Abstract
A new GADD45α isoform, GADD45α1, was identified in the cellular response to arsenic. DNA sequencing and biochemical analyses suggested that GADD45α1 is derived from an alternative splicing of the GADD45α mRNA by skipping the region corresponding to exon2 of the gadd45α gene during mRNA maturation. In addition to the size difference due to the lack of 34 amino acids encoded by exon2, GADD45α1 and GADD45α proteins differ in their effects on cell proliferation and cell cycle transition. Unlike GADD45α, the GADD45α1 is unable to attenuate cell growth. In over-expression experiments, the full length GADD45α, but not the GADD45α1, sensitized cells to arsenic-induced prometaphase arrest of the cell cycle. Furthermore, GADD45α1 appears to be able to antagonize the function of the GADD45α on the G2/M phase cell cycle arrest as demonstrated in co-transfection experiment. Thus, these data suggest that the generation of the GADD45α1 isoform may not only offset but also antagonize the effects of arsenic and GADD45α on cell growth and cell cycle regulation.
Keywords: GADD45α, GADD45α1, arsenic, alternative splicing, prometaphase
The growth arrest- and DNA damage-induced gene 45α (GADD45α) is highly inducible in response to chemical and physical stresses including genotoxic agents, UV irradiation, oxidative injury, osmotic stress, or nutrient deprivation [1]. The GADD45α protein plays pivotal roles in cell apoptosis, cell cycle transition, checkpoint responses, and DNA repair, which is largely achieved through direct interaction with PCNA, cyclin B/CDC2, p21cip1, Aurora-A kinase, histone proteins, etc. [2]. A still debatable issue is whether GADD45α is truly involved in either DNA repair or DNA demethylation. The gene promoter of the gadd45α has been shown previously to be heavily methylated in some tumor cells [3]. By using artificial reporter vectors that were transfected into HEK293 cells, Barreto et al. [4] showed that GADD45α overexpression activated a methylation-silenced reporter vector by promoting DNA demethylation. However, another research team using the same and/or similar experimental procedures failed to reproduce the results [5]. In fact, overexpression of the GADD45α appeared to enhance, rather than decrease the DNA methylation.
Transcription of the gadd45α gene can be either p53-dependent or p53-independent [2]. Many DNA damaging agents foster GADD45α expression through the binding of p53 to the consensus site located in the third intron region of the gadd45α gene. This p53-dependent regulation of GADD45α expression is antagonized by BRCA1 that interacts with a co-repressor protein named ZNF350 if DNA damage is not occurred [6, 7]. In the presence of DNA damage, however, the BRCA1 and ZNF350 complex may act as an activator for the gadd45α gene by cooperation with p53 and the recruitment of other transcription factors [7]. There are several factors contributing to p53-independent regulation of the gadd45α gene, among which NF-κB and JNK activation may play opposite roles in this regard [8]. Disruption of the activation pathway for NF-κB by expression of a kinase-mutated IKKβ induced an increased and prolonged expression of GADD45α in the cells treated with arsenic [8]. In contrast, activation of NF-κB attenuated GADD45α expression, possibly through the destabilization of the GADD45α mRNA or the induction of c-myc, a transcriptional repressor of the GADD45α [9, 10]. An enhanced JNK activation was frequently observed in the cells with deficiency in NF-κB signaling, whereas suppression of JNK reduced GADD45α expression [8], indicating that JNK signaling may be important for stress-induced GADD45α expression. In addition to its potential role on the transcription of the GADD45α gene, JNK may also be involved in the stabilization of the GADD45α mRNA. An earlier study implicated a requirement of JNK activation in nucleolin-mediated stabilization of the IL-2 mRNA [11]. In our recent studies, we demonstrated that nucleolin is indeed able to bind to and stabilize the GADD45α mRNA in the cells treated with arsenic [12]. Also, we demonstrated a translational regulation of the GADD45α protein in growth-arrested cells treated with arsenic, taxol or UV irradiation [13]. It appears that an internal ribosome entry site (IRES) in the 5′-UTR of the GADD45α mRNA is responsible for the cap-independent translation of the GADD45α.
We previously showed the presence of several alternatively spliced GADD45α mRNAs in a RT-PCR analysis using total RNA from the cells treated with arsenic [12]. Such alternative splicing of the GADD45α mRNA may provide an additional mechanism for the functional regulation of the GADD45α protein. This alternative splicing not only expands the diversity of the GADD45α protein by generating distinct GADD45α isoforms but also complicates the function of the GADD45α by producing functionally altered or inert isoforms. In the present report, we characterized one of these alternatively spliced GADD45α mRNAs, GADD45α1, by determining its molecular properties and roles played in cell growth and cell cycle transition. We demonstrated that in contrast to its normal counterpart GADD45α, GADD45α1 isoform with a deletion of the sequence encoded by exon2 is unable to impede cell proliferation and induce cell cycle arrest at the prometaphase.
Materials and Methods
Cell treatment and Western blotting
The BEAS-2B and HEK 293 cells were purchased from American Type Culture Collection (ATCC, Manassas, VA). The mouse embryo fibroblasts (MEFs) were gift of Dr. Karin at University of California at San Diego. The cells were maintained in DMEM supplemented with 5% or 10% fetal calf serum at 37°C, 5% CO2 in a humidified incubator. The cells were treated with the indicated concentrations of arsenic(III) chloride (As3+) (Sigma-Aldrich, St Louis, MO). The protein lysates from the cells cultured in the absence or presence of As3+ were analyzed by 14% SDS–PAGE and immunoblotted with the indicated antibodies. The antibodies against GADD45α and actin were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). The antibodies against myc-tag and phospho-histone H3 were purchased from Cell Signaling (Beverly, MA).
Reverse transcription-PCR (RT-PCR)
Total RNA from BEAS-2B cells was prepared using TRIzol Reagent (Invitrogen, CA). The GADD45α mRNAs in BEAS-2B cells treated by As3+ were determined by RT-PCR using the AccessQuick RT-PCR system (Promega, WI) with a temperature scale of 45°C for 50 min for reverse transcription, and 35 cycles of 94°C for 1 min, 56°C for 1 min, and 72°C for 1 min. The RT-PCR was carried out using either primer set 1 (5′-GGAGAGCAGAAGACCGAAA-3′ and 5′-TCACTGGAACCCATTGATC-3′) or primer set 2 (5′-AATATGACTTTGGAGGAATTC-3′ and 5′-TCACCGTTCAGGGAGATTAATC-3′). The PCR fragments were analyzed by 1.4% agarose and further separated by 5% polyacrylamide gel electrophoresis (PAGE). The slices of PAGE gel containing the amplified cDNA fragments were crushed by microcentrifuge and incubated in TE buffer at 4°C for overnight with constant shaking. The DNA was then retrieved by ethanol precipitation and cloned into the pCR4-TOPO vectors for DNA sequencing.
