Abstract
Cells of the CNS are constantly exposed to agents which damage DNA. Although much attention has been paid to the effects of this damage on nuclear DNA, the nucleus is not the only organelle containing DNA. Within each cell, there are hundreds to thousands of mitochondria. Within each mitochondria are multiple copies of the mitochondrial genome. These genomes are extremely vulnerable to insult and mutations in mitochondrial DNA (mtDNA) have been linked to several neurodegenerative diseases, as well as the normal process of aging. The principal mechanism utilized by cells to avoid DNA mutations is DNA repair. Multiple pathways of DNA repair have been elucidated for nuclear DNA. However, it appears that only base excision repair is functioning in mitochondria. This repair pathway is responsible for the removal of most endogenous damage including alkylation damage, depurination reactions and oxidative damage. Within the CNS, there are cell-specific differences mtDNA repair. Astrocytes exhibit efficient repair. Whereas, other glial cell types and neuronal cells exhibit a reduced ability to remove lesions from mtDNA. Additionally, a correlation was observed between those cells with reduced mtDNA repair and an increase in the induction of apoptosis. To demonstrate a causative relationship, a strategy of targeting DNA repair proteins to mitochondria to enhance mtDNA repair capacity was employed. Enhancement of mtDNA repair in oligodendrocytes provided protection from ROS- and cytokine- induced apoptosis. These experiments provide a novel strategy for protecting sensitive CNS cells from genotoxic insults and thus provide new treatment options for neurodegenerative diseases.
Keywords: DNA repair, mitochondrial DNA, apoptosis, CNS
Introduction
Cells within the CNS are routinely exposed to low concentrations of potentially deleterious genotoxic agents such as reactive oxygen species (ROS). Oxidative damage is the cytological consequence of an imbalance between the production of ROS such as hydrogen peroxide, superoxide radical, hydroxyl radical, and singlet oxygen, and the ability of the cell to defend against them. This damage can occur when there is an increase in the production of ROS, a decrease in the scavenging of these radicals, a decrease in the repair of oxidized macromolecules, or a combination of these processes working concomitantly. It has been theorized that the accumulation of oxidatively-modified macromolecules such as DNA can lead to altered cellular functions and progressive cell death. When this occurs in neural tissues, it has been proposed that various neurodegenerative maladies such as Alzheimer’s disease (AD, Bonilla et al., 1999; DiMauro and Davidzon, 2005) and Parkinson’s disease (PD, Jenner, 2003) can result. Additionally, accumulation of oxidative damage may contribute to the pathogenesis of amyotrophic lateral sclerosis (ALS, Borthwick et al., 1999) and Huntington’s disease (HD; Simonian and Coyle, 1996). Of the risk factors that have been associated with these diseases, age is the only one that appears to be common to all of them (Beal, 2005). For instance, in the case of AD, it has been reported that there is a prevalence of 47% in people over the age of 85 (Evans et al., 1989).
It has been established that the predominant source for the endogenous generation of ROS is through the oxidative phosphorylation pathway (Droge, 2002). Most of the oxygen that is consumed is reduced to H2O in the mitochondrial respiratory chain. This reduction must occur in four consecutive one-electron steps. During this reduction, a small proportion of the oxygen molecules (1–2%) are converted to superoxide anion radicals. It was accepted that there were two main sites in the respiratory chain where these reactions occur, Complex I and Complex IV(Turrens, 1997). However, recent studies localized at least nine submitochondrial ROS generating sites (Andreyev et.al., 2005). Hydrogen peroxide is then produced by the dismutation of superoxide either spontaneously or by superoxide dismutase (Cadenas and Davies, 2000). This species can diffuse through the cell and decompose to form noxious hydroxyl radicals which can injure the cell through interactions with macromolecules including lipids, proteins and DNA. Therefore, perturbations in mitochondria that lead to alterations in oxidative phosphorylation have particular relevance with respect to neurodegeneration. In support of the notion that oxidative phosphorylation is associated with disease processes are the findings that respiratory activity and mitochondrial complex IV activity are reduced in the aged rat brain (Harmon, et al., 1987; Curti, et al., 1990), and that the activities of mitochondrial complexes I and IV have been reported to be attenuated in aged rat muscle (Torii, et al., 1992). Additionally, experiments on non-human primate brains showed that there were age-related changes in the activities of complexes I and IV which correlate with a decrease in ATP production (Bowling, et al., 1993). Finally, studies in elderly humans have found increased numbers of myocytes with defective complex IV activity ( Müller-Hocker et al., 1992), reduced respiratory activity in liver mitochondria (Yen et al., 1989 ), and impaired electron transport chain function in skeletal muscle (Trounce et al., 1989; Cooper et al., 1992). In temporal association with these functional decrements in oxidative phosphorylation is an increase in markers of oxidative damage in DNA. It has been found that the mutagenic DNA adduct 8-oxo-2-deoxyguanosine (8O-dG) increases in several tissues from the rat (Fraga et al., 1990) and human (Hayakawa et al., 1991). Additionally, it has been discovered that this damage is 10- to 15-fold greater in human mtDNA than in nuclear DNA (nDNA) in a variety of tissues, including the brain (Hayakawa et al., 1992; Mecocci et al., 1993; Yakes and Van Houten, 1997; Santos et al., 2003). This finding suggests that mtDNA may be a more critical target for damage by ROS than is nDNA.
Each mitochondrion contains 4–10 molecules of DNA. Mammalian mtDNA is a circular double-stranded molecule that is 16.5 kb long and is not associated with histones. It codes for 22 tRNAs, 2 rRNAs and 13 polypeptides: ND1, ND2, ND3, ND4, ND5, and ND6 of complex I; cytochrome b of complex III; COI, COII, and COIII of complex IV; and subunits 6 and 8 (ABL) of complex V (Attardi and Schatz, 1988). The inheritance of mtDNA is almost completely maternal. However, a small paternal contribution has been identified in mice (Gyllensten et al., 1991). Consistent with the notion that mtDNA damage may contribute to neurodegeneration is the fact that this organelle is extremely vulnerable to damage from both endogenous and exogenous sources. Superoxide and hydrogen peroxide are normal byproducts of the mitochondrial respiratory chain and can cause significant oxidative damage to mtDNA (Martin et al., 1996). As would be expected, this increase in oxidative damage can be correlated with an increase in point mutations and deletions in mtDNA that are linked to neurodegenerative diseases and aging (Ozawa, 1997; Papa and Skulachev, 1997; Simonian and Coyle, 1996; Schon et al., 1997). In aging, the most common mtDNA lesion seen is a 4977 base pair (bp) deletion (Ozawa, 1997 ). However, other deletions also have been reported (Ozawa, 1997). In the case of the 4977 bp deletion, our laboratory, using the technique of ligation-mediated PCR (LM-PCR), has reported that it could be initiated by persistent oxidative damage at a single guanine at one of the break sites (Driggers et al., 1997). Point mutations and deletions, including in some cases the 4977 bp deletion found in aging, have been associated with AD (Hutchin and Cortopassi, 1995 ), PD (Bandmann et al., 1997; Schnopp et al., 1996; Wooten et al., 1997), and Huntington’s Disease, HD (Horton et al., 1995; Gu et al., 1996; Schapira, 1997), indicating that lesions in mtDNA also may play a role in these neurodegenerative diseases. As might be expected, these mutations have been correlated with an increase in oxidative damage in the brains of individuals with these diseases (Beal, 2005; Simonian and Coyle, 1996).
