Abstract
Voltage-dependent anion channels (VDACs) have been implicated as essential mediators of mitochondrial-dependent cell death by functioning as a channel-forming unit within the mitochondrial permeability transition (MPT) pore and the target of Bcl-2 family members. Here we report the effects of deletion of the 3 mammalian Vdac genes on mitochondrial-dependent cell death. Mitochondria from Vdac1-, Vdac3-, and Vdac1/Vdac3-null mice exhibited a Ca2+ and oxidative stress-induced MPT that was indistinguishable from wildtype mitochondria. Similarly, Ca2+ and oxidative-stress-induced MPT and cell death was unaltered or even exacerbated in fibroblasts lacking VDAC1, VDAC2, VDAC3, VDAC1/3, and VDAC1/2/3. Wildtype and Vdac-deficient mitochondria and cells also exhibited equivalent cytochrome c release, caspase cleavage, and cell death in response to Bax and Bid activation. These results indicate that VDACs are dispensable for both MPT and Bcl-2 family member-driven cell death.
Mitochondria are intracellular organelles that mediate high-energy phosphate production, fatty acid metabolism, porphyrin synthesis, ion homeostasis and apoptotic and necrotic cell death. Apoptotic cell death is mediated by both the “extrinsic” pathway; consisting of death receptor signaling constituents, as well as the “intrinsic” pathway; consisting of pro-death Bcl-2 family members functioning at the level of the mitochondria and endoplasmic reticulum (1). Mitochondria are also critically involved in necrotic cell death following Ca2+ overload, hypoxia, and oxidative damage, leading to swollen or ruptured mitochondria. The MPT pore, a protein complex that spans both the outer and inner mitochondrial membranes, is considered the mediator of this event and has been hypothesized to minimally consist of the VDAC in the outer membrane, the adenine nucleotide translocase (ANT) in the inner membrane, and cyclophilin-D in the matrix (2-4).
The VDAC is comprised of a family of evolutionarily conserved ion channels that are the most abundant proteins in the outer mitochondrial membrane. The physiologic function of VDACs is to control the movement of adenine nucleotides, NADH, and other metabolites across the outer membrane (5,6). However, VDACs have also been proposed to possess a pathological function as mediators of mitochondrial-dependent cell death through formation of the permeability pore (7,8). In addition, VDACs have been proposed to be essential binding partners for pro-apoptotic Bcl-2 family members (9-12), combining to form protein-permeable channels in the outer membrane that facilitate the release of cytochrome c and activation of the intrinsic apoptotic pathway (9,10).
Mammals possess three distinct Vdac genes, Vdac1, Vdac2, and Vdac3 (5,6), that from a metabolic standpoint, exhibit a high degree of functional redundancy (13). Mice deficient for either Vdac1 or Vdac3 are viable, although they exhibit deficiencies in mitochondrial respiration associated with abnormalities in mitochondrial ultrastructure (14,15). Mitochondria from Vdac1-null mice were also recently shown to have an intact MPT response, although redundancy with Vdac2 and Vdac3 was not addressed (16). Mice with a combined disruption of Vdac1 and Vdac3 are also viable, albeit at less than predicted Mendelian frequencies (17). In contrast, Vdac2 deletion in mice results in embryonic lethality and enhanced activation of the intrinsic apoptotic pathway associated with greater Bak activation at the mitochondria (18).
