Abstract
It has been reported that penile PDE5 expression was under androgen regulation. However it remained unknown whether the observed change in PDE5 expression in castrated animals was under direct androgen regulation or due to changes in smooth muscle content. In the present study we showed that castration of rats caused a reduction of penile size and cavernous smooth muscle content. Immunostaining detected concomitant reduction of PDE5 and alpha smooth muscle actin (α-SMA) expression in the corpus cavernosum of castrated rats. Real-time PCR and western blotting detected no change of PDE5 expression when normalized with α–SMA expression in castrated rats. Androgen receptor (AR) expression was increased while PDE5 expression remained unchanged in DHT-treated rat cavernous smooth muscle cells (CSMC). Prostate specific antigen (PSA) promoter activity was upregulated while PDE5A promoter activity remained unchanged in DHT-treated CSMC. Thus, PDE5 expression was not under direct androgen regulation.
Keywords: Penis, PDE5, androgen, cavernous smooth muscle
INTRODUCTION
Detumescence of the erect penis depends on the hydrolysis of cGMP by PDE5 [1; 2]. Pharmacological inhibition of PDE5’s cGMP-hydrolytic activity results in the temporary restoration of erectile function in the otherwise dysfunctional erectile tissue [2; 3]. Physiological or pathological regulation of PDE5 activity or PDE5 expression, particularly in the erectile tissue, is poorly understood. Downregulation of PDE5 expression has been observed in the penis of rats with ligated pudendal arteries [4] or mice with disrupted eNOS gene [5]. Reduced PDE5 expression has also been observed in the penis of male-to-female transsexual individuals and in orchiectomized animals, and these observations have led to the interpretation that PDE5 expression is positively regulated by androgens [6; 7; 8].
In year 2000 when we succeeded in cloning the human PDE5A promoter, we recognized in its upstream region a putative androgen responsive element (ARE) [9]. However, repeated efforts failed to demonstrate androgen regulation of PDE5 expression, and as such, the negative results were never published. In a 2006 review article [2] we mentioned the negative results and we cited the two studies that showed positive androgen regulation [6; 7]. However, it remains unclear why androgens, which are considered by these authors as positive regulators for erectile function, could also be positive regulators for PDE5, which is a key molecule for the termination of erection and is currently the best target for ED treatment [2]. This apparent self-contradiction and our earlier negative results prompted us to reexamine the effect of androgens on PDE5 expression. We repeated and expanded our earlier experiments of year 2000; we also conducted castrated animal experiments similar to those whose results were interpreted as evidence of androgen regulation of PDE5 [6; 7; 8]. We report here that the observed reduction of PDE5 expression in castrated animals is due to a reduction of smooth muscle content, not direct androgen regulation.
MATERIALS AND METHODS
Castration rat model
Twenty 8-week-old male Sprague-Dawley rats were equally and randomly divided into a control group and a castration group. Under anesthesia, rats of castration group underwent bilateral orchiectomy through a 1-cm midline scrotal incision. Rats of control group were sham operated. One month later, the rats’ body weight was measured and their penises harvested. After measurement of weight and length, each penis was divided into 3 parts for immunofluorescence, western blot, and RT-PCR. These animal experiments were approved by the Institutional Animal Care and Use Committee of the University of California at San Francisco.
Cell culture and androgen treatment
Rat cavernous smooth muscle cells (CSMC) were isolated and cultured as previously described [4]. For androgen treatment, CSMC were cultured in phenol red-free Dulbecco’s modified eagle’s medium with 10% charcoal-treated fetal bovine serum (HyClone Laboratories, Inc., Logan, UT). Cells were seeded at 1×106 cells per 10-cm dish for 24 h, treated with 30 nM dihydrotestosterone (DHT, Sigma-Aldrich Corporation, St. Louis, MO), and harvested at various time points as indicated in Results.
Immunofluorescence staining
Details of immunofluorescence staining have been described previously [10]. Briefly, frozen sections of the mid-penis were reacted to anti-α–SMA antibody (1:10000, Sigma, St Louis, MO) or anti-PDE5A antibody (1:1500, Santa Cruz Biotechnology, Santa Cruz, CA), followed by staining with FITC-conjugated goat anti-rabbit or Texas red-conjugated goat anti mouse secondary antibody (Jackson Immuno-Research, West Grove, PA). Nuclear staining was performed with 4′,6-diamidino-2-phenylindole (DAPI, 1 μg/ml, Sigma-Aldrich, St. Louis, MO). Five randomly selected fields per tissue were photographed and recorded using Retiga Q Image digital still camera and ACT-1 software (Nikon Instruments Inc., Melville, NY).