Construction of the expression vectors
The wild-type GADD45α (wt GADD45α) and two alternatively spliced GADD45α mRNA isoforms were amplified from the human GADD45α cDNA (SC118947, OriGene, MD) by PCR or Overlap Extension PCR with the indicated primer sets: wt GADD45α, forward: 5′-GTCAGAAGCTTCAATATGACTTTGGAGGAATTC-3′; reverse: 5′-GTCAGCTCGAGCCGTTCAGGGAGATTAATCAC-3′; ΔE2 GADD45α1, forward: 5′-GTCAGAAGCTTCAATATGACTTTGGAGGAATTC-3′; reverse: 5′-GTCAGCTCGAGCCGTTCAGGGAGATTAATCAC-3′; ΔE2 GADD45α1 Overlapping Extension PCR, forward: 5′-GCAGAAGACCGAAAGCGACCCCGATAACGTG-3′; reverse: 5′-CACGTTATCGGGGTCGCTTTCGGTCTTCTGC-3′; ΔE3 GADD45α, forward: 5′-GTCAGAAGCTTCAATATGACTTTGGAGGAATTC-3′; reverse: 5′-GTCAGCTCGAGAGGCAGGATCCTTCCATTGAG-3′; ΔE3 GADD45α Overlapping Extension PCR, forward: 5′-CCAAGCTGCTCAACGTAATCCACATTCATCTCAATG-3′; reverse: 5′-CATTGAGATGAATGTGGATTACGTTGAGCAGCTTGG-3′. (The underlined sequences indicate the HindIII and XhoI sites that were artificially added to the primers for cloning purpose). The amplified products were then individually cloned into the HindIII/XhoI sites of the mammalian expression vector pcDNA3.1/myc-HisA (Invitrogen).
Cell transfections, cell proliferation and flow cytometry
BEAS-2B cells were seeded into 6-well tissue culture plates at a concentration of 5 × 105 cells/well and cultured for 24 h. The transfections were performed using lipofectamine 2000 as suggested by the manufacturer (Invitrogen, Carlsbad, CA). The stable transfection of the cells with the GADD45α constructs was performed by culturing the transfected cells in G418 at a concentration of 600 μg/ml for 4 to 6 weeks. For cell proliferation assay, the cells were seeded onto 96-well tissue culture plates at a concentration of 1 × 104/ml in a volume of 100μl. The rate of cell growth was determined at day 1, day 2, day 3, and day 4 of culture by CellTiter 96 AQueous One Solution Cell Proliferation Assay reagent (Promega). Flow cytometry was performed by harvesting cells from 6-well tissue culture plates and labeling the cells with propidium iodide as described previously [8].
Immunofluorescence staining
The cells were seeded on chamber slides and maintained at 37 °C for 24 h. The cells were then either untreated or treated with As3+ for an additional 12 h. Immunofluorescence staining was performed as described previously [12]. Briefly, the slides were fixed in 10% buffer formaldehyde solution for 10 minutes at room temperature, permeabilized with 0.1% Triton X-100 and incubated with the primary antibodies overnight at 4°C. After extensive washing with PBS, the slides were further incubated with goat anti-mouse IgG conjugated with FITC, Alexa Fluor 488 or Alexa Fluor 546, or goat anti-rabbit IgG conjugated with Alexa Fluor 488 (Invitrogen) at room temperature for 1 hour. The slides were then mounted with ProLong® Gold antifade reagent with DAPI (Invitrogen). The Immunofluorescence images were captured using a Zeiss Axiovert100 microscope connected with a Pixera Pro150ES digital camera.
Results
New GADD45α isoform(s) in the cells treated with As3+
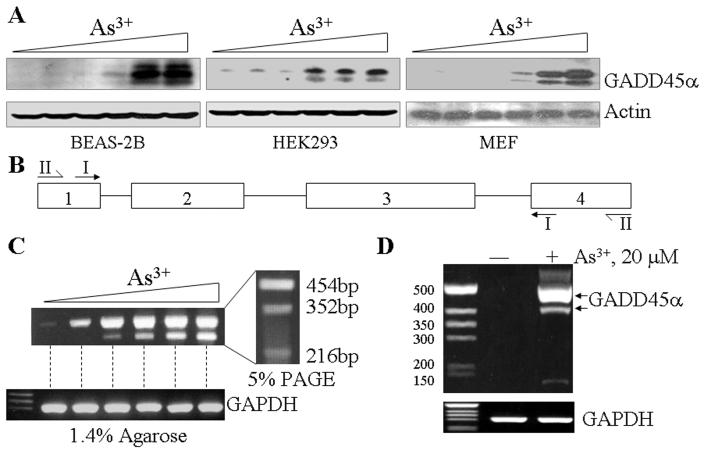
In our previous studies on the expression of the GADD45α, we had consistently observed one or two additional bands of the GADD45α protein in Western-blotting experiments using cell lysates from the bronchial epithelial cells (BEAS-2B) treated with As3+ [8, 9, 12, 13]. Furthermore, two additional cDNA bands could be detected in RT-PCR analyses using primers corresponding to the exon1 and exon4 regions of the GADD45α gene, respectively, suggesting possible alternative splicing of the GADD45α mRNA (GenBank ID DQ008445) [12]. To determine whether these phenomena are cell type specific, we evaluated the expression pattern of the GADD45α proteins in other types of the cells, such as HEK293 cells, mouse embryonic fibroblasts (MEF) and HeLa cells. Again, in the BEAS-2B cells, a dose-dependent induction of three GADD45α bands by As3+ was noted (Fig.1A), which is in agreement with our previous reports. In both HEK293 cells and MEFs, at least two bands of the GADD45α protein were detected (Fig. 1A). Accordingly, these data suggest to us there are indeed new isoform(s) of the GADD45α proteins in the cells treated with As3+. To further validate our previous and above observations, a new RT-PCR was conducted using the RNA from the cells treated with a various concentrations of As3+ and primer set 1 (depicted in Fig. 1B) to amplify the GADD45α mRNA from exon1 to exon4 region. Two cDNA bands were visualized when the PCR products were separated in a 1.4% agarose gel (Fig. 1C). To make a better resolution, the PCR products were re-loaded to a 5% PAGE gel. At least 3 cDNA bands could be seen in the PAGE gel (Fig. 1C, right panel). To rule out possible non-specific results of the above RT-PCR data, we used an additional primer set (set II) that encompass the entire GADD45a mRNA region from the beginning to the end as depicted in Fig. 1B for an additional RT-PCR analysis. Again, the alternatively spliced transcript is readily detectable in the As3+-treated cells (Fig. 1D).
