Abstract
Viruses employ different strategies to circumvent the antiviral actions of the innate immune response. SARS coronavirus (SARS-CoV), a virus that causes severe lung damage, encodes an array of proteins able to inhibit induction and signaling of type-I interferons. However, recent studies have demonstrated that interferons are produced during SARS-CoV infection in humans and macaques. Furthermore, nuclear translocation of activated STAT1 and a range of interferon-stimulated genes could be demonstrated in the lungs of SARS-CoV-infected macaques. In line with these observations, plasmacytoid dendritic cells have been shown to produce interferons upon SARS-CoV infection in vitro. Given the pivotal role of interferons during viral infections, (differential) induction of interferons may affect the outcome of the infection. Therefore, the functional implication of interferon production during SARS-CoV infection remains to be re-investigated.
Keywords: innate immunity, interferon, plasmacytoid dendritic cell, SARS coronavirus
SARS emerged late 2002–early 2003 in Guangdong Province, China, and spread rapidly to several countries in Asia, North America and Europe causing disease in 8096 people, of whom 774 died [101]. Although the number of deaths caused by SARS remained relatively low compared with, for example, flu pandemics, the impact of the SARS epidemic was quite high. Rapid spread of the virus owing to air travel, immediate media coverage all over the world and the globalization of the economy contributed to the high impact of the epidemic. The emergence of this new highly pathogenic virus led to a quick response from the scientific world; only a few months after the first emergence of SARS, a newly discovered coronavirus (CoV) was identified as its etiological agent [1–4]. Many of the people that were infected with SARS-CoV early during the epidemic had been in close contact with live animals on Chinese wet markets, suggesting that the virus came from an animal source [5]. The current hypothesis is that bats are the main natural reservoirs of SARS-CoV-like viruses, and virus transmission to humans most likely occurred via civet cats, which are common trade animals on these wet markets [6].
Infection with SARS-CoV in humans initially causes lower respiratory tract disease with clinical symptoms that include fever, malaise and lymphopenia [7–10]. Approximately 20–30% of SARS patients suffered from severe disease and needed to be transferred to intensive care units. Ultimately, the overall fatality rate approached 10–20% in adults, while children seemed to be relatively resistant to SARS [11]. The clinical course of SARS follows three phases. The first phase is characterized by active viral replication and patients experience the first symptoms of disease, such as fever and malaise. Virus levels start to decrease while antibodies, which are effective in controlling infection, increase in the second phase. Nonetheless, pneumonia and immunopathological injury also develop in this phase. Eventually, in the third phase, fatal cases of SARS develop severe pneumonia and acute respiratory distress syndrome (ARDS), characterized by the presence of diffuse alveolar damage [7]. Although a wide range of animal species are susceptible to experimental infection with SARS-CoV, nonhuman primates are the only animals in which pathology similar to that of human SARS patients has been observed so far. The lungs of SARS-CoV-infected macaques demonstrate type-II pneumocyte hyperplasia and multiple foci of acute diffuse alveolar damage, characterized by flooding of the alveoli with edema and infiltration of inflammatory cells [4]. Furthermore, these lungs demonstrate lesions that are quite similar to those observed in human SARS patients. While SARS does not seem to produce the severe illness in macaques that is seen in some, mainly elderly, human SARS patients, symptoms resemble those that are seen in children. Interestingly, more recent studies have demonstrated that aged macaques do show more severe disease than young macaques, which corroborates findings made in humans infected with SARS-CoV [Smits SL et al., Manuscript in Preparation].
ARDS is characterized by massive infiltration of immune cells in the lungs, edema formation and the induction of an array of inflammatory cytokines and chemokines, such as CXCL10, CCL2, IL-6, IL-8, IL-12, IL-1β and IFN-γ [12]. We also observed elevated levels of these inflammatory cytokines and chemokines in the lungs of cynomolgus macaques that were experimentally infected with SARS-CoV as evidenced by microarray analysis and quantitative reverse transcription-PCR [13]. Significant induction of these inflammatory agents was also observed in human SARS patients and has been described as a ‘cytokine storm’ [14–16].
Several of the earlier reports did not find evidence for the induction of type-I interferons in SARS patients [14–19]. Given the importance of interferons in antiviral defence, considerable efforts have been made to analyze whether SARS-CoV blocks induction of interferons. This review will focus on the apparent discrepancies in reports analyzing the induction of interferons by SARS-CoV.
Interferon induction during viral infection
Interferons are a group of cytokines that elicit distinct antiviral effects. Type-I interferons, IFN-α (13 human subtypes) and IFN-β (one human subtype), together with type-III interferons, IFN-λ1, IFN-λ 2 and IFN-λ 3 (IL-29, IL-28A and IL-18B, respectively) are induced in direct response to virus infection [20]. IFN-γ, a single type-II interferon, is not secreted in direct response to viral infection but produced by activated T cells and natural killer cells. In principal, most mammalian cells are capable of producing type-I interferons, however, the mechanism and amount of interferons that are induced can differ among cell types. While fibroblasts, epithelial cells and neurons mainly secrete IFN-β initially before they switch to IFN-α production, plasma-cytoid dendritic cells (pDCs) initially make large amounts of IFN-α followed by a switch to IFN-β. Interferons do not only function as direct antiviral proteins, they also have several other biological properties such as inhibition of cellular proliferation and immunomodulation, making their role in viral infections broader than just their direct antiviral activity.
