls at 24 wk of age and suggests a mechanism in females to inhibit bone resorption in the absence of the CTR.
The synthesis of calcitonin is controlled primarily by serum ionized calcium levels.(33) Despite total serum calcium, total protein, calculated ultrafiltrable calcium, and PTH levels all being unaltered in adult female and male global CTRKOs, serum calcitonin levels were highly elevated. The increased serum calcitonin levels in the absence of the CTR provides evidence for a possible role of the CTR in feedback regulation of calcitonin synthesis. Alternatively, this may be attributed to transient or immeasurable increases in serum ionized calcium levels in the absence of calcitonin action through the CTR, which lead to increased calcitonin secretion. Serum calcitonin levels have not been reported for the CT/CGRP mice, and serum total and ionized calcium and PTH are unaltered,(7) whereas these parameters have not been reported in the haploinsufficient CTR mice.(8)
Evidence provided by the phenotype of the global CTRKOs indicates that the CTR receptor plays a modest physiological role in the regulation of bone turnover and calcium homeostasis in the basal state in mice up to 6 mo of age. Therefore, to test the hypothesis that calcitonin acting through the CTR plays an important role in protecting the skeleton in times of calcium stress such as hypercalcemia, pregnancy, lactation, and in states of high bone turnover, we induced hypercalcemia in our global CTRKO mice. Calcitriol treatment of mice fed a low calcium diet for 2 wk induced hypercalcemia in male and female controls and global CTRKOs. The peak in serum total calcium after induced hypercalcemia, however, was greater by 44% and 21% in male and female global CTRKOs, respectively, compared with controls, showing for the first time a physiological role for the CTR in regulating calcium homeostasis in states of calcium stress, such as hypercalcemia. These data are also consistent with the observation >20 years ago in thyroidectomized patients that the decrease in serum calcium levels in response to short-term intravenous calcium infusion was delayed compared with patients with an intact thyroid, showing the role of calcitonin in the short-term control of bone resorption.(12,34)
The mechanism of the CTR in protecting against induced hypercalcemia is most likely to be mediated through its acute inhibition of bone resorption. However, the loss of calcitonin's action through the CTR in the kidney to increase calcium excretion(35) may also have contributed to the increased magnitude of hypercalcemia observed in the global CTRKOs compared with controls after calcitriol treatment. It is also possible that the response to exogenous calcitriol may be altered in global CTRKOs, thereby contributing to the increased magnitude of hypercalcemia observed in these mice. Because the basal mRNA levels of the genes involved in osteoclastogenesis (i.e., RANK, RANKL, and OPG) were unchanged in the global CTRKOs, we believe that any alteration in response to calcitriol in these mice is unlikely to be mediated through calcitriol's actions to upregulate RANKL.(36) However, further study is required to determine whether the response to calcitriol treatment is altered in global CTRKOs by measuring the expression of these and other calcitriol responsive genes after treatment with calcitriol.
The greater extent of induced hypercalcemia in global CTRKOs compared with controls after calcitriol treatment could potentially be caused by the actions of the other calcitonin-related peptides, such as amylin, which can also bind to the CTR in the presence of RAMPs 1, 2, or 3.(37) However, it is most likely that the regulation of calcium homeostasis under stress conditions is regulated by the action of calcitonin caused by its high affinity for the CTR,(38) and our findings in amylin KO and haploinsufficient CTR mice suggest that the CTR is not the main receptor through which amylin affects osteoclastogenesis.(8) In support of this is the observation that at comparable levels of PTH-induced hypercalcemia, thyroparathyroidectomized rats have low levels of serum calcitonin and trabecular bone loss, whereas parathyroidectomized rats have significantly higher serum levels of calcitonin with no trabecular bone loss, suggesting that endogenous calcitonin protects against the bone loss associated with PTH induced-hypercalcemia.(16)
Other studies have also supported a physiological role for calcitonin acting through the CTR in protecting in times of calcium stress, such as pregnancy and lactation. Mice lose 20–25% of their BMD during lactation(39); however, Woodrow et al.(40) showed that this loss of BMC during lactation was doubled at the spine in CT/CGRP KO mice compared with littermate controls. Daily treatment with salmon calcitonin, but not CGRP, normalized the bone loss in CT/CGRP KO mice during lactation, suggesting that one of the important physiological roles of calcitonin is to protect the maternal skeleton against excessive resorption and fragility during lactation.(40) Our data are consistent with these actions being mediated through the CTR.
