Abstract
The purpose of this article is to provide an overview of genetic research involving post-traumatic stress disorder (PTSD). First, we summarize evidence for genetic influences on PTSD from family investigations. Second, we discuss the distinct contributions to our understanding of the genetics of PTSD permitted by twin studies. Finally, we summarize findings from molecular genetic studies, which have the potential to inform our understanding of underlying biological mechanisms for the development of PTSD.
Keywords: post-traumatic stress disorder, trauma, genetics
INTRODUCTION
Prevalence estimates from epidemiological studies suggest that the majority of individuals (50–70%) have been exposed to at least one potentially-traumatic event (PTE) during their lifetime [Kessler et al., 1995; Breslau et al., 1998]. Although the majority of those exposed to PTEs are resilient or recover rapidly following exposure, a substantial minority develop chronic psychopathology, most commonly post-traumatic stress disorder (PTSD; [Kessler et al., 1995; Kilpatrick et al., 2003]). PTSD is characterized by symptoms of re-experiencing, avoidance, and hyperarousal persisting for more than 1 month post-trauma [American Psychiatric Association, 1994]. The present article provides an overview of genetic factors in the etiology of PTSD, with explicit goals of informing clinical conceptualization of PTSD as well as promoting further research in a pioneering area of study.
PTSD RUNS IN FAMILIES
If risk for PTSD is partially explained by genetic factors, biological relatives (family members) of individuals with PTSD should have a higher prevalence of PTSD than similarly trauma-exposed controls that did not develop PTSD. Consistent with a role for a genetic contribution in PTSD, adult children of Holocaust survivors with PTSD had a higher risk of PTSD following trauma compared to adult children of Holocaust survivors without PTSD [Yehuda et al., 2001]. Similarly, Cambodian refugee children whose parents both had PTSD were five times more likely to receive the diagnosis than refugee children whose parents did not have PTSD [Sack et al., 1995]. However, family studies cannot tell us whether a disorder, such as PTSD, runs in families for genetic or environmental reasons. Twin studies have been used to distinguish between genetic and environmental influences in disorder etiology.
PTSD IS HERITABLE
Twin studies have made four major contributions to our understanding of the genetic etiology of PTSD. First, they indicate that genetic factors influence exposure to PTEs. This is referred to as gene-environment correlation, whereby selection of environment, and subsequently potential for exposure to trauma, is partly determined by genetic factors [Kendler and Eaves, 1986]. For example, twin studies have demonstrated that genetic factors influence exposure to PTEs such as combat exposure [Lyons et al., 1993] and assaultive violence [Stein et al., 2002]. These gene-environmental correlations are likely due in part to individual differences in personality. Personality characteristics are moderately heritable and influence the tendency for individuals to select themselves into potentially harmful environments. For example, longitudinal investigations have found that childhood adjustment and neuroticism predicted subsequent stressful life events in adulthood [Van Os and Jones, 1999]. Similarly, research has found that childhood externalizing is prospectively associated with both risk of trauma exposure and with PTSD in adulthood [Koenen et al., 2007b]. One investigation found that genetic factors partially mediated the association between personality variables (such as antisocial personality traits, psychoticism, and openness to novelty) and exposure to violent traumatic events [Jang et al., 2003].
Second, twin studies suggest genetic influences explain a substantial proportion of vulnerability to PTSD even after accounting for genetic influences on trauma exposure. The first twin study to estimate heritability of PTSD was conducted by True et al. [1993] on members of the Vietnam Era Twin (VET) Registry. The authors found that approximately 30% of the variance in PTSD was accounted for by genetic factors, even after controlling for combat exposure. Genetic influences on PTSD were similar for twins who did not serve in Southeast Asia, suggesting heritability of PTSD has generalization to traumatic events other than combat exposure. The second twin study of PTSD was conducted on a sample of male and female civilian volunteers [Stein et al., 2002]. Consistent with True and colleagues, the authors found moderate heritability in PTSD symptoms, with additional variance accounted for by non-shared environmental factors. The findings from these two twin studies support suggest that genetic factors play a substantial role in vulnerability to developing PTSD.
