Abstract
Growing evidence has pointed to an interaction between the tetracycline antibiotic minocycline and drugs with abuse liability such as opioids and amphetamines. In this work, we tested the hypothesis that similar to its effects on methamphetamine-induced locomotor sensitization, minocycline may influence the behavioral effects of cocaine. Experiments were performed in male C57BL/6J mice using an automated system to measure locomotor activity. We found that 80 mg/kg minocycline significantly reduced locomotor activity when administered either alone or injected 30 min prior to cocaine, which increased locomotor activity. To investigate whether minocycline selectively affects the development of locomotor sensitization induced by four daily injections of 10 mg/kg cocaine, we sought a schedule of minocycline administration that does not per se affect locomotor activity. Thus, we selected 40 mg/kg minocycline administered 3 hours prior to cocaine; minocycline did not affect cocaine-stimulated locomotor activity on the first day of administration but prevented the development of cocaine sensitization. We also tested whether minocycline would affect an already established cocaine sensitization. After establishing the sensitization effect by four daily injections, cocaine treatment was discontinued and mice were treated with minocycline daily (days 5–11) or on day 11 only. There was no effect of minocycline treatment on the response of cocaine-sensitized mice to the challenge dose of cocaine on day 11. The mechanisms by which minocycline interferes with the development of cocaine sensitization needs to be characterized.
Keywords: Minocycline, cocaine, sensitization, addiction, behavior, glutamate, methamphetamine
The central nervous system (CNS)-penetrable tetracycline antibiotic minocycline [4] is being researched for its potentially beneficial non-antibiotic CNS effects. Among those effects, the neuroprotective actions of minocycline have attracted significant interest [6, 9, 12, 21, 24]. In addition, it was proposed that minocycline might provide antidepressant activity [17, 18], and that it could help in the treatment of schizophrenia [5, 7, 15].
Recently, growing evidence has pointed to an interaction between minocycline and drugs with abuse liability such as opioids and amphetamines. These studies show that minocycline attenuates morphine tolerance [14], potentiates morphine analgesia, and interferes with the rewarding actions of opioids [10]. Furthermore, a series of recent reports characterized the effects of minocycline on the behavioral and biochemical actions of methamphetamine [8, 16, 23]. For example, the administration of minocycline together with methamphetamine inhibited the development of methamphetamine-induced behavioral sensitization (i.e., increased locomotor activity due to a repeated daily administration of the same dose of methamphetamine) [16].
The proposed molecular mechanisms of minocycline’s CNS actions include the inhibition of poly(ADP-ribose) polymerase-1 (PARP-1) [1], inhibition of 5-lipoxygenase (5-LOX) [21], depression of glutamatergic calcium signaling [6], and potentiation of the phosphorylation and membrane insertion of the GluR1 (also known as GluR-A) subunits of ionotropic glutamate/AMPA (α-amino-3-hydroxy-5-methylisoxazole-4-propionate) receptors [11].
Since repeated daily injections of cocaine trigger behavioral sensitization similar to the sensitization induced by methamphetamine and because GluR1 receptors play a key role in these actions of cocaine [2], in this work, we tested the hypothesis that similar to its effects on methamphetamine-induced behaviors, minocycline may influence the behavioral effects of cocaine.
