Abstract
Experiments were performed to determine whether exogenous L-arginine could ameliorate angiotensin II-induced hypertension and renal damage. Rats were instrumented with chronic indwelling femoral venous and arterial catheters for infusions of drugs and measurement of conscious arterial pressure. Arterial blood pressure significantly increased from 124±1 to 199±4 mmHg, following 9 days of continuous infusion of angiotensin II (20 ng/kg/min; i.v; n=6–9). In contrast, the increase in arterial pressure after 9 days of angiotensin II infusion was significantly blunted by 45% (P = 0.0003) in rats co-administered L-arginine (300 µg/kg/min; i.v; n=7–9). The glomerular injury index was significantly greater in rats administered angiotensin II in comparison to rats administered saline vehicle (P<0.001). Co-infusion of L-arginine significantly increased plasma nitrate/nitrite concentrations (P<0.001), and completely prevented angiotensin II-induced glomerular damage (P<0.001). Angiotensin II infusion alone, and combined angiotensin II plus L-arginine infusion significantly increased urinary albumin excretion. Albuminuria in rats administered angiotensin II plus L-arginine is likely to be due to increased intraglomerular pressure. Our experiments demonstrate that L-arginine can blunt angiotensin II induced hypertension and associated renal damage. This latter observation is most exciting as it indicates that increasing nitric oxide bioavailability, in addition to lowering arterial pressure, can greatly reduce hypertension induced renal damage.
Keywords: Hypertension, angiotensin II, L-arginine, nitric oxide, kidney
INTRODUCTION
Angiotensin II is a potent vasoconstrictor and has been shown to cause hypertension when infused chronically into the systemic circulation 1, 2. This model of hypertension closely mimics increased intra-renal angiotensin II formation, as well as high bioavailability of superoxide and low bioavailability of nitric oxide (NO) observed in human essential hypertension 3–5. Angiotensin II can stimulate the production of reactive oxygen species 6, 7, which act as NO scavengers 1, 8 and uncouple NO synthase 1. Uncoupled NO synthase produces superoxide creating a vicious cycle that further reduces NO bioavailability in hypertension. It has been suggested that reduced bioavailability of NO makes a major contribution to the development and maintenance of hypertension, and in particular associated target organ damage and endothelial dysfunction 9, 10 1. Thus, treatments that increase the bioavailability of NO should be able to interrupt the vicious cycle that maintains hypertension and associated end organ damage. In fact, there is evidence that antihypertensive treatments that increase NO bioavailability as opposed to those that merely lower arterial pressure, exert additional beneficial effects to hypertensive patients, particularly in preventing target organ damage 9. Many recent studies have focused on strategies to reduce NO inactivation by superoxide, as a means of increasing NO bioavailability in hypertension 6, 8, 11. Although the formation of NO equally affects its bioavailability, the potential beneficial effects of increasing NO formation in hypertension have received less attention.
L-arginine is the substrate for vascular NO formation. 12. The intracellular level of L-arginine (100– 3800 µM) in cultured endothelial cells 13 far exceeds the Michaelis-Menten constant (Km) of NO synthase for L-arginine (<5 µM) 14. The ‘L-arginine paradox’ is that endogenous NO formation is dependent on the extracellular L-arginine concentration despite the extremely high intracellular L-arginine levels 15–17. For example, in the isolated perfused kidney preparation, renal perfusion flow rate, glomerular filtration rate, urine flow and sodium reabsorption all decreased when L-arginine was eliminated from the perfusate 18. In anesthetized Sprague Dawley rats, L-arginine increased total renal blood flow 17, renal medullary perfusion and NO content 15. The results of this latter study also indicated that infusion of amino acids that compete with L-arginine for cellular uptake reduced renal medullary perfusion and NO content in anesthetized rats 15. The data of these functional studies indicate that extracellular concentrations of L-arginine and the uptake mechanisms that transport L-arginine into intracellular compartments play important roles in modulating NO formation in the kidney under normal physiological conditions.
Under conditions of hypertension, increasing L-arginine concentration enhances NO-dependent vasorelaxation and other indices of endothelial function 19 20–24. For example, in Dahl salt sensitive rats chronic oral, intravenous or medullary interstitial administration of L-arginine prevented sodium dependent hypertension 20–23, 25. L-arginine has also been shown to be beneficial in reducing arterial pressure and improving endothelial function in essential hypertensive patients with mild to moderate hypertension 26, 27. Together, these data indicate that L-arginine exerts beneficial effects in different forms of hypertension.
The aim of the current study was to determine whether exogenous L-arginine can interrupt the vicious cycle which reduces NO bioavailability in angiotensin II induced hypertension. We hypothesized that exogenous L-arginine can increase the bioavailability of NO and ameliorate angiotensin II dependent hypertension and kidney damage.
METHODS
Animals
Fourty three male Sprague-Dawley rats weighing between 300 to 332 g were used in this study. The animals were purchased from Harlan Sprague Dawley (Madison, WI), and housed in the Animal Resource Centre at the Medical College of Wisconsin. Rats were provided with food and water ad libitum. All experiments were conducted in accordance with the Medical College of Wisconsin Institutional Animal Care and Use Committee’s guidelines.
Surgical preparations
Rats were deeply anesthetized with an intraperitoneal injection of ketamine (35 mg/kg,), xylazine (10 mg/kg) and acepromazine (0.5 mg/kg) with supplemental anesthesia administered as required. Catheters were placed in the femoral artery and vein as we described previously28. After recovery from anesthesia rats were placed in individual stainless steel cages which permitted conscious blood pressure measurements (protocol 1) or blood and urine collection (Protocol 2).
Protocol 1
After a one week recovery period, blood pressure was measured from 10 am to 1 pm daily. After 3 days of control blood pressure measurements, rats were randomly divided into three groups. The first group of rats (n = 6–9) received intravenous infusions of angiotensin II (20 ng/kg/min) in saline vehicle (1 ml/hr), and the second group of rats (n = 7–9) received intravenous infusions of angiotensin II (Sigma Chemical Co; 20 ng/kg/min) and L-arginine (Sigma Chemical Co; 300 µg/kg/min). All these infusions were continued for 9 days. The third group of rats (n = 5) received intravenous infusions of angiotensin II (20 ng/kg/min) only during the first 7 days. An intravenous infusion of L-arginine (300 µg/kg/min) was also commenced on day 8. Both L-arginine and angiotensin II infusions were continued until day 16. This latter group of rats allowed us to determine whether L-arginine can reverse angiotensin II induced hypertension.