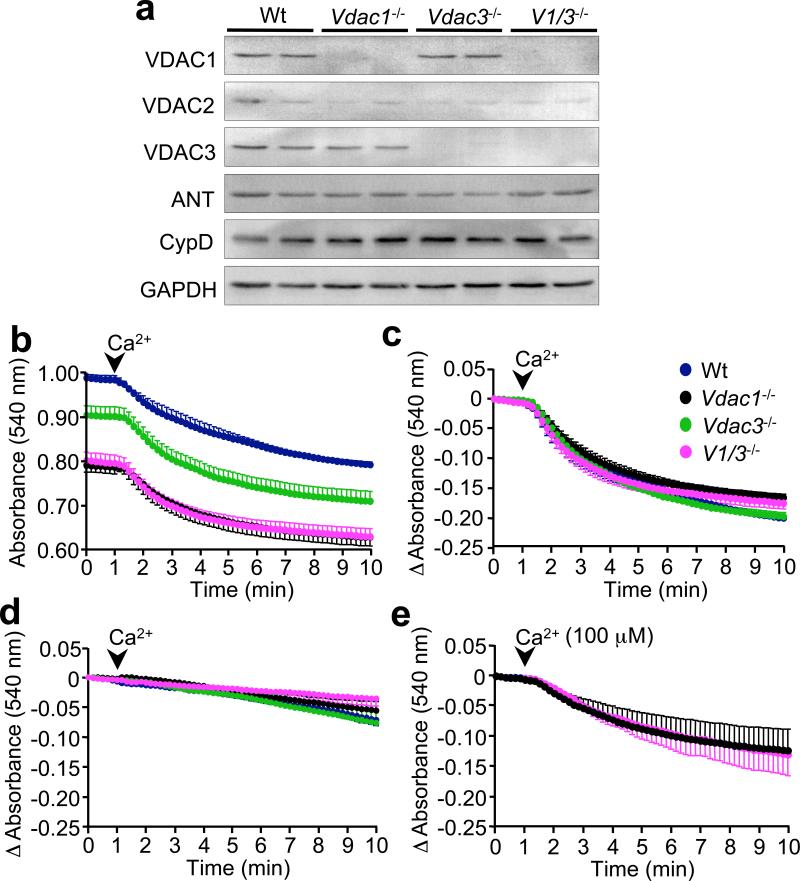
Here we assessed whether MPT was altered in Vdac1-, Vdac3-, and Vdac1/Vdac3-null mice. Western blotting of cardiac lysates from these mice showed the complete lack of the respective VDAC protein in each line without compensatory alterations in the other VDAC isoforms (Fig. 1a). There were also no significant changes in ANT and cyclophilin D, two other putative components of the MPT pore (Fig. 1a). Cardiac mitochondria isolated from wildtype, Vdac1-, Vdac3-, and Vdac1/Vdac3-null mice were assessed for their ability to undergo Ca2+-induced swelling, an index of MPT. Interestingly, both single- and double-null mitochondria exhibited a pronounced swelling at baseline compared to wildtype, most notably in mitochondria lacking Vdac1 (Fig. 1b). This is consistent with previous electron microscopy data demonstrating increased mitochondrial volume in Vdac-deficient cells (14,15). Despite these differences in baseline swelling, mitochondria deficient in Vdac1, Vdac3, or both, showed the same change in swelling after 250 μM Ca2+ (Fig. 1c), and this swelling was still inhibited with cyclosporine A, a blocker of MPT (Fig. 1d). A lower dosage of Ca2+ (100 μM) also induced an identical swelling response, albeit smaller, between wildtype and Vdac1/Vdac3-null mitochondria (Fig. 1e). Similarly, MPT-induced cytochrome c release caused by either Ca2+ or oxidative stress (tert-Butyl-hydroperoxide, [tBH]) was unaltered in Vdac-deficient mitochondria (Fig. 2a, b; also see Supplementary Information, Fig. S1a-d). These data indicate that neither VDAC1 nor VDAC3 are necessary for MPT, at least in vitro.
Figure 1.
Mitochondrial permeability transition in VDAC1- and 3-deficient mitochondria. (a) Western blotting for VDAC1, VDAC2, VDAC3, ANT, and cyclophilin-D (CypD) in wildtype, Vdac1-, Vdac3-, and Vdac1/Vdac3-null mouse hearts. GAPDH was used to demonstrate equivalent loading. (b) Mitochondrial swelling assay measured by absolute absorbance at baseline and after Ca2+ (250 μM) addition (arrowhead) in isolated cardiac mitochondria from each of the indicated genotypes (V1/3 represents Vdac1/Vdac3 double null). (c) The data shown in panel b were also plotted as change in absorbance, which shows that loss of VDAC1 and/or VDAC3 protein does not compromise MPT. (d) Change in absorbance of mitochondria after Ca2+ stimulation in the presence of cyclosporine A (1 μM) for each of the indicated genotypes. (e) Change in absorbance of mitochondrial with a lower dosage of Ca2+ (100 μM). The results represent the average values of at least 4 independent experiments. Error bars indicate s.e.m.
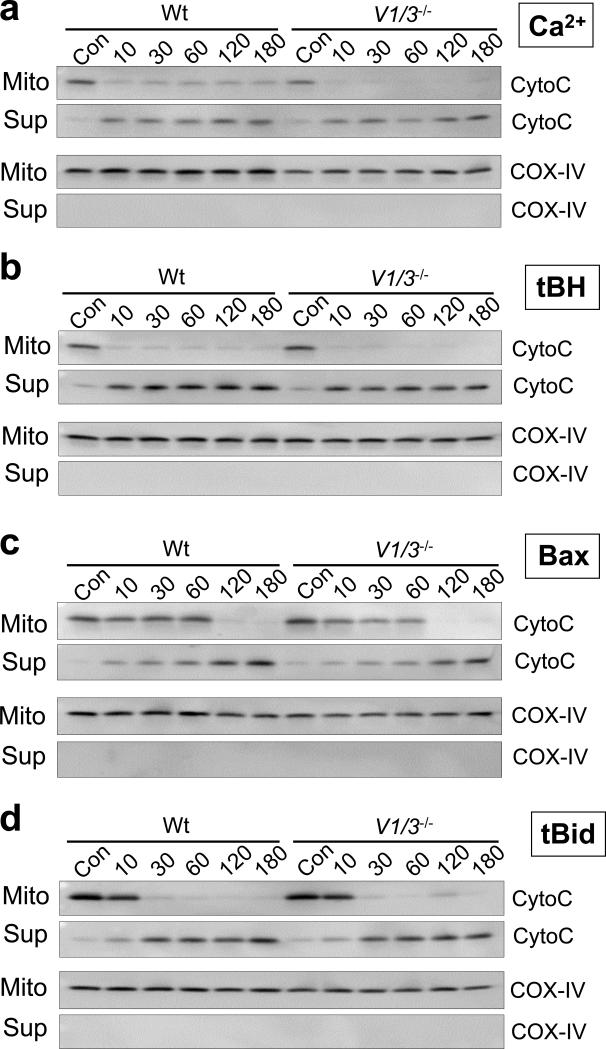
Figure 2.
Cytochrome c release induced by Ca2+, oxidative stress, Bax and tBid. (a-d) Isolated wildtype and Vdac1/Vdac3 null (V1/3−/−) liver mitochondria were incubated with 250 μM Ca2+ (a), 100 μM tert-butyl hydroperoxide (tBH; b), 1 μg GST-Bax (c) or 0.25 μg tBid (d) and incubated over time (in minutes). The control (Con) represents mitochondria treated with vehicle or GST over 180 min. The suspension was centrifuged and the resultant pellet (Mito) and supernatant (Sup) were subjected to Western blotting for cytochrome c (CytoC). COX-IV (cytochrome oxidase IV) was used as a control to show the integrity of the two protein fractions. The results shown are representative of 3 independent experiments.
