Abstract
To clarify the role of Angiotensin II in the regulation of peripheral sensory and motor systems, we initiated a study of the expression, localization and transport of Angiotensin II receptor types in the rat sciatic nerve pathway, including L4–L5 spinal cord segments, the corresponding dorsal root ganglia (DRGs) and the sciatic nerve.
We used quantitative autoradiography for AT1 and AT2 receptors, and in situ hybridization to detect AT1A, AT1B and AT2 mRNAs. We found substantial expression and discrete localization of Angiotensin II AT1 receptors, with much higher numbers in the grey than in the white matter. A very high AT1 receptor expression was detected in the superficial dorsal horns and in neuronal clusters of the DRGs. Expression of AT1A mRNA was significantly higher than that of AT1B. AT1 receptor binding and AT1A and AT1B mRNAs were especially prominent in ventral horn motor neurons, and in the DRG neuronal cells. Unilateral dorsal rhizotomy significantly reduced AT1 receptor binding in the ipsilateral side of the superficial dorsal horn, indicating that a substantial number of dorsal horn AT1 receptors have their origin in the DRGs. After ligation of the sciatic nerve, there was a high accumulation of AT1 receptors proximal to the ligature, a demonstration of anterograde receptor transport. We found inconsistent levels of AT2 receptor binding and mRNA.
Our results suggest multiple roles of Angiotensin II AT1 receptors in the regulation of sensory and motor functions.
Keywords: Renin Angiotensin System, Dorsal root ganglia, Spinal cord, Angiotensin II AT1 and AT2 receptor types, Sciatic nerve ligation, Dorsal rhizotomy
1. Introduction
The Renin-Angiotensin System (RAS), with its active principle Angiotensin II (Ang II) is a fundamental factor in blood pressure and fluid homeostasis in mammals (de Gasparo et al., 2000). Ang II mediates its effects through two receptor types, AT1 and AT2 (de Gasparo et al., 2000). Most of the physiological effects of Ang II are mediated through AT1 receptor stimulation. The role of AT2 receptors is still controversial but their stimulation may balance AT1 receptor effects (de Gasparo et al., 2000).
In addition to the circulatory system there are important local RAS systems in many organs, including the brain, where Ang II affects multiple functions (Saavedra, 1992; Paul et al., 2006). There is substantial evidence that Ang II, interacting with the autonomic system, participates in the central and peripheral regulation of sensory information. A well-studied mechanism located in the dorsomedial medulla controls autonomic homeostasis, and Ang II plays a fundamental role in this system by modulating the baroreceptor and chemoreceptor pathways (Paton and Kasparov, 2000). There is also strong evidence that Ang II is involved in the central and peripheral regulation of many sensory modalities, including nociception (Sakagawa et al., 2000; Pelegrini-da-Silva et al., 2005), taste (Tsuruoka et al., 2005) and vision (Wheeler-Schilling et al., 1999). An important role of Ang II has been demonstrated in salt-sensitive hypertension induced by sensory nerve degeneration (Huang and Wang, 2001). In addition, previous seminal work by other groups has demonstrated the expression and distribution of Ang II receptors in the spinal cord and dorsal root ganglia of all mammalian species studied (Mendelsohn et al., 1984; Gehlert et al., 1985; Besson and Chaouch, 1987; White et al., 1988; Oldfield et al., 1994; MacGregor et al., 1995; Ahmad et al., 2003).
This evidence led us to examine in further detail the role of Ang II in the sensory system. We focused on the pathway including the sciatic nerve, dorsal root ganglia (DRGs) and the lower lumbar spinal cord segments of the rat. The sciatic nerve contains a mixture of myelinated and unmyelinated, motor, sensory and sympathetic axons (Schmalbruch, 1986). Almost all primary sensory neurons of the sciatic nerve are located in the DRGs at the L4–L5 level of the spinal cord (Swett et al., 1991) and we selected these structures for our study. We used quantitative autoradiography and in situ hybridization, unilateral dorsal rhizotomy and sciatic nerve ligature, as initial steps to localize Ang II receptor types and to determine their cellular origin and transport.
2. Results
2. 1. Angiotensin II receptors in the lower lumbar spinal cord segments
The marked displacement of the [125I]-Sar1-Ang II receptor binding by the selective AT1 receptor ligand losartan revealed that the most of the Ang II receptors in the spinal cord are of the AT1 type. AT1 receptor binding was present throughout the grey and white matter, but it was strikingly higher in the grey matter (Fig. 1). The highest concentration of AT1 receptors was detected in the superficial dorsal horns, including the marginal zone and substantia gelatinosa (Rexed’s lamina I and II, respectively) (Table 1). Relatively high AT1 receptor binding was seen in the central canal region (lamina X) and in the anterior spinal artery and highly vascularized pia mater (Fig. 1). The selective AT2 receptor ligand, PD123319, did not displace the labeled ligand in the grey matter, indicating that AT2 receptors were absent or below the detection threshold of our method (Fig. 1).
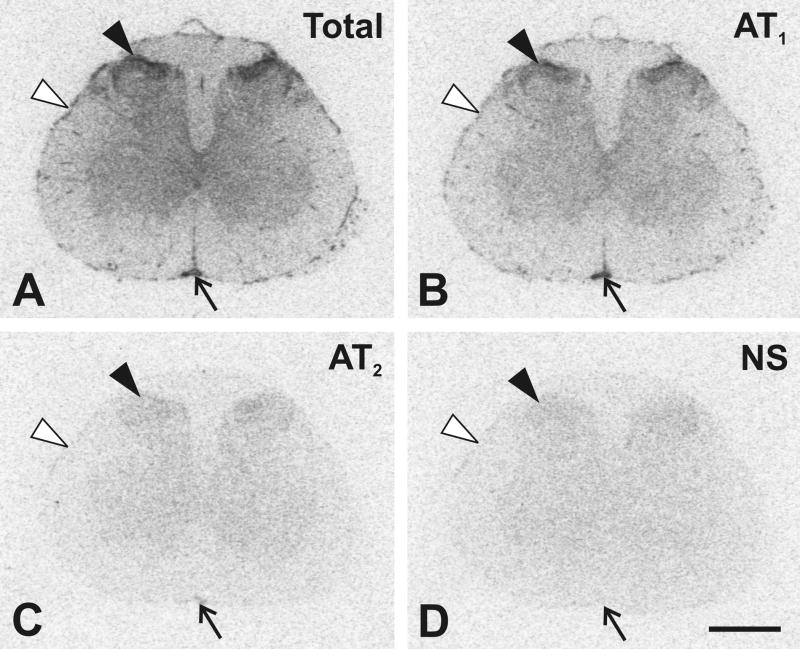
Fig. 1.
Representative autoradiograms showing regional distribution of Angiotensin II receptors in the lower L4 lumbar segment of the spinal cord. (A) Section showing total binding after incubation with [125I]-Sar1-Ang II. Consecutive sections showing binding as in A with addition of: (B) PD123319 to displace binding to AT2 receptors; (C) losartan to displace binding to AT1 receptors; (D) unlabeled Ang II displacing binding to AT1 and AT2 receptors (see Materials and Methods). Black arrowheads point to the superficial dorsal horn of the grey matter. Arrows point to the anterior spinal artery, and white arrowheads point to highly vascularized pia mater. Scale bar = 1 mm. NS, non specific.
TABLE 1.
Quantitative autoradiography of AT1 receptors in the grey and white matter regions of lower lumbar (L4–L5) spinal cord segments
| Grey Matter | White Matter | |||||
|---|---|---|---|---|---|---|
| Spinal Cord Segment | Superficial dorsal horn | Lamina X | Ventral horn | Dorsal funiculus | Lateral funiculus | Ventral funiculus |
| fmol/mg protein | ||||||
| L4 segment | 5.66 ± 0.96 | 2.87 ± 0.60 | 1.06 ± 0.26 | 0.33 ± 0.10 | 0.18 ± 0.05 | 0.23 ± 0.06 |
| L5 segment | 4.87 ± 0.69 | 2.92 ± 0.73 | 1.06 ± 0.24 | 0.40 ± 0.12 | 0.22 ± 0.07 | 0.24 ± 0.07 |
Values are means ± SEM from group of five animals, measured individually as described under Materials and Methods, and are expressed as fmol/mg protein.