RT-PCR and real-time PCR
RT-PCR was performed as previously described [11]. PDE5A1, PDE5A2, and β-actin primers used in these experiments were the same as those reported previously [12]. The real-time PCR procedure has also been described recently [13]. The reaction consisted of 4 μl cDNA, 4 μl primers [500 nM], 2 μl H2O and 10 μl SYBR Green PCR master mixture and was run in ABI7300 PCR System (Applied Biosystems, Foster City, CA) with settings of 1 cycle at 95°C for 10 min, 1 cycle at 95°C for 15s and 60°C for 1 min, 40 cycles at 95°C for 15s, 60°C for 30s, and 95°C for 15s. The primers used in real-time PCR were: α–SMA-forward: 5′-CGGGCTTTGCTGGTGATG-3′, α–SMA-reverse: 5′-GGTCAGGATCCCTCTCTTGCT-3′, PDE5-forward: 5′-CACAGTGCATGTTTGCTGCTCTGA-3′, and PDE5-reverse: 5′-CAATCAGCAATGCAAGCGTCTCCA-3′. GAPDH-forward: 5′-ATGATTCTACCCACGGCAAG-3′, and GAPDH-reverse: 5′-CTGGAAGATGGTGATGGGTT-3′. Each experiment was repeated three times.
Western blot analysis
Primary antibodies used in these experiments were anti-α–SMA (Sigma-Aldrich Corporation, St. Louis, MO), anti-AR, anti-PDE5 and anti-β-tubulin (Santa Cruz Biotech, Santa Cruz, CA). Details of the western blotting procedure have been described previously [4]. Briefly, equal amount of protein (20 μg) from each tissue or cell sample was electrophoresed in 8% SDS-PAGE, transferred to PVDF membrane (Millipore Corp., Bedford, MA), incubated with a primary antibody overnight at 4°C, and then incubated with HRP-conjugated secondary antibody (Pierce Chemical Company, Rockford, IL) for 1 h at room temperature. Detection of reactive antigens was performed with ECL kit (Amersham Life Sciences Inc., Arlington Heights, IL). The resulting image was analyzed with ChemiImager 4000 (Alpha Innotech, San Leandro, CA) for protein band densitometry.
Promoter assay
PDE5A promoter construct and details of promoter assay procedure have been described previously [9]. Prostate specific antigen (PSA) promoter construct [14] was used as positive control. Briefly, CSMC were seeded at approximately 2 × 105 per well in a 6-well plate. The next day, the seeded cells were transfected with 1 μg of PDE5A or PSA promoter construct and 20 ng of pRL-SV40 (Promega, Inc., Madison, WI). When indicated, cells were treated with 30 nM of DHT 24 h after transfection. The cells were further maintained for 24 h before being washed and lysed in 500 μl of lysis buffer (Promega Inc. Madison, WI). The lysates (20 μl) were assayed for firefly luciferase activity (PDE5A or PSA promoter activity) and Renilla luciferase activity (SV40 promoter activity) with the Dual-Luciferase reporter assay system (Promega Inc. Madison, WI) and the TD-20/20 luminometer (Turner Designs Inc., Sunnyvale, CA). The experiment was done in triplicates.
Statistical analysis
Data were analyzed with Prism 4 (GraphPad Software, Inc., San Diego, CA). T-test was used to analyze differences in mRNA or protein expression between castrated and control rats or between DHT and vehicle-treated cells. T-test was used to analyze differences in promoter activity between DHT and vehicle-treated cells. P value <0.05 was considered significant.
RESULTS
Effect of castration on general penile characteristics
Twenty 8-week-old male rats were randomly divided into a castration group of 10 rats and a control (sham-operated) group of 10 rats. One month later, after body weight measurements (Table 1), the rats were sacrificed and their penises harvested. Size differences in the penises of castration and control groups were readily identifiable upon gross inspection. Weight and length measurements confirmed such observations (Table 1). Measurements of the circumference of the fixed penile specimens also confirmed the difference between castration and control groups (Table 1).