A key question that remains to be answered is how alterations in the mitochondrial genome, which basically affect only electron transport, can cause different diseases. One mechanism that could lead to altered cellular function is related to differences in energy requirements in different cellular compartments. There is evidence that mitochondria are required to provide compartmentalized ATP to specific areas of the cell (Lindgren and Smith, 1986; Hevner et al., 1992; Morris and Hollenbeck, 1993; Stürmer et al., 1995). For instance, the cell membrane requires ATP to energize specialized processes such as ion pumping, electrical transmission across the membrane and neurotransmitter release (Schon et al., 1997 ). Since different cells, and more specifically different populations of CNS cells, have different energy requirements for these processes, it is likely that they would be functionally-affected in different ways by oxidative damage and subsequent mutations in mtDNA. Another way that oxidative stress and the concomitant rise in mutations in mtDNA could differentially affect cells is through progressive cell death. Neurodegenerative diseases are associated with a progressive loss of neurons that may span several decades, with neuronal degeneration often starting many years before the manifestation of symptoms (Simonian and Coyle, 1996; Beal, 2005).
Two pathways have been described for cell death in the CNS. Cell death following severe and sudden injury such as anoxia, hyperthermia, physical trauma, or chemical damage often occurs by necrosis. Necrosis proceeds as the plasma membrane loses its ability to regulate osmotic gradients. The cell swells and its contents are leaked into the surrounding tissue. However, this does not appear to be the mechanism of cell death associated with most neurodegenerative diseases and aging. Swollen neuronal nuclei are not usually observed. Instead, shrunken nuclei, characteristic of apoptosis, are seen. During apoptosis, the cell activates an intrinsic suicide program and systematically destroys itself. Early in the process the cell surface begins to bleb and express pro-phagocytic signals. The chromatin in the nucleus condenses and is specifically cleaved to internucleosomal-sized fragments. The entire apoptotic cell then fragments into membrane-bound vesicles that are rapidly disposed of by phagocytosis. Thus, there is minimal damage to the surrounding tissue. The biochemical components required for apoptotic cell death are constitutively present in virtually all mammalian cells, and can be activated by a wide range of extra- and intra-cellular signals. Interest in the initiation and regulation of these pathways has resulted in an exponential growth of apoptosis research in the last few years. Heterogeneous death signals precede a common effector phase during which cells pass a threshold of “no return”, and are engaged in a degradation phase that results in the disassembly of the cellular scaffolding. There have been numerous hypotheses which postulate that specific mediators of pathways are responsible for neuronal apoptosis. One area that has received much attention is a group of dimerizing proteins that belong to the Bcl-2 family. The localization of the Bcl-2 family of proteins to the mitochondrial membrane and other early changes that occur in the mitochondrion, such as loss of membrane potential, the opening of permeability transition (PT) pores, release of proapoptotic proteins and the translocation of cytochrome c, have led to the hypothesis that the mitochondrion plays a key role in the regulation of apoptosis (Skulachev, 1996; Ferri and Kroemer, 2001; Joza et al., 2001).
The upstream factors that precede the disruption of the mitochondrial membrane have not been fully elucidated. Previously, the involvement of oxidative damage has been suggested. Because cells are continuously bombarded by ROS, their survival depends upon a fine balance between radical production, damage repair, and antioxidant activity. The hypothesis that oxidative damage plays a role in apoptosis is supported by the observation that the addition of ROS or the removal of endogenous antioxidants causes, in some cases, apoptosis (Buttke and Sanstrom, 1994). Additionally, tumor necrosis factor-induced apoptosis is associated with increases in intracellular ROS levels (Goossens et al., 1995). In attempting to provide a mechanism by which oxidative damage might induce apoptosis, data has been reported which suggests a pathway which is initiated by oxidative damage which causes damage to mitochondrial DNA which leads to a bioenergetic crisis and disruption of mitochondrial membrane potential and ultimately induces apoptosis (Ozawa, 1997). In this scenario, mtDNA repair plays a critical early role.
Mitochondrial DNA repair
Studies performed in the mid 70’s to evaluate the repair of UV-induced pyrimidine dimers failed to detect nucleotide excision repair (NER) in mitochondrial DNA (Clayton et al., 1974; Prakash, 1975). These studies coupled with a large number of reports of more damage in mitochondrial DNA than in nuclear DNA led to the dogma that excision repair did not operate on the mitochondrial genome. However, with the development of techniques that allowed the assessment of repair in specific sequences and the consideration of the mitochondrial sequence as a unique DNA sequence, base excision repair was demonstrated for mtDNA within mammalian cells. In the initial studies, formation and repair of N-methylpurines, was shown in an insulinoma cell line (RINr 38) after exposure to the naturally-occurring nitrosoamide streptozotocin (SZ) (Pettepher et al., 1991). By the late 1990’s substantial corroborative evidence began to accumulate, from numerous laboratories, that DNA repair does operate in mtDNA (Bogenhagen et al., 2001; Bohr, 2002; Bohr and Anson, 1999; Dianov et al, 2001; Kang and Hamasake, 2002; LeDoux and Wilson, 2001; Mandaville, et al., 2001; Van Houten and Freidberg, 1999; Wei, 1998)1. Additionally, the repair proteins which are involved in mitochondrial DNA repair were beginning to be identified and Pinz and Bogenhagen were able to use proteins isolated from mitochondria from Xenopus to perform in vitro DNA repair (Pinz and Bogenhagen, 1998). Recent studies have revealed that mtDNA repair capacity, as well as the components of the mitochondrial BER machinery have tissue variations (Karahalil, et.al, 2002), and could be altered with age (Szczesny, et.al, 2003), mtDNA depletion (Stuart, et.al, 2004), and caloric restriction (Stuart, et.al, 2004(a)). One important question which remains to be answered is whether mitochondrial DNA repair plays a critical role in the cellular response to genotoxic insults.
If indeed mitochondrial DNA repair does play a key role, then it could be anticipated that there would be cell specific differences in mtDNA repair that would correlate with sensitivity to genotoxic agents. Furthermore, if a causative relationship exists between mtDNA repair and cellular sensitivity then enhancement of repair in should cause viability to increase. Conversely, if repair is disrupted sensitivity should increase. The focus of the remainder of this review will be on the data from cells in the CNS which support these predictions.
CNS Cell -specific Differences in Repair of Alkylation damage
Within the CNS, there is differential sensitivity to nitrosoureas with both normal and neoplastic cells of the oligodendrocyte lineage possessing increased sensitivity to nitrosoureas, as evidenced by the enhanced chemotherapeutic response seen in oligodendrogliomas and the selective induction of oligodendrogliomas following transplacental exposure of animals to ethylnitrosourea. To determine the reasons for these differences our lab utilized well characterized primary cultures of rat astrocytes, oligodendrocytes and microglia (McCathy and deVellis, 1980 ). Viability studies revealed an increased sensitivity to methylnitrosourea in both oligodendrocytes and microglia compared to astrocytes. Using a quantitative Southern blot procedure to assess formation and repair of N-methylpurines within mtDNA of astrocytes, oligodendrocytes and microglia, we found no differences in the initial formation of N-methylpurines within mtDNA among the three cell types. In contrast, repair experiments revealed, a significant decrease in repair capacity in oligos and microglia compared to astrocytes. Moreover, DNA fragmentation and quantitative morphological analysis of ultrastructural evaluations indicated that the induction of apoptosis correlated with this decrease in repair capacity. These studies were the first to demonstrate a cell-specific difference in repair of mtDNA damage in the CNS and indicated that this difference correlated with the induction of apoptosis ( LeDoux et al., 1998).