In situ hybridization using the AT1A and AT1B antisense riboprobes allowed us to detect separately mRNAs for the two AT1 receptor subtypes. Specific AT1A and AT1B receptor antisense probe hybridization was seen primarily in grey matter (Fig. 2). Visual examination and quantitative determination showed that the AT1A antisense probe gave the most intense labeling in the spinal cord (Fig. 2A, Table 2). A marked discrete localization of AT1A mRNA was seen in the mediolateral part of ventral horn, suggesting association with the spinal motor neurons (Fig. 2A,D). The hybridization signal for AT1B antisense probe in the grey matter was approximately 2–3 times weaker than for AT1A (Table 2). Quantitative measurement revealed a very low expression of both AT1A and AT1B mRNA in white matter regions (Table 2). The cellular localization of Ang II receptors by emulsion autoradiography revealed the presence of AT1A and AT1B receptor mRNAs in some neuronal cells scattered throughout the spinal grey matter, but especially prominent in ventral motor neurons (Fig. 2E,F).
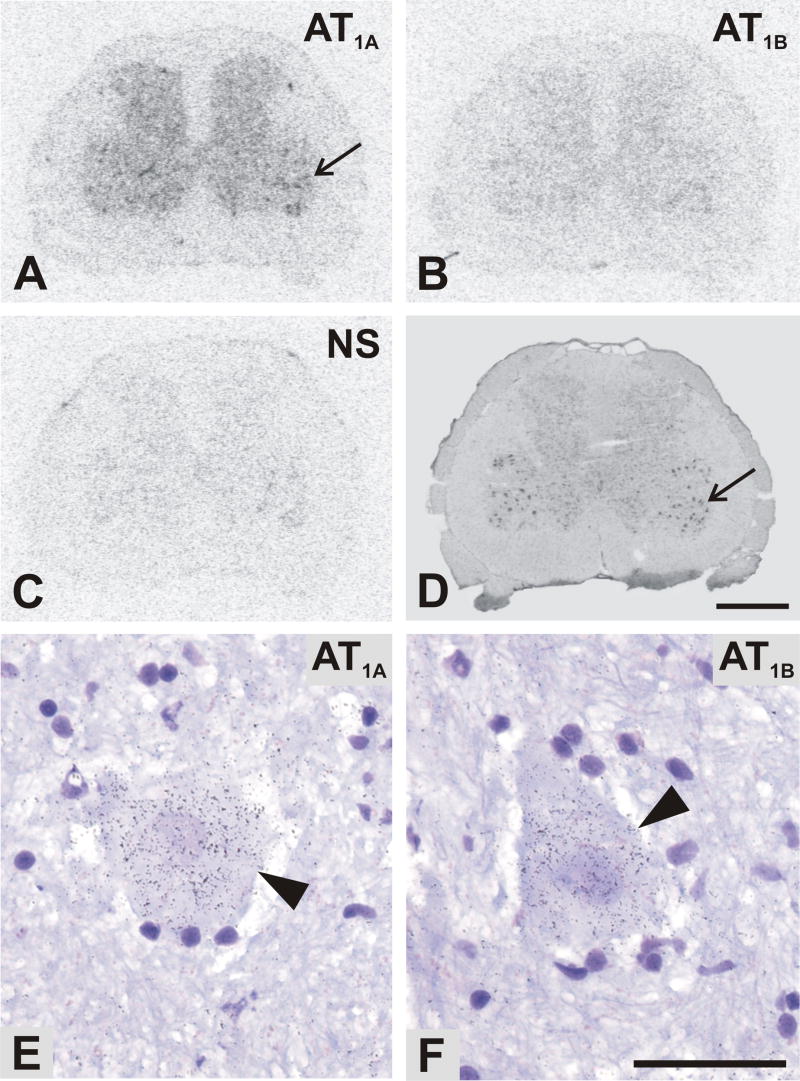
Fig. 2.
Representative autoradiograms showing regional distribution of Angiotensin II AT1A (A) and AT1B (B) receptor subtype mRNAs in consecutive sections of the L4 spinal cord segment, as revealed by in situ hybridization. (C) The AT1B sense control indicating the level of non-specific hybridization. AT1A sense probes showed a similar level of non-specific signal. (D) Cresyl violet staining. Note a marked discrete localization of AT1A mRNA in the ventral horn region (arrow in A and D). Bright-field microphotographs of emulsion autoradiography showed aggregations of silver grains of antisense AT1A (E) and AT1B (F) in the spinal cord motor neurones (arrowheads). Scale bar is 1mm in A–D and 50μm in E, F. NS, non specific.
TABLE 2.
Regional distribution of Angiotensin II AT1A and AT1B receptor subtype mRNA in the lower lumbar (L4–L5) spinal cord segments
| Grey Matter | White Matter | ||||||
|---|---|---|---|---|---|---|---|
| Spinal Cord Segment | Receptor Subtype | Superficial dorsal horn | Lamina X | Ventral horn | Dorsal funiculus | Lateral funiculus | Ventral funiculus |
| nCi/g | |||||||
| L4 segment | AT1A | 41.1 ± 1.9 | 47.4 ± 2.8 | 41.3 ± 1.9 | 6.5 ± 0.6 | 5.6 ± 0.4 | 5.6 ± 0.4 |
| AT1B | 19.8 ± 2.3 | 18.8 ± 1.4 | 20.4 ± 1.9 | 5.0 ± 1.1 | 3.3 ± 0.6 | 4.0 ± 0.7 | |
| L5 segment | AT1A | 49.7 ± 3.8 | 64.7 ± 6.3 | 51.8 ± 3.5 | 6.5 ± 0.7 | 5.2 ± 0.8 | 6.4 ± 0.9 |
| AT1B | 15.8 ± 0.5 | 18.2 ± 0.4 | 18.1 ± 1.7 | 6.1 ± 0.2 | 5.8 ± 0.3 | 6.0 ± 0.2 | |
Values are means ± SEM from group of five animals, measured individually as described under Materials and Methods, and are expressed as nCi/g tissue equivalent.
Unfortunately, we could not determine AT2 probe hybridization, because the sense probe showed a higher signal than the antisense probe in spinal cord sections (not shown).
2. 2. Selective dorsal rhizotomy
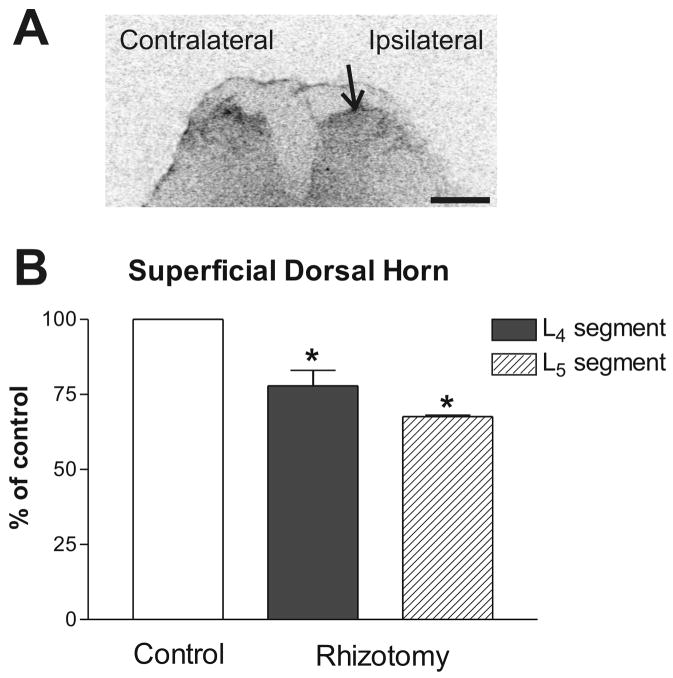
Twenty-four hours after unilateral dorsal rhizotomy, the AT1 receptor binding in the superficial dorsal horns of the lower lumbar segments on the side ipsilateral to the rhizotomy was significantly lower than the contralateral side (Paired Student t-test, t = 7.677 for L4 segment, t = 7.377 for L5 segment) (Fig. 3). No significant increase in AT1 receptor binding was found ipsilaterally in the DRGs (L4 level: 2.627±0.274 (ipsilateral side) vs. 3.078±0.409 (contralateral side); L5 level: 3.306±0.291 (ipsilateral side) vs. 3.492±0.263 (contralateral side)). No significant changes were detected in the sciatic nerve, when the ipsilateral and contralateral sides were compared after dorsal rhizotomy.
