Abstract
Tissue engineering of skeletal muscle from cultured cells has been attempted using a variety of synthetic and natural macromolecular scaffolds. Our study describes the application of artificial scaffolds (collagen sponges, CS) consisting of collagen-I with parallel pores (width 20–50 μm) using the permanent myogenic cell line C2C12. CS were infiltrated with a high-density cell suspension, incubated in medium for proliferation of myoblasts prior to further culture in fusion medium to induce differentiation and formation of multinucleated myotubes. This resulted in a parallel arrangement of myotubes within the pore structures. CS with either proliferating cells or with myotubes were grafted into the beds of excised anterior tibial muscles of immunodeficient host mice. The recipient mice were transgenic for enhanced green fluorescent protein (eGFP) to determine a host contribution to the regenerated muscle tissue. Histological analysis 14–50 days after surgery showed that donor muscle fibres had formed in situ with host contributions in the outer portions of the regenerates. The function of the regenerates was assessed by direct electrical stimulation which resulted in the generation of mechanical force. Our study demonstrated that biodegradable CS with parallel pores support the formation of oriented muscle fibres and are compatible with force generation in regenerated muscle.
Keywords: tissue engineering, collagen scaffold, oriented pore structure, cell culture, myoblast, transplantation, muscle regeneration
Introduction
The grafting of reconstructed or ‘engineered’ functional mammalian tissue may be of great medical importance, because it might compensate for missing tissue in the recipient, for example in cases of congenital malformations, diseases leading to tissue destruction, losses due to accidents or tissue ablation as a consequence of surgery. In contrast to the transplantation of donor organs, tissue engineering starts with cultured proliferating cells and aims at reconstituting a tissue-like structure in culture. In case of solid tissues, a mere injection of cell suspensions can rarely be expected to yield a functional replacement. Rather, a macroscopic three-dimensional coherent cell assembly is required for transplantation and this should have an ordered internal structure similar to that of the authentic tissue to be replaced. To achieve this, a biocompatible matrix or scaffold is required that allows single cells to distribute and adhere, to proliferate, and, if necessary, to finally differentiate and mature to a functional state. Ideally, the transplanted scaffold should slowly be degraded and replaced by an endogenous extracellular matrix. In the case of engineered skeletal muscle, a longitudinal order of the resulting muscle fibres is necessary for the graft to generate directed force.
Several matrix materials have been examined for growth and differentiation of myogenic cells in mass culture (cf. [1]) and for the use in grafts. These included alginate hydrogels [2], fibrin gels [3], polyglycolic acid meshes [4], poly(L-lactic acid) fibres [5], sponge-like composites of poly(L-lactic acid) and polylactic-glycolic acid [6], polypropylene fibres coated with laminin [7], UV-embossed/micropatterned polyurethane diacrylate [8] and a degradable block polyesterurethane [9]. In agreement with the experience of many authors (e.g.[10, 11]) collagen-based matrices or collagen coated surfaces supported growth and differentiation of myogenic cells both in mass culture [1] and in grafts.
The aim of the present work was to evaluate, in a first pilot study, a highly ordered collagen-I-based scaffold and culture conditions for myogenic cells as tools to efficiently graft muscle precursor cells or immature muscle fibres (myotubes) into recipient mice and to achieve the formation of skeletal muscle-like tissue in situ. Scaffold cultures were analysed directly or after grafting into a muscle bed of enhanced green fluorescent proteins (eGFP) labelled nude host mice. The recipient eGFP label served to distinguish host from donor cells [12]. The permanent mouse cell line C2C12 was used as a model myogenic cell.
Furthermore, the present study aimed at demonstrating the importance of the eGFP labelling of the host for the assessment of the host and donor contributions of regenerates, respectively.
Material and methods
Production and properties of collagen sponges with oriented pores (CS)
The preparation of collagen scaffolds with parallel oriented pores is based on a patented ‘unidirectional freezing’ process of an aqueous dispersion containing 1.5 wt% of porcine collagen fibres (mainly type I) and low amounts of elastin followed by a freeze-drying process [13–15]. The collagen dispersion is cooled and frozen using a defined temperature gradient that is maintained during cooling. The temperature gradient is induced by two heat sinks placed above and below the sample. By establishing two different homogeneous temperatures at the respective heat sinks (e.g. 35°C and 5°C), a one-dimensional heat flow is created in the direction of the temperature gradient. In order to freeze the dispersion, both heat sink temperatures are lowered with the same cooling rate, leading to a directional freezing of the ice crystals in the direction of the temperature gradient. By adding an organic acid (e.g. acetic acid), the ice crystals are induced to grow in a dendritic ice crystal morphology without side-branches. These finger-shaped ice crystals sublime during freeze-drying, and oriented pores result. The majority of the collagen fibres in the dispersion are excluded from the ice crystals, and are concentrated between the ice fingers thereby forming the final structure of the collagen scaffold.