There are several routes by which cells can recognize the presence of an invading virus and by which interferons can be produced subsequently. The relevance of a specific route depends on the type of virus, type of cell that is infected and the stage of infection in that same cell. The two major pathways through which cells sense invading viruses are the intracellular pathway and the endosomal pathway. In the intracellular pathway, IFN-β is induced when pathogen-associated molecular patterns, such as dsRNA and 5′-triphosphorylated ssRNA, are sensed by the RNA helicases RIG-I and MDA-5, the main intracellular receptors of viral RNA [21–24]. When viral RNA binds to these helicases, a signaling chain is induced, eventually leading to the phosphorylation of the transcription factor IRF-3, a molecule that plays an essential role in the activation of the IFN-β promoter [25–26]. As soon as IRF-3 is phosphorylated, the molecule homodimerizes and is translocated to the nucleus where it recruits the cofactors CPB and p300 and binds the IFN-β promoter in order to start IFN-β gene transcription. Ongoing viral replication subsequently induces the activation of transcription factors nuclear factor (NF)-κB and AP-1, which both bind the IFN-β promoter and further enhance IFN-β gene expression [27]. The induction of interferon leads to the production of IRF-7, which is only present in low amounts in most cells. Phosphorylated IRF-7 subsequently binds the IFN-β promoter, resulting in enhanced induction of IFN-β and the induction of the IFN-α genes through a positive feedback loop.
Induction of interferon through the endosomal pathway involves the Toll-like receptors (TLRs), such as TLR3, −7, −8 and −9, which can detect viruses in endosomal compartments as they enter cells. This pathway is used by pDCs, which are also known as ‘professional type-I interferon producers’ [28]. pDCs predominantly sense viral RNA through the endosomal TLR7 and TLR8. When these TLRs become activated, the adapter molecule MyD88 is recruited, which in turn recruits IRAK-4 and IRAK-1. Subsequently, through activation of several signaling molecules, IRF-7, NF-κB and IRF-3 are activated and translocated to the nucleus, leading to the production of IFN-α and IFN-β. Interestingly, pDC is the only known cell type that constitutively expresses high levels of IRF-7 and thus high levels of interferon can be produced by these cells [29].
Interferon induction by SARS-CoV
SARS-CoV typically does not induce an interferon response in infected cell cultures although substantial amounts of the interferon-inducing dsRNA are generated during infection [30–33]. These data suggest that SARS-CoV is able to inhibit the induction of interferons, either actively or passively. Several potential mechanisms through which SARS-CoV can inhibit interferon induction have been described recently. Double membrane vesicles are formed after CoV infection, and it is hypothesized that these complexes shield viral RNA from sensing molecules [34]. Another possibility is that one of the SARS-CoV viral proteins sequesters the genomic RNA in such a way that host-sensing proteins do not detect it as has been demonstrated for several other viruses, such as VP35 from Ebola and NS1 of influenza. It remains to be proven if SARS-CoV actively inhibits interferon production or just escapes from the induction of interferons. Spiegel and colleagues demonstrated that although SARS-CoV does not induce interferon, IRF-3 initially translocates to the nucleus of infected cells in SARS-CoV-infected cells [32]. However, at a later time point after infection, IRF-3 was again localized in the cytoplasm. Furthermore, SARS-CoV interfered with IRF-3 hyperphosphorylation, dimerization and binding to cofactor chromatin-binding protein. Also, studies using expression plasmids that contain one of the SARS-CoV proteins have demonstrated that SARS-CoV proteins open reading frame (ORF)3b, ORF6 and the N proteins are able to inhibit interferon production by affecting the IRF-3 activation pathway [35]. More recently, other groups demonstrated that SARS-CoV does not activate IRF-3 and induce NF-κB or interferon in infected cells, but that subsequent treatment with polyriboinosinic acid:polyribocytidylic acid or infection with Sendai virus still results in interferon production [36,37]. In addition, when recombinant SARS-CoV, deleted for one of their accessory ORFs, was used to infect cells, none of the deletion mutants were able to induce interferon, and Sendai virus infection did induce IFN-β production in these cells [37]. Apparently, SARS-CoV does not induce the interferon-sensing pathway, but also does not actively block sensing of other viral proteins or stimuli either.
Most studies that use tissue or blood samples from SARS patients did not find a strong interferon response in these patients [15–17,38,39]. This apparent absence of interferon induction in human SARS patients can be explained by the fact that good quality tissue and blood samples from SARS patients were not readily available and even more by the fact that samples were often taken at the time of death, for example, late during infection, making type-I interferon levels difficult to detect. However, induction of type-I interferons, both IFN-α and IFN-β, can be observed in SARS-CoV-infected cynomolgus macaques early during infection, both at the gene expression and protein level [13]. In accordance with our macaque data, a recent study using material from 40 clinically well described SARS cases revealed high levels of type-I interferon in plasma and robust interferon stimulated gene expression in precrisis SARS patients [40]. These data demonstrate that, although SARS-CoV blocks interferon induction in vitro, type-I interferons are still produced during SARS-CoV infections in vivo.
