Abstract
Systemic lupus erythematosus (SLE) is characterized by abnormal T-cell activation and death, processes which are crucially dependent on the controlled production of reactive oxygen intermediates (ROI) and of ATP in mitochondria. The mitochondrial transmembrane potential (Δψm) has conclusively emerged as a critical checkpoint of ATP synthesis and cell death. Lupus T cells exhibit persistent elevation of Δψm or mitochondrial hyperpolarization (MHP) as well as depletion of ATP and glutathione which decrease activation-induced apoptosis and instead predispose T cells for necrosis, thus stimulating inflammation in SLE. NO-induced mitochondrial biogenesis in normal T cells accelerates the rapid phase and reduces the plateau of Ca2+ influx upon CD3/CD28 co-stimulation, thus mimicking the Ca2+ signaling profile of lupus T cells. Treatment of SLE patients with rapamycin improves disease activity, normalizes CD3/CD28-induced Ca2+ fluxing but fails to affect MHP, suggesting that altered Ca2+ fluxing is downstream or independent of mitochondrial dysfunction. Understanding the molecular basis and consequences of MHP is essential for controlling T-cell activation and death signaling in SLE.
Lupus T cells exhibit mitochondrial dysfunction
Mitochondrial hyperpolarization (MHP) and ATP depletion predispose lupus T cells to death by necrosis which is pro-inflammatory
MHP is caused by depletion of glutathione and exposure to nitric oxide (NO)
NO-induced mitochondrial biogenesis regenerates the Ca2+ signaling profile of lupus T cells
Rapamycin treatment normalizes Ca2+ fluxing but not MHP, suggesting that the mammalian target of rapamycin, acts as a sensor and effector of MHP in SLE
Keywords: lupus, mitochondria, nitric oxide, glutathione, calcium
Introduction
Abnormal T cell activation and cell death underlie the pathology of SLE (1). Potentially autoreactive T and B lymphocytes are removed by apoptosis during development and after completion of an immune response. Paradoxically, lupus T cells exhibit both enhanced spontaneous apoptosis and defective activation-induced cell death (2). Increased spontaneous apoptosis has been linked to chronic lymphopenia (2) and compartmentalized release of nuclear autoantigens in patients with SLE (3). By contrast, defective activation-induced cell death (AICD) may be responsible for persistence of autoreactive cells (2).
Both cell proliferation and apoptosis are energy-dependent processes. Energy in the form of ATP is provided through glycolysis and oxidative phosphorylation. The mitochondrion, the site of oxidative phosphorylation, has long been identified as a source of energy and cell survival (2). The synthesis of ATP is driven by an electrochemical gradient across the inner mitochondrial membrane maintained by an electron transport chain and the membrane potential (Δψm, negative inside and positive outside). A small fraction of electrons react directly with oxygen and form reactive oxygen intermediates (ROI). The Δψm is regulated by the supply of reducing equivalents (NADH/NAD + NADPH/NADP + GSH) and the production of ROI and nitric oxide (NO) (4). Regeneration of GSH by glutathione reductase from its oxidized form, GSSG, and synthesis of NO depend on NADPH produced by the pentose phosphate pathway (PPP) (2). While disruption of the mitochondrial membrane potential Δψm has been proposed as the point of no return in apoptosis, elevation of Δψm or mitochondrial hyperpolarization (MHP) occurs prior to activation of caspases, phosphatidylserine (PS) externalization and disruption of Δψm in Fas- (5), H2O2- (6), and NO-induced apoptosis (7). MHP is also triggered by T-cell receptor (TCR) stimulation that is associated with transient inhibition of F0F1-ATPase, ATP depletion, and sensitization to necrosis, suggesting that Δψm elevation is a critical checkpoint of T cell fate decisions (8). Importantly, lupus T cells exhibit persistent MHP and ATP depletion which causes predisposition to death by necrosis that is highly pro-inflammatory (2). The mammalian target of rapamycin (mTOR) is located in the outer mitochondrial membrane and serves as a sensor of the Δψm in T cells (9). Focused on targeting MHP for treatment of lupus patients resistant or intolerant to conventional immunosuppressants, rapamycin improved disease activity and normalized baseline and T cell activation-induced Ca2+ fluxing without affecting MHP(10). These findings suggested that mTOR represents a gate keeper between MHP and altered Ca2+ signaling in SLE. The present review will focus on establishing the hierarchy of the metabolic pathways that underlie and mediate the consequences MHP and identify checkpoints that can be targeted for therapeutic intervention in SLE (Fig. 1).