The resulting parallel-layered structure of the dry collagen scaffold is interconnected by collagen strands. The distance between the layers corresponds to the smallest pore size in the collagen scaffold. For optimal cell seeding the pores have to be continuously interconnected throughout the collagen sponge, which is the case for ‘unidirectional solidification’, and the smallest pore size has to be large enough to allow the cells of interest to migrate into the pore. Typically, a pore size distribution between 20 μm and 50 μm was achieved (Fig. 1).
1.
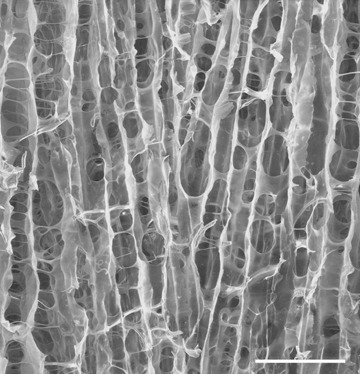
Scanning electron micrograph showing the structure of the collagen sponge used in this study. The orientation of the extended pores (vertical in the micrograph) defined the longitudinal axis of the internal structure of the sponge, in the transverse direction (horizontal in the micrograph) there are connecting bridges and holes. Scale bar 100 μm.
Cells and culture media
As a source of myogenic cells, the C2C12 subline (ATCC CRL 1772, [16]) of the permanent myogenic cell line C2 (derived from the C3H inbred mouse strain; [17]) was used.
The basal medium was Dulbecco's modified Eagle medium (DMEM) with high glucose content (4.5 g/l; PAA Laboratories, Pasching, Austria), supplemented with penicillin G (Biochrom AG Seromed, Berlin) and streptomycin sulphate (Sigma, Taufkirchen, Germany).
For expansion, the medium was supplemented with 10% foetal calf serum (FCS Gold, PAA) and termed proliferation medium (PM). To induce differentiation, that is fusion into myotubes, PM was replaced by fusion medium (FM) containing 2% FCS, 1%Insulin-Transferrin-Selenium-A (100x)“ containing 670 μg/l sodium selenite, 11 g/l sodium pyruvate, 1 g/l insulin and 550 mg/l transferrin (ITS-Supplement, Gibco Invitrogen, Carlsbad, USA).
For expansion, cells were cultured in monolayer in 175 cm2 Nunc EasYFlask™ tissue-culture flasks (Nunc, Roskilde, Denmark).
Three-dimensional Cell-Matrix-Cultures were cultivated in hydrophobic polystyrene Petri dishes of 40 mm diameter (Nunc).
The potential of the C2C12 cell line to yield spontaneously contractile myotubes upon change to differentiation medium was assessed by culturing in Matrigel™ (BD Biosciences, Bedford, USA), a reconstituted basement membrane matrix derived from murine tumour cells [18]. Sublines of C2C12 and the rat myogenic line L6 that did not develop into highly contractile myotubes on Matrigel™ tended to produce fast growing tumours when transplanted as collagen sponge (CS) cultures into the muscle bed; they were therefore excluded from experiments on muscle reconstitution.
Culture of myogenic cells in collagen sponges
Collagen-I sponges with pores of 20–50 μm diameter, oriented in parallel and running through their whole thickness were used as scaffolds. To colonize the CS with a maximum number of cells per volume, drops of high-density cell suspensions (4 × 107/ml) were directly applied to dry pieces of CS of approximately 6 mm × 3 mm × 2.5 mm size with a total of 1.5 × 105 or 1.2 × 106 cells seeded. These were subsequently incubated at 37°C in a humid chamber to improve adherence before they were covered with PM in plastic suspension culture dishes, which were not coated to prevent outward migration of cells. Proliferation was allowed to proceed for 1–7 days. Subsequently, the medium was changed to FM to induce the fusion of C2C12 cells, resulting in the differentiation to myotubes.
