Abstract
Long-term maintenance of the memory T-cell response is the hallmark of immune protection and, hence, constitutes one of the most important objectives of vaccine-development strategies. Persistent memory T cells, developed after vaccination or microbial infections, ensure the generation of an antimicrobial response upon re-exposure to the pathogen through rapid clonal proliferation and activation of effector functions. However, in the context of many pathogen infections, these memory T cells fail to persist and die. In this review, we will highlight recent exciting findings in studies of memory T cells, their generation, their lineage relationships and their survival pathways; indeed, survival of memory T cells and maintenance of their functionality are key features of the immune response in its quest to control disease progression and in the development of vaccines to persistent microbial infections.
Keywords: differentiation, FOXO3a, memory, signaling, survival, T cell
Generation of memory T cells
The hallmark of the adaptive immune system lies in its ability to develop immune memory enabling it to quickly react to previously encountered specific antigens (Ags) with higher potency and efficacy [1]. This protective immune memory persists for several years after the initia l antigenic exposure. The best example of persistent immune memory is the finding that Ag-specific CD4 and CD8 responses can still be identified 60 years following vaccination against smallpox, even though the virus was eliminated and that antigenic re-encounter was excluded [2]. The immune response to yellow fever vaccine provides further evidence that immune memory is long lasting [3,4]; herein, immunity to the vaccine was shown to last for up to 35 years. Several lines of evidence have shown that the induction of memory T cells and long-lived plasma cells are major components of the success of vaccines, as they can promote protection against re-infection and the development of infections [5,6].
However, the molecular mechanisms that lead to the generation of memory T cells after infection or vaccination are poorly understood. Te initial phase of the immune response is characterized by the expansion of Ag-reacting T cells. This first phase is an activation phase, where Ag-specific T lymphocytes undergo a clonal expansion and acquire peripheral tissue homing receptors, cytotoxic activity and upregulate the secretion of effector cytokines. Once Ag has been cleared, sustained effector function could result in harmful immune inflammation. Therefore, most effector cells die during the second phase, known as the contraction phase, which is characterized by a rapid decline in the frequency of Ag-specific cells that die by apoptosis via activation-induced cell death [7], or by neglect as a consequence of growth factor withdrawal [8]. Even though the molecular mechanisms involved in this massive contraction of the response are not totally deciphered, it has been shown that both Bim and Fas are involved in the CD8 contraction of Ag-specific CD8 T-cell responses after acute viral lymphocytic choriomeningitis virus infection [9].
In parallel, a small proportion of Ag-specific T cells survive this contraction phase and constitute the pool of memory T cells that are deemed to persist. Te adaptive immune system, hence, maintains T-cell oligoclonal specificities that were proven successful in controlling dissemination of a pathogen. Te third phase is the memory phase, during which designated Ag-specific cells that survived the second phase develop into immunologic memory cells [10,11]. These resting memory T cells have several key features that distinguish them from naive cells. First, they are formed at much higher frequencies than naive T cells that have the same T-cell receptor (TCR) clonotype [12]. Moreover, they undergo self-renewal as do hematopoietic stem cells [13]. In addition, they show enhanced ability of Ag-independent self-renewal [14,15], through homeostatic turnover in response to cytokines such as IL-2, IL-7, IL-15, and IL-21 [16–22], rapid clonal proliferation and functional activation upon re-encountering the same pathogen [23,24]. Te enhanced magnitude of the secondary response to Ag by memory T cells is correlated with the regulation of gene-expression profiles by genetic changes, such as DNA methylation, histone modification, chromatin structure re-organization [25,26] or the upregulation of specific transcription factors required for the secondary activation, such as BCL6b [27].
Different subsets of memory T cells: TCM & TEM
Human memory T cells, as well as mouse memory T cells, can be subdivided into multiple subsets based on the expression of different cell surface molecules underlying homing markers and chemokines as well cytokine receptors [28–32]. In the present paper we will focus on human memory T cells. Naive T cells are characterized by the expression of CD45RA, CCR7, the costimulatory receptors CD28 and CD27, and the lack of expression of cytolytic molecules, such as granzymes, perforin and CD107a. Long-lived central memory T cells (TCM) that home preferentially to lymph nodes, as they express the lymph node-homing markers CD62L and CCR7, share several phenotypic properties with naive T cells, except that they do not express CD45RA. TCM, as opposed to naive T cells, can rapidly differentiate into cells endowed with effector functions upon exposure to Ag [29]; moreover, they upregulate CD40L to a greater extent. TCM cells are also characterized by their ability to proliferate and to secrete high levels of IL-2. TABLE 1 provides an overview of some of the molecules commonly used to dissect T-cell subsets. Experiments performed in mouse models have suggested that TCM have a better capacity to reconstitute the memory T-cell pool and to mediate protective immunity when compared with relatively short-lived effector memory T cells (TEM) [24,33–37] In that context, it is important to conceive TCM as the stem cells of the Ag-specific immune response, which will allow its long-term persistence.
Table 1.
Enrichment markers for human memory T-cell subsets.
| Marker | Expression enriched in subsets of: |
Protein superfamily |
Ref. |
|---|---|---|---|
| Cluster of differentiation others molecules | |||
| CD11a (LFA-1) | TEM, TCM | Integrin | [122] |
| CD11b* | E | Integrin | [123] |
| IL-15Ra | TCM, TEM, E | [46,124] | |
| CD25 (IL-2Ra) | E | [1,46] | |
| IL-2/15Rb | N, TCM, TEM | [38,125] | |
| CD27 | N, TCM | TNF receptor | [123] |
| CD28 | N, TCM | Immunoglobulin like | [123] |
| CD44* | E, M | Cartilage link protein | [1,126,127] |
| CD45RA‡ | N, E | PTP receptor | [1,123] |
| CD45RO‡ | E, TCM, TEM | PTP receptor | [1] |
| CD49d* | E, TEM | Integrin | [98,123] |
| CD56 | E, M | Immunoglobulin like | [128,129] |
| CD57 | E | [123] | |
| CD62L | N, TCM | C-type lectin | [1,127] |
| CD69 | E | C-type lectin | [1] |
| CD95 (Fas) | E, TCM, TEM | TNF receptor | [123,130] |
| CD95L (FasL) | E, TCM, TEM | TNF | [123,130] |
| CD127 (IL_7Ra) | TCM, TEM | [124] | |
| CD137 (4–1BB) | E | TNF receptor | [131] |
| CD154 (CD40L) | E | TNF | [132] |
| CD134 (OX-40) | E | TNF receptor | [133] |
| CD152 (CTLA-4) | E | Immunoglobulin like | [134] |
| Chemokine receptors | |||
| CCR3 | TEM | GPCR | [28–31,135] |
| CCR5 | TEM, E | GPCR | [1,28–31,135–138] |
| CCR6 | E, TCM | GPCR | [1] |
| CCR7 | N, TCM | GPCR | [1] |
| CCR8 | M | GPCR | [135] |
| CXCR3 | E, M | GPCR | [1,135,138] |
| CXCR4 | N, M | GPCR | [1,136] |
| Others | |||
| ICOS | E | Immunoglobulin like | [139] |
| Ly6c | M | [1] | |
| PD-1 | TCM, TEM | [113,140] | |
| PNA ligand* | E, M | O-glycan | [141] |
| 1B11 ligand* | E | O-glycan | [142] |
Markers established for murine T cells only.
CD45RA and CD45RO are isoforms of the CD45 molecule.
E: Effector cell; GPCR: G protein-coupled receptor; M: Memory phenotype cells; N: Naive; PTP: Protein tyrosine phosphatase; TCM: Central memory T cell; TEM: Effector memory T cell.
Effector memory T cells migrate preferentially to peripheral tissues and do not express CD45RA or CCR7 and, depending on their state of differentiation, they may or may not express CD27/CD28 [30]. TEM cells possess immediate effector functions (cytotoxicity and IFN-γ secretion) but have a reduced ability to proliferate and to secrete IL-2. Another subset termed ‘late’ or ‘terminally’ differentiated TEM, which is mostly found in CD8 T cells, expresses CD45RA but lacks expression of CCR7, CD27 and CD28. These cells have potent effector functions but also have limited proliferative capacity and fail to produce IL-2 [29,38]. TEM cells are, thus, subdivided into two subsets: CD45RA− [30] and CD45RA+ (TEMRA). Gauduin et al also showed that the presence of the CD4 TEMRA subset is strongly associated with protection mediated by attenuated SIV [39]. Many other transitory memory subsets in both CD4 and CD8 compartments have been described. After TCR triggering of naïve and memory T cells, we have observed two transitory memory subsets, CD45RA−, CCR7−, CD27+ [40] and CD45RA−, CCR7+, CD27+ (yassine-diab b, npublished data). Effector memory CD8+ T cells (CD45RA−, CCR7−) have been divided into four distinct subpopulations based on the expression of CD27 and CD28 [41]. Te authors found that effector memory EM1 (CD27+CD28+) and EM2 (CD27− CD28+) cells express low levels of mediators of effector T-cell function, such as granzyme B and perforin, but high levels of CD127, the IL-7 receptor (IL-7R)-α. On the other hand, the subsets EM3 (CD27+CD28−) and EM4 (CD27−CD28−) exhibit more effector functions, such as cytotoxic activity [41]. Moreover, a recent study characterized 14 different human CD8+ T-cell subsets based on the combination of CCR7, CD45RA, CD27, CD28, CD11a and CD62L [42]. Te development of an ex vivo differentiation system and the use of system biology approach should allow a better characterization of functional memory T-cell subsets.
