Abstract
The amygdala has a pivotal role in deciphering the emotional significance of sensory stimuli enabling emotional memory formation. We have previously shown that prefrontal cortices in rhesus monkeys project to the amygdala mainly from their deep layers, suggesting feedback communication. If sensory areas convey signals pertinent to the state of the environment, they should issue feedforward projections to the amygdala, arising mainly from the upper layers, consistent with the flow of information from earlier-to later-processing sensory cortices. Here we addressed this hypothesis in cases with injection of tracers in sites of the amygdala known to have robust connections with prefrontal cortices, and mapped connections in insular and temporal cortices associated with sensory processing and memory. The medial temporal pole, the entorhinal and perirhinal areas, and the agranular and dysgranular insula had the densest connections with the amygdala, and the lateral temporal pole, the parahippocampal region, and the granular insula had sparser connections. Most areas projected to the amygdala predominantly from the upper layers, suggesting feedforward communication, and received reciprocal amygdalar innervation primarily in their superficial layers, suggesting feedback communication. In contrast, the entorhinal cortex issued projections to the amygdala from its deep layers, suggesting feedback communication, and received reciprocal amygdalar projections most densely in layers II–III, which project to the hippocampus. These findings may help explain how the amygdala can attach emotional value to environmental stimuli, participate in the sequence of information processing of emotions, and modulate the formation of emotional memories.
The amygdala has a role in extracting the affective significance of sensory stimuli and mediating the formation of emotional memories. It receives convergent information from all sensory systems, originating mainly in high-order sensory cortices associated with visual, auditory, olfactory, gustatory, somatosensory, visceral and polymodal processing [reviewed in (McDonald, 1998; Pitkanen, 2000; Zald, 2003)]. Sensory information is transmitted to the amygdala through modality-specific cortico-cortical cascades that originate in the primary sensory cortices and flow towards high-order association areas, or directly through the thalamus (Aggleton et al., 1980; McDonald, 1998). Projections from polymodal cortices to the amygdala originate in the perirhinal and hippocampal regions (McDonald, 1998) and the prefrontal cortex [e.g., (Aggleton et al., 1980; Porrino et al., 1981; Ottersen, 1982; Russchen, 1982)]. Behavioral responses are generated primarily through the central and medial nuclei of the amygdala which project to hypothalamic and brainstem centers involved in autonomic control (Price and Amaral, 1981; McDonald, 1998; Pitkanen, 2000; Barbas et al., 2003). The amygdala also projects to the cortex, targeting heavily polymodal areas such as the entorhinal and perirhinal areas (Pitkanen, 2000) and the prefrontal cortex (Aggleton et al., 1980; Porrino et al., 1981; Barbas and De Olmos, 1990; Carmichael and Price, 1995; Ghashghaei and Barbas, 2002; Ghashghaei et al., 2007). The connections between the amygdala and the entorhinal and perirhinal cortices as well as the hippocampus contribute to emotional memory (Cahill and McGaugh, 1998; McCaugh et al., 2000), while the prefrontal cortex has a role in the cognitive and emotional interpretation of valenced sensory stimuli, and in controlling the subsequent behavioral responses (Fuster, 1989).
We have recently shown that in rhesus monkeys caudal orbitofrontal and caudal medial prefrontal cortices are heavily innervated by the amygdala in a feedforward manner, which then send reciprocal projections to the amygdala that originate primarily from layer V, resembling “feedback” projections (Ghashghaei et al., 2007). In this study we investigated the laminar origin of neurons in sensory and polymodal cortices that project to the same sites in the amygdala. We reasoned that if sensory areas convey signals to the amygdala pertinent to the state of the environment, they should resemble feedforward connections and originate in the upper layers of the cortex, consistent with the flow of information from earlier to later processing sensory areas. To test this hypothesis, we studied amygdalar connections in temporal and insular cortices, using the same cases injected with bidirectional tracers in the amygdala to map prefrontal connections in a previous study (Ghashghaei et al., 2007). Using the opposite experimental approach, with injections of tracers in cortical areas, we had earlier found that the connections of temporal visual, auditory, and polymodal cortices overlap extensively in the amygdala with connections of prefrontal cortices (Ghashghaei and Barbas, 2002). Based on these findings, we focused on sensory association areas that may provide information about the status of the environment, including the anterior temporal areas, associated with processing high-order olfactory, auditory and visual signals, and the insula, associated with gustatory, visceral, and somatosensory processing. In addition, we studied the amygdalar connections in medial temporal entorhinal, perirhinal and parahippocampal regions, associated with long-term memory.
Materials and methods
Animals
Experiments for injection of multiple tracers in the amygdala were conducted on three young adult rhesus monkeys (Macaca mulatta; 2–2.5 years old; 2–3 kg body weight, of both sexes), obtained through the New England Regional Primate Research Center (NEPRC, MA, USA). All efforts were made to minimize animal suffering. Experiments were conducted in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publications 86–23 revised 1987 and 80–22 revised 1996). The experiments were approved by the Institutional Animal Care and Use Committee at Boston University School of Medicine, Harvard Medical School, and New England Primate Research Center.
Brain imaging
Prior to surgery for injection of tracers, the coordinates of the amygdala were calculated using magnetic resonance imaging (Brigham and Womens Hospital, Harvard Medical School, Boston, MA). The monkeys were sedated with ketamine hydrochloride (10 mg/kg, intramuscularly) and then anesthetized with sodium pentobarbital (30 mg/kg intravenously) through a femoral catheter. Animals were placed in a non-magnetic stereotaxic frame with hollow ear bars filled with betadine salve (H, Purdue Pharma, CT) that is visible on the MRI. A T1 weighted 3D SPGR (TR70 ms, TE6 ms, Flip 45°) was obtained through the amygdala using 512 × 384 matrices and 16 × 16 FOVs. The interaural line was used as reference, and the coordinates for the amygdala injections were measured in three dimensions: medio-lateral relative to the midline; antero-posterior relative to the interaural line, and dorso-ventral relative to the brain surface.
Surgical procedures
Surgery for injection of neural tracers was conducted immediately after, or one week after, the MRI. The monkeys were sedated with ketamine hydrochloride (10–15 mg/kg, intramuscularly) intubated and anesthetized with isoflurane until a surgical level of anesthesia was achieved. Surgery was performed under aseptic conditions, and heart rate, muscle tone, respiration, and pupillary dilatation were closely monitored. The monkeys were placed in the same stereotaxic apparatus used for imaging. A midline incision was made in the scalp and the skull was exposed. A small burr hole over the region of the cortex above the amygdala was made. The tracers were injected using a microsyringe (Hamilton 701, 26s gauge, Reno, NV, Cat #80383) mounted onto a microdrive. In all cases, 1–3 penetrations were made from the top of the brain to the calculated depth in the amygdala. The experiments comprised 6 tracer injection sites, which included six of the seven injection sites used previously to study the connections between the amygdala and the prefrontal cortex (Ghashghaei et al., 2007).
Neural tracers
We used a combination of retrograde (Fast Blue) and bidirectional (Fluoro Ruby; Biotinylated Dextran Amine) tracers, which are distinct and can be injected simultaneously in each animal. This approach maximizes the amount of information obtained with the least number of animals. Fast Blue is an excellent retrograde tracer, and Biotinylated Dextran Amine (BDA) and Fluoro Ruby are excellent bidirectional tracers that label neuronal cell bodies retrogradely and axons and terminal boutons anterogradely. Fast Blue (1μl of 2 mg/ml in dH2O; Mw 379.84, Sigma, St. Louis, MO, Cat #F5756) was injected in the amygdala in the left hemisphere of one animal (case BB-B), and Fluoro Ruby (dextran tetramethylrhodamine, 2.5 μl of 2 mg/ml in dH2O, Mw 3000, Molecular Probes; CAT#D-3308) was injected on the left side of another animal (case AX-L). Biotinylated Dextran Amine (BDA, Mw 3000, Molecular Probes, Eugene, OR, Cat # D-7135) was injected in both hemispheres of two monkeys (10μl of 10 mg/ml in dH2O; cases BB-R, BB-L, BD-R and BD-L). Connections between the cortex and the amygdala are ipsilateral in rhesus monkeys (Ghashghaei and Barbas, 2002), as they are in rats (Cassell et al., 1989), so injections in two hemispheres in the same animal can be considered independent.
Perfusion and tissue sectioning
After a survival period of 18 days, the monkeys were anesthetized with a lethal dose of sodium pentobarbital (>50 mg/kg, intravenously) and transcardially perfused with 4% paraformaldehyde in 0.1 M phosphate buffer (PB, pH 7.4). The brains were removed from the skull, photographed, and cryoprotected in graded solutions of sucrose (10–30%) in 0.01M PBS. The brains were frozen in −75°C isopentane (Fisher Scientific, Pittsburgh, PA) and cut on a freezing microtome in the coronal plane at 50 μm to produce ten series of 1/10 sections. One series was directly mounted on gelatin coated glass slides, stained for Nissl and coverslipped. In cases with fluorescent tracers, two series of sections were mounted directly on glass slides after sectioning, and were stored at 4°C until analysis.
Histological procedures
In experiments with BDA injections, we processed one series of sections to see labeled boutons and projection neurons as described previously (Ghashghaei et al., 2007). Briefly, free-floating sections were rinsed in 0.01M PBS and incubated for 1h in an avidin–biotin HRP complex (AB kit, Vector Laboratories, Burlingame, CA) diluted 1:100 in 0.01M PBS with 0.1% Triton X-100. The sections were then washed and processed for immunoperoxidase reaction using diaminobenzidine (DAB; Zymed, San Francisco, CA), washed, mounted and dried. Every other section was counterstained with thionin for Nissl, and all sections were coverslipped. Adjacent series of sections were stained for acetylcholinesterase to delineate the nuclei of the amygdala (Geneser-Jensen and Blackstad, 1971).
Data analysis
We marked the borders of architectonic areas of temporal and insular cortices and their layers in sections counterstained with thionin. Coronal sections through the rostro-caudal extent of the temporal cortex were numbered and architectonic areas within each section identified.
Retrograde analysis: Exhaustive sampling
Sections through the temporal cortex ipsilateral to the injection sites were viewed under a microscope (Olympus, BX60) using brightfield or fluorescence illumination. Labeled neuronal cell bodies were mapped quantitatively using a system designed in our laboratory, as described previously (Barbas and De Olmos, 1990), or using a semi-automated commercial system with a motorized stage and software (Neurolucida, Microbrightfield, Williston, VT). In both systems, projection neurons were mapped in precise register with respect to anatomical landmarks. We used systematic exhaustive mapping of labeled projection neurons after injection of tracers in the amygdala. We mapped and counted projection neurons in every other section in one series of each case, so that 1/20 sections through the temporal and insular cortices were used for quantitative analyses of labeled neurons.
Anterograde analysis: Stereology
We performed stereological analysis of BDA-labeled boutons using an Olympus BX60 microscope under brightfield illumination. We employed standard stereological procedures to estimate the areal and laminar density of boutons in the temporal and insular cortices, using the optical fractionator according to the method described previously [(Gundersen et al., 1988; West et al., 1991); reviewed in (Howard and Reed, 1998)]. Data for stereological analysis were obtained using a semi-automated commercial system and software (StereoInvestigator, MicroBrightfield, Williston, VT). We used 600× magnification, 50×50 μm counting frame, 200–1000 μm grid size (depending on the size of the architectonic areas), and 3–30 sampling sites for every layer in 3 sections for each injection site. These settings consistently resulted in reliable bouton estimates with a coefficient of error (CE) below 10%, in accordance with studies performed in the prefrontal cortex (Ghashghaei et al., 2007). The counting frames included exclusion and inclusion zones to avoid overestimating, as well as guard zones (2 μm each on top and bottom of the sections) to avoid error due to plucked boutons at the cut edge of sections (Williams and Rakic, 1988). For each area, three sections were selected using systematic random sampling to count boutons in different laminar compartments (layer I, layers II–III, layer IV (when present), and layers V–VI). The stereological data included volume calculations for each layer, which take into consideration the area of the layer and thickness of each section. The volume estimates along with the total estimates of boutons were used to calculate the density of boutons per unit volume (mm3).