Host mouse strain and surgery
The mouse strain C57BL/6 Cr Slc TgN(act-EGFP)OsbC15–001-FJ001 transgenic for the enhanced green fluorescent protein of the jelly fish Aequoria victoria[19] had been crossed into an NMRI-nu mouse strain. This hybrid strain shows high concentrations of eGFP in all muscle types [20]. In order to prevent graft rejection, nude (nu/nu) and eGFP transgenic individuals were used as hosts for transplantation experiments [12, 20].
Reconstituted muscles in CS were grafted into the bed of the previously excised anterior tibial muscle (TA) using the transplantation procedure previously described for donor muscles [12, 21]. The skin of the lower leg was opened and deflected and the tibialis anterior (TA) and extensor digitorum longus (EDL) muscles of the host animal were removed under deep anaesthesia, using blunt scissors to reduce bleeding. Subsequently, the CS (approximately of the size and shape of the TA) was positioned in the muscle bed, and fixed at both ends by suturing it to the remnant tissue under the knee and the distal tendon with a 6.0 thread. 100 μl of an analgetic (Metacam, Boehringer-Ingelheim, Ingelheim/Rhein, Germany, 50 μg/ml in 0.9% NaCl solution) were injected peritoneally during recovery from anaesthesia and again one day after surgery. Animal experimentation was performed according to the German law for the protection of animals, with a permit by the local authorities.
Physiology and histology
To assess the ability of the regenerates to generate force, isometric contraction measurements on collagen sponge grafts using direct stimulation were done as previously described for muscle grafts [22]. The graft regenerated in the bed of the host TA was freed from surrounding tissue up to the knee under deep anaesthesia. The (regenerated) tendon was tied to a force transducer (300B-LR lever system, Aurora Scientific, Vata Court, ON, Canada). Twitch (3–5 Hz), as well as incomplete (50 or 70 Hz) and complete (100 Hz) tetani were induced by direct stimulation at 10 Volts via silver plate electrodes. Signals were amplified and visualized on a HAMEG 205-3 (HAMEG GmbH, D-60528 Frankfurt, Germany) oscilloscope. Data were recorded using a 80286 PC and evaluated with SigmaPlot (Jandel Scientific, San Rafael, CA, USA) software.
Histology was done on fresh frozen sections as described elsewhere [12]. Nuclei were stained with Hoechst dye 33258 (bisbenzimide, Sigma; blue fluorescence). eGFP was fixed using the formaldehyde vapour method [20, 23]. Sarcomeric myosin heavy chain (MyHC) was stained on adjacent sections with the monoclonal murine antibody MF20 (ATCC [24]), laminin with the polyclonal rabbit antibody L9393 (Sigma). As secondary antibodies a TRITC-coupled goat antimouse (T 5393, Sigma) and a goat anti-rabbit antibody coupled to Alexa Fluor 488 (Molecular Probes, MoBiTec, Göttingen, Germany) were used.
Analysis and macroscopic observation was done using a Wild stereomicroscope and a UV hand lamp, histological analysis with a Zeiss Axiophot UV epifluorescence microscope (Zeiss, Jena, Germany), documentation was carried out in both cases with a Coolpix 990 camera (Nikon), image processing was done using Photoshop CS2 (Adobe, USA).
Results
Myotube formation in collagen sponges in culture
Sections of CS cultures (Fig. 2) revealed that myotubes had formed in a parallel orientation according to the pore structure of the scaffold (Fig. 2A). Differentiation of these myotubes was indicated by their content in sarcomeric myosin, as shown by staining with the monoclonal antibody MF20 (Fig. 2B). Furthermore, myotubes in the CS had synthesized and deposited laminin, and thus started to form their own extracellular matrix (Fig. 2C). Maintenance of myotubes derived from C2C12 cells in CS was possible for at least 30 days.
2.
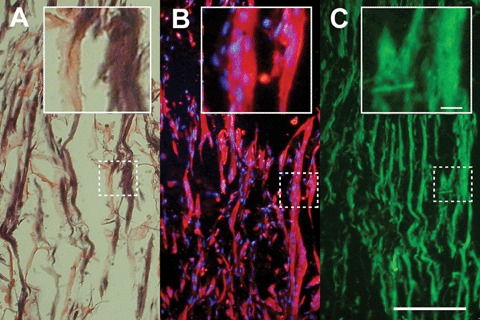
C2C12-derived myotubes grown in collagen sponge in culture. Serial longitudinal frozen sections of 8 μm thickness were analysed. A 6 mm × 3 mm × 2.5 mm collagen sponge was soaked with 1.2 × 106 C2C12 cells, and cultured for 2 days in proliferation medium (PM) and subsequently for 5 days in fusion medium (FM) to induce myotube differentiation. (A) Haematoxylin–eosin stain to show collagen matrix (pink) and myotubes (purple). (B) Fluorescence image combining anti-myosin heavy chain (MF20, monoclonal anti-MyHC, red) with Hoechst staining of nuclei (blue). (C) Fluorescence image of an anti-laminin stained section (green), showing the basal lamina of the myotubes. Scale bar in (C), 100 μm, 10 μm in inset.