Table 1.
Comparison of body weight and penile characteristics between castrated and control rats.
| Control group (N=10) | Castration group (N=10) | |
|---|---|---|
| Body weight (g) | 425.1 ± 11.7 | 400.8 ± 9.6 |
| Penile weight (mg) | 314.40 ± 8.66 | 192.7 ± 4.0 ** |
| Penile weight/body weight (×10−3) | 0.743 ± 0.024 | 0.484 ± 0.018 ** |
| Penile length (mm) | 21.60 ± 0.37 | 19.10 ± 0.28 ** |
| Penile circumference * (mm) | 10.74 ± 0.27 | 8.72 ± 0.20 ** |
Penile circumference was measured on tissue sections that were used for immunofluorescence analysis (Fig. 1).
P < 0.01 versus control group
Effect of castration on PDE5 expression
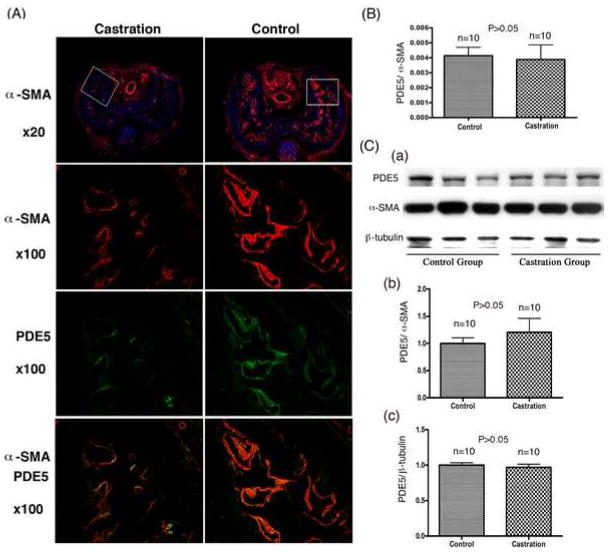
The middle part of each harvested penis was examined by immunofluorescence microscopy. Mirroring the above-described size differences, castrated rats had much reduced levels of α–SMA expression in the corpus cavernosum when compared to those of control rats (Fig. 1A). Interestingly, α–SMA was expressed at similar levels in the dorsal arteries and central vein of castrated and control rats (Fig. 1A). Castrated rats also had reduced levels of PDE5 expression in the corpus cavernosum when compared to control rats (Fig. 1A). For real-time PCR analysis, the distal part of each harvested penises was used. The results showed that the difference in PDE5 expression between castrated and control rats was statistically insignificant (Fig. 1B). For western blot analysis, the proximal part of each harvested penises was used. In these experiments equal amounts of protein (20 μg each) of the homogenized penises, regardless of the penile size, were examined. Under this condition, there was no significant difference in the expression of PDE5 or α–SMA between castrated and control rats (Fig. 1C). As an added layer of precaution, another control protein, β-tubulin, was also used for normalization of PDE5 expression, and the results again showed similar levels of PDE5 expression between castrated and control rats (Fig. 1C). Together, the staining, real-time PCR and western blotting data show that castration caused a reduction of cavernous smooth muscle content but not the relative amounts PDE5 or α–SMA (relative to the total protein content).
Fig. 1.
Effects of castration on PDE5 expression. (A) The mid-penis was examined by immunofluorescence microscopy for α–SMA (red) or PDE5 (green). Nuclear staining with DAPI (blue) was used to locate cells. Boxes in the 20x graphs indicate areas that are displayed in the 100x graphs. (B) The distal penis was examined by real-time PCR. The expression levels of PDE5 and α–SMA were each normalized with GAPDH. The normalized values were then presented as a ratio (PDE5/α-SMA). (C) The proximal penis was examined by western blot. Panel a shows the protein bands of PDE5, α–SMA, and β-tubulin of 3 rats from each group. Panel b shows the results of densitometric analysis of the protein bands of PDE5 and α–SMA of all 10 rats from each group. Panel c shows the results of densitometric analysis of the protein bands of PDE5 and β-tubulin of all 10 rats from each group.