In conclusion, the data presented here provide strong evidence for a biological role of the CTR in protecting against induced hypercalcemia in mice. Furthermore, we showed that the CTR plays a modest physiological role in the regulation of bone and calcium homeostasis in the basal state.
ACKNOWLEDGMENTS
The authors thank Patricia Russell of the University of Melbourne and Rebecca Sawyer of the Hanson Institute for excellent technical assistance. This work was supported by the National Health and Medical Research Council (Project Grant 454484), The Austin Hospital Medical Research Foundation, an Endocrine Research Grant provided by Eli Lilly Australia, and an Eva and Les Erdi Major Research Grant.
Footnotes
The authors state that they have no conflicts of interest.
REFERENCES
- 1.Findlay DM, Sexton PM. Calcitonin. Growth Factors. 2004;22:217–224. doi: 10.1080/08977190410001728033. [DOI] [PubMed] [Google Scholar]
- 2.Wallach S, Rousseau G, Martin L, Azria M. Effects of calcitonin on animal and in vitro models of skeletal metabolism. Bone. 1999;25:509–516. doi: 10.1016/s8756-3282(99)00200-8. [DOI] [PubMed] [Google Scholar]
- 3.Sexton PM, Findlay DM, Martin TJ. Calcitonin. Curr Med Chem. 1999;6:1067–1093. [PubMed] [Google Scholar]
- 4.Shinki T, Ueno Y, DeLuca HF, Suda T. Calcitonin is a major regulator for the expression of renal 25-hydroxyvitamin D3-1alpha-hydroxylase gene in normocalcemic rats. Proc Natl Acad Sci USA. 1999;96:8253–8258. doi: 10.1073/pnas.96.14.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singer FR, Aldred JP, Neer RM, Krane SM, Potts JT, Jr, Bloch KJ. An evaluation of antibodies and clinical resistance to salmon calcitonin. J Clin Invest. 1972;51:2331–2338. doi: 10.1172/JCI107044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Singer FR, Fredericks RS, Minkin C. Salmon calcitonin therapy for Paget's disease of bone. The problem of acquired clinical resistance. Arthritis Rheum. 1980;23:1148–1154. doi: 10.1002/art.1780231012. [DOI] [PubMed] [Google Scholar]
- 7.Hoff AO, Catala-Lehnen P, Thomas PM, Priemel M, Rueger JM, Nasonkin I, Bradley A, Hughes MR, Ordonez N, Cote GJ, Amling M, Gagel RF. Increased bone mass is an unexpected phenotype associated with deletion of the calcitonin gene. J Clin Invest. 2002;110:1849–1857. doi: 10.1172/JCI200214218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dacquin R, Davey RA, Laplace C, Levasseur R, Morris HA, Goldring SR, Gebre-Medhin S, Galson DL, Zajac JD, Karsenty G. Amylin inhibits bone resorption while the calcitonin receptor controls bone formation in vivo. J Cell Biol. 2004;164:509–514. doi: 10.1083/jcb.200312135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brain SD, Grant AD. Vascular actions of calcitonin gene-related peptide and adrenomedullin. Physiol Rev. 2004;84:903–934. doi: 10.1152/physrev.00037.2003. [DOI] [PubMed] [Google Scholar]
- 10.Gagel RF, Hoff AO, Huang SE, Cote GJ. Deletion of calcitonin/CGRP gene causes a profound cortical resorption phenotype in mice. J Bone Miner Res. 2007;22(S1):S35. [Google Scholar]
- 11.Martin TJ, Sims NA. Osteoclast-derived activity in the coupling of bone formation to resorption. Trends Mol Med. 2005;11:76–81. doi: 10.1016/j.molmed.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 12.Hirsch PF, Munson PL. Thyrocalcitonin. Physiol Rev. 1969;49:548–622. doi: 10.1152/physrev.1969.49.3.548. [DOI] [PubMed] [Google Scholar]