CNS Cell-specific Differences in Repair of Oxidative damage
Cells of the CNS frequently encounter ROS due to their high oxygen metabolism and susceptibility to certain pathological conditions. The oxidative stress that results has been implicated as a causal factor in a wide variety of neurodegenerative diseases. Within the CNS, there are cell-specific differences in sensitivity to oxidative stress, with oligodendrocytes, the glial cells responsible for myelination of axons, being extremely sensitive (Juurlink, 1997). Within the cell, the mitochondrion is the major producer of ROS. It has been estimated the 2% of the electrons that flow through the electron transport chain leak off and form superoxide. To explore the reasons for the selective sensitivity of oligodendrocytes to oxidative stress, menadione which redox cycles with complex I of the mitochondrial electron transport chain to generate superoxide within mitochondria was used to generate the ROS in primary cultures of oligodendrocytes, astrocytes and microglia. mtDNA is extremely sensitive to menadione-induced DNA damage because of its close proximity to the inner mitochondrial membrane where these ROS are generated and its lack of protection by histones. Using menadione as the ROS generator, it was possible to evaluate mtDNA repair at non-toxic concentrations where nuclear damage is below detectable levels (<1 adduct per 50 kb in nuclear DNA). The results from these studies showed that exposure to equimolar concentrations of menadione caused more initial mtDNA damage in oligodendrocytes and microglia as compared to astrocytes. Repair experiments then were performed using both equimolar concentrations and concentrations that caused comparable numbers of strand breaks. Under both conditions, astrocytes repaired the damage efficiently, with all of the lesions in mtDNA from astrocytes being removed by 6 hours as compared to approximately 60% repair in oligodendrocytes or microglia. ApoTag and Annexin V staining, DNA laddering and electron microscopy were used to evaluate whether the glial cells underwent apoptosis following exposure to menadione. There were no indications of apoptosis in any of the experiments with astrocytes, while oligodendrocyte and microglial cultures were positive in all cases. To determine whether the increased sensitivity was due to decreased antioxidants within the oligodendrocytes and microglia, enzyme activities for glutathione peroxidase, catalase, CuZn and MnSOD, along with total, reduced, and oxidized glutathione levels were measured. Cells were prepared exactly as for the repair studies. The results showed that under our culture conditions there were no significant differences in catalase, glutathione peroxidase or CuZnSOD between any of the cell types. With regard to glutathione, astrocytes had significantly lower levels of this antioxidant than oligodendrocytes or microglia. The same was true for MnSOD ( Hollensworth et al., 2000). Thus, while antioxidant and repair capacity are both involved in protecting cells from oxidant insults, it appears that cells with efficient mtDNA repair capacity may be spared, even in the presence of very low antioxidant levels, and that cells with less efficient repair are susceptible, even in the presence of higher levels of antioxidants.
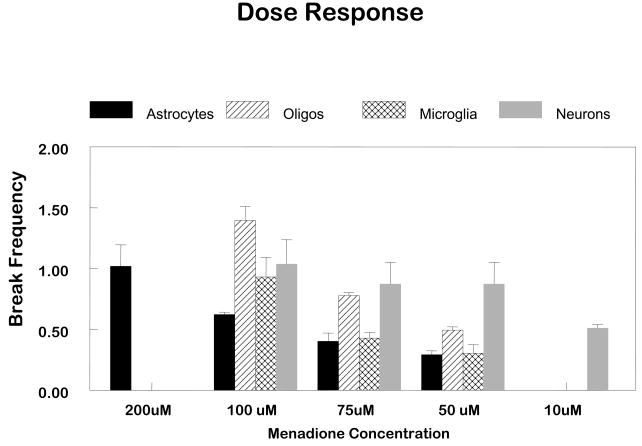
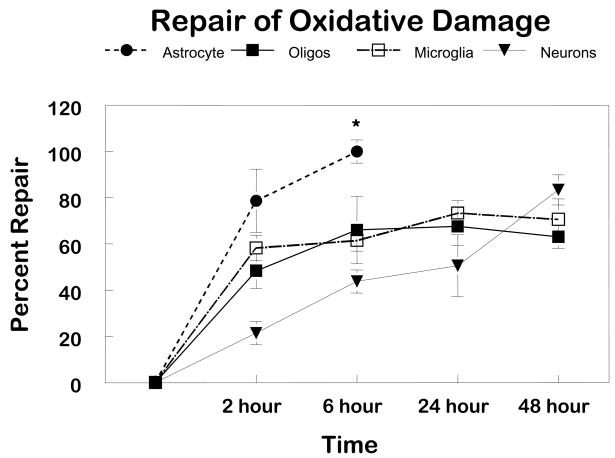
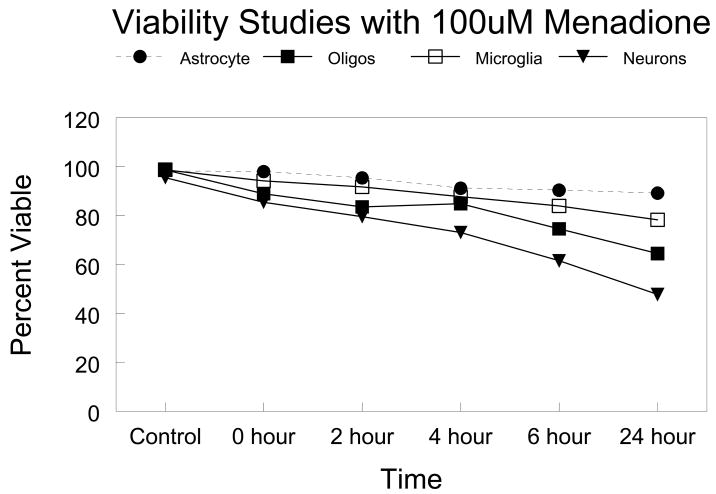
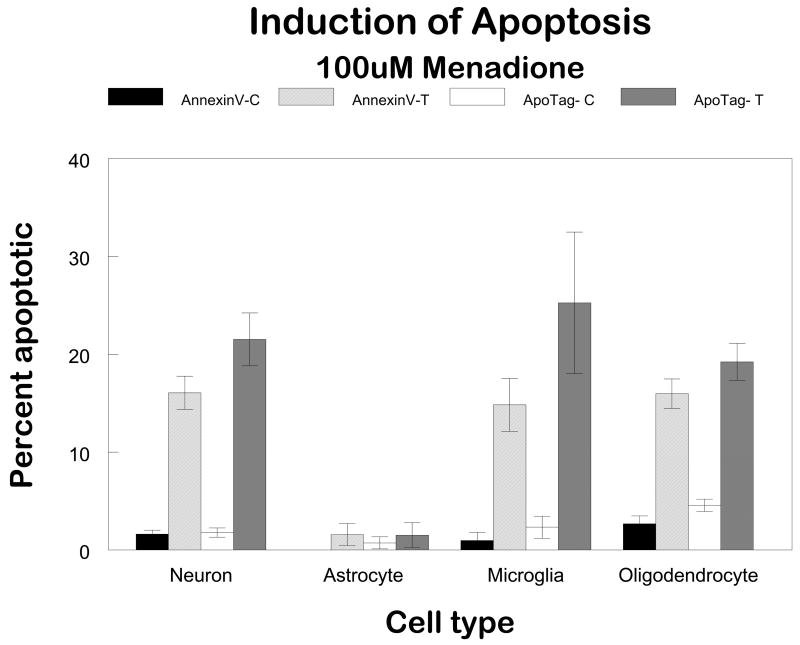
This observation of cell-specific differences in repair of oxidative damage among different populations of glial cells led to the obvious question “Is there proficient repair of oxidative damage to mtDNA in neurons?” To answer this question primary cultures of cerebellar granule cells were exposed to comparable concentrations of menadione. More initial damage was observed in the neuronal cultures compared to the glial cells (Figure 1). Therefore, to normalize the initial break frequency a 50 μM concentration was used for repair experiments in neurons. The results of these experiments revealed that the repair kinetics were slower in neurons as compared to glial cells (Figure 2). Viability assays using trypan blue dye exclusion revealed that cells with diminished mtDNA repair were more sensitive to cell killing (Figure 3). As in the glial cells studies, in cerebellar granule cells the induction of apoptosis was confirmed using either quantitative electron microscopic evaluation, annexin V positive staining, or Apotag positive staining following menadione exposure (Figure 4).
FIGURE 1. Analysis of the formation of oxidative damage to mtDNA of CNS cells.