In addition to their putative role in MPT, VDACs have been proposed as key elements in the intrinsic apoptotic pathway. Reports have indicated that pro-apoptotic Bcl-2 family members can interact with VDACs to form a protein-permeable pore in the outer mitochondrial membrane (9-12). In contrast, others have proposed that Bax/Bak/Bid pro-apoptotic Bcl-2 family members simply form outer membrane channels independent of VDAC (19-22). In light of this controversy, we sought to determine whether the pro-apoptotic effects of Bax (intrinsic pathway) and the active, cleaved form of Bid (tBid, extrinsic pathway) could be abrogated or reduced in the absence of VDACs. Incubation of isolated mitochondria with either recombinant Bax or tBid induced a time-dependent release of cytochrome c that was no different between wildtype, Vdac1-, Vdac3-, and Vdac1/Vdac3-null organelles (Fig. 2c, d; also see Supplementary Information, Fig. S2a-d). Finally, a dose response analysis for cytochrome c release from Vdac1/Vdac3 null mitochondria was performed with Bax, tBid, tBH and Ca2+ to exclude the possibility that a lower dosage might show a differential effect. However, lower dosages of these agents did not show a difference compared with wildtype in causing mitochondrial cytochrome c release (Supplementary Information, Fig. S3a).
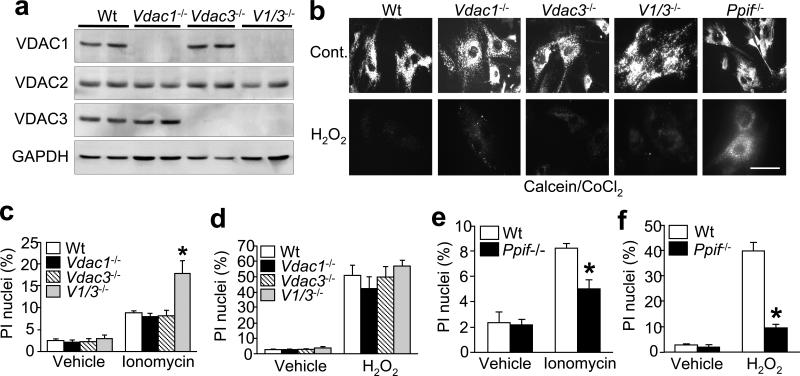
We next examined the effects associated with Vdac1/Vdac3 deletion on MPT in living cells. To this end, mouse embryonic fibroblasts (MEFs) from wildtype, Vdac1-, Vdac3-, and Vdac1/Vdac3-null embryos were generated and examined. Western blot analyses of cellular lysates again revealed loss of the respective VDAC isoforms in each genotype without compensatory changes in the other VDAC isoforms, ANT, or CypD (Fig. 3a, and data not shown). In order to assess MPT in living cells, we utilized the calcein/CoCl2 fluorescence technique (23). Treatment of wildtype MEFs with H2O2 resulted in a loss of calcein fluorescence indicative of MPT (Fig. 3b), whereas MEFs lacking Ppif (cyclophilin D encoding gene) were resistant to MPT and maintained mitochondrial fluorescence (Fig. 3b, and ref 24). In contrast, Vdac1-, Vdac3-, and Vdac1/Vdac3-null MEFs exhibited the same loss of mitochondrial fluorescence in response to H2O2 as wildtype cells. In parallel, TMRE-dependent mitochondrial transmembrane potential (ΔΨm) was measured as an indirect indicator of MPT, which demonstrated a similar level of dissipation between wildtype and Vdac-deficient cells (data not shown).
Figure 3.
Mitochondrial permeability transition and cell death in Vdac1- and Vdac3-null MEFs. (a) Western blotting for VDAC1, VDAC2, VDAC3, and GAPDH in wildtype, Vdac1-, Vdac3-, and Vdac1/Vdac3–null MEFs. (b) H2O2-induced MPT determined by calcein/CoCl2 fluorescence in Vdac1-, Vdac3-, Vdac1/Vdac3- and Ppif-null MEFs. Bar = 50 μm. (c, d) Propidium iodide (PI) staining in wildtype, Vdac1-, Vdac3-, and Vdac1/Vdac3-null MEFs treated with 10 μM ionomycin (c) for 18 h or 500 μM H2O2 (d) for 6 h. (e) PI staining in wildtype and Ppif-deficient MEFs treated with 10 μM ionomycin for 18 h or (f) 500 μM H2O2 for 6 h. Results are indicative of 3 independent experiments. Error bars are s.e.m., and the asterisk denotes P<0.05 versus wildtype.
We also examined the effects associated with Vdac1/Vdac3 deletion on cell death. Consistent with the MPT data discussed above, Vdac1-, Vdac3-, and Vdac1/Vdac3-null MEFs retained sensitivity to both ionomycin- and H2O2-induced death (Fig. 3c, d). In fact, MEFs lacking both Vdac1 and Vdac3 were more sensitive to Ca2+ overload-induced death than wildtype or single-null MEFs (Fig. 3c). However, MEFs lacking Ppif showed partial protection from both ionomycin- and H2O2-induced death (Fig. 3e, f). Similarly, Vdac1- and Vdac3-null mice were not protected in vivo against myocardial ischaemia/reperfusion injury (see Supplementary Information, Fig. S3b), in contrast to significant protection observed in mice lacking Ppif (24).
