Abstract
Ca2+-induced inhibition of α1C voltage-gated Ca2+ channels is a physiologically important regulatory mechanism that shortens the mean open time of these otherwise long-lasting high-voltage-activated channels. The mechanism of action of Ca2+ has been a matter of some controversy, as previous studies have proposed the involvement of a putative Ca2+-binding EF hand in the C terminus of α1C and/or a sequence downstream from this EF-hand motif containing a putative calmodulin (CaM)-binding IQ motif. Previously, using site directed mutagenesis, we have shown that disruption of the EF-hand motif does not remove Ca2+ inhibition. We now show that the IQ motif binds CaM and that disruption of this binding activity prevents Ca2+ inhibition. We propose that Ca2+ entering through the voltage-gated pore binds to CaM and that the Ca/CaM complex is the mediator of Ca2+ inhibition.
Keywords: heart, EF hand, IQ motif
Ca2+ entering through the cardiac voltage-gated Ca2+ channel is an essential event for the contraction of the heart muscle in response to a depolarizing stimulus. The opening of this channel is evident in the shape of the cardiac action potential and is responsible for the broad shoulder in its down stroke. The cardiac voltage-gated Ca2+ channel is of the long-lasting or L-type, showing only a minor voltage-induced inactivation component. Molecularly, it is made up of an α1 subunit, α1C, which is the pore-forming subunit proper, and β and α2δ regulatory or accessory subunits. The cardiac form of α1C (1), which is expressed also in smooth muscle (2) and neurons (3), displays inhibition by incoming Ca2+ (4), although there are certain neuronal splice variants that are inhibited (5). In the heart, Ca2+-induced inhibition insures an inactivation rate compatible with the length of the Ca2+-mediated plateau phase in the action potential, which would be predicted to be much longer if inactivation proceeded according to the inactivation kinetics seen with Ba2+ as the charge carrier. This fact places Ca2+-induced inhibition of the cardiac Ca2+ channel at the center of the mechanisms that operate to insure coordinated contraction and relaxation cycles of the heart muscle fiber.
Kinetic arguments have placed the site of action of Ca2+ very close to the conduction pathway, but the location of this site has remained unknown and is subject to some controversy. de Leon et al. (6) focused on the existence of a Ca2+-binding consensus sequence, an EF hand, near the beginning of the 660-aa C-terminal tail of α1C and tested for its participation in Ca2+ inhibition. They found that, although replacing the α1C EF hand with a homologous but less perfect EF hand from the Ca2+-insensitive α1E resulted in the loss of Ca2+ inhibition, the introduction into α1E of a 250-aa α1C segment, which included the 29-aa EF-hand motif, conferred Ca2+ sensitivity to the Ca2+-insensitive α1E. This result led them to propose this motif as the site to which Ca2+ binds to inhibit channel activity. We analyzed α1E/α1C chimeras, as did de Leon et al. (6), but we subdivided the transferred segments further and were unable to substantiate their proposal. Instead, we pinpointed a shorter amino acid segment located downstream of the EF hand as essential for Ca2+ inhibition (7). This segment contains 144 aa and was designated RL–VS, denoting the beginning and ending amino acids. Experiments in which we tested for direct binding of 45Ca2+ to the segment of α1C that was able to confer Ca2+ sensitivity to α1E were unsuccessful (N.Q. and L.B., unpublished results), leaving open the question as to how this segment conferred Ca2+ sensitivity to the channel and whether Ca2+ acted on the channel directly or indirectly.
Based on analysis of neuronal α1C splice variants for their voltage- and Ca2+-dependent inactivation and on properties of artificial deletion mutants, Reuter and coworkers (5, 8) concluded that Ca2+-induced inhibition of α1C depends on three amino acid sequences: (i) the EF-hand motif, (ii) two hydrophilic residues (N and E) 77–78 aa downstream of the EF hand, and, (iii) 40 aa downstream from these, an 8-aa sequence with an IQ motif. This last is a putative calmodulin (CaM)-binding motif located approximately in the middle of the RL–VS sequence. Zuehlke et al. (8) identified the three relevant sequences by the loss of function after their excision. In our previous studies, amino acid replacements within the EF-hand motif, which eliminated the motif but kept relative distances of the connected sequences undisturbed, preserved Ca2+ inhibition. This result led us to rule out the actual participation of the EF hand in Ca2+ inhibition. For this study, we directly tested the hypothesis that CaM binding to the IQ motif within the RL–VS sequence of α1C mediates Ca2+ inhibition. Here, we report that indeed RL–VS binds the Ca/CaM complex, whereas fragments of α1C without the IQ motif do not. Disruption of CaM binding by site-directed mutagenesis prevents Ca2+-mediated inhibition.