Normalization of data
The relative density of labeled projection neurons or boutons in each area was expressed as a percentage of the total number of labeled neurons or density of boutons mapped in temporal and insular cortices for each injection site. The relative laminar density of labeled projection neurons or boutons found in the upper layers (layers II–III for neurons; layer I and II–III for boutons) or in the deep layers (layers V–VI for neurons; and layers IV–VI for boutons) was expressed as a percentage of the total number of labeled neurons or relative density of boutons in each area. The laminar density normalization (i.e., within areas) does not reveal density differences across areas, whereas the regional density normalization (i.e., across areas) does. Only areas with significant label (>20 neurons or >50 boutons) were used for regional and laminar comparisons. For the regional analyses, an input/output density ratio (I/O) was generated to reflect the input from the amygdala to the temporal and insular cortices versus the reciprocal output from the temporal and insular cortices to the amygdala. A density sum (I+O) was generated to reflect the overall connection strength between the amygdala and a given area in the temporal and insular cortices.
Photography
Photomicrographs were captured directly from histological slides using a CCD camera mounted on an Olympus Optical microscope (BX60) connected to a personal computer, using a commercial imaging system (NeuroLucida, Virtual Slice software, MicroBrightfield, Williston, VT, USA). Images were imported into Adobe Photoshop for assembly, labeling, and adjustment of overall brightness, but were not retouched.
Results
Nomenclature
Amygdala
The nuclei of the amygdala were delineated from Nissl and acetylcholinesterase stains and the terminology used was based on the maps of Johnston (Johnston, 1923), complemented when necessary with the map of DeOlmos for the human (De Olmos, 1990) and Price et al. for the macaque monkey (Price et al., 1987). The borders of nuclei do not differ in the various maps. However, there is variation in the names used for some nuclei, and in those cases we mention all names for clarity. The basolateral complex includes the lateral nucleus (L), the basolateral nucleus (BL) and the basomedial nucleus (BM, also known as the accessory basal nucleus). The basolateral nucleus is further subdivided into magnocellular (BLmc), intermediate (BLi) and parvicellular (BLpc) divisions, and the basomedial nucleus is subdivided into magnocellular (BMmc) and parvicellular (BMpc) divisions. The cortical group is composed of an anterior part (ACo), a posterior part (PCo), as described by Price et al. (Price et al., 1987), and a ventral part (VCo), as described (Barbas and De Olmos, 1990).
Temporal and insular cortices
The nomenclature of the temporal and insular areas is varied in the literature, most notably in the temporal pole. We used a descriptive terminology for the temporal pole, which was divided into the medially located agranular temporal periallocortex (Tpall), the dysgranular dorsomedial temporal pole (TPdm), and the dysgranular ventromedial temporal pole (TPvm). The lateral (granular) parts of the temporal pole and the adjacent temporal gyrus include the rostral portions of the auditory association areas Ts1 and Ts2, and the rostral portion of visual association area TE1. Alternative terminologies used for the temporal pole include area 38 (Brodmann, 1909), area TG (Von Bonin and Bailey, 1947), area Pro (Pandya and Sanides, 1973; Seltzer and Pandya, 1978; Galaburda and Pandya, 1983), agranular/dysgranular temporal pole (TPa-p, TPdg) (Mesulam and Mufson, 1982a; Moran et al., 1987), and periallocortical/ventral proisocortical temporal pole [(TppAll, TpproV) (Gower, 1989)].
The medial temporal cortex consists of the entorhinal cortex (area 28), perirhinal areas 35 and 36, and parahippocampal areas TH and TF (Suzuki and Amaral, 1994; Suzuki and Amaral, 2003a). Area 36 was further subdivided into a medial (36M) and a lateral part (36L). The insular cortex has granular (Ig), dysgranular (Idg), and agranular (Iag) parts and the adjacent parainsular area PaI (Mesulam and Mufson, 1982a). These areas have also been called AI, DI and GI, respectively in the monkey, rat, and cat (McDonald, 1998).
Injection sites
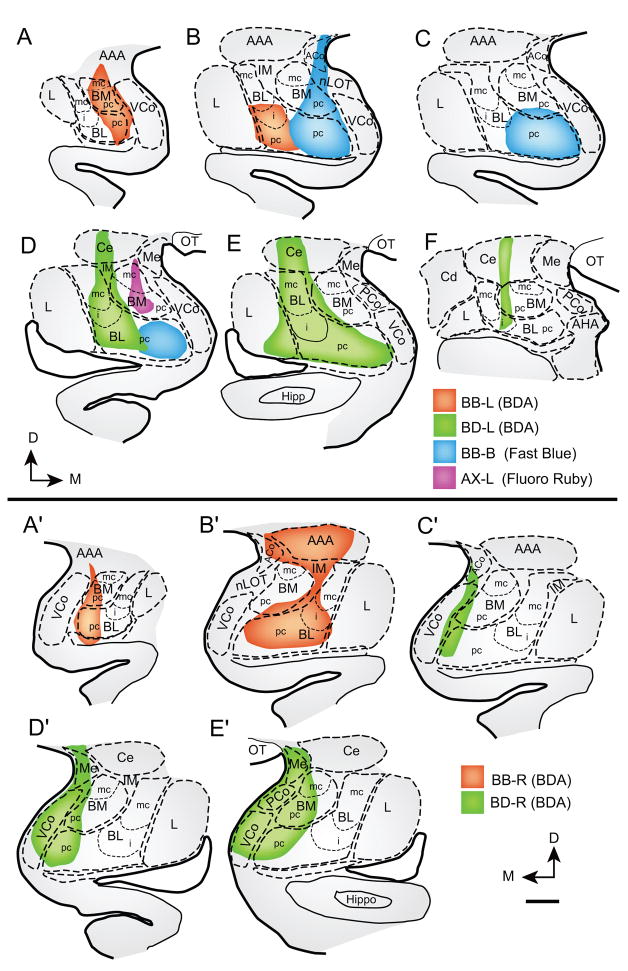
There were six injection sites in the amygdala, including bilateral injections of BDA in two monkeys, a unilateral injection of the fluorescent tracer Fast Blue in one monkey, and a unilateral injection of Fluoro Ruby in another monkey (Fig. 1). In the BDA cases, the tracer occupied extensive parts of the amygdala, centered mainly in the basolateral and basomedial nuclei, and to a lesser extent in the parts of the cortical (Co) and medial (Me) nuclei and the anterior amygdalar area (AAA). In the case with injection of the fluorescent tracer Fast Blue the tracer occupied restricted sites of the medial aspects of the basolateral and basomedial nuclei. In a case with injection of the fluorescent tracer Fluoro Ruby the tracer was restricted to the basomedial nucleus (also known as accessory basal), and included its magnocellular as well as the parvicellular sector.
Fig. 1.
Composites of tracer injection sites in the amygdala. Coronal sections through rostral to caudal levels in: A–F, left hemisphere; A′–E′, right hemisphere. Color key shows the corresponding cases. Scale bar: 1 mm. Abbreviations: AAA - anterior amygdalar area; ACo - anterior cortical nucleus; AHA – amygdalo-hippocampal area; BDA - biotinylated dextran amine; BM - basomedial nucleus (also known as accessory basal nucleus; mc - magnocellular; pc - parvicellular part); BL - basolateral nucleus (also known as basal nucleus; mc - magnocellular; pc - parvicellular; i - intermediate parts of nuclei); Ce - central nucleus; D -dorsal; Hippo - hippocampus; IM - intercalated masses; L - lateral nucleus; M - medial; Me - medial nucleus; nLOT - nucleus of the lateral olfactory tract; OT - optic tract; PCo - posterior cortical nucleus; VCo - ventral cortical nucleus.
Overview of amygdalar connections in temporal and insular cortices
The regions containing labeled neuronal cell bodies after injection of tracer in the amygdala were delineated and mapped throughout the temporal and insular cortices (Fig. 2). Labeled projection neurons and axon terminals varied in density in different areas and layers of temporal and insular cortices. The areas with the highest number of labeled projection neurons included the medial regions of the temporal pole (Tpall and TPvm), the entorhinal (area 28) and perirhinal areas (area 35 and 36) of the medial temporal cortex, the agranular (Iag) and dysgranular (Idg) parts of the insula, and the adjacent parainsular cortex (area PaI). Sparser projections originated in the auditory association cortices in the lateral parts of the temporal pole (areas Ts1 and Ts2), the high order visual association area TE1, the parahippocampal region (area TH and TF), and the granular insula (Ig). These regional patterns were observed in all cases in spite of the fact that the injection sites were centered in different parts of the amygdala (Figs. 1, 2).
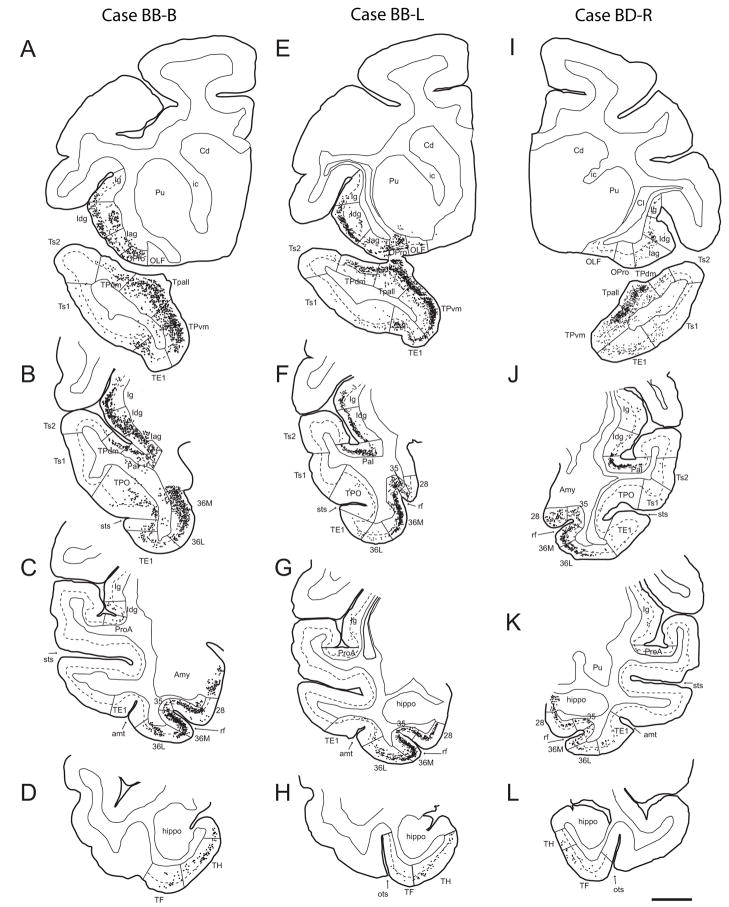
Fig. 2.