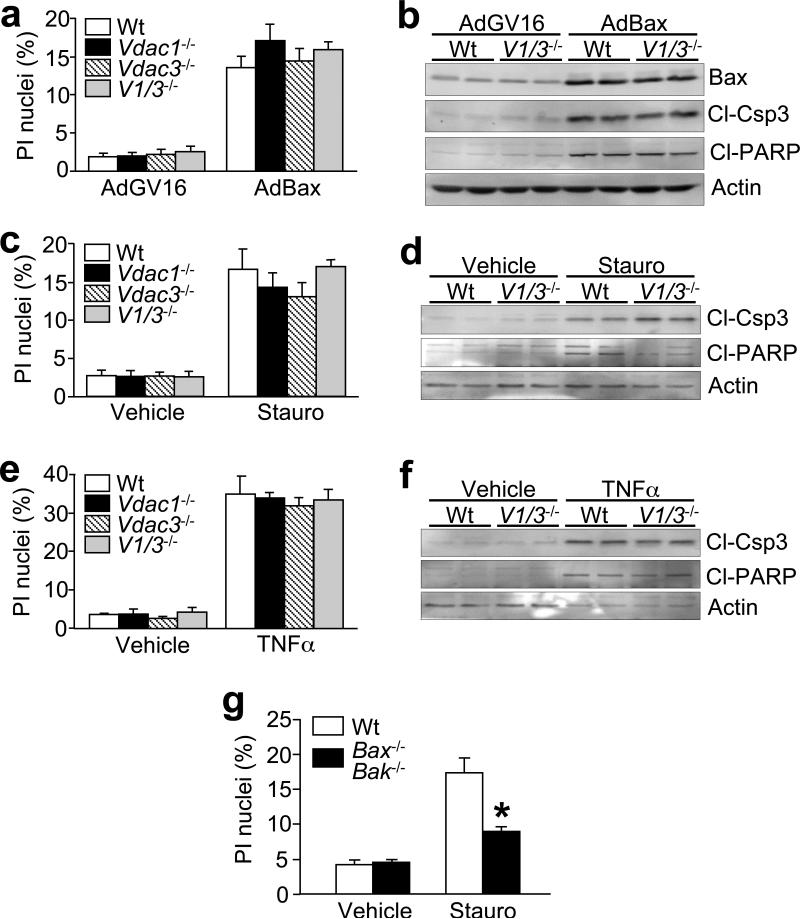
Other more classic inducers of apoptotic cell death were also investigated. Forced expression of Bax through adenoviral transduction elicited equivalent cell death, caspase-3 cleavage, and poly ADP-ribose polymerase (PARP) cleavage in wildtype, Vdac1-, Vdac3-, and Vdac1/Vdac3-null MEFs (Fig. 4a, b). Similarly, activation of endogenous Bax and Bid by staurosporine and tumour necrosis factor (TNF)-α, respectively, induced comparable degrees of cell death and protein cleavage between the different genotypes of MEFs (Fig. 4c-f). As an important control, Bax/Bak double null MEFs showed a significant reduction in staurosporine-induced death (Fig. 4g). Thus, the intrinsic and extrinsic apoptotic pathways appear to progress through a mechanism that is independent of VDAC1 and VDAC3.
Figure 4.
Cell death in Vdac-deficient MEFs. (a) Propidium iodide (PI) staining in wildtype, Vdac1-, Vdac3-, and Vdac1/Vdac3-null MEFs infected with adenoviruses encoding inducible Bax (AdBax). (b) Western blotting for Bax, cleaved caspase-3 (Cl-Csp3), cleaved PARP (Cl-PARP), and actin was performed in parallel. (c) PI staining in wildtype, Vdac1-, Vdac3-, and Vdac1/Vdac3-null MEFs treated with 300 nM staurosporine (Stauro) for 18 h. (d) Western blotting for cleaved caspase-3, cleaved PARP, and actin was also analyzed in parallel. (e) PI staining in wildtype, Vdac1-, Vdac3-, and Vdac1/Vdac3-null MEFs treated with 3 ng/mL TNFα plus 0.1 μg/mL actinomycin-D for 18 h, (f) and corresponding western blotting for caspase-3 and PARP cleavage. (g) PI staining in wildtype and Bax/Bak double-null MEFs following 300 nM staurosporine (Stauro) treatment for 18 h. All graphs show the average of 4 independent experiments. Error bars are s.e.m., and the asterisk denotes P<0.05 versus wildtype.
To confirm and extend the cell death data, we also performed a careful series of experiments in Vdac1/Vdac3 double-null MEFs using multiple dosages of apoptotic and necrotic agents to determine if protection occurs at lower levels of stimulation. Vdac1/Vdac3 double-null MEFs showed no protection from H2O2, ionomycin, staurosporine, or TNFα stimulation at any dosage used, and some dosages even showed a significant increase in cell death (see Supplementary Information Fig, S4a-f). Collectively, these results suggest that Vdac1 and Vdac3 are not primary regulators of cell death, further suggesting that VDACs are not essential components of the MPT in vivo.