Third, twin studies have demonstrated that genetic influences on PTSD overlap with those for other mental disorders. The extent of the overlap varies with the disorder studied. For example, genetic influences on major depression account for the majority of the genetic variance in PTSD [Fu et al., 2007; Koenen et al., 2007a]. Genetic influences common to generalized anxiety disorder and panic disorder symptoms account for approximately 60% of the genetic variance in PTSD [Chantarujikapong et al., 2001] and those common to alcohol and drug dependence [Xian et al., 2000] and nicotine dependence [Koenen et al., 2005a] account for over 40% of the variance associated with PTSD. Thus, the limited data available suggest that the majority of genes that affect risk for PTSD also influence risk for other psychiatric disorders and vice versa.
Fourth, twin studies can provide important information regarding possible biological “endophenotypes,” or intervening phenotypes inmultifactorial disorders that may be more proximal to genetic variants. Toward the examination of these endophenotypes, twin studies have employed monozygotic (MZ) discordant design, in which individuals with and without PTSD are compared across twin pairs on a specific biological correlate to determine whether that marker is associated with the PTSD diagnosis. The design includes four participant groups: (1) trauma-exposed index twins who developed PTSD; (2) their “high-risk” trauma-unexposed co-twins who did not have PTSD (considered high risk because their identical twin developed PTSD when exposed to trauma); (3) trauma-exposed index twins with no PTSD; and (4) “low risk” trauma unexposed co-twins who did not have PTSD (deemed low risk because their identical twin did not develop PTSD when exposed to trauma). Pitman et al. [2006] have used the MZ discordant design to test whether commonly found biological correlates of PTSD are actually risk factors for developing the disorder. Investigations have generally emerged from established concomitants of chronic PTSD [Kitayama et al., 2005; Smith, 2005] and have identified smaller hippocampal volume [Gilbertson et al., 2002] and abnormally large CSP [May et al., 2004] in twins with chronic PTSD and in their non-combat exposed cotwins, as compared to combat veterans who did not develop the disorder. Similarly, neurological soft signs (NSS), or subtle indices of neurological function thought to represent “subtle cortical dysfunction,” are more prevalent in combat exposed index twins with PTSD than those without PTSD and high-risk co-twins had greater NSS than low-risk co-twins [Gurvits et al., 2006]. These investigations provide important information regarding biological endophenotypes of PTSD that, given unique data afforded by MZ discordant design, likely represents pre-trauma risk factors rather than consequences of PTSD.
MOLECULAR GENETIC STUDIES OF PTSD
The primary limitation of twin studies is that they cannot tell us which genes are important in PTSD etiology. In contrast, molecular genetic studies have the potential to identify markers of vulnerability or resilience. To date, only 11 candidate gene studies of PTSD have been published (see Table I).
TABLE I.
