Abstract
Human S100A7 (psoriasin) is considered a marker for specific stages of breast cancer. hS100A15 is almost identical to hS100A7 and difficult to discriminate. We developed specific probes to distinguish hS100A7 and hS100A15, and demonstrate their differential distribution in normal breast tissue. Further, hS100A7 and S100A15 transcripts are elevated in ER/PR negative breast cancers, but hS100A15 protein is detected in all cancer specimens while hS100A7 protein is sporadically expressed. The differential regulation, expression and distribution of hS100A7 and hS100A15 and their reported distinct functions are compelling reasons to discriminate among these proteins in normal breast and breast cancers.
Keywords: mammary cancer, calcium binding proteins, S100, psoriasis, myoepithelial cells
Introduction
Early diagnosis of breast cancer has prompted the search for molecular markers to predict the risk of tumor progression. Upregulation of human S100A7 (hS100A7, psoriasin) is considered a useful marker for recognizing in-situ carcinomas and pre-invasive foci of the primary breast epithelial tumors. hS100A7 expression often decreases in invading tumor foci, however its persistent expression in invasive carcinomas is associated with poor prognosis [1–3].
hS100A7 belongs to the largest, multigenic family of calcium-binding EF-hand S100 proteins [4]. Despite their small size and conserved functional domains, gene duplications and variations throughout vertebrate evolution led to an increase in number and diversity within the S100 family [5]. Along with hS100A7 (psoriasin), the human S100A15 (hS100A15) was first identified as upregulated in psoriatic skin, where the S100A15 gene transcribes two alternative mRNA splice variants, S100A15-S (short) and hS100A15-L (long) [6;7]. hS100A7 and hS100A15 are both intracellular and secreted proteins and are believed to contribute to the inflammatory phenotype that characterizes psoriasis. Both hS100A7 and hS100A15 are encoded by separate genes within the highly recombinant region of S100 gene cluster on chromosome 1q21 (Epidermal Differentiation Complex). As evolutionary late genes, hS100A7 and hS100A15 are highly similar paralogs (93% sequence identity) diverging the least among all S100 gene family. Recent studies indicate that secreted hS100A7 and S100A15 proteins exert a pro-inflammatory function through distinct receptors and act synergistically as chemoattractants when co-expressed[8].
The high homology of hS100A15 and hS100A7 makes them difficult to distinguish when co-expressed. However, their distinct biological functions compel an analysis of their dual presence and potential contribution to normal breast and cancers. By using specific PCR-primers and antibodies, we demonstrate differential expression and distribution of hS100A7 and hS100A15 in both normal breast tissue and invasive carcinomas. The specific expression patterns suggest both proteins contribute unique functional elements for breast physiology and tumorigenesis.
Material and Methods
All the materials and methods used have been approved by the NCI. Breast tissues were obtained for research from B.K.V., NCI, NIH, Bethesda and the Manitoba Breast Tumor Bank, Canada under REB approved protocols.
Human breast cancer tissues
A cohort of invasive breast cancers was obtained from the Manitoba Breast Cancer Tumor Bank. Cases and controls were selected for this study on the basis of hS100A7 status and availability of frozen tissues to include ten hS100A7 positive and negative cases, respectively as previously determined by immunohistochemistry [9]. All cases were associated with frozen tissues containing invasive components that occupied more than 20% of the tumor section, while normal epithelial areas comprised no more than 10% of the epithelial content. ER/PR status were defined by ligand binding analysis of separate samples from each tumor (ER positive status> 3 fmol/mg protein, PR positive status > 15 fmol/mg protein). Tumor grade was determined using the Nottingham grading system.
Establishment of specific primers for hS100A7 and hS100A15 and quantitative reverse transcriptase-polymerase chain reaction (RT-PCR) was performed based on extracted RNA (1000 ng) that was reverse transcribed in a total volume of 20 μl as described previously [3]. qPCR was performed using fluorescence thermocycler (Biorad, Hercules, CA) and gene-specific primers (forward, reverse): hS100A7: (AGACGTGATGACAAGATTGAC,TGTCCTTTTTCTCAAAGACGTC); hS100A15-L: (ACGTCACTCCTGTCTCTCTTTGCT,TGATGAATCAACCCATTTCCTGGG), hS100A15-S: (CAAGTTCCTTCTGCTCCATCTTAG,AGCCTTCAGGAAATAAAGACAATC). The specificity of the hS100A7, hS100A15-L and hS100A15-S products was verified by sequencing. The ΔΔCt method was used to normalize transcript to hGAPDH.