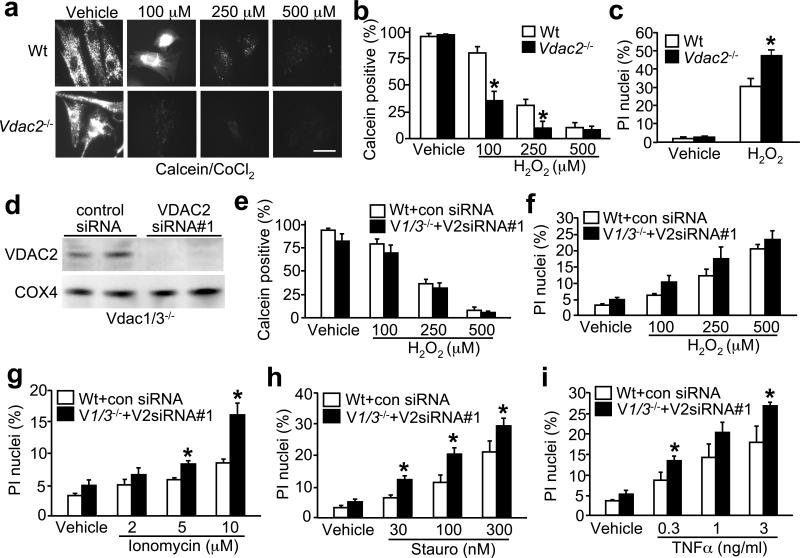
While the data presented to this point suggest that VDAC1/3 are not involved in MPT and cell death, we were concerned about a potential compensatory effect for VDAC2. However, VDAC2 has recently been shown to act as a cytoprotective agent through the specific sequestration and inactivation of the pro-death protein Bak (18). Indeed, Vdac2-null mice are embryonic lethal, presumably with excessive apoptosis (18). Despite these data, we addressed the issue of compensation by analyzing Vdac2-null mitochondria and MEFs for MPT and cell death, as well as MEFs lacking all three VDAC isoforms. Stimulation of Vdac2-null MEFs with H2O2 caused a reduction in calcein fluorescence and MPT at a dose (100 μM) that had only moderate effects on wildtype cells (Fig. 5a, b). Consistent with these data, Vdac2-null MEFs exhibited enhanced cell death in response to oxidative stress (Fig. 5c). However, to more directly address compensation by VDAC2, MEFs null for Vdac1/Vdac3 were treated with a siRNA against VDAC2, resulting in a greater than 98% reduction in total protein levels (Fig. 5d). Importantly, MEFs essentially lacking all three VDAC isforms showed no defect in MPT following multiple dosages of H2O2 stimulation (Fig. 5e). MEFs lacking all three VDAC isoforms also showed similar levels of cell death, or even enhanced death, following stimulation with H2O2, ionomycin, staurosporine, and TNFα (Fig. 5f-i).
Figure 5.
MPT and cell death in Vdac2-null fibroblasts and in MEFs deficient in VDAC1/2/3 protein. (a, b) Calcein/CoCl2 fluorescence in wildtype and Vdac2-null MEFs treated with 100, 250, and 500 μM H2O2. Bar = 50 μm. Graph shows the average of 4 independent experiments. Error bars are s.e.m and asterisk denotes P<0.05 versus wildtype. (c) PI staining for cell death in wildtype and vdac2-null MEFs treated with 500 μM H2O2 for 6 h. (d) Western blotting for VDAC2 protein from Vdac1/Vdac3 double null MEFs treated with a control scrambled siRNA or an siRNA (#1) against VDAC2. COX4 blotting shows equal amounts of protein and mitochondria in each sample. (e) Calcein fluorescence-based quantitation of MPT in MEFs transfected with the indicated siRNAs and treated with the indicated dosages of H2O2 for 6 h. (f) PI staining for cell death in wildtype and Vdac1/Vdac3-null MEFs transfected with the indicated siRNAs and treated with the indicated dosages of H2O2 for 6 h, (g) ionomycin for 18 h, (h) staurosporine for 18 h, and (i) TNFα plus 0.1 μg/mL actinomycin-D for 18 h. Graphs show the average of 3 independent experiments. Error bars are s.e.m., and asterisks denote P<0.05 versus wildtype of the same treatment condition.
A potential concern with the data presented above is the reliance on a single siRNA as a means of disrupting VDAC2 expression. Obviously it would be desirable to simply generate Vdac1/2/3 triple null MEFs from gene-targeted mice, but this is nearly impossible for technical reasons (Vdac2−/− are early embryonic lethal and Vdac1/3−/− have significant fertility issues, as do combinatorial heterozygotes). However, we did perform a series of experiments with an entirely independent siRNA against VDAC2 (#2), as well as a series of dose-response experiments (Supplementary Information, Fig. S5a-f). Both siRNAs (#1 and #2) produced an equivalent dosage-dependent decrease in endogenous VDAC2 mRNA and protein in Vdac1/Vdac3 deficient MEFs (Fig. S5a,b). Similar to the dose response data shown using siRNA#1 (Fig. 5e), siRNA#2 (120 nM) showed no reduction in MPT following multiple dosages of H2O2 stimulation in Vdac1/Vdac3 double null MEFs (Fig. S5c,d). Moreover Vdac1/Vdac3 double null MEFs treated with VDAC2 siRNA#2 showed similar levels of cell death, or even enhanced death, following stimulation with H2O2 and staurosporine (Fig. S5e,f). Collectively, these results indicate that loss of VDAC2 protein alone, or all three VDAC isoforms together, does not disrupt MPT function and provides no protection from multiple forms of necrotic or apoptotic cell death. Indeed, MEFs lacking all three VDAC isoforms sometimes showed enhanced cell death following stimulation with select agents.
The mitochondrial pore, a putative multimeric complex situated at mitochondrial contact sites, mediates MPT. On the basis of biochemical evidence the standard model for the MPT pore consists of VDAC in the outer mitochondrial membrane, ANT in the inner mitochondrial membrane and cyclophilin D in the matrix (2-4,25,26). Although recent genetic studies have confirmed a regulatory role for cyclophilin D in MPT (24,27-29), mice lacking Ant1/2 still exhibit a classical MPT response that was inhibited with cyclosporine A (30). Indeed, Ant1/2 null cells were only defective in the ability of adenine nucleotides to regulate MPT and exhibited a somewhat decreased sensitivity to Ca2+-induced MPT, suggesting that ANT may only act as a regulator of the MPT pore, but not as a bone fide pore-forming unit as originally proposed. (30). Thus, current genetic strategies indicate that only cyclophilin D serves as a necessary component of the MPT.