Effect of DHT treatment on PDE5 expression
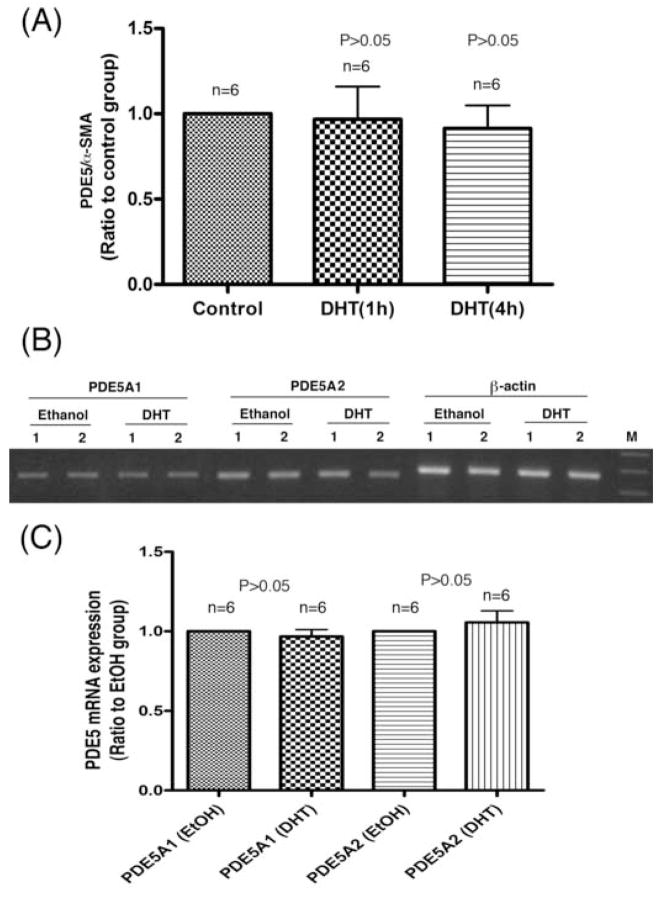
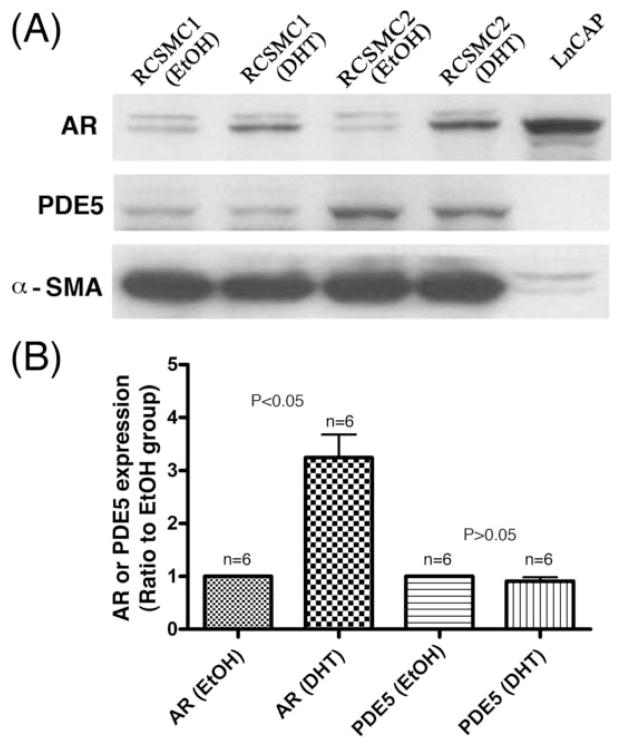
Testosterone was used in previous studies that reported androgen regulation of PDE5 expression [6; 7; 8]. However, testosterone can be converted into estrogen and its use thus can produce unintended effects. In addition, DHT has been shown to be the active androgen in erectile tissue [15]. Thus, DHT rather than testosterone was used throughout this study. Treatment of cavernous smooth muscle cells with DHT for 1 h or 4 h produced no increase of PDE5 mRNA expression, as assessed by real-time PCR (Fig. 2A). Treatment for 24 h also did not result in increased expression of either PDE5A1 or PDE5A2, as assessed by RT-PCR (Fig. 2B). Shorter treatment times (1 h and 4 h) produced similar RT-PCR results as the 24 h-treatment results (data not shown). The treated cells were also analyzed by western blotting. Because it has been reported that DHT treatment of CSMC caused increased expression of androgen receptor [16], we examined AR expression to ensure that our CSMC did respond to DHT treatment. We also included AR-expressing prostate cancer cell line LnCap as positive control. The results of 24 h DHT treatment showed that, while AR expression was clearly upregulated in DHT-treated cells, PDE5 expression was not (Fig. 3).