Establishment of antibodies specific for hS100A7 and hS100A15
Monospecific antisera to human S100A15 were prepared in rabbits by injecting a synthetic peptide which corresponds to the N-terminal amino acid sequence of the deduced hS100A15 protein (gene bank acc. number AAO40032). The antibodies were affinity purified using the synthetic peptide-coupled to Affigel-15 (Biorad, St. Louis, MS) [10]. The polyclonal anti-hS100A15 antibody 3923, several commercial (Imgenex, San Diego, CA; Abcam, Cambridge, MA; Exalpha, Maynard, MA) and custom anti-hS100A7 antibodies (Fig 1, 4) [1;9] were tested for specificity using recombinant hS100 proteins (custom, see above; Novus Biologicals, Littleton, CO; 50 ng/lane) and human keratinocyte lysate (20ug/lane). Preabsorption studies with the corresponding proteins blocked the appropriate S100 antibody staining as described previously[8].
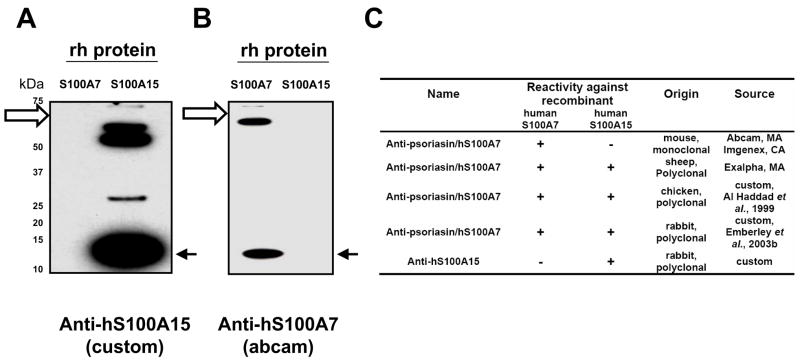
Figure 1. Characterization of the human S100A7 and S100A15 antibodies.
Indicated recombinant human S100 proteins (50 ng) were subjected to gel electrophoresis and transferred to membranes and immunoblotted with the affinity-purified polyclonal anti-hS100A15 antibody 3923 (custom, Fig 1A), or monoclonal anti-hS100A7 antibody (Abcam, MA; Fig 1B). Solid arrows indicate the migration of the corresponding hS100A15 and hS100A7 monomers. The immunoreactive bands above the recombinant S100 monomers (open arrows) represent uncleaved recombinant MBP-hS100A7 and MBP-hS100A15 fusion proteins. Specificity of tested commercial and custom antibodies generated against hS100A7 and hS100A15 and tested similarly is summarized in Fig 1C.
cDNA cloning, recombinant protein expression and purification of S100 gene family members
cDNA was prepared by PCR-cloning of full-length transcripts (hS100A7: NM_002963; hS100A15: AY189119) isolated from human skin [6]. Untagged proteins were expressed in Escherichia coli BL21 (DE3) cells harboring plasmid his6-MBP-tev-human S100A15/-human S100A7 and proteins were tev-cleaved and purified as described [11].
Immunoblotting and Immunohistochemistry
For immunoblot analysis, lysates of normal breast tissue and invasive carcinomas were prepared using 1% Triton-containing lysis buffer (Cell Signaling). Proteins were separated using a 12% polyacrylamide gel, transferred to reinforced nitrocellulose membranes and visualized by Ponceau stain. Membranes were incubated with blocking buffer (TBS, pH 7.4, with 5% milk, 0.1% Tween 20) for 30 min, primary antibody (anti-hS100A15, 1μg/mL; monoclonal mouse anti-hS100A7 antibodies, 1μg/mL; polyclonal chicken anti-hS100A7, 1:2000 [1]; polyclonal rabbit anti-hS100A7, 1:2000 [9] overnight, and secondary antibody was applied for 1 h with several washes (TBS, pH 7.4, 0.1% Tween 20) between incubations.
