Abstract
Diastolic dysfunction is usually identified by the combination of characteristic mitral and pulmonary vein flow patterns. However, obtaining a complete set of echocardiographic parameters can be technically difficult and data may conflict. We hypothesized that, as a stand alone variable, diastolic (ventricular diastole) dominant pulmonary vein flow predicts heart failure (HF) hospitalizations and cardiovascular (CV) death. Standard transthoracic echocardiograms were performed in 906 participants from the Heart and Soul Study, a prospective study of the effects of depression on coronary heart disease. Pulmonary vein flow pattern was determined by the dominant velocity time integral. Cardiac events were determined by two independent adjudicators and Cox proportional hazards models were used. Systolic dominant pulmonary vein flow was present in 89% of the participants, and diastolic dominant in the remaining 11%. During an average 4.1 years of follow-up, participants with diastolic dominant pulmonary vein flow had a 25% rate of HF hospitalization and 9% rate of CV death. After multivariate adjustment including left ventricular ejection fraction, diastolic pulmonary vein flow was associated with a three-fold risk for HF hospitalization (p=0.001) and a two-fold risk for HF hospitalization or death (p=0.004). In conclusion, diastolic dominant pulmonary vein flow pattern is a stand alone predictor of adverse cardiac events and its presence is associated with significantly higher rates of HF hospitalizations and CV death.
Keywords: pulmonary vein flow, echocardiography, heart failure, prognosis
INTRODUCTION
Normal pulmonary vein flow pattern in middle age and beyond is systolic (ventricular systole) dominant. 1,2 Although diastolic dominant pulmonary vein flow pattern has been validated as diagnostic of increased LV filling pressures, 3–5 its prognostic significance as an independent variable has not been evaluated. In addition, some studies have found that pulmonary vein flow pattern may not correlate well with LV filling pressures in patients with preserved LV ejection fraction. 6,7 To further define the value of pulmonary vein flow, we examined these flow patterns in ambulatory patients with coronary heart disease and hypothesized that diastolic dominant pulmonary vein flow is a stand alone echocardiographic parameter that independently predicts heart failure (HF) hospitalization and cardiovascular (CV) death.
METHODS
Participants were enrolled in the Heart and Soul Study, a prospective cohort study investigating the influence of psychosocial factors on cardiovascular events. Methods have been described previously. 8 Administrative databases were used to identify outpatients with documented coronary artery disease at two Department of Veterans Affairs (VA) medical center databases (San Francisco and Palo Alto, California), one University-based medical center (University of California Medical Center–San Francisco), and 9 public health clinics in the Community Health Network of San Francisco, California. Criteria for enrollment included one of the following: (1) history of myocardial infarction (MI); (2) angiographic evidence of at least 50% stenosis in at least one coronary vessel; (3) evidence of exercise-induced ischemia by treadmill electrocardiogram or stress nuclear perfusion imaging; (4) or history of coronary revascularization. Participants were excluded if they deemed themselves unable to walk one block, had an acute coronary syndrome in the prior 6 months, or were planning to move out of the local area within 3 years.
The study protocol was approved by the institutional review boards at each of the participating sites, and all participants provided written informed consent.