MATERIALS AND METHODS
Channel Expression in Xenopus Oocytes
The cDNAs encoding β2a, α2δ, and DN 60 (α1C lacking amino acids 2–60), have been described (9–11), as have the methods for the preparation of cRNAs, the expression of these cRNAs in Xenopus oocytes, and the electrophysiological recording techniques (12–14).
Manipulation of cDNAs and Construction of Expression Vectors
The standard molecular-biology techniques that we (7, 11) and others (15) have described were used throughout. The nucleotide compositions of the final constructs were confirmed by double sequencing of double-stranded DNA by using the dideoxy chain-termination method (16).
Protein–Protein Interaction Tests
Glutathione S-Transferase (GST)-CaM Fusion Protein.
The GST-CaM fusion plasmid was based on pGEX-4T-1 (Amersham Pharmacia) and was constructed by fusing the C terminus of GST to the N terminus of CaM. After transfection into Escherichia coli BL21, synthesis of the fusion protein was induced with 0.2 mM isopropyl β-d-thiogalactoside in a liquid culture grown to OD at 1.0 nm. After 2–3 h at 37°C, the cells were collected by centrifugation, resuspended in NETN lysis buffer (0.5% Nonidet P-40/1 mM EDTA/20 mM Tris⋅HCl, pH 8.0/100 mM NaCl; 1.0 ml of buffer per 20 ml of culture), and lysed by sonication. The lysate was cleared by centrifugation at 10,000 × g for 10 min at 4°C. GST-CaM in the supernatant was adsorbed for 30 min at room temperature to Agarose-glutathione (GSH) beads (Amersham Pharmacia) [1 vol of lysate/1 vol of 50% (vol/vol) slurry of Agarose-GSH beads in NETN]. Finally, the beads were washed with binding buffer A (20 mM Tris⋅HCl, pH 7.5/100 mM NaCl/0.5% Triton X-100).
Synthesis of 35S-Labeled α1C Fragments by in Vitro Translation.
35S-labelled forms of α1C fragments having the compositions given in Results (see also Figs. 1–4) were synthesized with the TNT (transcription/translation) Coupled Rabbit Reticulocyte Lysate System (Promega) in the presence of [35S]methionine following manufacturer’s protocols. Aliquots of the incubation mixtures were used directly either for analysis by SDS/PAGE to confirm synthesis of proteins of the desired size or for the ability of the newly synthesized proteins to bind to GST or GST-CaM.
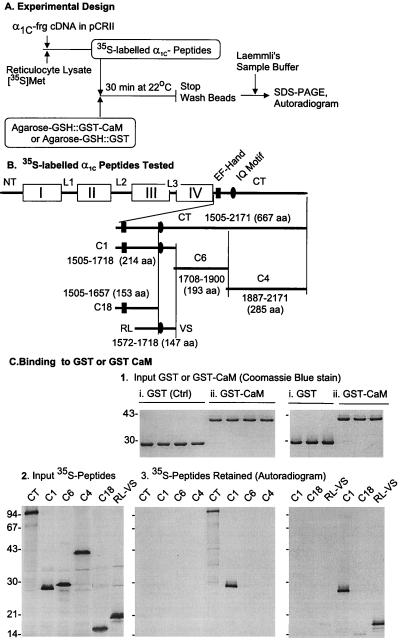
Figure 1.