Fig. 1.
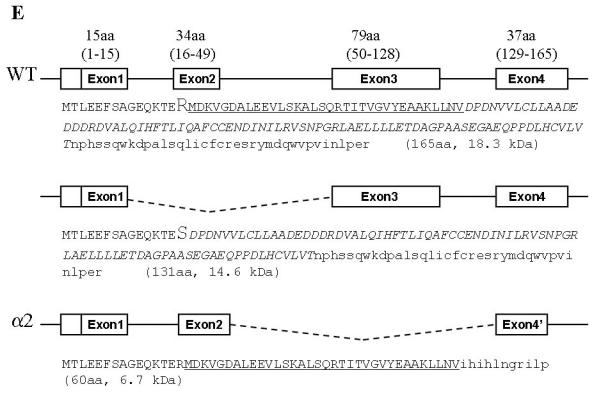
GADD45α isoforms were identified in the cells treated with As3+. A. Western-blottings were performed using cell lysates from the BEAS2-2B, HEK293 and MEF cells treated with 0, 2.5, 5, 10, 20, or 40 μM arsenic (As3+) for 12 hours. The expression of the GADD45α proteins was measured using anti-GADD45α antibody. An equal protein loading was verified by the use of anti-actin antibody in Western-blotting. B. Schematic illustration of the PCR primer sets corresponding to the indicated exon regions. C. RT-PCR analysis of the GADD45α mRNA using total RNA extracted from the BEAS-2B and PCR primer set I as indicated in B. The cells were treated as in A. Right panel shows further separation of the amplified GADD45α mRNAs in 5% PAGE. D. RTPCR analysis using primer set II and total RNA from control or As3+-treated cells. E. Schematic illustration of the full-length GADD45α and the alternatively spliced GADD45α isoforms generated from skipping exon2 and exon3, respectively, during pre-mRNA splicing. The deduced amino acid (aa) sequences for each mRNA were indicated along with the putative molecular weight (kD).
To determine the nature of these RT-PCR products, the amplified cDNA bands were excised from the PAGE gel and cloned for DNA sequencing. The data suggest that the top three bands are the products of the full-length GADD45α, GADD45α without exon2 and the GADD45α in which the entire exon3 region was skipped (Fig. 1E). Thus, the protein products from these three GADD45α mRNAs would have a deduced molecular weight of 18.3, 14.6 (ΔE2 isoform, GADD45α1) and 6.7 kDa (ΔE3), respectively. Alternative splicing of the GADD45α mRNA by skipping exon2 generates a GADD45α protein without 34 amino acids (aa) encoded by exon2 (GADD45α1). The arginine residue at position 15 (15R) in GADD45α1 is converted to serine (15S) due to the code change from AGG to AGC resulted from exon1-exon3 splicing. Alternative splicing of the GADD45α mRNA by skipping exon3 not only generated a protein without the region encoded by exon3 (79aa) but also caused the shift of the open-reading-frame (ORF) of exon4 that encodes 11aa with a new stop code (Fig. 1E).
GADD45α1 is functionally inert in cell growth and cell cycle
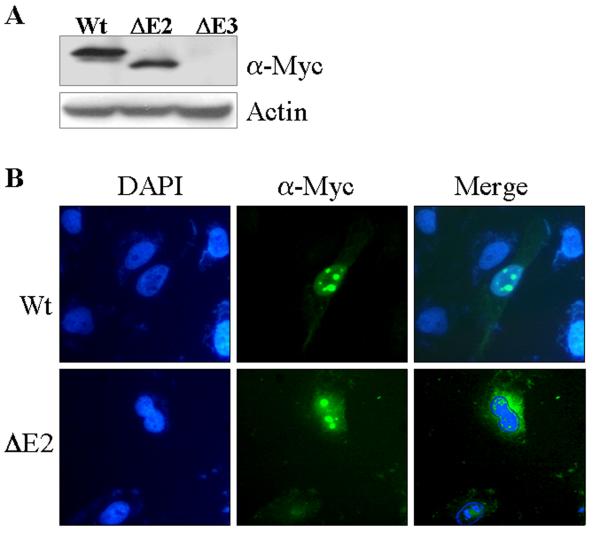
To determine whether or not the protein products of the GADD45α isoforms derived from alternative splicing are functional, expression vectors for full-length GADD45α (wt), GADD45α1 (ΔE2) and GADD45α2 (ΔE3) were constructed using pcDNA3.1/myc-HisA vectors. The expression and intracellular distribution of these c-terminal myc-tagged GADD45α isoforms were examined following transient transfection of these vectors into the BEAS-2B cells. The expression of GADD45α and GADD45α1 was detected by Western-blotting using an antibody against the myc tag (Fig. 2A). We failed to detect the expression of the GADD45α2 (ΔE3) in several experiments using either transient or stable transfection techniques, possibly due to the fact that GADD45α2 mRNA is not satisfactory for protein translation. The intracellular distribution of the GADD45α and GADD45α1 was investigated by immunofluorescence staining using anti-myc tag antibody. GADD45α is exclusively nuclear localized with an enrichment in nucleoli (Fig. 2B). Although a small fraction of the cells transfected with GADD45α1 exhibited nuclear localization, the majority of the cells expressing GADD45α1 showed both nuclear as well as cytoplasmic localization of this isoform (Figs 2B & 2C). It is very likely, thus, that deletion of the exon2-encoded region by alternative splicing weakens the ability of the GADD45α protein for nuclear distribution.
Fig. 2.