- 13.Martin TJ, Melick RA. The acute effects of porcine calcitonin in man. Australas Ann Med. 1969;18:258–263. doi: 10.1111/imj.1969.18.3.258. [DOI] [PubMed] [Google Scholar]
- 14.Cooper CW, Hirsch PF, Toverud SU, Munson PL. An improved method for the biological assay of thyrocalcitonin. Endocrinology. 1967;81:610–616. doi: 10.1210/endo-81-3-610. [DOI] [PubMed] [Google Scholar]
- 15.Zhu LJ, Bagchi MK, Bagchi IC. Attenuation of calcitonin gene expression in pregnant rat uterus leads to a block in embryonic implantation. Endocrinology. 1998;139:330–339. doi: 10.1210/endo.139.1.5707. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto M, Seedor JG, Rodan GA, Balena R. Endogenous calcitonin attenuates parathyroid hormone-induced cancellous bone loss in the rat. Endocrinology. 1995;136:788–795. doi: 10.1210/endo.136.2.7835311. [DOI] [PubMed] [Google Scholar]
- 17.Davey RA, MacLean HE, McManus JF, Findlay DM, Zajac JD. Genetically modified animal models as tools for studying bone and mineral metabolism. J Bone Miner Res. 2004;19:882–892. doi: 10.1359/JBMR.040206. [DOI] [PubMed] [Google Scholar]
- 18.Davey RA, MacLean HE. Current and future approaches using genetically modified mice in endocrine research. Am J Physiol Endocrinol Metab. 2006;291:E429–E438. doi: 10.1152/ajpendo.00124.2006. [DOI] [PubMed] [Google Scholar]
- 19.Schwenk F, Baron U, Rajewsky K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 1995;23:5080–5081. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chiu WS, McManus JF, Notini AJ, Cassady AI, Zajac JD, Davey RA. Transgenic mice that express Cre recombinase in osteoclasts. Genesis. 2004;39:178–185. doi: 10.1002/gene.20041. [DOI] [PubMed] [Google Scholar]
- 21.Davey RA, Hahn CN, May BK, Morris HA. Osteoblast gene expression in rat long bones: Effects of ovariectomy and dihydrotestosterone on mRNA levels. Calcif Tissue Int. 2000;67:75–79. doi: 10.1007/s00223001100. [DOI] [PubMed] [Google Scholar]
- 22.Itoh K, Udagawa N, Katagiri T, Iemura S, Ueno N, Yasuda H, Higashio K, Quinn JM, Gillespie MT, Martin TJ, Suda T, Takahashi N. Bone morphogenetic protein 2 stimulates osteoclast differentiation and survival supported by receptor activator of nuclear factor-kappaB ligand. Endocrinology. 2001;142:3656–3662. doi: 10.1210/endo.142.8.8300. [DOI] [PubMed] [Google Scholar]
- 23.Davey RA, Moore AJ, Chiu MW, Notini AJ, Morris HA, Zajac JD. Effects of amylin deficiency on trabecular bone in young mice are sex-dependent. Calcif Tissue Int. 2006;78:398–403. doi: 10.1007/s00223-005-0286-2. [DOI] [PubMed] [Google Scholar]
- 24.Notini AJ, McManus JF, Moore A, Bouxsein M, Jimenez M, Chiu WS, Glatt V, Kream BE, Handelsman DJ, Morris HA, Zajac JD, Davey RA. Osteoblast deletion of exon 3 of the androgen receptor gene results in trabecular bone loss in adult male mice. J Bone Miner Res. 2007;22:347–356. doi: 10.1359/jbmr.061117. [DOI] [PubMed] [Google Scholar]
- 25.O'Loughlin PD, Morris HA. Oestrogen deficiency impairs intestinal calcium absorption in the rat. J Physiol. 1998;511:313–322. doi: 10.1111/j.1469-7793.1998.313bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morony S, Capparelli C, Lee R, Shimamoto G, Boone T, Lacey DL, Dunstan CR. A chimeric form of osteoprotegerin inhibits hypercalcemia and bone resorption induced by IL-1beta, TNF-alpha, PTH, PTHrP, and 1, 25(OH)2D3. J Bone Miner Res. 1999;14:1478–1485. doi: 10.1359/jbmr.1999.14.9.1478. [DOI] [PubMed] [Google Scholar]