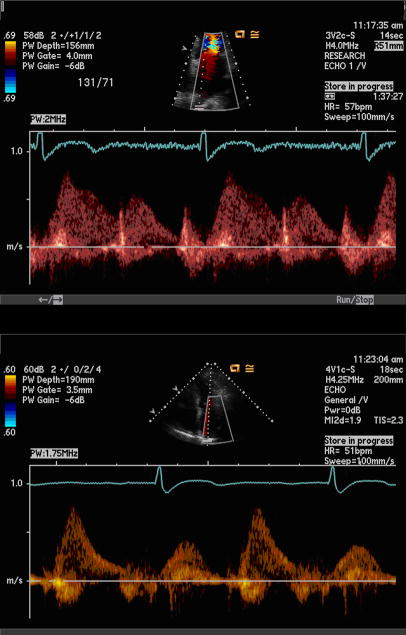
We performed echocardiography in the standard left lateral recumbent and supine positions with a commercially available ultrasound system with harmonic imaging (Acuson Sequoia, Siemens Corp, Mountain View, California). From the standard apical four-chamber view, pulse-wave Doppler signal of the right superior pulmonary vein was obtained according to guidelines of the American Society of Echocardiography. 9 The pulmonary vein flow was first visualized using color Doppler at a low velocity scale (< 40 cm/s). 9 Using a small sample volume and low wall filter, the pulmonary vein flow velocity was recorded with pulse-wave Doppler. (Figure 1) Maximum velocity time integral was used to determine flow pattern dominance. We excluded participants with non-sinus rhythm, non-native valves, at least moderate mitral or aortic regurgitation, and technically difficult pulmonary vein Doppler signal.
Figure 1.
Pulse-wave Doppler of systolic dominant pulmonary vein flow (top) and diastolic dominant pulmonary vein flow (bottom).
Prespecified end-points included all-cause mortality, incident hospitalization for heart failure, and death from heart disease during follow-up. We conducted annual telephone follow-up interviews with participants (or their proxy) to ask about death or hospitalization for “heart trouble.” For any reported event, medical records, EKGs, death certificates, and coroner’s reports were retrieved and reviewed by two independent and blinded adjudicators. If the adjudicators agreed on the outcome classification, their classification was binding. In the event of disagreement, the adjudicators conferred, reconsidered their classification, and requested consultation from a third blinded adjudicator.
All-cause mortality was determined by review of death certificates. Myocardial infarction (MI) was defined using standard diagnostic criteria. 10 Death was considered due to coronary heart disease if: (a) the participant died during the same hospitalization in which acute MI was the primary diagnosis; or (b) the participant experienced sudden coronary heart disease death defined as an unexpected, otherwise unexplained fatality within one hour of the onset of terminal symptoms.
Heart failure was defined as hospitalization for a clinical syndrome involving at least two of the following: paroxysmal nocturnal dyspnea, orthopnea, elevated jugular venous pressure, pulmonary rales, third heart sound, cardiomegaly on chest radiography, or pulmonary edema on chest radiograph. 11 These clinical signs and symptoms must have represented a clear change from the normal clinical state of the patient, and must have been accompanied by either failing cardiac output as determined by peripheral hypoperfusion (hypotension in the absence of other causes such as sepsis or dehydration) or peripheral or pulmonary edema. Supportive documentation of reduced cardiac index, rising pulmonary capillary wedge pressure, falling oxygen saturation and end-organ hypoperfusion, if available, were included in adjudication.
Each participant completed a detailed questionnaire that included age, sex, race, medical history, level of physical activity, current smoking, and level of alcohol consumption. Study personnel recorded all current medications and measured height, weight, and blood pressure. Medication categories were categorized using Epocrates Rx (San Mateo, CA). Left ventricular ejection fraction was measured quantitatively using the 2-D echocardiography biplane method of disks. 12,13 We defined left ventricular hypertrophy as left ventricular mass index of >90 g/m2 based on the 2-D echocardiography truncated ellipse method. 14 A symptom-limited, graded exercise treadmill test was performed, and we used stress echocardiography to seek inducible ischemia, defined as the presence of cardiac wall motion abnormality at peak exercise that was not present at rest. A single cardiologist (N. B. S.), blinded to clinical and laboratory information, evaluated all of the echocardiograms. Total cholesterol, low-density lipoprotein (LDL) and high-density lipoprotein (HDL) were measured from fasting serum samples. Creatinine clearance was determined by 24-hour urine.