Cells were exposed to concentrations of menadione ranging from 50μM to 200μM for one hour and lysed immediately. Control cultures were incubated in drug diluent only (HBSS). High molecular weight DNA was isolated and digested to completion with Bam H1. Samples were exposed to 0.1N NaOH prior to Southern blot analysis and hybridized with a PCR generated mitochondrial probe. Quantitation of the hybridization was performed using a Bio-Rad phosphoimager. The fraction of fragments free of damage (zero class) was determined by dividing the band intensity of the treated sample by the intensity of the nontreated sample. The number of lesions per fragment was determined using the Poisson expression. The values represent the mean for at least three separate experiments.
FIGURE 2. Comparison of mtDNA repair in CNS cells.
Using quantitative Southern Blot analysis, autoradiographic bands representing a 10.8 kb DNA fragment were densitometrically scanned. The break frequency per fragment was determined using the Poisson expression (s=-lnP0; where s is the number of breaks per fragment and P0 is the fraction of fragments free of breaks). Repair is determined by BF0-BFt/BF0, where BF0 is the break frequency at 0 hours of repair and BFt is the break frequency at the time of interest. The values represent the mean ± SEM for at least three separate experiments.
FIGURE 3. Viability of CNS cells after treatment with 100μM menadione.
Cells were plated into 24-well plates and exposed to 100μM menadione for a one-hour period. Following this incubation, cells were rinsed with HBSS and normal media was replaced for 2, 4, 6, or 24 hours. For control and 0-hour samples, a 10% trypan blue solution was added to the wells and viable cells counted using a light microscope.
FIGURE 4. Quantitation of apoptotic CNS cells following menadione exposure.
In order to quantitate apoptosis, two commercially available kits were employed. The ApoTag kit from Oncor detects the 3′-OH groups generated during apoptosis while the Annexin-V-FITC kit from Trevigen (Gaithersburg, MD, USA) labels the phosphatidylserines that have been flipped to the outside of the cell membrane.
Cells were plated into Labtech slides and treated with 100μM menadione for one hour. The cells were rinsed with HBSS and culture media replaced for 6 hours. At this time, cells were rinsed with cold 1XPBS and the ApoTag/Annexin V assays performed. Using an immunofluorescent microscope, cells in five random fields were counted and the number of positive-staining cells was divided by the total number of cells in the field to give a % of apoptotic cells.
Since menadione redox cycles with complex I of the electron transport chain to produce superoxide, it is possible that this process will cause the induction of the apoptosis through the mitochondrial pathway and thus activate of caspase 9. The release of cytochrome c from the mitochondrial intermembrane space into the cytosol is required for this activation. Western blots performed using mitochondrial and cytosolic proteins and a monoclonal antibody to cytochrome c revealed that no cytochrome c protein was detected in the cytosolic fraction prior to menadione treatment, indicating that the cell fractionation procedure had not disrupted the outer mitochondrial membrane. However, two hr after exposure to 100 μM menadione, there was a substantial decrease in the intensity of the mitochondrial cytochrome c band with a concomitant increase in the cytosolic band in oligodendrocytes, microglia and neurons. As expected, astrocytes, which show no evidence of cell death in response to exposure to menadione, showed no increase in the cytosolic cytochrome c band. Therefore, it can be concluded that release of cytochrome c from mitochondria into the cytosol correlates with induction of apoptosis in CNS cells.
To determine whether this release of cytochrome c initiated the activation of caspase 9, a colorimetric activity assay based on cleavage of a caspase 9 specific substrate was performed. For a positive control, staurosporine, which has been shown to cause cytochrome c release in cerebellar granule neurons, was employed. After three hr, caspase 9 activity in oligodendrocytes, microglia, neurons and Jurkat cells, which served as positive controls for apoptosis induction, exposed to menadione was elevated compared to control cells. No detectable increase in astrocyte caspase 9 activity was found. Subsequent experiments were performed using xanthine oxidase and hypoxanthine to generate the ROS. Again activation of caspase 9 was observed.
Because it has been suggested that caspase-8 may play a role in the mitochondrial pathway for apoptosis, its activity following menadione exposure was tested in each of the cell types. Activation of caspase-8 has been well documented in the Fas-pathway of apoptosis; thus, Jurkat cells treated with an anti-Fas antibody were used for a positive control. No caspase-8 activity was detected in any of the cell types following exposure to menadione. Following treatment with the anti-Fas antibody, however, oligodendrocytes and microglia demonstrated caspase-8 activity. Conversely, astrocytes remained unresponsive to induction of apoptosis and, therefore, showed no caspase-8 activity. These studies were the first to demonstrate a cell-specific difference in the repair of oxidative damage in the CNS and indicated that this difference correlated with the induction of apoptosis (Hollensworth et al., 2000).
Effects of enhancing mtDNA repair
To establish that there is a causal relationship between decreased mtDNA repair and sensitivity of oxidative damage, it is necessary to alter the ability of the cell to repair mtDNA. To accomplish this a gene targeting strategy was employed. For these studies, the human 8-oxoguanine DNA glycosylase (hOGG1) gene was fused to a mitochondrial targeting sequence (MTS) from human MnSOD and cloned into a pcDNA3 vector. After sequencing the construct for confirmation, it was linearized and HeLa cells were stably transfected by electroporation. For the intial studies a HeLa cell isolate was used because it exhibited a limited capacity to repair oxidative damage to its mtDNA. Selection with 400 μg/mL G418 was started 24 hours after transfection. Confirmation of the presence of the transfected gene was accomplished by Southern blot analysis. Western blot analysis of mitochondrial extracts showed that the recombinant protein was indeed targeted to this organelle. Additionally, enzyme activity assays showed that mitochondrial extracts from cells transfected with the construct had increased enzyme activity for 8-oxoguanine as compared to cells transfected with the vector only. Nuclear enzyme activities from either cell type were the same. Repair assays showed that there was enhanced repair of oxidative lesions in mtDNA from construct transfected cells. Additional studies revealed that this augmented repair led to enhanced cellular viability as determined by trypan blue dye exclusion and clonogenic assays. (Dobson et al., 2000.)
To fully evaluate the potential of using recombinant repair enzymes to alter mtDNA repair and to exclude the possibility that in the stable transfection study the effects we observed were due to the site of integration of the construct into the genome, a series of experiments were conducted in which the expression of the repair gene was under the control of an inducible promoter. The tetracycline (Tet)-regulated expression system using control elements of the tetracycline resistance operon encoded in Tn10 of E. coli was used. This system relies on the presence or absence of Tet or a commonly used analog, doxycyclin, to control gene expression. For these experiments, we constructed a vector containing the sequence for hOGG1 downstream of the MnSOD mitochondrial targeting sequence under the control of the Tet-response element and introduced it into HeLa cells transfected previously with pTet-Off plasmid. As in the previous study, the presence of the transfected gene was confirmed using Southern blot analysis and the appropriate control by doxycyclin of expression of the protein in the mitochondria was confirmed by Western blot analysis. Doxycyclin-regulated hOGG1 activity was demonstrated using an in vitro oligonucleotide assay. In vivo repair activity showed that conditional expression of hOGG1 led to enhanced repair of oxidative lesions and increased cell survival. Thus, these findings support our previous work using cells that constitutively overexpress hOGG1 (Rachek et al. 2002)
To determine whether the overexpression of other glycosylase/AP lyases which predominantly remove oxidized pyrimidines from DNA could improve mtDNA repair and cell survival, we constructed vectors containing sequences for two bacterial glycosylase/AP lyases. The coding sequence for Endo III and Endo VIII were placed downstream of the mitochondrial targeting sequence (MTS) from MnSOD. The coding sequences were placed under the control of the tetracycline (Tet)-response element. Successful integrations of MTS-Endo III or MTS-Endo VIII into the HeLa Tet-On genome were confirmed by Southern blot. Western blots of mitochondrial extracts from MTS-Endo III and MTS-Endo VIII clones revealed that the recombinant proteins were targeted into mitochondria and their expression was doxycycline (Dox)-dependent. Enzyme activity assays and mtDNA repair studies showed that the conditionally expressed MTS-Endo III and MTS-Endo VIII were functional, and both MTS-Endo III and MTS-Endo VIII (Dox+) clones were significantly more proficient at repairing oxidative damage in their mtDNA when compared to the same cells in which the genes were not expressed. This enhanced repair led to increased cellular resistance to oxidative stress. Because these two enzymes and OGG1 have different specificities for base adducts but all have AP lyase activity, it is likely that it is the AP lyase activity that is enhancing the repair capacity and providing the protection from oxidative damage (Rachek, et al., 2004)
On the other hand, targeting of specific repair enzymes into mitochondria also can lead to an imbalance in base excision repair thus making otherwise resistant cells more vulnerable to the effects of cytotoxic drugs. Expressing the bacterial Exonuclease III in mitochondria of breast cancer cells renders them more susceptible to oxidative stress (Shokolenko et.al, 2003). Also, expression of the mitochondrially targeted N-methylpurine DNA glycosylase dramatically increases the sensitivity of breast cancer cells to alkylation damage (Fishel et.al, 2003). Thus mtDNA repair plays an important role in normal cell physiology, making it a potential target for therapeutic intervention.