Protocol 2
Rats were allowed a one week period to recover from surgery. At the end of week one, rats were randomly divided into three treatment groups. The first group of rats received an intravenous infusion of saline vehicle (1 ml/hr) only (n =6). The second group of rats received intravenous infusions of angiotensin II (20 ng/kg/min) and saline (n=7) while the third group of rats received intravenous infusions of angiotensin II (20 ng/kg/min) plus L-arginine (300 µg/kg/min) (n=6). Urine samples were collected overnight from 4 pm to 8 am on the day before infusions commenced and also on the ninth (i.e last) day of the infusions. Blood samples were also collected on these days. Urinary albumin was quantified with Albumin Blue 580 dye (Molecular probes) and a fluorescent plate reader (FL600, Bio-Tek). The plasma samples were filtered and analyzed using a nitrate/nitrite fluorometric assay kit (Cayman, Ann Arbor, Michigan). The plates were read in a Synergy HT Spectrometer, using an excitation wavelength of 360–340 nm and an emission wavelength of 460–440 nm. At the end of the 9th day of infusions, the kidneys were collected for histological analysis as described below.
Histological analysis of kidney tissues
Rats were anesthetized with sodium pentobarbital (50 mg/kg, IP). Kidney tissues were prepared as we described previously 28. A Nikon E-400 fitted with a spot insight camera was used to photograph the slides. Digital micrographs were taken at different magnifications. Thirty to 40 glomeruli per rat were evaluated using the semi quantitative index method of Raij et al 29 and scored from 0 (best) to 4 (worst) on the basis of glomerulosclerosis and mesangial expansion as we described previously 30, 31.
Statistical Analysis
All data are presented as mean ± SEM. Unpaired or paired t-tests, and 2-way repeated measures ANOVAs with Tukey post hoc tests were used as appropriate, to evaluate the effects of saline vehicle, angiotensin II, L-arginine and combined angiotensin II plus L-arginine treatments.
RESULTS
Protocol 1
Conscious blood pressure measurements
Control MAP (before administration of any drugs) averaged 124 ± 1 mmHg, 115 ± 1 mmHg and 121 ± 1 mmHg in rats that subsequently received angiotensin II (20 ng/kg/min; IV) only (group 1), angiotensin II plus L-arginine (300 µg/kg/min; IV) (group 2), and angiotensin II and then L-arginine (group 3), respectively. Basal MAP was higher in the group of rats that received angiotensin II only, compared with the group of rats that received angiotensin II plus L-arginine (P = 0.03). In the group of rats that were administered angiotensin II only, 9 days of continuous angiotensin II infusion increased MAP from 124 ± 1 mmHg to 199 ± 4 mmHg (P = 0.005). In contrast, in the group of rats that were co-administered L-arginine, MAP only increased up to 149 ± 1 mmHg (P = 0.005) (Figure 1). During the last 5 days of the infusions, MAP increased by 62 ± 4 mmHg in the group of rats that received angiotensin II alone, and by 34 ± 1 mmHg in the group of rats that received angiotensin II plus L-arginine. Co-administration of L-arginine blunted hypertension by 45% (P = 0.0003). In the group of rats that were administered angiotensin II for seven days followed by angiotensin II plus L-arginine for an additional 9 days, MAP was significantly increased from control values of 121±1 to 170±1 by angiotensin II (P = 0.009). Subsequent co-administration of L-arginine for 9 days prevented a further increase in MAP (Figure 2).
Figure 1.
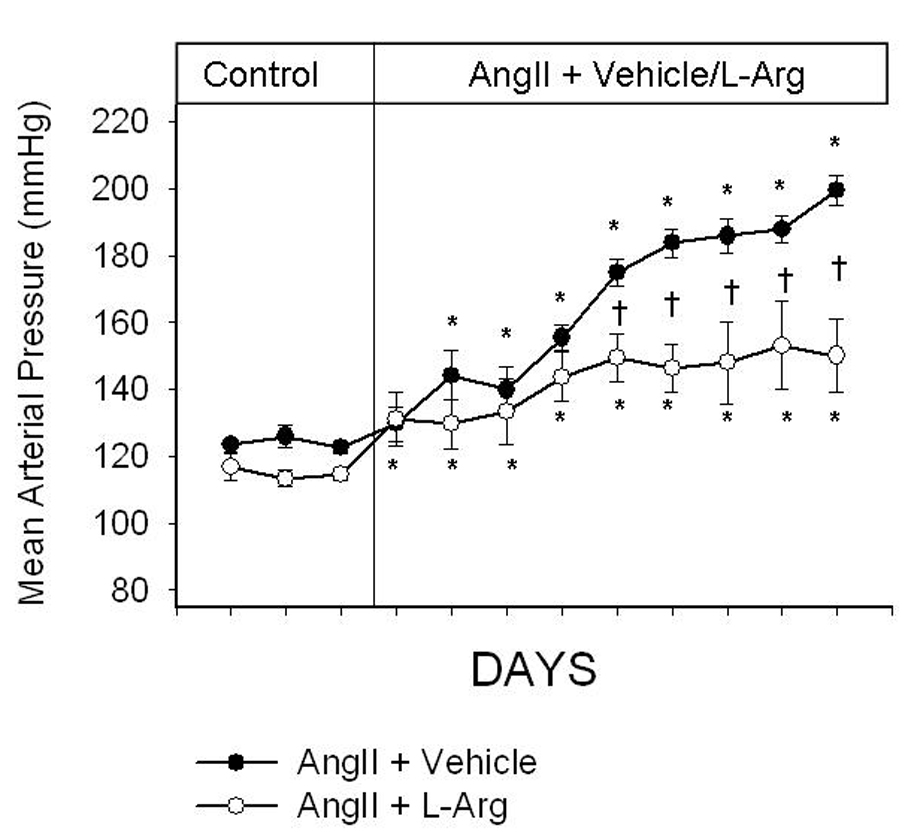
Daily conscious mean arterial pressure (MAP) in Sprague Dawley rats administered either angiotensin II (20 ng/kg/min; IV; n=6–9) or combined angiotensin II plus L-arginine (300 µg/kg/hr; IV; n=7–9) for 9 days. All infusions were administered into the femoral vein. Dotted line indicates the commencement of infusions. * indicates P<.05 from the final control day; † indicates P<.05 from the other group on the same day. (two-way ANOVA followed by a Holm-Sidak post-hoc test).
Figure 2.