Review of Published Case-Control Candidate Gene Associations Studies of PTSD
| First author | Year | Trauma exposed controls? |
Trauma type | Gene name (symbol) | Finding |
|---|---|---|---|---|---|
| Comings | 1991 | No | Combat | Dopamine receptor D2 (DRD2) | Excess D2A1 allele in PTSD cases P = 0.007 |
| Comings | 1996 | Yes | Combat | Dopamine receptor D2 (DRD2) | Excess D2A1 allele in PTSD cases P = 0.041 |
| 1996 | Yes | Combat | Dopamine receptor D2 (DRD2) | Excess D2A1 allele in PTSD cases P = 0.002 |
|
| Gelernter | 1999 | No | Combat | Dopamine receptor D2 (DRD2) | No significant association between D2A1 allele/DRD2 haplotypes and PTSD |
| Lappalainen | 2002 | No | Combat | Neuropeptide Y (NPY) | No significant association between Leu7Pro polymorphism and PTSD |
| Segman | 2002 | Yes | Various | Dopamine transporter (DAT1) | Excess 9-repeat allele in PTSD cases P = 0.012 |
| Young | 2002 | No | Combat | Dopamine receptor D2 (DRD2) | Excess D2A1 allele only in PTSD cases with harmful drinking P < 0.001 |
| Bachman | 2005 | Yes | Combat | Glococorticoid receptor (GCCR) | No significant association between GCCR polymorphisms and PTSD |
| Lee | 2005 | No | Various | Serotonin transporter (SLC6A4) | Excess s allele in PTSD cases P = 0.04 |
| Zhang | 2006 | Not specified | Not specified | Brain derived neurotrophic factor (BDNF) | No significant association between three BDNF variants and PTSD |
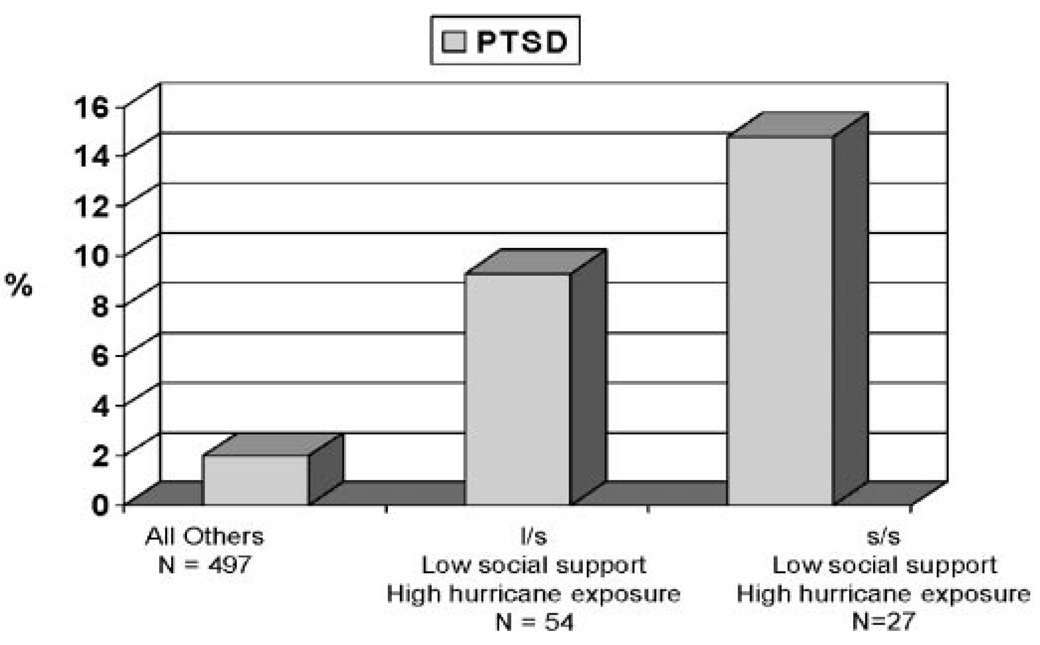
| Kilpatrick | 2007 | Yes | Hurricane | Serotonin transporter (SLC6A4) | Significant association between s/s genotype and PTSD in adults with high hurricane exposure and low social support |
PTSD, post-traumatic stress disorder; D2DA1, Al one allele of DRD2 gene; s allele, short version (versus long) of the serotonin transporter promoter polymorphism.
Most studies have focused on dopamine (DA) system genes. Both animal and human studies have implicated the DA system in the etiology of PTSD [Yehuda et al., 1992; Inglis and Moghaddam, 1999]. Five out of the six investigations of DA system genes studied the association between marker alleles at the D2 DA receptor gene (DRD2) and PTSD. Whereas initial investigations found a positive association with the DRD2A1 allele [Comings et al., 1991, 1996], a subsequent investigation, that did not assess for trauma exposure in the control group, found no association with the DRD2A1 allele or with any combination of alleles for the DRD2 locus [Gelernter et al., 1999]. Of note, Comings et al. [1996] investigation consisted of a relatively small sample of substance abusers with PTSD (N = 37) compared with substance abusers without PTSD (N = 19), limiting generalizability to a substance abusing population. Comorbid PTSD and substance abuse was also addressed in an investigation of combat veterans with and without PTSD, with analyses revealing a positive association between DRD2A1 and PTSD only in the subset of PTSD cases who engaged in harmful drinking [Young et al., 2002]. The final study examined a slightly different facet of DA transmission in patients with chronic PTSD and trauma-exposed healthy controls, reporting a positive association between of the DA transporter SLC6A3 (DAT1) 3′ polymorphism and chronic PTSD [Segman et al., 2002].