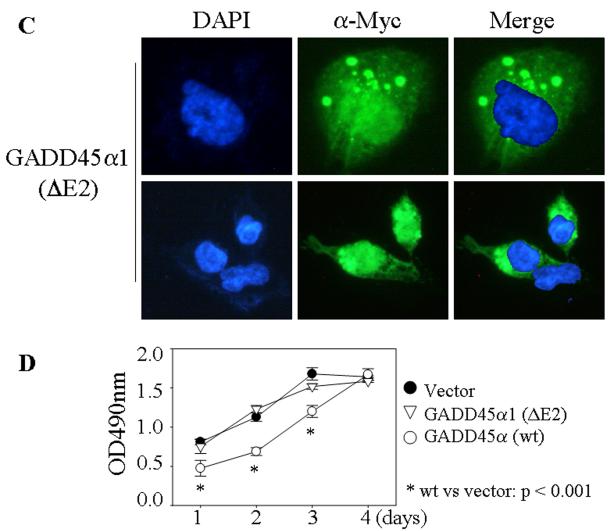
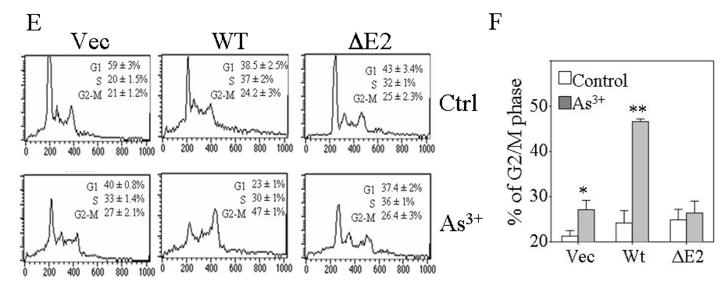
GADD45α1 differs from GADD45α in cell growth and cell cycle. A. Transient transfection of the wild type (wt), ΔE2 or ΔE3 GADD45α in BEAS-2B cells. The Myc-tagged GADD45α proteins were detected by Western-blotting using anti-myc tag antibody (α-Myc). B. Intracellular distribution of the GADD45α isoforms was determined by immunofluorescence staining as detailed in the Materials and Methods. C. GADD45α1 is located in both cytoplasm and nucleus. D. Cell proliferation assay for the cells transfected with a control vector, GADD45α1 or GADD45α. Data are means ± SD (n=3). E. Flow cytometry analyses of the cells expressing the indicated vectors and cultured in the absence or presence of 20 μM As3+ for 12 h. F. Average percentages of the cells in G2/M phase of these cells transfected with the indicated vectors in the absence or presence of As3+. Data are means ± SD (n=3). * p = 0.016; ** p < 0.001.
The involvement of GADD45α and GADD45α1 in cell growth and cell cycle transition was determined in the cells stably transfected with these myc-tagged constructs. Equal number of the cells transfected with the indicated vectors was seeded and the cell growth rate was measured at 1, 2, 3, and 4 day intervals by a MTS-based colorimetric cell proliferation assay. The cells expressing control vector and the ΔE2 GADD45α (GADD45α1) showed similar growth kinetics at the tested time ranges (Fig. 2D). Except at day 4, an appreciable decrease in cell growth at day 1, day 2 and day 3 was noted in the cells expressing wt GADD45α relative to the cells expressing control vector or the GADD45α1 (Fig. 2D). As depicted in Fig. 2E, all of these transfected cells exhibited a similar cell cycle profile under the basal condition (Fig. 2E). However, the cells expressing wt GADD45α, but not the ΔE2 GADD45α (GADD45α1), showed a remarkable increase of the cells in G2/M phase following 20 μM As3+ treatment (Figs. 2E and 2F). These data clearly suggest that the GADD45α1 isoform is unable to modulate cell growth and the cell cycle transition at the G2/M phase.
GADD45α, but not GADD45α1, sensitizes As3+-induced histone H3 phosphoylation
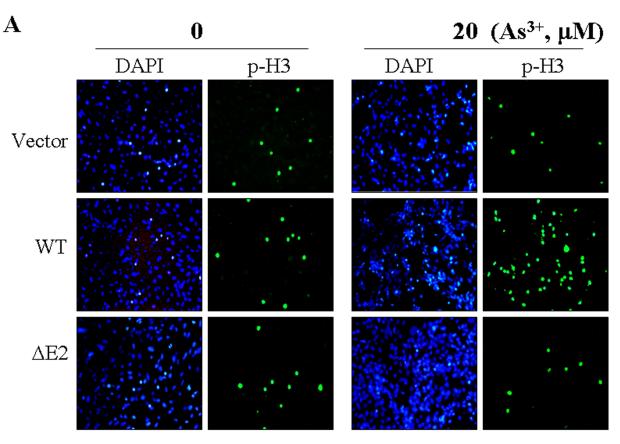
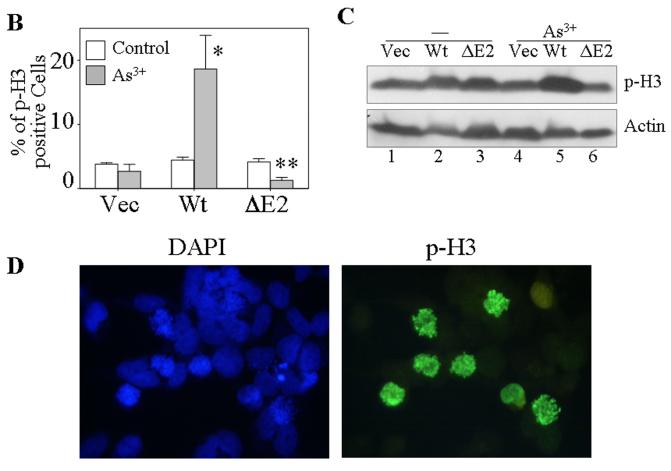
One of the most notable markers for the mitotic entry of the cells is the phosphoylation of histone H3 (phospho-H3, p-H3), which plays an important role in the initiation of chromosome condensation and cell mitosis. To define the role of the new GADD45α isoform, GADD45α1, on a specific mitotic phase rather than generally on G2/M phase of the cell cycle, the phosphoylation status of histone H3 was determined by immunofluorescence staining. Under basal conditions, the cells stably transfected with a control vector, the wt GADD45α or the GADD45α1 (ΔE2) showed a similar level of the p-H3 positive cells (Fig. 3A). Strikingly, wt GADD45α-transfected cells exhibited a very high level of p-H3 relative to the vector-transfected and GADD45α1-tranfected cells in the presence of 20 μM As3+ for 12 hours. Quantification of the p-H3 positive cells indicates that about 3-5% of the cells transfected with these indicated vectors are in the mitotic phase under the control condition (Fig. 3B). In the presence of As3+, the percentages of the p-H3 positive cells are 4, 19 and 2% for the cells expressing the control vector, wt GADD45α and GADD45α1, respectively (Fig. 3B). It is intriguing to note that As3+ treatment reduced, rather than increased the level of p-H3 for the cells expressing GADD45α1 (ΔE2, Fig. 3B), which suggests a potential antagonistic role of this isoform for the endogenous wt GADD45α-induced mitotic arrest. In addition to immunofluorescence staining, the levels of p-H3 in these transfected cells in the absence or presence of As3+ were also examined by immunoblotting. As depicted in Fig. 3C, the level of p-H3 was roughly equal among the cells transfected with a control vector, GADD45α (wt) and GADD45α1 (ΔE2) without As3+ treatment. As3+ treatment elevated the level of p-H3 in the GADD45α (wt) cells, but not in the vector- or GADD45α1 (ΔE2)-transfected cells. In agreement with the data from immunofluorescence staining, As3+ treatment in fact reduced the level of p-H3 in the GADD45α1-transfected cells (compare lane 6 with lane 3 in Fig. 3C). Morphological analysis of the DAPI-stained nuclei revealed that the majority of the p-H3 positive GADD45α expressing cells are in prometaphase in response to As3+ (Fig. 3D). Accordingly, these data suggest that GADD45α, but not the GADD45α1, enhances mitotic arrest of the cells at prometaphase.