An explanation for the differences seen in SARS-CoV-induced interferon production in cell culture and in vivo experiments was given by Cervantes-Barragan and coworkers [41]. They found that, in contrast to earlier reports from studies performed in in vitro cell cultures, interferon induction is not inhibited in pDCs after SARS-CoV infection. While interferons cannot be detected in SARS-CoV-infected myeloid DCs, pDCs rapidly produce and secrete considerable amounts of IFN-α and IFN-β upon SARS-CoV infection. Furthermore, using mouse hepatitis virus as a CoV model, it was observed that rapid interferon induction in pDCs was dependent on TLR7 and MyD88 [41]. These sensing molecules induce expression of IFN-α upon viral infection through IRF-7, constitutively expressed in pDCs, suggesting that the pDC restricted TLR7/IRF-7 interferon induction pathway is not affected by the virus. It is still unclear whether DCs are infected by SARS-CoV, and so far no conclusive evidence on infection of DCs by SARS-CoV has been reported [42–45]. On the other hand, active viral replication may not be necessary to mount an efficient interferon response. Castilletti and coworkers observed that SARS-CoV induces IFN-α and IFN-γ in peripheral blood mononuclear cells (PBMCs) from healthy donors and that this interferon response was even more robust when PBMCs were incubated with (paraformaldehyde) fixed SARS-CoV-infected Vero cells [43]. In addition, Spiegel and coworkers found that both live and UV-inactivated SARS-CoV were able to induce type-I interferons in DC cultures [46].
pDCs are known to be the most potent producers of IFN-α in the human body, producing 10–100 times more type-I interferons than other cell types, and type-I and type-III interferons account for 60% of the total genes expressed in activated pDCs [47]. Although there is no proof as yet that pDCs are responsible for the induction of interferons during SARS-CoV infection in vivo, the fact that pDCs are able to produce type-I interferons upon SARS-CoV infection in vitro is suggestive for their role in vivo. However, it is still possible that another cell type is accountable for the interferon production seen in humans and macaques during SARS. For example, for several other RNA viruses it has been shown that alveolar macrophages, not pDCs, are the main type-I interferon producers early in infection [48].
SARS-CoV blocks interferon signaling
As soon as IFN-α and IFN-β bind to the common type-I interferon receptor (composed of the products of the IFNAR1 and the IFNAR2 genes), conformational changes in the intracellular part of the receptor lead to the activation of the JAK/signal transducers and activators of transcription (STAT) signaling pathway. The cytoplasmic tails of IFNAR1 and IFNAR2 associate with tyrosine kinase 2 and tyrosine kinase janus 1, respectively, leading to binding and activation of STAT1 and STAT2. After STAT1 and STAT2 have formed a stable heterodimer, the complex is translocated into the nucleus where it associates with IRF-9 to form the heterotrimer ISGF3. This complex binds to the interferon-stimulated response element resulting in transcription of ISGs, such as PKR, 2′5′-OAS, MX, ISG15, ISG20 and ISG54 [49].
More than 300 ISGs are induced by IFN-α/β, leading to a wide array of biological effects in the host cell. For example, the G1/S phase-specific cyclin-dependent kinase inhibitor p21 triggers cell cycle arrest, while proteins such as procaspases, PKR and OAS have an antiviral effect by causing apoptosis. Additionally, ISGs can have immunomodulatory effects by promoting the presentation of viral antigens through upregulated expression of the MHC class-I molecules, by promoting maturation of DCs or by upregulating the activities of NK cells. The combined expression of these ISGs by a cell is collectively known as an ‘antiviral state’ [49].
Recently, it was demonstrated that SARS-CoV does not only prevent the induction of type-I interferons, but that several SARS-CoV proteins also block different steps in the interferon signaling pathway. For example, the ORF7a protein and nsp-1 were observed to inhibit cellular protein synthesis and promote host cell mRNA degradation, respectively [50,51]. In addition, the SARS-CoV proteins ORF3b and ORF6 inhibit interferon signaling through the JAK/STAT pathway both passively and actively [35]. Inhibition of interferon signaling by ORF6 was described in detail by Frieman and coworkers [52]. They demonstrated that ORF6 tethers karyopherin α2 and karyopherin β1 to the endoplasmic reticulum–Golgi membrane, inhibiting the nuclear import of STAT1 and thus preventing transcription of the ISGs.