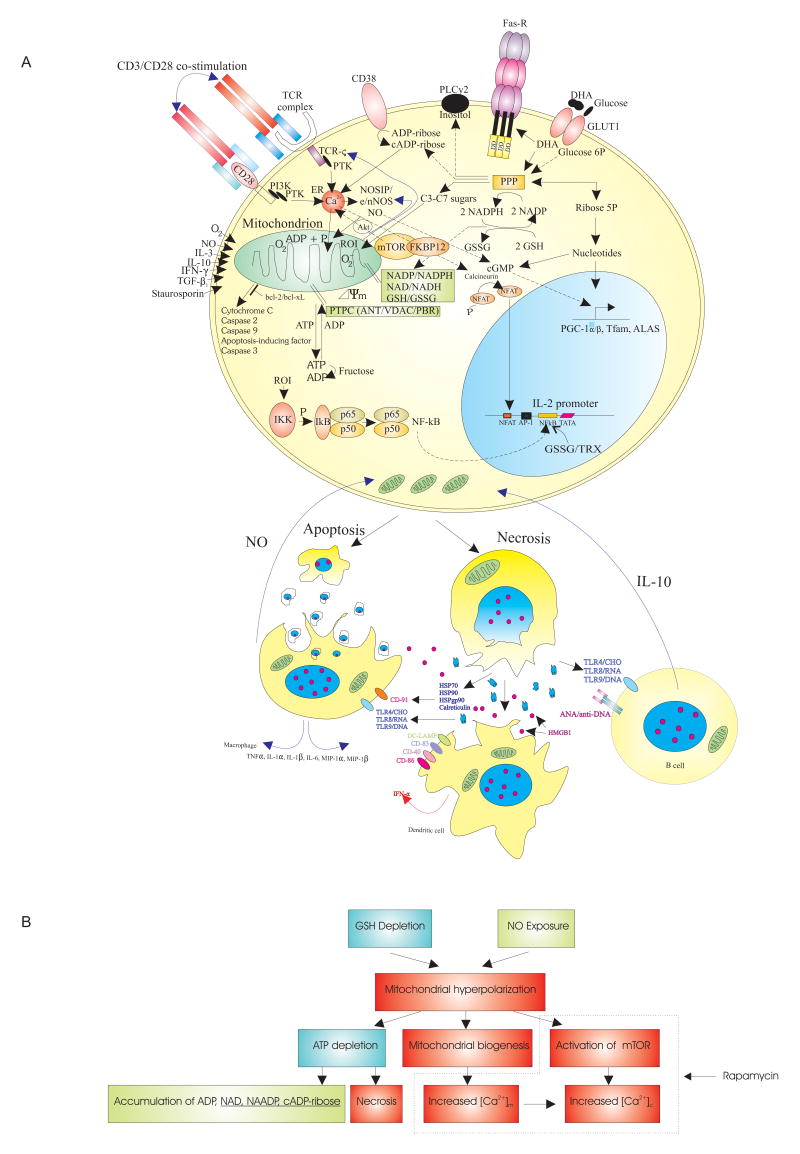
Fig 1.