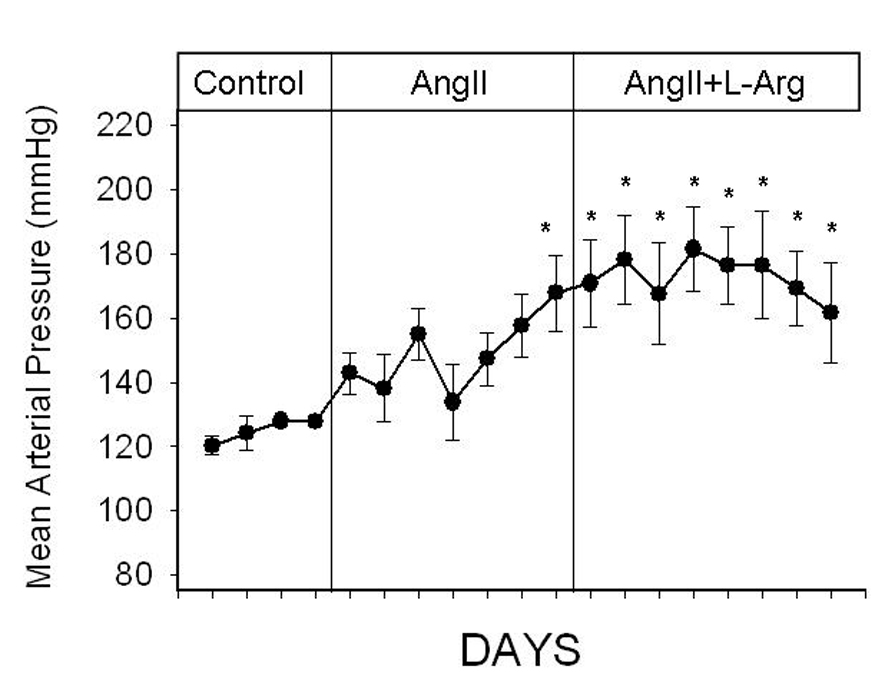
Daily conscious mean arterial pressure (MAP) in Sprague Dawley rats administered angiotensin II (20 ng/kg/min; IV; n=6) alone during the first 7 days and subsequently L-arginine (300 µg/kg/hr; IV; n=7–9) for the remaining 9 days. All infusions were administered into the femoral vein. Dotted line indicates the commencement of infusions. * indicates P<.05 from the the final control day. (by one-way ANOVA and Holm-Sidak test)
Protocol 2
Effects of angiotensin II and combined angiotensin II plus L-arginine treatments on glomerular damage
When compared to the kidneys of rats administered saline vehicle, there was significantly greater level of glomerular damage (represented by blue fibrotic tissue and collapsed capillary structure) in the kidneys of rats administered angiotensin II. However, co-infusion of L-arginine significantly and visibly reduced blue fibrotic tissue formation and also improved the collapsed nature of capillary structures in the glomeruli (Figure 3).
Figure 3.
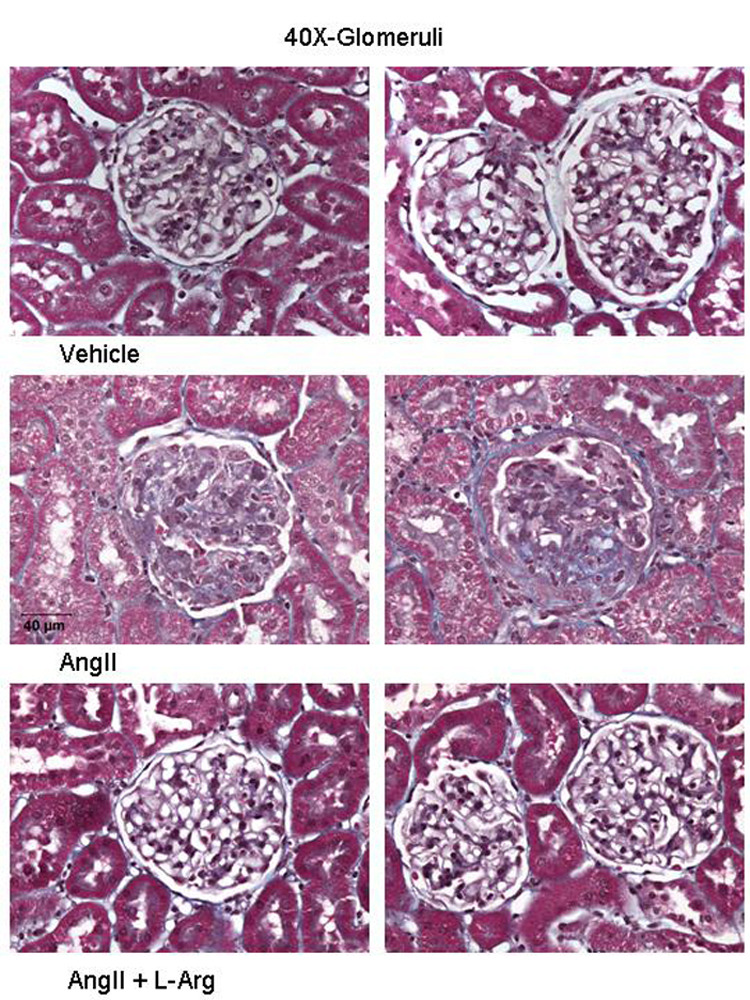
Light micrographs of glomeruli of the kidneys of Sprague Dawley rats administered saline vehicle (1ml/hr; n=6), angiotensin II (20ng/kg/min; n=7) or combined angiotensin II plus L-arginine (300 µg/kg/min; n=6) for 9 days. Glomerular sclerosis (blue fibrotic tissue and collapsed capillary structure) is present in the kidneys of rats administered angiotensin II. There is visibly less glomerular injury in rats co-administered L-arginine, compared with rats administered angiotensin II alone.
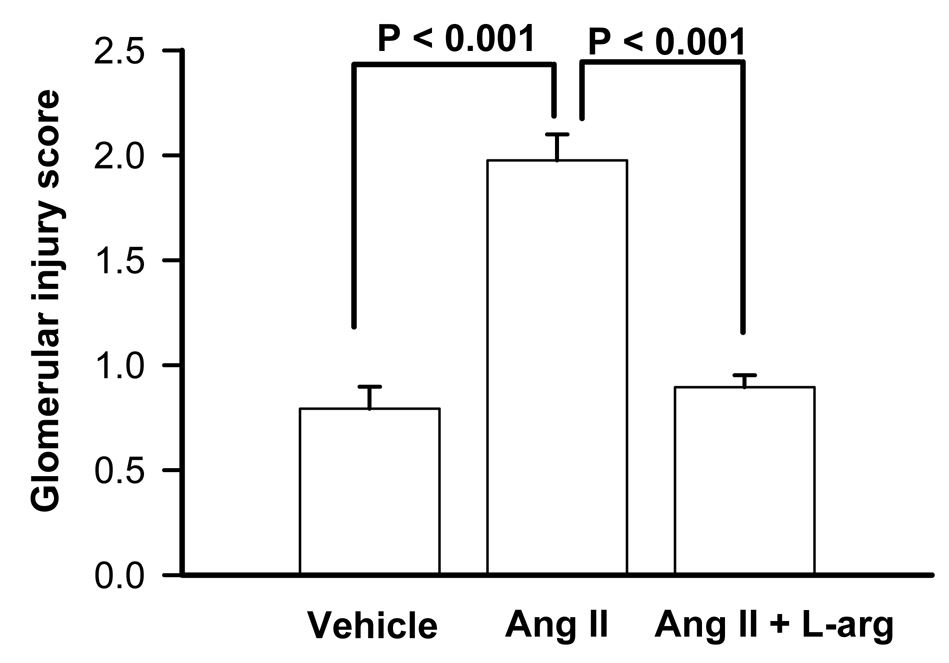
The glomerular injury score was 0.8 ± 0.05 in rats administered saline vehicle (n = 5). The glomerular injury index was significantly greater in rats administered angiotensin II when compared with the glomeruli of rats administered saline vehicle (P < 0.001). Co-infusion of L-arginine significantly reduced the glomerular injury score (P < 0.001) (Figure 4).