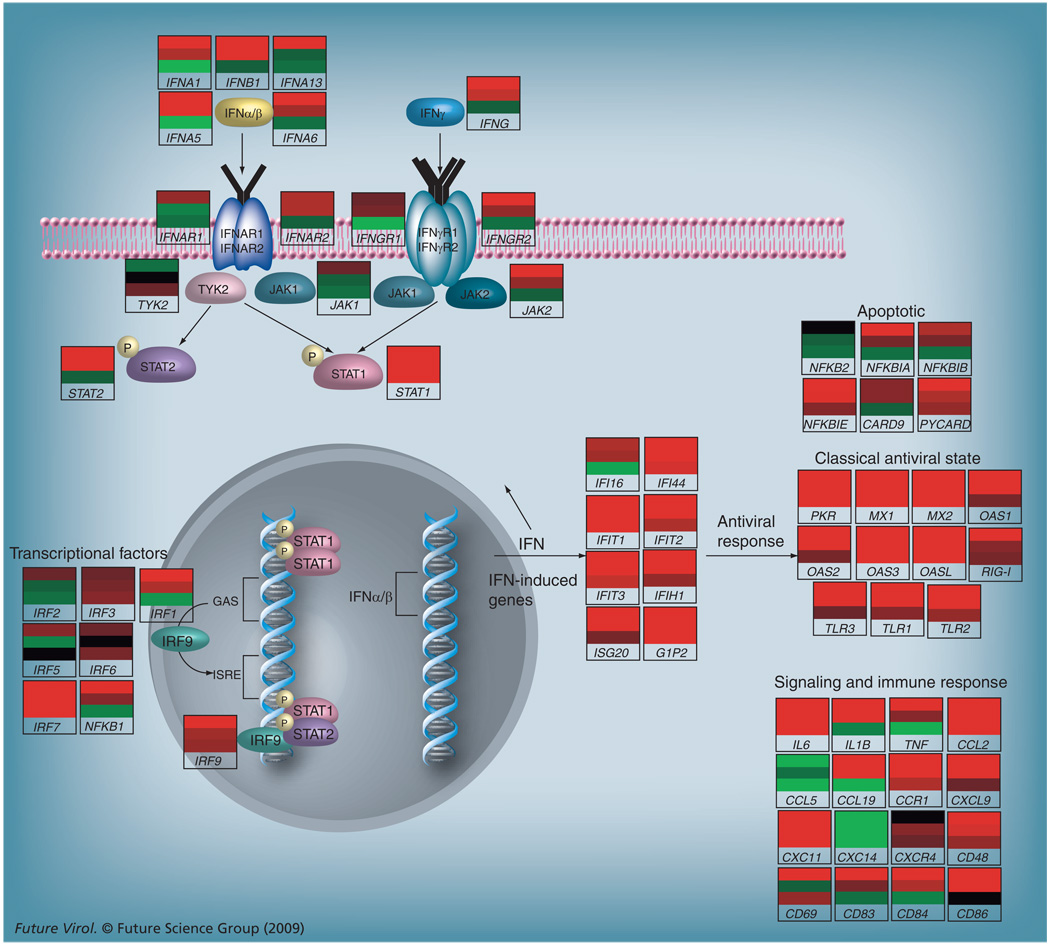
Despite a wealth of evidence that SARS-CoV blocks interferon signaling, we were able to visualize interferon signaling in the lungs of SARS-CoV-infected macaques by immunohistochemical staining of phosphorylated STAT1 [13]. In line with the observations made with single SARS-CoV genes, activated STAT1 was not observed in SARS-CoV-infected cells, while the nuclei of surrounding cells displayed nuclear translocation of activated STAT1. The induction of interferons and the subsequent activation of the JAK/STAT signaling pathway, resulting in transcription of several ISGs, is schematically represented in Figure 1. Cynomolgus macaques were infected with SARS-CoV and subsequently euthanized either at day 1 or day 4 after infection, after which pieces of lung were sampled randomly from each macaque [13]. RNA was isolated from these lung pieces and used for microarrays, from which genomic data are incorporated in Figure 1. Each gene-included pathway is illustrated with an individual heatmap, representing differential expression of that particular gene in a SARS-CoV-infected macaque compared with a mock-infected (phosphate-buffered saline) macaque. A red bar indicates that the particular gene is upregulated in SARS-CoV-infected macaques, as compared with mock-infected macaques, while a green bar indicates that the gene is downregulated. At day 1, represented by the upper bars in the heatmaps, an induction of several IFN-α subtypes and IFN-β was observed, leading to induced expression of numerous ISGs, such as PKR, MX molecules, OAS molecules and RIG-I, and cytokines such as IL-6 and CCL2. Furthermore, induced expression of IRF-7, part of the positive feedback loop for interferon production, as described earlier, was also observed. At day 4, represented by the middle bars, expression of these genes remained upregulated, except for some of the IFN-α subtypes. In pieces of lung from the infected macaques where no viral RNA could be detected, the lower bars in the individual gene heatmaps, no expression of type-I interferons was observed but various ISGs were upregulated, suggesting paracrine stimulation. Overall, these data demonstrate that interferons are produced after SARS-CoV infection and are capable of activating neighboring cells.
Figure 1. Schematic representation of the JAK/STAT signaling pathway in lungs of SARS-coronavirus-infected macaques.
Micorarray experiments were performed on lung material from six SARS coronavirus (SARS-CoV)-infected macaques [13]. Individual heatmaps were created for each gene by comparing gene expression in the SARS-CoV-infected animal to expression of that gene in a pool of mock-infected animals. The top bar of each heatmap represents differential gene expression at day 1, the middle bar represents differential gene expression at day 4 and the lower bar represents differential gene expression at day 4 in lung areas where no virus was detected by TaqMan®. Genes were considered differentially expressed when showing a fold change greater than two and a p-value <0.0001.