A) Schematic outline of the metabolic pathways controlling (PPP,GSH, NO) and sensing (mTOR) mitochondrial hyperpolarization (MHP) of lupus T cells. In normal T cells, MHP and mitochondrial biogenesis is mediated via production of NO by eNOS or nNOS (28)and up-regulation of transcription factors PGC-1α, Tfam, and ALAS (39). NO production by eNOS may be compartmentalized to the T cell synapse (29). NO causes transient MHP via reversible inhibition of complex IV/cytochrome c oxidase (7) and persistent MHP via S-nitrosylation of complex I of the ETC in a state of GSH depletion (24). The PPP regulates the Δψm by producing 1) NADPH that serves as a reducing equivalent for GSH regeneration from its oxidized form GSSG and for production of NO by NOS and 2) ribose 5-phosphate for biosynthesis of nucleotides, ADP, ATP, NAD, NAADP, (c)ADP-ribose, and cGMP, the latter is a second messenger of NO. NAADP and (c) ADP-ribose induce Ca2+ release from the endoplasmic reticulum (ER) via ryanodine receptors (RyR). mTOR senses Δψm and regulates IP3R-mediated Ca2+ release (40). The intracellular rapamycin receptor FKBP12 directly binds the RyR. The Bcl-2 family proteins control permeability of the outer mitochondrial membrane and release of apoptosis-inducing factors. Necrosis-prone T cells release oxidized DNA and HMGB1 which stimulate B cells, macrophages, and dendritic cells (DC). In turn, B cells produce IL-10 and macrophages and DC produce NO which stimulate MHP of T cells. B) Proposed hierarchy of metabolic pathways upstream and downstream of MHP in lupus T cells. Broken line demarcates checkpoints affected by rapamycin.
Metabolic control of T cell activation and Ca2+ Fluxing
ROI modulate T cell activation, cytokine production, and proliferation at multiple levels (2). The antigen-binding αβ or γδTCR is associated with a multimeric receptor module comprised of the CD3 γδε and ζ chains. The cytoplasmic domain of CD3ζ chain contains an immunoglobulin receptor family tyrosine-based activation motif (ITAM) which is crucial for coupling of intracellular tyrosine kinases (11). Expression of CD3ζ is suppressed by ROI (12). Binding of p56lck to CD4 or CD8 attracts this kinase to the TCR-CD3 complex, leading to phosphorylation of ITAM. Phosphorylation of both tyrosines of each ITAM is required for SH-2-mediated binding by zeta-associated protein-70 (ZAP-70) or the related SYK. ZAP-70 is activated through phosphorylation by p56lck. Activated ZAP-70 and SYK target two key adaptor proteins LAT and SLP-76 (11). Phosphorylated LAT binds directly to phospholipase C-γ1 that controls hydrolysis of phosphatydilinositol-4,5-biphosphate (PIP2) to inositol-1,4,5-triphosphate (IP3) and diacylglycerol (DAG). Phosphorylation of inositol lipid second messengers is mediated by phosphatidylinositol 3′hydroxyl kinase (PI3K). The stimulatory effect of the TCR alone on PI3K activity is small. Concurrent triggering of the CD28 co-stimulatory molecule by its ligands CD80 or CD86 is required for optimal PI3K activation. IP3 binds to its receptors in the endoplasmic reticulum (ER), opening Ca2+ channels that release Ca2+ to the cytosol. Decreased ER Ca2+ concentration activates the Ca2+ release-activated Ca2+ channel (CRAC) in the cell membrane. The resultant Ca2+ influx activates the phosphatase calcineurin which dephosphorylates the transcription factor, NFAT. Dephosphorylated NFAT can translocate to the nucleus where it promotes transcription of IL-2 in concert with AP-1, NFκB, and Oct-1. While activities of AP-1 and NFκB are increased by oxidative stress (13), both thiol insufficiency and H2O2 treatment suppresses calcineurin-mediated activation of NFAT (14). Thus, expression of cytokines, i.e. IL-2 (with AP-1 and NFAT motif-containg promoter) and IL-4 (with AP-1-less NFAT enhancer), can be selectively regulated by oxidative stress depending on the relative expression level of transcription factors involved (Fig. 1).
The calcium signal is dysregulated in SLE T-cells in a number of ways. SLE T-cells have elevated intracellular and mitochondrial calcium at baseline (15), although the amount of Ca2+ present in the endoplasmic reticulum is normal (16). And while the T-cell activation-induced calcium flux is elevated initially, the plateau phase is reduced relative to activated control T-cells (15). Dysregulation of NO may play a role, since normal T cells pre-treated with NO donors recapitulate the Ca2+fluxing abnormalities observed in SLE T cells (15). Considering that the Ca2+ is important for activation of PKC, calcineurin, NFAT, and production of IL-2, the altered Ca2+ fluxing may account for the inappropriate activation of T cells in SLE, or conversely, may contribute to their inability to produce adequate amounts of IL-2 (1).