Origin of projection neurons directed to the amygdala from temporal and insular cortices. Projection neurons (black dots) shown on coronal sections at four representative rostro-caudal levels (top to bottom in each column) from three injection sites, mapped after injection of bidirectional or retrograde tracers in the amygdala. A–D: Case BB-B; Fast Blue injection localized to BLpc, BMpc, nLOT, ACo. E–H: Case BB-L; BDA injection localized to BMmc, BMpc, BLi, BLpc, AAA. I–L: Case BD-R; BDA injection localized to Me, BMmc, PCo, VCo, BMpc, BLpc (abbreviations of amygdalar nuclei are as in Fig. 1). The dotted line through the cortex shows the upper border of layer V. Scale bar: 5 mm. Abbreviations: 28 - entorhinal cortex; 35 -perirhinal area 35; 36M - medial perirhinal area 36; 36L - lateral perirhinal area 36; amt - anterior middle temporal dimple; Amy - amygdala; Cd - caudate nucleus; Hippo - hippocampus; Iag - agranular insular cortex; Idg - dysgranular insular cortex; Ig - granular insular cortex; OLF - olfactory area; OPro - orbital proisocortex (dysgranular cortex); PaI - parainsular area; ProA - prokoniocortical auditory area; Pu -putamen; rf - rhinal fissure; sts - superior temporal sulcus; TE1 – inferior temporal visual association area 1; TF - area TF of the temporal cortex; TH - area TH of the temporal cortex; Tpall - temporal periallocortex; TPdm - dorsomedial area of the temporal pole; TPvm - ventromedial area of the temporal pole; Ts1 –superior temporal auditory association area 1; Ts2 – superior temporal auditory association area 2.
Scattered projection neurons were observed in the caudally located auditory, visual and polymodal association cortices in the superior and inferior temporal gyri. A few labeled neurons and terminals were seen in occipital areas as well, consistent with previous findings (Amaral et al., 2003), but these were not included in the analyses.
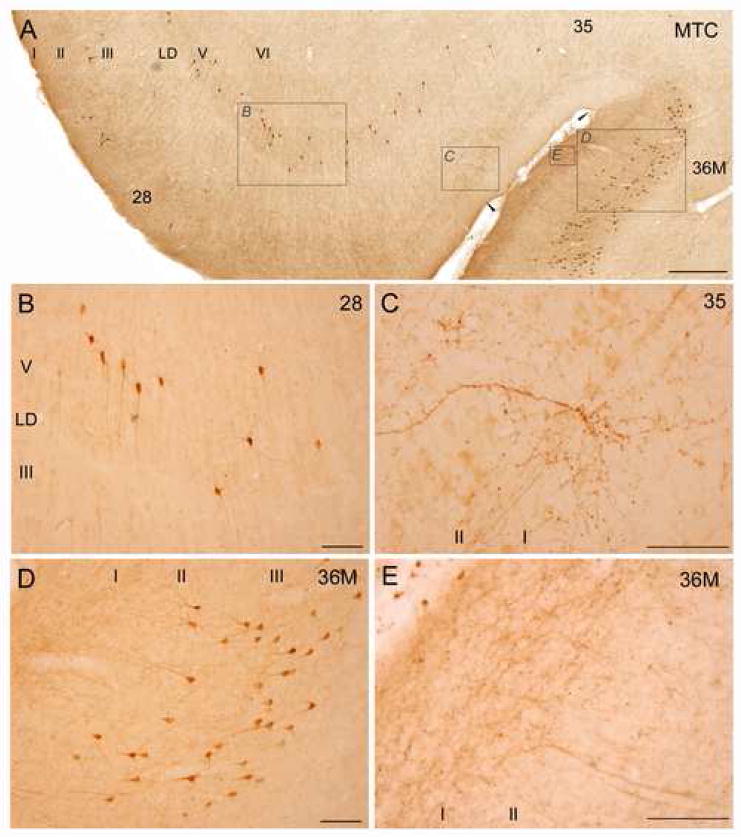
Figure 3 shows low magnification photomicrographs of the key regions that displayed the densest connections with the amygdala (the temporal pole, the medial temporal cortex, and the insula). At higher magnification, various morphological features of these regions are shown in Figure 4 (temporal pole), Figure 5 (medial temporal cortex), and Figure 6 (insula). Although all labeled neurons that projected to the amygdala appeared to belong to the pyramidal class of neurons, coronal sections through the temporal pole showed diverse morphologies that may be associated with cortical folding (Hilgetag and Barbas, 2006). Some projection neurons had prominent labeled dendrites resembling Golgi labeled neurons (Fig. 4). In the medial temporal cortex there was an abrupt change in the laminar distribution of labeled projection neurons, as well as the innervation pattern of amygdalar input, when progressing laterally from area 28 through areas 35 to 36 (Fig. 5). Most notably, in areas 28 and 35 labeled projection neurons were found primarily in the deep layers, while in area 36 labeled projection neurons were found predominantly in the upper layers. At the border between areas 35 and 36M there was a dramatically higher innervation of layer I in area 36M compared to area 35 (Fig 5A, E).
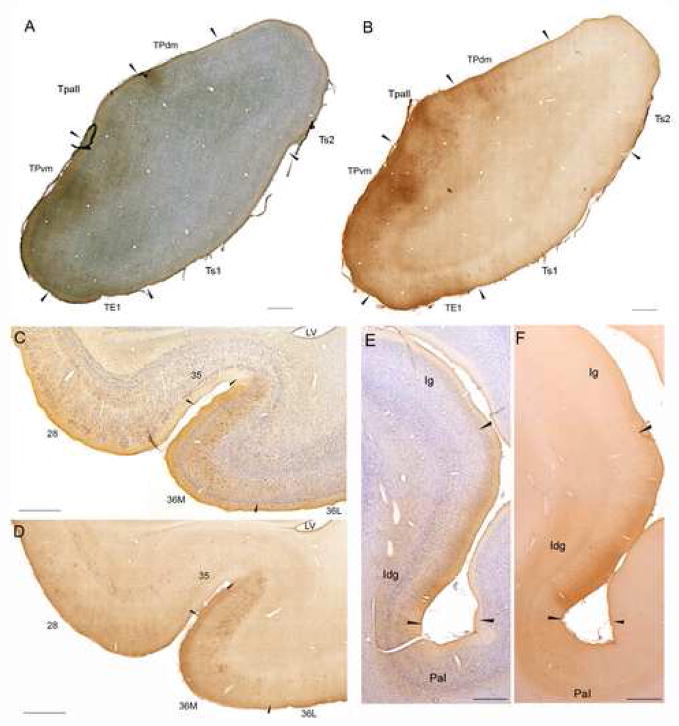
Fig. 3.
Overview of connections of the temporal and insular cortices with the amygdala. Brightfield photomicrographs showing examples of coronal sections through temporal and insular areas with labeled projection neurons and axon terminals after injection of BDA in the amygdala. A: Temporal pole: BDA label with Nissl counterstain, B: Temporal pole: BDA label, adjacent section. C: Medial temporal cortex: BDA label with Nissl counterstain, D: Medial temporal cortex: BDA label, adjacent section. E: Insula: BDA label with Nissl counterstain, F: Insula: BDA label, adjacent section. Scale bars: 1 mm. For all panels, medial is to the left and dorsal at top. Abbreviations: 28 - entorhinal cortex; 35 - perirhinal area 35; 36M - medial perirhinal area 36; 36L - lateral perirhinal area 36; Iag - agranular insular cortex; Idg - dysgranular insular cortex; Ig - granular insular cortex; PaI - parainsular area; TE1 - temporal visual association area 1; Tpall -temporal periallocortex; TPdm - dorsomedial area of the temporal pole; TPvm - ventromedial area of the temporal pole; Ts1 - temporal auditory association area 1; Ts2 - temporal auditory association area 2.
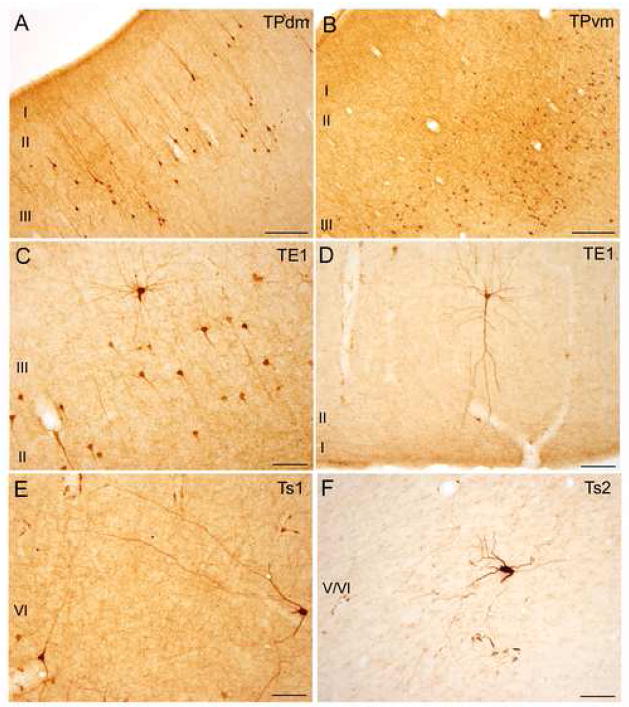
Fig. 4.
Examples of connections between the temporal pole and the amygdala. Brightfield photomicrographs of areas showing projection neurons and axonal fibers and terminations after BDA injection in the amygdala. A: Area TPdm layers I–III. Labeled neurons in layers II–III with long apical dendrites extending to layer I. B: Area TPvm layers I–III. Labeled neurons in layers II–III. C: Rostral part of area TE1. Labeled neurons in layers II–III. D: Rostral part of area TE1. Distinct labeled pyramidal neuron in layer II with prominent apical dendrite reaching layer I. E: Rostral part of area Ts1. Labeled neuron in layer V displaying long dendritic branching. F: Rostral part of area Ts2. Large labeled neuron in layer V/VI. Scale bars: 250 μm (A–B) and 100 μm (C–F). For all panels, medial is to the left and dorsal at top.
Fig. 5.
Examples of connections between the medial temporal cortex and the amygdala. Brightfield photomicrographs of coronal sections through areas showing labeled projection neurons and axonal fibers and terminations after BDA injection in the amygdala. A: Areas 28 (entorhinal cortex), and 35–36 (perirhinal cortex). Labeled neurons in areas 28 and 35 are seen in layers V–VI, while labeled neurons in area 36M are found in layers II–III. Note the faint cell-free band (Lamina Dissecans, LD) in area 28, and the abrupt change in the axonal innervation of layer I at the border between areas 35 and 36M, and a shift of labeled neurons from the deep to the upper layers. Insets in panel A are shown in B–D at higher magnification. B: Area 28. Labeled neurons in layers V–VI. C: Area 35. Prominent branching of a labeled axon at the border between layers I–II. D: Medial part of area 36. Labeled neurons in layers II–III. E: Area 36M. Dense innervation of axonal terminals in layer I. Scale bars: 500 μm (A) and 100 μm (B–E). For all panels, medial is to the left and dorsal at top.
Fig. 6.
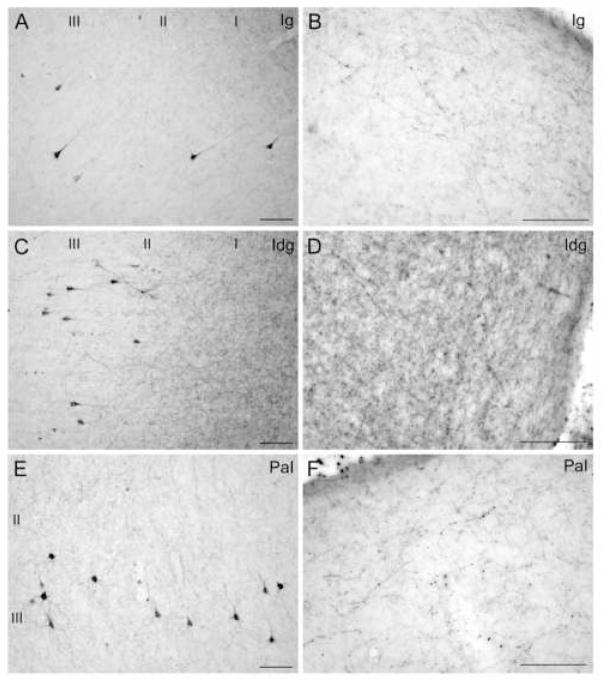
Examples of connections between the insula and the amygdala. Brightfield photomicrographs of coronal sections through areas showing labeled projection neurons and axonal fibers and terminals after BDA injection in the amygdala. A–B: Area Ig, granular insula. C–D: Area Idg, dysgranular insula. E–F: Area PaI, parainsular area. Panels A, C and E show labeled projection neurons in layers II–III; panels B, D and F show labeled axonal terminations in layer I. Scale bars: 100 μm. For all panels, medial is to the left and dorsal at top.