Identification of the RL–VS segment of the α1C C terminus as an attachment site for CaM. (A) The strategy of the experiments. frg, fragment; [35S]Met, [35S]methionine. (B) Diagram of main structural motifs of α1C and placement of C-terminal fragments in relation to each other. I–IV, homologous repeat sequences forming transmembrane domains of the Ca2+ channel; NT, N terminus; L1–L3, connecting loops; CT, C terminus. (C) Only RL–VS containing fragments of the C terminus bind CaM. Molecular mass markers are expressed in kDa. (C, 1) Input GST or GST-CaM visualized by Coomassie blue staining of polyacrylamide gels resulting from the electrophoresis of proteins eluted from Agarose-GSH∷GST or GST-CaM that had been incubated with [35S]α1C fragments (partial view). (C, 2) Autoradiogram of SDS/PAGE analysis of in vitro translated α1C fragments tested for CaM-binding activity. (C, 3) Autoradiogram of the gel shown in C, 1.
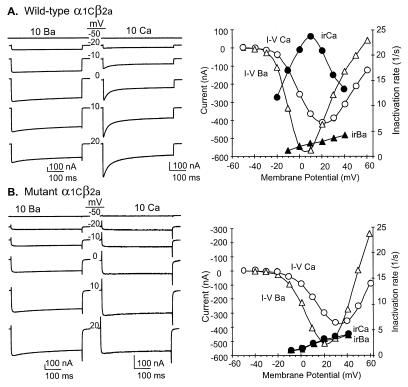
Figure 4.
Loss of Ca2+-mediated inactivation in mutant α1C, which is unable to bind CaM. β2a and either wild-type α1C (α1C[1,654-IQEYFRKFKKRK-1,665]) or mutant α1C (α1C[1,654-IQEYFEEFEEEE-1,665]) were coexpressed in Xenopus oocytes, and their activation and inactivation in the presence of either 10 mM Ca2+ or 10 mM Ba2+ as charge carriers were measured at the indicated test potentials. (A) Activation/inactivation time courses. Test potentials were set to the indicated voltages from a holding potential of −90 mM with either 10 mM Ba2+ or 10 mM Ca2+. (B) Current–voltage (I–V) and inactivation rate–voltage (ir-V) relations. Peak current values and initial inactivation rates are shown as a function of test potentials. Time constants of inactivation in 10 mM Ba2+ were derived from fitting the decay phases of the time courses shown in A with a single-exponential decay function. The inactivation rate (irCa and irBa in the figure) is the inverse of the time constant of current decays. For wild-type α1Cβ2a, the time constants in 10 mM Ca2+ were obtained from double-exponential fits. The fast component of inactivation was used for the inactivation rate. For mutant α1Cβ2a, the time constants in 10 mM Ca2+ were obtained from single-exponential fits. For wild type, the results from a single representative oocyte are shown. For mutant α1C, the means of seven oocytes from two frogs were averaged for both the 10 mM Ca2+ and the 10 mM Ba2+ conditions to give the I–V and inactivation rate–voltage relations shown. SEMs of inactivation rates were smaller than the symbols used to depict the inactivation rates on the figure. Error bars of I–V curves are not shown.
Protein–Protein Interactions.
Slurries of Agarose-GSH beads [50% (vol/vol)] with ≈1 μg of GST or GST-CaM were incubated for 30 min at room temperature in a final volume of 200 μl of binding buffer A with 1 mM CaCl2 and 10 μl of lysate that had been incubated with a template for the synthesis of α1C fragments and contained 0.3–0.5 fmol of these fragments (the final concentration in the presence of GST or GST-CaM was 3–5 nM). At the end of the incubations, the beads were washed three times with 1.0 ml of binding buffer, resuspended in 15 μl of 2× Laemmli’s sample buffer, and analyzed for retention by the GST-CaM fusion protein bound to Agarose-GSH with either a scintillation counter or by SDS/PAGE followed by autoradiography.