The goal of this study was to examine the association of pulmonary venous flow pattern with cardiovascular outcomes. Differences in participant characteristics by pattern of pulmonary venous flow were determined using analysis of variance for continuous variables and χ2 tests for dichotomous variables. We used Cox proportional hazards models to evaluate the independent association of diastolic dominant pulmonary venous flow pattern with cardiovascular events after adjusting for all variables in Table 1. For these analyses, we report hazard ratios (HR) with 95% confidence intervals (CI). Analyses were performed using Statistical Analysis Software (Version 9, SAS Institute Inc, Cary, NC).
Table 1.
Characteristics of study participants.
| Variable | Systolic Dominant (n=803) | Diastolic Dominant (n=103) | P value |
|---|---|---|---|
| Age (years) | 66.5±10.3 | 67.3±11.6 | 0.4 |
| Male sex | 655 (82%) | 89 (86%) | 0.3 |
| White race | 470 (59%) | 68 (66%) | 0.1 |
| Current smoker | 166 (21%) | 19 (18%) | 0.6 |
| Regular alcohol use | 231 (29%) | 30 (29%) | 0.9 |
| Not physically active | 290 (36%) | 34 (33%) | 0.5 |
| Hypertension | 569 (71%) | 78 (76%) | 0.3 |
| Diabetes mellitus | 200 (25%) | 40 (39%) | 0.003 |
| Prior myocardial infarction | 419 (53%) | 62 (60%) | 0.1 |
| Prior stroke | 110 (14%) | 17 (17%) | 0.5 |
| Prior revascularization | 451 (56%) | 83 (81%) | <0.0001 |
| Beta blocker | 462 (58%) | 71 (69%) | 0.03 |
| Angiotensin converting enzyme inhibitor or angiotensin receptor blocker | 392 (49%) | 57 (55%) | 0.2 |
| Statin | 520 (65%) | 71 (69%) | 0.4 |
| Aspirin | 641 (80%) | 87 (84%) | 0.3 |
| Left ventricular hypertrophy | 426 (54%) | 67 (65%) | 0.03 |
| Left ventricular mass index | 96.7±24.8 | 101.4±28.4 | 0.08 |
| Left ventricular ejection fraction | 62.5±9.0 | 59.2±11.9 | 0.003 |
| Left atrial volume | 60.4±19.5 | 74.0±26.8 | <0.0001 |
| Inducible ischemia | 160 (22%) | 23 (25%) | 0.004 |
| Body mass index (kg/m2) | 28.3±5.0 | 28.5±6.1 | 0.7 |
| LDL (mg/dl)| | 105.6±34.0 | 98.9±28.7 | 0.09 |
| HDL (mg/dl) | 45.9±14.1 | 45.5±13.7 | 0.6 |
| SBP (mm Hg) | 132.7±20.5 | 135.3±23.1 | 0.3 |
| DBP (mm Hg) | 74.9±11.4 | 72.5±11.0 | 0.05 |
| Heart rate (beats per min) | 68.0±11.8 | 62.7±11.6 | <0.0001 |
| Creatinine clearance | 83.2±27.8 | 77.0±30.4 | 0.04 |
| Log N-terminal prohormone brain natriuretic peptide | 5.0±1.2 | 6.0±1.4 | <0.0001 |
| Subsequent revascularization | 109 (14%) | 13 (13%) | 0.79 |
RESULTS
Between September 2000 and December 2002, a total of 1024 participants were enrolled in the Heart and Soul Study. After exclusions, the analytic sample was 906 participants.
Systolic dominant pulmonary vein flow was present in 89% of the participants, and diastolic dominant in the remaining 11%. Participants with diastolic dominant pulmonary vein flow were more likely to have diabetes mellitus, lower left ventricular ejection fraction, inducible ischemia, beta-blocker therapy, and lower creatinine clearance. They were also more likely to have undergone coronary revascularization. (Table 1)
During an average of 4.1±1.1 years of follow-up, participants with diastolic dominant pulmonary vein flow had a higher rate of hospitalization for heart failure (25% vs. 8%; p<0.0001), hospitalization for heart failure or death (36% vs. 18%; p<0.0001), cardiovascular death (9% vs. 4%; p=0.04), and all-cause death (28% vs. 15%; p=0.0007) than those with systolic dominant pulmonary vein flow. (Table 2).