Figure 4.
Effects of continuous infusion of saline (1 ml/hr; n=6), angiotensin II (20 ng/kg/min; IV; n= 7) or angiotensin II plus L-arginine (300 µg/kg/min; n=6) for 9 consecutive days on glomerular injury score. P values indicate the outcomes of unpaired t-tests. ANG II; angiotensin II, L-Arg; L-arginine.
Effects of angiotensin II and combined angiotensin II plus L-arginine treatments on plasma nitrate/nitrite concentrations
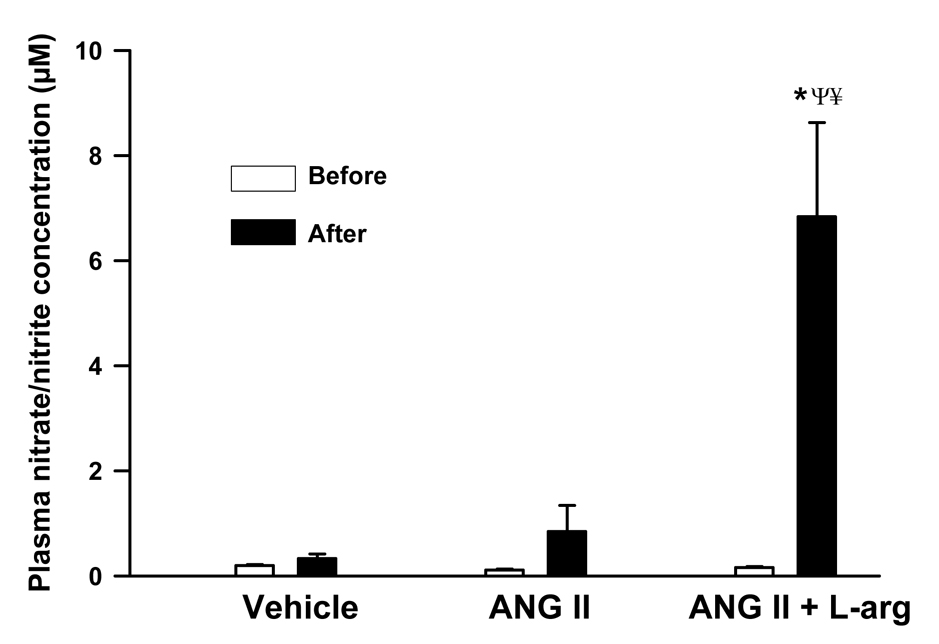
Control plasma nitrate/nitrite concentrations in rats administered saline (n = 6), angiotensin II (n=7) and combined angiotensin II plus L-arginine (n = 6) were 0.19 ± 0.02, 0.11 ± 0.02 and 0.16 ± 0.02 µM, respectively. The control plasma nitrate/nitrite concentrations were not significantly different between the three treatment groups (P = 0.07). Saline or angiotensin II infusion had little effect on plasma nitrate/nitrite concentrations. In contrast, co-infusion of L-arginine significantly increased plasma nitrate/nitrite concentrations to 7 ± 2 µM (P < 0.001) (Figure 5).
Figure 5.
Plasma nitrate/nitrite concentrations (µM) in Sprague Dawley rats administered saline vehicle (1 ml/hr; n = 6), angiotensin II (20 ng/kg/min; n = 7) or angiotensin II plus L-arginine (300 µg/kg/min; n = 6). All infusions were administered into the femoral vein. P values indicate the outcomes of 2-way repeated measures ANOVA with Tukey post hoc tests. * indicates P < 0.05 vs. before treatment in the same group of rats. Ψ indicates P < 0.05 vs rats administered saline vehicle. ¥ indicates P < 0.05 vs rats administered angiotensin II.
Effects of angiotensin II and combined angiotensin II plus L-arginine on albumin excretion
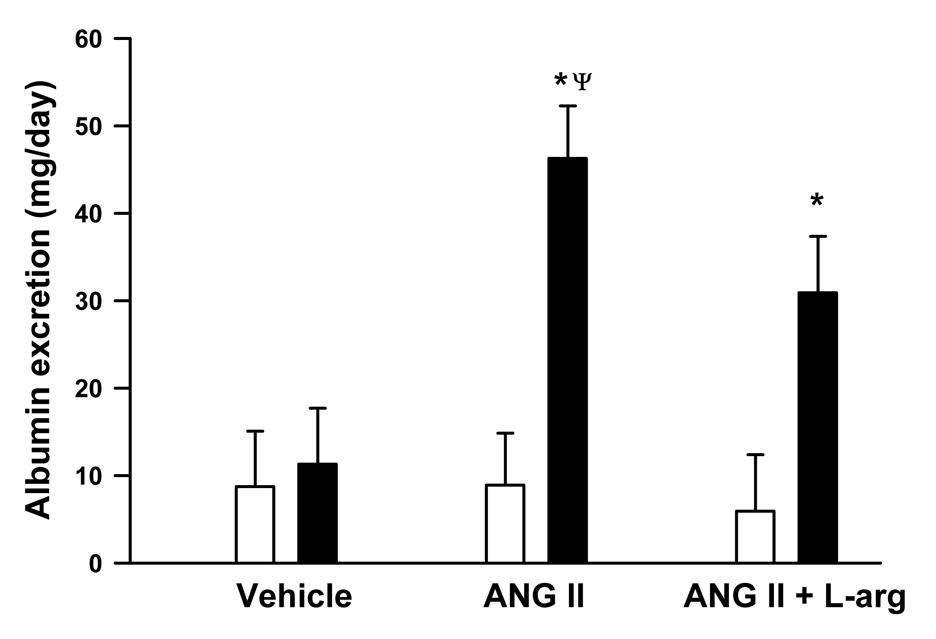
Albumin excretion, an index of kidney disease, is illustrated in Figure 6. Baseline albumin excretion before administration of any treatments, averaged 8.7 ± 6, 8.8 ± 5.9 and 5.9 ± 6.4 mg/day in rats that subsequently received saline vehicle (n = 6), angiotensin II (n = 7) or angiotensin II plus L-arginine (n = 6) respectively. These baseline albumin excretion levels did not differ between different treatment groups (P ≥ 0.5). Saline vehicle had little effect on urinary albumin excretion. In contrast, angiotensin II infusion significantly increased albumin excretion to 46 ± 6 mg/day (P < 0.001), which was significantly greater than the albumin excretion levels observed after saline infusion (P = 0.007). Combined angiotensin II plus L-arginine infusion also increased albumin excretion to 31 ± 6 mg/day (P = 0.007) (Figure 6). Daily protein excretion in rats administered saline vehicle, angiotensin II or angiotensin II plus L-arginine followed a similar pattern to that of albumin excretion (data not shown).