Myotube formation and muscle regeneration in transplanted CS
CS colonized with C2C12 cells were transplanted into the bed of an excised TA of nude host mice, either in the non-fused, proliferating state or after induction to fusion in culture. The host mice were transgenic for eGFP. Due to technical limitations at the time of the experiments only a fraction of the sponges had long enough pores to be transplanted with the pores running longitudinally in the muscle bed. However, properties other than contractility could be studied in transversely oriented CS (cf. Table 1).
1.
Grafting experiments using C2C12 myogenic cells in collagen sponges and the bed of the anterior tibial (TA) muscle as a graft site
| Cells | + | + | 0 | 0 |
| CS | + | + | + | 0 |
| Orient. | Transv. | Longit. | Longit. | − |
| No. of Expt's | 15 | 3 | 2 | 1 |
| Myotubes/myofibres | ++ | ++ | 0 | 0 |
| Immig. | + | (+) | (+) | 0 |
| Muscle reg. | ++ | ++ | 0 | 0 |
| Tumours | 0 | 0 | 0 | 0 |
External appearance and function
Recipient animals resumed normal gait after about 10 days post-operatively (p.o.). They were sacrificed between 14 and 50 days after surgery. When the skin was deflected from the operated leg, the surface of the graft appeared reddish due to extensive vascularization; it was distinct from that of the surrounding host muscles by the absence of intense green fluorescence (Fig. 3A). The tendon of the TA to which the graft had been fixed, was shown to have regenerated (50 days p.o.). Force measurement upon direct electrical stimulation revealed twitch and tetanic tensions (Fig. 3B and C) in the range of 5–20% of an authentic adult TA and were comparable to those of regenerated transplanted TAs [22]. Time-to-peak and half-relaxation times corresponded to those of fast muscle of the mouse.
3.
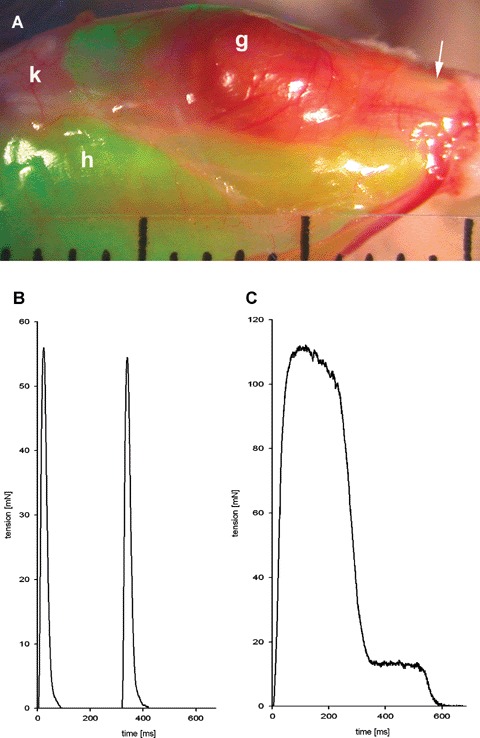
Anatomy and physiology of a regenerated graft of C2C12 cells cultured in collagen sponge (CS). The collagen sponge (8 mm × 3 mm × 2.5 mm) was seeded with 1.2 × 106 C2C12 cells, cultured for 1 day in PM and for 5 days in FM, and grafted into the anterior tibial (TA) bed of the enhanced green fluorescent protein (eGFP) transgenic host mouse with its pores longitudinally oriented. The graft was analysed 50 d p.o. (A) External aspect of the regenerate derived from the C2C12-CS graft; g, graft; h, host; k, knee; arrow, regenerated tendon. Divisions of scale 1 mm. (B, C) Force generation upon direct electrical stimulation in situ of regenerate shown in (A). (B) twitch, stimulation 10 V, 3 Hz. (C) tetanus, stimulation 10 V, 100Hz for 500 ms.