Future perspective
The presence of type-I interferons and ISGs in SARS-CoV-infected humans and macaques raises the question to what extent these pathways play a role in SARS pathogenesis. Pretreatment of cells with interferon before infection prevents SARS-CoV replication in these cells [53]. Interestingly, it has been demonstrated that IFN-γ downregulates expression of ACE2, the cellular SARS-CoV receptor, on the cell surface, resulting in decreased infection in these cells [54]. In addition, Haagmans and coworkers showed that IFN-α protects type-I pneumocytes from SARS-CoV infection in macaques [55]. In line with these observations, interferon treatment starting early after onset of clinical symptoms may also be effective in humans [56]. Especially in individuals that produce low levels of endogenous interferon, interferon therapy might be quite effective in preventing severe disease. Definitive evidence for a protective role of interferons in mice is lacking at this moment. Hogan and coworkers found that mice deficient for STAT1 showed slightly more pathological changes compared with wild-type mice, suggesting a role of the JAK/STAT signaling pathway in the outcome of infection [57]. A limited role of STAT1 signaling in this model can be explained by the fact that no significant IFN-α production has been reported in SARS-CoV mouse models thus far [58]. It remains to be investigated whether the pathogenesis of SARS-CoV in different animal models is linked to varying amounts of IFN-α produced.
The mortality rate of SARS was significantly higher in the elderly than in young adults and children, who rarely succumbed to the disease. The reason for this difference is still unclear. However, it is hypothesized that SARS pathology is caused by an disproportional immune response, characterized by the induction of several inflammatory cytokines [11]. It has been shown that inflammatory cytokines like IL-1, IL-6 and IL-8 are produced at higher levels in elderly people as compared with the young, making them more vulnerable for the development of ARDS after SARS-CoV infection. Furthermore, it could be envisaged that pDC numbers or their function decreases with age, leading to a less efficient interferon response at the time of infection or that pDCs become less productive at a higher age.
ARDS is a severe form of acute lung failure and cannot only be triggered by SARS-CoV, but also by other pathogens such as H5N1 avian influenza and anthrax. Furthermore, a variety of other conditions like sepsis, pancreatitis and severe trauma can lead to ARDS. Despite todays modern intensive care medicine, no pharmacological therapies have been developed as yet that can alleviate ARDS symptoms, and the mortality associated with ARDS remains very high. Insights in the complex regulation of the interferon response during SARS-CoV infection may provide clues for intervention strategies based on the use of interferons.
Executive summary
Introduction
SARS coronavirus (SARS-CoV) emerged in 2002 as the first coronavirus that causes severe disease in humans. More than 8000 people were infected of whom nearly 10% died. SARS-CoV spread rapidly from China to several other countries across the globe and its emergence had a significant economical impact.
Interferon induction during viral infection
Type-I interferons are induced in direct response to viral infection and play an important role in innate immunity. Although most mammalian cells are capable of producing type-I interferons, plasmacytoid dendritic cells, also known as ‘professional’ interferon producers, produce much higher amounts of IFN-α than other cell types.
Invading viruses are sensed by the host cell either though a cytoplasmatic pathway, using the RNA helicases RIG-I and MDA-5, or by the endosomal pathway, in which Toll-like receptors are involved.
After viral RNA is detected by the host cell, activation of several signaling pathways ultimately leads to phosphorylation of IRF-3 and IRF-7, which are subsequently translocated to the nucleus where they activate the interferon promoter together with other cofactors, resulting in the production of type-I interferons.
Interferon induction by SARS-CoV
SARS-CoV employs several mechanisms that inhibit induction of type-I interferons. As a result, most cell types do not produce type-I interferons upon SARS-CoV infection.
Despite blocks on interferon production, type-I interferons are detected in SARS-CoV-infected macaques and humans.
Plasmacytoid dendritic cells are able to produce interferons upon SARS-CoV infection and are a possible source for interferon production in vivo.
SARS-CoV blocks interferon signaling
When interferons activate the JAK/STAT signaling pathway, more than 300 interferon-stimulated genes are activated.
SARS-CoV not only blocks interferon induction but also interferon signaling through the JAK/STAT pathway.
Interferons produced during in vivo SARS-CoV infection activate STAT1 in uninfected cells.
Future perspective
Patients with severe cases of SARS develop pneumonia and acute respiratory distress syndrome (ARDS). Although ARDS-associated mortality remains very high, no successful pharmacological therapies have been developed for ARDS as yet.
Insights into the complex regulation of the interferon response during SARS-CoV may provide clues for intervention strategies based on the use of interferons. However, more research in appropriate SARS animal models should be performed to address this issue.
Acknowledgments
Financial & competing interests disclosure
The authors research is supported by the NIH grant HL080621-01A1 and the Virgo consortium, an Innovative Cluster approved by the Netherlands Genomics Initiative, and partially funded by the Dutch Government (BSIK 03012). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Footnotes
Website
World Health Organization statistics. www.who.int/csr/sars/country/table2004_04_21/en/index.html
Contributor Information
Anna de Lang, Department of Virology, Erasmus Medical Center, Rotterdam, The Netherlands.
Tracey Baas, Department of Microbiology, School of Medicine, University of Washington, Seattle, WA, USA.
Saskia L Smits, Department of Virology, Erasmus Medical Center, Rotterdam, The Netherlands.
Michael G Katze, Department of Microbiology, School of Medicine, University of Washington, Seattle, WA, USA.
Albert DME Osterhaus, Department of Virology, Erasmus Medical Center, Rotterdam, The Netherlands.
Bart L Haagmans, Department of Virology, Erasmus MC, PO box 2040, 3000 CA Rotterdam, The Netherlands, Tel.: +31 107 044 004;, Fax: +31 107 044 760; b.haagmans@erasmusmc.nl.