The five remaining studies explored genetic polymorphisms across alternative neurobiological pathways, with the majority of studies reporting no association between specific genes and chronic PTSD. One investigation found no association between polymorphisms in the brain derived neurotrophic factor (BDNF) gene and chronic PTSD [Zhang et al., 2006]. Similarly, no significant association was found between chronic PTSD and either the Leu7Pro polymorphism in the neuropeptide Y (NPY) gene [Lappalainen et al., 2002] or two glucocorticoid receptor polymorphisms (N363S and BclI) [Bachmann et al., 2005].
Investigations of the serotonergic system have proven slightly more fruitful. One investigation examined an insertion/deletion polymorphism in the promoter region of the serotonin transporter (SLC6A4, locus 5-HTTLPR), reporting an excess of s/s genotypes in Korean PTSD patients compared with normal controls [Lee et al., 2005]. Kilpatrick et al. [2007] also documented a significant association between the 5-HTTLPR genotype and PTSD in a sample of hurricane-exposed adults (see Fig. 1). However, 5-HTTLPR genotype was only associated with increased risk of PTSD among adults with high stress exposure. This is the first report of a significant genotype-environment interaction in PTSD.
Figure 1.
Prevalence of post-hurricane PTSD by SLC6A4 genotype, level of social support, and level of hurricane exposure in adults exposed to 2004 Florida Hurricanes.
Another promising approach to identification of candidate genes is investigation of the association between genetic polymorphisms and a range of phenotypes evidenced by individuals diagnosed with PTSD. For example, Koenen et al. [2005b] have found that polymorphisms in FKBP5 are associated with peritraumatic dissociation pediatric injury patients. Lawford et al. [2006] reported that the DRD2 A1 allele was associated to comorbidity in male veterans with PTSD. Given evidence supporting both an association between APOE and cognitive disturbances [Gallagher-Thompson et al., 2001] and PTSD and neurocognitive deficits [Gilbertson et al., 2006], Freeman et al. [2005] reported that patients with the APOE allele reported increased symptoms of re-experiencing and demonstrated poorer performance on facets of memory function relative to patients without the APOE allele.
Unfortunately, as the hypothesis-driven candidate gene association approach is reliant on extant literature regarding the biological endophenotype of PTSD, candidate gene association strategies are significantly limited by the relative paucity of information regarding the biological underpinnings of PTSD. Further, the majority of published investigations have been conducted cross-sectionally, with limited to no efforts to address duration of time since trauma (which may be associated with remission or change in symptoms), age at first trauma exposure (which may be associated with differences in endophenotype), or repeated traumatic exposure. Finally, stratification effects have rarely been considered in candidate gene association investigations of PTSD [for an exception see Lappalainen et al., 2002].
CONCLUSIONS
In sum, converging evidence from diverse research designs supports a role for genetic influences in the etiology of PTSD. Family studies have laid the foreground for research in this area, indicating increased risk for PTSD in relatives. Twin studies further support the heritability of PTSD, yielding three main findings related to the genetic influences on: the likelihood of exposure to PTEs, the development of PTSD, and the existence of comorbidity. Although these studies support a role for genetics, little information is provided regarding the specific genetic underpinnings. Partially filling this void are candidate gene studies, which attempt to identify specific genes related to the etiology of PTSD. The few candidate gene studies completed to date, though limited by small samples, point to potential roles for genetic influences in the DA and serotonergic systems, though evidence for genotype–environment interactions and for possibly distinct influences for comorbid disorders highlight both the complexity of candidate gene studies and the importance of further study using this methodology. Finally, as the aforementioned limitations of candidate gene investigations may be intensified in small samples, it will be important for future investigations to replicate published findings.