Table 2.
Outcomes - number (proportion of column).
| Variable | Systolic dominant (n=803) | Diastolic dominant (n=103) | P value |
|---|---|---|---|
| All-cause mortality | 120 (15%) | 29 (28%) | 0.0007 |
| Heart failure Hospitalization | 62 (8%) | 25 (25%) | <0.0001 |
| Heart failure hospitalization or death | 145 (18%) | 37 (36%) | <0.0001 |
| Cardiovascular Death | 33(4%) | 9(9%) | 0.04 |
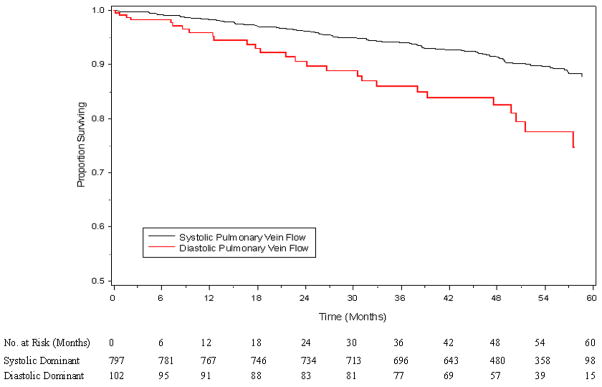
After multivariable adjustment for baseline characteristics in Table 1, diastolic dominant pulmonary vein flow pattern has almost a three-fold risk for hospitalization for heart failure (p=0.001) and a two-fold risk for hospitalization for heart failure or death (p=0.004). (Table 3) Diastolic dominant pulmonary vein flow pattern resulted in a statistically significant decrease in survival free of heart failure during follow-up. (Figure 2)
Table 3.
| Variable | Hazard Ratio (95% CI) All-cause mortality | P value | Hazard Ratio (95% CI) Heart failure hospitalization | P value | Hazard Ratio (95% CI) Heart failure hospitalization or death | P value |
|---|---|---|---|---|---|---|
| Unadjusted | 1.9 (1.2–2.8) | 0.003 | 3.4 (2.2–5.5) | <0.0001 | 2.2(1.5–3.2) | <0.0001 |
| Adjusted for demographic characteristics | 1.8 (1.2–2.8) | 0.003 | 3.6 (2.3–5.8) | <0.0001 | 2.2(1.5–3.2) | <0.0001 |
| Adjusted for above plus medical history | 1.8 (1.2–2.7) | 0.006 | 3.1 (1.9–5.1) | <0.0001 | 2.1(1.4–3.0) | 0.0002 |
| Adjusted for above plus medication use | 1.8 (1.2–2.7) | 0.008 | 3.1 (1.9–5.1) | <0.0001 | 2.0(1.3–2.9) | 0.0005 |
| Adjusted for above plus disease severity | 1.4(0.8–2.5) | 0.24 | 3.1 (1.5–6.1) | 0.001 | 2.1(1.3–3.4) | 0.004 |
Figure 2.
Survival free of heart failure or death, according to pulmonary vein flow, adjusted for all variables in Table 1 (p=0.0002).
DISCUSSION
We found that diastolic dominant pulmonary vein flow pattern is present in 11% of stable ambulatory participants with coronary heart disease and predicts heart failure hospitalization or death. Our results highlight the importance of obtaining pulmonary vein flow patterns in standard echocardiograms. Furthermore, because there are no consensus criteria for categorization of diastolic dysfunction and because Doppler patterns of diastolic functions may conflict, 11,15,16 pulmonary vein flow pattern provides a stand-alone measurement that identifies patients at risk for cardiovascular events. Additionally, pulmonary vein flow is obtained with high-yield (1001 out of 1024 participants), demonstrating robust clinical utility.