Differentiation of memory subsets: lineage relationships
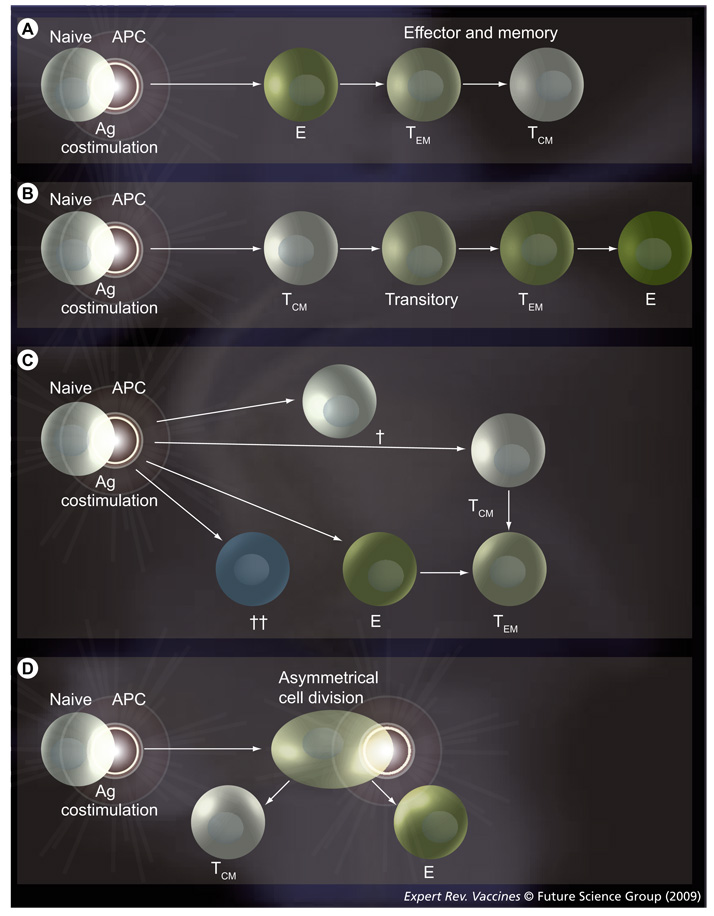
Different models have been developed to study the lineage relationship of TCM, TEM and naive T cells following Ag encounter [11,43]. The first model suggests that naive CD4 T cells upon activation by cognate Ag–MHC complexes differentiate into effector cells and then into memory cells [44]. Wherry et al. proposed a lineage relationship in memory CD8 T cells, where TCM and TEM do not necessarily represent distinct subsets, but are part of a continuum in a linear naive → effector → TEM → TEM differentiation pathway (FIGURE 1A) [24]. A second model states that memory T cells are generated directly from naive T cells upon Ag receptor triggering, therefore, bypassing an effector stage (FIGURE 1B) [45]. A third model was also proposed based on progressive differentiation of memory T cells according to signal-strength during the interaction of an Ag-presenting cell (APC) with a T cell. In this model, TCM differentiated to TEM upon Ag stimulation (FIGURE 1C) [28]. The differentiation of T cells was dependent on the concentration of Ag, costimulatory molecules, cytokines and of the ratio of T cells and APCs [46]. In addition, our unpublished experiments and that of another group [47] showed that TEM do not differentiate back to TCM. Thus, we showed that stimulation of highly purifed TEM doe s not gener ate a pool of TCM. However, Schwendemann et al. have demonstrated that on activation, human CCR7−CD62L− peripheral blood CD8+ and CD4+ TEM cells exhibit a dynamic differentiation, involving transient as well as stable changes to TCM phenotype and properties [48].
Figure 1. Suggested models for memory T-cell differentiation lineages.
(A) Dedifferentiation model. Upon contact with cognate MHC–peptide complexes on APCs, naive T cells become effector cells that are highly functional. These effector cells can differentiate into TEM and later into TCM. (B) Progressive differentiation model. After antigen activation, T cells differentiate directly into TCM then acquire a TEM phenotype. (C) Signal strength model. This model is based on the strength of the signal generated upon the interaction between naive T cells and APCs. The strength of the signal is represented by the thickness of the arrows. The cross represents the cells that die by neglect (single cross) or by activation-induced cell death (double cross) upon triggering with a very strong signal. (D) Asymmetric division model. In this model the daughter cell adjacent to the immunological synapse gives rise to an effector cytotoxic T lymphocyte, whereas that derived from the distal pole differentiates into a long-lived memory cell. APC: Antigen-presenting cell; E: Effector cell; TCM: Central memory cell; TEM: Effector memory cell; Transitory: Intermediate transitory stages of memory cell.
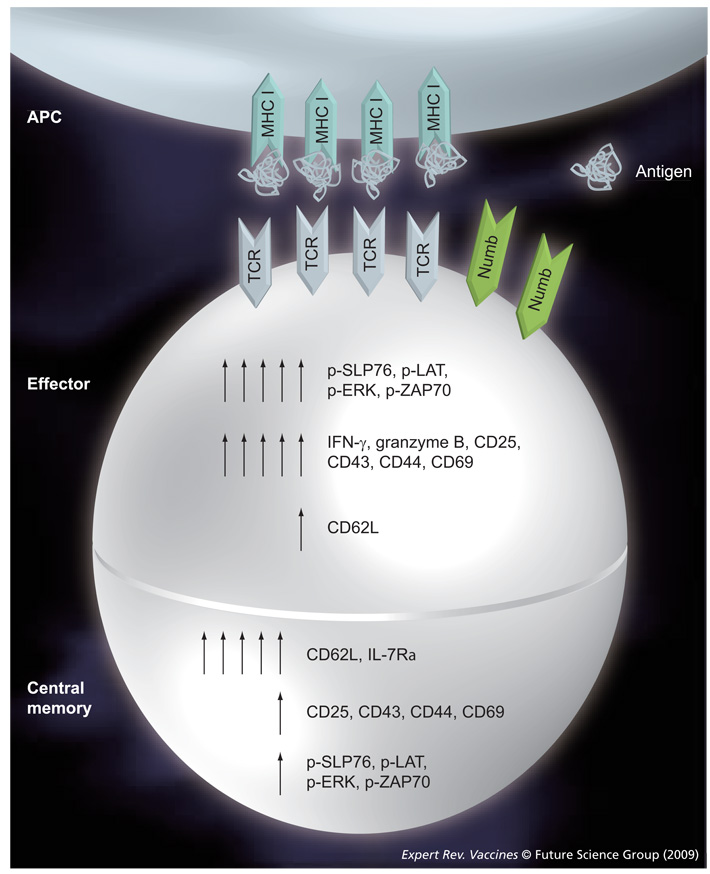
Recently, Chang et al. proposed an asymmetric division model where effector and memory cells are formed simultaneously upon the first cell division of naive progenitor T cells (FIGURE 1D) [49]. In this model, the daughter cell adjacent to the immunological synapse gave rise to an effector cytotoxic T lymphocyte, whereas those derived from the distal pole differentiated into a long-lived memory T cell (FIGURE 2). Hence, effector cells were generated from the daughter cell that received more Ag and cytokine signals, while the cell at the opposite side of the synapse kept the capacity to self-renew and to differentiate into effector cells at subsequent contacts with the Ag [49,50]. Following the contact of a T cell and an APC, a bipolar segregation of immune receptors, such as CD3, CD4 or CD8 and LFA-1 from one side and PKCζ from the opposite side, were observed early during mitosis possibly yielding a distinct phenotype of the proximal and distal daughter cells. Te proximal daughter cell was larger in size and expressed markers of an effector cell, such as CD69, CD43, CD25, CD44 and low levels of CD62L. Functionally, the proximal daughter T cell expressed IFN-γ and granzyme B. On the other hand, the distal daughter was smaller in size and expressed high levels of CD62L and lower levels of CD69, CD43, CD25 and CD44 and elevated mRNA for IL-7Rα, being more compatible with TCM. Te relevant difference between these four models could be due to several reasons, including the use of different species (human versus mice); the use of different markers to define the memory subsets; and experiments being performed either in vivo or in vitro. In our view, the asymmetric division is a very interesting model, as it shows the possibility to generate phenotypic and functionally distinct T-cell lineages from a single naive CD8 T cell following the contact with an Ag presented by an APC in the context of MHC molecules. In this regard, whether or not other important cell decisions, such as the CD4 T-cell differentiation to Th1, Th2, Th17 or Treg cells would ft into this model are still open to experimental evidence [51,52]. Interestingly, several data have provided evidence for a major role for Notch during the asymmetric division process. This mechanism was well described in Drosophila melanogaster, especially during asymmetric division of larval neuroblasts and sensory organ precursors [53–55]. This process implicates the Numb protein, which is a negative regulator of Notch signaling. Notch is a highly conserved family of receptors, constituted by four members (Notch1–4), interacting with theirs ligands (Δ-like1, 2, 4 and Jagged 1–2). Notch signaling is implicated in the maintenance and the self renewal of hematopoietic stem cells [56]. Te differential segregation of Numb between the two daughters of dividing cell regulates the Notch-dependent binary cell fates [57]. The repartition of Numb in unconjugated T helper cells is predominantly perimembranous, as the conjugation of a T cell with an APC leads to a rapid polarization of Numb to the immunologic synapse of T cell (FIGURE 2) [58,59]. Chang et al., however, showed a co-segregation of Numb and CD8 in the same daughter cell upon T-cell activation [49]. Te asymmetrical division of a memory T cell provides evidence for the generation and maintenance of T-cell memory and the differentiation into an effector cell could, hence, imply Numb. Some preliminary data generated in our laboratory have shown different levels of Numb and its negative regulator Musashi-1 (MSI-1) into two subpopulations of activated central memory CD4 T cells. This difference could allow us to discriminate a population enriched in ‘long-term TCM’.
Figure 2. Schematic model for TCR-coupled signaling during asymmetric division.
The first two daughter T cells display phenotypic and functional indicators of being differentially fated toward effector and memory lineages. The numbers of arrows indicate levels of expression.
APC: Antigen-presenting cell; TCR: T-cell receptor.
Our recent unpublished results suggest a model for the development of subsets of memory T cells that integrates the differentiation of naive T cells into a TCM cell model [28] and the asymmetric division model (FIGURE 2) [49]. We have used sorted cells (naive [CD45RA+CCR7+CD27+], TCM [CD45R A− CCR7+CD27+] and TEM cells [CD45RA−CCR7−CD27−]) co-cultured with dendritic cells (DCs) in the presence of Staphylococcal enterotoxin B (SEB) for 6 days to define the lineage relationships of memory cells. The maturation phenotype of proliferating cells (CFSE-low) was followed by staining with a battery of cell surface markers expressed by memory T cells. Our results showed that after activation with SEB, naive T cells and TCM differentiated into all subsets of memory T cells, including transitory stages of differentiation; however, TEM cells did not differentiate into TCM. Based on these results, we cannot exclude the possibility that naive cells in the presence of Ag generate simultaneously TCM and effector cells as observed in the asymmetric division model [49]; indeed, we identified both phenotypes after stimulation of naive cells with Ag. Additionally, through analysing sjTREC expression on naive T cells and subsets of memory T cells obtained from healthy donors, we observed, as expected, that the levels of sjTREC on naive cells are the highest. Of note, TCM also expressed levels of sjTREC almost comparable to those observed in naive T cells indicating that they underwent a minimal number of cell divisions upon TCR encounter with peptide–MHC complexes. By contrast, TEM cells and other memory differentiated phenotypes showed almost undetectable levels of sjTREC, indicating that these cells have undergone clonal expansion and differentiation. These results suggest that TCM can be differentiated directly from naive cells and have undergone a smaller number of cell divisions when compared with TEM. As such, these data do not ft with the lineage model of T-cell differentiation proposed by Wherry et al. [24]. It should be noted, however, that our results were predominantly obtained for CD4 cells, while Wherry et al. predominantly studied CD8 cells.