Internal structure of C2C12-CS grafts
In cross-sections of regenerates, numerous immature and mature muscle fibres were observed. Histology revealed residual CS up to 50 days after surgery, the cellular material in which was largely eGFP negative, that is, of donor origin, but partially invaded by regenerated host (eGFP positive) muscle fibres, regardless of whether proliferating or pre-fused C2C12 cells had been transplanted.
Host and donor contributions to the regenerated muscle at the graft site as well as possible co-fusion between host and donor cells were scored on the basis of eGFP content. (Fig. 4A–E; cf. [12]). There were large regions of myotubes and immature muscle fibres devoid of eGFP and thus of solely donor origin (Fig. 4A and B). Regenerated fibres, regardless of their origin, had a much wider distribution of diameters than original muscle fibres and the distributions of donor, mixed and host-derived muscle fibres showed a broad overlap (Fig. 5). Most likely the smaller mean diameter of donor fibres reflects a loss of the permanent C2C12 cell line to achieve the stage of a fully mature muscle fibre (c.f. [25]). Fig. 5 suggests that a high density of seeding the C2C12 cells and the possibility to proliferate after transplantation is more important for the calibre of resulting muscle fibres than fusion prior to transplantation.
4.
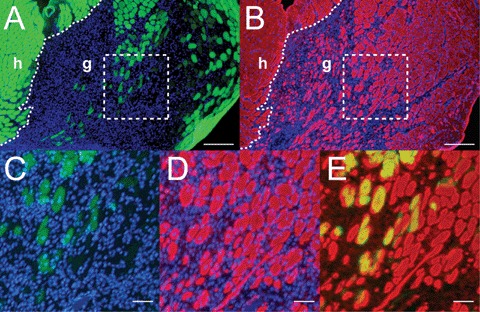
Donor and host contributions to regenerated muscle derived from a graft of C2C12-CS (8 mm × 3 mm × 2.5 mm) seeded with 1.5 × 105 cells, cultured for 2 days in PM and for 5 days in FM. Analysis 14 days after surgery. (A, B) Adjacent cross sections of the regenerated graft; (A) formaldehyde vapour fixed to show eGFP fluorescence (host label, green); (B) unfixed for immunostaining of myofibrillar myosin (red). Original host muscle (h) is situated to the left, regenerated graft (g) to the right. Regenerating host muscle fibres have invaded the middle und right outer portion of the graft, but myotubes and myofibres in large areas are eGFP negative and hence derived from donor- C2C12 cells only. (C, D) Enlargements of areas indicated by dotted outline in (A) and (B), respectively. (E) Overlay of (C) and (D). In (C), regenerated myofibres, largely of host origin are recognizable by high eGFP fluorescence and centrally located nuclei, indicating regeneration. They are surrounded by large numbers of eGFP-negative and hence donor-derived immature muscle fibres and myotubes (D) and (E). Scale bar, 200 μm in (A) and (B), 50 μm in (C, D) and (E).
5.
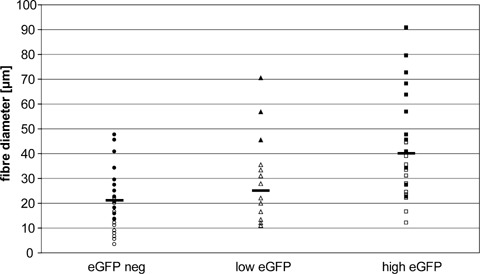
Diameters of muscle fibres and myotubes within regenerated C2C12-CS grafts, 14 days post-operatively (p.o.). Frozen sections stained with antibodies against sarcomeric myosin heavy chain (MF20) and laminin were used for measurements of fibre diameters. 255 fibres (from two experiments) were classified according to their eGFP fluorescence: eGFP negative (donor derived, circles), intermediate (triangles, derived from co-fusion of host and donor cells), high eGFP (squares, host derived). Experiment 1 (full symbols): seeding of CS with 1.2 × 106 cells, cultured for 7 days in PM; experiment 2 (empty symbols): CS seeded with 1.5 × 105 cells, cultured for 2 days in PM followed by 5 days in FM; same experiment as shown in Figure 4.
Controls
No tumour formation was observed when using differentiation competent C2C12 cells, either proliferating or pre-fused in culture (Table 1).
In the absence of the C2C12-CS graft no muscle regeneration was observed in the muscle bed after removal of the TA [26].
When a CS without cells was implanted, there was some immigration by host cells from the margins but no myotube formation.