Fig. 3.
GADD45α1 is unable to sensitize As3+ induced mitotic arrest. A. The BEAS-2B cells stably expressing a control vector, GADD45α (WT) or GADD45α1 (ΔE2) were cultured in the absence or presence of 20 μM As3+ for 12 h. The cell mitosis was indicated by immunofluorescence staining using anti-phospho-histone H3 antibody (p-H3) and counter stained with DAPI to visualize the nuclei. B. Average percentage of the p-H3 positive cells of the vector-, GADD45α (wt)- or GADD45α1 (ΔE2)-transfected cells cultured in the absence or presence of 20 μM A3+ for 12 h. Data are means ± SD (n=3). *: p < 0.01; **:p < 0.005. C. Western-blotting analysis of the p-H3 in the cells transfected with the indicated vectors and cultured in the absence or presence of As3+. D. Morphological analysis suggested that As3+ treatment arrests cells in prometaphase.
GADD45α1 antagonizes wild-type GADD45α in G2/M phase arrest
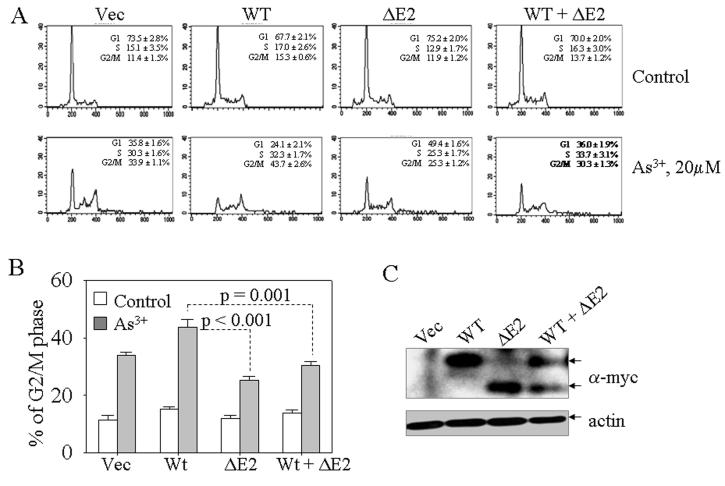
Analyses of p-H3 in both immunofluorescence and immunoblotting indicated a possible antagonistic role of GADD45α1 on wild-type GADD45α (Fig. 3). To validate such antagonistic effect directly, we performed co-transfection experiments with GADD45α (WT) and GADD45α1 (ΔE2) followed by flow cytometry of the transfected cells. Again, a similar cell cycle profile was noted in all of these transfected cells without As3+ treatment (Fig. 4A). In the presence of As3+, about 44% of the WT GADD45α expressing cells were arrested at the G2/M phase. The G2/M phase arresting effect of WT GADD45α was substantially decreased when the cells were co-transfected with ΔE2 (Figs. 4A and 4B). The expression of the transfected GADD45α and GADD45α1 was confirmed by Western-blotting using anti-myc antibody (Fig. 4C).
Fig. 4.
GADD45α1 antagonizes wild-type GADD45α. A. transient transfection of the cells with the indicated vectors for 36h followed by treatment of the cells with 20 μM As3+. Flow cytometry was performed at the end of the culture. B. Average percentage of the cells in G2/M phase for each transfected cells. C. Western-blotting shows expression of the exogenously transfected proteins.
GADD45α1 failed to arrest cells at prometaphase
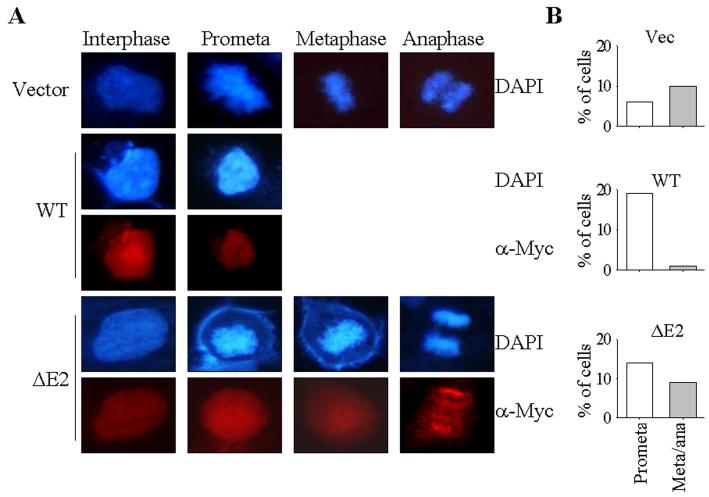
To further determine the mitotic effects of the GADD45α isoform, GADD45α1, we next evaluated the mitotic progress of the cells stably expressing the wt GADD45α and GADD45α1, respectively. Without As3+ treatment, the cells in prophase, prometaphase, metaphase, and anaphase are visualized in the cells transfected with a control vector or the GADD45α1, suggesting that the mitotic progress in these cells was normal (Fig. 5A). In contrast, in the cells expressing GADD45α, only the prophase or prometaphase cells could be detected. The cells in metaphase or anaphase are barely detected in the cells expressing GADD45α (Fig. 5A). Partial quantification of the cells in prometaphase (promate) and metaphase/anaphase (meta/ana) suggests that around 10% of the non-synchronized vector- or GADD45α1 (ΔE2)-transfected cells are in metaphase and/or anaphase. However, less than 1% of the GADD45α-transfected cells are in metaphase and anaphase (Fig. 5B).