Differential T-cell receptor signaling in naive, effector & memory T cells
Memory T cells can persist at relatively constant numbers after infection or vaccination [60]. A characteristic of memory T cells is their ability to undergo Ag-independent homeostatic turnover and, thus, to maintain a stable pool of Ag-specific memory T cells [60]. Survival and death are balanced in this homeostatic proliferation, resulting in stable numbers of memory T cells [61]. Studies have demonstrated that TCR-mediated signals are required for memory T-cell survival [62–64], while others have shown that, once generated, memory T cells become independent of further TCR triggering [65,66]. However, some studies have shown that memory T cells that survive in the absence of MHC engagement become nonfunctional [67].
The proper development of functional memory T cells requires signaling not only through the TCR, but also via costimulatory molecules such as CD28. Moreover, signaling through CD27, CD40, 4-1BB or an inducible costimulatory molecule has also been shown to promote continued expansion, survival or memory differentiation of CD8 T cells [68].
The interaction of the α,β-TCR with cognate peptide–MHC complexes leads to the immediate phosphorylation of the TCR-associated CD3 chains on tyrosine residues in specific immunoreceptor tyrosine-based activation motif (ITAM) sequences by Src family kinase members, such as Lck and Fyn. Phosphorylation of CD3 molecules triggers the recruitment and activation of the ZAP-70 kinase to the CD3ζ signaling subunit [69]. ZAP-70 subsequently phosphorylates linker/adapter molecules SLP-76 [70] and LAT [71], which serve as molecular scaffolds to couple proximal phosphorylation to distal signaling events. LAT couples TCR to the Ras pathway and Ca2+ flux [71], while SLP-76 binds several adaptors including LAT, leading to Ca2+ flux and activation of the MAPK, and the guanine nucleotide exchange factor Vav, resulting in actin reorganization [72]. Activated MAPKs, in turn, phosphorylate and activate transcription factors, such as jun and nuclear factor of activated T cells (NFAT), for translocation into the nucleus [73].
Analysis of purified naive, effector and memory T cells allow us to detect specific alterations in TCR signaling in both human and mouse memory T-cell subsets [74–77]. In general, naive CD4 T cells follow the generalized signaling scheme identified in T-cell lines and clones, and exhibit a low level of tyrosine phosphorylation at the resting state [75,77]. On the other hand, effector CD4 T cells generated upon the activation and differentiation of resting CD4 T cells show profound amplifications in signaling, with overall increases in total intracellular tyrosine phosphorylation when analyzed directly or upon stimulation by TCR/CD3 crosslinking [78,75]. Tese signaling amplifications occur at the linker/adapter and MAP kinase levels, as detected by the enhanced phosphorylation of SLP-76, LAT and Erk1/2 (FIGURE 2) [76], with a concomitant alteration in the proximal TCR configuration and kinase activation [74]. In contrast to effector cells, human memory T cells are characterized by a profound dampening of TCR-coupled signaling, manifested by lower levels of tyrosine phosphorylation both at the resting and activated states [76–78] and by a decrease in the activation of the proximal ZAP-70 kinase (FIGURE 2) [77]. Relative to naive CD4 T cells, mouse memory CD4 T cells exhibit a profound decrease in the expression of the linker/adapter molecule SLP-76, while effector CD4 T cells express normal to elevated levels of SLP-76 [76]. Tese effector CD4 T cells show increased associations of SLP-76 to phosphorylated linkers and, downstream, hyperphosphorylate Erk1/2 MAPK [76].
These findings lead to a model in which signaling changes drive the differentiation of effector and memory T cells. Tus, the amplification of signaling at the linker/adapter and distal levels drives effector T-cell differentiation, and dampening of signals, possibly by Numb, may determine whether activated T cells persist as memory T cells.
Survival pathways in memory T cells: the role of FOXO3a, cytokines, chemokines, IGF, TGF-β & osteopontin; do they converge to FOXO3a?
The memory T-cell pool functions as a repository of heterogeneous long-lived T cells that had been previously in contact with an Ag. The understanding of how the pool of memory T cells can be maintained during the lifetime of an individual is fundamental to the development of vaccines. To date, no mediator has been described as a unique inducer of memory T-cell survival. As such, it is possible that multiple signals with redundant or partially overlapping functions could be involved to ensure the optimal survival of memory T cells. The identification of these mechanisms is also fundamental to decipher the dysfunction of memory cells, which is often observed in several chronic diseases, including viral infections or tumors. They also pave the way for the development of strategies aimed at restoring the functionality of these signal transduction cascades, through the generation of immunotherapeutics and adjuvants that target these pathways, thereby promoting the establishment of a long-lived memory T-cell pool.
Role of FOXO3a in the generation & persistence of memory T cells
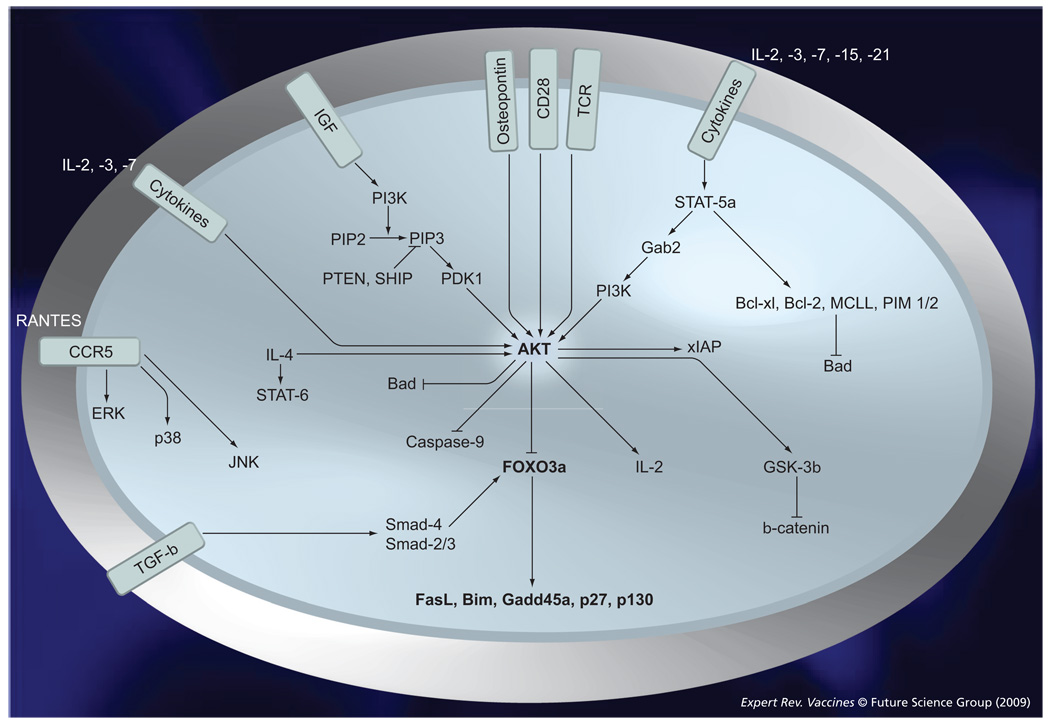
Recently, our group has shown that the long-term survival of TCM is critically dependent on the phosphorylation status of FOXO3a, a transcription factor that regulates proliferation and cell death [40]. Interestingly, we have shown that the persistence of TCM cells from elite controller subjects is a direct consequence of the inactivation of the FOXO3a pathway [79]. Upon TCR and cytokine receptor engagement in TCM cells, FOXO3a is highly phosphorylated by the kinases AKT and IKK at multiple residues, including S253, S315 and T32. The unphosphorylated, transcriptionally active form of FOXO3a translocates to the nucleus and induces the transcription of genes encoding proapoptotic and antiproliferative proteins, such as FasL, Bim and p130 (FIGURE 3) [79]. In addition, three different phosphorylation sites (S294, S344 and S425) have been newly described [80]. Phosphorylation of FOXO3a at the latter residues is ERK mediated, and promoted physical interaction of FOXO3a with MDM2 and its subsequent ubiquitination and proteasomal degradation; this consequently enhanced cell proliferation and led to tumorigenesis. On the other hand, we have shown that, in healthy subjects, ex vivo TCM express higher levels of the transcriptionally inactive phosphorylated forms of FOXO3a compared with TEM and concomitantly lower levels of the pro-apoptotic FOXO3a target Bim, Fas-L and PUMA. Experiments aimed at blocking the phosphorylation of FOXO3a by Akt and IKK-β phosphorylation confirmed the role of this phosphoprotein in protecting TCM from apoptosis. For the first time in humans, these results provided an insight into the molecular mechanisms that could be responsible for the longevity and persistence of CD4 TCM [40]. Other important players in the long-term survival of TCM include cytokines, chemokines, IGF, TGF-β and osteopontins (OPNs) (FIGURE 3). However, there is a paucity of data to show their direct link to FOXO3a and their direct role in the maintenance of the memory T-cell pool.
Figure 3. TCR and cytokine signaling leads to FOXO3a phosphorylation and drives the survival of CD4+ central memory T cells.
T-cell receptor and γ cytokine (IL-2 and IL-7) signaling induces FOXO3a phosphorylation, thereby preventing the transcription of proapoptotic molecules, such as FasL and Bim; moreover, γ-c cytokines allow the activation of STAT5a and the concomitant induction of several antiapoptotic molecules (Bcl-2, PIM-1 and PIM-2). IGF, TCR and osteopontin mediate their cellular effects by activating AKT; whereas, the chemokine receptor CCR5 mediates its effect through the MAPKs ERK, p38 and JNK. TGF-β induces translocation of SMAD-4 and SMAD2/3 proteins to the nucleus where they interact with FOXO3a to induce its transcription activity.
TCR: T-cell receptor.
Role of cytokines in the maintenance of memory T cells
Several studies have emphasized the role of cytokines that signal through the common γ-chain receptor (IL-2, IL-4, IL-7, IL-9, IL-15 and IL-21) in the survival and homeostatic proliferation of lymphocyte populations (FIGURE 3) [62,81,82]. The involvement of IL-7 and IL-15 in the survival of memory CD8 T cells has been shown by several groups [21,83–85]. However, the requirement of these cytokines in the persistence of CD4 memory T cells remains controversial [62–64]. IL-15 is known to mediate T-cell turnover, while IL-7 provides a signal to rescue survival by increasing the levels of the Bcl-2 and Bcl-XL anti-apoptotic molecules through STAT5a phosphorylation [19,86] and also by phosphorylating FOXO3a at T32 [40]. IL-2 has also been shown to mediate a critical role in the maintenance of memory T cells, as shown by Blattman et al. [87]. Indeed, when compared with naive CD8 T cells, mouse memory CD8 T cells were found to upregulate survival genes, such as Bcl-2, intracellular signaling molecules, such as MAPKs, and specific transcription factors involved in cell-cycle regulation, such as the forkhead transcription factor (FKHR) [88,89] upon engagement of γc cytokine receptor with its ligand. Engagement of TCRs and the γc cytokines IL-2 and IL-7 induces specific FOXO3a phosphorylation at distinct sites, S315 and T32, respectively, suggesting that the convergence of both signals is required to induce optimal FOXO3a phosphorylation (quantitatively and qualitatively) and the subsequent inhibition of its transcriptional upregulation of the proapoptotic machinery.