Male C57BL/6J mice, 2 months old and weighing 25–30 g, were purchased from Jackson Laboratories (Bar Harbor, ME) and were housed in groups of six in a temperature controlled room on a 12-h light/dark cycle (lights on at 7 AM). They had free access to laboratory chow and water except during behavioral experiments. Experiments were performed during the morning starting at 10 AM. The experimental protocol was approved by the Institutional Animal Care Committee. Cocaine hydrochloride (Sigma Chemical, St. Louis, MO) was dissolved in sterile saline and administered intraperitoneally (i.p.) in an injection volume of 0.05 ml/25 g body weight. Minocycline hydrochloride (Sigma) was also dissolved in sterile saline with a pH adjustment [11] and injected in the same volume as cocaine. Controls received the same volume of a corresponding vehicle. Locomotor sensitization was assayed as previously described [22]. Briefly, mice were accustomed to experimental conditions by 3 daily i.p. saline injections. On day four, (i.e., day 1 of experiment) mice that had not been previously exposed to the testing monitors were placed individually in activity cages (Cage Rack Photobeam Activity Measurement System, San Diego Instruments, San Diego, CA) equipped with computer-monitored photobeam frames for a 25-min adaptation period. Immediately after this 25-min adaptation period, animals received the experimental i.p. injections and were returned to the activity cages for another 30-min measurement period. The movement of each animal in this 30-min period was recorded as the number of beam interruptions every 5 min and is reported as locomotor activity. Thereafter, mice were returned to their home cages. Statistical analyses were performed using SPSS software (SPSS Inc., Chicago, IL). Data (shown as mean ± S.E.M.) were analyzed by one-way analysis of variance (ANOVA) or repeated measures ANOVA followed by Dunnett’s multiple comparison test or t-test for two-group comparisons. Significance was accepted at p<0.05.
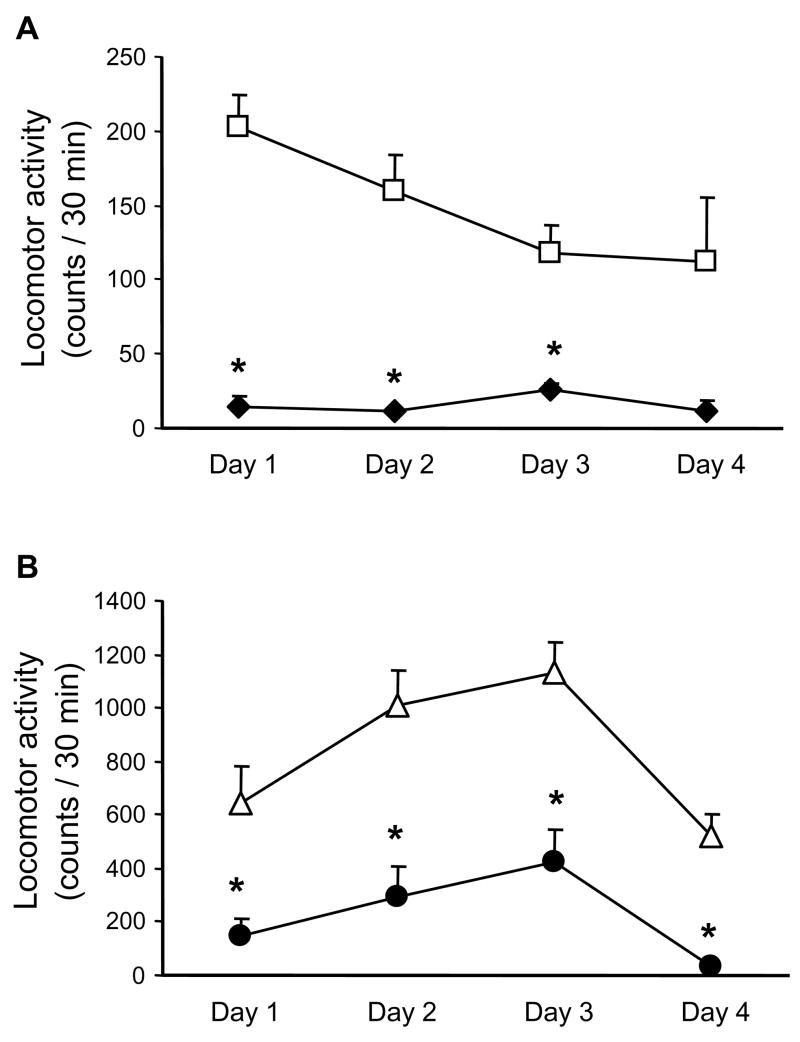
In our previous experiments with mice, minocycline was injected at a dose of 80 mg/kg [11], whereas locomotor sensitization was typically induced by daily injections of 20 mg/kg cocaine [22]. We selected these doses for the initial investigations of the effects of minocycline on the cocaine-induced increase in locomotor activity (Fig. 1). We found that 80 mg/kg minocycline significantly reduced locomotor activity when administered alone; this suppressive effect was evident on all four days of minocycline treatment (significant on days 1–3; Fig. 1A). Similar to previous observations [20], daily injections of 20 mg/kg cocaine resulted in a bell-shaped locomotor response; a gradual increase up to day 3 followed by a reduced response on day 4 (Fig. 1B). The locomotor suppressive action of 80 mg/kg minocycline persisted in the presence of 20 mg/kg cocaine. Although the first injection of cocaine resulted in increased locomotor activity compared to vehicle treated controls (counts/30 min; control = 203 ± 21; cocaine = 646 ± 139; p<0.05; t-test), minocycline reduced the stimulatory effect of cocaine on all 4 days of treatment (Fig. 1B).