PTSD is unique among the psychiatric disorders in that a diagnostic prerequisite is exposure to a PTE, by definition necessitating an environmental influence on the development of PTSD. However, only one published study has examined whether the role of genotype-environment interaction in the etiology of PTSD [Kilpatrick et al., 2007]. Future genetic research in PTSD should carefully consider factors related to trauma exposure that may modify genetic effects. Factors such as the type of traumatic event, perceived life threat during the trauma, post-trauma social support, peritraumatic dissociation, peritraumatic emotional responses, additional life stress, time-lapsed since trauma exposure, prior trauma exposure are particularly important [Brewin et al., 2000; Ozer et al., 2003].
In short, research in the genetics of PTSD is in its infancy and the areas of possible contribution are immense. Furthermore, the potential impact of genetically informed studies of trauma is substantial. Interested readers are pointed to more detailed recommendations for genetic research in PTSD [Segman and Shalev, 2003; Koenen, 2007], and for gene–environment interaction in PTSD [Koenen et al., in press].
ACKNOWLEDGMENTS
Dr. Koenen is supported in part by US-NIMH K08 MH070627. Ananda Amstadter is supported by US-NIAAA T32 AA007474.
Grant sponsor: US-NIMH; Grant number: K08 MH070627; Grant sponsor: US-NIAAA; Grant number: T32 AA007474; Grant sponsor: US-NIMH; Grant number: MH078928.
Contributor Information
Nicole R. Nugent, T32 Postdoctoral Fellow at Brown Medical School and at the Bradley/Hasbro Children’s Research Center. Dr. Nugent has previously published research in biological predictors of posttraumatic stress disorder (PTSD) in pediatric populations and is presently conducting research examining psychiatric genetics of depression and PTSD as related to medical adherence and health outcomes in HIV-infected youth.
Ananda B. Amstadter, Clinical Psychology Intern at the National Crime Victims Research and Treatment Center of the Medical University of South Carolina. Her research focuses on identification of risk and resilience factors related to post-trauma psychopathology.
Karestan C. Koenen, Assistant Professor of Society, Human Development and Health and Epidemiology at the Harvard School of Public Health. Her research interests lie in understanding the etiology of post-traumatic stress disorder with a specific focus on the how early childhood and genetic factors shape vulnerability and resilience to traumatic events..
REFERENCES
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington, DC: 1994. [Google Scholar]
- Bachmann AW, Sedgley TL, Jackson RV, Gibson JN, Young RM, Torpy DJ. Glucocorticoid receptor polymorphisms and posttraumatic stress disorder. Psychoneuroendocrinology. 2005;30:297–306. doi: 10.1016/j.psyneuen.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Breslau N, Kessler R, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: The 1996 Detroit area survey of trauma. Arch Gen Psychiatry. 1998;55:626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- Brewin CR, Andrews B, Valentine JD. Meta-analysis of risk factors for posttraumatic stress disorder in trauma-exposed adults. J Consult Clin Psychol. 2000;68:317–336. doi: 10.1037//0022-006x.68.5.748. [DOI] [PubMed] [Google Scholar]
- Chantarujikapong SI, Scherrer JF, Xian H, Eisen SA, Lyons MJ, Goldberg J, Tsuang M, True WR. A twin study of generalized anxiety disorder symptoms, panic disorder symptoms and post-traumatic stress disorder in men. Psychiatry Res. 2001;103:133–145. doi: 10.1016/s0165-1781(01)00285-2. [DOI] [PubMed] [Google Scholar]