Role of chemokines in the generation & maintenance of memory T cells
The human chemokine superfamily currently includes at least 46 ligands, which bind to 18 functionally signaling G-protein-coupled receptors, such as C-C chemokine receptor (CCR)5 and CCR7. They are critical mediators of cell migration during routine immune surveillance, inflammation, and development. By binding to G-protein coupled receptors, chemokines cause conformational changes that trigger intracellular signaling pathways involved in cell movement and activation [90]. Recently, it has been shown that CCR5 plays a crucial role in the early memory CD8+ T-cell response to respiratory virus infections by accelerating the recruitment of memory CD8 T cells to the lung airways [91]. CCR5 mediates its effect by activating different MAPKs, such as ERK, p38 and JNK [92]. CCR7, another chemokine receptor, is the essential chemokine receptor responsible for lymphocyte and DC homing to secondary lymphoid organs, where they receive and/or provide the signals critical for the maintenance of T-cell memory. In addition to its well-established role in chemotaxis, recent published data have shown that CCR7 also induces antiapoptotic signaling in mature DCs through activation of the AKT pathway [93]. Although there is no direct evidence for a role of CCR7 on memory T cell survival, Ziegler et al. have shown that CCR7 signaling inhibits T-cell proliferation through delayed degradation of the cyclin-dependent kinase (CDK) inhibitor p27(Kip1) and the downregulation of CDK1 [94]. This shows that CCR7 signaling can be linked to cell cycle control and that sustained engagement of CCR7, either by high concentrations of soluble ligands or by high density of immobilized ligands, is capable of inducing cell cycle arrest in TCR-stimulated cells [94]. Recently, a group described a critical role of FOXO3a in chemokine-induced B-chronic lymphocytic leukemia (B-CLL) cell survival [95]. Their data support the notion that homeostatic chemokines contribute to the resistance of B-CLL cells to cell death through inactivation of the transcription factor FOXO3a. Chemokines, therefore, also appear to regulate the survival of many T cells. Whether they are critical for the maintenance of long-term memory remains to be addressed.
Role of IGF in memory T-cell survival
IGF is synthesized in response to growth hormone and it induces the activation of Akt in promoting T-lymphocyte survival [96]; however, no role for IGF has been described in the development and persistence of the memory T-cell pool. Nevertheless, low IGF concentrations were associated with low TCM counts, low CD4 lymphocyte counts, low naive T-lymphocyte counts and a smaller thymus [97]. Different studies showed that FOXO3a is downstream of IGF, although none of these studies were performed on lymphocytes [98].
Role of TGF-β in memory T-cell survival
TGF-β, a member of the TGF-β superfamily, which includes activins and inhibins, and is an important factor involved in the homeostasis of several cellular functions, such as proliferation, cell differentiation, apoptosis, cell motility and cell adhesion [99]. One of the critical functions of TGF-β is to maintain immunotolerance [100,101]. Along with the regulation of chemotaxis, activation and survival of lymphocytes, TGF-β inhibits the inflammatory response [102]. TGF-β signals through Smad-dependent and-independent pathways. TGF-β blocks the proliferation of T cells by triggering Smad3 to inhibit IL-2 production and by downregulating expression of cyclin D2, CDK4 and c-myc. On the other hand, TGF-β inhibits the differentiation of T helper and CTL cells by suppressing T-bet/Stat4 and GATA-3/NFAT signaling. In addition, TGF-β is also involved in the activation of MAPK and PI3K pathways. Smad7, one of the targets of TGF-β, acts as a negative feedback mechanism for TGF-β activation, by competing with Smad2-and Smad3-mediated signaling [103]. Unpublished data from our laboratory shows that the differentiation of human CD4+ TCM to TEM is inhibited by TGF-β. This inhibition can be partially rescued by IL-2. Recently, it has been demonstrated that TGF-β inhibits effector/memory peripheral blood T lymphoblast proliferation and IL-2 production. TGF-β priming resulted in ERK 1/2-independent p21 induction and decreased cyclin D1 expression, leading to accumulation of T cells in G0/G1 phases, as well as cell cycle arrest [104]. Interestingly, the impact of TGF-β on the TCR signaling pathway was not generalized to cytokine (IL-2, IL-7 and IL-15) signaling [104]. Other reports have also shown an inhibitory effect of TGF-β on IL-12 and IL-2 induced activities that are unlikely to be due to inhibition of JAK or STAT activation [105]. Recently, TGF-β-activated pathways, which are FOXO3a dependent, have been shown to impact the regulation of the hematopoietic progenitor cycle status [106]. Therefore, rather than blocking signal transduction, TGF-β might act through an undefined pathway to redirect functional TCR signal transduction. This altered signal transduction could indirectly impact memory T-cell survival.
Role of osteopontin
Osteopontin, a secreted glycophosphoprotein, acts on a variety of cellular processes, including bone resorption, extracellular matrix remodeling, immune cell activation and inhibition of apoptosis. OPN is expressed by several immune cell types, particularly in pathological situations. These include macrophages, T cells, B cells, natural killer cells and platelets [107]. In addition, OPN is involved in the enhanchment of Th1 cytokine (IFN-γ and TNF) levels and the inhibition of Th2 cytokine (IL-4 and IL-10) levels [108]. Moreover, OPN has been shown to promote the survival of activated T cells by inhibiting the transcription factor Foxo3a [109]. However, a role for OPN in memory T-cell maintenance has yet to be defined.
Programmed death-1 role in memory T-cell exhaustion
During chronic viral infections or in the presence of persisting Ag, Ag-specific CD8+ T cells initially acquire effector functions but gradually become less functional as the infection progresses. This loss of function, known as ‘exhaustion’, consists of a decreased capacity to proliferate and produce effector cytokines [110], and an increased sensitivity to apoptosis. Thus, early control of chronic infections may allow the development of highly functional and self-renewing memory T cells, while a prolonged exposure to Ag-specific stimulation may prevent optimal memory T-cell differentiation [110]. It is currently unclear whether these defective memory T cells arise from functionally exhausted T cells or whether they emerge through a different pathway [110].
Sustained negative signaling pathways during chronic infection have been proposed as one of the major factors for T-cell exhaustion [111]. Moreover, studies to elucidate the cause of impaired T-cell function have pointed to seven distinct inhibitory receptors [112], signaling primarily through programmed death (PD)-1 [111]. We and others have recently demonstrated that upregulation of PD-1 on HIV-specific CD8 T cells leads to an exhaustion status that is reversible by PD-1 blockade [113–115]. Shin and Wherry have suggested that immunoregulatory pathways, such as PD-1, may alter memory CD8 T-cell differentiation, as well as suppressing effector function [110]. Of note, during chronic viral infections, the expression of CD127 and CD62L markers of normal memory CD8+ T cell differentiation remained low [112], which could suggest that these cells do not receive the proper survival signals as their CD127 (IL-7R) levels are low, or these cells do not migrate to the lymph node where they interact with DCs that can also supply survival signals.
Expert commentary & five-year view
Understanding the mechanisms involved in the generation and survival of memory T cells is of fundamental importance for the development of successful vaccines. The well-succeeded vaccines stimulate long-term T-and B-cell responses and their maintenance is dependent on the preservation of long-lived memory T and B cells. In chronic infection, such as HCV and HIV the correlates of protection are not fully known, but there is no doubt that memory T cells constitute a major mechanism in controlling disease progression. Badr et al. have indicated that the preservation of long-lived memory T cells could correlate with a better protection against HCV infection [116]. In addition, Esser et al. have proposed that understanding the mechanisms by which long-lived cellular immune responses are generated following vaccination should facilitate the development of safe and effective vaccines against emerging diseases, such as malaria, TB, HIV and HCV [117]. Moreover, in the context of HIV infection, elite controllers as well as long-term nonprogressors exhibit a sustained protection against HIV disease progression because their TCM cells are capable of self-renewing and of differentiating into efficient TEM cells. In this regard, it is of high importance to develop a strategy to increase the expression of the survival factors enumerated previously, such as p-Foxo3a, IGF and osteopontin, and cytokines, such as IL-7 and IL-15. Moreover, the triggering of survival pathways as for AKT pathway [118] and Wnt [119] should also be considered as a strategy to enhance memory T cells. Along with increasing survival signals, it is essential to decrease or dampen factors that are involved in cell death or dysfunction in HIV infection, such as PD-1 receptor, Bim [120] and CTLA-4 [121]. Therefore, administration of a cocktail of molecules including adjuvants that trigger signal transduction pathways upstream of FOXO3a and/or siRNA that block ex vivo the inhibitory signals of survival seems to be the promising challenge in the future, in order to slow down HIV disease progression and improve the development of human vaccines for chronic infections and tumors.
Key issues
Long-term maintenance of the memory T-cell response is the hallmark of immune protection.
Upon contact with cognate MHC–peptide complexes on antigen-presenting cells, the daughter cell adjacent to the immunological synapse gives rise to an effector cytotoxic T lymphocyte; whereas, that derived from the distal pole differentiates into a long-lived memory T cell.
Naive T cells and central memory T cells differentiate into all subsets of memory T cells, including transitory stages of differentiation; however, effector memory T cells do not differentiate into central memory T cells.
It is of high importance to develop strategies to increase the expression of survival factors, such as p-Foxo3a, IGF and osteopontin, and cytokines, such as IL-7 and-15, in order to overcome T-cell death in the context of HIV infection.
Footnotes
Financial & competing interests disclosure
Grants awarded to Rafick-Pierre Sékaly from the US NIH, the Canadian Institutes of Health Research, the Canadian Network for Vaccine and Immunotherapeutics, Genome Québec, Genome Canada, and the Fonds de la recherche en Santé du Québec AIDS network. Rafick-Pierre Sékaly is the Canada Research Chair in Human Immunology. Andre Tanel holds a scholarship from Fonds de la recherche en Santé du Québec. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
Contributor Information
Andre Tanel, Laboratoire d’Immunologie, Centre de, Recherche du Centre Hospitalier de, l’Université de Montréal (CR-CHUM), Saint-Luc, 264 Rene Levesque Est, Montréal, Québec H2X 1P1, Canada; Laboratoire d’Immunologie, Département, de Microbiologie et d’Immunologie, Université de Montréal, Québec, Canada; INSERM U743, CR-CHUM, Université, de Montréal, 264 Rene Levesque Est, Montréal, Québec H2X1P1, Canada, Tel.: +1 514 890 8000 ext. 35246, Fax: +1 514 412 7415 andre.tanel@umontreal.ca.