Bibliography
Papers of special note have been highlighted as:
▪of interest
▪▪of considerable interest
- 1.Drosten C, Gunther S, Preiser W, et al. Identification of a novel coronavirus in patients with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1967–1976. doi: 10.1056/NEJMoa030747. [DOI] [PubMed] [Google Scholar]
- 2.Ksiazek TG, Erdman D, Goldsmith CS, et al. A novel coronavirus associated with severe acute respiratory syndrome. N. Engl. J. Med. 2003;348:1953–1966. doi: 10.1056/NEJMoa030781. [DOI] [PubMed] [Google Scholar]
- 3.Peiris JS, Lai ST, Poon LL, et al. Coronavirus as a possible cause of severe acute respiratory syndrome. Lancet. 2003;361:1319–1325. doi: 10.1016/S0140-6736(03)13077-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kuiken T, Fouchier RA, Schutten M, et al. Newly discovered coronavirus as the primary cause of severe acute respiratory syndrome. Lancet. 2003;362:263–270. doi: 10.1016/S0140-6736(03)13967-0. Demonstrates that SARS coronavirus (SARS-CoV)-infected macaques develop pneumonia and diffuse alveolar damage, similar to human SARS patients.
- 5.Xu RH, He JF, Evans MR, et al. Epidemiologic clues to SARS origin in China. Emerg. Infect. Dis. 2004;10:1030–1037. doi: 10.3201/eid1006.030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li W, Shi Z, Yu M, et al. Bats are natural reservoirs of SARS-like coronaviruses. Science. 2005;310:676–679. doi: 10.1126/science.1118391. [DOI] [PubMed] [Google Scholar]
- 7.Nicholls JM, Poon LL, Lee KC, et al. Lung pathology of fatal severe acute respiratory syndrome. Lancet. 2003;361:1773–1778. doi: 10.1016/S0140-6736(03)13413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Denison MR. Severe acute respiratory syndrome coronavirus pathogenesis, disease and vaccines: an update. Pediatr. Infect. Dis. J. 2004;23:S207–S214. doi: 10.1097/01.inf.0000144666.95284.05. [DOI] [PubMed] [Google Scholar]
- 9.Peiris JS, Chu CM, Cheng VC. Clinical progression and viral load in a community outbreak of coronavirus-associated SARS pneumonia: a prospective study. Lancet. 2003;361:1767–1772. doi: 10.1016/S0140-6736(03)13412-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leung CW, Chiu WK. Clinical picture, diagnosis, treatment and outcome of severe acute respiratory syndrome (SARS) in children. Paediatr. Respir. Rev. 2004;5:275–288. doi: 10.1016/j.prrv.2004.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hon KL, Leung CW, Cheng WT, et al. Clinical presentations and outcome of severe acute respiratory syndrome in children. Lancet. 2003;361:1701–1703. doi: 10.1016/S0140-6736(03)13364-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ware LB, Matthay MA. The acute respiratory distress syndrome. N. Engl. J. Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. Extensive review in which the pathogenesis of acute respiratory distress syndrome is discussed
- 13. de Lang A, Baas T, Teal T, et al. Functional genomics highlights differential induction of antiviral pathways in the lungs of SARS-CoV-infected macaques. PLoS Pathog. 2007;3:e112. doi: 10.1371/journal.ppat.0030112. First paper in which functional genomics in combination with immunohistochemical techniques are used to study host responses in SARS-CoV-infected macaques
- 14.Zhang Y, Li J, Zhan Y, et al. Analysis of serum cytokines in patients with severe acute respiratory syndrome. Infect. Immun. 2004;72:4410–4415. doi: 10.1128/IAI.72.8.4410-4415.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang KJ, Su IJ, Theron M, et al. An interferon-γ-related cytokine storm in SARS patients. J. Med. Virol. 2005;75:185–194. doi: 10.1002/jmv.20255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reghunathan R, Jayapal M, Hsu LY, et al. Expression profile of immune response genes in patients with severe acute respiratory syndrome. BMC Immunol. 2005;6:2. doi: 10.1186/1471-2172-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong CK, Lam CW, Wu AK, et al. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004;136:95–103. doi: 10.1111/j.1365-2249.2004.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang Y, Xu J, Zhou C, et al. Characterization of cytokine/chemokine profiles of severe acute respiratory syndrome. Am. J. Respir. Crit. Care Med. 2005;171:850–857. doi: 10.1164/rccm.200407-857OC. [DOI] [PubMed] [Google Scholar]
- 19.Theron M, Huang KJ, Chen YW, Liu CC, Lei HY. A probable role for IFN-γ in the development of a lung immunopathology in SARS. Cytokine. 2005;32:30–38. doi: 10.1016/j.cyto.2005.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onoguchi K, Yoneyama M, Takemura A, et al. Viral infections activate types I and III interferon genes through a common mechanism. J. Biol. Chem. 2007;282:7576–7581. doi: 10.1074/jbc.M608618200. [DOI] [PubMed] [Google Scholar]