Calcium Buffering and Calculation of Free Ca2+ Ion
For incubations in which free Ca2+ was varied, Agarose-GSH∷GST-CaM beads first were equilibrated in binding buffers with the desired concentrations of free Ca2+ ion, resuspended as a 50% (vol/vol) slurry, and then mixed with 1 volume of reticulocyte lysate incubates (40–60 nM α1C C-terminal RL–VS peptide) and 18 volumes of binding buffer B (0.5% Triton X-100, 2.5 or 5.0 mM EGTA/CaCl2 mixtures to give the indicated concentration of free Ca2+, 100 mM NaCl, and 50 mM Bistris propane {1,3-bis[tris(hydroxymethyl)methylamino]propane, Sigma}, pH 7.0). The final pH was adjusted again to 7.0 with 1 M NaOH after adding the required amounts of CaCl2. Free Ca2+ was calculated as described for free Mg2+ (17) by using the following equilibrium association constants: pKa EGTA4−⋅Ca2+/CaEGTA2− = 11.00 and pK1 to pK4 = 2.0, 2.68, 8.85, and 9.43, respectively (18). Taking into consideration that only EGTA4− chelates Ca2+, the corrected affinity constant of EGTA for Ca2+ at any given pH, K′a[pH] is calculated by applying the formula K′a = Ka/(1 + H/K1 + H2/K1K2 + H3/K1K2K3 + H4/K1K2K3K4), where −log values of Ka and K1 to K4 were given above and H denotes the concentration of H+. At pH 7.0, pK′a for the EGTA–Ca2+ interaction is 6.91. This value corresponds to an equilibrium dissociation constant (K′d) of 123 nM. The affinity of EGTA for Ca2+ at pH 7.0 was validated experimentally by obtaining the same dependence on calculated free Ca2+ at two different total concentrations of EGTA (2.5 and 5.0 mM), requiring addition of different concentrations of CaCl2 to obtain the same Ca2+.
RESULTS
To test for the CaM-binding capacity of the IQ motif in RL–VS fragments of α1C, we first attempted to prepare a GST-(RL–VS) fusion protein with the intention of adsorbing it to Agarose-GSH for incubation with CaM. However, we found that fusion proteins of proximal α1C C-terminal fragments (i.e., fragments containing the first 200 aa after the last transmembrane segment of the channel), including GST-(RL–VS), are extremely insoluble under nondenaturing conditions. This fact made it impossible to study their CaM-binding activity. Therefore, we inverted the design of the experiment, fusing recombinant CaM to GST and testing whether GST-CaM would bind α1C fragments made in radioactive form by in vitro translation in reticulocyte lysates in the presence of [35S]methionine. Fig. 1 summarizes our findings, showing that although none of the α1C C-terminal fragments bind to GST, the complete α1C C terminus (α1C[1,505–2,171]) and fragments that contain the IQ motif bind to CaM. These fragments include RL–VS (α1C[1,572–1,718]). Fragments of the C terminus that did not harbor the IQ motif did not bind to GST-CaM. CaM binding was resistant to 500 mM KCl (data not shown).
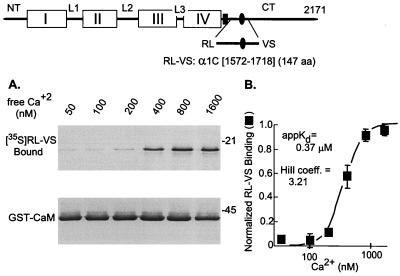
CaM binding to α1C fragments depended on not only the presence of the IQ motif in the peptide but also the concentration of Ca2+. As determined with RL–VS, half-maximal binding was obtained at 0.37 μM (Fig. 2) and was highly cooperative (Hill coefficient = 3.21). This result indicates that only CaM⋅Ca42+ binds to the channel peptide.
Figure 2.
Dependence of the CaM–(RL–VS) interaction on Ca2+. (Top) Diagram depicting placement of RL–VS within the α1C sequence. (A) Binding of [35S]RL–VS (Upper) to GST-CaM (Lower). (B) Average of four experiments, two at 2.5 mM total EGTA and two at 5 mM total EGTA. For quantification, the [35S]RL–VS bound to GST-CAM was counted in a liquid scintillation counter. Data were normalized to [35S]RL–VS bound at 1.6 μM Ca2+.