Effects of enhancing mtDNA repair in oligodendrocytes
To determine whether there was a causal link between decreased mtDNA repair and the induction of apoptosis in oligodendrocytes, cells were transiently transfected using cationic liposomes with a vector containing the MTS from MnSOD upstream of the sequence for hOGG1. The efficiency of transfection and the localization of recombinant protein were determined by fluorescence microscopy and by Western blot analysis. Subsequent mtDNA repair studies, employing menadione to produce reactive oxygen species, showed a significant enhancement in repair of oxidative lesions in mtDNA from MTS-hOGG1 transfected oligodendrocytes compared to cells transfected with vector only. Additional experiments were conducted to determine the effect of changing mtDNA repair capacity on menadione-induced apoptosis in oligodendrocytes. These experiments showed that targeting hOGG1 to mitochondria significantly reduced the release of cytochrome c from the intermitochondrial space and the activation of caspase 9 in oligodendrocytes after exposure to menadione. Therefore, the results of these experiments established that a causal relationship exits between inefficient mtDNA repair of oxidative damage and the induction of apoptosis. (Druzhyna et al., 2003.)
Cytokines are important mediators in the inflammatory demyelination observed in human multiple sclerosis, as well as in animal models of MS and in the inflammation that occurs following ischemic insults to the CNS. Oligodendrocytes can be stimulated to express inducible nitric oxide synthase by inflammatory cytokines. Based on our previous data using pancreatic beta cells (Wilson et al., 1997), we hypothesized that mtDNA would be one of the critical targets for NO-induced damage. In this study, a combination of tumor necrosis factor (TNF;50 ng/ml) and γ interferon (INF;25 ng/ml) caused elevated NO production in cultured oligodendrocytes. Western blot analysis revealed a strong enhancement of inducible nitric oxide synthase (iNOS) expression 48 h after cytokine treatment. Within this same time period, NO-mediated mtDNA damage was shown by Southern blot analysis. The damaging species was identified to be peroxynitrite as determined by ligation mediated PCR. Targeting the DNA repair enzyme hOGG1 to mitochondria of oligodendrocytes enhanced repair of this cytokine-mediated mtDNA damage. Moreover, studies using a TUNEL assay showed that following treatment with cytokines MTS-hOGG1 transfected oligodendrocytes had fewer apoptotic cells (17% ± 5.2) compared to (31% ± 3.6) in nontransfected cells. Subsequent experiments revealed that targeting hOGG1 to mitochondria reduces the activation of caspase 9 showing that this recombinant protein works to reduce apoptosis that is occurring through a mitochondrial pathway. The significance of these findings is that they indicate that cytokine-mediated apoptosis involves both the receptor-mediated pathway with caspase 8 activation and the mitochondrial pathway with activation of caspase 9. The finding that enhancement of mtDNA repair by targeting of hOGG1 to the mitochondria indicated that mtDNA damage is a stimulus that can activate caspase-9. Therefore, it appears that activation of apoptosis through the two pathways is additive and that removal of the stimulus for caspase 9 activation provides protection. Consequently, these results suggest that targeting of mtDNA repair proteins may provide a means of protecting these sensitive cells from the detrimental effects of cytokines released during an inflammatory processes. (Druzhyna et al., 2005).
Effects of imbalancing of mtDNA repair in neuronal cells
The normal physiological function of neurons requires that they carry a heavy oxidative burden. The increased rates of transcription and translation required for the specific duties of neurons require high metabolic rates, and thus increased ROS production. In order to maintain ‘redox homeostasis’, neuronal cells must balance the effects of increased oxidative stress with various antioxidants or reductants such as SOD and GSH as well as maintain the lowest advantageous rate of mutation in the mitochondrial genome by efficiently repairing oxidative mtDNA damage. Thus, chronic increased oxidative stress may be an important factor contributing to the increased sensitivity of neuronal cells to oxidative challenges. To evaluate the antioxidant defenses for the various CNS cell types, steady-state levels of intracellular prooxidants were determined using an oxidation sensitive probe. These studies revealed that granule cells and oligodendrocytes have significantly elevated steady-state levels of intracellular prooxidants with cerebellar granule cells exhibiting a mean fluorescence index (MFI) 6 fold greater than that of astrocytes. The significant increase in fluorescence of the oxidation sensitive probe is indicative of an increased oxidative state in both cerebellar granule cells and oligodendrocytes compared to astrocytes.
This increase in ROS production results in an elevated oxidation state within these cells and the increase in expression of key proteins involved in protecting cells from oxidative insults. One of the proteins whose expression is redox regulated is Apurinic/apyrimidinic endonuclease/redox effector factor-1 (APE/Ref-1). This multifunctional enzyme influences a variety of cellular processes. In addition to its DNA repair function, which includes 5′-AP site incision as well as 3′-diesterase activity, APE/Ref-1 also functions as a major cellular reduction/oxidation (redox) factor that maintains many important transcription factors, such as AP-1, NF-kB and p53 in a reduced (active) state. To determine the role of APE in neural mtDNA repair, the relative AP lyase and APE activities were assessed for a proficient BER pathway (astrocyte mitochondrial extracts) as well as an inefficient BER pathway (cerebellar granule cell mitochondrial extracts). The rate at which mitochondrial lysates cleaved an APE-specific (tetrahydrofuran [THF] AP site containing oligonucleotide) substrate was used to determine the level of APE activity. Pure AP sites in DNA can be processed by either APE/Ref-1 or AP lyases. Because the lyase cannot cleave a THF, the APE actiivity is reflected in the cleavage of the THF containing oligo and the lyase activity could be calculated by subtracting the enzyme activity using the THF oligo from that using a pure abasic site. Interestingly, the mitochondrial activity of both AP lyase and APE was significantly higher in cerebellar granule cell cultures compared to astrocytes. To determine whether AP lyase or APE activities in astrocytes are inducible under oxidative stress, cells were pretreated with 100 μM menadione for 1 h, washed with fresh media, and then harvested 2 h later for nuclear and mitochondrial protein fractionation. Mitochondrial APE and AP lyase activities were not induced under these conditions in either cell type. Western blot analysis confirmed that levels of OGG1 and APE/Ref-1 were higher in cerebellar granule cell mitochondrial extracts compared to astrocytes. This increased expression and activity of APE is likely to inadvertently affect mtDNA repair. The increase in the persistence of the single strand breaks in neuronal mtDNA observed in the quantitative Southern analysis suggests that the rate-limiting factor in neuronal BER is downstream of the incision of the phosphate backbone, thus POL γ levels were assessed. Western blot analysis revealed that granule cells contain substantially lower levels of POL γ, and activity assays with both lysates and immunoprecipitates using POL γ specific antibodies confirmed that there is decreased POL γ activity in cerebellar granule cells compared to astrocytes. This suggests that decreased levels of POL γ may contribute to inefficient mtDNA repair in neuronal cells (Harrison et al., 2005).