Metabolic control of cell death
Programmed cell death (PCD) or apoptosis is a physiological mechanism for elimination of autoreactive lymphocytes during development. Signaling through the Fas or structurally related TNF family of cell surface death receptors represent dominant pathways in elimination of unwanted cells under physiological and disease conditions (2). Fas stimulation leads to sequential activation of caspases resulting in the cleavage of cellular substrates and the characteristic morphologic and biochemical changes of apoptosis. Apically, trimerization of cell surface Fas receptors through formation of disulfide bonds is required for activation of the death-inducing signaling complex and cleavage of caspase 8 (2). Importantly, GSH is required for activity of caspases and its depletion can prevent Fas-dependent apoptosis (17). ATP is also required for apoptosis and its deficiency predisposes to necrosis (18). Autophagy mediates the bulk degradation of cytoplasmic contents, proteins and organelles including mitochondria, in lysosomes; this process is induced by rapamycin through inactivating mTOR (19). Interestingly, rapamycin treatment in vivo did not affect MHP or mitochondrial mass of lupus T cells (10).
MHP and ATP depletion predispose lupus T cells to necrosis
The mitochondrion is the site of ATP synthesis via oxidative phosphorylation. The synthesis of ATP is driven by an electrochemical gradient across the inner mitochondrial membrane maintained by an electron transport chain and the Δψm. Activity of caspases require ATP to the extent that depletion of ATP by inhibition of F0F1-ATPase with oligomycin (18) or exhaustion of intracellular ATP stores by prior apoptosis signals, Fas stimulation or H2O2 pretreatment, leads to necrosis. Thus, intracellular ATP concentration is a key switch in the cell's decision to die via apoptosis or necrosis (18).
Apoptosis is a physiological process that results in nuclear condensation and break-up of the cell into membrane-enclosed apoptotic bodies suitable for phagocytosis by macrophages thus preventing inflammation. By contrast, necrosis is a pathological process that results in cellular swelling, followed by lysis and release of proteases, oxidizing molecules, and other pro-inflammatory and chemotactic factors resulting in inflammation and tissue damage (20). Swollen lymph nodes of patients with SLE harbour increased numbers of necrotic T lymphocytes and dendritic cells (DC) (21). Necrotic, but not apoptotic, cell death generates inflammatory signals necessary for the activation and maturation of DCs, the most potent antigen-presenting cells (2;22). High mobility group B1 (HMGB1) protein, an abundant DNA-binding protein, remains immobilized on chromatin of apoptotic bodies, however, it is released from necrotic cells (2). Necrotic but not apoptotic cells also release heat shock proteins (HSPs), HSPgp96, hsp90. hsp70, and calreticulin. Mature DCs express high levels of the DC-restricted markers, CD83 and lysosome-associated membrane glycoprotein (DC-LAMP) and the costimulatory molecules CD40 and CD86 (2), which may contribute to altered intercellular signaling in SLE (Fig. 1). Their activation is driven by circulating interferon-α (IFN-α) that may come from one of the DC subsets, i.e., plasmacytoid dendritic cells (PDC) that infiltrate lupus skin lesions. SLE patients harbour activated PDC which may be responsible for increased production of IFN-α in SLE (23).
Targeting of metabolic checkpoints of T cell activation for therapeutic intervention in SLE
Depletion of intracellular glutathione
Reduced glutathione (GSH) levels are profoundly depleted in lymphocytes SLE patients (8). Low GSH in T cells over-expressing transaldolase predispose to MHP (5). GSH depletion is robust trigger of MHP via S-nitrosylation of complex I upon exposure to NO (24). Thus, the effect of NO on MHP is tightly related to GSH levels. Diminished production of GSH in face of MHP and increased ROI production is suggestive of a metabolic defect in de novo GSH synthesis or maintenance of its reduced state due to deficiency of NADPH (25). Recent studies showed diminished GSH/GSSG ratios in the kidneys of 8-month-old versus 4 month-old (NZB × NZW) F1 mice; treatment with N-acetylcysteine (NAC), a precursor of GSH and stimulator of its de novo biosynthesis, prevented the decline of GSH/GSSG ratios, reduced autoantibody production and development of glomerulopnephritis (GN) and prolonged the survival of (NZB × NZW) F1 mice (26). Oral NAC has been used to treat oxidative stress in patients with idiopathic pulmonary fibrosis (IPF) (27). In a one-year study of IPF patients treated with prednisone and azathioprine, addition of NAC (3 × 600 mg/d) improved vital capacity and reduced myelotoxicity in comparison to placebo. Therefore, prospective clinical studies appear justified to assess whether NAC treatment can reverse GSH depletion, correct T-cell signaling defects and provide clinical benefit to patients with lupus.