Fig. 2.
Effect of androgen on PDE5 mRNA expression. Two rat CSMC strains (indicated as 1 and 2 in panel B) were used in these experiments. Panel A shows the results of real-time PCR analysis of cells treated with DHT or vehicle (ethanol, noted as control) for 1 h or 4 h. The expression levels of PDE5 and α–SMA were each normalized with GAPDH. The normalized values were then presented as a ratio (PDE5/α–SMA). Panel B shows the results of RT-PCR analysis of cells treated with DHT or vehicle (ethanol) for 24 h. Lane M is size marker (500, 400, and 300 bp). Panel C shows compiled results of densitometric analysis of 3 experiments with 2 CSMC strains (n=6) as done in panel B.
Fig. 3.
Effect of androgen on PDE5 protein expression. (A) Two rat CSMC strains (1 and 2) were treated with DHT or vehicle (ethanol, EtOH) for 24 h and then analyzed by western blotting. Prostate cancer cells (LnCAP) were included as positive androgen receptor (AR) control. (B) Compiled results of densitometric analysis of 3 experiments with 2 CSMC strains (n=6) as done in panel A.
Effect of DHT treatment on PDE5A promoter activity
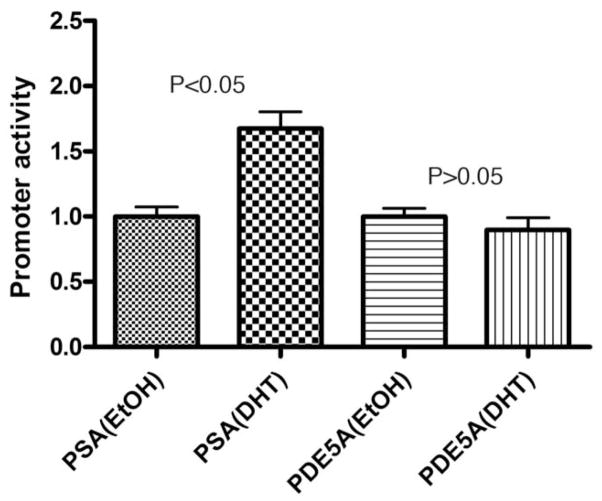
We previously reported the identification of a putative ARE in the upstream region of human PDE5A promoter [9]. Thus a reporter construct containing this ARE was used for the detection of possible androgen regulation of PDE5A promoter. To ensure the effectiveness of DHT treatment and the efficacy of the promoter assay system, a reporter construct containing the androgen-responsive PSA promoter [14] was used as a positive control. Indeed, treatment of CSMC with DHT resulted in an upregulation of PSA promoter (Fig. 4). However, under identical treatment conditions, the activity of PDE5A promoter remained unchanged (Fig. 4).
Fig. 4.
Effect of androgen on PDE5A promoter activity. CSMC were transfected with 1 μg of PDE5A or PSA promoter construct and 20 ng of SV40 promoter construct (transfection control). The transfected cells were then treated with 30 nM of DHT or vehicle (ethanol, EtOH) for 24 h. The cells were assayed for firefly luciferase activity (PDE5A or PSA promoter activity) and Renilla luciferase activity (SV40 promoter activity). The ratio between firefly and Renilla luciferase activities of vehicle-treated cells is expressed as 1.0 on the y-axis. The ratio between firefly and Renilla luciferase activities of DHT-treated cells is expressed relative to the respective (PSA or PDE5A) vehicle-treated value on the y-axis. The experiment was done in triplicates.
DISCUSSION
Between 2004 and 2006 three studies reported that PDE5 was positively regulated by androgens [6; 7; 8]. In addition, 5 recent review articles cited these studies as evidence for androgen regulation of PDE5 [17; 18; 19; 20; 21]. However, if indeed androgens upregulate PDE5 expression, wouldn’t they undermine the effectiveness of PDE5 inhibitors? The fact is that, on the contrary, many published studies have shown that androgens increased the effectiveness of PDE5 inhibitors, as summarized in a recent review article [22].