Fig. 3.
Representative autoradiogram of the AT1 receptor binding in the superficial dorsal horns of the lower lumbar spinal cord segment after unilateral selective dorsal rhizotomy. (A) Autoradiogram showing the AT1 receptor binding on the ipsilateral (lesion) and contralateral (intact) side. Arrow points to the superficial dorsal horn on the lesioned side. (B) Quantitative autoradiography of the AT1 receptor binding. Values are means ± SEM from groups of four animals, measured individually as described under Materials and Methods, and expressed as percentage of the control. *p<0.05 vs. contralateral side (control). Scale bar = 1 mm.
2. 3. Angiotensin II receptors in the DRGs
Similarly as in the spinal cord, a quantitative determination and the marked displacement of the labeled ligand by the selective AT1 receptor ligand losartan revealed that most of the Ang II receptors in the lower lumbar DRGs are of the AT1 receptor type. AT1 receptor binding was localized in small clusters asymmetrically spread throughout the ganglia, suggesting association with neuronal clusters (Fig. 4). There was no consistent displacement of [125I]-Sar1-Ang II with PD123319, a selective AT2 ligand (Fig. 4C).
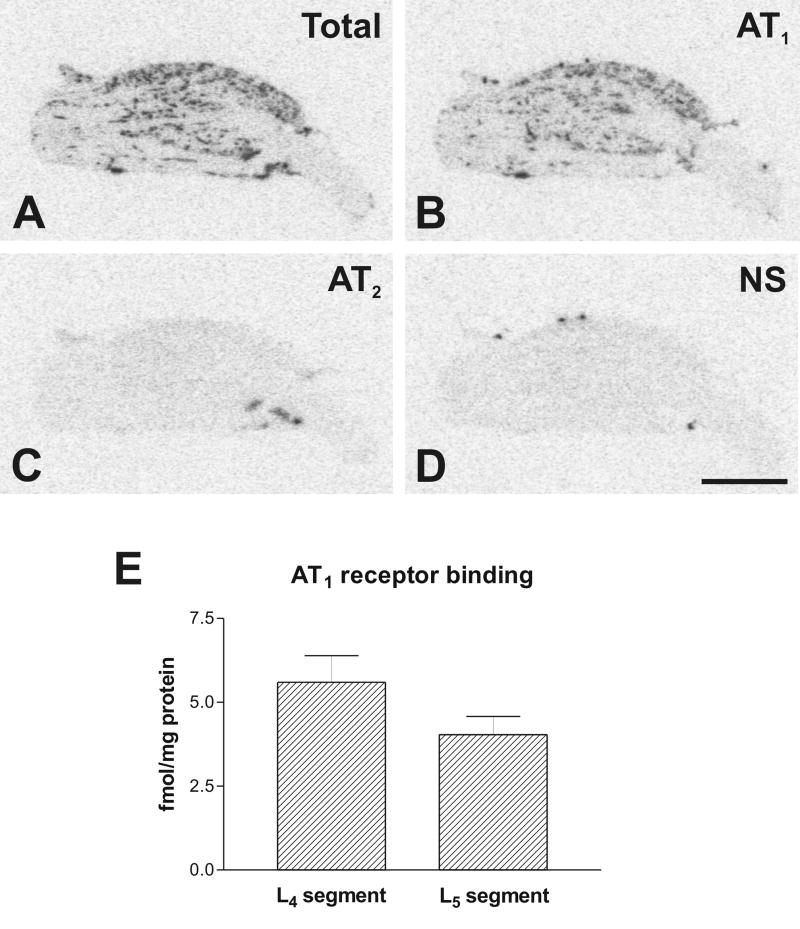
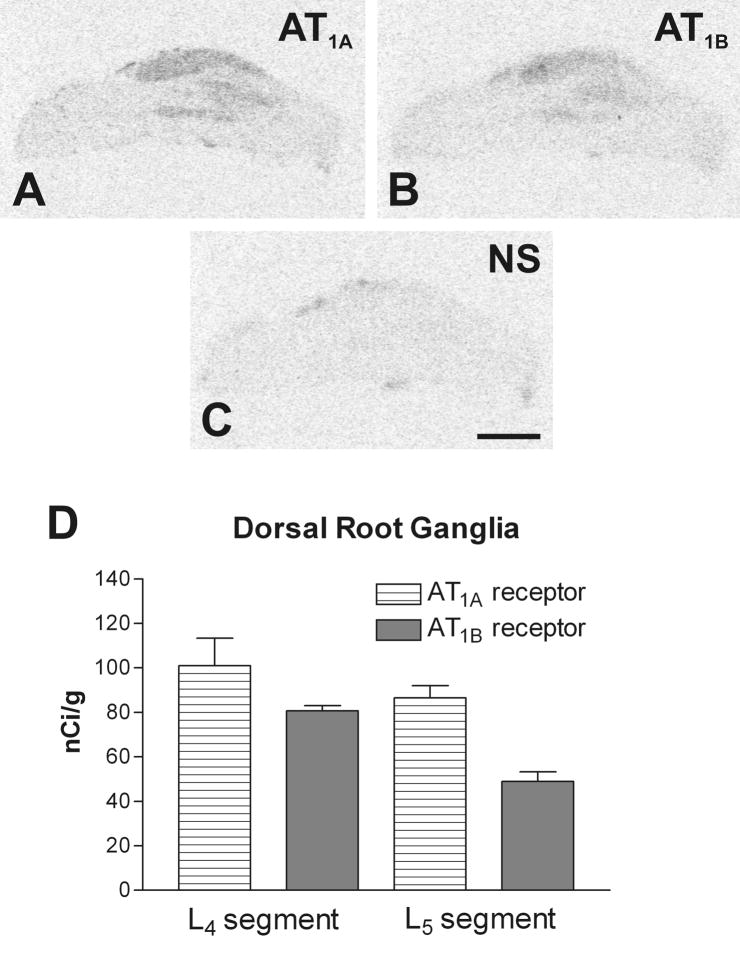
Fig. 4.
Representative pictures of Angiotensin II receptor binding in DRG from lower L4 lumbar level (A) Section showing total binding in the presence of [125I]-Sar1-Ang II. Consecutive sections showing binding as in A with addition of: (B) PD123319 to displace binding to AT2 receptors; (C) losartan to displace binding to AT1 receptors; (D) unlabeled Ang II displacing binding to AT1 and AT2 receptors. (E) Quantitative autoradiography of AT1 receptors in the DRGs. Values are means ± SEM from group of five animals, measured individually as described under Materials and Methods, and expressed as fmol/mg protein. Scale bar is 1 mm. NS, non specific.