Figure 6.
Effects of infusion of saline (1ml/hr; n=6), angiotensin II (20ng/kg/min; n=7) and angiotensin II plus L-arginine (300 µg/kg/min; n=6) for 9 days, on albumin excretion (mg/day) in Sprague Dawley rats. All infusions were administered into the femoral vein. P values indicate the outcomes of 2-way repeated measures ANOVA with Tukey post hoc tests. * indicates P < 0.05 vs. before treatment in the same group of rats. Ψ indicates P < 0.05 vs rats administered saline vehicle.
Effects of angiotensin II and combined angiotensin II plus L-arginine on creatinine clearance (as an index of glomerular filtration rate)
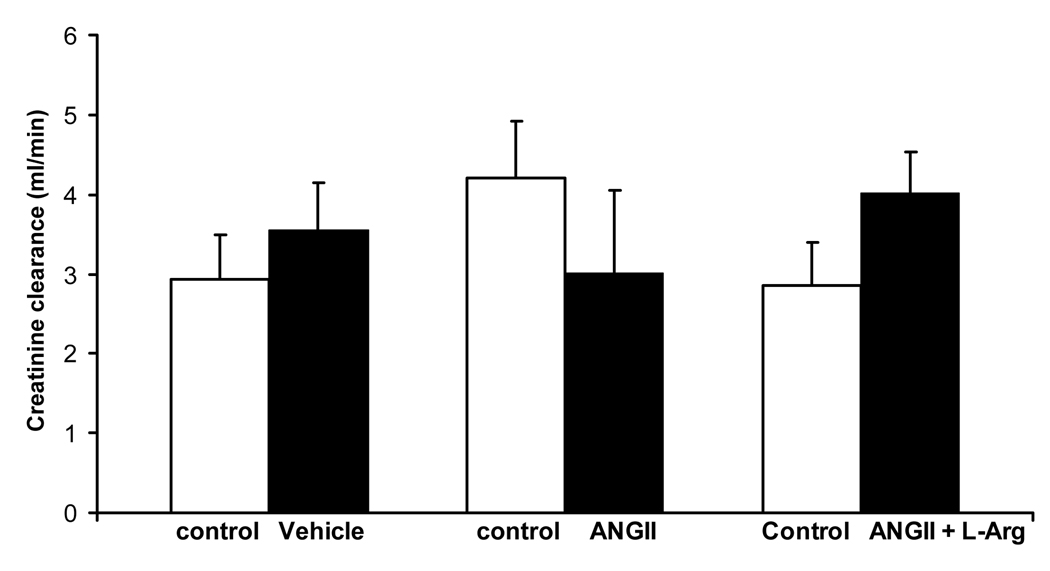
Creatinine clearance, as an index of glomerular filtration rate is illustrated in Figure 8. Baseline creatinine excretion levels before administration of any treatments, averaged 3 ± 1, 4 ± 1 and 3 ± 1 ml/min, in rats that subsequently received saline vehicle (n = 6), angiotensin II (n = 7) or angiotensin II plus L-arginine (n = 6) respectively. The baseline creatinine clearance levels did not differ between different treatment groups (P ≥ 0.17). Saline vehicle, angiotensin II and angiotensin II plus L-arginine had little effect on creatinine excretion levels (P ≥ 0.16) (Figure 7).
Figure 7.
Effects of infusion of saline (1ml/hr; n=6), angiotensin II (20ng/kg/min; n=7) and angiotensin II plus L-arginine (300 µg/kg/min; n=6) for 9 days, on creatinine excretion (ml/min) in Sprague Dawley rats. All infusions were administered into the femoral vein. P values indicate the outcomes of paired t-tests. * indicates P < 0.05 vs. before treatment in the same group of rats. Ψ indicates P < 0.05 vs rats administered saline vehicle.
DISCUSSION
Our study has a number of novel findings. Firstly, our data demonstrate that exogenous L-arginine can significantly blunt angiotensin II dependent hypertension and associated renal damage. This latter observation is most exciting as it stresses the importance of increasing NO bioavailability in addition to merely lowering arterial pressure in preventing hypertension induced target organ damage. Secondly, our data provide evidence that under conditions of hypertension, albuminuria can occur in the absence of significant glomerular damage. Thirdly, our present data indicate that L-arginine infusion significantly increased plasma nitrate/nitrite levels, which is an index of plasma NO content. These data are in agreement with our previous data 15, 16 and those of others 32, 33 which indicate that exogenous L-arginine can increase endogenous NO levels. Finally, our present data indicate that L-arginine can halt the development of angiotensin II induced hypertension.
Our current study indicates that L-arginine can increase NO bioavailability during chronic angiotensin II infusion. We have previously shown that L-arginine can increase renal NO bioavailability under normal physiological conditions15, and that manipulation of L-arginine transport mechanisms result in alterations in NO levels in rats 34. Collectively, these data indicate that cellular L-arginine transport is an important determinant of endogenous NO bioavailability. This is paradoxical since the Km of NO synthase for L-arginine is < 5 µM ( Proc Nat Acad Sci 87:8612–8616, 1990 ) and the intracellular L-arginine concentrations are in the range of 100–3800 µM in cultured endothelial cells35. However, the Km of NO synthase for L-arginine under in vivo conditions is unknown, but is likely to be much higher than demonstrated from the in vitro experiments. Presence of endogenous NO synthase inhibitors and compartmentalization of intracellular L-arginine pools are likely to increase Km of NO synthase for L-arginine under in vivo conditions 19. This could at least in part explain our observation, that exogenous L-arginine increased endogenous NO bioavailability.
Our current data indicate that L-arginine can ameliorate angiotensin II induced hypertension and related renal damage. We did not examine the effects of other amino acids that are not precursors to NO production in this model of hypertension. However, we have previously shown that chronic infusion of L-lysine or L-ornithine reduced renal NO bioavailability and initiated hypertension while L-arginine reversed these effects in SD rats. This indicates that amino acids that are not pre-cursors to NO production, do not have the same effect on long term blood pressure control as L-arginine.