Immunohistochemistry was performed on serial 5 μm frozen sections of human normal breast and invasive carcinomas fixed in acetone. The sections were treated with 96% methanol and 4% hydrogen peroxide to exhaust endogenous peroxidase activity, blocked in 10% normal goat serum, and incubated overnight with anti-hS100A15 or monoclonal mouse anti-hS100A7 (5 μg/ml each). Slides were then treated the next day with biotinylated anti-rabbit or anti-mouse IgG (H+L) (1:1000), followed by an Avidin-Biotin Complex incubation (Elite Vectastain). Samples were exposed using the Vector DAB Kit and mounted. All reagents for immunostaining were from Vector Laboratories, Burlington, CA. Serial dilution competition assays were performed in the absence and presence of blocking peptide as indicated to determine the optimal working concentration and specificity of the primary hS100A15 antibody using both immunohistochemistry and immunoblot analysis.
For immunofluorescence, donkey anti-rabbit cy3 (1:250) or donkey anti-mouse FITC (1:250) (Jackson Laboratory, Bar Harbor, MI) was used as a secondary antibody for hS100A15 or hS100A7, respectively. When sections were co-stained, monoclonal mouse anti-hS100A7 or smooth muscle actin (1:25, Serotec, Raleigh, NC) were mixed with the primary hS100A15 antibody. All sections were nuclear stained with DAPI (Sigma) and mounted.
In preliminary studies, we tested antibodies previously used to study the expression of hS100A7 in breast cancer [1;9;12;13]. Both polyclonal chicken (Fig 1SA) and rabbit (Fig 1SB) hS100A7 antibodies did not cross-react with hS100A8 and hS100A10 proteins but recognized both hS100A7 and hS100A15 proteins. Further, both antibodies were able to detect corresponding native S100 proteins in human keratinocyte lysates (Fig 1SA, B). Sensitivity and specificity of tested commercial and custom antibodies generated against hS100A7 and hS100A15 are summarized in Fig 1C.
Results
hS100A7 and hS100A15 can be discriminated
Using antibodies generated in rabbits to a unique N-terminal sequence in human S100A15 (hS100A15), immunoblotting revealed a single monomer band of recombinant hS100A15 distinct from hS100A7 as well as corresponding uncleaved recombinant protein (Fig 1A). The hS100A15 antibody did not detect the highly homologous hS100A7 protein. Similarly, the monoclonal hS100A7 antibodies (Abcam, Imgenex) revealed specific staining of the hS100A7 monomer in addition to high molecular weight bands of uncleaved recombinant hS100A7 protein (Fig 1B and not shown). In contrast, the commercial polyclonal hS100A7 antibody (Exalpha Biologicals) detects both recombinant hS100A7 and hS100A15 proteins but not related hS100A8 and hS100A10 (data not shown). Specificity of tested commercial and custom antibodies generated against hS100A7 and hS100A15 are summarized in Fig 1 and Fig 1S.
Human S100A7 and S100A15 are differentially expressed in normal breast tissue
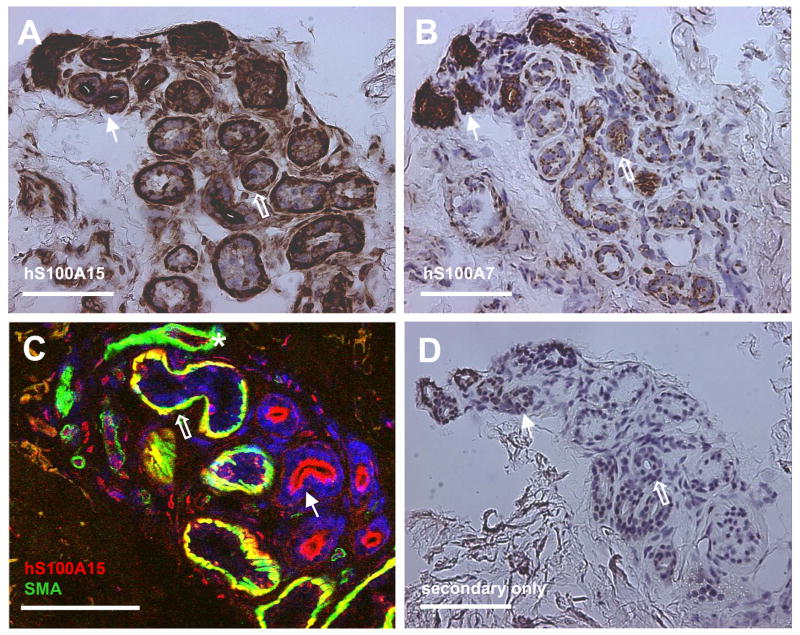
Because of the previous lack of a specific hS100A15 antibody, cell type specific expression of hS100A7 and hS100A15 in normal breast structures has not been reported. Using hS100A7- and hS100A15-specific antibodies, we analyzed the differential expression and distribution of these highly homologous proteins in normal breast tissue. Both hS100A15 (Fig 2A, C) and hS100A7 (Fig 2B) were expressed in normal lobular epithelial cells and more prominently by breast ducts. Further, hS100A15 is expressed by epithelium-derived myoepithelial cells (smooth muscle actin positive, Fig 2C) surrounding the breast alveoli, where hS100A7 could not be detected. In the stromal compartment, hS100A15 staining was noted in endothelial cells interior to vascular smooth muscle cells. This broader expression of hS100A15 and its presence in endothelial cells and smooth muscle cells but absence in stromal fibroblasts has been noted previously in human skin sections and speaks to specific functions of this protein in multiple specialized cell types [8].