Maps of areas with the densest connections provided the basis for our selection of areas of interest to examine quantitatively using stereological methods. Quantitative data of labeled projection neurons were obtained from five injection sites (four BDA, one Fast Blue), and stereological data of labeled boutons were obtained from four injection sites of BDA, as described below. A case with an injection site restricted to the basomedial nucleus (case AX-L) showed the same pattern of connections as the other cases, and was used as a confirmatory case but is not included in the quantitative analyses.
Temporo-amygdalar projections: retrograde label
Regional distribution
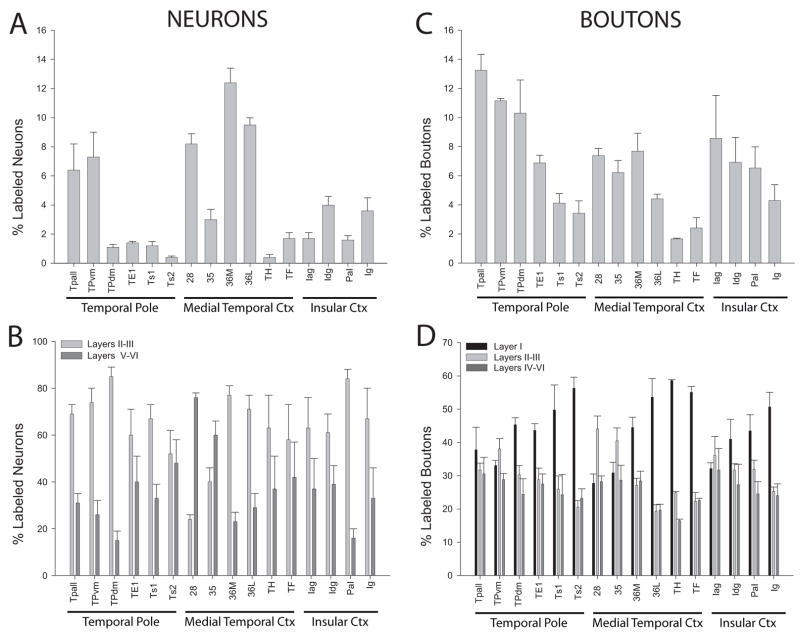
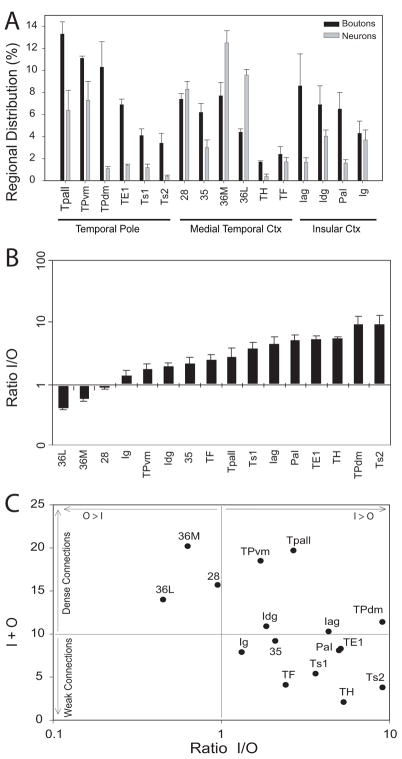
Most labeled projection neurons directed to the amygdala were found in the temporal pole, the medial temporal cortex and the insula (Fig. 7A). In the temporal pole, the highest densities of labeled neurons were found in the temporal periallocortex (Tpall) and in the ventromedial temporal pole (TPvm). Fewer projection neurons were found in the dorsomedial temporal pole (TPdm), the rostral visual association area TE1, and in the laterally situated auditory association areas Ts1 and Ts2. In the medial temporal cortices, which comprise the entorhinal cortex (area 28) and perirhinal cortices (areas 35 and 36), area 36 had the highest density of projection neurons, followed by area 28. Comparatively fewer projection neurons were found in perirhinal area 35 and in the parahippocampal areas TH and TF. In the insula, the dysgranular (Idg) area had the highest densities of projection neurons, followed by the rostral part of the granular insula (Ig). The adjacent agranular insula (Iag) and the parainsular area (PaI) had a moderate density of labeled neurons.
Fig. 7.
Distribution of labeled projection neurons and boutons in the temporal and insular cortices after tracer injection in the amygdala. A: Normalized areal distribution of neurons expressed as a percentage of the total number of labeled neurons in temporal and insular cortices for each injection site; B: Normalized laminar distribution of labeled neurons in the superficial (layers II–III) and deep (V–VI) layers, expressed as percentages of the total number of labeled neurons in each area; C: Normalized areal distribution of labeled boutons expressed as a percentage of the total density in temporal and insular cortices for each injection site; D: Normalized distribution of boutons in laminar compartments (layers I, II–III and IV–VI) expressed as percentages of the total density for each area. Abbreviations are as in Fig. 2.
Laminar distribution
The laminar distribution of labeled neurons is shown in Figure 7B. In most regions, the majority of labeled projection neurons were found in the upper layers. This pattern was seen in the medial parts of the temporal pole, which had the highest overall density of projection neurons, although the deep layers also had a significant proportion of labeled neurons. In the lateral temporal pole (rostral TE1 and rostral areas Ts1 and Ts2) the overall density of labeled neurons was lower, but the laminar distribution pattern was also mainly in the upper layers. In the parainsular area and in the parahippocampal areas (TH and TF) projection neurons were likewise primarily found in the upper layers. We noted the same pattern of preferential origin in the upper layers in the agranular, dysgranular and granular sectors of the insula, though significant numbers of projection neurons were also found in the deep layers. Overall, the insula showed the highest variability in the laminar origin of projection neurons among cases with moderate labeling. The highest variability was seen in areas with sparse projections. In area 36, which had dense connections, most projection neurons were found in the upper layers, exhibiting the same laminar pattern as the more rostrally adjacent polar region (TPvm). In sharp contrast to all other areas examined, in medial temporal areas 28 and 35, the majority of the labeled projection neurons were found in the deep layers, exhibiting a dramatic shift in the laminar distribution at the area 35–36 border (Fig. 5A).
Amygdalo-temporal projections: anterograde label
Regional distribution
As with the distribution of labeled projection neurons, the axonal terminations from the amygdala were most dense in the temporal pole, the medial temporal cortex and the insula (Fig. 7C). In the temporal pole, most labeled boutons from the amygdala were noted in the temporal periallocortex (Tpall), the dorsomedial (TPdm) and ventromedial (TPvm) portions of the temporal pole, and the rostral portions of area TE1. Fewer labeled boutons were found in the rostral parts of the laterally located auditory association areas Ts1 and Ts2. In the medial temporal cortex, the entorhinal and perirhinal areas received the heaviest projections from the amygdala, while the parahippocampal region received sparser projections. Axonal terminations in the insula were most densely distributed in its agranular and dysgranular parts, and in the adjacent parainsular area, and were moderately distributed in the granular insula.
Laminar distribution
The laminar distribution of labeled boutons is shown in Figure 7D. In the temporal pole, labeled boutons in Tpall and TPvm were found throughout all layers; these areas also had the highest density of boutons and projection neurons. In the dorsomedial temporal pole (TPdm) and in area TE1, labeled boutons were found mainly in the upper layers, especially layer I. This pattern was even more prominent in areas Ts1 and Ts2, and in the parahippocampal areas TH and TF (Fig. 7D). In the medial temporal areas 28 and 35 the majority of labeled boutons were found in layers II–III, the two areas where the majority of labeled projection neurons were found in the deep layers. When progressing from area 35 towards area 36M, there was an abrupt change in the laminar innervation pattern at the border between area 35 and area 36M. In layer I of area 35 there was a moderate proportion of labeled boutons, in contrast to a dense proportion in area 36M (Fig. 5A). No other region showed a similarly abrupt change in innervation pattern at areal borders. In the insula, the agranular area showed an equal distribution of boutons among layers, while in the dysgranular and granular areas terminations were concentrated in the upper layers, primarily layer I.
Comparison of the laminar distribution of labeled neurons and boutons
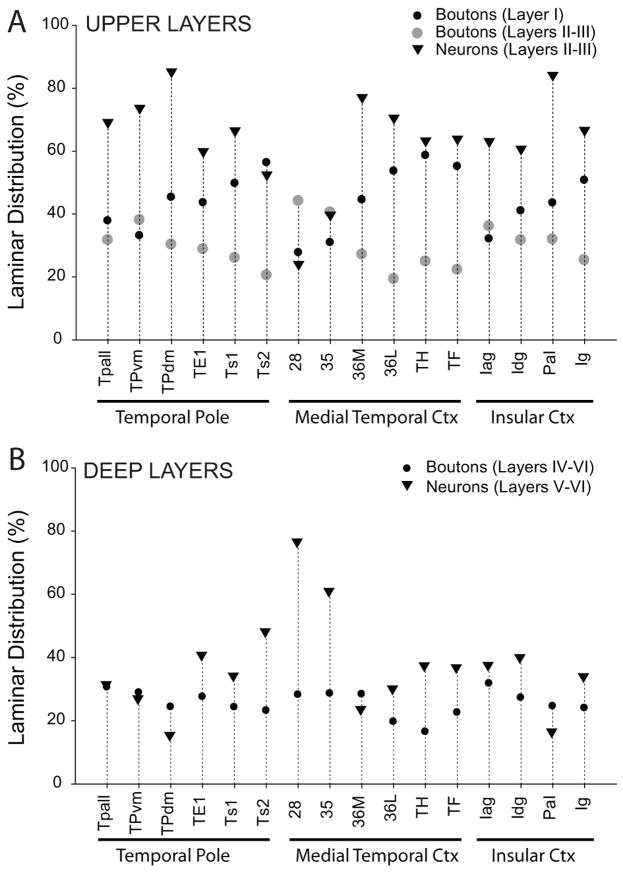
We then compared the relative laminar densities of labeled neurons and boutons (Fig. 8). In most areas the majority of projection neurons were found in the upper layers, consistent with feedforward projections. In marked contrast, in areas 28 and 35 a large majority of projection neurons were found in the deep layers, consistent with feedback projections. Moreover, areas 28 and 35 had the lowest proportion of labeled boutons in layer I among those studied (Fig. 8A). In contrast, all other areas had labeled boutons primarily in the upper layers, including significant proportions in layer I. Interestingly, areas 28 and 35 had the highest proportion of labeled boutons in layers II and III, which project to the hippocampus.
Fig. 8.
Laminar distribution of labeled projection neurons and boutons in temporal and insular cortices after tracer injection in the amygdala. A: Label in the upper layers (I–III). Normalized data showing the average proportion of labeled boutons from the amygdala terminating in layer I or layers II–III, and labeled projection neurons from layers II–III directed to the amygdala. B: Label in the deep layers (IV–VI). Average proportion of labeled boutons from the amygdala terminating in layers IV–VI, and labeled projection neurons from layers V–VI directed to the amygdala. Abbreviations are as in Fig. 2.