Discussion
The role of the collagen matrix in our experiments is twofold:
First, it allows a large number of single cells to be placed into a defined position in a muscle bed. This would obviously be impossible using a cell suspension without a solid support to which the cells adhere.
Second, the internal geometry of the matrix serves as an ordered scaffold for the formation of a solid anisotropic tissue from previously randomly suspended cells.
Specifically, our results suggest that CS with parallel pores are promising scaffolds for muscle tissue engineering. This stands in contrast to previous studies attempting to engineer the ordered structure of skeletal muscle from cells suspended in gels. In one attempt, C2C12 cells were embedded in single solid cylinders (diameters <1mm) consisting of a solidifying collagen type I gel [11], in which the orientation of myotubes in parallel was achieved in a second step by mechanical stretching. In another study, myotubes resulting from in fibrin gel seeded myoblasts oriented themselves through self-contraction [3]. However, it is unclear if such an unstructured gel construct would also support subsequent alignment of host-derived myotubes resulting from immigrated host myoblasts upon grafting in situ. In contrast the application of CS with oriented internal pores, results in an artificial tissue section with hundreds or thousands of host, as well as graft derived myotubes automatically oriented in parallel in a global scale.
In the present study, C2C12 cells pre-cultured in CS (non-fused or fused) when grafted into a muscle bed were shown to develop into more mature stages than in culture and to contribute to regenerating muscle, capable of producing contractile force. In order to simulate a clinically relevant situation, we have not used γ irradiation of the recipient limb to eliminate a contribution by host myogenic cells to the regeneration of the excised muscle. In case of a muscle deficit due to a hereditary muscle disease it is desirable that the normal genetic constitution of implanted cells should, by co-fusion with host myoblasts, convey that condition to regenerating host tissue (e.g.[27]).
In contrast to most transplantation experiments, the present work uses a ‘negative labelling’ of the donor cells, that is absence of the eGFP label (cf. [12]). In conjunction with cell fusion during myogenesis and the high diffusibility of eGFP within a given muscle fibre [12] the eGFP labelling of host muscle provides an extremely sensitive measure for host contributions to the regenerated muscle fibres at the graft site. Fibres of mixed donor-host origin can be recognized by their intermediate levels of eGFP. Ideally there should be complementary markers for donor and recipient, for example nLacZ and eGFP transgenes [12]. Due to the phenomenon of co-fusion in muscle grafts, a host label, especially with the highly diffusible eGFP allows for a more stringent evaluation of the donor contribution than a donor label (cf. [12, 20]).
Outlook
In future experiments, expanded satellite cells (myogenic stem cells) rather than a permanent cell line should be used in order to achieve a higher degree of maturation in the CS. At the same time, CS with wider and longer pores, which are in the process of being developed, should be used; in principle it should be possible to achieve pore sizes up to 100 μm by applying appropriate freezing parameters in the directional freezing process [28]. A further refinement would be the use of ordered CS, as described in this paper, in conjunction with either a supplementation with growth factors [29, 30], or non-myogenic cells (e.g. fibroblasts) [6] or both to improve the integration at the host site and to promote vascularization. Furthermore, appropriate host nerves should be surgically directed into the graft in order to achieve motor and sensory innervation.
Conclusions
In this pilot study we have shown that the use of a novel collagen sponge with oriented pores offers a most promising method for the engineering of at least small muscles, provided that it is seeded at very high cell density. In future experiments, expanded satellite cells (myogenic stem cells) rather than a permanent cell line should be used in order to achieve a higher degree of maturation in CS with wider and longer pores.
Acknowledgments
The present work was partially financed by the Stem Cell Network North Rhine Westphalia, Ministry of Science and Research NRW, and by the Fonds der Chemischen Industrie. We thank Drs. Petra Budde, BioVision GmbH, Hannover, Ute Wiesemann, RWTH Aachen and Professors Anna Starzinski-Powitz, Human Genetics, University of Frankfurt, Jürgen Lehmann, Bielefeld University and Willi Jahnen-Dechent, RWTH Aachen for support of this work, and Dr. Garrit Koller, Dental Institute, King's College London, for helpful comments.