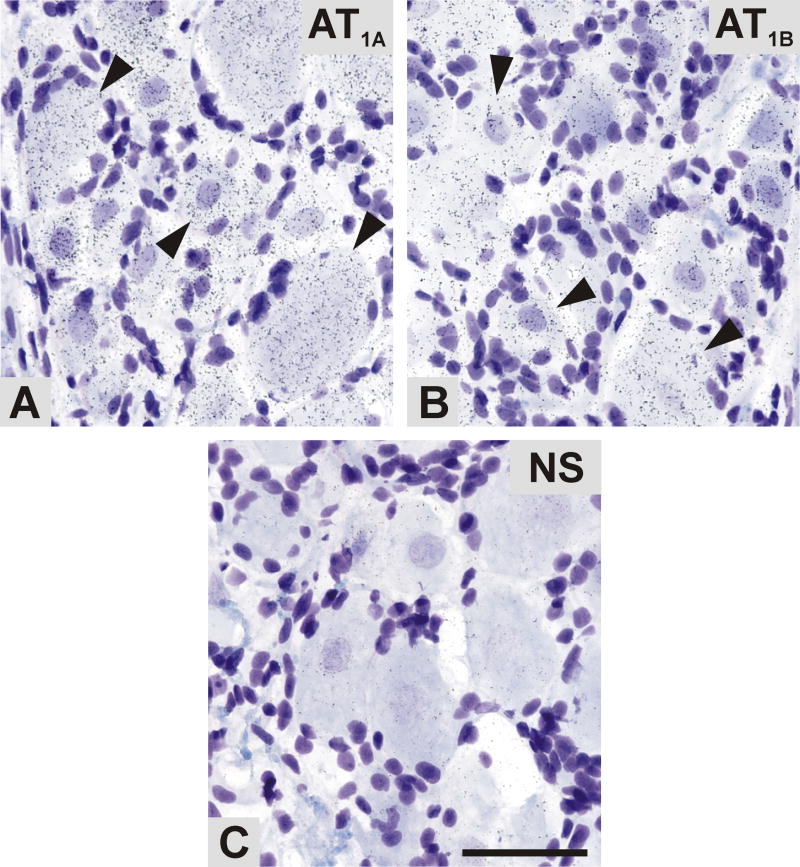
The film autoradiography of the hybridized DRG sections revealed clear signals for AT1A and AT1B mRNA (Fig. 5A,B). As in spinal cord, AT1A mRNA predominated over AT1B in the DRGs corresponding to L4–L5 spinal cord segments (Fig. 5D). The hybridization signal was detected in areas corresponding with the localization of DRG neurons. These findings were confirmed by emulsion autoradiography. Silver grains were selectively accumulated over all DRG neuronal cell bodies whose nuclei were lightly stained with cresyl violet (Fig. 6A,B). Conversely, AT2 mRNA hybridization signal was not consistently detectable or detectable only at very low levels (results not shown).
Fig. 5.
Representative autoradiograms showing the presence of Angiotensin II AT1A (A), and AT1B (B) receptor subtype mRNAs in consecutive DRG sections, as revealed by in situ hybridization. (C) The AT1B sense control indicating the level of non-specific hybridization. The AT1A sense probe showed a same level of non-specific signal. (D) Quantitative autoradiography of Ang II receptor types mRNA. Values are means ± SEM (n=5), measured individually as described in Materials and Methods, and expressed as nCi/mg tissue equivalent. Scale bar=1mm. NS, non specific.
Fig. 6.
Bright-field microphotographs representing emulsion autoradiography of AT1A and AT1B mRNA in the DRG, as revealed by in situ hybridization. Aggregations of silver grains of antisense AT1A (A) and AT1B (B) riboprobes were detectable in the DRG neuronal cells (arrowheads). (D) AT1A sense control indicating the level of non-specific hybridization. Scale bar= 50μm. NS, non specific.
2. 4. Angiotensin II receptors in the sciatic nerve
A low level of AT1 receptor binding (0.304 ± 0.033 fmol/mg protein) was expressed throughout the sciatic nerve (Fig. 7A). Visual examination and quantitative measurements from autoradiograms using [125I]-Sar1-Ang II did not reveal consistent binding to AT2 receptors (results not shown). This result indicated that AT2 receptors were either absent from the nerve, or present in numbers below the sensitivity of our autoradiography binding assays.
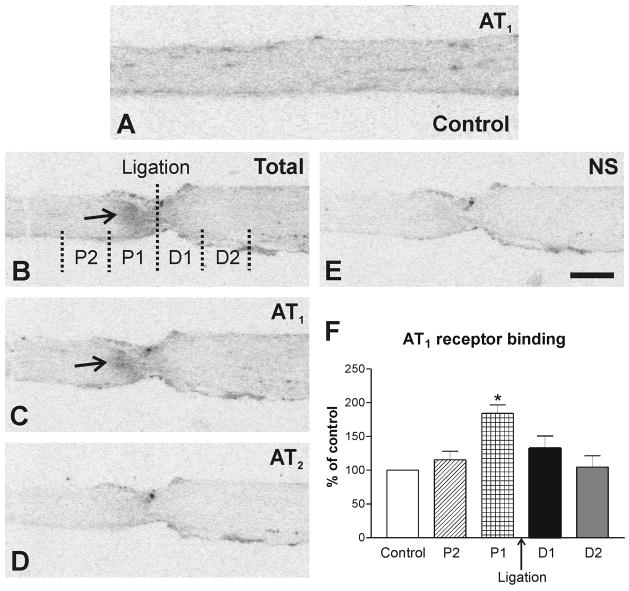
Fig. 7.
Autoradiography of Angiotensin II receptor binding in longitudinal consecutive sections of the ligated sciatic nerve. (A) Autoradiogram showing AT1 receptor binding in intact sciatic nerve considered as a control; (B) Sections showing total binding in the presence of [125I]-Sar1-Ang II. Consecutive sections showing binding as in A with addition of: (C) PD123319 to displace binding to AT2 receptors; (D) losartan to displace binding to AT1 receptors; (E) unlabeled Ang II displacing binding to AT1 and AT2 receptors (see Methods). Binding in the ligated sciatic nerve was studied in 2 proximal (P1 and P2) and 2 distal (D1 and D2) regions at 1mm intervals. Arrows point to the proximal (P1) region of the sciatic nerve revealing a high accumulation of AT1 receptor binding. (F) Quantitative determination of Ang II receptor binding in proximal and distal regions of the ligated sciatic nerve. *p<0.05 vs. all other groups. Scale bar = 1 mm. NS, non specific.
In contrast with DRG and spinal cord, neither film nor emulsion autoradiography showed significant mRNA hybridization signals in the sciatic nerve sections incubated with the AT1A, AT1B or AT2 antisense riboprobes (data not shown).
2. 5. Unilateral sciatic nerve ligation
Densitometric analysis of autoradiograms after the sciatic nerve ligation revealed significant differences between proximal and distal regions. A very high accumulation of the AT1 receptor binding was detected in the proximal (P1) region, closest to the ligature (Fig. 7).
Discussion
We used a combination of quantitative autoradiography and in situ hybridization to study the expression, cellular localization and transport of Ang II receptor types and subtypes in the sciatic sensory motor system of the rat. These methods have advantages, but are not without limitations. Autoradiography allows quantitative measurement of binding sites in discrete tissue areas, but its power of resolution does not permit cellular localization of receptor binding. In rodents, AT1 receptors are of two subtypes, AT1A and AT1B, and AT1A receptors are the predominant subtype in the brain (Jöhren et al., 1995; De Gasparo et al., 2000). Because of very high homology in their coding regions and similar ligand affinities, binding to AT1A and AT1B receptors cannot be differentiated by autoradiography (Inagami et al., 1993). In situ hybridization using riboprobes corresponding to the non-coding regions of the receptor subtypes allowed us not only a quantitative determination of Ang II receptor mRNAs, but also a discrimination of AT1A and AT1B receptor subtypes (Jöhren et al., 1995). Moreover, in situ hybridization using emulsion techniques provided a cellular localization of the mRNA (Wisden and Morris, 1994). The combination of autoradiography and in situ hybridization in alternative tissue sections allows the comparative localization of receptor binding and mRNA, and therefore the areas and/or cells where receptors are formed and the areas where the receptors are expressed.
Our comprehensive analysis of Ang II receptor types showed a strong predominance of the AT1 receptors in the spinal cord and in the DRGs, supporting the hypothesis of a role for Ang II, and its AT1 receptors, in processing and regulating sensory information and motor function. There is general agreement between the present results and the previously reported distribution of Ang II receptors in the spinal cord and DRGs of all mammalian species studied (Mendelsohn et al., 1984; Gehlert et al., 1985; Besson and Chaouch, 1987; White et al., 1988; Oldfield et al., 1994; MacGregor et al., 1995; Ahmad et al., 2003; Tang et al., 2008).