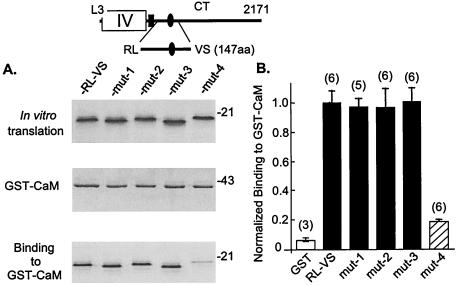
These results suggested that CaM may mediate the inhibitory action of Ca2+ on α1C. To substantiate this hypothesis, we mutated RL–VS to interfere with its CaM-binding activity and then tested whether loss of CaM binding would cause a functional correlate loss of Ca2+ inhibition. CaM binding was surprisingly resistant to amino acid substitutions in RL–VS. For example, changing IQ to AA, or IQEYFRK to AAAAAAA, had little if any effect (Fig. 2). CaM binding was reduced severely when IQEYFRKFKKRK was changed to IQEYFEEFEEEE, i.e., when a peptide in which the cluster of positively charged amino acids located after the IQ doublet was replaced with negatively charged amino acids (Fig. 3). Fig. 4 shows that loss of CaM binding led to a loss of inhibition of the channel by the incoming Ca2+.
Figure 3.
Binding of RL–VS and mutant forms of RL–VS to GST-CaM. RL–VS (α1C[1,572–1,718]) contains α1C[1,654-IQEYFRKFKKRK-1,665]. mut-1, RL–VS with IQ → AA; mut-2, RL–VS with IQEYFR → IQEYFE; mut-3, RL–VS with IQEYFRK → AAAAAAA; mut-4, RL–VS with IQEYFRKFKKRK → IQEYFEEFEEEE. (A) Representative result. (B) Normalized binding to GST-CaM averaged from the number of experiments shown on top of the bars.
DISCUSSION
Previous structure-function studies with α1C had pointed clearly to the proximal region of the C terminus as being important for Ca2+-induced inhibition of this channel. This deduction was made both from deletion (excision) experiments and, to a lesser extent, from amino acid replacement experiments. Our previous deletion studies (7) together with those of Zuehlke and Reuter (8) have shown that although the presence of a peptide backbone chain is required in the place of the EF hand, a putative Ca2+-binding function is not. It was determined that the region downstream of the EF hand is responsible for meditating Ca2+ inhibition by using a gain-of-function approach in which segments from the Ca2+-sensitive α1C were introduced into the Ca2+-insensitive α1E (7). The deletion mutants used by Zuehlke and Reuter (8) helped to narrow possible responsible amino acids to just a few, including a set that forms part of a consensus CaM-binding motif. We now have shown that the putative CaM-binding motif is real and that disruption of its CaM binding ability by amino acid replacement leads to loss of the inhibitory effect of Ca2+.
The Ca2+ dependence for CaM binding is surprising, however. To account for Ca2+ inhibition of the channel, the dependence of CaM binding on Ca2+ suggests a reaction sequence in which Ca2+ enters through the pore, as suggested by the relation between the initial rate of inactivation and the activating voltage (4, 14), and then binds to CaM. CaM, in turn, on changing its conformation, would bind to the channel and initiate the intramolecular process that culminates in inactivation. Can the time in which the fluxing Ca2+ leads to inactivation accommodate this multistep process that involves establishment of a protein–protein interaction? It is unlikely, given that Ca2+ inhibition is installed within the first millisecond of channel opening (14).
It follows that CaM binding to the channel should precede Ca2+ binding. Therefore, the CaM binding site uncovered in RL–VS should be “incomplete”. The binding of CaM to larger fragments of the C terminus, including the complete 667-aa C-terminal fragment, was also Ca2+-dependent. Thus, it would seem that in addition to the CaM-binding IQ motif of the C terminus identified here, there should exist one or more additional points of contact between the α1C Ca2+ channel and CaM to enable CaM to become the constitutive Ca2+-sensing subunit. The possibility must be considered as well that the binding site identified here bears no relation to the site of action of Ca2+, in spite of being a region of α1C that confers Ca2+ sensitivity to the Ca2+-insensitive α1E. The present study should allow for a more focused design for future experiments dealing with the molecular basis of Ca2+ inhibition of the cardiac α1C Ca2+ channel.
Acknowledgments
We thank John P. Adelman (Vollum Institute, Portland, OR) for pCaM[DEF2,3A] (19). L.B. thanks Roger Zuehlke (University of Bern, Bern, Switzerland) for an enlightening discussion that led to the experiments reported here. This work was supported in part by National Institutes of Health Grant AR43411 to L.B., by a National Institutes of Health National Research Service Award to N.Q., and by American Heart Association grants to R.O. and N.Q.