References
- 1.Bardouille C, Lehmann J, Heimann P, Jockusch H. Growth and differentiation of permanent and secondary mouse myogenic cell lines on microcarriers. Appl Microbiol Biotechnol. 2001;55:556–62. doi: 10.1007/s002530100595. [DOI] [PubMed] [Google Scholar]
- 2.Rowley JA, Madlambayan G, Mooney DJ. Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials. 1999;20:45–53. doi: 10.1016/s0142-9612(98)00107-0. [DOI] [PubMed] [Google Scholar]
- 3.Huang YC, Dennis RG, Larkin L, Baar K. Rapid formation of functional muscle in vitro using fibrin gels. J Appl Physiol. 2005;98:706–13. doi: 10.1152/japplphysiol.00273.2004. [DOI] [PubMed] [Google Scholar]
- 4.Saxena AK, Marler J, Benvenuto M, Willital GH, Vacanti JP. Skeletal muscle tissue engineering using isolated myoblasts on synthetic biodegradable polymers: preliminary studies. Tissue Eng. 1999;5:525–32. doi: 10.1089/ten.1999.5.525. [DOI] [PubMed] [Google Scholar]
- 5.Cronin EM, Thurmond FA, Bassel-Duby R, Williams RS, Wright WE, Nelson KD, Garner HR. Protein-coated poly(L-lactic acid) fibers provide a substrate for differentiation of human skeletal muscle cells. J Biomed Mater Res A. 2004;69:373–81. doi: 10.1002/jbm.a.30009. [DOI] [PubMed] [Google Scholar]
- 6.Levenberg S, Rouwkema J, Macdonald M, Garfein ES, Kohane DS, Darland DC, Marini R, Van Blitterswijk CA, Mulligan RC, D’Amore PA, Langer R. Engineering vascularized skeletal muscle tissue. Nat Biotechnol. 2005;23:879–84. doi: 10.1038/nbt1109. [DOI] [PubMed] [Google Scholar]
- 7.Neumann T, Hauschka SD, Sanders JE. Tissue engineering of skeletal muscle using polymer fiber arrays. Tissue Eng. 2003;9:995–1003. doi: 10.1089/107632703322495637. [DOI] [PubMed] [Google Scholar]
- 8.Shen JY, Chan-Park MB, Feng ZQ, Chan V, Feng ZW. UV-embossed microchannel in biocompatible polymeric film: application to control of cell shape and orientation of muscle cells. J Biomed Mater Res B Appl Biomater. 2006;77:423–30. doi: 10.1002/jbm.b.30449. [DOI] [PubMed] [Google Scholar]
- 9.Riboldi SA, Sampaolesi M, Neuenschwander P, Cossu G, Mantero S. Electrospun degradable polyesterurethane membranes: potential scaffolds for skeletal muscle tissue engineering. Biomaterials. 2005;26:4606–15. doi: 10.1016/j.biomaterials.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 10.Vandenburgh HH, Karlisch P, Farr L. Maintenance of highly contractile tissue-cultured avian skeletal myotubes in collagen gel. In Vitro Cell Dev Biol. 1988;24:166–74. doi: 10.1007/BF02623542. [DOI] [PubMed] [Google Scholar]
- 11.Okano T, Matsuda T. Tissue engineered skeletal muscle: preparation of highly dense, highly oriented hybrid muscular tissues. Cell Transplant. 1998;7:71–82. doi: 10.1177/096368979800700110. [DOI] [PubMed] [Google Scholar]
- 12.Jockusch H, Voigt S. Migration of adult myogenic precursor cells as revealed by GFP/nLacZ labelling of mouse transplantation chimeras. J Cell Sci. 2003;116:1611–6. doi: 10.1242/jcs.00364. [DOI] [PubMed] [Google Scholar]
- 13.Bozkurt A, Brook GA, Moellers S, Lassner F, Sellhaus B, Weis J, Woeltje M, Tank J, Beckmann C, Fuchs P, Damink LO, Schügner F, Heschel I, Pallua N. In vitro assessment of axonal growth using dorsal root ganglia explants in a novel three-dimensional collagen matrix. Tissue Eng. 2007;13:2971–9. doi: 10.1089/ten.2007.0116. [DOI] [PubMed] [Google Scholar]
- 14.Möllers S, Heschel I, Olde Damink LHH, Schügner F, Deumens R, Müller B, Bozkurt A, Noth J, Brook GA. Preparation, characterisation and in vitro cytocompati-bility of a novel, longitudinally microstructured collagen scaffold intended for nerve tissue repair unpublished. [DOI] [PubMed]
- 15.Schoof H, Apel J, Heschel I, Rau G. Control of pore structure and size in freezedried collagen sponges. J Biomed Mater Res. 2001;58:352–7. doi: 10.1002/jbm.1028. [DOI] [PubMed] [Google Scholar]