Fig. 1. Effects of minocycline (80 mg/kg, i.p.) and cocaine (20 mg/kg, i.p.) on locomotor activity.
Mice (n = 5–6) received daily i.p. injections of (A) vehicle + vehicle (open squares) and minocycline + vehicle (closed diamonds) or (B) vehicle + cocaine (open triangles) and minocycline + cocaine (closed circles). The first daily injection was administered 30 min before and the second immediately prior to the measurement of locomotor activity (for 30 min). Note the scale difference in (A) vs. (B) due to the cocaine-induced greater locomotor activity in (B). (A) Minocycline significantly reduced locomotor activity compared with vehicle-treated controls [repeated ANOVA: day, F (3, 27) = 3.09, p<0.05; group, F (1, 9) = 37.34, p<0.05; day × group, F (3, 27) = 3.52, p<0.05; *p<0.05 vs. corresponding control, t-test]. (B) Minocycline treatment suppressed locomotor activity of cocaine-treated mice [repeated ANOVA: day, F (3, 24) = 9.27, p<0.05; group, F (1, 8) =45.42, p<0.05; day × group, F (3, 24) = 0.77, p>0.05); * p<0.05 vs. corresponding cocaine-treated group, t-test].
To investigate whether minocycline selectively affects the development of cocaine sensitization, we sought a schedule of minocycline administration that per se does not affect locomotor activity. We found that a lower doses of minocycline, 20 and 40 mg/kg (used in studies with methamphetamine [16]), still reduce locomotor activity measured immediately after the injection or 30 min after the drug administration. However, 210 min after administration of 20 and 40 mg/kg minocycline, locomotor activity of treated mice did not differ from controls (Table 1). The dose of 80 mg/kg was locomotor suppressive even at the late time point (210 min) after drug injection (data not shown). Thus, we selected 40 mg/kg minocycline and the late time point for the subsequent minocycline + cocaine experiments designed to investigate the effects of minocycline on development of cocaine sensitization. Furthermore, to avoid the bell-shaped sensitization induced by 20 mg/kg cocaine, in subsequent experiments we used a dose of 10 mg/kg cocaine.
Table 1.
Suppressive effects of minocycline on locomotor activity: dose-dependence and time-course.
| Time after i.p. (min) | 0 | 30 | 210 |
| Locomotor activity (counts/30 min) | |||
| Vehicle | 227 ± 27 | 234 ± 50 | 184 ± 25 |
| Minocycline (20 mg/kg) | 144 ± 41* | 43.3 ± 13* | 244 ± 28 |
| Minocycline (40 mg/kg) | 122 ± 51* | – | 198 ± 14 |
Locomotor activity was measured for 30 min starting at indicated times after i.p. injection.
p<0.05 v.s. corresponding vehicle-treated control (Dunnett’s test and t-test; n = 4–6).