Since the kidney plays an important role in angiotensin II induced hypertension, the antihypertensive effects of L-arginine are likely mediated at least partly within the kidney. It has been shown that angiotensin II receptors in the kidney are obligatory for the development of hypertension in angiotensin II induced hypertension model 36. In addition, the pressure natriuresis mechanism is shifted to the right (greater levels of arterial pressure) in all forms of hypertension studied to date 37 and all antihypertensive treatments must shift the pressure natriuresis mechanism to the left (lower levels of arterial pressure) to ameliorate hypertension chronically37. In fact, there is evidence that NO can shift the pressure natriuresis mechanism to the left 38. As our data indicate that L-arginine can increase NO production, it is likely that L-arginine shifts the pressure natriuresis mechanism to the left by increasing NO bioavailability. It is doubtful that angiotensin II dependent hypertension is mediated by angiotensin II induced vasoconstriction in the systemic circulation, because if the pressure natriuresis mechanism remains unaltered then this would reduce arterial pressure by reducing extracellular fluid volume 37.
Kidney tissue damage (as indicated by the glomerular injury index) in rats administered angiotensin II was significantly higher than rats administered saline vehicle. Co-infusion of L-arginine completely prevented angiotensin II dependent glomerular damage, despite the fact that arterial pressure in these animals were still well above the levels of normotensive rats (~ 150 mmHg). This intriguing observation indicates that L-arginine can prevent glomerular damage under conditions of hypertension. There is evidence that antihypertensive treatments which also increase NO bioavailability exert additional beneficial effects to hypertensive patients, particularly in preventing target organ damage 9. Our current data are in strong agreement with this notion and provide support for the use of L-arginine as an adjunct to current antihypertensive treatments. L-arginine did not significantly blunt angiotensin II induced increases in albumin excretion. At first glance, this seems to be at odds with our histological data. Under conditions of hypertension, albuminuria is caused by two main factors; increased intraglomerular pressure and glomerular injury 39. This latter factor is likely to be related to endothelial dysfunction39, which occurs as a consequence of low NO bioavailability observed in hypertension9, 40. Since L-arginine significantly increased NO levels in rats administered angiotensin II, it is likely that this treatment improved endothelial dysfunction and thereby prevented glomerular damage in these rats. However, L-arginine blunted angiotensin II induced hypertension by only 45%, and MAP of rats administered L-arginine plus angiotensin II, was still ~ 150 mmHg. Thus, increased intraglomerular pressure (but not glomerular damage) must have contributed to albuminuria observed in these rats. Also, L-arginine can increase urinary albumin excretion independent of parallel increases in glomerular filtration rate, potentially by reducing proximal tubular protein reabsorption 41. In this regard, we are not surprised that L-arginine was able to completely prevent glomerular damage but was unable to significantly blunt albuminuria. Regardless, of the mechanism(s) responsible for albuminuria in rats administered angiotensin II plus L-arginine, our data provide evidence that albuminuria can occur under conditions of hypertension, in the absence of significant glomerular damage.
Our data indicate that L-arginine can halt but not reverse angiotensin II induced hypertension. However, it should be noted that even in the group of rats that simultaneously received angiotensin II plus L-arginine, approximately 5 days of chronic L-arginine infusion was required before an effect of L-arginine on blood pressure could be observed (Figure 1). Thus, it is likely that in the group of rats that received angiotensin II and then L-arginine, the effects of L-arginine did not start to have an effect until 5 days after commencement of this infusion. In fact, blood pressure continued to increase within the first 5 days after commencement of L-arginine reaching a maximum of 184 mmHg on day 15 (Figure 3). This increase in blood pressure likely reflects the predominating effects of angiotensin II during this period. The reduction in blood pressure from day 16 is likely to reflect the effects of L-arginine. In fact, the average blood pressure during the last 3 days (i.e days 18–20) of the infusions was significantly less than the average blood pressure during days 15–17 (P = 0.03). These data provide evidence that L-arginine has the potential to reverse established hypertension.
Chronic angiotensin II infusion has been shown to increase superoxide levels 42. This is suggested to largely contribute to the systemic and renal effects observed during chronic angiotensin II infusion 43. Reactive oxygen species can scavenge NO 44 and uncouple NOS 1, 3. Under these conditions, NO synthase will not produce NO, but will rather produce superoxide creating a vicious cycle which further reduces NO 3. Low bioavailability of NO has been suggested to play a major role in maintaining hypertension, and the development of associated target organ damage 9. Therefore, a treatment regimen that interrupts the vicious cycle which reduces NO bioavailability in hypertension, should in turn be able to ameliorate hypertension and prevent associated target organ damage. It has previously been shown that administration of the superoxide dismutase mimetic, tempol 45 reduced superoxide bioavailability and ameliorated hypertension induced by chronic angiotensin II infusion. Although these authors did not measure NO content, it is likely that the effects of tempol were dependent on increased NO bioavailability.
PERSPECTIVES
We speculate that L-arginine inhibits the cascade of events which creates the vicious cycle leading to low NO bioavailability in hypertension, upstream to angiotensin II induced superoxide formation. There is evidence that L-arginine has antioxidant properties 46 47. It has been shown that L-arginine can counteract high salt diet induced increases in the expression of NADPH subunits gp91phox and p47phox as well as thromboxane B2 excretion 46. It is suggested that L-arginine can increase NO formation, under conditions of increased superoxide bioavailability (such as under conditions of hypertension) by three mechanisms. Firstly, L-arginine can increase NO formation via the conventional pathway by intact NO synthase. This mechanism is well supported by recent studies 15–17. Secondly, there is evidence that L-arginine can ‘recouple’ the uncoupled NO synthase 47. Finally, there is evidence that L-arginine can produce NO via a non enzymatic pathway by reacting with reactive oxygen species 47. All these actions should increase NO bioavailability under conditions of hypertension. Our current results do not allow us to directly determine the effects of L-arginine on superoxide production. We speculate that L-arginine reduces superoxide bioavailability by increasing the bioavailability of NO. However, we cannot completely exclude the possibility that antihypertensive and reno-protective effects of L-arginine might be partly mediated by NO independent pathways. This hypothesis merits investigation in the future.
ACKNOWLEDGEMNTS
None
SOURCES OF FUNDING
NW Rajapakse is a recipient of a NMHRC (Australia) CJ Martin Fellowship (ID: 384299). This work was partially supported by NIH (US) grants HL-29587 and DK-62803 to DL Mattson.