Receptor binding assays revealed a higher level of AT1 receptor localization in the grey than in the white matter, and a particularly high expression in lamina I and II of the spinal cord and in the DRGs. The very high AT1 expression in the superficial dorsal horn (laminae I–II), the termination sites of polymodal nociceptive C- and Aδ-fibers (Besson and Chaouch, 1987), supports the hypothesis of a role of Ang II in the regulation of nociception (Irvine et al., 1995; Toma et al., 1997). AT1A receptors clearly predominate over AT1B receptors in the tissues studied. In situ hybridization using the emulsion technique detected a strong hybridization signal for AT1A and much lower for AT1B mRNA, predominantly over neurons localized in spinal ventral horns (motor neurons), but also in neurons scattered throughout the grey matter. Our finding supplements a previous study demonstrating the presence of AT1A and AT1B transcripts by real time-PCR in the spinal cord and AT1-immunoreactivity in spinal motor neurons (Ahmad et al., 2003). The large number of AT1 receptors located in spinal cord motor neurons agrees with recent reports indicating that Ang II may have direct access to locomotor network elements, and that AT1 receptor stimulation increases motoneuron excitability (Barriere et al., 2005; Oz et al., 2005). AT1 receptor stimulation may also be responsible for the proposed trophic effect of Ang II in the ventral horn of the spinal cord (Iwasaki et al., 1991).
In addition, we found significant numbers of Ang II AT1 receptors in large spinal cord arteries and in the highly vascularized pia mater surrounding the spinal cord. Our results support earlier observations (Zhou et al., 2006) of high AT1 receptor localization in the endothelium of cerebral microvessels and the endothelium and smooth muscle of brain arterioles. We conclude that AT1 receptors may participate in the regulation of blood flow to the spinal cord, as in the rest of the central nervous system.
AT1 receptor binding and mRNA were localized to numerous neurons in the DRGs. These results implicate Ang II as a possible regulatory factor for sensory information in the DRGs, perhaps in combination with other neuropeptides synthesized in DRG neurons such as vasoactive intestinal polypeptide (Noguchi et al., 1989; Kashiba et al., 1992), cholecystokinin (Xu et al., 1993), neuropeptide Y (Wakisaka et al., 1991; Noguchi et al., 1993), substance P (Noguchi et al., 1994), galanin (Wiesenfeld-Hallin and Xu, 1998), and calcitonin gene-related peptide (Cottrell et al., 2005). Some support for such an interaction comes from double label immunohistochemistry studies suggesting co-localization of AT1 and calcitonin gene-related peptide in rat DRG (Tang et al. 2008). These neuropeptides have been postulated to be involved in multiple functions and pathological processes, including nociception, development of peripheral inflammation and axonal regeneration.
Since AT1A and AT1B mRNA are localized to spinal motor and DRG neurons as determined by emulsion in situ hybridization, it is possible that the receptor subtypes are colocalized in specific neuronal groups. A definite answer to this question can only be obtained by the simultaneous use of fluorescence-labeled selective riboprobes.
In the superficial dorsal horn, we detected very high AT1 receptor binding, but markedly lower AT1A and/or AT1B receptor mRNA. Our experiment with unilateral dorsal rhizotomy was carried out to clarify this discrepancy and to establish the origin of the AT1 receptors in the superficial dorsal horn. The significant, but partial decline in AT1 receptor binding in the superficial dorsal horns ipsilateral to the rhizotomy indicates that at least part of the AT1 receptors present in the superficial dorsal horn originates in the DRG neuronal cells and are transported through projecting fibers to this region of the spinal cord. The rhizotomy-induced reduction in AT1 receptor binding in the superficial dorsal horn was not complete, and a period of 24hrs may not be sufficient to detect the maximum effect of the operation on AT1 receptor level in this region. Alternatively, axonal transport of the AT1 receptors from the DRGs may not be the sole source of the receptors in the superficial dorsal horn. Physiological significance and relative importance of transported and locally produced AT1 receptors are matters for further investigation.
The sciatic nerve expressed low but significant numbers of AT1 receptors in the absence of detectable mRNA, suggesting again an origin by axonal transport. The accumulation of AT1 receptor binding in the proximal, but not the distal, region close to a sciatic nerve ligature indicates anterograde, but not retrograde, axonal transport of the AT1 receptors. This transport may be proceeding either along sensory fibers, originating in DRG neurons, or along motor fibers originating in motor neurons of the ventral horns (Ahmad et al. 2003, Tang et al., 2008 and present results).
The absence of AT2 receptor binding, and AT1A, AT1B and AT2 mRNA hybridization signals in the sciatic nerve is in contrast with earlier reports of the presence of AT1 and AT2 receptors in cultured Schwann cells obtained from a human schwannoma (Bleuel et al., 1995) and from the observation of time-dependent increase of AT1A, AT1B and AT2 receptor mRNA expression in the sciatic nerve and DRG following axotomy and crush-induced lesions, as determined by RT-PCR (Gallinat et al., 1998). These discrepancies can be best explained by differential AT2 receptor expression in cultured cells (Heemskerk et al., 1999) or in cells of neoplastic origin (Bleuel et al. 1995), and by the very high sensitivity of the RT-PCR method, 1 copy/1 million cells when including hot start modification (Nuovo et al., 1994).
We demonstrate here that AT1 receptors are highly expressed in the spinal cord and DRGs, and at a much lower level in the sciatic nerve. We hypothesize that AT1 receptors may be involved in sensory and motor function. The relevance of a second Ang II receptor type, the AT2 receptor, is still questionable. It has been proposed that AT1 and AT2 receptor types interact at the cellular level, under the assumption of same-cell localization (Gelband et al., 1997). However, in the rat brain, AT1 and AT2 receptors are distinctly localized to different neurons, and in most cases to different pathways (Tsutsumi and Saavedra, 1991; Jöhren et al., 1995). We attempted to determine the presence and number of AT2 receptors in the sciatic nerve pathway. Our studies were hampered by the limited number of tools available for AT2 receptor studies. Using antisense riboprobes for AT2 receptor mRNA, we found inconsistent and very low signals. In addition the signals obtained with sense AT2 riboprobes were similar or higher than those found with the antisense probes (results not shown). For this reason we could not determine the presence of AT2 receptor mRNA in our preparations. Using autoradiography, signals obtained by displacement of [125I]-Sar1-Ang II with the selective AT2 antagonist PD123319 were very low and inconsistent, and in most cases they were not significantly higher than background signals. Whether or not low numbers of AT2 receptors, undetectable using the techniques available to us, exert meaningful physiological actions, cannot be presently determined. Nonetheless, we lean toward the view that AT2 receptors play a comparatively minor role in the spinal cord - DRG - sciatic nerve pathway.
In conclusion, our observations demonstrate the anatomical and cellular basis for multiple and complex functions of Ang II in the spinal cord, DRGs and the sciatic nerve. These results suggest a major role for AT1 receptors, and a secondary, still undetermined and uncertain role for the AT2 receptor type. We demonstrated for the first time that locally formed AT1 receptors are axonally transported through the sciatic nerve to the periphery, and from the DRGs to the superficial dorsal horns. On the basis of our results and those in the literature, we hypothesize that AT1 receptors may play multiple roles regulating sensory and motor functions. Blood flow to the spinal cord may be regulated by locally formed as well as circulating Ang II, stimulating endothelial and smooth muscle cell AT1 receptors. Locally formed Ang II may control the trophic state and excitability of motor neurons, and regulate sensory transmission.
4. Experimental procedures
4. 1. Experimental animals
We used male adult (250–320g) Sprague-Dawley rats purchased from Charles River Laboratories, Wilmington, MA and kept at 22 ± 0.5°C in a 12h dark/light cycle with free access to a normal rat diet and tap water. Experimental protocols were approved by the NIMH Animal Care and Use Committee with the aim of minimizing the number of animals used and their suffering, according to the NIH Guide for the Care and Use of Laboratory Animals, NIH Publication N°80-23, revised 1996. Animals were divided into three experimental groups: 1) control animals (n=5), 2) animals subjected to unilateral sciatic nerve ligation (n = 5), 3) animals subjected to selective unilateral dorsal rhizotomy (n = 4).
4. 2. Sciatic nerve ligation
Rats were anesthetized with sodium pentobarbital (45 mg/kg, i.p.), supplemented with smaller doses (5mg/kg, i.p.) as needed. The skin of the left thigh was shaved and disinfected with 1.75% iodine and the left sciatic nerves were exposed. The nerve was then tied above the knee with autoclaved sterile silk suture (5-0, 1.0 metric), 5 to 10 mm above the bifurcation of its tibial and peroneal branches. The skin incision was then closed with sterile sutures. To prevent “autotomy” (gnawing at the foot on the transected side), a cloth bandage was wrapped around the foot on the operated side. Rats were euthanized with sodium pentobarbital (250 mg/kg, i.p.) after 20–24 hrs, and the lower (L4–L5) spinal cord segments, corresponding DRGs and the ligated sciatic nerves were quickly dissected, along with the contralateral nerves as experimental controls. Then tissues were immediately frozen in isopentane cooled by dry ice, and stored in −80°C until used.