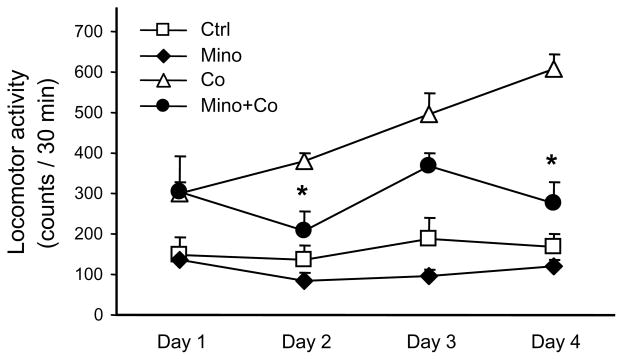
Applied in a manner described in Table 1, even repeated daily administration of 40 mg/kg minocycline did not alter the locomotor activity of mice (Fig. 2). Furthermore, when 10 mg/kg cocaine was administered on day 1 in the presence of minocycline, locomotor activity did not differ from the cocaine-induced activity in the absence of minocycline (Fig. 2). However, whereas the daily administration of cocaine in the absence of minocycline resulted in a gradual increase of the locomotor response (i.e., sensitization), minocycline prevented the development of cocaine sensitization (Fig. 2).
Fig. 2. Inhibition of the development of cocaine-induced locomotor sensitization by daily pre-treatment with 40 mg/kg minocycline.
Minocycline (Mino) or its vehicle (Ctrl) were administrated daily 3 h before i.p. injection of 10 mg/kg cocine (Co) or its vehicle (n = 6). Locomotor activity was measured for 30 min starting immediately after cocaine administration. Repeated ANOVA revealed that significant difference occurred between Co and Co + Mino groups, but not Ctrl and Mino groups [day, F (1, 20) = 4.63, p<0.05; group, F (3, 20) = 78.24, p<0.05; day × group, F (3, 20) = 2.85, p>0.05); * p<0.05 vs. corresponding Cocaine-treated group, t-test].
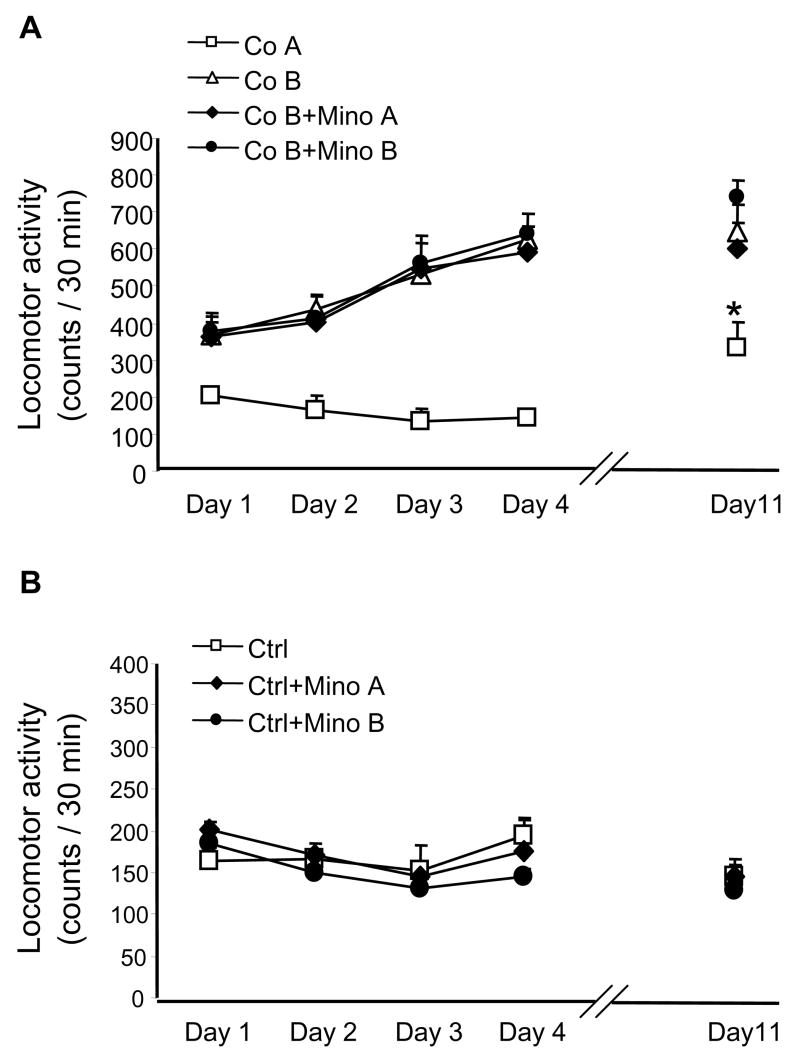
To test whether minocycline would affect an already established cocaine sensitization, we performed the experiments described in Fig. 3. Cocaine (10 mg/kg) was administered daily for 4 days to three groups of mice; the fourth group was treated with vehicle. Locomotor activity was measured daily. In all three groups, cocaine treatment resulted in the development of locomotor sensitization (Fig. 3A). Thereafter, cocaine treatment was discontinued and mice were treated with minocycline (40 mg/kg; daily from day 5–11 or only on day 11) or vehicle (see figure legend for details). On day 11, all cocaine-sensitized groups received a challenge dose of 10 mg/kg cocaine. There was no effect of minocycline treatment on the response of cocaine-sensitized mice to the challenge dose of cocaine and all three sensitizd groups showed greater locomotor activity vs. the fourth group which received cocaine only on day 11 (Fig. 3A). The corresponding minocycline treatment without cocaine did not alter locomotor activity, compared with vehicle treated controls (Fig. 3B)