Conclusion
In conclusion, there are CNS cell-specific differences in the repair of both alkylation and oxidative damage in mtDNA. Furthermore, inefficient mtDNA repair correlates with a decrease in viability following genotoxic insults. In order to demonstrate a causative relationship, mtDNA repair efficiency was modulated using a gene targeting approach. Targeting of hOGG1 to mitochondria of oligodendrocytes resulted in enhancement of mtDNA repair and a decrease in the induction of apoptosis following exposure to either an oxidative stress or cytokines. Thus, it can be concluded that mtDNA repair is a critical player in the response of CNS cells to genotoxic insults. Future challenges include the development of therapeutic approaches which modulate mtDNA repair and block or retard the pathogenesis of neurodegenerative diseases.
Abbreviations
- CNS
central nervous system
- ROS
reactive oxygen species
- AD
Alzheimer’s disease
- PD
Parkinson’s disease
- ALS
amyotrophic lateral sclerosis
- mtDNA
mitochondrial DNA
- HD
Huntington’s disease
- PT
permeability transition
- NER
nucleotide excision repair
- SZ
streptozotocin
- hOGG1
human 8-oxoguaine glycosylase
- MTS
mitochondrial tarteting sequence
- MnSOD
manganese superoxide dismutase
- POL γ
polymerase γ
Footnotes
This research was supported by National Institutes of Health Grants ES03456, ES05865, NS047208, and AG19602.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Andreyev AYu, Kushnareva YuE, Starkov AA. Mitochondrial metabolism of reactive oxygen species. Biochemistry (Moscow) 2005;70(2):200–214. doi: 10.1007/s10541-005-0102-7. [DOI] [PubMed] [Google Scholar]
- Attardi G, Schatz G. Biogenesis of mitochondria. Annu Rev Cell Biol. 1988;4:289–333. doi: 10.1146/annurev.cb.04.110188.001445. [DOI] [PubMed] [Google Scholar]
- Bandmann O, Sweeney MG, Daniel SE, Marsden CD, Wood NW. Mitochondrial DNA polymorphisms in pathologically proven Parkinson’s disease. J Neurol. 1997;244:262–265. doi: 10.1007/s004150050082. [DOI] [PubMed] [Google Scholar]
- Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann Neurol. 2005;58:495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- Bogenhagen DF, Pinz KG, Peres-Jannotti RM. Enzymology of mitochondrial base excision repair. Prog Nucleic Acid Res Mol Biol. 2001;68:257–271. doi: 10.1016/s0079-6603(01)68105-4. [DOI] [PubMed] [Google Scholar]
- Bohr VA. Repair of oxidative DNA damage in nuclear and mitochondrial DNA, and some changes with aging in mammalian cells. Free Radic Biol Med. 2002;32:804–812. doi: 10.1016/s0891-5849(02)00787-6. [DOI] [PubMed] [Google Scholar]
- Bohr VA, Anson RM. Mitochondrial DNA repair pathways. J Bioenerg Biomembr. 1999;31:391–398. doi: 10.1023/a:1005484004167. [DOI] [PubMed] [Google Scholar]
- Bonilla E, Tanji K, Hirano M, Vu TH, DiMauro S, Schon EA. Mitochondrial involvement in Alzheimer’s disease. Biochim Biophys Acta. 1999;1410:171–182. doi: 10.1016/s0005-2728(98)00165-0. [DOI] [PubMed] [Google Scholar]
- Borthwick GM, Johnson MA, Ince PG, Shaw PJ, Turnbull DM. Mitochohdrial enzyme activity in amyotrophic lateral sclerosis. Ann Neurol. 1999;46:787–790. doi: 10.1002/1531-8249(199911)46:5<787::aid-ana17>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Bowling AC, Mutisya EM, Walker LC, Price DL, Cork LC, Beal MF. Age-dependent impairment of mitochondrial function in primate brain. J Neurochem. 1993;60:1964–1967. doi: 10.1111/j.1471-4159.1993.tb13430.x. [DOI] [PubMed] [Google Scholar]
- Buttke TM, Sandsrtrom PA. Oxidative stress as a mediator of apoptosis. Immunol Today. 1994;15:7–10. doi: 10.1016/0167-5699(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Cadenas E, Davies KJA. Mitochondrial free radical generation, oxidative stress, and aging. Free Radic Biol Med. 2000;29:222–230. doi: 10.1016/s0891-5849(00)00317-8. [DOI] [PubMed] [Google Scholar]
- Clayton DA, Doda JN, Friedberg EC. The absence of a pyrimidine dimer repair mechanism in mammalian mitochondria. Proc Natl Acad Sci USA. 1974;71:2777–2781. doi: 10.1073/pnas.71.7.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper JM, Mann VM, Schapira AHV. Analyses of mitochondrial respiratory chain function and mitochondrial DNA deletion in human skeletal muscle:effect of aging. J Neurol Sci. 1992;13:91–98. doi: 10.1016/0022-510x(92)90270-u. [DOI] [PubMed] [Google Scholar]
- Curti D, Giangare MC, Redolfi ME, Fugaccia I, Benzi G. Age related modifications of cytochrome c oxidase activity in discrete brain regions. Mech Ageing Dev. 1990;55:171–180. doi: 10.1016/0047-6374(90)90024-a. [DOI] [PubMed] [Google Scholar]
- Dianov GL, Souza-Pinto N, Nyaga SG, Thybo T, Stevnsner T, Bohr VA. Base excision repair in nuclear and mitochondrial DNA. Prog Nucleic Acid Res Mol Biol. 2001;68:285–297. doi: 10.1016/s0079-6603(01)68107-8. [DOI] [PubMed] [Google Scholar]
- DiMauro S, Davidzon G. Mitochondrial DNA and disease. Ann Med. 2005;37(3):222–233. doi: 10.1080/07853890510007368. [DOI] [PubMed] [Google Scholar]
- Dobson AW, Xu Y, Kelley MR, LeDoux SP, Wilson GL. Enhanced mtDNA Repair and Cellular Survival Following Oxidative Stress by Targeting the hOGG Repair Enzyme to Mitochondria. J Biol Chem. 2000;275:37518–37523. doi: 10.1074/jbc.M000831200. [DOI] [PubMed] [Google Scholar]
- Driggers WJ, Holmquist GP, LeDoux SP, Wilson GL. Mapping frequencies of endogenous oxidative damage and the kinetic response to oxidative stress in a region of rat mtDNA. Nucl Acids Res. 1997;25:4362–4369. doi: 10.1093/nar/25.21.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Druzhyna NM, Hollensworth SB, Kelley MR, Wilson GL, LeDoux SP. Targeting Human 8-Oxoguanine Glycosylase to Mitochondria of Oligodendrocytes Protects Against Menadione-Induced Oxidative Stress. Glia. 2003;42:370–378. doi: 10.1002/glia.10230. [DOI] [PubMed] [Google Scholar]
- Druzhyna NM, Musiyenko SI, Wilson GL, LeDoux SP. Cytokines induce NO-mediated mtDNA damage and apoptosis in oligodendrocytes. Protective role of targeting 8-oxoguanine glycosylase to mitochondria. J Biol Chem. 2005;280:21673–21679. doi: 10.1074/jbc.M411531200. [DOI] [PubMed] [Google Scholar]
- Evans DA, Funkenstein HH, Albert MS, Scher PA, Cook NR, Chown MJK, Hebert LE, Henneken SCH, Taylor JO. Prevalance of Alzheimer’s disease in a community population of older persons. Higher than previously reported. JAMA. 1989;262:2551–2556. [PubMed] [Google Scholar]