Upon closer examination of the 3 original studies [6; 7; 8] we found that they did not investigate the smooth muscle content and did not use smooth muscle actin as control in western blotting and/or RT-PCR analyses. This lack of smooth muscle control raised the question whether the observed reduction of PDE5 expression in castrated animals was a result of reduced smooth muscle content. Indeed, several studies have shown that PDE5 is primarily expressed in smooth muscle and castration causes a reduction of penile smooth muscle content [23; 24; 25; 26; 27]. As such, we suspected that the observed reduction of PDE5 expression was due to a reduced smooth muscle content.
In the present study we noted that, at one month post-orchiectomy, the penises of castrated rats were much smaller than those of control rats. Similar observations have been reported previously [28]. Immunostaining further showed that castrated rats had significantly reduced levels of PDE5 expression in the corpus cavernosum. Similar observations have also been reported previously [6; 7; 8]. However, while these studies did not investigate changes in smooth muscle content, we performed immunostaining for α–SMA and obtained data showing a significant reduction of this smooth muscle marker in the corpus cavernosum of castrated rats. Thus, the reduced PDE5 expression in castrated animals paralleled the reduced penile size as well as the reduced smooth muscle content. Interestingly, no significant difference in α–SMA expression was found in the central vein and dorsal arteries of castrated and control rats. To our knowledge, such a differential effect of castration has not been reported and may warrant further investigation.
While our above-described PDE5 staining data are largely in agreement with the 3 previous studies [6; 7; 8], our PCR and western blotting data for PDE5 expression differ sharply from those reported in these previous studies. Specifically, in our hands, neither PCR nor western blotting detected a downregulation of PDE5 expression in the castrated rats’ penises. It should be noted that, while staining analysis has the ability to examine the overall structure of a rat penis (i.e., its cross section), PCR and western blotting analyses do not. As illustrated in Table 1, castration caused a significant reduction of penile size, and this change was readily detectable by staining (Fig. 1A). Furthermore, a concomitant reduction of both the smooth muscle content and PDE5 expression in the castrated rats’ penises was also detectable by staining. On the other hand, there is no practical way to analyze the whole rat penis by PCR or western blotting. Rather, protein and RNA samples were compared at equal input regardless of tissue/organ sizes. As such, the reduction in penile size and the concomitant reduction in PDE5 expression could not and were not detected by PCR or western blotting analysis. Thus, while the staining experiments showed that castration caused a reduction of smooth muscle content and PDE5 expression, the PCR and western blotting analyses showed that the castration-associated reduction of PDE5 expression was due to a castration-associated reduction of smooth muscle content, not because of a reduction of PDE5 gene activity.
In addition to the above-described animal studies, we also assessed the effect of androgens on PDE5 expression more directly at the cellular level. By using AR expression as positive control [16], we showed that DHT had essentially no effect on PDE5 expression in CSMC. By using PSA promoter as positive control [14], we also showed that DHT had essentially no effect on PDE5A promoter. These negative results regarding possible androgen regulation of PDE5 expression were further supported by 3 recent studies in which genome-wide searches for androgen receptor-bound genes were performed [29; 30; 31]. Specifically, while these searches identified approximately 1,500 positive genes, PDE5 was not one of them. Thus, whether it was at gene, RNA, or protein level, PDE5 expression was not directly under androgen regulation.