Comparison of input and output zones
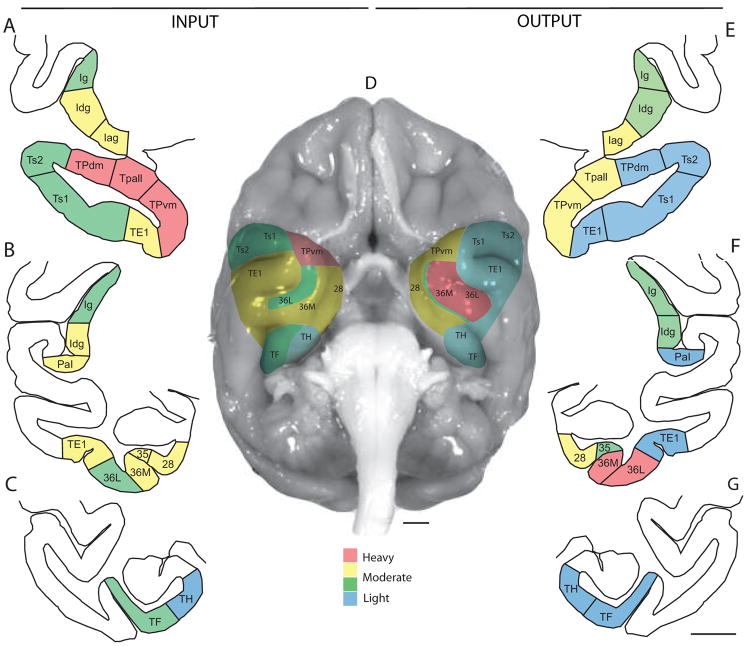
We then compared the relative density of projection neurons to the relative density of axonal terminals in the temporal and insular cortices, in order to determine to what extent the different areas were primarily recipients of input from the amygdala, or primarily providers of output to the amygdala. We expressed data for each area as a proportion of the entire series of temporal and insular areas examined, and used the normalized data to generate an input/output (I/O) ratio of connections in the cortex with the amygdala, and an overall input+output (I+O) parameter; the latter was considered to be a marker for the overall “connection strength”. As shown in Figure 9, area 36 had comparatively more output projections than input fibers (O>I), although it received dense innervation from the amygdala. This pattern suggests that area 36 may be regarded primarily as a “sender” of cortical information to the amygdala. In area 28 the relative input to relative output were approximately equal. In the remaining temporal areas analyzed the relative input was greater than the relative output (I>O), including areas with robust connections (Tpall, TPvm and TPdm in the temporal pole, and insular areas Idg and Iag), as well as in areas with sparser connections (medial temporal areas 35, TH and TF, temporal polar areas TE1, Ts1 and Ts2, and insular areas PaI and Ig). All of these areas thus may be regarded primarily as “receivers” of information from the amygdala. The individual cases did not differ significantly in their input/output ratios, in spite of the differences in the injection sites within the amygdala. Figure 10 summarizes schematically the regional densities of the input (boutons) and output (neurons) distribution patterns in medial temporal and insular cortices at different rostro-caudal levels, using color-coded ranks of increasing densities (blue-green-yellow to red).
Fig. 9.
Relative proportions of input and output connections between the amygdala and temporal and insular cortices. A: Regional distribution of boutons (input), or projection neurons (output) in temporal and insular cortices originating from, or projecting to, the amygdala. Values are expressed as percentages of the total number of labeled boutons or neurons in the temporal and insular areas for each injection site. Note that only area 36 (and to a lesser extent area 28) displayed comparatively more output (neurons) than input (boutons). B: Ratios of boutons (I=input) to projection neurons (O=output) in the cortex. A low I/O ratio implies more output than input, and a high I/O ratio implies more input than output. Only area 36 (and to a lesser extent area 28) displayed comparatively more output than input. C: Input/output ratios (I/O) to the overall sum of input and output (I+O). Densely interconnected areas with high I+O values are seen in the upper squares; areas with lighter connections have low I+O values and are seen in the lower squares. Areas to the left (areas 28 and 36M and 36L), have proportionately more output (O>I) projections to the amygdala, while areas to the right have proportionately higher input from the amygdala than output to the amygdala (I>O). Abbreviations are as in Fig. 2.
Fig. 10.
Summary of the distribution of input and output linking temporal and insular cortices with the amygdala. A–C: Input (labeled boutons) shown on coronal sections through insular and temporal cortices; E–G: Output (labeled neurons) in the temporal and insular cortices connected with the amygdala. Densitfies are color-coded based on ranks and mapped onto schematic coronal sections at three different rostro-caudal levels (top to bottom). Blue-green-yellow to red show differences in densities based on percentages of the total number of labeled boutons or neurons averaged across cases, as follows: blue: <2%; green: 2–5%; yellow: 5–10%; red: >10 %. D: Surface view of areas visible on the basal surface; input is shown on the left and output on the right. Scale bars: 5 mm. Abbreviations are as in Fig. 2.
Discussion
Classical studies have revealed a large number of connections between the amygdala and the cerebral cortex. In particular, agranular and dysgranular (limbic) parts of different cortical regions, such as the posterior orbitofrontal cortex, the anterior cingulate, the temporal pole and the anterior insula, heavily project to the amygdala [e.g., (Nauta, 1962; Herzog and Van Hoesen, 1976; Aggleton et al., 1980; Turner et al., 1980; Mufson et al., 1981; Porrino et al., 1981; Van Hoesen, 1981; Amaral and Price, 1984; Iwai et al., 1987; Iwai and Yukie, 1987; Barbas and De Olmos, 1990; Carmichael and Price, 1995) from the upper and deep layers (Aggleton et al., 1980; Turner et al., 1980; Mufson et al., 1981; Saper, 1982; Mesulam and Mufson, 1982b; Friedman et al., 1986; Amaral and Insausti, 1992; Stefanacci and Amaral, 2000; Stefanacci and Amaral, 2002). The present study confirms and extends these findings by showing that the strongest connections of the amygdala were found in the medial temporal pole, medial temporal entorhinal and perirhinal areas (areas 35 and 36), and the agranular and dysgranular areas of the anterior insula. Weaker connections with the amygdala were found in the lateral temporal pole, the parahippocampal region, and the granular insula, consistent with previous findings in the monkey, cat and rat [e.g., (McDonald, 1998; Pikkarainen and Pitkanen, 2001; Pitkanen et al., 2002)]. Moreover, we found that sensory association areas projected to the amygdala mostly from their upper layers, while medial temporal areas, which are associated with long-term memory, projected to the amygdala from their deep layers. These findings suggest a laminar-based sequence of information processing for emotions and the formation of emotional memory, as elaborated below.
Sensory processing for emotions
Convergent information from all sensory modalities originating in high-order visual, auditory, olfactory, gustatory, visceral, somatosensory and polymodal cortices reaches the amygdala [reviewed in (McDonald, 1998; Pitkanen, 2000)]. Information from these sensory cortices is preferentially issued from areas associated with complex sensory processing with emotional significance. For example, in anterior inferior temporal cortex (area TE1), neurons respond to complex visual stimuli, such as faces and facial expressions, and bilateral lesions of the amygdala impair recognition of facial emotional expressions [e.g., (Baylis et al., 1987; Adolphs et al., 1994; Kim et al., 2004)]. Similarly, within the auditory modality, species specific calls have special emotional significance (Poremba et al., 2004), and the amygdala is activated in association with non-linguistic emotional vocalizations (Sander and Scheich, 2005; Fecteau et al., 2007). These functions may depend on the strong bidirectional connections between the amygdala and anterior temporal visual, auditory, and polymodal association cortices in the temporal pole (Pribram and MacLean, 1953; Markowitsch et al., 1985; Moran et al., 1987). In view of these interconnections between the temporal pole and the amygdala, it is possible that high-order sensory information becomes linked to emotional value (Olson et al., 2007).
The insula, and especially its agranular and dysgranular parts, had prominent connections with the amygdala as well, in line with previous observations (Aggleton et al., 1980; Turner et al., 1980; Mufson et al., 1981; Saper, 1982; Mesulam and Mufson, 1982b; Friedman et al., 1986; Amaral and Insausti, 1992; Stefanacci and Amaral, 2000; Stefanacci and Amaral, 2002). The insula has been implicated in a variety of somatosensory, gustatory and visceral functions [e.g., (Mufson and Mesulam, 1982; Saper, 1982; Mesulam and Mufson, 1982b)], and in the complex processing accompanying the experience of disgust and the visual perception of disgust in others (Phillips et al., 1997; Wicker et al., 2003). The insula and the orbitofrontal cortex also send projections to the temporal pole (Pandya and Kuypers, 1969; Nauta, 1972; Mesulam and Mufson, 1982a; Mesulam and Mufson, 1982b; Markowitsch et al., 1985; Kondo et al., 2003; Barbas et al., 2005), in circuits thought to have a role in visceral-autonomic function and gustatory-olfactory integration (Mesulam and Mufson, 1982a).
Sequence of sensory processing for emotions
Previous studies have shown that the lateral nucleus of the amygdala is the major recipient of projections from sensory association cortices. Intrinsic connections link the lateral nucleus with other parts of the basal complex of the amygdala, which then project to the central nucleus, the main output of the amygdala to the hypothalamus and brainstem [reviewed in (LeDoux, 1995)]. However, projections from sensory association cortices extend beyond the lateral nucleus, reaching the basolateral, basomedial (also known as accessory basal), and the cortical nuclei of the amygdala [e.g., (Mufson et al., 1981; Ghashghaei and Barbas, 2002; reviewed in (McDonald, 1998; Pitkanen et al., 2000a)]. Further, the connections of the prefrontal cortex with the amygdala are highly distributed, and include not only the basolateral and basomedial nuclei, but also the lateral and cortical nuclei, albeit to a lesser extent [e.g., (Barbas and De Olmos, 1990; Carmichael and Price, 1995)]. Moreover, the connections of orbitofrontal and anterior cingulate cortices overlap extensively in the amygdala with the connections of temporal sensory association cortices, suggesting widespread influence of prefrontal cortices on sensory and intrinsic processing in the amygdala (Ghashghaei and Barbas, 2002).
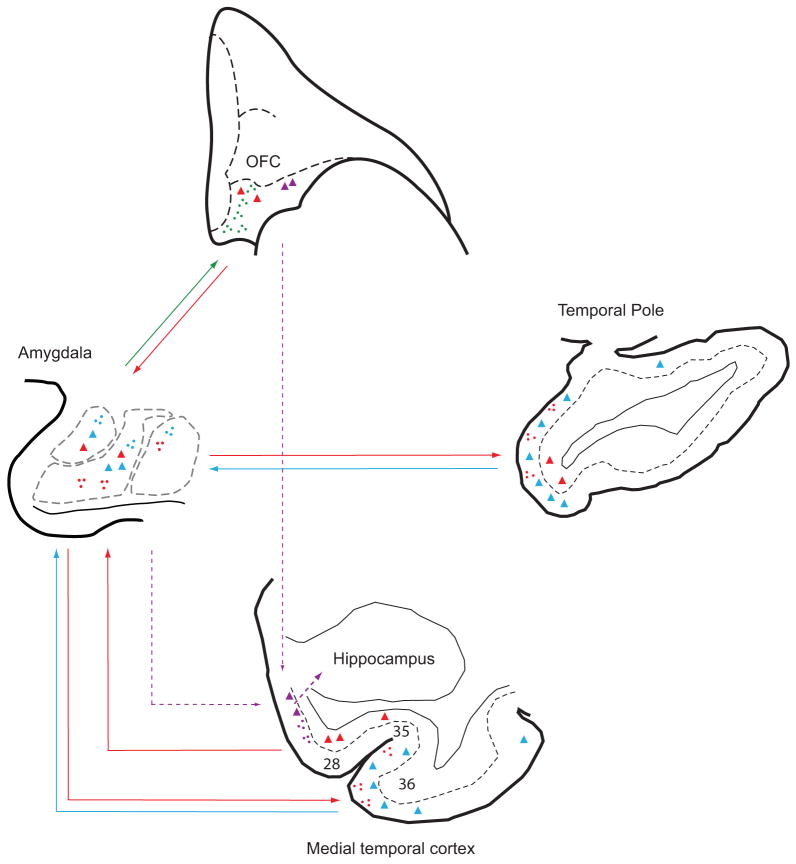
The laminar pattern of connections provides a novel approach to deciphering information processing for emotions through sensory cortices, the amygdala and the prefrontal cortex. This was accomplished by comparing how the same sites in the amygdala are connected with sensory cortices, shown in this study, and with the prefrontal cortex, shown in a previous study (Ghashghaei et al., 2007). We hypothesized that if polymodal and sensory association areas convey signals to the amygdala pertinent to the state of the environment, they should resemble feedforward connections, arising mainly from the upper layers (II–III), consistent with the flow of information from earlier- to later-processing sensory areas (Felleman and Van Essen, 1991). Our findings support this hypothesis, since in most sensory association and polymodal areas labeled projection neurons directed to the amygdala were indeed found primarily in the upper layers, resembling feedforward projections. The perirhinal area 36 in the medial temporal cortex, in particular, had dense connections with the amygdala, and a comparison of its input and output patterns showed that it issued comparatively more projections to the amygdala than it received, acting as a primary “sender” of feedforward information to the amygdala. These findings indicate that a large variety of sensory association and polymodal cortices issue feedforward projections to the amygdala. In the reverse direction, axonal terminations from the amygdala to the temporal and insular cortices, targeted preferentially the upper layers, most prominently layer I, consistent with feedback cortico-cortical projections.