Pulmonary vein flow is systolic dominant in healthy adults and correlates with LV filling pressures. 1,3,4 Although some studies have shown that pulmonary vein flow pattern may not correlate with pulmonary artery wedge pressure in patients with normal LV ejection fraction, 6,7 our study suggest that pulmonary vein flow is predictive of cardiovascular events even after adjustment for LV ejection fraction. In addition, diastolic dominant pulmonary vein flow was predictive of outcomes even after adjusting for variables known to be associated with increased LV filling pressures, such as left atrial volume, LV mass, and NT-proBNP. Thus in our study population, diastolic dominant pulmonary vein flow pattern likely represents elevated LV filling pressure due to coronary artery disease and its comorbidities.
We believe that the unique features of this study include its large sample size, comprehensive measurement of potential confounding variables, and meticulous long-term follow-up. Nonetheless, limitations should be considered. First, our study excluded participants with arrhythmias and valvular disease. However, including these participants would likely improve the predictability of diastolic dominant pulmonary vein flow because these participants would probably have higher event rates. Second, other echocardiographic parameters of diastolic dysfunction such as Doppler tissue imaging for mitral annular velocity and Valsalva maneuver were not performed. However, the purpose of our study was to use pulmonary vein flow pattern as a simple, high-yield, stand-alone method to predict outcome. Finally, our study participants were primarily older men with stable coronary heart disease, and our results may be less applicable to other populations.
Acknowledgments
This work was supported by grants from the Department of Veterans Affairs, Washington, DC, the National Heart Lung and Blood Institute (R01 HL079235), the American Federation for Aging Research (Paul Beeson Scholars Program), New York, NY, the Robert Wood Johnson Foundation (Faculty Scholars Program), Princeton, NJ, the Ischemia Research and Education Foundation, and the Nancy Kirwan Heart Research Fund, San Francisco, CA.
Footnotes
There are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Sohn DW, Chai IH, Lee DJ, Kim HC, Kim HS, Oh BH, Lee MM, Park YB, Choi YS, Seo JD, Lee YW. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol. 1997;30:474–480. doi: 10.1016/s0735-1097(97)88335-0. [DOI] [PubMed] [Google Scholar]
- 2.Kuecherer HF, Muhiudeen IA, Kusumoto FM, Lee E, Moulinier LE, Cahalan MK, Schiller NB. Estimation of mean left atrial pressure from transesophageal pulsed Doppler echocardiography of pulmonary venous flow. Circulation. 1990;82:1127–1139. doi: 10.1161/01.cir.82.4.1127. [DOI] [PubMed] [Google Scholar]
- 3.Keren G, Sherez J, Megidish R, Levitt B, Laniado S. Pulmonary venous flow pattern--its relationship to cardiac dynamics. A pulsed Doppler echocardiographic study. Circulation. 1985;71:1105–1112. doi: 10.1161/01.cir.71.6.1105. [DOI] [PubMed] [Google Scholar]
- 4.Nishimura RA, Abel MD, Hatle LK, Tajik AJ. Relation of pulmonary vein to mitral flow velocities by transesophageal Doppler echocardiography. Effect of different loading conditions. Circulation. 1990;81:1488–1497. doi: 10.1161/01.cir.81.5.1488. [DOI] [PubMed] [Google Scholar]
- 5.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 6.Rivas-Gotz C, Manolios M, Thohan V, Nagueh SF. Impact of left ventricular ejection fraction on estimation of left ventricular filling pressures using tissue Doppler and flow propagation velocity. Am J Cardiol. 2003;91:780–784. doi: 10.1016/s0002-9149(02)03433-1. [DOI] [PubMed] [Google Scholar]