ABBREVIATIONS
- CaM
calmodulin
- GSH
glutathione
- GST
glutathione S-transferase
Note Added in Proof
Injection of cRNA encoding a mutant CaM partially defective in Ca2+ binding to its second and third EF hand (Cam[DEF2,3A]; ref. 19) caused marked loss of Ca2+ inhibition at α11C·β2a channels. This strongly argues for a prebound Ca2+ sensor and strengthens our argument that Ca2+ inhibition is mediated by CaM.
References
- 1.Mikami A, Imoto K, Tanabe T, Niidome T, Mori Y, Takeshima H, Narumiya S, Numa S. Nature (London) 1989;340:230–233. doi: 10.1038/340230a0. [DOI] [PubMed] [Google Scholar]
- 2.Biel M, Ruth P, Bosse E, Hullin R, Stühmer W, Flockerzi V, Hofmann F. FEBS Lett. 1990;269:409–412. doi: 10.1016/0014-5793(90)81205-3. [DOI] [PubMed] [Google Scholar]
- 3.Snutch T P, Tomlinson W J, Leonard J P, Gilbert M M. Neuron. 1991;7:45–57. doi: 10.1016/0896-6273(91)90073-9. [DOI] [PubMed] [Google Scholar]
- 4.Neely A, Olcese R, Wei X, Birnbaumer L, Stefani E. Biophys J. 1994;66:1895–1903. doi: 10.1016/S0006-3495(94)80983-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Soldatov N M, Zuehlke R D, Bouron A, Reuter H. J Biol Chem. 1997;272:3560–3566. doi: 10.1074/jbc.272.6.3560. [DOI] [PubMed] [Google Scholar]
- 6.de Leon M, Wang Y, Jones L, Perez-Reyes E, Wei X, Soong T W, Snutch T P, Yue D T. Science. 1995;270:1502–1506. doi: 10.1126/science.270.5241.1502. [DOI] [PubMed] [Google Scholar]
- 7.Zhou J, Olcese R, Qin N, Noceti F, Birnbaumer L, Stefani E. Proc Natl Acad Sci USA. 1997;94:2301–2305. doi: 10.1073/pnas.94.6.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zuehlke R D, Reuter H. Proc Natl Acad Sci USA. 1998;95:3287–3294. doi: 10.1073/pnas.95.6.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wei X, Perez-Reyes E, Lacerda A E, Schuster G, Birnbaumer L, Brown A M. J Biol Chem. 1991;266:21943–21947. [PubMed] [Google Scholar]
- 10.Wei X, Neely A, Olcese R, Stefani E, Birnbaumer L. Recept Channels. 1996;4:205–215. [PubMed] [Google Scholar]
- 11.Qin N, Platano D, Olcese R, Stefani E, Birnbaumer L. Proc Natl Acad Sci USA. 1997;94:8866–8871. doi: 10.1073/pnas.94.16.8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taglialatela M, Stefani E. Proc Natl Acad Sci USA. 1993;90:4758–4762. doi: 10.1073/pnas.90.10.4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noceti F, Baldelli P, Wei X, Qin N, Toro L, Birnbaumer L, Stefani E. J Gen Physiol. 1996;108:143–155. doi: 10.1085/jgp.108.3.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Noceti F, Olcese R, Qin N, Zhou J, Stefani E. J Gen Physiol. 1998;111:463–475. doi: 10.1085/jgp.111.3.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 16.Sanger F, Nicklen S, Coulson A B. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Birnbaumer L, Desmier M, Stengel D, Hanoune J. Eur J Biochem. 1983;136:107–112. doi: 10.1111/j.1432-1033.1983.tb07712.x. [DOI] [PubMed] [Google Scholar]
- 18.Raaflaub J. Methods Biochem Anal. 1956;3:301–324. doi: 10.1002/9780470110195.ch10. [DOI] [PubMed] [Google Scholar]
- 19.Xia X-M, Falker B, Rivard A, Wayman G, Johnson-Pais T, Keen J E, Ishil T, Hirschberg B, Bond C T, Lutsenko S, et al. Nature (London) 1998;395:503–507. doi: 10.1038/26758. [DOI] [PubMed] [Google Scholar]