Simone G Fonseca, Laboratoire d’Immunologie, Centre de, Recherche du Centre Hospitalier de, l’Université de Montréal (CR-CHUM), Saint-Luc, 264 Rene Levesque Est, Montréal, Québec H2X 1P1, Canada; Laboratoire d’Immunologie, Département, de Microbiologie et d’Immunologie, Université de Montréal, Québec, Canada; INSERM U743, CR-CHUM, Université, de Montréal, 264 Rene Levesque Est, Montréal, Québec H2X1P1, Canada, Tel.: +1 514 890 8000 ext. 35246, Fax: +1 514 412 7415, fonseca.simone@gmail.com.
Bader Yassine-Diab, Laboratoire d’Immunologie, Centre de, Recherche du Centre Hospitalier de, l’Université de Montréal (CR-CHUM), Saint-Luc, 264 Rene Levesque Est, Montréal, Québec H2X 1P1, Canada; Laboratoire d’Immunologie, Département, de Microbiologie et d’Immunologie, Université de Montréal, Québec, Canada; INSERM U743, CR-CHUM, Université, de Montréal, 264 Rene Levesque Est, Montréal, Québec H2X1P1, Canada, Tel.: +1 514 890 8000 ext. 35246, Fax: +1 514 412 7415, bader.yassine-diab@umontreal.ca.
Rebeka Bordi, Laboratoire d’Immunologie, Centre de, Recherche du Centre Hospitalier de, l’Université de Montréal (CR-CHUM), Saint-Luc, 264 Rene Levesque Est, Montréal, Québec H2X 1P1, Canada; Laboratoire d’Immunologie, Département, de Microbiologie et d’Immunologie, Université de Montréal, Québec, Canada; INSERM U743, CR-CHUM, Université, de Montréal, 264 Rene Levesque Est, Montréal, Québec H2X1P1, Canada, Tel.: +1 514 335 8350 ext. 54935, Fax: +1 514 333 0542, rebeka.bordi@umontreal.ca.
Joumana Zeidan, Laboratoire d’Immunologie, Centre de Recherche du Centre Hospitalier de l’Université de Montréal (CR-CHUM) Saint-Luc, 264 Rene Levesque Est, Montréal, Québec H2X 1P1, Canada; Laboratoire d’Immunologie, Département de Microbiologie et d’Immunologie, Université de Montréal, Québec, Canada; INSERM U743, CR-CHUM, Universitfé, de Montréal, 264 Rene Levesque Est, Montréal, Québec H2X1P1, Canada, Tel.: +1 514 890 8000 ext. 35246, Fax: +1 514 412 7415, joumana.zeidan@mail.mcgill.ca.
Yu Shi, Laboratoire d’Immunologie, Centre de Recherche du Centre Hospitalier de l’Université de Montréal (CR-CHUM) Saint-Luc, 264 Rene Levesque Est, Montréal, Québec H2X 1P1, Canada; Laboratoire d’Immunologie, Département de Microbiologie et d’Immunologie, Université de Montréal, Québec, Canada; INSERM U743, CR-CHUM, Université de Montréal, 264 Rene Levesque Est, Montréal, Québec H2X1P1, Canada, Tel.: +1 514 890 8000 ext. 35246, Fax: +1 514 412 7415 yu.shi@umontreal.ca.
Clarisse Benne, Laboratoire d’Immunologie, Centre de, Recherche du Centre Hospitalier de, l’Université de Montréal (CR-CHUM), Saint-Luc, 264 Rene Levesque Est, Montréal, Québec H2X 1P1, Canada; Laboratoire d’Immunologie, Département, de Microbiologie et d’Immunologie, Université de Montréal, Québec, Canada; INSERM U743, CR-CHUM, Université, de Montréal, 264 Rene Levesque Est, Montréal, Québec H2X1P1, Canada, Tel.: +1 514 890 8000 ext. 35246, Fax: +1 514 412 7415, clarisse.benne@gmail.com.
Rafick-Pierre Sékaly, Laboratoire d’Immunologie, Centre de, Recherche du Centre Hospitalier de, l’Université de Montréal (CR-CHUM), Saint-Luc, 264 Rene Levesque Est, Montréal, Québec H2X 1P1, Canada; Laboratoire d’Immunologie, Département, de Microbiologie et d’Immunologie, Université de Montréal, Québec, Canada; INSERM U743, CR-CHUM, Université, de Montréal, 264 Rene Levesque Est, Montréal, Québec H2X1P1, Canada; Department of Microbiology and Immunology, 3775 University Street, McGill University, Montréal, Québec, H3A 2B4, Canada, Tel.: +1 514 890 8000 ext. 35289, Fax: +1 514 412 7415, rafck-pierre.sekaly@umontreal.ca.
References
Papers of special note have been highlighted as:
• of interest
- 1.Dutton RW, Bradley LM, Swain SL. T cell memory. Annu. Rev. Immunol. 1998;16:201–223. doi: 10.1146/annurev.immunol.16.1.201. [DOI] [PubMed] [Google Scholar]
- 2.Combadiere B, Boissonnas A, Carcelain G, et al. Distinct time effects of vaccination on long-term proliferative and IFN-γ-producing T cell memory to smallpox in humans. J. Exp. Med. 2004;199(11):1585–1593. doi: 10.1084/jem.20032083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Courtois G. Duration of immunity after yellow fever vaccination. Ann. Soc. Belg. Med. Trop. (1920) 1954;34(1):9–12. [PubMed] [Google Scholar]
- 4.Poland JD, Calisher CH, Monath TP, Downs WG, Murphy K. Persistence of neutralizing antibody 30–35 years after immunization with 17D yellow fever vaccine. Bull. World Health Organ. 1981;59(6):895–900. [PMC free article] [PubMed] [Google Scholar]
- 5.Sant AJ, Chaves FA, Krafcik FR, et al. Immunodominance in CD4 T-cell responses: implications for immune responses to influenza virus and for vaccine design. Expert Rev. Vaccines. 2007;6(3):357–368. doi: 10.1586/14760584.6.3.357. [DOI] [PubMed] [Google Scholar]
- 6.Wrammert J, Ahmed R. Maintenance of serological memory. Biol. Chem. 2008;389(5):537–539. doi: 10.1515/bc.2008.066. [DOI] [PubMed] [Google Scholar]
- 7.Sabbagh L, Kaech SM, Bourbonniere M, et al. The selective increase in caspase-3 expression in effector but not memory T cells allows susceptibility to apoptosis. J. Immunol. 2004;173(9):5425–5433. doi: 10.4049/jimmunol.173.9.5425. [DOI] [PubMed] [Google Scholar]
- 8.Diaz-Guerra E, Vernal R, del Prete MJ, Silva A, Garcia-Sanz JA. CCL2 inhibits the apoptosis program induced by growth factor deprivation, rescuing functional T cells. J. Immunol. 2007;179(11):7352–7357. doi: 10.4049/jimmunol.179.11.7352. [DOI] [PubMed] [Google Scholar]
- 9.Weant AE, Michalek RD, Khan IU, Holbrook BC, Willingham MC, Grayson JM. Apoptosis regulators Bim and Fas function concurrently to control autoimmunity and CD8+ T cell contraction. Immunity. 2008;28(2):218–230. doi: 10.1016/j.immuni.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 10.Kaech SM, Ahmed R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2001;2(5):415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaech SM, Wherry EJ. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity. 2007;27(3):393–405. doi: 10.1016/j.immuni.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murali-Krishna K, Altman JD, Suresh M, et al. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8(2):177–187. doi: 10.1016/s1074-7613(00)80470-7. [DOI] [PubMed] [Google Scholar]
- 13.Luckey CJ, Bhattacharya D, Goldrath AW, Weissman IL, Benoist C, Mathis D. Memory T and memory B cells share a transcriptional program of self-renewal with long-term hematopoietic stem cells. Proc. Natl Acad. Sci. USA. 2006;103(9):3304–3309. doi: 10.1073/pnas.0511137103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rivino L, Messi M, Jarrossay D, Lanzavecchia A, Sallusto F, Geginat J. Chemokine receptor expression identifies pre-T helper (Th)1, pre-Th2, and nonpolarized cells among human CD4+ central memory T cells. J. Exp. Med. 2004;200(6):725–735. doi: 10.1084/jem.20040774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murali-Krishna K, Lau LL, Sambhara S, Lemonnier F, Altman J, Ahmed R. Persistence of memory CD8 T cells in MHC class I-deficient mice. Science. 1999;286(5443):1377–1381. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- 16.Goldrath AW, Sivakumar PV, Glaccum M, et al. Cytokine requirements for acute and Basal homeostatic proliferation of naive and memory CD8+ T cells. J. Exp. Med. 2002;195(12):1515–1522. doi: 10.1084/jem.20020033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaech SM, Tan JT, Wherry EJ, Konieczny BT, Surh CD, Ahmed R. Selective expression of the interleukin 7 receptor identifies effector CD8 T cells that give rise to long-lived memory cells. Nat. Immunol. 2003;4(12):1191–1198. doi: 10.1038/ni1009. [DOI] [PubMed] [Google Scholar]
- 18.Belkaid Y, Piccirillo CA, Mendez S, Shevach EM, Sacks DL. CD4+CD25+ regulatory T cells control Leishmania major persistence and immunity. Nature. 2002;420(6915):502–507. doi: 10.1038/nature01152. [DOI] [PubMed] [Google Scholar]
- 19.Kieper WC, Tan JT, Bondi-Boyd B, et al. Overexpression of interleukin (IL)-7 leads to IL-15-independent generation of memory phenotype CD8+ T cells. J. Exp. Med. 2002;195(12):1533–1539. doi: 10.1084/jem.20020067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Becker TC, Wherry EJ, Boone D, et al. Interleukin 15 is required for proliferative renewal of virus-specific memory CD8 T cells. J. Exp. Med. 2002;195(12):1541–1548. doi: 10.1084/jem.20020369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat. Immunol. 2000;1(5):426–432. doi: 10.1038/80868. • IL-7 and IL-15 are key cytokines in the survival of memory CD8 T cells.