Fig. 3.
Failure of 40 mg/kg minocycline to reverse the established cocaine sensitization. Panel (A), cocaine (10 mg/kg) was administered daily for 4 days to three groups of mice (Co B; cocaine sensitization) or on day 11 only (Co A; not sensitized control) (n = 5). After the discontinuation of Co B treatment, i.e., after the fourth cocaine injection, mice were treated with minocycline (40 mg/kg; Mino A = daily from day 5–11, or Mino B = only on day 11) or its vehicle. On day 11, all three cocaine-sensitized groups received a challenge dose of 10 mg/kg cocaine (3 h after vehicle or minocycline). Locomotor activity was measured for 30 min starting immediately after cocaine administration. No significant differences were observed between three Co B groups; p<0.05 vs. all corresponding, i.e., day 11 Co B groups (Dunnett’s test). Panel (B), mice were treated with vehicle for 11 days (Ctrl); vehicle from day 1–4, plus minocycline from day 5–11 (Mino A); and vehicle from day 1–10, plus minocycline on day 11 (Mino B). On day 11, all three groups received an additional injection of vehicle 30 min prior to locomotor activity measurement. No significant differences were observed between these three groups (n = 5).
Minocycline is a relatively old antibiotic currently widely available in a generic form. Its use has been evaluated in a variety of infection-related and infection-unrelated conditions. Recently, Hashimoto [8] proposed that minocycline could be considered for the therapy of methamphetamine abuse disorders. This group of researchers reported that minocycline significantly attenuated behavioral abnormalities and neurotoxicity in the brains of mice and monkeys treated with methamphetamine [9, 23, 24] and that minocycline displays an antipsychotic action in animal models (dizocilpine-induced prepulse inhibition deficits and phencyclidine-induced cognitive deficits) of schizophrenia [5, 25].