- Comings DE, Comings BG, Muhleman D, Dietz G, Shabbahrami B, Tast D. The dopamine D2 receptor locus as a modifying gene in neuropsychiatric disorder. JAMA. 1991;266:1793–1800. [PubMed] [Google Scholar]
- Comings DE, Muhleman D, Gysin R. Dopamine D2 receptor (DRD2) gene and susceptibility to posttraumatic stress disorder: A study and replication. Biol Psychiatry. 1996;40:368–372. doi: 10.1016/0006-3223(95)00519-6. [DOI] [PubMed] [Google Scholar]
- Freeman T, Roca V, Guggenheim F, Kimbrell T, Griffin WS. Neuropsychiatric associations of apolipoprotein E alleles in subjects with combat-related posttraumatic stress disorder. J Neuropsychiatry Clin Neurosci. 2005;17:541–543. doi: 10.1176/jnp.17.4.541. [DOI] [PubMed] [Google Scholar]
- Fu Q, Koenen KC, Miller MW, Heath AC, Bucholz KK, Lyons MJ, Eisen SA, True WR, Goldberg J, Tsuang MT. Differential etiology of posttraumatic stress disorder with conduct disorder and Major depression in male veterans. Biol Psychiatry. 2007;62:1088–1094. doi: 10.1016/j.biopsych.2007.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher-Thompson D, O’Hara R, Simmons A, Kraemer HC, Murphy GM., Jr Apolipoprotein E epsilon4 allele affects the relationship between stress and depression in caregivers of patients with Alzheimer’s disease. J Geriatr Psychiatry Neurol. 2001;14:115–119. doi: 10.1177/089198870101400303. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Southwick S, Goodson S, Morgan A, Nagy L, Charney DS. No association between D2 dopamine receptor (DRD2) ‘A’ system alleles, or DRD2 haplotypes, and posttraumatic stress disorder. Biol Psychiatry. 1999;45:620–625. doi: 10.1016/s0006-3223(98)00087-0. [DOI] [PubMed] [Google Scholar]
- Gilbertson MW, Shenton ME, Ciszewski A, Kasai K, Lasko NB, Orr SP, Pitman RK. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci. 2002;5:1242–1247. doi: 10.1038/nn958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson MW, Paulus LA, Williston SK, Gurvits TV, Lasko NB, Pitman RK, Orr SP. Neurocognitive function in monozygotic twins discordant for combat exposure: Relationship to posttraumatic stress disorder. J Abnorm Psychol. 2006;115:484–495. doi: 10.1037/0021-843X.115.3.484. [DOI] [PubMed] [Google Scholar]
- Gurvits TV, Metzger LJ, Lasko NB, Cannistraro PA, Tarhan AS, Gilbertson MW, Orr SP, Charbonneau AM, Wedig MM, Pitman RK. Subtle neurologic compromise as a vulnerability factor for combat-related posttraumatic stress disorder: Results of a twin study. Arch Gen Psychiatry. 2006;63:571–576. doi: 10.1001/archpsyc.63.5.571. [DOI] [PubMed] [Google Scholar]
- Inglis FM, Moghaddam B. Dopaminergic innervation of the amygdala is highly responsive to stress. J Neurochem. 1999;72:1088–1094. doi: 10.1046/j.1471-4159.1999.0721088.x. [DOI] [PubMed] [Google Scholar]
- Jang KL, Stein MB, Taylor S, Asmundson GJ, Livesley WJ. Exposure to traumatic events and experiences: Aetiological relationships with personality function. Psychiatry Res. 2003;120:61–69. doi: 10.1016/s0165-1781(03)00172-0. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Eaves LJ. Models for the joint effects of genotype and environment on liability to psychiatric illness. Am J Psychiatry. 1986;143:279–289. doi: 10.1176/ajp.143.3.279. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Arch Gen Psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Ruggiero KJ, Acierno R, Saunders BE, Resnick HS, Best CL. Violence and risk of PTSD, major depression, substance abuse/dependence, and comorbidity: Results from the National Survey of Adolescents. J Consult Clin Psychol. 2003;71:692–700. doi: 10.1037/0022-006x.71.4.692. [DOI] [PubMed] [Google Scholar]