With respect to the VDAC, there is a paucity of data supporting the concept that it serves as a functional component of MPT. While reconstitution of a complex containing VDAC, ANT, and cyclophilin D does indeed yield an MPT-like pore in proteoliposomes (31), a similar channel can be reconstituted in the absence of VDAC (32). Moreover, a role for the VDAC in MPT has often been inferred from the use of inhibitory antibodies and putative chemical blockers (7,8), the specificity of which has been questioned (5,16) and more definitive genetic data have been lacking. Here we observed that the absence of one or more VDAC isoforms does not inactivate the mitochondrial pore, and in some cases actually increases the sensitivity of the MPT response. However, this increase in sensitivity to pore opening and cell death in the absence of VDAC1/2/3 may reflect a defect in the metabolic function of mitochondria, since the VDACs also maintain homeostasis in ions and other small metabolites. Moreover, premature closure of VDAC, a state that is likely pheno-copied in our Vdac-deleted cells, has been shown to promote premature death due to defects in metabolite homeostasis and coupled respiration (33-35). Thus, it remains plausible that loss of VDAC1/2/3 predisposes to cell death for reasons that are independent of MPT and likely related to the metabolic function of mitochondria.
Bcl-2 family members have been reported to interact with VDAC, thereby inducing a VDAC-Bcl protein-containing channel that, at least in liposomes, is sufficiently large enough to allow cytochrome c permeation (9-12). However, multiple studies have failed to recapitulate either a physical or functional cooperativity between pro-death Bcl-2 family members and VDACs (19-21), and the cytotoxic actions of Bax were unaltered in VDAC-deficient yeast (19,20). Indeed, Bax and Bak can form cytochrome c-permeant channels in the absence of other proteins (22). Our data confirm a VDAC-independent model of Bcl-2 family member-mediated cell killing. Neither VDAC1 nor VDAC3 appear to appreciably influence Bax or tBid-induced cytochrome c release and cell death, and VDAC2 was even shown to be cytoprotective (also see ref 18). These results challenge our concept of VDACs as specific mediators of any cell death program, and suggest that alternative proteins and/or mechanisms must play a role in mitochondrial-dependent cell mortality through the MPT pore.
METHODS
Vdac-deficient mice
The gene-targeting strategies for all three Vdac genes have been described previously (13).
Mitochondrial isolation and analyses
Mouse heart and liver mitochondria were isolated by differential centrifugation. Briefly, mouse organs were homogenized with a Dounce in buffer containing 250 mM Sucrose, 10 mM Tris (pH 7.4) and 1 mM EDTA. The homogenates were spun at 1,300 g for 10 min at 4°C to pellet nuclei and cell debris. The supernatant was then spun at 12,000 g for 30 min at 4°C to pellet the mitochondria. After washing twice in homogenization buffer (minus EDTA), the mitochondria were resuspended in buffer containing 120 mM KCl, 10 mM Tris (pH 7.4), and 5 mM KH2PO4 for analysis. Pore opening was determined by mitochondrial swelling (8,16,20), where the light scattering of 250 μg of cardiac mitochondria in 1 mL was measured at 540 nm. Swelling was induced by addition of 100 μM or 250 μM CaCl2. For cytochrome c release experiments, 40 μg of liver mitochondria in 60 μL were incubated at 30°C with 250 μM CaCl2, 100 μM tert-Butyl-hydroperoxide, 1 μg recombinant GST-Bax, or 0.25 μg recombinant tBid. The mitochondria were then pelleted and both the pellet and supernatant subjected to Western blotting for cytochrome c.
Western blotting
Proteins were resolved on 10−15% SDS-PAGE gels, transferred onto PVDF membranes, and immunoblotted using the following commercially available antibodies: anti-cyclophilin D (Affinity Bioreagents), anti-VDAC1, -ANT, and -Bax (Santa Cruz Biotechnology), anti-GAPDH (Research Diagnostics), anti-cytochrome c and anti-HSP60 (BD Pharmingen), anti-cytochrome oxidase IV (COX-IV, Molecular Probes), anti-actin (Sigma), anti-PARP and anti-cleaved caspase-3 (Cell Signaling). The anti-VDAC2 and VDAC3 antibodies were raised as described previously (15). Membranes were then incubated with the appropriate alkaline phosphatase-linked secondary antibody and visualized by enhanced chemifluorescence (Amersham).
Cell culture and drug treatment
Primary cultured wildtype, Vdac1-, Vdac2, Vdac3-, Vdac1/Vdac3- Ppif, and Bax/Bak-null mouse embryonic fibroblasts (MEFs) were obtained from E13.5−15.5 embryos by trypsin digestion as previously described (18). The MEFs were then maintained in DMEM medium supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 U/mL penicillin, and 0.1 mg/mL streptomycin. MEFs were treated with 30−300 nM staurosporine, 2−10 μM ionomycin, 0.3−3 ng/mL TNFα plus 0.1 mg/mL actinomycin-D for 18 h, or 100−500 μM H2O2 for 6 h, and cell death assayed by propidium iodide exclusion (24). For siRNA experiments, MEFs were transfected separately with a non-targeting RNAi or two separate VDAC2 RNAi (Invitrogen, catalog #MSS247970, MSS247971) using Lipofectamine 2000 (Invitrogen). The VDAC2 RNAi#1 sequence was GACCAAGUACAAAUGGUGUGAGUAU, and VDAC2 RNAi#2 was GGCUCAUCUAAUACAGACACUGGUA
Adenovirus construction and transduction
The binary Bax adenoviral system has been previously described (36). MEFs were typically infected with adenovirus at a multiplicity of infection of 100 plaque-forming units for 2 h at 37°C. Cells were then cultured for another 24 h in virus-free media before analysis.