Footnotes
DISCLOSURES
None
REFERENCES
- 1.Reckelhoff JF, Romero JC. Role of oxidative stress in angiotensin-induced hypertension. Am J Physiol. 2003;284:R893–R912. doi: 10.1152/ajpregu.00491.2002. [DOI] [PubMed] [Google Scholar]
- 2.Navar LG, Ichihara A, Chin SY, Imig JD. Nitric oxide-angiotensin II interactions in angiotensin II-dependent hypertension. Acta Physiol Scand. 2000;168:139–147. doi: 10.1046/j.1365-201x.2000.00630.x. [DOI] [PubMed] [Google Scholar]
- 3.Romero JC, Reckelhoff JF. Role of angiotensin and oxidative stress in essential hypertension. Hypertension. 1999;34:943–949. doi: 10.1161/01.hyp.34.4.943. [DOI] [PubMed] [Google Scholar]
- 4.Johnson RJ, Rodriguez-Iturbe B, Kang D-H, Feig DI, Herrera-Acosta J. A unifying pathway for essential hypertension. Am J Hypertens. 2005;18:431–440. doi: 10.1016/j.amjhyper.2004.08.035. [DOI] [PubMed] [Google Scholar]
- 5.Kobori H, Nangaku M, Navar LG, Nishiyama A. The intrarenal renin-angiotensin system: from physiology to the pathobiology of hypertension and kidney disease. Pharmacol Rev. 2007;59:251–287. doi: 10.1124/pr.59.3.3. [DOI] [PubMed] [Google Scholar]
- 6.Patzak A, Persson EG. Angiotensin II-nitric oxide interaction in the kidney. Curr Opin Nephrol Hypertens. 2007;16:46–51. doi: 10.1097/MNH.0b013e328011a89b. [DOI] [PubMed] [Google Scholar]
- 7.Sachse A, Wolf G. Angiotensin II induced reactive oxygen species and the kidney. J Am Soc Nephrol. 2007;18:2439–2446. doi: 10.1681/ASN.2007020149. [DOI] [PubMed] [Google Scholar]
- 8.Ritz E, Haxsen V. Angiotensin II and oxidative stress: an unholy alliance. J Am Soc Nephrol. 2003;14:2985–2987. doi: 10.1097/01.asn.0000096784.86791.21. [comment]. [DOI] [PubMed] [Google Scholar]
- 9.Portaluppi F, Boari B, Manfredini R. Oxidative stress in essential hypertension. Curr Pharml Des. 2004;10:1695–1698. doi: 10.2174/1381612043384619. [DOI] [PubMed] [Google Scholar]
- 10.Romero JC, Reckelhoff JF. Oxidative stress may explain how hypertension is maintained by normal levels of angiotensin II. Braz J Med Biol Res. 2000;33:653–660. doi: 10.1590/s0100-879x2000000600006. [DOI] [PubMed] [Google Scholar]
- 11.Majid DSA, Kopkan L. Nitric oxide and superoxide interactions in the kidney and their implication in the development of salt-sensitive hypertension. Clin Exp Pharmacol Physiol. 2007;34:946–952. doi: 10.1111/j.1440-1681.2007.04642.x. [DOI] [PubMed] [Google Scholar]
- 12.Tousoulis D, Antoniades C, Tentolouris C, Goumas G, Stefanadis C, Toutouzas P. L-arginine in cardiovascular disease: dream or reality? Vasc Med. 2002;7:203–211. doi: 10.1191/1358863x02vm434ra. [DOI] [PubMed] [Google Scholar]
- 13.Baydoun AR, Emery PW, Pearson JD, Mann GE. Substrate-dependent regulation of intracellular amino acid concentrations in cultured bovine aortic endothelial cells. Biochem Biophys Res Commun. 1990;173:940–948. doi: 10.1016/s0006-291x(05)80876-9. [DOI] [PubMed] [Google Scholar]
- 14.Pollock JFU, Mitchell JA, Warner TD, Schmidt HHHW, Nakane M, Murad F. Purification and characterization of particulate endothelium derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc Natl Acad Sci U S A. 1990;87:8612–8616. doi: 10.1073/pnas.88.23.10480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kakoki M, Kim H-S, Arendshorst WJ, Mattson DL. L-Arginine uptake affects nitric oxide production and blood flow in the renal medulla. Am J Physiol. 2004;287:R1478–R1485. doi: 10.1152/ajpregu.00386.2004. [DOI] [PubMed] [Google Scholar]
- 16.Kakoki M, Kim H-S, Edgell C-JS, Maeda N, Smithies O, Mattson DL. Amino acids as modulators of endothelium-derived nitric oxide. Am J Physiol - Renal Physiol. 2006;291:F297–F304. doi: 10.1152/ajprenal.00417.2005. [DOI] [PubMed] [Google Scholar]
- 17.Deng X, Welch WJ, Wilcox CS. Renal vasodilation with L-arginine. Effects of dietary salt. Hypertension. 1995;26:256–262. doi: 10.1161/01.hyp.26.2.256. [DOI] [PubMed] [Google Scholar]
- 18.Radermacher J, Klanke B, Kastner S, Haake G, Schurek HJ, Stolte HF, Frolich JC. Effect of arginine depletion on glomerular and tubular kidney function: studies in isolated perfused rat kidneys. Am J Physiol. 1991;261:F779–F786. doi: 10.1152/ajprenal.1991.261.5.F779. [DOI] [PubMed] [Google Scholar]
- 19.Gokce N. L-arginine and hypertension. J Nutr. 2004;134:2807S–2811S. doi: 10.1093/jn/134.10.2807S. discussion 2818S–2819S. [DOI] [PubMed] [Google Scholar]
- 20.Hu L, Manning RD., Jr Role of nitric oxide in regulation of long-term pressure-natriuresis relationship in Dahl rats. Am J Physiol. 1995;268:H2375–H2383. doi: 10.1152/ajpheart.1995.268.6.H2375. [DOI] [PubMed] [Google Scholar]
- 21.Chen PY, Sanders PW. Role of nitric oxide synthesis in salt sensitive hypertension in Dahl/Rapp rats. Hypertension. 1993;22:812–818. doi: 10.1161/01.hyp.22.6.812. [DOI] [PubMed] [Google Scholar]
- 22.Patel AR, Granger JP, Krichner KA. L-Arginine improves transmission of perfusion pressure to the renal interstitium in Dahl salt-sensitive rats. Am J Physiol. 1994;266:R1730–R1735. doi: 10.1152/ajpregu.1994.266.6.R1730. [DOI] [PubMed] [Google Scholar]
- 23.Miyata N, Zou AP, Mattson DL, Cowley AW., Jr Renal medullary interstitial infusion of L-arginine prevents hypertension in Dahl salt-sensitive rats. Am J Physiol. 1998;275:R1667–R1673. doi: 10.1152/ajpregu.1998.275.5.R1667. [DOI] [PubMed] [Google Scholar]
- 24.Siani A, Pagano E, Iacone R, Iacoviello L, Scopacasa F, Strazzullo P. Blood pressure and metabolic changes during dietary L-arginine supplementation in humans. Am J Hypertens. 2000;13:547–551. doi: 10.1016/s0895-7061(99)00233-2. [DOI] [PubMed] [Google Scholar]