The same sites of the amygdala that receive feedforward information from temporal and insular sensory association and polymodal cortices, as shown in this study, are also robustly connected with prefrontal cortices. In a previous study, using the same cases, the densest and most specialized connections of the amygdala were found in caudal orbitofrontal cortex and the anterior cingulate (Ghashghaei et al., 2007). The amygdala innervates all layers of orbitofrontal and anterior cingulate cortices in complex laminar patterns, including the middle layers, suggesting a strong feedforward innervation. In the reverse direction, a vast majority of prefrontal projection neurons targeting the amygdala originate in layer V, resembling feedback projections. Previous studies have shown that the same sensory association and polymodal cortices that project to the amygdala also project to prefrontal cortices (Barbas and Mesulam, 1985; Barbas et al., 1999; Rempel-Clower and Barbas, 2000; Barbas et al., 2005) in a feedforward manner as well (Rempel-Clower and Barbas, 2000), providing the circuits for direct and indirect feedforward sensory input to orbitofrontal cortex [reviewed in (Barbas, 1995; Barbas, 2007)]. These findings suggest a possible route through which sensory signals with emotional significance are forwarded from temporal and insular structures to the amygdala, and are then relayed through feedforward projections to orbitofrontal areas and the anterior cingulate, as summarized in Figure 11. In turn, the caudal orbitofrontal cortex issues specialized projections to specific regions of the amygdala, including the intercalated masses of the amygdala (Ghashghaei and Barbas, 2002), which may be recruited in emotional arousal (Barbas et al., 2003).
Fig. 11.
Model summarizing the sequence of emotional processing through laminar-specific connections between the amygdala, the temporal and insular areas, and the posterior orbitofrontal cortex. The amygdala receives feedforward projections from the upper layers (blue arrows) of the temporal pole as well as from area 36 of the medial temporal cortex, and sends reciprocal feedback projections (red arrows) to the upper layers of the same areas. This pattern was also seen in the insula, adjacent temporal sensory association areas, and the parahippocampal areas (not shown). The amygdala also projects to all layers, including the middle layers, in a feedforward fashion to the posterior orbitofrontal (green arrow) and medial prefrontal cortices (not shown), which send mostly feedback projections from their deep layers (red arrow) to the amygdala. The amygdala projects robustly to entorhinal layers II and III (purple hatched arrows), which, in turn, project to the hippocampus (purple hatched arrows) and can thus be considered ‘feedforward’ in this system. The posterior orbitofrontal cortex projects to the middle layers of the entorhinal cortex as well (purple hatched arrows). These combined pathways from the amygdala and the posterior orbitofrontal cortex may facilitate transmission of motivationally relevant signals, enabling the formation of emotional memories. Dots: axonal terminals. Triangles: projection neurons.
Pathways for emotional memories
The medial temporal entorhinal (area 28) and perirhinal (areas 36, 35) cortices, which have a key role in long-term memory (Zola-Morgan et al., 1989; Squire and Zola-Morgan, 1991; Meunier et al., 1993; Suzuki et al., 1993; Malkova et al., 1995; Buckley and Gaffan, 1998; Murray and Bussey, 1999; Eichenbaum, 2000; Simons and Spiers, 2003), had robust connections with the amygdala, consistent with previous findings in rats and monkeys (Krettek and Price, 1977; Ottersen, 1982; McDonald and Jackson, 1987; Amaral et al., 1992; Petrovich et al., 1996; Pitkanen et al., 2000b; Stefanacci and Amaral, 2002). These medial temporal cortices are high-order polymodal association areas and recipients of unimodal sensory and polymodal signals [e.g., (Jones and Powell, 1970; Van Hoesen et al., 1975; Van Hoesen and Pandya, 1975a; Suzuki and Amaral, 1994; Suzuki and Amaral, 2003a); reviewed in (Suzuki and Amaral, 2003b; Saleem et al., 2007)]. Convergent projections from sensory association cortices are sent in cortico-cortical cascades to areas 36, 35 and 28, and then to the hippocampus (Van Hoesen and Pandya, 1975b). This evidence suggests that medial temporal cortices provide a gateway between the sensory cortices and the hippocampus (de Curtis and Pare, 2004). These sensory-related pathways and their connections with the amygdala provide the structural basis for the linkage of sensory signals with emotional context and the formation of long-term memories. The convergence of sensory signals and amygdalar connections in medial temporal cortices may help explain the high correlation between the degree of amygdala activation at encoding and subsequent recall of information (Cahill et al., 1996), and why memory for emotionally arousing events is better than for neutral events (Adolphs et al., 1997; Dolcos et al., 2004; McGaugh, 2004; McGaugh, 2006). Consistent with these findings, humans with lesions of the amygdala lack arousal for emotional events (Kilpatrick and Cahill, 2003).
Sequence of mnemonic processing for emotions
In sharp contrast to the feedforward projections from polymodal area 36 to the amygdala, there was an abrupt shift in the laminar origin and termination of connections at the border between areas 36 and 35, so that perirhinal area 35 and entorhinal area 28 issued projections to the amygdala primarily from the deep layers, predominantly from layer V, resembling the pattern of projection of other limbic areas in cortico-cortical connections (Barbas, 1986; Barbas and Rempel-Clower, 1997). In the reverse direction, axons from the amygdala terminated in several layers of areas 35 and 28, but mostly in layers II-III, and sparsely in layer I, consistent with previous findings (Pitkanen et al., 2002). Interestingly, layers II and III of the entorhinal cortex, which were the primary targets of projections from the amygdala, project to the hippocampus (Van Hoesen and Pandya, 1975b; Steward and Scoville, 1976; Witter et al., 1989; Witter and Amaral, 1991). This laminar specific pathway from the amygdala to the entorhinal cortex may be the anatomical substrate for the enhanced memory for emotionally significant events, although the physiological mechanism is unknown.
Recent studies suggest that transfer of information from the neocortex to the hippocampus occurs with low probability (Pelletier et al., 2004), due to powerful inhibitory control by the rhinal cortices (de Curtis and Pare, 2004). The amygdala may facilitate declarative memory by enhancing transfer of sensory cues during behaviorally salient events to the hippocampus (Pelletier et al., 2005; Paz et al., 2006). Interestingly, a robust projection from the caudal orbitofrontal cortex terminates in the entorhinal cortex in a feedforward manner as well (Rempel-Clower and Barbas, 2000). Like the amygdala, the orbitofrontal cortex receives rich multimodal information from all sensory association cortices (Barbas, 1993; Barbas, 2000; Cavada et al., 2000; Barbas et al., 2002), and may thus provide an additional route through which pre-processed sensory information reaches the amygdala as well as the entorhinal cortex. Our findings thus suggest that dual pathways may participate in transferring this information to the entorhinal cortex, as summarized in Figure 11. A direct pathway from the amygdala reaches layers II and III of the entorhinal cortex, as shown in this study, and a robust feedforward pathway from the posterior orbitofrontal cortex projects to the entorhinal cortex as well (Rempel-Clower and Barbas, 2000). Together, these dual mechanisms of activation may help open the gate to the hippocampus allowing the formation of memories for emotionally significant events. Abnormal activation of these pathways may underlie several psychiatric disorders associated with anxiety, such as post-traumatic stress disorder.