- 16.Blau HM, Pavlath GK, Hardeman EC, Chiu CP, Silberstein L, Webster SG, Miller SC, Webster C. Plasticity of the differentiated state. Science. 1985;230:758–66. doi: 10.1126/science.2414846. [DOI] [PubMed] [Google Scholar]
- 17.Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270:725–7. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- 18.Kleinman HK, McGarvey ML, Hassell JR, Star VL, Cannon FB, Laurie GW, Martin GR. Basement membrane complexes with biological activity. Biochemistry. 1986;25:312–8. doi: 10.1021/bi00350a005. [DOI] [PubMed] [Google Scholar]
- 19.Okabe M, Ikawa M, Kominami K, Nakanishi T, Nishimune Y. ‘Green mice’ as a source of ubiquitous green cells. FEBS Lett. 1997;407:313–9. doi: 10.1016/s0014-5793(97)00313-x. [DOI] [PubMed] [Google Scholar]
- 20.Jockusch H, Voigt S, Eberhard D. Localization of GFP in frozen sections from unfixed mouse tissues: immobilization of a highly soluble marker protein by formaldehyde vapor. J Histochem Cytochem. 2003;51:401–4. doi: 10.1177/002215540305100315. [DOI] [PubMed] [Google Scholar]
- 21.Jockusch H, Fuchtbauer EM, Fuchtbauer A, Leger JJ, Leger J, Maldonado CA, Forssmann WG. Long-term expression of isomyosins and myoendocrine functions in ectopic grafts of atrial tissue. Proc Natl Acad Sci USA. 1986;83:7325–9. doi: 10.1073/pnas.83.19.7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Füchtbauer EM, Reininghaus J, Jockusch H. Developmental control of the excitability of muscle: transplantation experiments on a myotonic mouse mutant. Proc Natl Acad Sci U S A. 1988;85:3880–4. doi: 10.1073/pnas.85.11.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jockusch H, Eberhard D. Green Fluorescent Protein as a Tracer in Chimeric Tissues. The Power of Vapor Fixation. In: Anson D, editor. Methods in Molecular Biology, Vol 411: Reporter Genes: A Practical Guide. Totowa, NJ: Humana Press Inc; 2008. pp. 145–54. [DOI] [PubMed] [Google Scholar]
- 24.Bader D, Masaki T, Fischman DA. Immunochemical analysis of myosin heavy chain during avian myogenesis in vivo and in vitro. J Cell Biol. 1982;95:763–70. doi: 10.1083/jcb.95.3.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dennis RG, Kosnik PE, 2nd, Gilbert ME, Faulkner JA. Excitability and contractility of skeletal muscle engineered from primary cultures and cell lines. Am J Physiol Cell Physiol. 2001;280:C288–95. doi: 10.1152/ajpcell.2001.280.2.C288. [DOI] [PubMed] [Google Scholar]
- 26.Jockusch H, Mehrke G, Füchtbauer EM. Beating heart muscle in a skeletal muscle bed. Exp Neurol. 1983;81:749–55. doi: 10.1016/0014-4886(83)90341-2. [DOI] [PubMed] [Google Scholar]
- 27.Partridge TA, Morgan JE, Coulton GR, Hoffman EP, Kunkel LM. Conversion of mdx myofibres from dystrophin-negative to -positive by injection of normal myoblasts. Nature. 1989;337:176–9. doi: 10.1038/337176a0. [DOI] [PubMed] [Google Scholar]
- 28.Kuberka M, Von Heimburg D, Schoof H, Heschel I, Rau G. Magnification of the pore size in biodegradable collagen sponges. Int J Artif Organs. 2002;25:67–73. doi: 10.1177/039139880202500111. [DOI] [PubMed] [Google Scholar]
- 29.Barbero A, Benelli R, Minghelli S, Tosetti F, Dorcaratto A, Ponzetto C, Wernig A, Cullen MJ, Albini A, Noonan DM. Growth factor supplemented matrigel improves ectopic skeletal muscle formation–a cell therapy approach. J Cell Physiol. 2001;186:183–92. doi: 10.1002/1097-4652(200102)186:2<183::AID-JCP1020>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 30.Hill E, Boontheekul T, Mooney DJ. Regulating activation of transplanted cells controls tissue regeneration. Proc Natl Acad Sci USA. 2006;103:2494–9. doi: 10.1073/pnas.0506004103. [DOI] [PMC free article] [PubMed] [Google Scholar]