4. 3. Selective unilateral dorsal rhizotomy
Under deep anesthesia induced with isoflurane (1.5–2%) in oxygen delivered through a face mask, the animals were placed in a rodent stereotaxic frame and the vertebral spiny protuberances on L4 and L5 were identified as landmarks. After skin incision, spinal dorsal roots and adjacent dura matter on the left side at L4–L5 level were carefully exposed by hemi-laminectomy. Lateral bone removal was necessary to provide full access to the dorsal roots without spinal cord damage. After dura matter incision, the dorsal roots were identified and cut just inside the intervertebral foramen proximal to the dorsal root ganglia with fine scissors under an operating microscope. The surgical area was then sutured in layers and the skin around the incision sterilized with Betadyne solution. Completeness of the rhizotomy was determined 1) by microscopic inspection during surgery and prior to tissue removal, 2) by the absence of behavioral responses to intense noxious stimulation (pinch) of the deafferented hindlimb. The operation resulted in complete deafferentation, but not paralysis of the hind limb. After surgery and full recovery from anesthesia, the animals were returned to their cages with solid floors covered with 3–6cm of soft bedding (sawdust). Regular rat food and water was provided ad libitum throughout the recovery period. Twenty four hrs after surgery the rats were euthanized by decapitation, and the DRGs, spinal cord and sciatic nerve were removed, frozen and stored at −80°C until used.
4. 4. Angiotensin II receptor binding
For receptor autoradiography, 16-μm thick longitudinal or transverse sections were cut in a cryostat at −20°C, thaw-mounted on precleaned Polysine™-microscope slides (Erie Scientific Company, Portsmouth, NH), dried overnight in a desiccator at 4°C, and stored at −80°C until used. Before the procedure, the sections were dried in a desiccator at room temperature and then preincubated for 15 min at 22°C in freshly prepared 10 mM sodium phosphate buffer, pH 7.4, containing 120 mM NaCl, 5 mM Na2EDTA, 0.005% bacitracin (Sigma Chemical, St. Louis, MO) and 0.2% proteinase-free bovine serum albumin (Sigma Chemical). To quantify the total binding of Ang II to AT1 and AT2 receptor types, the sections were labeled in vitro for 120 min in buffer containing 0.5 nM of [125I]-Sarcosine1-Ang II ([125I]-Sar1-Ang II, Peninsula Laboratories, Belmont, CA), which was iodinated by the Peptide Radioiodination Service Center (School of Pharmacy, University of Mississippi) to a specific activity of 2176 Ci/mmol. To determine specific and non-specific binding, consecutive sections were incubated as above in the presence of 5μM (excess) unlabeled Ang II (Peninsula). Non-specific binding was defined as the binding which remained in the presence of the excess unlabeled agonist. Specific receptor binding was the difference between the total and non-specific binding. To determine selective binding to the Ang II receptor types, we incubated consecutive sections with 0.5 nM [125I]-Sar1-Ang II in the presence of 10-μM losartan (DuPont-Merck, Wilmington, DE, USA), a selective AT1 receptor antagonist, or 10-μM PD123319 (Sigma), a selective AT2 receptor antagonist. The 10-μM concentration of the antagonists had been demonstrated to give maximum specific displacement (Tsutsumi and Saavedra, 1991). The binding to AT1 and AT2 receptors was the binding displaced by losartan and PD123319, respectively (Tsutsumi and Saavedra, 1991).
After incubation, slides were rinsed four consecutive times for 1 min each in fresh ice-cold 50 mM Tris-(hydroxymethyl-aminomethane) HCl buffer, pH 7.5, followed by dipping in ice-cold distilled water, and drying under air (Tsutsumi and Saavedra, 1991). Sections were exposed to Kodak Biomax MR film (Eastman Kodak Company, Rochester, NY) together with 14C microscales (American Radiolabeled Chemicals, St. Louis, MO). The films were developed in GBX developer (Eastman Kodak) for 4 min, fixed in Kodak GBX fixer for 4 min, and rinsed in water for 15 min. The films were exposed for different times, depending on the amount of binding present, to obtain film images within the linear portion of the standard curve. Optical densities of autoradiograms generated by incubation with the 125I ligands for 4 days were normalized after comparison with 14C standards as described (Miller and Zahniser, 1987), by computerized densitometry using the Scion Image 4.0.2 Program (Scion Corporation) based on the NIH Image Program of the National Institutes of Health. The optical densities were then transformed to the corresponding values of fmol/mg protein (Miller and Zahniser, 1987; Nazarali et al., 1989).
4. 5. Probe synthesis and In Situ Hybridization
In situ hybridization was performed using [35S]-labeled antisense and sense (control) riboprobes for AT1A, AT1B, and AT2 receptors. The preparation of subclones, rAT1A-S2, rAT1B-S1, and rAT2-S1 was described previously (Jöhren et al. 1995). Briefly, fragments from the 3′-non-coding regions of the AT1A, AT1B and AT2 cDNAs were subcloned into the polylinker site of the pBluescripts KS(+) vector (Stratagene, La Jolla, CA). A 368bp PalI fragment of the rat AT1A cDNA was ligated into the EcoRV site of the pBluescript vector. The orientation of the insert was determined by restriction analysis with AvaII, which cuts asymmetrically within the subcloned AT1A cDNA fragment. A 396bp HindIII/EcoRI fragment of the rat AT1B cDNA was subcloned into the pBluescript vector restricted with the same enzymes and a 371bp XbaI/BglII fragment of the rat AT2 cDNA was subcloned into the Bluescript vector restricted with BamHI and XbaI. The subclones containing plasmids were linearized with HindIII or EcoRI for the rAT1A-S2 and AT1B-S1 or with XbaI or EcoRI for the AT2-S1, respectively, to generate [35S]-labeled sense and antisense probes. Radiolabeled probes were prepared by in vitro transcription in the presence of [35S]-UTP (Amersham, Arlington Heights, IL), 1μg of linearized subclone plasmid and T7 or T3 RNA polymerase, using a MAXIScript® T7/T3 kit (Ambion, Austin, TX) according to the protocol of the manufacturer. After transcription, the template DNA was digested with DNase I for 15min at 37°C. Unincorporated nucleotides were removed by centrifugation through ProbeQuant™ G-50 Micro Columns (Amersham Biosciences, Little Chalfont Buckinghamshire, England). The labeling of riboprobes was monitored with liquid scintillation counting and the specificity verified by a preliminary experiment with adrenal gland, the paraventricular nucleus, and pituitary gland as a positive controls (Jöhren et al., 1995; Lenkei et al., 1999).
For in situ hybridization, sections (16 μm) were thaw-mounted onto Superfrost®/Plus microslides, dried at 50°C for 5 min on a slide warmer and stored at −80°C until hybridization. Prior to use, the sections were warmed in a dessicator at room temperature and than fixed in 4% formaldehyde in 1XPBS for 10 min. After two washes in 1XPBS, they were acetylated for 10 min in 0.1M triethanolamine HCl, 0.9% NaCl, containing 0.25% acetic anhydride, delipidated in ethanol and chloroform, and air-dried. Each slide-mounted section was covered with 150 μl hybridization buffer containing 50% formamide, 0.3 M NaCl, 1mM EDTA, 20mM Tris, pH 7.5, 1x Denhardt’s solution, 10% dextran sulfate, 100 μg/ml salmon testes DNA, 250 μg/ml yeast RNA, 250 μg/ml yeast tRNA, 150 mM DTT, 0.2% SDS, 0.2% sodium tiosulfate (NTS) and 2×107 cpm/ml sense or antisense probe, and then slide was covered with glass cover slip. After hybridization for 18 hrs at 54°C, coverslips were removed and sections were rinsed several times in 4X standard saline citrate (SSC). Non-hybridized probes were digested by incubation with 40 μg/ml RNase A (Sigma Chemical, St. Louis, MO) for 30 min at 37°C. After a final high stringency wash in 0.1X SSC at 65°C for 60 min, sections were dehydrated in graded ethanols containing 0.3M ammonium acetate, air dried and exposed to Biomax MR film (Kodak, Rochester, NY) for 11 days. Films were developed as described above. The mRNA expression was analysed by measuring optical film densities using the Scion Image Program 4.0.2 (Scion Corporation) based on the NIH Image Program of the National Institutes of Health. The intensities of hybridization signals were expressed as nCi/g tissue equivalent after calibration with [14C]-microscales (Miller, 1991), and after subtraction of the values obtained in the same areas of adjacent sections hybridized with sense (control) probes, which represent a nonspecific hybridization. Data were expressed as means ± SEM, for groups of five animals, measured individually.