Inhibiting NO production
NO is a particularly interesting molecule in this context because it provides a link between seemingly dissociated features of T cell activation and mitochondrial function. NO is produced by nitric oxide synthases (NOS) that require Ca2+ to function and use NADPH and arginine as substrates. Thee isoforms exist: endothelial NOS, neuronal NOS, and inducible NOS, of which T cells express the former two (28). NO induces MHP and mitochondrial biogenesis, increases Ca2+ in the cytosol and mitochondria of normal T cells, and recapitulates the enhanced CD3/CD28-induced Ca2+ fluxing of lupus T cells (15). As recently reported, eNOS is recruited to the site of T-cell receptor engagement, locally increasing NO at the immunological synapse in a Ca2+ and PI3K-dependent manner, resulting in reduced IL-2 production(29) which is characteristic of SLE (1).
NO contributes to the development of GN in the MRL/lpr lupus mouse model (30). Inactivation of iNOS does not block the development of lupus (31), suggesting a role for eNOS and nNOS isoforms expressed in T cells. However, given the widespread expression of these isoforms in vascular smooth muscle and brain, it will be necessary to develop T-cell-specific approaches for inhibiting NOS to avoid potentially deleterious side effects.
Rapamycin
The mammalian target of rapamycin (mTOR) is associated with the outer mitochondrial membrane and senses mitochondrial dysfunction and changes in the Δψm of T cells (9). Rapamycin normalized T cell mitogen-stimulated splenocyte proliferation and IL-2 production, prevented the typical rise in anti-double-stranded DNA antibody and urinary albumin levels and GN, and prolonged survival of lupus-prone MRL/lpr mice (32). With a focus on mitochondrial dysfunction, we began to utilize rapamycin for treatment of SLE patients resistant or intolerant to conventional medications. We observed normalization of baseline Ca2+ levels in the cytosol and mitochondria and of CD3/CD28-induced Ca2+ fluxing as well as persistence of MHP (10), indicating that increased Ca2+ fluxing is downstream or independent of MHP in the pathogenesis of T-cell dysfunction in SLE. The effectiveness of rapamycin in murine and human SLE suggest that mTOR is a potential sensor and down-stream effector of MHP and mediator of T cell dysfunction and autoimmunity in SLE. Rapamycin can selective expand CD4+/CD25+/Foxp3+ regulatory T cells (33) which appear to be deficient in patients with SLE (34). Therefore, understanding the mechanism of persistent MHP that leads to mTOR activation and enhanced Ca2+ fluxing may be fundamental to the pathogenesis of T cell dysfunction and autoimmunity in SLE.
Table 1.