- 22.King C, Ilic A, Koelsch K, Sarvetnick N. Homeostatic expansion of T cells during immune insufficiency generates autoimmunity. Cell. 2004;117(2):265–277. doi: 10.1016/s0092-8674(04)00335-6. [DOI] [PubMed] [Google Scholar]
- 23.Barber DL, Wherry EJ, Ahmed R. Cutting edge: rapid in vivo killing by memory CD8 T cells. J. Immunol. 2003;171(1):27–31. doi: 10.4049/jimmunol.171.1.27. [DOI] [PubMed] [Google Scholar]
- 24.Wherry EJ, Teichgraber V, Becker TC, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat. Immunol. 2003;4(3):225–234. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- 25.Kersh EN, Fitzpatrick DR, Murali-Krishna K, et al. Rapid demethylation of the IFN-γ gene occurs in memory but not naive CD8 T cells. J. Immunol. 2006;176(7):4083–4093. doi: 10.4049/jimmunol.176.7.4083. [DOI] [PubMed] [Google Scholar]
- 26.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat. Rev. Immunol. 2002;2(12):933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 27.Manders PM, Hunter PJ, Telaranta AI, et al. BCL6b mediates the enhanced magnitude of the secondary response of memory CD8+ T lymphocytes. Proc. Natl Acad. Sci. USA. 2005;102(21):7418–7425. doi: 10.1073/pnas.0501585102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu. Rev. Immunol. 2004;22:745–763. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 29.Sallusto F, Lenig D, Forster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401(6754):708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 30.Hazenberg MD, Otto SA, Hamann D, et al. Depletion of naive CD4 T cells by CXCR4-using HIV-1 variants occurs mainly through increased T-cell death and activation. AIDS. 2003;17(10):1419–1424. doi: 10.1097/00002030-200307040-00001. [DOI] [PubMed] [Google Scholar]
- 31.Masopust D, Vezys V, Marzo AL, Lefrancois L. Preferential localization of effector memory cells in nonlymphoid tissue. Science. 2001;291(5512):2413–2417. doi: 10.1126/science.1058867. [DOI] [PubMed] [Google Scholar]
- 32.Willinger T, Freeman T, Hasegawa H, McMichael AJ, Callan MF. Molecular signatures distinguish human central memory from effector memory CD8 T cell subsets. J. Immunol. 2005;175(9):5895–5903. doi: 10.4049/jimmunol.175.9.5895. [DOI] [PubMed] [Google Scholar]
- 33.Bouneaud C, Garcia Z, Kourilsky P, Pannetier C. Lineage relationships, homeostasis, and recall capacities of central-and effector-memory CD8 T cells in vivo. J. Exp. Med. 2005;201(4):579–590. doi: 10.1084/jem.20040876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Castiglioni P, Gerloni M, Zanetti M. Genetically programmed B lymphocytes are highly effcient in inducing anti-virus protective immunity mediated by central memory CD8 T cells. Vaccine. 2004;23(5):699–708. doi: 10.1016/j.vaccine.2004.06.028. [DOI] [PubMed] [Google Scholar]
- 35.Klebanoff CA, Gattinoni L, Torabi-Parizi P, et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc. Natl Acad. Sci. USA. 2005;102(27):9571–9576. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu CY, Kirman JR, Rotte MJ, et al. Distinct lineages of Th1 cells have differential capacities for memory cell generation in vivo. Nat. Immunol. 2002;3(9):852–858. doi: 10.1038/ni832. [DOI] [PubMed] [Google Scholar]
- 37.Zaph C, Uzonna J, Beverley SM, Scott P. Central memory T cells mediate long-term immunity to Leishmania major in the absence of persistent parasites. Nat. Med. 2004;10(10):1104–1110. doi: 10.1038/nm1108. [DOI] [PubMed] [Google Scholar]
- 38.Geginat J, Lanzavecchia A, Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 2003;101(11):4260–4266. doi: 10.1182/blood-2002-11-3577. [DOI] [PubMed] [Google Scholar]
- 39.Gauduin MC, Yu Y, Barabasz A, et al. Induction of a virus-specific effector-memory CD4+ T cell response by attenuated SIV infection. J. Exp. Med. 2006;203(12):2661–2672. doi: 10.1084/jem.20060134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Riou C, Yassine-Diab B, Van Grevenynghe J, et al. Convergence of TCR and cytokine signaling leads to FOXO3a phosphorylation and drives the survival of CD4+ central memory T cells. J. Exp. Med. 2007;204(1):79–91. doi: 10.1084/jem.20061681. • FOXO3a plays a key role in the survival of central memory T cells (TCM).
- 41.Romero P, Zippelius A, Kurth I, et al. Four functionally distinct populations of human effector-memory CD8+ T lymphocytes. J. Immunol. 2007;178(7):4112–4119. doi: 10.4049/jimmunol.178.7.4112. [DOI] [PubMed] [Google Scholar]
- 42.Monteiro M, Evaristo C, Legrand A, Nicoletti A, Rocha B. Cartography of gene expression in CD8 single cells: novel CCR7-subsets suggest differentiation independent of CD45RA expression. Blood. 2007;109(7):2863–2870. doi: 10.1182/blood-2006-06-027060. [DOI] [PubMed] [Google Scholar]
- 43.Tough DF. Deciphering the relationship between central and effector memory CD8+ T cells. Trends Immunol. 2003;24(8):404–407. doi: 10.1016/s1471-4906(03)00169-8. [DOI] [PubMed] [Google Scholar]
- 44.Hu H, Huston G, Duso D, Lepak N, Roman E, Swain SL. CD4+ T cell effectors can become memory cells with high efficiency and without further division. Nat. Immunol. 2001;2(8):705–710. doi: 10.1038/90643. [DOI] [PubMed] [Google Scholar]
- 45.Farber DL. Differential TCR signaling and the generation of memory T cells. J. Immunol. 1998;160(2):535–539. [PubMed] [Google Scholar]
- 46.Gett AV, Sallusto F, Lanzavecchia A, Geginat J. T cell fitness determined by signal strength. Nat. Immunol. 2003;4(4):355–360. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- 47.Huster KM, Koffler M, Stemberger C, Schiemann M, Wagner H, Busch DH. Unidirectional development of CD8+ central memory T cells into protective Listeria-specific effector memory T cells. Eur J. Immunol. 2006;36(6):1453–1464. doi: 10.1002/eji.200635874. [DOI] [PubMed] [Google Scholar]
- 48.Schwendemann J, Choi C, Schirrmacher V, Beckhove P. Dynamic differentiation of activated human peripheral blood CD8+ and CD4+ effector memory T cells. J. Immunol. 2005;175(3):1433–1439. doi: 10.4049/jimmunol.175.3.1433. [DOI] [PubMed] [Google Scholar]
- 49. Chang JT, Palanivel VR, Kinjyo I, et al. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315(5819):1687–1691. doi: 10.1126/science.1139393. • The results obtained by Chang et al. confirmed our observations that naive T cells differentiate into effector and TCM cells. They proposed an asymmetric division model where effector and memory cells are formed simultaneously upon the first cell division of naive progenitor T cells.
- 50.Reiner SL, Sallusto F, Lanzavecchia A. Division of labor with a workforce of one: challenges in specifying effector and memory T cell fate. Science. 2007;317(5838):622–625. doi: 10.1126/science.1143775. [DOI] [PubMed] [Google Scholar]
- 51.Stockinger B, Veldhoen M. Differentiation and function of Th17 T cells. Curr. Opin. Immunol. 2007;19(3):281–286. doi: 10.1016/j.coi.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 52.Intlekofer AM, Takemoto N, Kao C, et al. Requirement for T-bet in the aberrant differentiation of unhelped memory CD8+ T cells. J. Exp. Med. 2007;204(9):2015–2021. doi: 10.1084/jem.20070841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rhyu MS, Jan LY, Jan YN. Asymmetric distribution of numb protein during division of the sensory organ precursor cell confers distinct fates to daughter cells. Cell. 1994;76(3):477–491. doi: 10.1016/0092-8674(94)90112-0. [DOI] [PubMed] [Google Scholar]
- 54.Lee CY, Andersen RO, Cabernard C, et al. Drosophila Aurora-A kinase inhibits neuroblast self-renewal by regulating aPKC/Numb cortical polarity and spindle orientation. Genes Dev. 2006;20(24):3464–3474. doi: 10.1101/gad.1489406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang H, Somers GW, Bashirullah A, Heberlein U, Yu F, Chia W. Aurora-A acts as a tumor suppressor and regulates self-renewal of Drosophila neuroblasts. Genes Dev. 2006;20(24):3453–3463. doi: 10.1101/gad.1487506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chiba S. Notch signaling in stem cell systems. Stem Cells. 2006;24(11):2437–2447. doi: 10.1634/stemcells.2005-0661. [DOI] [PubMed] [Google Scholar]
- 57.Spana EP, Kopczynski C, Goodman CS, Doe CQ. Asymmetric localization of numb autonomously determines sibling neuron identity in the Drosophila CNS. Development. 1995;121(11):3489–3494. doi: 10.1242/dev.121.11.3489. [DOI] [PubMed] [Google Scholar]
- 58.Anderson AC, Kitchens EA, Chan SW, et al. The Notch regulator Numb links the Notch and TCR signaling pathways. J. Immunol. 2005;174(2):890–897. doi: 10.4049/jimmunol.174.2.890. [DOI] [PubMed] [Google Scholar]
- 59.Luty WH, Rodeberg D, Parness J, Vyas YM. Antiparallel segregation of notch components in the immunological synapse directs reciprocal signaling in allogeneic Th:DC conjugates. J. Immunol. 2007;179(2):819–829. doi: 10.4049/jimmunol.179.2.819. [DOI] [PubMed] [Google Scholar]
- 60.Kaech SM, Wherry EJ, Ahmed R. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2002;2(4):251–262. doi: 10.1038/nri778. [DOI] [PubMed] [Google Scholar]
- 61.Sprent J, Cho JH, Boyman O, Surh CD. T cell homeostasis. Immunol. Cell. Biol. 2008;86(4):312–319. doi: 10.1038/icb.2008.12. [DOI] [PubMed] [Google Scholar]
- 62.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J. Exp. Med. 2002;195(12):1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kondrack RM, Harbertson J, Tan JT, McBreen ME, Surh CD, Bradley LM. Interleukin 7 regulates the survival and generation of memory CD4 cells. J. Exp. Med. 2003;198(12):1797–1806. doi: 10.1084/jem.20030735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seddon B, Tomlinson P, Zamoyska R. Interleukin 7 and T cell receptor signals regulate homeostasis of CD4 memory cells. Nat. Immunol. 2003;4(7):680–686. doi: 10.1038/ni946. [DOI] [PubMed] [Google Scholar]
- 65.Swain SL, Hu H, Huston G. Class II-independent generation of CD4 memory T cells from effectors. Science. 1999;286(5443):1381–1383. doi: 10.1126/science.286.5443.1381. [DOI] [PubMed] [Google Scholar]
- 66.Zamoyska R, Basson A, Filby A, Legname G, Lovatt M, Seddon B. The influence of the src-family kinases, Lck and Fyn, on T cell differentiation, survival and activation. Immunol. Rev. 2003;191:107–118. doi: 10.1034/j.1600-065x.2003.00015.x. [DOI] [PubMed] [Google Scholar]
- 67.Kassiotis G, Garcia S, Simpson E, Stockinger B. Impairment of immunological memory in the absence of MHC despite survival of memory T cells. Nat. Immunol. 2002;3(3):244–250. doi: 10.1038/ni766. [DOI] [PubMed] [Google Scholar]
- 68.Curtsinger JM, Johnson CM, Mescher MF. CD8 T cell clonal expansion and development of effector function require prolonged exposure to antigen, costimulation, and signal 3 cytokine. J. Immunol. 2003;171(10):5165–5171. doi: 10.4049/jimmunol.171.10.5165. [DOI] [PubMed] [Google Scholar]