- 25.Chen PY, Sanders PW. L-arginine abrogates salt-sensitive hypertension in Dahl/Rapp rats. J Clin Invest. 1991;88:1559–1567. doi: 10.1172/JCI115467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pagnotta P, Germano G, Grutter G, Leonardo F, Rosano G, Chierchia S. Oral L-arginine supplementation improves essential arterial hypertension. Circulation. 1997;96:538-I. [Google Scholar]
- 27.Higashi Y, Oshima T, Ozono R, Wantanabe M, Matsuura H, Kajiyama G. Effects of L-arginine infusion on renal hemodynamics in patients with mild essential hypertension. Hypertension. 1995;25:898–902. doi: 10.1161/01.hyp.25.4.898. [DOI] [PubMed] [Google Scholar]
- 28.Mattson DL, James L, Berdan EA, Meister CJ. Immune suppression attenuates hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension. 2008;48:149–156. doi: 10.1161/01.HYP.0000228320.23697.29. [DOI] [PubMed] [Google Scholar]
- 29.Raij L, Azar S, Keane W. Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int. 1984;26:137–143. doi: 10.1038/ki.1984.147. [DOI] [PubMed] [Google Scholar]
- 30.Mattson DL, Kunert MP, Kaldunski ML, Greene AS, Roman RJ, Jacob HJ, Cowley AW., Jr Influence of diet and genetics on hypertension and renal disease in Dahl salt-sensitive rats. Phys Genomics. 2004;16:194–203. doi: 10.1152/physiolgenomics.00151.2003. [DOI] [PubMed] [Google Scholar]
- 31.Mattson DL, Meister CJ, Marcelle ML. Dietary protein source determines the degree of hypertension and renal disease in the Dahl salt-sensitive rat. Hypertension. 2005;45:736–741. doi: 10.1161/01.HYP.0000153318.74544.cc. [DOI] [PubMed] [Google Scholar]
- 32.Bruins MJ, Soeters PB, Lamers WH, Meijer AJ, Deutz NEP. L-arginine supplementation in hyperdynamic endotoxemic pigs: effect on nitric oxide synthesis by the different organs. Crit Care Med. 2002;30:508–517. doi: 10.1097/00003246-200203000-00003. [see comment]. [DOI] [PubMed] [Google Scholar]
- 33.Fike CD, Kaplowitz MR, Rehorst-Paea LA, Nelin LD. L-Arginine increases nitric oxide production in isolated lungs of chronically hypoxic newborn pigs. J Appl Physiol. 2000;88:1797–1803. doi: 10.1152/jappl.2000.88.5.1797. [DOI] [PubMed] [Google Scholar]
- 34.Kakoki M, Wang W, Mattson DL. Cationic amino acid transport in the renal medulla and blood pressure regulation. Hypertension. 2002;39:287–292. doi: 10.1161/hy0202.102700. [DOI] [PubMed] [Google Scholar]
- 35.Closs EI, Scheld JS, Sharafi M, Forstermann U. Substrate supply for nitric oxide synthase in macrophages and endothelial cells: role of cationic amino acid transporters. Mol Pharmacol. 2000;57:68–74. [PubMed] [Google Scholar]
- 36.Crowley SD, Gurley SB, Herrera MJ, Ruiz P, Griffiths R, Kumar AP, Kim H-S, Smithies O, Le TH, Coffman TM. Angiotensin II causes hypertension and cardiac hypertrophy through its receptors in the kidney. Proc Natl Acad Sci USA. 2006;103:17985–17990. doi: 10.1073/pnas.0605545103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hall JE, Brands MW, Henegar JR. Angiotensin II and long-term arterial pressure regulation: the overriding dominance of the kidney. J Am Soc Nephrol. 1999;10:S258–S265. [PubMed] [Google Scholar]
- 38.Mount PF, Power DA. Nitric oxide in the kidney: functions and regulation of synthesis. Acta Physiol (Oxf) 2006;187:433–446. doi: 10.1111/j.1748-1716.2006.01582.x. [DOI] [PubMed] [Google Scholar]
- 39.Crippa G. Microalbuminuria in essential hypertension. J Hum Hypertens. 2002;16:S74–S77. doi: 10.1038/sj.jhh.1001348. [DOI] [PubMed] [Google Scholar]
- 40.Kurowska EM. Nitric oxide therapies in vascular diseases. Curr Pharmac Des. 2002;8:155–166. doi: 10.2174/1381612023396429. [DOI] [PubMed] [Google Scholar]
- 41.Bello E, Caramelo C, Lopez MD, Soldevilla MJ, Gonzalez-Pacheco FR, Rovira A, Delgado RG, Alcazar JM, Martell N, Gonzalez J, Ruilope LM, Casado S. Induction of microalbuminuria by l-arginine infusion in healthy individuals: an insight into the mechanisms of proteinuria. Am J Kid Dis. 1999;33:1018–1025. doi: 10.1016/S0272-6386(99)70137-X. [DOI] [PubMed] [Google Scholar]
- 42.Rajagopalan S, Laursen JB, Borthayre A, Kurz S, Keiser J, Haleen S, Giaid A, Harrison DG. Role for endothelin-1 in angiotensin II-mediated hypertension. Hypertension. 1997;30:29–34. doi: 10.1161/01.hyp.30.1.29. [DOI] [PubMed] [Google Scholar]
- 43.Laplante M-A, Wu R, Moreau P, de Champlain J. Endothelin mediates superoxide production in angiotensin II-induced hypertension in rats. Free Radic Biol Med. 2005;38:589–596. doi: 10.1016/j.freeradbiomed.2004.11.026. [DOI] [PubMed] [Google Scholar]
- 44.Szentivanyi M, Jr, Zou A-P, Mattson DL, Soares P, Moreno C, Roman RJ, Cowley AW., Jr Renal medullary nitric oxide deficit of Dahl S rats enhances hypertensive actions of angiotensin II. Am J Physiol. 2002;283:R266–R272. doi: 10.1152/ajpregu.00461.2001. [DOI] [PubMed] [Google Scholar]
- 45.Ortiz MC, Manriquez MC, Romero JC, Juncos LA. Antioxidants block angiotensin II-induced increases in blood pressure and endothelin. Hypertension. 2001;38:655–659. doi: 10.1161/01.hyp.38.3.655. [DOI] [PubMed] [Google Scholar]
- 46.Fujii SZL, Igarashi J, Kosaka H. L-arginine reverses p47phox and gp91phox expression induced by high salt in Dahl rats. Hypertension. 2003;42:1014–1020. doi: 10.1161/01.HYP.0000094557.36656.D0. [DOI] [PubMed] [Google Scholar]
- 47.Chowienczyk P, Ritter J. Arginine: NO more than a simple amino acid? Lancet. 1997;350:901–902. doi: 10.1016/S0140-6736(05)63263-1. [DOI] [PubMed] [Google Scholar]