Acknowledgments
We thank Ola Alade for technical assistance, Ron Killiany for help with brain imaging, and Linda Fernsten for surgical assistance at the New England Primate Research Center. Research was supported by grants from NIMH and NINDS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adolphs R, Cahill L, Schul R, Babinsky R. Impaired declarative memory for emotional material following bilateral amygdala damage in humans. Learn Mem. 1997;4:291–300. doi: 10.1101/lm.4.3.291. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Tranel D, Damasio H, Damasio A. Impaired recognition of emotion in facial expressions following bilateral damage to the human amygdala. Nature. 1994;372:669–672. doi: 10.1038/372669a0. [DOI] [PubMed] [Google Scholar]
- Aggleton JP, Burton MJ, Passingham RE. Cortical and subcortical afferents to the amygdala of the rhesus monkey (Macaca mulatta) Brain Research. 1980;190:347–368. doi: 10.1016/0006-8993(80)90279-6. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Behniea H, Kelly JL. Topographic organization of projections from the amygdala to the visual cortex in the macaque monkey. Neuroscience. 2003;118:1099–1120. doi: 10.1016/s0306-4522(02)01001-1. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Insausti R. Retrograde transport of D-[3H]-aspartate injected into the monkey amygdaloid complex. Experimental Brain Research. 1992;88:375–388. doi: 10.1007/BF02259113. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL. Amygdalo-cortical projections in the monkey (Macaca fascicularis) Journal of Comparative Neurology. 1984;230:465–496. doi: 10.1002/cne.902300402. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkänen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The Amygdala: neurobiological aspects of emotion, memory, and mental dysfunction. Wiley-Liss; New York: 1992. pp. 1–66. [Google Scholar]
- Barbas H. Pattern in the laminar origin of corticocortical connections. Journal of Comparative Neurology. 1986;252:415–422. doi: 10.1002/cne.902520310. [DOI] [PubMed] [Google Scholar]
- Barbas H. Organization of cortical afferent input to orbitofrontal areas in the rhesus monkey. Neuroscience. 1993;56:841–864. doi: 10.1016/0306-4522(93)90132-y. [DOI] [PubMed] [Google Scholar]
- Barbas H. Anatomic basis of cognitive-emotional interactions in the primate prefrontal cortex. Neuroscience & Biobehavioral Reviews. 1995;19:499–510. doi: 10.1016/0149-7634(94)00053-4. [DOI] [PubMed] [Google Scholar]
- Barbas H. Connections underlying the synthesis of cognition, memory, and emotion in primate prefrontal cortices. Brain Research Bulletin. 2000;52:319–330. doi: 10.1016/s0361-9230(99)00245-2. [DOI] [PubMed] [Google Scholar]
- Barbas H. Flow of information for emotions through temporal and orbitofrontal pathways. Journal of Anatomy. 2007;211:237–249. doi: 10.1111/j.1469-7580.2007.00777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, De Olmos J. Projections from the amygdala to basoventral and mediodorsal prefrontal regions in the rhesus monkey. Journal of Comparative Neurology. 1990;301:1–23. doi: 10.1002/cne.903000409. [DOI] [PubMed] [Google Scholar]
- Barbas H, Ghashghaei H, Dombrowski SM, Rempel-Clower NL. Medial prefrontal cortices are unified by common connections with superior temporal cortices and distinguished by input from memory-related areas in the rhesus monkey. Journal of Comparative Neurology. 1999;410:343–367. doi: 10.1002/(sici)1096-9861(19990802)410:3<343::aid-cne1>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- Barbas H, Ghashghaei H, Rempel-Clower N, Xiao D. Anatomic basis of functional specialization in prefrontal cortices in primates. In: Grafman J, editor. Handbook of Neuropsychology. 2. Elsevier Science B.V.; Amsterdam: 2002. pp. 1–27. [Google Scholar]
- Barbas H, Medalla M, Alade O, Suski J, Zikopoulos B, Lera P. Relationship of prefrontal connections to inhibitory systems in superior temporal areas in the rhesus monkey. Cerebral Cortex. 2005;15:1356–1370. doi: 10.1093/cercor/bhi018. [DOI] [PubMed] [Google Scholar]
- Barbas H, Mesulam MM. Cortical afferent input to the principalis region of the rhesus monkey. Neuroscience. 1985;15:619–637. doi: 10.1016/0306-4522(85)90064-8. [DOI] [PubMed] [Google Scholar]
- Barbas H, Rempel-Clower N. Cortical structure predicts the pattern of corticocortical connections. Cerebral Cortex. 1997;7:635–646. doi: 10.1093/cercor/7.7.635. [DOI] [PubMed] [Google Scholar]
- Barbas H, Saha S, Rempel-Clower N, Ghashghaei T. Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neurosci. 2003;4:25. doi: 10.1186/1471-2202-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylis GC, Rolls ET, Leonard CM. Functional subdivisions of the temporal lobe neocortex. Journal of Neuroscience. 1987;7:330–342. doi: 10.1523/JNEUROSCI.07-02-00330.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodmann K. Vergleichende Lokalizationslehre der Grosshirnrinde in ihren Prinizipien dargestelt auf Grund des Zellenbaues. Barth; Leipzig: 1909. [Google Scholar]
- Buckley MJ, Gaffan D. Perirhinal cortex ablation impairs visual object identification. Journal of Neuroscience. 1998;18:2268–2275. doi: 10.1523/JNEUROSCI.18-06-02268.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, Fallon J, Alkire MT, Tang C, Keator D, Wu J, McGaugh JL. Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proc Natl Acad Sci USA. 1996;93:8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, McGaugh JL. Mechanisms of emotional arousal and lasting declarative memory. Trends in Neuroscience. 1998;21:294–299. doi: 10.1016/s0166-2236(97)01214-9. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. Journal of Comparative Neurology. 1995;363:615–641. doi: 10.1002/cne.903630408. [DOI] [PubMed] [Google Scholar]
- Cassell MD, Chittick CA, Siegel MA, Wright DJ. Collateralization of the amygdaloid projections of the rat prelimbic and infralimbic cortices. Journal of Comparative Neurology. 1989;279:235–248. doi: 10.1002/cne.902790207. [DOI] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the macaque monkey orbitofrontal cortex. A review. Cerebral Cortex. 2000;10:220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Pare D. The rhinal cortices: a wall of inhibition between the neocortex and the hippocampus. Prog Neurobiol. 2004;74:101–110. doi: 10.1016/j.pneurobio.2004.08.005. [DOI] [PubMed] [Google Scholar]
- De Olmos JS. Amygdala. In: Paxinos G, editor. The Human Nervous System. Academic Press, Inc.; San Diego: 1990. pp. 583–710. [Google Scholar]
- Dolcos F, LaBar KS, Cabeza R. Interaction between the amygdala and the medial temporal lobe memory system predicts better memory for emotional events. Neuron. 2004;42:855–863. doi: 10.1016/s0896-6273(04)00289-2. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H. A cortical-hippocampal system for declarative memory. Nat Rev Neurosci. 2000;1:41–50. doi: 10.1038/35036213. [DOI] [PubMed] [Google Scholar]
- Fecteau S, Belin P, Joanette Y, Armony JL. Amygdala responses to nonlinguistic emotional vocalizations. Neuroimage. 2007;36:480–487. doi: 10.1016/j.neuroimage.2007.02.043. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cerebral Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Friedman DP, Murray EA, O’Neill JB, Mishkin M. Cortical connections of the somatosensory fields of the lateral sulcus of macaques: evidence for a corticolimbic pathway for touch. Journal of Comparative Neurology. 1986;252:323–347. doi: 10.1002/cne.902520304. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The Prefrontal Cortex. 2. Raven Press; New York: 1989. [Google Scholar]
- Galaburda AM, Pandya DN. The intrinsic architectonic and connectional organization of the superior temporal region of the rhesus monkey. Journal of Comparative Neurology. 1983;221:169–184. doi: 10.1002/cne.902210206. [DOI] [PubMed] [Google Scholar]
- Geneser-Jensen FA, Blackstad TW. Distribution of acetyl cholinesterase in the hippocampal region of the guinea pig. Z Zellforsch Mikrosk Anat. 1971;114:460–481. doi: 10.1007/BF00325634. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotions: Interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower EC. Efferent projections from limbic cortex of the temporal pole to the magnocellular medial dorsal nucleus in the rhesus monkey. Journal of Comparative Neurology. 1989;280:343–358. doi: 10.1002/cne.902800303. [DOI] [PubMed] [Google Scholar]
- Gundersen HJG, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, West MJ. The new stereological tools: disector, fractionator, nucleator and point sample intercepts and their use in pathological research and diagnosis. Acta Pathologica, Microbiologica et Immunologica Scandinavica. 1988;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Van Hoesen GW. Temporal neocortical afferent connections to the amygdala in the rhesus monkey. Brain Reserch. 1976;115:57–69. doi: 10.1016/0006-8993(76)90822-2. [DOI] [PubMed] [Google Scholar]
- Hilgetag CC, Barbas H. Role of mechanical factors in the morphology of the primate cerebral cortex. PLoS Comput Biol. 2006;2:e22. doi: 10.1371/journal.pcbi.0020022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard CV, Reed MG. Unbiased Stereology, Three-dimensional Measurement in Microscopy. 1. BIOS Scientific Publishers Limited; Oxford: 1998. [Google Scholar]
- Iwai E, Yukie M. Amygdalofugal and amygdalopetal connections with modality- specific visual cortical areas in macaques (Macaca fuscata, M. mulatta, and M. fascicularis) Journal of Comparative Neurology. 1987;261:362–387. doi: 10.1002/cne.902610304. [DOI] [PubMed] [Google Scholar]
- Iwai E, Yukie M, Suyama H, Shirakawa S. Amygdalar connections with middle and inferior temporal gyri of the monkey. Neuroscience Letters. 1987;83:25–29. doi: 10.1016/0304-3940(87)90210-2. [DOI] [PubMed] [Google Scholar]
- Johnston JB. Further contributions to the study of the evolution of the forebrain. Journal of Comparative Neurology. 1923;35:337–481. [Google Scholar]
- Jones EG, Powell TPS. An anatomical study of converging sensory pathways within the cerebral cortex. Brain. 1970;93:793–820. doi: 10.1093/brain/93.4.793. [DOI] [PubMed] [Google Scholar]
- Kilpatrick L, Cahill L. Amygdala modulation of parahippocampal and frontal regions during emotionally influenced memory storage. Neuroimage. 2003;20:2091–2099. doi: 10.1016/j.neuroimage.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Kim H, Somerville LH, Johnstone T, Polis S, Alexander AL, Shin LM, Whalen PJ. Contextual modulation of amygdala responsivity to surprised faces. J Cogn Neurosci. 2004;16:1730–1745. doi: 10.1162/0898929042947865. [DOI] [PubMed] [Google Scholar]
- Kondo H, Saleem KS, Price JL. Differential connections of the temporal pole with the orbital and medial prefrontal networks in macaque monkeys. J Comp Neurol. 2003;465:499–523. doi: 10.1002/cne.10842. [DOI] [PubMed] [Google Scholar]
- Krettek JE, Price JL. Projections from the amygdaloid complex and adjacent olfactory structures to the entorhinal cortex and to the subiculum in the rat and cat. Journal of Comparative Neurology. 1977;172:723–752. doi: 10.1002/cne.901720409. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion: clues from the brain. Annual Review of Psychology. 1995;46:209–235. doi: 10.1146/annurev.ps.46.020195.001233. [DOI] [PubMed] [Google Scholar]
- Malkova L, Mishkin M, Bachevalier J. Long-term effects of selective neonatal temporal lobe lesions on learning and memory in monkeys. Behavioral Neuroscience. 1995;109:212–226. doi: 10.1037//0735-7044.109.2.212. [DOI] [PubMed] [Google Scholar]
- Markowitsch HJ, Emmans D, Irle E, Streicher M, Preilowski B. Cortical and subcortical afferent connections of the primate’s temporal pole: a study of rhesus monkeys, squirrel monkeys, and marmosets. Journal of Comparative Neurology. 1985;242:425–458. doi: 10.1002/cne.902420310. [DOI] [PubMed] [Google Scholar]
- McCaugh JL, Ferry B, Vazdarjanova A, Roozendaal B. Amygdala: role in modulation of memory storage. In: Aggleton JP, editor. The Amygdala. A functional analysis. 2. Oxford Univ. Press book; 2000. [Google Scholar]
- McDonald AJ. Cortical pathways to the mammalian amygdala. Progress in Neurobiology. 1998;55:257–332. doi: 10.1016/s0301-0082(98)00003-3. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Jackson TR. Amygdaloid connections with posterior insular and temporal cortical areas in the rat. J Comp Neurol. 1987;262:59–77. doi: 10.1002/cne.902620106. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Make mild moments memorable: add a little arousal. Trends Cogn Sci. 2006;10:345–347. doi: 10.1016/j.tics.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. I: Architectonics in the insulo- orbito-temporal component of the paralimbic brain. Journal of Comparative Neurology. 1982a;212:1–22. doi: 10.1002/cne.902120102. [DOI] [PubMed] [Google Scholar]