Fig. 5.
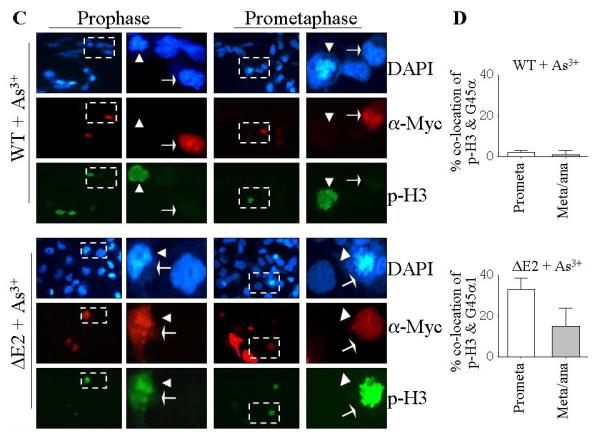
GADD45α1 failed to arrest cells at prometaphase. A. The cells in interphase, prometaphase (prometa), metaphase, and anaphase were determined by the morphology of the nuclear chromosomal DNA in DAPI staining of the transfected cells. The expression of the myc-tagged GADD45α or GADD45α1 was determined by immunofluorescence staining using anti-myc tag (α-Myc) antibody. B. Quantification of the cells in prometaphase (prometa) and metaphase/anaphase (meta/ana). C. Co-localization analyses of the phosphohistone H3 (p-H3) and the exogenous GADD45α in the transfected cells. Arrow heads indicate cells in prophase or prometaphase; white arrows indicate cells expressing exogenous GADD45α or GADD45α1. The area bounded by the dash-lined rectangle is enlarged on the right of each panel showing either prophase cells or prometaphase cells. D. Quantification for the co-localization of p-H3 and the myc-tagged GADD45α (upper panel) and GADD45α1 (lower panel).
To elucidate whether the stress condition changes the mitotic progress in these cells expressing wt GADD45α or the ΔE2 isoform, GADD45α1, we next measured the cell mitosis for these GADD45α- and GADD45α1-expressing cells treated with As3+ for 12 hours (Figs. 5C & 5D). Again, the cells in each mitotic phase were observed for the cells expressing the GADD45α1. Although a remarkable increase of the cells in prometaphase was noted in the GADD45α-, but not GADD45α1-expressing cells treated with As3+ (Figs. 3A and 3D), the cells in metaphase and anaphase were still hardly detected in these cells (Figs. 5C & 5D). It was surprising to note that the p-H3 positive and wt GADD45α expression are mutually exclusive in the prophase or prometaphase cells (Fig. 5C, the cells pointed with arrow heads and white arrows, respectively). It appeared that the wt GADD45α was expressed preferentially in the interphase cells in response to As3+. In contrast, a co-localization of ΔE2 isoform, GADD45α1, with p-H3 was observed in the prophase, prometaphase (Figs.5C & 5D), metaphase, and anaphase cells (data not shown). These data, thus, demonstrated that the wt GADD45α, but not the ΔE2 isoform, GADD45α1, is able to block the mitotic entry (prophase or prometaphase) from interphase of the cells.
As3+ induces accumulation of the exogenous GADD45α and GADD45α1
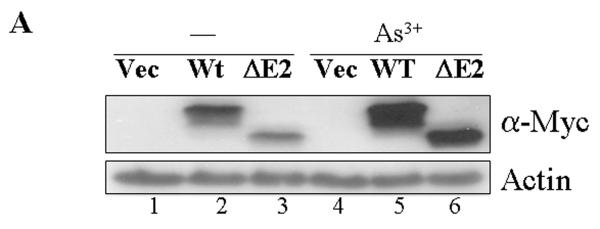
Our previous reports suggested that As3+ up-regulates GADD45α through either mRNA stabilization or an IRES-dependent protein translation [9, 12, 13]. To evaluate whether As3+ is able to modulate the levels of the exogenously transfected GADD45α, the protein levels of the myc-tagged GADD45α and GADD45α1 were examined in the transfected cells treated with As3+. An appreciable increase of the GADD45α (Wt) and GADD45α1 (ΔE2) was observed in the cells treated with 20 μM As3+ (Fig. 6A, compare lanes 4, 5 and 6 with 1, 2 and 3). It is very likely that this induction of the GADD45α proteins by As3+ is achieved through the mechanism of mRNA stabilization, since there is no 5′-UTR regions of the GADD45α mRNAs in these expression vectors and the cells were in logarithmical growth condition. This increase in the GADD45α expression induced by As3+, again, mainly occurred in the interphase cells in immunofluorescence staining experiments (Fig. 6B), which also showed a mutual exclusion of the GADD45α and p-H3 (Fig. 6B).
Fig. 6.
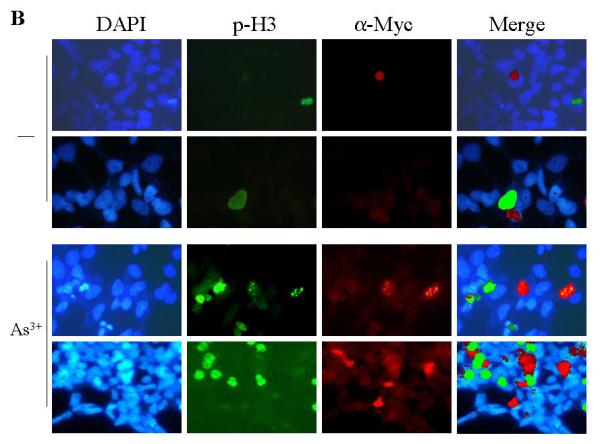
As3+ induces expression of exogenous GADD45α proteins. A. Western-blotting shows that As3+ enhances expression of the exogenous GADD45α proteins. B. Mutual exclusion of the GADD45α and p-H3 in the cells cultured in the absence (-) or presence of 20 μM As3+ for 12 hours. The exogenously transfected GADD45α was detected with anti-myc tag antibody.