For cellular localization, slides were dipped in Ilford Nuclear Research Emulsion K5D, exposed for 4–6 weeks, developed in Kodak D-19 developer for 4 min at 15°C, fixed for 4 min, washed in running water for 10 min, and counterstained with cresyl violet. Slides were dehydrated in graded ethanol, then coverslipped with Cytoseal 60 and analyzed under a Zeiss microscope. Positive hybridization signals were evaluated by the cellular localization of silver grains.
4. 6. Statistical analysis
Results are expressed as means ± SEM. Data from ligated sciatic nerve were statistically evaluated using one-way ANOVA followed by Newman-Keuls posthoc test. Differences between the contralateral and ipsilateral sides for rhizotomized animals were tested using the paired Student’s t-test. The level of significance in all cases is presented as p<0.05. All data were analyzed with Prism 3.03 software (GraphPad software for Science, San Diego, CA, USA).
Acknowledgments
This study was supported by the Division of Intramural Research Programs, National Institutes of Mental Health, National Institutes of Health, Department of Health and Human Services, USA.
Abbreviations
- RAS
the renin-angiotensin system
- Ang II
Angiotensin II
- DRGs
dorsal root ganglia
- AT1
Angiotensin II receptor type 1
- AT2
Angiotensin II receptor type 2
Footnotes
Classification terms: Section. 6. Regulatory Systems, Senior Editor: Alan F. Sved (Pittsburg, PA, USA); Associate Editors: Timothy H. Moran (Baltimore, MD, USA)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature references
- Ahmad Z, Milligan CJ, Paton JF, Deuchars J. Angiotensin type 1 receptor immunoreactivity in the thoracic spinal cord. Brain Res. 2003;985:21–31. doi: 10.1016/s0006-8993(03)03112-3. [DOI] [PubMed] [Google Scholar]
- Barriere G, Bertrand S, Cazalets JR. Peptidergic neuromodulation of the lumbar locomotor network in the neonatal rat spinal cord. Peptides. 2005;26:277–286. doi: 10.1016/j.peptides.2004.09.002. [DOI] [PubMed] [Google Scholar]
- Besson JM, Chaouch A. Peripheral and spinal mechanisms of nociception. Physiol Rev. 1987;67:67–186. doi: 10.1152/physrev.1987.67.1.67. [DOI] [PubMed] [Google Scholar]
- Bleuel A, de Gasparo M, Whitebread S, Püttner I, Monard D. Regulation of protease nexin-1 expression in cultured Schwann cells is mediated by Angiotensin II receptors. J Neurosci. 1995;15:750–761. doi: 10.1523/JNEUROSCI.15-01-00750.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cottrell GS, Roosterman D, Marvizon JC, Song B, Wick E, Pikios S, Wong H, Berthelier C, Tang Y, Sternini C, Bunnet NW, Grady EF. Localization of calcitonon receptor-like receptor and receptor activity modifying protein 1 in enteric neurons, dorsal root ganglia, and the spinal cord of the rat. J Comp Neurol. 2005;490:239–255. doi: 10.1002/cne.20669. [DOI] [PubMed] [Google Scholar]
- De Gasparo M, Catt KJ, Inagami T, Wright JW, Unger T. International Union of Pharmacology. XXIII. The Angiotensin II receptors. Pharmacol Rev. 2000;52:415–472. [PubMed] [Google Scholar]
- Gallinat S, Yu M, Dorst A, Unger T, Herdegen T. Sciatic nerve transecion evokes lasting up-regulation of Angiotensin AT2 and AT1 receptor mRNA in adult rat dorsal root ganglia and sciatic nerves. Mol Brain Res. 1998;57:111–122. doi: 10.1016/s0169-328x(98)00079-5. [DOI] [PubMed] [Google Scholar]
- Gehlert DR, Speth RC, Wamsley JK. Quantitative autoradiography of angiotensin II receptors in brain and kidney: focus on cardiovascular implications. Prog Clin Biol Res. 1985;192:241–249. [PubMed] [Google Scholar]
- Gelband CH, Zhu M, Lu D, Reagan LP, Fluharty SJ, Posner P, Raizada MK, Sumners C. Functional interaction between neuronal AT1 and AT2 receptors. Endocrinology. 1997;138:2195–2198. doi: 10.1210/endo.138.5.5236. [DOI] [PubMed] [Google Scholar]
- Heemskerk FM, Zorad S, Xu N, Gutkind SJ, Saavedra JM. Characterization of AT2 receptor expression in NIH 3T3 fibroblasts. Cell Mol Neurobiol. 1999;19:277–288. doi: 10.1023/A:1006985329240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Wang DH. Role of AT1 and AT2 receptor subtypes in salt-sensitive hypertension induced by sensory nerve degeneration. J Hypertens. 2001;19:1841–1846. doi: 10.1097/00004872-200110000-00019. [DOI] [PubMed] [Google Scholar]
- Inagami T, Iwan N, Sasaki K, Guo DF, Faruta H, Yamano Y, Bardhan S, Chaki S, Makito N, Badr K. Angiotensin II receptors: cloning and regulation. Arzneimittelforschung. 1993;43:226–228. [PubMed] [Google Scholar]
- Irvine RJ, White JM, Head RJ. The rennin angiotensin system and nociception in spontaneously hypertensive rats. Life Sci. 1995;56:1073–1078. doi: 10.1016/0024-3205(95)00043-6. [DOI] [PubMed] [Google Scholar]
- Iwasaki Y, Kinoshita M, Ikeda K, Shiojima T, Kurihara T, Appel SH. Trophic effect of angiotensin II, vasopressin and other peptides on the cultured ventral spinal cord of rat embryo. J Neurol Sci. 1991;103:151–155. doi: 10.1016/0022-510x(91)90158-4. [DOI] [PubMed] [Google Scholar]
- Jöhren O, Inagami T, Saavedra JM. AT1A, AT1B and AT2 angiotensin II receptor subtype gene expresion in rat brain. Neuroreport. 1995;6:2549–2552. doi: 10.1097/00001756-199512150-00024. [DOI] [PubMed] [Google Scholar]
- Kashiba H, Senba E, Ueda Y, Tohyama M. Co-lozalized but target-unrelated expression of vasoactive intestinal polypeptide and galanin in rat dorsal root ganglion neurons after peripheral nerve crush injury. Brain Res. 1992;582:47–57. doi: 10.1016/0006-8993(92)90315-z. [DOI] [PubMed] [Google Scholar]
- Lenkei Z, Nuyt AM, Grouselle D, Corvol P, Llorens-Cortes C. Identification of endocrine cell populations expressing the AT1B subtype of Angiotensin II receptors in the anterior pituitary. Endocrinology. 1999;140:472–477. doi: 10.1210/endo.140.1.6397. [DOI] [PubMed] [Google Scholar]
- MacGregor DP, Murone C, Song K, Allen AM, Paxinos G, Mendelsohn AO. Angiotensin II receptor subtypes in the human central nervous system. Brain Res. 1995;675:231–240. doi: 10.1016/0006-8993(95)00076-3. [DOI] [PubMed] [Google Scholar]
- Mendelsohn FA, Quirion R, Saavedra JM, Aguilera G, Catt KJ. Autoradiographic localization of angiotensin II receptors in rat brain. Proc Natl Acad Sci USA. 1984;81:1575–1579. doi: 10.1073/pnas.81.5.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller JA, Zahniser NR. The use of 14C-labeled tissue standards for the calibration of 125I-labeled ligands in quantitative autoradiography. Neurosci Lett. 1987;81:345–350. doi: 10.1016/0304-3940(87)90408-3. [DOI] [PubMed] [Google Scholar]