Figure 2. Human S100A7 and S100A15 proteins are differentially expressed in normal breast tissue.
Immunohistochemical staining of frozen sections of normal adult breast tissue stained with hS100A15 custom antibodies (A, C) and hS100A7 monoclonal antibody (B). Co-staining of hS100A15 with smooth muscle actin (C). Hollow arrows indicate breast acini, and full arrows breast ducts. Nuclei were stained with DAPI (C, blue). Secondary antibody control (D). Bar sizes: 100 μm
Human S100A7 and S100A15 are differentially regulated in ductal breast carcinoma
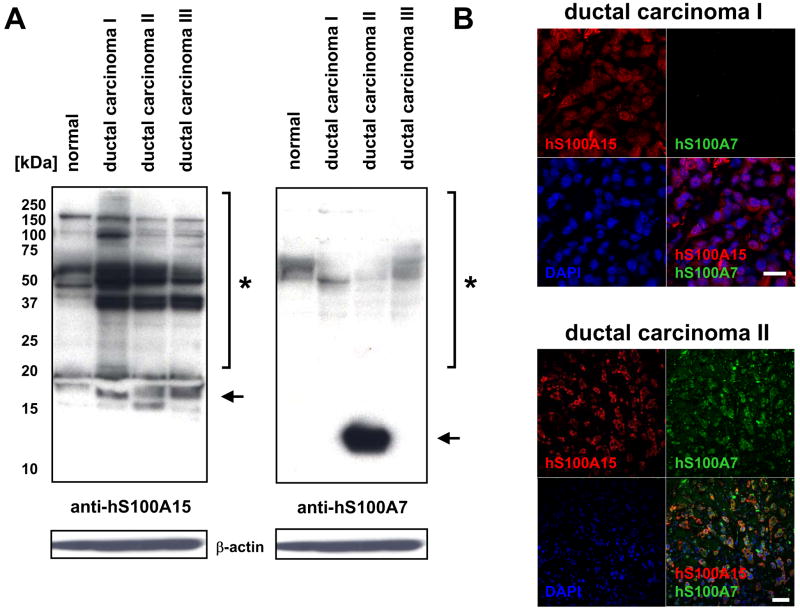
Previous studies suggest hS100A7 is upregulated in selective invasive carcinomas of the breast. To study the expression of these highly homologous proteins, lysates and histological sections of a pilot cohort of several ductal carcinomas was analyzed by immunoblotting and immunostaining. Both low and high molecular weight forms of hS100A15 were detected in normal breast lysates, and these were increased in invasive carcinomas along with additional S100A15 multimers unique to tumors (Fig 3A, left panel). The absence of the 11kD hS100A15 monomer in normal breast and breast tumors is unexplained from these studies but consistent with findings in keratinocyte lysates as previously reported [8]. In contrast, one of the investigated tumors showed a pronounced expression of the hS100A7 monomer, and the corresponding multimers were less prominent in both normal tissue and carcinomas. The hS100A7 band pattern is distinct from that of hS100A15 (Fig 3A, right panel). Immunostaining also revealed differential detection of hS100A7 and hS100A15 in tumor sections (Fig 3B). Although low levels of hS100A7 were detected in immunoblot analysis of ductal carcinoma I, only hS100A15 was detected by immunostaining. However, both hS100A7 and hS100A15 were detected in ductal carcinoma II stained sections, consistent with distinct detection of both proteins in immunoblot analysis (compare Fig 3A).
Figure 3. Human S100A7 and S100A15 are differentially regulated in invasive breast carcinoma.