Crippled interaction of the GADD45α1 with CDC2/cyclin B complex
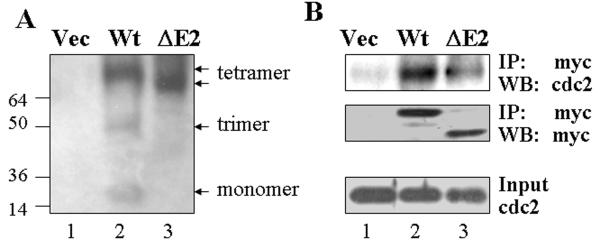
Studies on intracellular distribution, cell growth and cell mitosis suggested a notable functional deficiency of the GADD45α1 in arresting the cells at G2/M phase or prometaphase (Figs. 2 & 3). Co-transfection experiment even showed that GADD45α1 appeared to be capable of antagonizing the cell cycle arresting effect of the WT GADD45α in the cells treated with As3+ (Fig. 4). It had been previously demonstrated that the mitotic arrest of the GADD45α was achieved largely through direct interaction with and inhibition of the CDC2/cyclin B complex that is required for mitotic entry and completion [14]. To understand the mechanism of the above observations for the new GADD45α isoform, GADD45α1, we next investigated the molecular characteristics of the GADD45a1 in native gel and also determined its interaction with CDC2 in co-immunoprecipitation experiments. Total cell lysates were prepared from WT GADD45α- and GADD45α1-transfected cells followed by separation in 4-12% native gel. The expression of the transfected GADD45α proteins were detected by Western-blotting using anti-myc tag antibody. As implicated in Fig. 7A, the monomer, trimer and tetramer of WT GADD45α were visualized in this native gel. By contrast, only tetramer, but neither monomer nor trimer could be seen for the GADD45α1, indicating that this isoform is extremely aggregative. It is very likely, accordingly, that GADD45α1 may be functionally different from its WT counterpart in interaction with partner proteins. To seek such possibility, co-immunoprecipitation was performed using anti-myc tag antibody followed by Western-blotting for both CDC2 and myc-tagged GADD45α proteins, respectively. A notable weakness in interaction with CDC2 of the GADD45α1 was observed in this assay (lane 3, Fig. 7B). This result is in agreement with other reports suggesting that the central region of the GADD45α is responsible for interaction with the CDC2/cyclin B complex. Deletion of the region corresponding to exon2 of the gadd45α gene due to alternative splicing by exon2 skipping will result in the missing of amino acids 16-49, the region contributing to CDC2 interaction, of the GADD45α protein.
Fig. 7.
GADD45α1 forms tetramer that weakens interaction with CDC2/cyclin B complex. A. The expression of WT or ΔE2 GADD45α was determined in the 4-12% native gel. Arrows from top to bottom on the right of the panel indicate WT tetramer, ΔE2 tetramer, WT trimer, and WT monomer. B. Coimmunoprecipitation of the WT or ΔE2 GADD45α with CDC2 protein. The GADD45α or GADD45α1 was immunoprecipitated with α-myc antibody followed by Western-blotting using antibodies against CDC2 (top panel) or myctag (middle). Bottom panel shows total CDC2 protein in the lysates of the cells transfected with the indicated vectors.
Discussion
GADD45α has been generally viewed as a checkpoint protein that is inducibly expressed in cellular responses to DNA damage or stress signals [14]. The expression of the GADD45α protein may provide the cells with sufficient time to repair the damaged DNA and consequently prevent malignant transformation of the cells [15]. A robust induction of the GADD45α protein was seen in the cells treated with As3+, an unequivocally established human carcinogen, and other DNA damaging reagents. However, these cells remained in danger of tumorigenic transformation. It has been proposed that such transformation is due either to insufficient induction of the checkpoint proteins, such as GADD45α, or to the accompanying impairment of the DNA repairing machinery. The identification of a new GADD45α isoform, GADD45α1, may provide an alternative reason to explain why the cells exhibited tumorigenic potential when the GADD45α was induced. The failure of the GADD45α1 to reduce cell growth and arrest the cells at the prometaphase as demonstrated in the present report is indicative for the carcinogenic effects of As3+. The expression of the GADD45α1 may not only exhaust the checkpoint mechanism by generating a functionally inert protein in the control of cell growth and cell cycle, but interfere with the normal function of the counterpart protein, GADD45α also. The latter is more likely the case as evidenced by the observation that expression of GADD45α1 was unable to arrest the cells at mitosis, but rather, decreased the number of the cells in mitotic phase in response to As3+ (Figs. 3B & 3C). This assumption is further supported by a co-transfection experiment in which the expression of the GADD45α1 clearly weakened the G2/M arresting effect of the WT GADD45α (Fig. 4).
This report is the first to demonstrate the presence of an alternatively spliced GADD45α isoform, GADD45α1. Since our first deposition of the cDNA sequence of this new GADD45α isoform, GADD45α1, into the GenBank in 2005 (April 14, 2005, GenBank protein ID AAY25021, updated on January 20, 2006) [12], this isoform was also deposited by human genome projects of Celera Genomics (GenBank ID EAX06488, submitted on July 5, 2005) and Wellcome Trust Sanger Institute (GenBank ID CAI23495, submitted on April 20, 2008), respectively. Furthermore, this isoform had also been predicted in chimpanzee (XP_001164496) and rhesus (XP_001095295), respectively, according to the GenBank databases. In addition to As3+ treatment, the GADD45α1 may also occurred in the cells treated with other DNA damaging agents, including UV irradiation, cisplatin and taxol based on the presence of multiple GADD45α protein bands in immunoblotting [13]. Thus, we strongly believe that this isoform is truly present under many circumstances. Analyzing the genomic structure of the human gadd45α gene revealed several unique characteristics that form the biological bases of the alternative splicing of the mRNA by skipping out of the exon2. First, the exon2 contains a strong exonic splicing suppressor that suppressing constitutive splicing in PESX assay; Secondly, the 3′ splicing site of intron1 is the weakest splicing site as determined by both Splice Site Score Calculation and GeneSplicing programs (data not shown); Thirdly, the intron1 lacks the conserved branch site and the polypyrimidine tract (PPT), both of which are required for sufficient binding of the U2 and U2AF to the 3′-splicing site during splicing. Accordingly, under certain stress conditions that limited the assembly and availability of the spliceosomes, the exon2 might be skipped out due to the poor compatibility for spliceosome binding.