- 69.Chan AC, Iwashima M, Turck CW, Weiss A. ZAP-70: a 70 kd protein-tyrosine kinase that associates with the TCRζ chain. Cell. 1992;71(4):649–662. doi: 10.1016/0092-8674(92)90598-7. [DOI] [PubMed] [Google Scholar]
- 70.Bubeck Wardenburg J, Fu C, Jackman JK, et al. Phosphorylation of SLP-76 by the ZAP-70 protein-tyrosine kinase is required for T-cell receptor function. J Biol. Chem. 1996;271(33):19641–19644. doi: 10.1074/jbc.271.33.19641. [DOI] [PubMed] [Google Scholar]
- 71.Zhang W, Sloan-Lancaster J, Kitchen J, Trible RP, Samelson LE. LAT: the ZAP-70 tyrosine kinase substrate that links T cell receptor to cellular activation. Cell. 1998;92(1):83–92. doi: 10.1016/s0092-8674(00)80901-0. [DOI] [PubMed] [Google Scholar]
- 72.Jordan MS, Singer AL, Koretzky GA. Adaptors as central mediators of signal transduction in immune cells. Nat. Immunol. 2003;4(2):110–116. doi: 10.1038/ni0203-110. [DOI] [PubMed] [Google Scholar]
- 73.Kuo CT, Leiden JM. Transcriptional regulation of T lymphocyte development and function. Annu. Rev. Immunol. 1999;17:149–187. doi: 10.1146/annurev.immunol.17.1.149. [DOI] [PubMed] [Google Scholar]
- 74.Krishnan S, Warke VG, Nambiar MP, Tsokos GC, Farber DL. The FcR γ subunit and Syk kinase replace the CD3ζ-chain and ZAP-70 kinase in the TCR signaling complex of human effector CD4 T cells. J. Immunol. 2003;170(8):4189–4195. doi: 10.4049/jimmunol.170.8.4189. [DOI] [PubMed] [Google Scholar]
- 75.Krishnan S, Warke VG, Nambiar MP, Wong HK, Tsokos GC, Farber DL. Generation and biochemical analysis of human effector CD4 T cells: alterations in tyrosine phosphorylation and loss of CD3ζ expression. Blood. 2001;97(12):3851–3859. doi: 10.1182/blood.v97.12.3851. [DOI] [PubMed] [Google Scholar]
- 76. Hussain SF, Anderson CF, Farber DL. Differential SLP-76 expression and TCR-mediated signaling in effector and memory CD4 T cells. J. Immunol. 2002;168(4):1557–1565. doi: 10.4049/jimmunol.168.4.1557. • Memory cells have lower levels of tyrosine phosphorylation than effector cells.
- 77.Farber DL, Acuto O, Bottomly K. Differential T cell receptor-mediated signaling in naive and memory CD4 T cells. Eur J. Immunol. 1997;27(8):2094–2101. doi: 10.1002/eji.1830270838. [DOI] [PubMed] [Google Scholar]
- 78.Ahmadzadeh M, Hussain SF, Farber DL. Effector CD4 T cells are biochemically distinct from the memory subset: evidence for long-term persistence of effectors in vivo. J. Immunol. 1999;163(6):3053–3063. [PubMed] [Google Scholar]
- 79. van Grevenynghe J, Procopio FA, He Z, et al. Transcription factor FOXO3a controls the persistence of memory CD4+ T cells during HIV infection. Nat. Med. 2008;14(3):266–274. doi: 10.1038/nm1728. • Persistence of TCM cells from elite controller subjects is a direct consequence of the inactivation of the FOXO3a pathway.
- 80.Yang JY, Zong CS, Xia W, et al. ERK promotes tumorigenesis by inhibiting FOXO3a via MDM2-mediated degradation. Nat. Cell Biol. 2008;10(2):138–148. doi: 10.1038/ncb1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khaled AR, Durum SK. Lymphocide: cytokines and the control of lymphoid homeostasis. Nat. Rev. Immunol. 2002;2(11):817–830. doi: 10.1038/nri931. [DOI] [PubMed] [Google Scholar]
- 82.Migliaccio M, Alves PM, Romero P, Rufer N. Distinct mechanisms control human naive and antigen-experienced CD8+ T lymphocyte proliferation. J. Immunol. 2006;176(4):2173–2182. doi: 10.4049/jimmunol.176.4.2173. [DOI] [PubMed] [Google Scholar]
- 83. Schluns KS, Williams K, Ma A, Zheng XX, Lefrancois L. Cutting edge: requirement for IL-15 in the generation of primary and memory antigen-specific CD8 T cells. J. Immunol. 2002;168(10):4827–4831. doi: 10.4049/jimmunol.168.10.4827. • IL-7 and IL-15 are key cytokines in the survival of memory CD8 T cells.
- 84.Mueller YM, Petrovas C, Bojczuk PM, et al. Interleukin-15 increases effector memory CD8+ T cells and NK cells in simian immunodeficiency virus-infected macaques. J. Virol. 2005;79(8):4877–4885. doi: 10.1128/JVI.79.8.4877-4885.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Purton JF, Tan JT, Rubinstein MP, Kim DM, Sprent J, Surh CD. Antiviral CD4+ memory T cells are IL-15 dependent. J. Exp. Med. 2007;204(4):951–961. doi: 10.1084/jem.20061805. • IL-7 and IL-15 are key cytokines in the survival of memory CD8 T cells.
- 86.Akashi K, Kondo M, von Freeden-Jeffry U, Murray R, Weissman IL. Bcl-2 rescues T lymphopoiesis in interleukin-7 receptor-deficient mice. Cell. 1997;89(7):1033–1041. doi: 10.1016/s0092-8674(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 87.Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat. Med. 2003;9(5):540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- 88.Kaech SM, Hemby S, Kersh E, Ahmed R. Molecular and functional profiling of memory CD8 T cell differentiation. Cell. 2002;111(6):837–851. doi: 10.1016/s0092-8674(02)01139-x. [DOI] [PubMed] [Google Scholar]
- 89.Grayson JM, Zajac AJ, Altman JD, Ahmed R. Cutting edge: increased expression of Bcl-2 in antigen-specific memory CD8+ T cells. J. Immunol. 2000;164(8):3950–3954. doi: 10.4049/jimmunol.164.8.3950. [DOI] [PubMed] [Google Scholar]
- 90.Allen SJ, Crown SE, Handel TM. Chemokine: receptor structure, interactions, and antagonism. Annu. Rev. Immunol. 2007;25:787–820. doi: 10.1146/annurev.immunol.24.021605.090529. [DOI] [PubMed] [Google Scholar]
- 91.Kohlmeier JE, Miller SC, Smith J, et al. The chemokine receptor CCR5 plays a key role in the early memory CD8+ T cell response to respiratory virus infections. Immunity. 2008;29(1):101–113. doi: 10.1016/j.immuni.2008.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Oppermann M. Chemokine receptor CCR5: insights into structure, function, and regulation. Cell. Signal. 2004;16(11):1201–1210. doi: 10.1016/j.cellsig.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 93.Sanchez-Sanchez N, Riol-Blanco L, de la Rosa G, et al. Chemokine receptor CCR7 induces intracellular signaling that inhibits apoptosis of mature dendritic cells. Blood. 2004;104(3):619–625. doi: 10.1182/blood-2003-11-3943. [DOI] [PubMed] [Google Scholar]
- 94.Ziegler E, Oberbarnscheidt M, Bulfone-Paus S, Forster R, Kunzendorf U, Krautwald S. CCR7 signaling inhibits T cell proliferation. J. Immunol. 2007;179(10):6485–6493. doi: 10.4049/jimmunol.179.10.6485. [DOI] [PubMed] [Google Scholar]
- 95.Ticchioni M, Essafi M, Jeandel PY, et al. Homeostatic chemokines increase survival of B-chronic lymphocytic leukemia cells through inactivation of transcription factor FOXO3a. Oncogene. 2007;26(50):7081–7091. doi: 10.1038/sj.onc.1210519. [DOI] [PubMed] [Google Scholar]
- 96.Walsh PT, Smith LM, O’Connor R. Insulin-like growth factor-1 activates Akt and Jun N-terminal kinases (JNKs) in promoting the survival of T lymphocytes. Immunology. 2002;107(4):461–471. doi: 10.1046/j.1365-2567.2002.01525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Congote LF. Monitoring insulin-like growth factors in HIV infection and AIDS. Clin. Chim. Acta. 2005;361(1–2):30–53. doi: 10.1016/j.cccn.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 98.Brennan FM, Smith NM, Owen S, et al. Resting CD4+ effector memory T cells are precursors of bystander-activated effectors: a surrogate model of rheumatoid arthritis synovial T-cell function. Arthritis Res. Ther. 2008;10(2):R36. doi: 10.1186/ar2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Danielpour D, Song K. Cross-talk between IGF-I and TGF-β signaling pathways. Cytokine Growth Factor Rev. 2006;17(1–2):59–74. doi: 10.1016/j.cytogfr.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 100.Marie JC, Letterio JJ, Gavin M, Rudensky AY. TGF-β1 maintains suppressor function and Foxp3 expression in CD4+CD25+ regulatory T cells. J. Exp. Med. 2005;201(7):1061–1067. doi: 10.1084/jem.20042276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Alard P, Clark SL, Kosiewicz MM. Mechanisms of tolerance induced by TGF β-treated APC: CD4 regulatory T cells prevent the induction of the immune response possibly through a mechanism involving TGF β. Eur J. Immunol. 2004;34(4):1021–1030. doi: 10.1002/eji.200324547. [DOI] [PubMed] [Google Scholar]
- 102.Nakamura K, Kitani A, Fuss I, et al. TGF-β 1 plays an important role in the mechanism of CD4+CD25+ regulatory T cell activity in both humans and mice. J. Immunol. 2004;172(2):834–842. doi: 10.4049/jimmunol.172.2.834. [DOI] [PubMed] [Google Scholar]
- 103.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-β regulation of immune responses. Annu. Rev. Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 104.Das L, Levine AD. TGF-β inhibits IL-2 production and promotes cell cycle arrest in TCR-activated effector/memory T cells in the presence of sustained TCR signal transduction. J. Immunol. 2008;180(3):1490–1498. doi: 10.4049/jimmunol.180.3.1490. [DOI] [PubMed] [Google Scholar]
- 105.Sudarshan C, Galon J, Zhou Y, O’Shea JJ. TGF-β does not inhibit IL-12-and IL-2-induced activation of Janus kinases and STATs. J. Immunol. 1999;162(5):2974–2981. [PubMed] [Google Scholar]
- 106.Chabanon A, Desterke C, Rodenburger E, et al. A CROSS-TALK between SDF-1 and TGF-β controls the quiescence/cycling switch of CD34+ progenitors through FoxO3 and mTOR. Stem Cells. 2008;26(12):3150–3161. doi: 10.1634/stemcells.2008-0219. [DOI] [PubMed] [Google Scholar]
- 107.Giachelli CM, Lombardi D, Johnson RJ, Murry CE, Almeida M. Evidence for a role of osteopontin in macrophage infiltration in response to pathological stimuli in vivo. Am. J Pathol. 1998;152(2):353–358. [PMC free article] [PubMed] [Google Scholar]
- 108.Ashkar S, Weber GF, Panoutsakopoulou V, et al. Eta-1 (osteopontin): an early component of type-1 (cell-mediated) immunity. Science. 2000;287(5454):860–864. doi: 10.1126/science.287.5454.860. [DOI] [PubMed] [Google Scholar]
- 109.Hur EM, Youssef S, Haws ME, Zhang SY, Sobel RA, Steinman L. Osteopontin-induced relapse and progression of autoimmune brain disease through enhanced survival of activated T cells. Nat. Immunol. 2007;8(1):74–83. doi: 10.1038/ni1415. [DOI] [PubMed] [Google Scholar]
- 110.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr. Opin. Immunol. 2007;19(4):408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 111. Ha SJ, West EE, Araki K, Smith KA, Ahmed R. Manipulating both the inhibitory and stimulatory immune system towards the success of therapeutic vaccination against chronic viral infections. Immunol. Rev. 2008;223:317–333. doi: 10.1111/j.1600-065X.2008.00638.x. • Programmed death (PD)-1 is a regulator of T-cell survival in HIV infection.