A) Protein lysates of normal breast tissue and invasive breast carcinomas (20 μg), subjected to immunoblotting by incubation with polyclonal anti-hS100A15 antibody 3923 or monoclonal anti-hS100A7. Shown are low (arrow) and high (*) molecular weight forms in breast tissue lysates. B) Co-staining of hS100A15 (red) with hS100A7 (green) in corresponding ductal carcinomas (A) using indicated antibodies. Nuclei were stained with DAPI (blue). Bar sizes: 20 μm
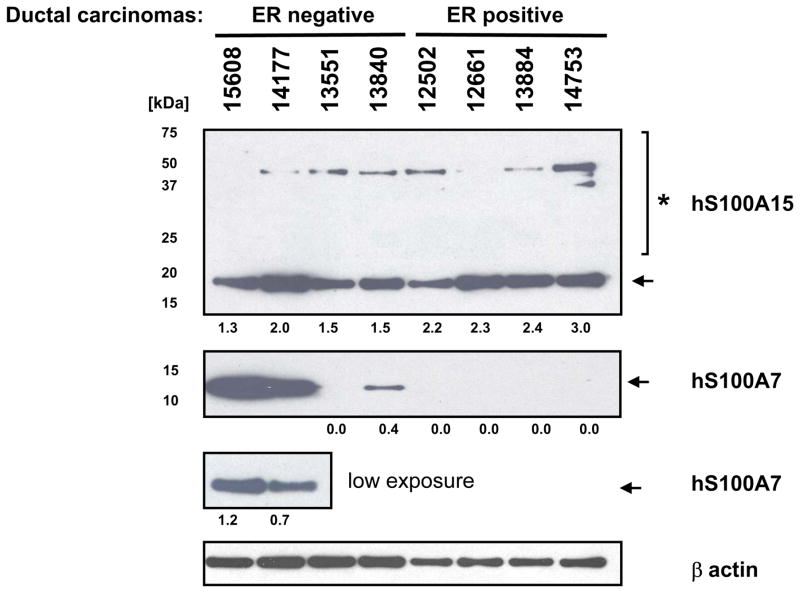
The differential regulation of hS100A7 and hS100A15 mRNAs and proteins was confirmed in a larger previously documented sample case cohort of breast tumors (see material and methods) using specific primers for qPCR analysis and antibodies for immunoblotting (summarized in Table I and Fig 4). hS100A7 and hS100A15 transcript levels varied among the investigated samples but both were co-upregulated in invasive carcinomas (9/20 cases) particularly in ER/PR negative tumors (9/10). There, hS100A15 expression was associated with the long S100A15-mRNA isoform, hS100A15-L, (9/10 ER/PR negative cases), whereas the short RNA transcript, hS100A15-S, was elevated in only a few ER/PR negative cases (2/10, data not shown). In cancer cell lysates, the hS100A15 protein was expressed ubiquitously at similar levels in ER positive and ER negative invasive carcinomas and both low molecular weight bands and multimers are detected (Fig 4). In contrast, hS100A7 protein expression followed closely the corresponding RNA levels, suggesting a strong transcriptional regulation of the S100A7 protein compared with hS100A15. In these samples, only hS100A7 low molecular weight bands were detected.
Table I. S100A7 and S100A15 transcript expression in invasive carcinomas.
Relative transcript levels of hS100A7 and hS100A15-L (long isoform) in invasive breast carcinomas
| # | case | a hS100A7 | b S100A15-L | c ER | d PR |
|---|---|---|---|---|---|
| 1 | 13895 | 4 | 4 | neg | neg |
| *2 | 15608 | 4 | 2 | neg | neg |
| 3 | 14664 | 3 | 4 | neg | neg |
| *4 | 14177 | 4 | 2 | neg | neg |
| 5 | 15021 | 2 | 3 | neg | neg |
|
| |||||
| 6 | 14744 | 2 | 2 | neg | neg |
| 7 | 14232 | 2 | 1 | neg | neg |
| *8 | 13551 | 2 | 2 | neg | neg |
| *9 | 13840 | 2 | 3 | neg | neg |
| 10 | 15170 | 2 | 2 | neg | pos |
|
| |||||
| 11 | 11186 | 1 | 1 | pos | neg |
| 12 | 11429 | 1 | 1 | pos | pos |
| 13 | 11945 | 1 | 1 | pos | neg |
| *14 | 12502 | 1 | 1 | pos | neg |
| *15 | 12661 | 1 | 1 | pos | neg |
|
| |||||
| 16 | 13212 | 1 | 1 | pos | pos |
| 17 | 13642 | 1 | 1 | pos | neg |
| *18 | 13884 | 1 | 1 | pos | pos |
| 19 | 13918 | 2 | 1 | pos | neg |
| *20 | 14753 | 1 | 1 | pos | pos |
hS100A7 expression by qRT-PCR relative to hGAPDH (1: ≤ 10, 2: >10-2000, 3: >2000-10.000, 4: >10000)
hS100A15-long transcript expression by qRT-PCR PCR relative to hGAPDH (1: ≤ 10, 2: >10-200, 3: >200-500, 4: >500
ER −ve >3 fmol/mg protein
PR −ve >15 fmol/mg protein
confirmed by immunoblot analysis (see Fig 4)
Figure 4. Human S100A7 and S100A15 are distinctly expressed in invasive breast carcinoma.