Acknowledgments
We are grateful to Dr. Hongwu Chen for providing the PSA promoter construct. This work was supported in part by grants from the California Urology Foundation, Mr. Arthur Rock and the Rock Foundation, and the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lin CS, Lin G, Lue TF. Cyclic nucleotide signaling in cavernous smooth muscle. J Sex Med. 2005;2:478–91. doi: 10.1111/j.1743-6109.2005.00080.x. [DOI] [PubMed] [Google Scholar]
- 2.Lin CS, Lin G, Xin ZC, Lue TF. Expression, distribution and regulation of phosphodiesterase 5. Curr Pharm Des. 2006;12:3439–57. doi: 10.2174/138161206778343064. [DOI] [PubMed] [Google Scholar]
- 3.Lin CS, Xin ZC, Lin G, Lue TF. Phosphodiesterases as therapeutic targets. Urology. 2003;61:685–691. doi: 10.1016/s0090-4295(02)02439-1. [DOI] [PubMed] [Google Scholar]
- 4.Lin G, Xin ZC, Lue TF, Lin CS. Up and down-regulation of phosphodiesterase-5 as related to tachyphylaxis and priapism. J Urol. 2003;170:S15–8. doi: 10.1097/01.ju.0000075500.11519.e8. discussion S19. [DOI] [PubMed] [Google Scholar]
- 5.Champion HC, Bivalacqua TJ, Takimoto E, Kass DA, Burnett AL. Phosphodiesterase-5A dysregulation in penile erectile tissue is a mechanism of priapism. Proc Natl Acad Sci U S A. 2005;102:1661–6. doi: 10.1073/pnas.0407183102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morelli A, Filippi S, Mancina R, Luconi M, Vignozzi L, Marini M, Orlando C, Vannelli GB, Aversa A, Natali A, Forti G, Giorgi M, Jannini EA, Ledda F, Maggi M. Androgens regulate phosphodiesterase type 5 expression and functional activity in corpora cavernosa. Endocrinology. 2004;145:2253–63. doi: 10.1210/en.2003-1699. [DOI] [PubMed] [Google Scholar]
- 7.Zhang XH, Morelli A, Luconi M, Vignozzi L, Filippi S, Marini M, Vannelli GB, Mancina R, Forti G, Maggi M. Testosterone Regulates PDE5 Expression and in vivo Responsiveness to Tadalafil in Rat Corpus Cavernosum. Eur Urol. 2005;47:409–16. doi: 10.1016/j.eururo.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 8.Armagan A, Kim NN, Goldstein I, Traish AM. Dose-response relationship between testosterone and erectile function: evidence for the existence of a critical threshold. J Androl. 2006;27:517–26. doi: 10.2164/jandrol.05157. [DOI] [PubMed] [Google Scholar]
- 9.Lin CS, Chow S, Lau A, Tu R, Lue TF. Identification and regulation of human PDE5A gene promoter. Biochem Biophys Res Com. 2001;280:684–92. doi: 10.1006/bbrc.2000.4220. [DOI] [PubMed] [Google Scholar]
- 10.Lin G, Garcia M, Ning H, Banie L, Guo YL, Lue TF, Lin CS. Defining stem and progenitor cells within adipose tissue. Stem Cells Dev. 2008;17:1053–63. doi: 10.1089/scd.2008.0117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin CS, Lau A, Tu R, Lue TF. Expression of three isoforms of cGMP-binding cGMP-specific phosphodiesterase (PDE5) in human penile cavernosum. Biochem Biophys Res Commun. 2000;268:628–35. doi: 10.1006/bbrc.2000.2187. [DOI] [PubMed] [Google Scholar]
- 12.Lin CS, Lin G, Lue TF. Isolation of two isoforms of phosphodiesterase 5 from rat penis. Int J Impot Res. 2003;15:129–36. doi: 10.1038/sj.ijir.3900983. [DOI] [PubMed] [Google Scholar]
- 13.Banie L, Lin G, Ning H, Wang G, Lue TF, Lin CS. Effects of estrogen, raloxifene and levormeloxifene on alpha1A-adrenergic receptor expression. J Urol. 2008;180:2241–6. doi: 10.1016/j.juro.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 14.Louie MC, Yang HQ, Ma AH, Xu W, Zou JX, Kung HJ, Chen HW. Androgen-induced recruitment of RNA polymerase II to a nuclear receptor-p160 coactivator complex. Proc Natl Acad Sci U S A. 2003;100:2226–30. doi: 10.1073/pnas.0437824100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lugg JA, Rajfer J, Gonzalez-Cadavid NF. Dihydrotestosterone is the active androgen in the maintenance of nitric oxide-mediated penile erection in the rat. Endocrinology. 1995;136:1495–501. doi: 10.1210/endo.136.4.7534702. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez-Cadavid N, Vernet D, Fuentes Navarro A, Rodriguez JA, Swerdloff RS, Rajfer J. Up-regulation of the levels of androgen receptor and its mRNA by androgens in smooth-muscle cells from rat penis. Mol Cell Endocrinol. 1993;90:219–29. doi: 10.1016/0303-7207(93)90155-d. [DOI] [PubMed] [Google Scholar]