Collectively, our data revealed that minocycline, administered to mice in a dose and schedule that does not affect locomotor activity and does not diminish the locomotor-stimulatory effect of the first injection of cocaine administered to naïve mice, prevents the development of behavioral cocaine sensitization produced by subsequent daily injections of cocaine. In contrast, administered to mice that had previously been sensitized to cocaine, minocycline was unable to reverse this sensitization. These results are similar to previously reported effects of minocycline on locomotor sensitization induced by methamphetamine. Yamada and colleagues [16] found that post-treatment with minocycline failed to affect an already established methamphetamine sensitization, whereas co-treatment with minocycline and methamphetamine inhibited the development of methamphetamine-induced behavioral sensitization.
Our results show that minocycline affects locomotor activity even in the absence of psychostimulants such as methamphetamine and cocaine. A similar effect was previously reported; minocycline suppressed both the baseline locomotor activity and the amphetamine-induced hyperactivity [13]. These authors confirmed that the behavioral effects of minocycline are mediated by a central action of this drug but were not able to link this action of minocycline to a hypothetical involvement of the cyclic adnosine monophosphate (cAMP) signaling system. Alternative mechanisms for attenuation of locomotor activity could involve minocycline-mediated inhibition of dopamine release [25] and glutamate release [6]. Nevertheless, when schedules of minocycline administration were adjusted so that the baseline locomotor activity was not altered (our results and [16]), minocycline was still able to prevent the development of behavioral sensitization to the psychostimulants methamphetamine and cocaine. Although minocycline is generally considered a safe drug, lager doses of minocycline (for example 360 mg/kg/day) were shown to be capable of enhancing the toxicity of compound such as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) and 3-nitropropionic acid (3-NP) [3]. It remains to be evaluated whether the transient behavior-impairing effects of minocycline observed in our study are associate with putative drug toxicity.
The CNS effects of minocycline appear to involve drug-mediated alterations of the phosphorylation states of several signaling systems including the extracellular-signal-regulated kinase (ERK 1/2) and the GluR1 receptors. For example, minocycline-mediated modifications of ERK 1/2 activity may participate in the beneficial effects of minocycline on methamphetamine-induced impairment of recognition memory but are not involved in the actions of minocycline on psychostimulant-induced behavioral sensitization [16]. The latter action of minocycline may involve minocycline-mediated increased phosphorylation of GluR1 [11]. Namely, these receptors play the crucial role in cocaine sensitization. In recent experiments, Bachtell et al. [2] used a viral-mediated gene transfer of wild-type GluR1 (wt-GluR1) or a dominant-negative pore-dead GluR1 (pd-GluR1, which reduces AMPA receptor activity), respectively, and found that transient increases in wt-GluR1 during cocaine treatment diminished the development of cocaine sensitization, while pd-GluR1 expression exacerbated cocaine sensitization. These authors suggested that GluR1-dependent elevated AMPA glutamate receptor function could be responsible for the attenuation of the development of cocaine sensitization. Since increased GluR1 phosphorylation also leads to greater membrane insertion of GluR1 and increased AMPA receptor function [19], it is possible that minocycline-attenuated development of cocaine sensitization involves minocycline-stimulated GluR1 phosphorylation [11].
In conclusion, our results provide further evidence for the ability of minocycline to interfere with the behavioral effects of repeated exposure to psychostimulants such as methamphetamine and cocaine. Along with previous work by others [8, 9, 10, 13, 14, 16, 23, 24], our data suggest that further research is needed to explore whether these effects of minocycline hold potential for clinical use as a therapy for drug abuse.