A) Protein lysates of invasive carcinomas (5 μg), subjected to immunoblotting by incubation with polyclonal anti-hS100A15 antibody 3923 or monoclonal anti-hS100A7. Shown are S100 low (arrow) and high (*) molecular weight forms in breast tissue lysates. hS100A7 high molecular forms could not be detected in these samples (not shown). Numbers indicate the expression of hS100A7 and hS100A15 relative to beta-actin.
Discussion
We have been able to discriminate specific expression patterns for almost identical hS100A7 and hS100A15 paralogs in human breast by overcoming the specificity issues inherent in previous reports that used cross-reacting antibodies and primers. Upregulation of hS100A7 has been previously associated with breast cancer, and it was identified by microarray analysis to be expressed in normal breast, preferentially in myoepithelial cells [14]. However, much of this previous work on hS100A7 has been at the mRNA level [3;14] and precedes the discovery of the highly related hS100A15 gene. Here we use antibodies that specifically distinguish hS100A7 and hS100A15 and first demonstrate that both proteins can be detected in specific cellular subsets of alveolar and small duct luminal cells within normal breast. In addition, hS100A15 but not hS100A7 is detected in myoepithelial cells and endothelial cells of the stroma. In skin, both proteins have antimicrobial functions as part of an innate host defense controlling and precedes the discovery of the highly related hS100A15 gene. Here we use antibodies that specifically distinguish hS100A7 and hS100A15 and first demonstrate that both proteins can be detected in specific cellular subsets of alveolar and small duct luminal cells within normal breast. In addition, hS100A15 but not hS100A7 is detected in myoepithelial cells and endothelial cells of the stroma. In skin, both proteins have antimicrobial functions as part of an innate host defense controlling E. coli growth [15;16]. Thus, one potential function of these proteins in normal breast tissue could be to regulate the microbial homeostasis within the host as well as in the digestive tract of nursing newborns. That hS100A15 is expressed by epithelial-derived myoepithelial cells around acini and in surrounding blood vessels may reflect its biological diversity from hS100A7 with additional, distinct functions for hS100A15 [8]. In particular the proteins have been shown to attract specific subtypes of inflammatory cells that may play an important function in normal homeostasis and disease [8].
While little is known about the intracellular functions of hS100A15, hS100A7 has been reported to regulate the expression of pro-survival genes in breast cancer through interaction with Jab1 [17]. Identification of the Jab1-binding motif in the N-terminal sequence of hS100A7 that is different in hS100A15 suggests that Jab-1 might not be an intracellular target for hS100A15. Similarly the binding of extracellular hS100A7 to the pattern receptor RAGE while S100A15 binds to a G protein coupled receptor[8] indicates further distinct functions for these closely related homologues. Because of the previous lack of awareness of the cross-reactivity of certain hS100A7 antibodies with the recently discovered hS100A15, and similar problems with RNA and DNA probes (unpublished data), hS100A15 might have contributed to the reported hS100A7 association with tumor progression. However, mechanistic contributions of these proteins inside the tumor cells as well as in the tumor stroma remain to be determined [18;19]. In pre-neoplastic breast lesions, increased hS100A7 has been associated with atypical apocrine differentiation [20]. Further study will now be required to explore the contribution of hS100A15 in this situation.