- 7.Yamamoto K, Nishimura RA, Chaliki HP, Appleton CP, Holmes DR, Jr, Redfield MM. Determination of left ventricular filling pressure by Doppler echocardiography in patients with coronary artery disease: critical role of left ventricular systolic function. J Am Coll Cardiol. 1997;30:1819–1826. doi: 10.1016/s0735-1097(97)00390-2. [DOI] [PubMed] [Google Scholar]
- 8.Ruo B, Rumsfeld JS, Hlatky MA, Liu H, Browner WS, Whooley MA. Depressive symptoms and health-related quality of life: the Heart and Soul Study. Jama. 2003;290:215–221. doi: 10.1001/jama.290.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Quinones MA, Otto CM, Stoddard M, Waggoner A, Zoghbi WA. Recommendations for quantification of Doppler echocardiography: a report from the Doppler Quantification Task Force of the Nomenclature and Standards Committee of the American Society of Echocardiography. J Am Soc Echocardiogr. 2002;15:167–184. doi: 10.1067/mje.2002.120202. [DOI] [PubMed] [Google Scholar]
- 10.Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, Goldberg RJ, Hand MM, Jaffe AS, Julian DG, Levy D, Manolio T, Mendis S, Mensah G, Pajak A, Prineas RJ, Reddy KS, Roger VL, Rosamond WD, Shahar E, Sharrett AR, Sorlie P, Tunstall-Pedoe H. Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–2549. doi: 10.1161/01.CIR.0000100560.46946.EA. [DOI] [PubMed] [Google Scholar]
- 11.Bursi F, Weston SA, Redfield MM, Jacobsen SJ, Pakhomov S, Nkomo VT, Meverden RA, Roger VL. Systolic and diastolic heart failure in the community. Jama. 2006;296:2209–2216. doi: 10.1001/jama.296.18.2209. [DOI] [PubMed] [Google Scholar]
- 12.Quinones MA, Waggoner AD, Reduto LA, Nelson JG, Young JB, Winters WL, Jr, Ribeiro LG, Miller RR. A new, simplified and accurate method for determining ejection fraction with two-dimensional echocardiography. Circulation. 1981;64:744–753. doi: 10.1161/01.cir.64.4.744. [DOI] [PubMed] [Google Scholar]
- 13.Cheitlin MD, Alpert JS, Armstrong WF, Aurigemma GP, Beller GA, Bierman FZ, Davidson TW, Davis JL, Douglas PS, Gillam LD, Lewis RP, Pearlman AS, Philbrick JT, Shah PM, Williams RG, Ritchie JL, Eagle KA, Gardner TJ, Garson A, Gibbons RJ, O’Rourke RA, Ryan TJ. ACC/AHA guidelines for the clinical application of echocardiography: executive summary. A report of the American College of Cardiology/American Heart Association Task Force on practice guidelines (Committee on Clinical Application of Echocardiography). Developed in collaboration with the American Society of Echocardiography. J Am Coll Cardiol. 1997;29:862–879. doi: 10.1016/s0735-1097(96)90000-5. [DOI] [PubMed] [Google Scholar]
- 14.Schiller NB, Shah PM, Crawford M, DeMaria A, Devereux R, Feigenbaum H, Gutgesell H, Reichek N, Sahn D, Schnittger I, et al. Recommendations for quantitation of the left ventricle by two-dimensional echocardiography. American Society of Echocardiography Committee on Standards, Subcommittee on Quantitation of Two-Dimensional Echocardiograms. J Am Soc Echocardiogr. 1989;2:358–367. doi: 10.1016/s0894-7317(89)80014-8. [DOI] [PubMed] [Google Scholar]
- 15.Yu CM, Lin H, Yang H, Kong SL, Zhang Q, Lee SW. Progression of systolic abnormalities in patients with “isolated” diastolic heart failure and diastolic dysfunction. Circulation. 2002;105:1195–1201. doi: 10.1161/hc1002.105185. [DOI] [PubMed] [Google Scholar]
- 16.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. Jama. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]