Signaling alterations underlying abnormal cell death of T cells in patients with SLE
| Signal | Effect | Reference |
|---|---|---|
| Spontaneous apoptosis ↑ | Compartmentalized autoantigen release, disease activity ↑ | (3;8) |
| AICD ↓ | Persistence of autoreactive cells | (35;36) |
| FasL ↑ | Spontaneous apoptosis ↑ | (37) |
| IL-10 ↑ | Selective induction of apoptosis in SLE | (36;37) |
| Δψm ↑ | ROI ↑, ATP ↓ | (8) |
| GSH ↓ | ROI ↑, Spontaneous apoptosis ↑ | (8;38) |
| ATP ↓ | AICD ↓, Predisposes for necrosis ↑ | (8;18) |
| NO ↑ | Disease activity ↑,Δψm ↑, mitochondrial biogenesis ↑, enhanced baseline and activation-induced Ca2+ flux | (15;28) |
| ROI ↑ | Spontaneous apoptosis ↑, IL-10 production ↑ | (8;36) |
| IL-10 blockade | Spontaneous apoptosis ↓, ROI ↓ | (36;37) |
| IL-12 | Spontaneous apoptosis ↓, ROI ↓ | (36) |
increase;
decrease;
Acknowledgments
This work was supported in part by grants AI 048079, AI 061066, and AI 072648 from the National Institutes of Health and the Central New York Community Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kyttaris VC, Juang YT, Tsokos GC. Immune cells and cytokines in systemic lupus erythematosus: an update. Curr Opin Rheumatol. 2005;17:518–522. doi: 10.1097/01.bor.0000170479.01451.ab. [see comment]. [Review] [29 refs] [DOI] [PubMed] [Google Scholar]
- 2.Perl A, Gergely P, Jr, Nagy G, Koncz A, Banki K. Mitochondrial hyperpolarization: a checkpoint of T cell life, death, and autoimmunity. Trends Immunol. 2004;25:360–367. doi: 10.1016/j.it.2004.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casciola-Rosen LA, Anhalt G, Rosen A. Autoantigens targeted in systemic lupus erythematosus are clustered in two populations of surface structures on apoptotic keratinocytes. J Exp Med. 1994;179:1317–1330. doi: 10.1084/jem.179.4.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nagy G, Koncz A, Fernandez D, Perl A. Nitric oxide, mitochondrial hyperpolarization, and T cell activation. Free Radic Biol Med. 2007;42:1625–1631. doi: 10.1016/j.freeradbiomed.2007.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banki K, Hutter E, Gonchoroff N, Perl A. Elevation of mitochondrial transmembrane potential and reactive oxygen intermediate levels are early events and occur independently from activation of caspases in Fas signaling. J Immunol. 1999;162:1466–1479. [PMC free article] [PubMed] [Google Scholar]
- 6.Puskas F, Gergely P, Banki K, Perl A. Stimulation of the pentose phosphate pathway and glutathione levels by dehydroascorbate, the oxidized form of vitamin C. FASEB J. 2000;14:1352–1361. doi: 10.1096/fj.14.10.1352. [DOI] [PubMed] [Google Scholar]
- 7.Almeida A, Almeida J, Bolanos JP, Moncada S. Different responses of astrocytes and neurons to nitric oxide: the role of glycolytically generated ATP in astrocyte protection. Proc Natl Acad Sci USA. 2001;98(26):15294–15299. doi: 10.1073/pnas.261560998. 2001 Dec 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gergely PJ, Grossman C, Niland B, Puskas F, Neupane H, Allam F, et al. Mitochondrial hyperpolarization and ATP depletion in patients with systemic lupus erythematosus. Arth Rheum. 2002;46:175–190. doi: 10.1002/1529-0131(200201)46:1<175::AID-ART10015>3.0.CO;2-H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Desai BN, Myers BR, Schreiber SL. FKBP12-rapamycin-associated protein associates with mitochondria and senses osmotic stress via mitochondrial dysfunction. Proc Natl Acad Sci USA. 2002;99:4319–4324. doi: 10.1073/pnas.261702698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernandez D, Bonilla E, Mirza N, Perl A. Rapamycin reduces disease activity and normalizes T-cell activation-induced calcium fluxing in patients with systemic lupus erythematosus. Arth Rheum. 