The ability to distinguish the closely related hS100A7 and hS100A15 at the RNA and protein level has revealed significant distinctions in their regulation. The breast cancer specimens we have studied link high expression levels of both hS100A7 and hS100A15 transcripts with ER negativity and imply a correlation to clinical outcome as previously indicated for S100A7 [9]. However, while hS100A7 protein expression closely follows corresponding RNA levels, hS100A15 protein is ubiquitous in invasive carcinomas and appears to be preferentially modified/cross-linked, and thus providing a more stable and potentially longer lived protein even when transcript levels are low. Interestingly, neither protein is detected as 11kD monomer when extracted from tissues and analyzed by immunoelectrophoresis, suggesting the proteins are modified in vivo by as yet undefined mechanisms.
The coincident but differential expression and intracellular localization of these almost identical paralogs could have significant biological implications for normal breast and breast cancer. Co- expression of transcripts for both S100A7 and hS100A15 proteins in ER/PR negative tumors suggests a joint regulation related to tumor progression. While the secreted proteins have distinct roles as chemoattractants [8], they also act synergistically to enhance inflammation and thus could influence both normal breast epithelium and breast tumors.
This study shows that it is important to discriminate the highly related hS100A7 and hS100A15 paralogs and opens the opportunity to further dissect their differential roles in normal tissue and their use as distinct markers in breast cancer pathogenesis.
Supplementary Material
A,B) Indicated recombinant human S100 proteins (50 ng) and human keratinocyte lysates (20 μg) subjected to immunoblotting by incubation with chicken or rabbit polyclonal anti-hS100A7 antibody. Both antibodies detect high (*) molecular weight forms in keratinocytes (A, B).
Acknowledgments
We thank Dr Akira Oshima for histological evaluation. This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. Ronald Wolf is funded by the German Research Foundation (DFG), Emmy-Noether Program (Wo 843/2-1).
Footnotes
Conflict of interest statement
Authors declare no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Al Haddad S, Zhang Z, Leygue E, Snell L, Huang A, Niu Y, Hiller-Hitchcock T, Hole K, Murphy LC, Watson PH. Psoriasin (S100A7) expression and invasive breast cancer. American Journal of Pathology. 1999;155:2057–2066. doi: 10.1016/S0002-9440(10)65524-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Emberley ED, Alowami S, Snell L, Murphy LC, Watson PH. S100A7 (psoriasin) expression is associated with aggressive features and alteration of Jab1 in ductal carcinoma in situ of the breast. Breast Cancer Res. 2004;6:R308–R315. doi: 10.1186/bcr791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leygue E, Snell L, Hiller T, Dotzlaw H, Hole K, Murphy LC, Watson PH. Differential expression of psoriasin messenger RNA between in situ and invasive human breast carcinoma. Cancer Res. 1996;56:4606–4609. [PubMed] [Google Scholar]
- 4.Schafer BW, Heizmann CW. The S100 family of EF-hand calcium-binding proteins: functions and pathology. TIBS. 1996:134–140. doi: 10.1016/s0968-0004(96)80167-8. [DOI] [PubMed] [Google Scholar]
- 5.Kulski JK, Lim CP, Dunn DS, Bellgard M. Genomic and phylogenetic analysis of the S100A7 (Psoriasin) gene duplications within the region of the S100 gene cluster on human chromosome 1q21. J Mol Evol. 2003;56:397–406. doi: 10.1007/s00239-002-2410-5. [DOI] [PubMed] [Google Scholar]
- 6.Wolf R, Mirmohammadsadegh A, Walz M, Lysa B, Tartler U, Remus R, Hengge U, Michel G, Ruzicka T. Molecular cloning and characterization of alternatively spliced mRNA isoforms from psoriatic skin encoding a novel member of the S100 family. FASEB J. 2003;17:1969–1971. doi: 10.1096/fj.03-0148fje. [DOI] [PubMed] [Google Scholar]