Acknowledgments
This study was supported by the National Institutes of Health grant from the National Institute on Drug Abuse (NIDA) 1R21 DA024099-01 (H.M.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alano CC, Kauppinen TM, Valls AV, Swanson RA. Minocycline inhibits poly(ADP-ribose) polymerase-1 at nanomolar concentrations. Proc Natl Acad Sci USA. 2006;103:9685–9690. doi: 10.1073/pnas.0600554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachtell RK, Choi KH, Simmons DL, Falcon E, Monteggia LM, Neve RL, Self DW. Role of GluR1 expression in nucleus accumbens neurons in cocaine sensitization and cocaine-seeking behavior. Eur J Neurosci. 2008;27:2229–2240. doi: 10.1111/j.1460-9568.2008.06199.x. [DOI] [PubMed] [Google Scholar]
- 3.Diguet E, Fernagut PO, Wei X, Du Y, Rouland R, Gross C, Bezard E, Tison F. Deleterious effects of minocycline in animal models of Parkinson’s disease and Huntington’s disease. Eur J Neurosci. 2004;12:3266–3276. doi: 10.1111/j.0953-816X.2004.03372.x. [DOI] [PubMed] [Google Scholar]
- 4.Fagan SC, Edwards DJ, Borlongan CV, Xu L, Arora A, Feuerstein G, Hess DC. Optimal delivery of minocycline to the brain: implication for human studies of acute neuroprotection. Exp Neurol. 2004;186:248–251. doi: 10.1016/j.expneurol.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 5.Fujita Y, Ishima T, Kunitachi S, Hagiwara H, Zhang L, Iyo M, Hashimoto K. Phencyclidine-induced cognitive deficits in mice are improved by subsequent subchronic administration of the antibiotic drug minocycline. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:336–339. doi: 10.1016/j.pnpbp.2007.08.031. [DOI] [PubMed] [Google Scholar]
- 6.González JC, Egea J, Del Carmen Godino M, Fernandez-Gomez FJ, Sánchez-Prieto J, Gandía L, García AG, Jordán J, Hernández-Guijo JM. Neuroprotectant minocycline depresses glutamatergic neurotransmission and Ca(2+) signalling in hippocampal neurons. Eur J Neurosci. 2007;26:2481–2495. doi: 10.1111/j.1460-9568.2007.05873.x. [DOI] [PubMed] [Google Scholar]
- 7.Hashimoto K. Reply to: Minocycline, schizophrenia and GluR1 glutamate receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2008a doi: 10.1016/j.pnpbp.2008.11.004. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto K. Minocycline as a therapeutic drug for methamphetamine use disorders. Nihon Shinkei Seishin Yakurigaku Zasshi. 2008b;28:19–22. [PubMed] [Google Scholar]
- 9.Hashimoto K, Tsukada H, Nishiyama S, Fukumoto D, Kakiuchi T, Iyo M. Protective effects of minocycline on the reduction of dopamine transporters in the striatum after administration of methamphetamine: a positron emission tomography study in conscious monkeys. Biol Psychiatry. 2007;61:577–581. doi: 10.1016/j.biopsych.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 10.Hutchinson MR, Northcutt AL, Chao LW, Kearney JJ, Zhang Y, Berkelhammer DL, Loram LC, Rozeske RR, Bland ST, Maier SF, Gleeson TT, Watkins LR. Minocycline suppresses morphine-induced respiratory depression, suppresses morphine-induced reward, and enhances systemic morphine-induced analgesia. Brain Behav Immun. 2008 doi: 10.1016/j.bbi.2008.07.008. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imbesi M, Uz T, Manev R, Sharma RP, Manev H. Minocycline increases phosphorylation and membrane insertion of neuronal GluR1 receptors. Neurosci Lett. 2008;447:134–137. doi: 10.1016/j.neulet.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim HS, Suh YH. Minocycline and neurodegenerative diseases. Behav Brain Res. 2008 doi: 10.1016/j.bbr.2008.09.040. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 13.Kofman O, van Embden S, Alpert C, Fuchs I. Central and peripheral minocycline suppresses motor activity in rats. Pharmacol Biochem Behav. 1993;44:397–402. doi: 10.1016/0091-3057(93)90481-8. [DOI] [PubMed] [Google Scholar]