- Mesulam MM, Mufson EJ. Insula of the old world monkey. III: Efferent cortical output and comments on function. Journal of Comparative Neurology. 1982b;212:38–52. doi: 10.1002/cne.902120104. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M, Murray EA. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. Journal of Neuroscience. 1993;13:5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran MA, Mufson EJ, Mesulam MM. Neural inputs into the temporopolar cortex of the rhesus monkey. Journal of Comparative Neurology. 1987;256:88–103. doi: 10.1002/cne.902560108. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM. Insula of the old world monkey. II. Afferent cortical input and comments on the claustrum. Journal of Comparative Neurology. 1982;212:23–37. doi: 10.1002/cne.902120103. [DOI] [PubMed] [Google Scholar]
- Mufson EJ, Mesulam MM, Pandya DN. Insular interconnections with the amygdala in the rhesus monkey. Neuroscience. 1981;6:1231–1248. doi: 10.1016/0306-4522(81)90184-6. [DOI] [PubMed] [Google Scholar]
- Murray EA, Bussey TJ. Perceptual-mnemonic functions of the perirhinal cortex. Trends Cogn Sci. 1999;3:142–151. doi: 10.1016/s1364-6613(99)01303-0. [DOI] [PubMed] [Google Scholar]
- Nauta WJH. Neural associations of the amygdaloid complex in the monkey. Brain. 1962;85:505–520. doi: 10.1093/brain/85.3.505. [DOI] [PubMed] [Google Scholar]
- Nauta WJH. Neural associations of the frontal cortex. Acta Neurobiologiae Experimentalis. 1972;32:125–140. [PubMed] [Google Scholar]
- Olson IR, Plotzker A, Ezzyat Y. The Enigmatic temporal pole: a review of findings on social and emotional processing. Brain. 2007;130:1718–1731. doi: 10.1093/brain/awm052. [DOI] [PubMed] [Google Scholar]
- Ottersen OP. Connections of the amygdala of the rat IV: corticoamygdaloid and intraamygdaloid connections as studied with axonal transport of horseradish peroxidase. Journal of Comparative Neurology. 1982;205:30–48. doi: 10.1002/cne.902050104. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Kuypers HGJM. Cortico-cortical connections in the rhesus monkey. Brain Reserch. 1969;13:13–36. doi: 10.1016/0006-8993(69)90141-3. [DOI] [PubMed] [Google Scholar]
- Pandya DN, Sanides F. Architectonic parcellation of the temporal operculum in rhesus monkey and its projection pattern. Z Anat Entwickl-Gesch. 1973;139:127–161. doi: 10.1007/BF00523634. [DOI] [PubMed] [Google Scholar]
- Paz R, Pelletier JG, Bauer EP, Pare D. Emotional enhancement of memory via amygdala-driven facilitation of rhinal interactions. Nature Neuroscience. 2006;9:1321–1329. doi: 10.1038/nn1771. [DOI] [PubMed] [Google Scholar]
- Pelletier JG, Apergis J, Pare D. Low-probability transmission of neocortical and entorhinal impulses through the perirhinal cortex. J Neurophysiol. 2004;91:2079–2089. doi: 10.1152/jn.01197.2003. [DOI] [PubMed] [Google Scholar]
- Pelletier JG, Apergis-Schoute J, Pare D. Interaction between amygdala and neocortical inputs in the perirhinal cortex. J Neurophysiol. 2005;94:1837–1848. doi: 10.1152/jn.00260.2005. [DOI] [PubMed] [Google Scholar]
- Petrovich GD, Risold PY, Swanson LW. Organization of projections from the basomedial nucleus of the amygdala: a PHAL study in the rat. Journal of Comparative Neurology. 1996;374:387–420. doi: 10.1002/(SICI)1096-9861(19961021)374:3<387::AID-CNE6>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Phillips ML, Young AW, Senior C, Brammer M, Andrew C, Calder AJ, Bullmore ET, Perrett DI, Rowland D, Williams SC, Gray JA, David AS. A specific neural substrate for perceiving facial expressions of disgust. Nature. 1997;389:495–498. doi: 10.1038/39051. [DOI] [PubMed] [Google Scholar]
- Pikkarainen M, Pitkanen A. Projections from the lateral, basal and accessory basal nuclei of the amygdala to the perirhinal and postrhinal cortices in rat. Cerebral Cortex. 2001;11:1064–1082. doi: 10.1093/cercor/11.11.1064. [DOI] [PubMed] [Google Scholar]
- Pitkanen A. Connectivity of the rat amygdaloid complex. In: Aggleton JP, editor. The Amygdala, A funtional analysis. 2. Oxford Univ. Press book; 2000. [Google Scholar]
- Pitkanen A, Jolkkonen E, Kemppainen S. Anatomic heterogeneity of the rat amygdaloid complex. Folia Morphol (Warsz) 2000a;59:1–23. [PubMed] [Google Scholar]
- Pitkanen A, Kelly JL, Amaral DG. Projections from the lateral, basal, and accessory basal nuclei of the amygdala to the entorhinal cortex in the macaque monkey. Hippocampus. 2002;12:186–205. doi: 10.1002/hipo.1099. [DOI] [PubMed] [Google Scholar]
- Pitkanen A, Pikkarainen M, Nurminen N, Ylinen A. Reciprocal connections between the amygdala and the hippocampal formation, perirhinal cortex, and postrhinal cortex in rat. A review. Annals of the New York Academy of Sciences. 2000b;911:369–391. doi: 10.1111/j.1749-6632.2000.tb06738.x. [DOI] [PubMed] [Google Scholar]
- Poremba A, Malloy M, Saunders RC, Carson RE, Herscovitch P, Mishkin M. Species-specific calls evoke asymmetric activity in the monkey’s temporal poles. Nature. 2004;427:448–451. doi: 10.1038/nature02268. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Crane AM, Goldman-Rakic PS. Direct and indirect pathways from the amygdala to the frontal lobe in rhesus monkeys. Journal of Comparative Neurology. 1981;198:121–136. doi: 10.1002/cne.901980111. [DOI] [PubMed] [Google Scholar]
- Pribram KH, MacLean PD. Neuronographic analysis of medial and basal cerebral cortex. II. Monkey. J Neurophysiol. 1953;16:324–340. doi: 10.1152/jn.1953.16.3.324. [DOI] [PubMed] [Google Scholar]
- Price JL, Amaral DG. An autoradiographic study of the projections of the central nucleus of the monkey amygdala. Journal of Neuroscience. 1981;1:1242–1259. doi: 10.1523/JNEUROSCI.01-11-01242.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price JL, Russchen FT, Amaral DG. The limbic region. II. The amygdaloid complex. In: Björklund A, Hökfelt T, Swanson LW, editors. Handbook of Chemical Neuroanatomy. Vol 5, Integrated Systems of the CNS, Part I. Elsevier; Amsterdam: 1987. pp. 279–381. [Google Scholar]
- Rempel-Clower NL, Barbas H. The laminar pattern of connections between prefrontal and anterior temporal cortices in the rhesus monkey is related to cortical structure and function. Cerebral Cortex. 2000;10:851–865. doi: 10.1093/cercor/10.9.851. [DOI] [PubMed] [Google Scholar]
- Russchen FT. Amygdalopetal projections in the cat. I. Cortical afferent connections. A study with retrograde and anterograde tracing techniques. Journal of Comparative Neurology. 1982;206:159–179. doi: 10.1002/cne.902060206. [DOI] [PubMed] [Google Scholar]
- Saleem KS, Price JL, Hashikawa T. Cytoarchitectonic and chemoarchitectonic subdivisions of the perirhinal and parahippocampal cortices in macaque monkeys. JComp Neurol. 2007;500:973–1006. doi: 10.1002/cne.21141. [DOI] [PubMed] [Google Scholar]
- Sander K, Scheich H. Left auditory cortex and amygdala, but right insula dominance for human laughing and crying. J Cogn Neurosci. 2005;17:1519–1531. doi: 10.1162/089892905774597227. [DOI] [PubMed] [Google Scholar]
- Saper CB. Convergence of autonomic and limbic connections in the insular cortex of the rat. Journal of Comparative Neurology. 1982;210:163–173. doi: 10.1002/cne.902100207. [DOI] [PubMed] [Google Scholar]
- Seltzer B, Pandya DN. Afferent cortical connections and architectonics of the superior temporal sulcus and surrounding cortex in the rhesus monkey. Brain Reserch. 1978;149:1–24. doi: 10.1016/0006-8993(78)90584-x. [DOI] [PubMed] [Google Scholar]
- Simons JS, Spiers HJ. Prefrontal and medial temporal lobe interactions in long-term memory. Nat Rev Neurosci. 2003;4:637–648. doi: 10.1038/nrn1178. [DOI] [PubMed] [Google Scholar]
- Squire LR, Zola-Morgan S. The medial temporal lobe memory system. Science. 1991;253:1380–1386. doi: 10.1126/science.1896849. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Amaral DG. Topographic organization of cortical inputs to the lateral nucleus of the macaque monkey amygdala: a retrograde tracing study. Journal of Comparative Neurology. 2000;421:52–79. doi: 10.1002/(sici)1096-9861(20000522)421:1<52::aid-cne4>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Amaral DG. Some observations on cortical inputs to the macaque monkey amygdala: an anterograde tracing study. J Comp Neurol. 2002;451:301–323. doi: 10.1002/cne.10339. [DOI] [PubMed] [Google Scholar]
- Steward O, Scoville SA. Cells of origin of entorhinal cortical afferents to the hippocampus and fascia dentata of the rat. Journal of Comparative Neurology. 1976;169:347–370. doi: 10.1002/cne.901690306. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: Cortical afferents. Journal of Comparative Neurology. 1994;350:497–533. doi: 10.1002/cne.903500402. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: cytoarchitectonic and chemoarchitectonic organization. J Comp Neurol. 2003a;463:67–91. doi: 10.1002/cne.10744. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Where are the perirhinal and parahippocampal cortices? A historical overview of the nomenclature and boundaries applied to the primate medial temporal lobe. Neuroscience. 2003b;120:893–906. doi: 10.1016/s0306-4522(03)00281-1. [DOI] [PubMed] [Google Scholar]
- Suzuki WA, Zola-Morgan S, Squire LR, Amaral DG. Lesions of the perirhinal and parahippocampal cortices in the monkey produce long-lasting memory impairment in the visual and tactual modalities. Journal of Neuroscience. 1993;13:2430–2451. doi: 10.1523/JNEUROSCI.13-06-02430.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner BH, Mishkin M, Knapp M. Organization of the amygdalopetal projections from modality-specific cortical association areas in the monkey. Journal of Comparative Neurology. 1980;191:515–543. doi: 10.1002/cne.901910402. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW. The differential distribution, diversity and sprouting of cortical projections to the amygdala of the rhesus monkey. In: Ben-Ari Y, editor. The Amygdaloid complex. Elsevier/North Holland Biomedical Press; Amsterdam: 1981. pp. 77–90. [Google Scholar]
- Van Hoesen GW, Pandya DN. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. I. Temporal lobe afferents. Brain Reserch. 1975a;95:1–24. doi: 10.1016/0006-8993(75)90204-8. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Pandya DN. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. III. Efferent connections. Brain Reserch. 1975b;95:39–59. doi: 10.1016/0006-8993(75)90206-1. [DOI] [PubMed] [Google Scholar]
- Van Hoesen GW, Pandya DN, Butters N. Some connections of the entorhinal (area 28) and perirhinal (area 35) cortices of the rhesus monkey. II. Frontal lobe afferents. Brain Reserch. 1975;95:25–38. doi: 10.1016/0006-8993(75)90205-x. [DOI] [PubMed] [Google Scholar]
- Von Bonin G, Bailey P. The Neocortex of Macaca mulatta. The University of Illinois Press; Urbana: 1947. [Google Scholar]
- West MJ, Slomianka L, Gundersen HJG. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anatomical Record. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Wicker B, Keysers C, Plailly J, Royet JP, Gallese V, Rizzolatti G. Both of us disgusted in My insula: the common neural basis of seeing and feeling disgust. Neuron. 2003;40:655–664. doi: 10.1016/s0896-6273(03)00679-2. [DOI] [PubMed] [Google Scholar]
- Williams RW, Rakic P. Three-dimensional counting: An accurate and direct method to estimate numbers of cells in sectioned material. Journal of Comparative Neurology. 1988;278:344–352. doi: 10.1002/cne.902780305. [DOI] [PubMed] [Google Scholar]
- Witter MP, Amaral DG. Entorhinal cortex of the monkey: V. Projections to the dentate gyrus, hippocampus, and subicular complex. Journal of Comparative Neurology. 1991;307:437–459. doi: 10.1002/cne.903070308. [DOI] [PubMed] [Google Scholar]
- Witter MP, Van Hoesen GW, Amaral DG. Topographical organization of the entorhinal projection to the dentate gyrus of the monkey. Journal of Neuroscience. 1989;9:216–228. doi: 10.1523/JNEUROSCI.09-01-00216.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH. The human amygdala and the emotional evaluation of sensory stimuli. Brain Res Brain Res Rev. 2003;41:88–123. doi: 10.1016/s0165-0173(02)00248-5. [DOI] [PubMed] [Google Scholar]
- Zola-Morgan S, Squire LR, Amaral DG, Suzuki WA. Lesions of perirhinal and parahippocampal cortex that spare the amygdala and hippocampal formation produce severe memory impairment. Journal of Neuroscience. 1989;9:4355–4370. doi: 10.1523/JNEUROSCI.09-12-04355.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]