- 112. Blackburn SD, Shin H, Haining WN, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat. Immunol. 2009;10(1):29–37. doi: 10.1038/ni.1679. • PD-1 is a regulator of T-cell survival in HIV infection.
- 113. Trautmann L, Janbazian L, Chomont N, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat. Med. 2006;12(10):1198–1202. doi: 10.1038/nm1482. • PD-1 is a regulator of T-cell survival in HIV infection.
- 114. Brenchley JM, Price DA, Schacker TW, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat. Med. 2006;12(12):1365–1371. doi: 10.1038/nm1511. • PD-1 is a regulator of T-cell survival in HIV infection.
- 115. Petrovas C, Casazza JP, Brenchley JM, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J. Exp. Med. 2006;203(10):2281–2292. doi: 10.1084/jem.20061496. • PD-1 is a regulator of T-cell survival in HIV infection.
- 116.Badr G, Bedard N, Abdel-Hakeem MS, et al. Early interferon therapy for hepatitis C virus infection rescues polyfunctional, long-lived CD8+ memory T cells. J. Virol. 2008;82(20):10017–10031. doi: 10.1128/JVI.01083-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Esser MT, Marchese RD, Kierstead LS, et al. Memory T cells and vaccines. Vaccine. 2003;21(5–6):419–430. doi: 10.1016/s0264-410x(02)00407-3. [DOI] [PubMed] [Google Scholar]
- 118.Fruman DA. Phosphoinositide 3-kinase and its targets in B-cell and T-cell signaling. Curr. Opin. Immunol. 2004;16(3):314–320. doi: 10.1016/j.coi.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 119.Staal FJ, Luis TC, Tiemessen MM. WNT signalling in the immune system: WNT is spreading its wings. Nat. Rev. Immunol. 2008;8(8):581–593. doi: 10.1038/nri2360. [DOI] [PubMed] [Google Scholar]
- 120.Wang C, Wen T, Routy JP, Bernard NF, Sekaly RP, Watts TH. 4-1BBL induces TNF receptor-associated factor 1-dependent Bim modulation in human T cells and is a critical component in the costimulation-dependent rescue of functionally impaired HIV-specific CD8 T cells. J. Immunol. 2007;179(12):8252–8263. doi: 10.4049/jimmunol.179.12.8252. [DOI] [PubMed] [Google Scholar]
- 121.Kaufmann DE, Kavanagh DG, Pereyra F, et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat. Immunol. 2007;8(11):1246–1254. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 122.Hedlund G, Hansson J, Ericsson PO, Sjogren HO, Dohlsten M. Expression of CD11a and CD45R isoforms defines distinct subsets of CD8+ TCR αβ and TCR γδ CTL in vivo. Immunol. Rev. 1995;146:82–94. doi: 10.1111/j.1600-065x.1995.tb00685.x. [DOI] [PubMed] [Google Scholar]
- 123.Hamann D, Baars PA, Rep MH, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J. Exp. Med. 1997;186(9):1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Lanzavecchia A, Sallusto F. Understanding the generation and function of memory T cell subsets. Curr. Opin. Immunol. 2005;17(3):326–332. doi: 10.1016/j.coi.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 125.Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4+ T cells. J. Exp. Med. 2001;194(12):1711–1719. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Yamada H, Matsuzaki G, Chen Q, Iwamoto Y, Nomoto K. Reevaluation of the origin of CD44high “memory phenotype” CD8 T cells: comparison between memory CD8 T cells and thymus-independent CD8 T cells. Eur. J. Immunol. 2001;31(6):1917–1926. doi: 10.1002/1521-4141(200106)31:6<1917::aid-immu1917>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 127.Tough DF, Sun S, Zhang X, Sprent J. Stimulation of naive and memory T cells by cytokines. Immunol. Rev. 1999;170:39–47. doi: 10.1111/j.1600-065x.1999.tb01327.x. [DOI] [PubMed] [Google Scholar]
- 128.Cohavy O, Targan SR. CD56 marks an effector T cell subset in the human intestine. J. Immunol. 2007;178(9):5524–5532. doi: 10.4049/jimmunol.178.9.5524. [DOI] [PubMed] [Google Scholar]
- 129.Pittet MJ, Speiser DE, Valmori D, Cerottini JC, Romero P. Cutting edge: cytolytic effector function in human circulating CD8+ T cells closely correlates with CD56 surface expression. J. Immunol. 2000;164(3):1148–1152. doi: 10.4049/jimmunol.164.3.1148. [DOI] [PubMed] [Google Scholar]
- 130.Gupta S, Gollapudi S. CD95-mediated apoptosis in naive, central and effector memory subsets of CD4+ and CD8+ T cells in aged humans. Exp. Gerontol. 2008;43(4):266–274. doi: 10.1016/j.exger.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 131.Cannons JL, Lau P, Ghumman B, et al. 4-1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy. J. Immunol. 2001;167(3):1313–1324. doi: 10.4049/jimmunol.167.3.1313. [DOI] [PubMed] [Google Scholar]
- 132.Mackey MF, Barth RJ, Jr, Noelle RJ. The role of CD40/CD154 interactions in the priming, differentiation, and effector function of helper and cytotoxic T cells. J. Leukoc. Biol. 1998;63(4):418–428. doi: 10.1002/jlb.63.4.418. [DOI] [PubMed] [Google Scholar]
- 133.Ishii T, Ohnuma K, Murakami A, et al. CD26-mediated signaling for T cell activation occurs in lipid rafts through its association with CD45RO. Proc. Natl Acad. Sci. USA. 2001;98(21):12138–12143. doi: 10.1073/pnas.211439098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Slifka MK, Whitton JL. Activated and memory CD8+ T cells can be distinguished by their cytokine profiles and phenotypic markers. J. Immunol. 2000;164(1):208–216. doi: 10.4049/jimmunol.164.1.208. [DOI] [PubMed] [Google Scholar]
- 135.Holse M, Assing K, Poulsen LK. CCR3, CCR5, CCR8 and CXCR3 expression in memory T helper cells from allergic rhinitis patients, asymptomatically sensitized and healthy individuals. Clin. Mol. Allergy. 2006;4:6. doi: 10.1186/1476-7961-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nicholson JK, Browning SW, Hengel RL, et al. CCR5 and CXCR4 expression on memory and naive T cells in HIV-1 infection and response to highly active antiretroviral therapy. J. Acquir. Immune Defic. Syndr. 2001;27(2):105–115. doi: 10.1097/00126334-200106010-00002. [DOI] [PubMed] [Google Scholar]
- 137.Fukada K, Sobao Y, Tomiyama H, Oka S, Takiguchi M. Functional expression of the chemokine receptor CCR5 on virus epitope-specific memory and effector CD8+ T cells. J. Immunol. 2002;168(5):2225–2232. doi: 10.4049/jimmunol.168.5.2225. [DOI] [PubMed] [Google Scholar]
- 138.Lepej SZ, Rode OD, Jeren T, Vince A, Remenar A, Barsic B. Increased expression of CXCR3 and CCR5 on memory CD4+ T-cells migrating into the cerebrospinal fluid of patients with neuroborreliosis: the role of CXCL10 and CXCL11. J. Neuroimmunol. 2005;163(1–2):128–134. doi: 10.1016/j.jneuroim.2005.03.005. [DOI] [PubMed] [Google Scholar]
- 139.Hutloff A, Dittrich AM, Beier KC, et al. ICOS is an inducible T-cell co-stimulator structurally and functionally related to CD28. Nature. 1999;397(6716):263–266. doi: 10.1038/16717. [DOI] [PubMed] [Google Scholar]
- 140.Sharpe AH, Wherry EJ, Ahmed R, Freeman GJ. The function of programmed cell death 1 and its ligands in regulating autoimmunity and infection. Nat. Immunol. 2007;8(3):239–245. doi: 10.1038/ni1443. [DOI] [PubMed] [Google Scholar]
- 141.Galvan M, Murali-Krishna K, Ming LL, Baum L, Ahmed R. Alterations in cell surface carbohydrates on T cells from virally infected mice can distinguish effector/memory CD8+ T cells from naive cells. J. Immunol. 1998;161(2):641–648. [PubMed] [Google Scholar]
- 142.Harrington LE, Galvan M, Baum LG, Altman JD, Ahmed R. Differentiating between memory and effector CD8 T cells by altered expression of cell surface O-glycans. J. Exp. Med. 2000;191(7):1241–1246. doi: 10.1084/jem.191.7.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]