- Kilpatrick DG, Koenen KC, Ruggiero KJ, Acierno R, Galea S, Resnick HS, Roitzsch J, Boyle J, Gelernter J. The serotonin transporter genotype and social support and moderation of posttraumatic stress disorder and depression in hurricane-exposed adults. Am J Psychiatry. 2007;164:1693–1699. doi: 10.1176/appi.ajp.2007.06122007. [DOI] [PubMed] [Google Scholar]
- Kitayama N, Vaccarino V, Kutner M, Weiss P, Bremner JD. Magnetic resonance imaging (MRI) measurement of hippocampal volume in posttraumatic stress disorder: A meta-analysis. J Affect Disord. 2005;88:79–86. doi: 10.1016/j.jad.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Koenen KC. Genetics of posttraumatic stress disorder: Review and recommendations for future studies. J Trauma Stress. 2007;20:737–750. doi: 10.1002/jts.20205. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Hitsman B, Lyons MJ, Niaura R, McCaffery J, Goldberg J, Eisen SA, True W, Tsuang M. A twin registry study of the relationship between posttraumatic stress disorder and nicotine dependence in men. Arch Gen Psychiatry. 2005a;62:1258–1265. doi: 10.1001/archpsyc.62.11.1258. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Saxe G, Purcell S, Smoller JW, Bartholomew D, Miller A, Hall E, Kaplow J, Bosquet M, Moulton S, Baldwin C. Polymorphisms in FKBP5 are associated with peritraumatic dissociation in medically injured children. Mol Psychiatry. 2005b;10:1058–1059. doi: 10.1038/sj.mp.4001727. [DOI] [PubMed] [Google Scholar]
- Koenen KC, Fu QJ, Ertel K, Lyons MJ, Eisen SA, True WR, Goldberg J, Tsuang MT. Common genetic liability to major depression and posttraumatic stress disorder in men. J Affect Disord. 2007a;105:109–115. doi: 10.1016/j.jad.2007.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Moffitt TE, Poulton R, Martin J, Caspi A. Early childhood factors associated with the development of posttraumatic stress disorder: Results from a longitudinal birth cohort. Psychol Med. 2007b;37:181–192. doi: 10.1017/S0033291706009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen KC, Nugent NR, Amstadter A. Posttraumatic stress disorder: A review and agenda for gene-environment interaction Research in trauma. Eur Arch Psychiatry Clin Neurosci. 2008 doi: 10.1007/s00406-007-0787-2. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappalainen J, Kranzler HR, Malison R, Price LH, Van Dyck D, Krystal JH, Gelernter J. A functional neuropeptide Y Leu7Pro polymorphism associated with alcohol dependence in a large population sample from the United States. Arch Gen Psychiatry. 2002;59:825–831. doi: 10.1001/archpsyc.59.9.825. [DOI] [PubMed] [Google Scholar]
- Lawford BR, Young R, Noble EP, Kann B, Ritchie T. The D2 dopamine receptor (DRD2) gene is associated with comorbid depression, anxiety and social dysfunction in untreated veterans with posttraumatic stress disorder. Eur Psychiatry. 2006;21:180–185. doi: 10.1016/j.eurpsy.2005.01.006. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Lee MS, Kang RH, Kim H, Kim SD, Kee BS, Kim YH, Kim YK, Kim JB, Yeon BK, Oh KS, Oh BH, Yoon JS, Lee C, Jung HY, Chee IS, Paik IH. Influence of the serotonin transporter promoter gene polymorphism on susceptibility to posttraumatic stress disorder. Depress Anxiety. 2005;21:135–139. doi: 10.1002/da.20064. [DOI] [PubMed] [Google Scholar]
- Lyons MJ, Goldberg J, Eisen SA, True W, Tsuang MT, Meyer JM. Do genes influence exposure to trauma? A twin study of combat. Am J Med Genet. 1993;48:22–27. doi: 10.1002/ajmg.1320480107. [DOI] [PubMed] [Google Scholar]
- May FS, Chen QC, Gilbertson MW, Shenton ME, Pitman RK. Cavum septum pellucidum in monozygotic twins discordant for combat exposure: Relationship to posttraumatic stress disorder. Biol Psychiatry. 2004;55:656–658. doi: 10.1016/j.biopsych.2003.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer EJ, Best SR, Lipsey TL, Weiss DS. Predictors of posttraumatic stress disorder and symptoms in adults: A meta-analysis. Psychol Bull. 2003;129:52–73. doi: 10.1037/0033-2909.129.1.52. [DOI] [PubMed] [Google Scholar]