- Miller JA. The calibration of 35S or 32P with 14C-labeled brain paste or 14C-plastic standards for quantitative autoradiography using LKB Ultrafilm or Amersham Hyperfilm. Neurosci Lett. 1991;121:211–214. doi: 10.1016/0304-3940(91)90687-o. [DOI] [PubMed] [Google Scholar]
- Nazarali AJ, Gutkind JS, Saavedra JM. Calibration of 125I-polymer standards with 125I-brain paste standards for use in quantitative receptor autoradiography. J Neurosci Methods. 1989;30:247–253. doi: 10.1016/0165-0270(89)90135-0. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Senba E, Morita Y, Sato M, Tohyama M. Prepro-VIP and preprotachykinin mRNAs in the rat dorsal root ganglion cells following peripheral axotomy. Brain Res Mol Brain Res. 1989;6:327–330. doi: 10.1016/0169-328x(89)90077-6. [DOI] [PubMed] [Google Scholar]
- Noguchi K, De Leon M, Nahin RL, Senba E, Ruda MA. Quantification of axotomy-induced alteration of neuropeptide mRNAs in dorsal root ganglion neurons with special reference to neuropeptide Y mRNA and the effects of neonatal capsaicin treatment. J Neurosci Res. 1993;35:54–66. doi: 10.1002/jnr.490350108. [DOI] [PubMed] [Google Scholar]
- Noguchi K, Dubner R, De Leon M, Senba E, Ruda MA. Axotomy induces preprotachykinin gene expression in a subpopulation of dorsal root ganglion neurons. J Neurosci Res. 1994;37:596–603. doi: 10.1002/jnr.490370506. [DOI] [PubMed] [Google Scholar]
- Nuovo GJ. Vol. Vol. 33. Humana Press; Totowa, NJ, USA: 1994. PCR In situ hybridization. In Methods in Molecular Biology: In situ hybridization protocols; pp. 223–241. [DOI] [PubMed] [Google Scholar]
- Oldfield BJ, Allen AM, Hards DK, McKinley MJ, Schlawe I, Mendelsohn FAO. Distribution of angiotensin II receptor binding in the spinal cord of the sheep. Brain Res. 1994;650:40–48. doi: 10.1016/0006-8993(94)90204-6. [DOI] [PubMed] [Google Scholar]
- Oz M, Yang KH, O’donovan MJ, Renaud LP. Presynaptic angiotensin II AT1 receptors enhance inhibitory and excitatory synaptic neurotransmission to motoneurons and other ventral horn neurons in neonatal rat spinal cord. J Neurophysiol. 2005;94:1405–1412. doi: 10.1152/jn.00165.2005. [DOI] [PubMed] [Google Scholar]
- Paton JF, Kasparov S. Sensory channel specific modulation in the nucleus of the solitary tract. J Auton Nerv Syst. 2000;80:117–129. doi: 10.1016/s0165-1838(00)00077-1. [DOI] [PubMed] [Google Scholar]
- Paul M, Poyan Mehr A, Kreutz R. Physiology of local renin-angiotensin systems. Physiol Rev. 2006;86:747–803. doi: 10.1152/physrev.00036.2005. [DOI] [PubMed] [Google Scholar]
- Pelegrini-da-Silva A, Martins AR, Prado WA. A new role for the renin-angiotensin system in the rat periaqueductal gray matter: angiotensin receptor-mediated modulation of nociception. Neuroscience. 2005;132:453–463. doi: 10.1016/j.neuroscience.2004.12.046. [DOI] [PubMed] [Google Scholar]
- Saavedra JM. Brain and Pituitary Angiotensin. Endocr Rev. 1992;13:324–380. doi: 10.1210/edrv-13-2-329. [DOI] [PubMed] [Google Scholar]
- Sakagawa T, Okuyama S, Kawashima N, Hozumi S, Nakagawasai O, Tadano T, Kisara K, Ichiki T, Inagami T. Pain threshold, learning and formation of brain edema in mice lacking the angiotensin II type 2 receptor. Life Sci. 2000;67:2577–2585. doi: 10.1016/s0024-3205(00)00841-9. [DOI] [PubMed] [Google Scholar]
- Schmalbruch H. Fiber composition of the rat sciatic nerve. Anat Rec. 1986;215:71–81. doi: 10.1002/ar.1092150111. [DOI] [PubMed] [Google Scholar]
- Swett JE, Torigoe Y, Elie VR, Bourassa CM, Miller PG. Sensory neurons of the rat sciatic nerve. Exp Neurol. 1991;114:82–103. doi: 10.1016/0014-4886(91)90087-s. [DOI] [PubMed] [Google Scholar]
- Tang H, Pavel J, Saavedra JM, Brimijoin S. Angiotensin II type 1 receptors may not influence response of spinal neurons to axonal damage. Neurol Res. 2008 doi: 10.1179/174313208X298020. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toma N, Sgambato V, Couture R. Effect of angiotensin II on a spinal nociceptive reflex in the rat: receptor and mechanism of action. Life Sci. 1997;61:503–513. doi: 10.1016/s0024-3205(97)00410-4. [DOI] [PubMed] [Google Scholar]
- Tsuruoka S, Wakaumi M, Ioka T, Yamamoto H, Ando H, Sugimoto K, Fujimura A. Angiotensin II receptor blocker-induces blunted taste sensitivity: comparison of candesartan and valsartan. Br J Clin Pharmacol. 2005;60:204–207. doi: 10.1111/j.1365-2125.2005.02394.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsumi K, Saavedra JM. Quantitative autoradiography reveals different angiotensin II receptor subtypes in selected rat brain nuclei. J Neurochem. 1991;56:348–351. doi: 10.1111/j.1471-4159.1991.tb02602.x. [DOI] [PubMed] [Google Scholar]
- Wakisaka S, Kajander KC, Bennet GJ. Increased neuropeptide Y (NPY)-like immunoreactivity in rat sensory neurons following peripheral axotomy. Neurosci Lett. 1991;124:200–203. doi: 10.1016/0304-3940(91)90093-9. [DOI] [PubMed] [Google Scholar]
- Wheeler-Schilling TH, Kohler K, Sautter M, Guenther E. Angiotensin II receptor subtype gene expression and cellular localization in the retina and non-neuronal ocular tissues of the rat. Eur J Neurosci. 1999;11:3387–3394. doi: 10.1046/j.1460-9568.1999.00787.x. [DOI] [PubMed] [Google Scholar]
- White SR, Penner JD, Speth RC, Chan JY. Angiotensin II receptors in the lumbar spinal cord of the rat. Brain Res. 1988;441:195–201. doi: 10.1016/0006-8993(88)91398-4. [DOI] [PubMed] [Google Scholar]
- Wiesenfeld-Hallin Z, Xu XJ. Galanin in somatosensory function. Ann N Y Acad Sci. 1998;863:383–389. doi: 10.1111/j.1749-6632.1998.tb10708.x. [DOI] [PubMed] [Google Scholar]
- Wisden W, Morris BJ. In situ hybridization with synthetic oligonucleotide probes. In: Wisden W, Morris BJ, editors. In situ hybridization protocols for the brain. Academic Press; San Diego, CA: 1994. pp. 9–34. [Google Scholar]
- Xu XJ, Puke MJ, Verge VM, Wiesenfeld-Hallin Z, Hughes J, Hokfelt T. Up-regulation of cholecystokinin in primary sensory neurons is associated with morphine insensitivity in experimental neuropathic pain in the rat. Neurosci Lett. 1993;152:129–132. doi: 10.1016/0304-3940(93)90500-k. [DOI] [PubMed] [Google Scholar]
- Zhou J, Pavel J, Macova M, Yu ZX, Imboden H, Ge L, Nishioku T, Dou J, Delgiacco E, Saavedra JM. AT1 receptor blockade regulates the local angiotensin II system in cerebral microvessels from spontaneously hypertensive rats. Stroke. 2006;37:1271–1276. doi: 10.1161/01.STR.0000217404.64352.d7. [DOI] [PubMed] [Google Scholar]