Immunofluorescence microscopy
For measurements of mitochondrial ΔΨ, MEFs seeded on chamber slides were treated with 100−500 μM H2O2 for 6 h, and then incubated with 50 nM TMRE (Molecular Probes) in HBSS for 30 min at 37°C. Mitochondrial pore opening was assessed using the calcein/CoCl2 method (23). Briefly, treated MEFs were incubated with 1 μM calcein-AM (Molecular Probes) plus 1 mM CoCl2 in HBSS for 30 min at 37°C. Both TMRE and calcein-loaded cells were then washed twice with HBSS and fluorescence images collected using an inverted fluorescent microscope (Olympus IX70) connected to a digital SPOT camera (Diagnostic Instruments).
Ischaemia/reperfusion injury model
The mouse model of ischaemia/reperfusion injury has been described previously (37). Briefly, mice were anaesthetised with isofluorane and intubated. A left-lateral thoracotomy was performed and an 8−0 silk ligature tied around the left-anterior descending coronary artery using a slipknot. The thoracotomy was closed and the mice were allowed to recover. The mice were subjected to 30 min ischaemia followed by 24 h reperfusion by tying and releasing the slipknot, respectively. The ischaemic area and the infarcted region were then identified by postmortem perfusion of Evans blue and tetrazolium chloride, respectively (37).
Acknowledgements
We are very grateful to B. Fang for the generous gift of the Bax adenovirus, R. Gottlieb for recombinant Bax and tBid, and the late Stanley Korsmeyer for Bax/Bak gene-targeted mice. This work was supported by grants from the National Institutes of Health (J.D.M, W.J.C), an American Heart Association Scientist Development Grant (C.P.B.) and Established Investigator Grant (J.D.M.), a National Institutes of Health NRSA award (R.A.K.), The Children's Hospital Research Foundation (C.P.B.), and the Fondation Leducq (Heart failure network grant to J.D.M).
References
- 1.Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- 2.Zamzami N, Kroemer G. The mitochondrion in apoptosis: how Pandora's box opens. Nat. Rev. Mol. Cell. Biol. 2001;2:67–71. doi: 10.1038/35048073. [DOI] [PubMed] [Google Scholar]
- 3.Crompton M, Barksby E, Johnson N, Capano M. Mitochondrial intermembrane junctional complexes and their involvement in cell death. Biochimie. 2002;84:143–152. doi: 10.1016/s0300-9084(02)01368-8. [DOI] [PubMed] [Google Scholar]
- 4.Halestrap AP. Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem. Soc. Trans. 2006;34:232–237. doi: 10.1042/BST20060232. [DOI] [PubMed] [Google Scholar]
- 5.Rostovtseva TK, Tan W, Colombini M. On the role of VDAC in apoptosis: fact and fiction. J. Bioenerg. Biomembr. 2005;37:129–142. doi: 10.1007/s10863-005-6566-8. [DOI] [PubMed] [Google Scholar]
- 6.Blachly-Dyson E, Forte M. VDAC channels. IUBMB Life. 2001;52:113–118. doi: 10.1080/15216540152845902. [DOI] [PubMed] [Google Scholar]
- 7.Zheng Y, et al. Essential role of the voltage-dependent anion channel (VDAC) in mitochondrial permeability transition pore opening and cytochrome c release induced by arsenic trioxide. Oncogene. 2004;23:1239–1247. doi: 10.1038/sj.onc.1207205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cesura AM, et al. The voltage-dependent anion channel is the target for a new class of inhibitors of the mitochondrial permeability transition pore. J. Biol. Chem. 2003;278:49812–49818. doi: 10.1074/jbc.M304748200. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee J, Ghosh S. Bax increases the pore size of rat brain mitochondrial voltage-dependent anion channel in the presence of tBid. Biochem. Biophys. Res. Commun. 2004;323:310–314. doi: 10.1016/j.bbrc.2004.08.094. [DOI] [PubMed] [Google Scholar]
- 10.Shimizu S, Ide T, Yanagida T, Tsujimoto Y. Electrophysiological study of a novel large pore formed by Bax and the voltage-dependent anion channel that is permeable to cytochrome c. J. Biol. Chem. 2000;275:12321–12325. doi: 10.1074/jbc.275.16.12321. [DOI] [PubMed] [Google Scholar]
- 11.Sugiyama T, Shimizu S, Matsuoka Y, Yoneda Y, Tsujimoto Y. Activation of mitochondrial voltage-dependent anion channel by a pro-apoptotic BH3-only protein Bim. Oncogene. 2002;21:4944–4956. doi: 10.1038/sj.onc.1205621. [DOI] [PubMed] [Google Scholar]
- 12.Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- 13.Wu S, Sampson MJ, Decker WK, Craigen WJ. Each mammalian mitochondrial outer membrane porin protein is dispensable: effects on cellular respiration. Biochim. Biophys. Acta. 1999;1452:68–78. doi: 10.1016/s0167-4889(99)00120-2. [DOI] [PubMed] [Google Scholar]
- 14.Anflous K, Armstrong DD, Craigen WJ. Altered mitochondrial sensitivity for ADP and maintenance of creatine-stimulated respiration in oxidative striated muscles from VDAC1-deficient mice. J. Biol. Chem. 2001;276:1954–1960. doi: 10.1074/jbc.M006587200. [DOI] [PubMed] [Google Scholar]
- 15.Sampson MJ, et al. Immotile sperm and infertility in mice lacking mitochondrial voltage-dependent anion channel type 3. J. Biol. Chem. 2001;276:39206–39212. doi: 10.1074/jbc.M104724200. [DOI] [PubMed] [Google Scholar]