- Pitman RK, Gilbertson MW, Gurvits TV, May FS, Lasko NB, Metzger LJ, Shenton ME, Yehuda R, Orr SP. Clarifying the origin of biological abnormalities in PTSD through the study of identical twins discordant for combat exposure. Ann N Y Acad Sci. 2006;1071:242–254. doi: 10.1196/annals.1364.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sack WH, Clarke GN, Seeley J. Posttraumatic stress disorder across two generations of Cambodian refugees. J Am Acad Child Adolesc Psychiatry. 1995;34:1160–1166. doi: 10.1097/00004583-199509000-00013. [DOI] [PubMed] [Google Scholar]
- Segman RH, Shalev AY. Genetics of posttraumatic stress disorder. CNS Spectr. 2003;8:693–698. doi: 10.1017/s1092852900008889. [DOI] [PubMed] [Google Scholar]
- Segman RH, Cooper-Kazaz R, Macciardi F, Goltser T, Halfon Y, Dobroborski T, Shalev AY. Association between the dopamine transporter gene and posttraumatic stress disorder. Mol Psychiatry. 2002;7:903–907. doi: 10.1038/sj.mp.4001085. [DOI] [PubMed] [Google Scholar]
- Smith ME. Bilateral hippocampal volume reduction in adults with posttraumatic stress disorder: A meta-analysis of structural MRI studies. Hippocampus. 2005;15:798–807. doi: 10.1002/hipo.20102. [DOI] [PubMed] [Google Scholar]
- Stein MB, Jang KJ, Taylor S, Vernon PA, Livesley WJ. Genetic and environmental influences on trauma exposure and posttraumatic stress disorder: A twin study. Am J Psychiatry. 2002;159:1675–1681. doi: 10.1176/appi.ajp.159.10.1675. [DOI] [PubMed] [Google Scholar]
- True WJ, Rice J, Eisen SA, Heath AC, Goldberg J, Lyons MJ, Nowak J. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch Gen Psychiatry. 1993;50:257–264. doi: 10.1001/archpsyc.1993.01820160019002. [DOI] [PubMed] [Google Scholar]
- Van Os J, Jones PB. Early risk factors and adult person-environment relationships in affective disorder. Psychol Med. 1999;29:1055–1067. doi: 10.1017/s0033291799001026. [DOI] [PubMed] [Google Scholar]
- Xian H, Chantarujikapong SI, Shrerrer JF, Eisen SA, Lyons MJ, Goldberg J, Tsuang M, True W. Genetic and environmental influences on posttraumatic stress disorder, alcohol, and drug dependence in twin pairs. Drug Alcohol Depend. 2000;61:95–102. doi: 10.1016/s0376-8716(00)00127-7. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Southwick S, Giller EL, Ma X, Mason JW. Urinary catecholamine excretion and severity of PTSD symptoms in Vietnam combat veterans. J Nerv Ment Dis. 1992;180:321–325. doi: 10.1097/00005053-199205000-00006. [DOI] [PubMed] [Google Scholar]
- Yehuda R, Halligan SL, Bierer LM. Relationship of parental trauma exposure and PTSD to PTSD, depressive and anxiety disorders in offspring. J Psychiatr Res. 2001;35:261–270. doi: 10.1016/s0022-3956(01)00032-2. [DOI] [PubMed] [Google Scholar]
- Young BR, Lawford BR, Noble EP, Kanin B, Wilkie A, Ritchie T, Arnold L, Shadforth S. Harmful drinking in military veterans with posttraumatic stress disorder: Association with the D2 dopamine receptor A1 allele. Alcohol Alcohol. 2002;37:451–456. doi: 10.1093/alcalc/37.5.451. [DOI] [PubMed] [Google Scholar]
- Zhang H, Ozbay F, Lappalainen J, Kranzler HR, van Dyck CH, Charney DS, Price LH, Southwick S, Yang BZ, Rasmussen A, Gelernter J. Brain derived neurotrophic factor (BDNF) gene variants and Alzheimer’s disease, affective disorders, posttraumatic stress disorder, schizophrenia, and substance dependence. Am J Med Genet Part B Neuropsychiatr Genet. 2006;141B:387–393. doi: 10.1002/ajmg.b.30332. [DOI] [PMC free article] [PubMed] [Google Scholar]