- 27.Morris HA, Porter SJ, Durbridge TC, Moore RJ, Need AG, Nordin BE. Effects of oophorectomy on biochemical and bone variables in the rat. Bone Miner. 1992;18:133–142. doi: 10.1016/0169-6009(92)90853-6. [DOI] [PubMed] [Google Scholar]
- 28.Chambers TJ, Magnus CJ. Calcitonin alters behaviour of isolated osteoclasts. J Pathol. 1982;136:27–39. doi: 10.1002/path.1711360104. [DOI] [PubMed] [Google Scholar]
- 29.Seck T, Pellegrini M, Florea AM, Grignoux V, Baron R, Mierke DF, Horne WC. The delta e13 isoform of the calcitonin receptor forms a six-transmembrane domain receptor with dominant-negative effects on receptor surface expression and signaling. Mol Endocrinol. 2005;19:2132–2144. doi: 10.1210/me.2004-0472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shyu JF, Inoue D, Baron R, Horne WC. The deletion of 14 amino acids in the seventh transmembrane domain of a naturally occurring calcitonin receptor isoform alters ligand binding and selectively abolishes coupling to phospholipase C. J Biol Chem. 1996;271:31127–31134. doi: 10.1074/jbc.271.49.31127. [DOI] [PubMed] [Google Scholar]
- 31.Hayman AR, Macary P, Lehner PJ, Cox TM. Tartrate-resistant acid phosphatase (Acp 5): Identification in diverse human tissues and dendritic cells. J Histochem Cytochem. 2001;49:675–684. doi: 10.1177/002215540104900601. [DOI] [PubMed] [Google Scholar]
- 32.Beamer WG, Donahue LR, Rosen CJ, Baylink DJ. Genetic variability in adult bone density among inbred strains of mice. Bone. 1996;18:397–403. doi: 10.1016/8756-3282(96)00047-6. [DOI] [PubMed] [Google Scholar]
- 33.Garrett JE, Tamir H, Kifor O, Simin RT, Rogers KV, Mithal A, Gagel RF, Brown EM. Calcitonin-secreting cells of the thyroid express an extracellular calcium receptor gene. Endocrinology. 1995;136:5202–5211. doi: 10.1210/endo.136.11.7588259. [DOI] [PubMed] [Google Scholar]
- 34.Williams GA, Hargis GK, Galloway WB, Henderson WJ. Evidence for thyrocalcitonin in man. Proc Soc Exp Biol Med. 1966;122:1273–1276. doi: 10.3181/00379727-122-31380. [DOI] [PubMed] [Google Scholar]
- 35.Pondel M. Calcitonin and calcitonin receptors: Bone and beyond. Int J Exp Pathol. 2000;81:405–422. doi: 10.1046/j.1365-2613.2000.00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kitazawa R, Kitazawa S. Vitamin D(3) augments osteoclastogenesis via vitamin D-responsive element of mouse RANKL gene promoter. Biochem Biophys Res Commun. 2002;290:650–655. doi: 10.1006/bbrc.2001.6251. [DOI] [PubMed] [Google Scholar]
- 37.Muff R, Buhlmann N, Fischer JA, Born W. An amylin receptor is revealed following co-transfection of a calcitonin receptor with receptor activity modifying proteins-1 or -3. Endocrinology. 1999;140:2924–2927. doi: 10.1210/endo.140.6.6930. [DOI] [PubMed] [Google Scholar]
- 38.Lerner UH. Deletions of genes encoding calcitonin/alpha-CGRP, amylin and calcitonin receptor have given new and unexpected insights into the function of calcitonin receptors and calcitonin receptor-like receptors in bone. J Musculoskelet Neuronal Interact. 2006;6:87–95. [PubMed] [Google Scholar]
- 39.Kovacs CS, Kronenberg HM. Maternal-fetal calcium and bone metabolism during pregnancy, puerperium, and lactation. Endocr Rev. 1997;18:832–872. doi: 10.1210/edrv.18.6.0319. [DOI] [PubMed] [Google Scholar]
- 40.Woodrow JP, Sharpe CJ, Fudge NJ, Hoff AO, Gagel RF, Kovacs CS. Calcitonin plays a critical role in regulating skeletal mineral metabolism during lactation. Endocrinology. 2006;147:4010–4021. doi: 10.1210/en.2005-1616. [DOI] [PubMed] [Google Scholar]