- Ferri KF, Kroemer G. Mitochondria-the suicide organelles. Bioessays. 2001;23:111–115. doi: 10.1002/1521-1878(200102)23:2<111::AID-BIES1016>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Fishel ML, Seo YR, Smith ML, Kelley MR. Imbalancing the DNA base excision repair pathway in the mitochondria; targeting and overexpressing N-methylpurine DNA glycosylase in mitochondria leads to enhanced cell killing. Cancer Res. 2003;63:608–615. [PubMed] [Google Scholar]
- Fraga CG, Shigenaga MK, Park J-W. Oxidative damage to DNA during aging: 8-hydroxy-2′-deoxyguanosine in rat organ DNA and urine. Proc Natl Acad Sci USA. 1990;87:4533–4537. doi: 10.1073/pnas.87.12.4533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens V, Grooten J, De Vos K, Fiers W. Direct evidence for tumor necrosis factor-induced mitochondrial reactive oxygen intermediates and their involvement in cytotoxicity. Proc Natl Acad Sci USA. 1995;92:8115–8119. doi: 10.1073/pnas.92.18.8115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu M, Gash MT, Mann VM, Javoy-Agid F, Cooper JM, Schapira AH. Mitochondrial defect in Huntington’s disease caudate nucleus. Ann Neurol. 1996;39:385–89. doi: 10.1002/ana.410390317. [DOI] [PubMed] [Google Scholar]
- Gyllensten U, Wharton D, Josefisson A, Wilson AC. Paternal inheritance of mitochondrial DNA in mice. Nature. 1991;352:255–257. doi: 10.1038/352255a0. [DOI] [PubMed] [Google Scholar]
- Harmon HJ, Nank S, Floyd RA. Age-dependent changes in rat brain mitochondria of synaptic and non-synaptic origins. Mech Ageing Dev. 1987;38:167–177. doi: 10.1016/0047-6374(87)90076-5. [DOI] [PubMed] [Google Scholar]
- Harrison JF, Hollensworth SB, Spitz DR, Copeland WC, Wilson GL, LeDoux SP. Oxidative stress-induced apoptosis in neurons correlates with mitochondrial DNA base excision repair pathway imbalance. Nucleic Acids Res. 2005;22(14):4660–4671. doi: 10.1093/nar/gki759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayakawa M, Hattori K, Sugiyama S, Ozawa T. Age-associated oxygen damage and mutations in mitochondrial DNA in human hearts. Biochem Biophys Res Comm. 1992;189:979–985. doi: 10.1016/0006-291x(92)92300-m. [DOI] [PubMed] [Google Scholar]
- Hayakawa M, Torii K, Sugiyama S, Tanaka M, Ozawa T. Age associated accumulation of 8-hydroxydeoxyguanine in mitochondrial DNA of human diaphragm. Biochem Biophys Res Comm. 1991;179:1023–1029. doi: 10.1016/0006-291x(91)91921-x. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Duff RS, Wong-Riley MT. Coordination of ATP production and consumption in brain: parallel regulation of cytochrome oxidase and Na+, K+ ATPase. Neurosci Lett. 1992;138:188–192. doi: 10.1016/0304-3940(92)90502-x. [DOI] [PubMed] [Google Scholar]
- Hollensworth BS, Shen C, Sim JE, Spitz DR, Wilson GL, LeDoux SP. Glial Cell Type-Specific Responses to Menadione-Induced Oxidative Stress. Free Radic Biol Med. 2000;28:1161–1174. doi: 10.1016/s0891-5849(00)00214-8. [DOI] [PubMed] [Google Scholar]
- Horton TM, Graham BH, Corral-Debrinski M, Shoffner JM, Kaufman AE, Beal MF, Wallace DC. Marked increase in mitochondrial DNA deletion levels in the cerebral cortex of Huntington’s disease patients. Neurology. 1995;45:1879–1883. doi: 10.1212/wnl.45.10.1879. [DOI] [PubMed] [Google Scholar]
- Hutchin T, Cortopassi G. A mitochondrial DNA clone is associated with increased risk for Alzheimer’s disease. Proc Natl Acad Sci USA. 1995;92:6892–6895. doi: 10.1073/pnas.92.15.6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner P. Oxidative stress in Parkinson’s disease. Ann Neurol. 2003;53(Suppl 3):S26–S36. doi: 10.1002/ana.10483. [DOI] [PubMed] [Google Scholar]
- Joza A, Susin SA, Daugas E, Stanford WL, Cho SK, Li CYJ, Sasaki T, Ella AJ, Chang HY, Ravagnan L, Ferri KF, Zanzami N, Wakeham A, Hakem R, Yoshida H, Kong Y, Mak T, Zuniga-Pflucker JC, Kroemer G, Penninger JM. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature. 2001;410:549–554. doi: 10.1038/35069004. [DOI] [PubMed] [Google Scholar]
- Juurlink B. Response of glial cells to ischemia: roles of reactive oxygen species and glutathione. Neurosci Biobehav Rev. 1997;21:151–166. doi: 10.1016/s0149-7634(96)00005-x. [DOI] [PubMed] [Google Scholar]
- Kang D, Hamasaki N. Maintenance of mitochondrial DNA integrity: repair and degradation. Curr Genet. 2002;41:311–322. doi: 10.1007/s00294-002-0312-0. [DOI] [PubMed] [Google Scholar]
- Karahalil B, Hogue BA, Souza-Pinto NC, Bohr VA. Base-excision repair capacity in mitochondria and nuclei: tissue-specific variations. FASEB J. 2002;16(14):1895–1902. doi: 10.1096/fj.02-0463com. [DOI] [PubMed] [Google Scholar]
- LeDoux SP, Shen C, Grishko VI, Fields PA, Gard AL, Wilson GL. Glial Cell-specific Differences in Response to Alkylation Damage. Glia. 1998;24:304–312. [PubMed] [Google Scholar]
- LeDoux SP, Wilson GL. Base excision repair of mitochondrial DNA damage in mammalian cells. Prog Nucleic Acid Res Mol Biol. 2001;68:273–284. doi: 10.1016/s0079-6603(01)68106-6. [DOI] [PubMed] [Google Scholar]
- Lindgren CA, Smith DO. Increased presynaptic ATP levels coupled to synaptic activity at the crayfish neuromuscular junction. J Neurosci. 1986;6:2644–2652. doi: 10.1523/JNEUROSCI.06-09-02644.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandavilli BS, Santos JH, Van Houten B. Mitochondrial DNA repair and aging. Mutat Res. 2002;509:127–151. doi: 10.1016/s0027-5107(02)00220-8. [DOI] [PubMed] [Google Scholar]
- Martin GM, Austad SN, Johnson TE. Genetic analysis of ageing: role of oxidative damage and environmental stresses. Nature Genetics. 1996;13:25–34. doi: 10.1038/ng0596-25. [DOI] [PubMed] [Google Scholar]
- McCarthy KD, deVellis J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J Cell Biol. 1980;85:890–902. doi: 10.1083/jcb.85.3.890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecocci P, Macgarvey U, Kaufman AE, Koontz D, Shoffner JM, Wallace DC, Beal MF. Oxidative damage to mitochondrial DNA shows marked age-dependent increases in human brain. Ann Neurol. 1993;34:609–616. doi: 10.1002/ana.410340416. [DOI] [PubMed] [Google Scholar]
- Morris RL, Hollenbeck PJ. The regulaiton of bidirectional mitochondrial transport is coordinated with axonal outgrowth. J Cell Sci. 1993;104:917–927. doi: 10.1242/jcs.104.3.917. [DOI] [PubMed] [Google Scholar]
- Müller-Hocker J, Schneiderbanger K, Stefani FH, Kadenbach B. Progressive loss of cytochrome-c oxidase in the human extraocular muscles in ageing-acytochemical-immunohistochemical study. Mut Res. 1992;275:115–124. doi: 10.1016/0921-8734(92)90016-i. [DOI] [PubMed] [Google Scholar]