2006;54(9):2983–2988. doi: 10.1002/art.22085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koretzky GA, Boerth NJ. The role of adapter proteins in T cell activation. Cell Mol Life Sci. 1999;56:1048–1060. doi: 10.1007/s000180050492. [Review] [137 refs] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Otsuji M, Kimura Y, Aoe T, Okamoto Y, Saito T. Oxidative stress by tumor-derived macrophages suppresses the expression of CD3 zeta chain of T-cell receptor complex and antigen-specific T-cell responses. Proc Natl Acad Sci USA. 1996;93:13119–13124. doi: 10.1073/pnas.93.23.13119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beiqing L, Chen M, Whisler RL. Sublethal levels of oxidative stress stimulate transcriptional activation of c-jun and suppress IL-2 promoter activation in Jurkat T cells. J Immunol. 1996;157:160–169. [PubMed] [Google Scholar]
- 14.Furuke K, Shiraishi M, Mostowski HS, Bloom ET. Fas ligand induction in human NK cells is regulated by redox through a calcineurin-nuclear factors of activated T cell-dependent pathway. J Immunol. 1999;162:1988–1993. [PubMed] [Google Scholar]
- 15.Nagy G, Barcza M, Gonchoroff N, Phillips PE, Perl A. Nitric Oxide-Dependent Mitochondrial Biogenesis Generates Ca2+ Signaling Profile of Lupus T Cells. J Immunol. 2004;173(6):3676–3683. doi: 10.4049/jimmunol.173.6.3676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vassilopoulos D, Kovacs B, Tsokos GC. TCR/CD3 complex-mediated signal transduction pathway in T cells and T cell lines from patients with systemic lupus erythematosus. J Immunol. 1995;155(4):2269–2281. [PubMed] [Google Scholar]
- 17.Hentze H, Kunstle G, Volbracht C, Ertel W, Wendel A. CD95-Mediated murine hepatic apoptosis requires an intact glutathione status. Hepatology. 1999;30:177–185. doi: 10.1002/hep.510300111. [DOI] [PubMed] [Google Scholar]
- 18.Leist M, Single B, Castoldi AF, Kuhnle S, Nicotera P. Intracellular Adenosine Triphosphate (ATP) Concentration: A Switch in the Decision Between Apoptosis and Necrosis. J Exp Med. 1997;185:1481–1486. doi: 10.1084/jem.185.8.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kundu M, Thompson CB. Macroautophagy versus mitochondrial autophagy: a question of fate? Cell Death Differ. 2005;12(Suppl2):1484–1489. doi: 10.1038/sj.cdd.4401780. [Review] [58 refs] [DOI] [PubMed] [Google Scholar]
- 20.Fiers W, Beyaert R, Declercq w, Vandenabeele P. More than one way to die: apoptosis, necrosis and reactive oxygen damage. Oncogene. 1999;18:7719–7730. doi: 10.1038/sj.onc.1203249. [DOI] [PubMed] [Google Scholar]
- 21.Kojima M, Nakamura S, Morishita Y, Itoh H, Yoshida K, Ohno Y, et al. Reactive follicular hyperplasia in the lymph node lesions from systemic lupus erythematosus patients: a clinicopathological and immunohistological study of 21 cases. Pathol Int. 2000;50(4):304–312. doi: 10.1046/j.1440-1827.2000.01052.x. [DOI] [PubMed] [Google Scholar]
- 22.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of Cell Death: Exposure to Necrotic Tumor Cells, but Not Primary Tissue Cells or Apoptotic Cells, Induces the Maturation of Immunostimulatory Dendritic Cells. J Exp Med. 2000;191(3):423–434. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pascual V, Banchereau J, Palucka AK. The central role of dendritic cells and interferon-alpha in SLE. Curr Opin Rheumatol. 2003;15(5):548–556. doi: 10.1097/00002281-200309000-00005. [Review] [79 refs] [DOI] [PubMed] [Google Scholar]
- 24.Clementi E, Brown GC, Feelisch M, Moncada S. Persistent inhibition of cell respiration by nitric oxide: crucial role of S-nitrosylation of mitochondrial complex I and protective action of glutathione. Proc Natl Acad Sci USA. 1998;95(13):7631–7636. doi: 10.1073/pnas.95.13.7631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perl A, Gergely P, Jr, Puskas F, Banki K. Metabolic switches of T-cell activation and apoptosis. Antiox Redox Signal. 2002;4(5):427–443. doi: 10.1089/15230860260196227. [DOI] [PubMed] [Google Scholar]
- 26.Suwannaroj S, Lagoo A, Keisler D, McMurray RW. Antioxidants suppress mortality in the female NZB × NZW F1 mouse model of systemic lupus erythematosus (SLE) Lupus. 2001;10(4):258–65. doi: 10.1191/096120301680416940. [DOI] [PubMed] [Google Scholar]