- 7.Wolf R, Lewerenz V, Buchau AS, Walz M, Ruzicka T. Human S100A15 splice variants are differentially expressed in inflammatory skin diseases and regulated through Th1 cytokines and calcium. Exp Dermatol. 2007;16:685–691. doi: 10.1111/j.1600-0625.2007.00587.x. [DOI] [PubMed] [Google Scholar]
- 8.Wolf R, Howard OMZ, Dong HF, Voscopoulos C, Boeshans K, Winston J, Divi R, Gunsior M, Goldsmith P, Ahvazi B, Chavakis T, Oppenheim JJ, Yuspa SH. Chemotactic activity of S100A7 (psoriasin) is mediated by RAGE and potentiates inflammation with highly homologous but functionally distinct S100A15. J Immunol. 2008;181:1499–1506. doi: 10.4049/jimmunol.181.2.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Emberley ED, Niu Y, Njue C, Kliewer EV, Murphy LC, Watson PH. Psoriasin (S100A7) expression is associated with poor outcome in estrogen receptor-negative invasive breast cancer. Clin Cancer Res. 2003;9:2627–2631. [PubMed] [Google Scholar]
- 10.Goldsmith P, Gierschik P, Milligan G, Unson CG, Vinitsky R, Malech HL, Spiegel AM. Antibodies directed against synthetic peptides distinguish between GTP-binding proteins in neutrophil and brain. J Biol Chem. 1987;262:14683–14688. [PubMed] [Google Scholar]
- 11.Boeshans KM, Wolf R, Voscopoulos C, Gillette W, Esposito D, Mueser TC, Yuspa SH, Ahvazi B. Purification, crystallization and preliminary X-ray diffraction of human S100A15. Acta Crystallograph Sect F Struct Biol Cryst Commun. 2006;62:467–470. doi: 10.1107/S1744309106012838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enerback C, Porter DA, Seth P, Sgroi D, Gaudet J, Weremowicz S, Morton CC, Schnitt S, Pitts RL, Stampl J, Barnhart K, Polyak K. Psoriasin expression in mammary epithelial cells in vitro and in vivo. Cancer Res. 2002;62:43–47. [PubMed] [Google Scholar]
- 13.Jiang WG, Watkins G, Douglas-Jones A, Mansel RE. Psoriasin is aberrantly expressed in human breast cancer and is related to clinical outcomes. Int J Oncol. 2004;25:81–85. [PubMed] [Google Scholar]
- 14.Jones C, Mackay A, Grigoriadis A, Cossu A, Reis-Filho JS, Fulford L, Dexter T, Davies S, Bulmer K, Ford E, Parry S, Budroni M, Palmieri G, Neville AM, O’Hare MJ, Lakhani SR. Expression profiling of purified normal human luminal and myoepithelial breast cells: identification of novel prognostic markers for breast cancer. Cancer Res. 2004;64:3037–3045. doi: 10.1158/0008-5472.can-03-2028. [DOI] [PubMed] [Google Scholar]
- 15.Buchau AS, Hassan M, Kukova G, Lewerenz V, Kellermann S, Wurthner JU, Wolf R, Walz M, Gallo RL, Ruzicka T. S100A15, an Antimicrobial Protein of the Skin: Regulation by E. coli through Toll-Like Receptor 4. J Invest Dermatol. 2007 doi: 10.1038/sj.jid.5700946. [DOI] [PubMed] [Google Scholar]
- 16.Glaser R, Harder J, Lange H, Bartels J, Christophers E, Schroder JM. Antimicrobial psoriasin (S100A7) protects human skin from Escherichia coli infection. Nat Immunol. 2005;6:57–64. doi: 10.1038/ni1142. [DOI] [PubMed] [Google Scholar]
- 17.Emberley ED, Niu Y, Leygue E, Tomes L, Gietz RD, Murphy LC, Watson PH. Psoriasin interacts with Jab1 and influences breast cancer progression. Cancer Res. 2003;63:1954–1961. [PubMed] [Google Scholar]
- 18.Emberley ED, Murphy LC, Watson PH. S100A7 and the progression of breast cancer. Breast Cancer Res. 2004;6:153–159. doi: 10.1186/bcr816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Krop I, Marz A, Carlsson H, Li X, Bloushtain-Qimron N, Hu M, Gelman R, Sabel MS, Schnitt S, Ramaswamy S, Kleer CG, Enerback C, Polyak K. A putative role for psoriasin in breast tumor progression. Cancer Res. 2005;65:11326–11334. doi: 10.1158/0008-5472.CAN-05-1523. [DOI] [PubMed] [Google Scholar]
- 20.Celis JE, Gromova I, Gromov P, Moreira JM, Cabezon T, Friis E, Rank F. Molecular pathology of breast apocrine carcinomas: a protein expression signature specific for benign apocrine metaplasia. FEBS Letters. 2006;580:2935–2944. doi: 10.1016/j.febslet.2006.03.080. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A,B) Indicated recombinant human S100 proteins (50 ng) and human keratinocyte lysates (20 μg) subjected to immunoblotting by incubation with chicken or rabbit polyclonal anti-hS100A7 antibody. Both antibodies detect high (*) molecular weight forms in keratinocytes (A, B).