- Ozawa T. Genetic and functional changes in mitochondria associated with aging. Physiol Revs. 1997;77:425–464. doi: 10.1152/physrev.1997.77.2.425. [DOI] [PubMed] [Google Scholar]
- Papa S, Skulachev VP. Reactive oxygen species, mitochondria, apoptosis and aging. Mol Cell Biochem. 1997;174:305–319. [PubMed] [Google Scholar]
- Pettepher CC, LeDoux SP, Bohr VA, Wilson GL. Repair of alkali labile sites within the mitochondrial DNA of RINr 38 cells after exposure to the nitrosourea streptozotocin. J Biol Chem. 1991;266:3113–3117. [PubMed] [Google Scholar]
- Pinz KG, Bogenhagan DF. Efficient repair of abasic sites in DNA by mitochondrial enzymes. Mol Cell Biol. 1998;18:1257–1265. doi: 10.1128/mcb.18.3.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash L. Repair of pyrimidine dimers in nuclear and mitochondrial DNA of yeast irradiated with low doses of ultraviolet light. J Mol Biol. 1975;98:781–795. doi: 10.1016/s0022-2836(75)80010-6. [DOI] [PubMed] [Google Scholar]
- Rachek LI, Grishko VI, Musiyenko SI, Kelley MR, LeDoux SP, Wilson GL. Conditional targeting of the DNA repair enzyme hOGG1 into mitochondria. J Biol Chem. 2002;277:44932–7. doi: 10.1074/jbc.M208770200. [DOI] [PubMed] [Google Scholar]
- Rachek LI, Grishko VI, Alexeyev MF, Pastukh VV, LeDoux SP, Wilson GL. Endonuclease III and endonuclease VIII conditionally targeted into mitochondria enhance mitochondrial DNA repair and cell survival following oxidative stress. Nucleic Acids Res. 2004;32:3240–7. doi: 10.1093/nar/gkh648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos JH, Hunakova L, Chen Y, Bortner C, Van Houten B. Cell Sorting Experiments Link Persistent Mitochondrial DNA Damage with Loss of Mitochondrial Membrane Potential and Apoptotic Cell Death. J Biol Chem. 2003;278:1728–1734. doi: 10.1074/jbc.M208752200. [DOI] [PubMed] [Google Scholar]
- Schapira AH. Mitochondrial disorders. Curr Opin Neurol. 1997;10:43–47. doi: 10.1097/00019052-199702000-00009. [DOI] [PubMed] [Google Scholar]
- Schapira AH, Cooper JM. Mitochondrial function in neurodegeneration and ageing. Mutat Res. 1992;275:133–143. doi: 10.1016/0921-8734(92)90018-k. [DOI] [PubMed] [Google Scholar]
- Schnopp NM, Kosel S, Egensperger R, Graeber MB. Regional heterogeneity of mtDNA heteroplasmy in parkinsonian brain. Clin Neuropathol. 1996;15:348–352. [PubMed] [Google Scholar]
- Schon EA, Bonilla E, Dimauro S. Mitochondrial DNA mutations and pathogenesis. J Bioernerg And Biomemb. 1997;29:131–149. doi: 10.1023/a:1022685929755. [DOI] [PubMed] [Google Scholar]
- Shokolenko IN, Alexeyev MF, Robertson FM, LeDoux SP, Wilson GL. The expression of Exonuclease III from E. coli in mitochondria of breast cancer cells diminishes mitochondrial DNA repair capacity and cell survival after oxidative stress. DNA Repair (Amst) 2003;2:471–482. doi: 10.1016/s1568-7864(03)00019-3. [DOI] [PubMed] [Google Scholar]
- Simonian NA, Coyle T. Oxidative stress in neurodegenerative diseases. Annu Rev Pharmacol Toxicol. 1996;36:83–106. doi: 10.1146/annurev.pa.36.040196.000503. [DOI] [PubMed] [Google Scholar]
- Skulachev VP. Why are mitochondria involved in apoptosis? Permeability transition pores and apoptosis as selective mechanisms to eliminate superoxide-producing mitochondria and cells. FEBS Letters. 1996;397:7–10. doi: 10.1016/0014-5793(96)00989-1. [DOI] [PubMed] [Google Scholar]
- Stuart JA, Hashiguchi K, Wilson DM, III, Copeland WC, Souza-Pinto NC, Bohr VA. DNA base excision repair activities and pathway function in mitochondrial and cellular lysates from cells lacking mitochondrial DNA. Nucleic Acids Res. 2004;32(7):2181–2192. doi: 10.1093/nar/gkh533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuart JA, Karahalil B, Hogue BA, Souza-Pinto NC, Bohr VA. (a), Mitochondrial and nuclear DNA base excision repair are affected differently by caloric restriction. FASEB J. 2004;18(3):595–597. doi: 10.1096/fj.03-0890fje. [DOI] [PubMed] [Google Scholar]
- Stürmer K, Baumann O, Walz B. Actin-dependent light-induced translocation of mitochondria and ER cisternae in the photoreceptor cells of the lowest Schistocerca gregria. J Cell Sci. 1995;108:2273–2283. doi: 10.1242/jcs.108.6.2273. [DOI] [PubMed] [Google Scholar]
- Szczesny B, Hazra TK, Papaconstantinou J, Mitra S, Boldogh I. Age-dependent deficiency in import of mitochondrial DNA glycosylases required for repair of oxidatively damaged bases. PNAS. 2003;100(19):10670–10675. doi: 10.1073/pnas.1932854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torii K, Sugiyama S, Takagi K, Satake T, Ozawa T. Age-related decrease in respiratory muscle mitochondrial function in rats. Am J Resp Cell Molec Biol. 1992;6:88. doi: 10.1165/ajrcmb/6.1.88. [DOI] [PubMed] [Google Scholar]
- Trounce I, Byrne E, Marzuki S. Decline in skeletal muscle mitochondrial respiratory chain function: possible factor in ageing. Lancet. 1989;i:637–639. doi: 10.1016/s0140-6736(89)92143-0. [DOI] [PubMed] [Google Scholar]
- Turrens JF. Superoxide production by the mitochondrial respiratory chain. Biosci Rep. 1997;17:3–8. doi: 10.1023/a:1027374931887. [DOI] [PubMed] [Google Scholar]
- Van Houten B, Friedberg EC. DNA Repair: Mitochondrial DNA Damage and Repair. Elsevier; New York, NY: 1999. [Google Scholar]
- Wei YH. Oxidative stress and mitochondrial DNA mutations in human aging. Proc Soc Exp Biol Med. 1998;217:53–63. doi: 10.3181/00379727-217-44205. [DOI] [PubMed] [Google Scholar]
- Wilson GL, Patton NJ, LeDoux SP. Mitochondrial DNA in β-cells is a sensitive target for damage by nitric oxide. Diabetes. 1997;46:1291–1295. doi: 10.2337/diab.46.8.1291. [DOI] [PubMed] [Google Scholar]
- Wooten GF, Currie LJ, Bennett JP, Harrison MB, Trugman JM, Parker WD. Maternal inheritance in Parkinson’s disease. Ann Neurol. 1997;41:265–268. doi: 10.1002/ana.410410218. [DOI] [PubMed] [Google Scholar]
- Yakes FM, Van Houten B. Mitochondrial DNA damage is more extensive and persists longer than nuclear DNA damage in human cells following oxidative stress. Proc Natl Acad Sci USA. 1997;94:514–519. doi: 10.1073/pnas.94.2.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen T-C, Chen Y-S, King KL, Yeh S-J, Wei Y-H. Liver mitochondrial respiratory functions decline with age. Biochem Biophys Res Commun. 1989;165:994–1003. doi: 10.1016/0006-291x(89)92701-0. [DOI] [PubMed] [Google Scholar]