- 17.Burnett AL. Molecular pharmacotherapeutic targeting of PDE5 for preservation of penile health. J Androl. 2008;29:3–14. doi: 10.2164/jandrol.107.003483. [DOI] [PubMed] [Google Scholar]
- 18.Yassin AA, Saad F. Testosterone and erectile dysfunction. J Androl. 2008;29:593–604. doi: 10.2164/jandrol.107.004630. [DOI] [PubMed] [Google Scholar]
- 19.Hwang TI, Lin YC. The relationship between hypogonadism and erectile dysfunction. Int J Impot Res. 2008;20:231–5. doi: 10.1038/sj.ijir.3901633. [DOI] [PubMed] [Google Scholar]
- 20.Traish AM. Androgens Play a Pivotal Role in Maintaining Penile Tissue Architecture and Erection: A Minireview. J Androl. 2008 doi: 10.2164/jandrol.108.006007. [DOI] [PubMed] [Google Scholar]
- 21.Gurbuz N, Mammadov E, Usta MF. Hypogonadism and erectile dysfunction: an overview. Asian J Androl. 2008;10:36–43. doi: 10.1111/j.1745-7262.2008.00375.x. [DOI] [PubMed] [Google Scholar]
- 22.Blute M, Hakimian P, Kashanian J, Shteynshluyger A, Lee M, Shabsigh R. Erectile dysfunction and testosterone deficiency. Front Horm Res. 2009;37:108–22. doi: 10.1159/000176048. [DOI] [PubMed] [Google Scholar]
- 23.Palese MA, Crone JK, Burnett AL. A castrated mouse model of erectile dysfunction. J Androl. 2003;24:699–703. doi: 10.1002/j.1939-4640.2003.tb02729.x. [DOI] [PubMed] [Google Scholar]
- 24.Traish AM, Munarriz R, O’Connell L, Choi S, Kim SW, Kim NN, Huang YH, Goldstein I. Effects of medical or surgical castration on erectile function in an animal model. J Androl. 2003;24:381–7. doi: 10.1002/j.1939-4640.2003.tb02686.x. [DOI] [PubMed] [Google Scholar]
- 25.Traish AM, Park K, Dhir V, Kim NN, Moreland RB, Goldstein I. Effects of castration and androgen replacement on erectile function in a rabbit model. Endocrinology. 1999;140:1861–8. doi: 10.1210/endo.140.4.6655. [DOI] [PubMed] [Google Scholar]
- 26.Traish AM, Toselli P, Jeong SJ, Kim NN. Adipocyte accumulation in penile corpus cavernosum of the orchiectomized rabbit: a potential mechanism for veno-occlusive dysfunction in androgen deficiency. J Androl. 2005;26:242–8. doi: 10.1002/j.1939-4640.2005.tb01091.x. [DOI] [PubMed] [Google Scholar]
- 27.Liu WJ, Xin ZC, Xin H, Yuan YM, Tian L, Guo YL. Effects of icariin on erectile function and expression of nitric oxide synthase isoforms in castrated rats. Asian J Androl. 2005;7:381–8. doi: 10.1111/j.1745-7262.2005.00066.x. [DOI] [PubMed] [Google Scholar]
- 28.Takane KK, George FW, Wilson JD. Androgen receptor of rat penis is down-regulated by androgen. Am J Physiol. 1990;258:E46–50. doi: 10.1152/ajpendo.1990.258.1.E46. [DOI] [PubMed] [Google Scholar]
- 29.Bolton EC, So AY, Chaivorapol C, Haqq CM, Li H, Yamamoto KR. Cell- and gene-specific regulation of primary target genes by the androgen receptor. Genes Dev. 2007;21:2005–17. doi: 10.1101/gad.1564207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Massie CE, Adryan B, Barbosa-Morais NL, Lynch AG, Tran MG, Neal DE, Mills IG. New androgen receptor genomic targets show an interaction with the ETS1 transcription factor. EMBO Rep. 2007;8:871–8. doi: 10.1038/sj.embor.7401046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Q, Li W, Liu XS, Carroll JS, Janne OA, Keeton EK, Chinnaiyan AM, Pienta KJ, Brown M. A hierarchical network of transcription factors governs androgen receptor-dependent prostate cancer growth. Mol Cell. 2007;27:380–92. doi: 10.1016/j.molcel.2007.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]