An additional interesting observation in the present report is the mutual exclusion between the induced expression of the GADD45α and the cell mitotic marker, p-H3 (Figs. 5C & 6B). Upon As3+ treatment, the interphase cells, rather than the mitotic cells, exhibited expression of the GADD45α protein. It is perhaps difficult to understand how a protein arresting cells at mitosis is expressed preferentially in the interphase cells. Our explanation is that the interphase, such as G2 phase, expression of the GADD45α protein may prevent the activation of the CDC2/cyclin B kinase complex whose activity arose in G2 phase for driving cells into mitotic entry and the completion of the mitosis [16]. Absence of the region encoded by the exon2 of the gadd45α gene, which resulted from the alternative splicing of the GADD45α mRNA, may cripple such capability in CDC2/cyclin B suppression. Further experimentation is underway to demonstrate whether GADD45α1 acts as a true antagonist of the normal GADD45α in genomic stabilization, centrosome duplication and interaction with Aurora-A, PCNA and others.
Acknowledgement
This work was supported by the intramural research grant of the NIOSH/CDC (9270036) to F Chen. X. Shi was supported by NIH grants 5R01CA119028 and 5R01CA116697. We thank Dr. Murali Rao in the Pathology and Physiology Research Branch at the NIOSH for critical reading of and suggestions to this manuscript.
Footnotes
Publisher's Disclaimer: Disclaimer: The opinions expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Institute for Occupational Safety and Health, Centers for Disease Control and Prevention of the USA.
References
- 1.Hoffman B, Liebermann DA. Role of gadd45 in myeloid cells in response to hematopoietic stress. Blood Cells Mol Dis. 2007;39(3):344–7. doi: 10.1016/j.bcmd.2007.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhan Q. Gadd45a, a p53- and BRCA1-regulated stress protein, in cellular response to DNA damage. Mutat Res. 2005;569(1-2):133–43. doi: 10.1016/j.mrfmmm.2004.06.055. [DOI] [PubMed] [Google Scholar]
- 3.Wang W, Huper G, Guo Y, Murphy SK, Olson JA, Jr., Marks JR. Analysis of methylation-sensitive transcriptome identifies GADD45a as a frequently methylated gene in breast cancer. Oncogene. 2005;24(16):2705–14. doi: 10.1038/sj.onc.1208464. [DOI] [PubMed] [Google Scholar]
- 4.Barreto G, Schafer A, Marhold J, et al. Gadd45a promotes epigenetic gene activation by repair-mediated DNA demethylation. Nature. 2007;445(7128):671–5. doi: 10.1038/nature05515. [DOI] [PubMed] [Google Scholar]
- 5.Jin SG, Guo C, Pfeifer GP. GADD45A does not promote DNA demethylation. PLoS Genet. 2008;4(3):e1000013. doi: 10.1371/journal.pgen.1000013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Wang RH, Li W, et al. Genetic interactions between Brca1 and Gadd45a in centrosome duplication, genetic stability, and neural tube closure. J Biol Chem. 2004;279(28):29606–14. doi: 10.1074/jbc.M312279200. [DOI] [PubMed] [Google Scholar]
- 7.Fabbro M, Henderson BR. BARD1 regulates BRCA1-mediated transactivation of the p21(WAF1/CIP1) and Gadd45 promoters. Cancer Lett. 2008;263(2):189–96. doi: 10.1016/j.canlet.2008.01.001. [DOI] [PubMed] [Google Scholar]
- 8.Chen F, Lu Y, Zhang Z, et al. Opposite effect of NF-kappa B and c-Jun N-terminal kinase on p53-independent GADD45 induction by arsenite. J Biol Chem. 2001;276(14):11414–9. doi: 10.1074/jbc.M011682200. [DOI] [PubMed] [Google Scholar]
- 9.Zheng X, Zhang Y, Chen YQ, Castranova V, Shi X, Chen F. Inhibition of NF-kappaB stabilizes gadd45alpha mRNA. Biochem Biophys Res Commun. 2005;329(1):95–9. doi: 10.1016/j.bbrc.2005.01.105. [DOI] [PubMed] [Google Scholar]
- 10.Zerbini LF, Wang Y, Czibere A, et al. NF-kappa B-mediated repression of growth arrest- and DNA-damage-inducible proteins 45alpha and gamma is essential for cancer cell survival. Proc Natl Acad Sci USA. 2004;101(37):13618–23. doi: 10.1073/pnas.0402069101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen CY, Gherzi R, Andersen JS, et al. Nucleolin and YB-1 are required for JNK-mediated interleukin-2 mRNA stabilization during T-cell activation. Genes Dev. 2000;14(10):1236–48. [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Bhatia D, Xia H, Castranova V, Shi X, Chen F. Nucleolin links to arsenic-induced stabilization of GADD45alpha mRNA. Nucleic Acids Res. 2006;34(2):485–95. doi: 10.1093/nar/gkj459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chang Q, Bhatia D, Zhang Y, et al. Incorporation of an internal ribosome entry site-dependent mechanism in arsenic-induced GADD45 alpha expression. Cancer Res. 2007;67(13):6146–54. doi: 10.1158/0008-5472.CAN-07-0867. [DOI] [PubMed] [Google Scholar]
- 14.Amanullah A, Azam N, Balliet A, et al. Cell signalling: cell survival and a Gadd45-factor deficiency. Nature. 2003;424(6950):741. doi: 10.1038/424741b.; discussion 2.
- 15.Hollander MC, Sheikh MS, Bulavin DV, et al. Genomic instability in Gadd45a-deficient mice. Nat Genet. 1999;23(2):176–84. doi: 10.1038/13802. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan M, Morgan DO. Finishing mitosis, one step at a time. Nat Rev Mol Cell Biol. 2007;8(11):894–903. doi: 10.1038/nrm2276. [DOI] [PubMed] [Google Scholar]