- 16.Krauskopf A, Eriksson O, Craigen WJ, Forte MA, Bernardi P. Properties of the permeability transition in VDAC1−/− mitochondria. Biochim. Biophys. Acta. 2006;1757:590–595. doi: 10.1016/j.bbabio.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 17.Weeber EJ, et al. The role of mitochondrial porins and the permeability transition pore in learning and synaptic plasticity. J. Biol. Chem. 2002;277:18891–18897. doi: 10.1074/jbc.M201649200. [DOI] [PubMed] [Google Scholar]
- 18.Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- 19.Priault M, Chaudhuri B, Clow A, Camougrand N, Manon S. Investigation of bax-induced release of cytochrome c from yeast mitochondria permeability of mitochondrial membranes, role of VDAC and ATP requirement. Eur. J. Biochem. 1999;260:684–691. doi: 10.1046/j.1432-1327.1999.00198.x. [DOI] [PubMed] [Google Scholar]
- 20.Polcic P, Forte M. Response of yeast to the regulated expression of proteins in the Bcl-2 family. Biochem. J. 2003;374:393–402. doi: 10.1042/BJ20030690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rostovtseva TK, et al. Bid, but not Bax, regulates VDAC channels. J. Biol. Chem. 2004;279:13575–13583. doi: 10.1074/jbc.M310593200. [DOI] [PubMed] [Google Scholar]
- 22.Antonsson B, Montessuit S, Lauper S, Eskes R, Martinou JC. Bax oligomerization is required for channel-forming activity in liposomes and to trigger cytochrome c release from mitochondria. Biochem. J. 2000;345:271–278. [PMC free article] [PubMed] [Google Scholar]
- 23.Petronilli V, et al. Transient and long-lasting openings of the mitochondrial permeability transition pore can be monitored directly in intact cells by changes in mitochondrial calcein fluorescence. Biophys. J. 1999;76:725–734. doi: 10.1016/S0006-3495(99)77239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baines CP, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 25.Bernardi P, et al. The mitochondrial permeability transition from in vitro artifact to disease target. FEBS. J. 2006;273:2077–2099. doi: 10.1111/j.1742-4658.2006.05213.x. [DOI] [PubMed] [Google Scholar]
- 26.Halestrap AP, McStay GP, Clarke SJ. The permeability transition pore complex: another view. Biochimie. 2002;84:153–166. doi: 10.1016/s0300-9084(02)01375-5. [DOI] [PubMed] [Google Scholar]
- 27.Nakagawa T, et al. Cyclophilin D-dependent mitochondrial permeability transition regulates some necrotic but not apoptotic cell death. Nature. 2005;434:652–658. doi: 10.1038/nature03317. [DOI] [PubMed] [Google Scholar]
- 28.Basso E, et al. Properties of the permeability transition pore in mitochondria devoid of cyclophilin D. J. Biol. Chem. 2005;280:18558–18561. doi: 10.1074/jbc.C500089200. [DOI] [PubMed] [Google Scholar]
- 29.Schinzel AC, et al. Cyclophilin D is a component of mitochondrial permeability transition and mediates neuronal cell death after focal cerebral ischemia. Proc. Natl. Acad. Sci. 2005;102:12005–12010. doi: 10.1073/pnas.0505294102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kokoszka JE, et al. The ADP/ATP translocator is not essential for the mitochondrial permeability transition pore. Nature. 2004;427:461–465. doi: 10.1038/nature02229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crompton M, Virji S, Ward JM. Cyclophilin-D binds strongly to complexes of the voltage-dependent anion channel and the adenine nucleotide translocase to form the permeability transition pore. Eur. J. Biochem. 1998;258:729–735. doi: 10.1046/j.1432-1327.1998.2580729.x. [DOI] [PubMed] [Google Scholar]
- 32.Woodfield K, Ruck A, Brdiczka D, Halestrap AP. Direct demonstration of a specific interaction between cyclophilin-D and the adenine nucleotide translocase confirms their role in the mitochondrial permeability transition. Biochem. J. 1998;336:287–290. doi: 10.1042/bj3360287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vander Heiden MG, et al. Outer mitochondrial membrane permeability can regulate coupled respiration and cell survival. Proc. Natl. Acad. Sci. USA. 2000;97:4666–4671. doi: 10.1073/pnas.090082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vander Heiden MG, et al. Bcl-xL promotes the open configuration of the voltage-dependent anion channel and metabolite passage through the outer mitochondrial membrane. J. Biol. Chem. 2001;276:19414–19419. doi: 10.1074/jbc.M101590200. [DOI] [PubMed] [Google Scholar]
- 35.Lai JC, et al. A pharmacologic target of G3139 in melanoma cells may be the mitochondrial VDAC. Proc. Natl. Acad. Sci. USA. 2006;103:7494–7499. doi: 10.1073/pnas.0602217103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kagawa S, et al. A binary adenoviral vector system for expressing high levels of the proapoptotic gene bax. Gene Ther. 2000;7:75–79. doi: 10.1038/sj.gt.3301048. [DOI] [PubMed] [Google Scholar]
- 37.Kaiser RA, et al. Targeted inhibition of p38 mitogen-activated protein kinase antagonizes cardiac injury and cell death following ischemia-reperfusion in vivo. J. Biol. Chem. 2004;279:15524–15530. doi: 10.1074/jbc.M313717200. [DOI] [PubMed] [Google Scholar]