- 27.Demedts M, Behr J, Buhl R, Costabel U, Dekhuijzen R, Jansen HM, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N Engl J Med. 2005;353:2229–2242. doi: 10.1056/NEJMoa042976. see comment. [DOI] [PubMed] [Google Scholar]
- 28.Nagy G, Koncz A, Perl A. T cell activation-induced mitochondrial hyperpolarization is mediated by Ca2+- and redox-dependent production of nitric oxide. J Immunol. 2003;171:5188–5197. doi: 10.4049/jimmunol.171.10.5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ibiza S, Victor VM, Bosca I, Ortega A, Urzainqui A, O'Connor JE, et al. Endothelial Nitric Oxide Synthase Regulates T Cell Receptor Signaling at the Immunological Synapse. Immunity. 2006;24(6):753–765. doi: 10.1016/j.immuni.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Weinberg JB, Granger DL, Pisetsky DS, Seldin MF, Misukonis MA, Mason SN, et al. The role of nitric oxide in the pathogenesis of spontaneous murine autoimmune disease: Increased nitric oxide production and nitric oxide synthase expression in MRL-lpr/lpr mice, and reduction of spontaneous glomerulonephritis and arthritis by orally administered NG -monomethyl-L-arginine. J Exp Med. 1994;179:651–660. doi: 10.1084/jem.179.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilkeson GS, Mudgett JS, Seldin MF, Ruiz P, Alexander AA, Misukonis MA, et al. Clinical and serologic manifestations of autoimmune disease in MRL-lpr/lpr mice lacking nitric oxide synthase type 2. J Exp Med. 1997;186:365–373. doi: 10.1084/jem.186.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Warner LM, Adams LM, Sehgal SN. Rapamycin prolongs survival and arrests pathophysiologic changes in murine systemic lupus erythematosus. Arth Rheum. 1994;37:289–297. doi: 10.1002/art.1780370219. [DOI] [PubMed] [Google Scholar]
- 33.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin Promotes Expansion of Functional CD4+CD25+FOXP3+ Regulatory T Cells of Both Healthy Subjects and Type 1 Diabetic Patients. J Immunol. 2006;177(12):8338–8347. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 34.Valencia X, Yarboro C, Illei G, Lipsky PE. Deficient CD4+CD25high T Regulatory Cell Function in Patients with Active Systemic Lupus Erythematosus. J Immunol. 2007;178(4):2579–2588. doi: 10.4049/jimmunol.178.4.2579. [DOI] [PubMed] [Google Scholar]
- 35.Kovacs B, Vassilopoulos D, Vogelgesang SA, Tsokos GC. Defective CD3-mediated cell death in activated T cells from patients with systemic lupus erythematosus: role of decreased intracellular TNF-alpha. Clin Immunol Immunopathol. 1996;81:293–302. doi: 10.1006/clin.1996.0192. [DOI] [PubMed] [Google Scholar]
- 36.Gergely PJ, Niland B, Gonchoroff N, Pullmann R, Jr, Phillips PE, Perl A. Persistent mitochondrial hyperpolarization, increased reactive oxygen intermediate production, and cytoplasmic alkalinization characterize altered IL-10 signaling in patients with systemic lupus erythematosus. J Immunol. 2002;169:1092–1101. doi: 10.4049/jimmunol.169.2.1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Georgescu L, Vakkalanka RK, Elkon KB, Crow MK. Interleukin-10 promotes activation-induced cell death of SLE lymphocytes mediated by Fas ligand. J Clin Invest. 1997;100:2622–2633. doi: 10.1172/JCI119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Banki K, Hutter E, Colombo E, Gonchoroff NJ, Perl A. Glutathione Levels and Sensitivity to Apoptosis Are Regulated by changes in Transaldolase expression. J Biol Chem. 1996;271:32994–33001. doi: 10.1074/jbc.271.51.32994. [DOI] [PubMed] [Google Scholar]
- 39.Nisoli E, Carruba MO. Nitric oxide and mitochondrial biogenesis. J Cell Sci. 2006;119(14):2855–2862. doi: 10.1242/jcs.03062. [DOI] [PubMed] [Google Scholar]
- 40.MacMillan D, Currie S, Bradley KN, Muir TC, McCarron JG. In smooth muscle, FK506-binding protein modulates IP3 receptor-evoked Ca2+ release by mTOR and calcineurin. J Cell Sci. 2005;(23):5443–5451. doi: 10.1242/jcs.02657. [DOI] [PubMed] [Google Scholar]