- 14.Mika J, Wawrzczak-Bargiela A, Osikowicz M, Makuch W, Przewlocka B. Attenuation of morphine tolerance by minocycline and pentoxifylline in naive and neuropathic mice. Brain Behav Immun. 2009;23:75–84. doi: 10.1016/j.bbi.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 15.Miyaoka T, Yasukawa R, Yasuda H, Hayashida M, Inagaki T, Horiguchi J. Minocycline as adjunctive therapy for schizophrenia: an open-label study. Clin Neuropharmacol. 2008;31:287–292. doi: 10.1097/WNF.0b013e3181593d45. [DOI] [PubMed] [Google Scholar]
- 16.Mizoguchi H, Takuma K, Fukakusa A, Ito Y, Nakatani A, Ibi D, Kim HC, Yamada K. Improvement by minocycline of methamphetamine-induced impairment of recognition memory in mice. Psychopharmacology (Berl) 2008;196:233–241. doi: 10.1007/s00213-007-0955-0. [DOI] [PubMed] [Google Scholar]
- 17.Molina-Hernández M, Tellez-Alcántara NP, Pérez-García J, Olivera-Lopez JI, Jaramillo-Jaimes MT. Antidepressant-like actions of minocycline combined with several glutamate antagonists. Prog Neuropsychopharmacol Biol Psychiatry. 2008a;32:380–386. doi: 10.1016/j.pnpbp.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 18.Molina-Hernández M, Téllez-Alcántara NP, Pérez-García J, Olivera-Lopez JI, Jaramillo-Jaimes MT. Desipramine or glutamate antagonists synergized the antidepressant-like actions of intra-nucleus accumbens infusions of minocycline in male Wistar rats. Prog Neuropsychopharmacol Biol Psychiatry. 2008b;32:1660–1666. doi: 10.1016/j.pnpbp.2008.06.010. [DOI] [PubMed] [Google Scholar]
- 19.Palmer CL, Cotton L, Henley JM. The molecular pharmacology and cell biology of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Pharmacol Rev. 2005;57:253–277. doi: 10.1124/pr.57.2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Prinssen EP, Kleven MS, Vignon J, Kamenka JM, Koek W. Effects of repeated administration of N-[1-(2-benzo(b)-thiophenyl)cyclohexy]piperidine and cocaine on locomotor activity in C57BL/6 mice. J Pharmacol Exp Ther. 1996;276:904–911. [PubMed] [Google Scholar]
- 21.Song Y, Wei EQ, Zhang WP, Ge QF, Liu JR, Wang ML, Huang XJ, Hu X, Chen Z. Minocycline protects PC12 cells against NMDA-induced injury via inhibiting 5-lipoxygenase activation. Brain Res. 2006;1085:57–67. doi: 10.1016/j.brainres.2006.02.042. [DOI] [PubMed] [Google Scholar]
- 22.Uz T, Akhisaroglu M, Ahmed R, Manev H. The pineal gland is critical for circadian Period1 expression in the striatum and for circadian cocaine sensitization in mice. Neuropsychopharmacology. 2003;28:2117–2123. doi: 10.1038/sj.npp.1300254. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, Kitaichi K, Fujimoto Y, Nakayama H, Shimizu E, Iyo M, Hashimoto K. Protective effects of minocycline on behavioral changes and neurotoxicity in mice after administration of methamphetamine. Prog Neuropsychopharmacol Biol Psychiatry. 2006a;30:1381–1393. doi: 10.1016/j.pnpbp.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 24.Zhang L, Shirayama Y, Shimizu E, Iyo M, Hashimoto K. Protective effects of minocycline on 3,4-methylenedioxymethamphetamine-induced neurotoxicity in serotonergic and dopaminergic neurons of mouse brain. Eur J Pharmacol. 2006b;544:1–9. doi: 10.1016/j.ejphar.2006.05.047. [DOI] [PubMed] [Google Scholar]
- 25.Zhang L, Shirayama Y, Iyo M, Hashimoto K. Minocycline attenuates hyperlocomotion and prepulse inhibition deficits in mice after administration of the NMDA receptor antagonist dizocilpine. Neuropsychopharmacology. 2007;32:2004–2010. doi: 10.1038/sj.npp.1301313. [DOI] [PubMed] [Google Scholar]