- 21.Yoneyama M, Kikuchi M, Matsumoto K, et al. Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J. Immunol. 2005;175:2851–2858. doi: 10.4049/jimmunol.175.5.2851. [DOI] [PubMed] [Google Scholar]
- 22.Andrejeva J, Childs KS, Young DF, et al. The V proteins of paramyxoviruses bind the IFN-inducible RNA helicase, MDA-5, and inhibit its activation of the IFN-β promoter. Proc. Natl Acad. Sci. USA. 2004;101:17264–17269. doi: 10.1073/pnas.0407639101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kato H, Takeuchi O, Sato S, et al. Differential roles of MDA5 and RIG-I helicases in the recognition of RNA viruses. Nature. 2006;441:101–105. doi: 10.1038/nature04734. [DOI] [PubMed] [Google Scholar]
- 24.Yoneyama M, Kikuchi M, Natsukawa T, et al. The RNA helicase RIG-I has an essential function in double-stranded RNA-induced innate antiviral responses. Nat. Immunol. 2004;5:730–737. doi: 10.1038/ni1087. [DOI] [PubMed] [Google Scholar]
- 25.Hiscott J. Triggering the innate antiviral response through IRF-3 activation. J. Biol. Chem. 2007;282:15325–15329. doi: 10.1074/jbc.R700002200. [DOI] [PubMed] [Google Scholar]
- 26.Fitzgerald KA, McWhirter SM, Faia KL, et al. IKKε and TBK1 are essential components of the IRF-3 signaling pathway. Nat. Immunol. 2003;4:491–496. doi: 10.1038/ni921. [DOI] [PubMed] [Google Scholar]
- 27.Paladino P, Cummings DT, Noyce RS, Mossman KL. The IFN-independent response to virus particle entry provides a first line of antiviral defense that is independent of TLRs and retinoic acid-inducible gene I. J. Immunol. 2006;177:8008–8016. doi: 10.4049/jimmunol.177.11.8008. [DOI] [PubMed] [Google Scholar]
- 28.Fitzgerald-Bocarsly P, Dai J, Singh S. Plasmacytoid dendritic cells and type I IFN: 50 years of convergent history. Cytokine Growth Factor Rev. 2008;19:3–19. doi: 10.1016/j.cytogfr.2007.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Prakash A, Smith E, Lee CK, Levy DE. Tissue-specific positive feedback requirements for production of type I interferon following virus infection. J. Biol. Chem. 2005;280:18651–18657. doi: 10.1074/jbc.M501289200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber F, Wagner V, Rasmussen SB, Hartmann R, Paludan SR. Double-stranded RNA is produced by positive-strand RNA viruses and DNA viruses but not in detectable amounts by negative-strand RNA viruses. J. Virol. 2006;80:5059–5064. doi: 10.1128/JVI.80.10.5059-5064.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cinatl J, Jr, Hoever G, Morgenstern B, et al. Infection of cultured intestinal epithelial cells with severe acute respiratory syndrome coronavirus. Cell. Mol. Life Sci. 2004;61:2100–2112. doi: 10.1007/s00018-004-4222-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Spiegel M, Pichlmair A, Martinez-Sobrido L, et al. Inhibition of B interferon induction by severe acute respiratory syndrome coronavirus suggests a two-step model for activation of interferon regulatory factor 3. J. Virol. 2005;79:2079–2086. doi: 10.1128/JVI.79.4.2079-2086.2005. Demonstrates that SARS-CoV interferes with the activation of interferon regulatory factor-3, preventing the induction of type-I interferons
- 33.Tang BS, Chan KH, Cheng VC, et al. Comparative host gene transcription by microarray analysis early after infection of the Huh7 cell line by severe acute respiratory syndrome coronavirus and human coronavirus 229E. J. Virol. 2005;79:6180–6193. doi: 10.1128/JVI.79.10.6180-6193.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Snijder EJ, van der Meer Y, Zevenhoven-Dobbe J, et al. Ultrastructure and origin of membrane vesicles associated with the severe acute respiratory syndrome coronavirus replication complex. J. Virol. 2006;80:5927–5940. doi: 10.1128/JVI.02501-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kopecky-Bromberg SA, Martinez-Sobrido L, Frieman M, Baric RA, Palese P. Severe acute respiratory syndrome coronavirus open reading frame (ORF) 3b, ORF 6, and nucleocapsid proteins function as interferon antagonists. J. Virol. 2007;81:548–557. doi: 10.1128/JVI.01782-06. SARS-CoV proteins ORF3b and ORF6 actively inhibit interferon signaling
- 36.Versteeg GA, Bredenbeek PJ, van den Worm SH, Spaan WJ. Group 2 coronaviruses prevent immediate early interferon induction by protection of viral RNA from host cell recognition. Virology. 2007;361:18–26. doi: 10.1016/j.virol.2007.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Frieman M, Heise M, Baric R. SARS coronavirus and innate immunity. Virus Res. 2008;133:101–112. doi: 10.1016/j.virusres.2007.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu SY, Hu YW, Liu XY, Xiong W, Zhou ZT, Yuan ZH. Gene expression profiles in peripheral blood mononuclear cells of SARS patients. World J. Gastroenterol. 2005;11:5037–5043. doi: 10.3748/wjg.v11.i32.5037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jones BM, Ma ES, Peiris JS, et al. Prolonged disturbances of in vitro cytokine production in patients with severe acute respiratory syndrome (SARS) treated with ribavirin and steroids. Clin. Exp. Immunol. 2004;135:467–473. doi: 10.1111/j.1365-2249.2003.02391.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cameron MJ, Ran L, Xu L, et al. Interferon-mediated immunopathological events are associated with atypical innate and adaptive immune responses in patients with severe acute respiratory syndrome. J. Virol. 2007;81:8692–8706. doi: 10.1128/JVI.00527-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cervantes-Barragan L, Zust R, Weber F, et al. Control of coronavirus infection through plasmacytoid dendritic-cell-derived type I interferon. Blood. 2007;109:1131–1137. doi: 10.1182/blood-2006-05-023770. Human plasmacytoid dendritic cells, but not myeloid dendritic cells, produce type-I interferon upon SARS-CoV infection
- 42.Ziegler T, Matikainen S, Ronkko E, et al. Severe acute respiratory syndrome coronavirus fails to activate cytokine-mediated innate immune responses in cultured human monocyte-derived dendritic cells. J. Virol. 2005;79:13800–13805. doi: 10.1128/JVI.79.21.13800-13805.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Castilletti C, Bordi L, Lalle E, et al. Coordinate induction of IFN-α and -γ by SARS-CoV also in the absence of virus replication. Virology. 2005;341:163–169. doi: 10.1016/j.virol.2005.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Law HK, Cheung CY, Ng HY, et al. Chemokine up-regulation in SARS-coronavirus-infected, monocyte-derived human dendritic cells. Blood. 2005;106:2366–2374. doi: 10.1182/blood-2004-10-4166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tseng CT, Perrone LA, Zhu H, Makino S, Peters CJ. Severe acute respiratory syndrome and the innate immune responses: modulation of effector cell function without productive infection. J. Immunol. 2005;174:7977–7985. doi: 10.4049/jimmunol.174.12.7977. [DOI] [PubMed] [Google Scholar]
- 46.Spiegel M, Schneider K, Weber F, Weidmann M, Hufert FT. Interaction of severe acute respiratory syndrome-associated coronavirus with dendritic cells. J. Gen. Virol. 2006;87:1953–1960. doi: 10.1099/vir.0.81624-0. [DOI] [PubMed] [Google Scholar]
- 47.Ito T, Kanzler H, Duramad O, Cao W, Liu YJ. Specialization, kinetics, and repertoire of type 1 interferon responses by human plasmacytoid predendritic cells. Blood. 2006;107:2423–2431. doi: 10.1182/blood-2005-07-2709. [DOI] [PubMed] [Google Scholar]
- 48.Kumagai Y, Takeuchi O, Kato H, et al. Alveolar macrophages are the primary interferon-α producer in pulmonary infection with RNA viruses. Immunity. 2007;27:240–252. doi: 10.1016/j.immuni.2007.07.013. [DOI] [PubMed] [Google Scholar]
- 49.Uddin S, Platanias LC. Mechanisms of type-I interferon signal transduction. J. Biochem. Mol. Biol. 2004;37:635–641. doi: 10.5483/bmbrep.2004.37.6.635. [DOI] [PubMed] [Google Scholar]
- 50.Kamitani W, Narayanan K, Huang C, et al. Severe acute respiratory syndrome coronavirus nsp1 protein suppresses host gene expression by promoting host mRNA degradation. Proc. Natl Acad. Sci. USA. 2006;103:12885–12890. doi: 10.1073/pnas.0603144103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kopecky-Bromberg SA, Martinez-Sobrido L, Palese P. 7a protein of severe acute respiratory syndrome coronavirus inhibits cellular protein synthesis and activates p38 mitogen-activated protein kinase. J. Virol. 2006;80:785–793. doi: 10.1128/JVI.80.2.785-793.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Frieman M, Yount B, Heise M, Kopecky-Bromberg SA, Palese P, Baric RS. Severe acute respiratory syndrome coronavirus ORF6 antagonizes STAT1 function by sequestering nuclear import factors on the rough endoplasmic reticulum/Golgi membrane. J. Virol. 2007;81:9812–9824. doi: 10.1128/JVI.01012-07. Describes one of the mechanisms by which SARS-CoV inhibits interferon signaling in detail.
- 53.Cinatl J, Morgenstern B, Bauer G, Chandra P, Rabenau H, Doerr HW. Treatment of SARS with human interferons. Lancet. 2003;362:293–294. doi: 10.1016/S0140-6736(03)13973-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.de Lang A, Osterhaus AD, Haagmans BL. Interferon-γ and interleukin-4 downregulate expression of the SARS coronavirus receptor ACE2 in Vero E6 cells. Virology. 2006;353:474–481. doi: 10.1016/j.virol.2006.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Haagmans BL, Kuiken T, Martina BE, et al. Pegylated interferon-α protects type 1 pneumocytes against SARS coronavirus infection in macaques. Nat. Med. 2004;10:290–293. doi: 10.1038/nm1001. Paper showing that IFN-α reduces viral replication and pulmonary damage in SARS-CoV-infected macaques.
- 56.Loutfy MR, Blatt LM, Siminovitch KA, et al. Interferon alfacon-1 plus corticosteroids in severe acute respiratory syndrome: a preliminary study. JAMA. 2003;290:3222–3228. doi: 10.1001/jama.290.24.3222. [DOI] [PubMed] [Google Scholar]
- 57.Hogan RJ, Gao G, Rowe T, et al. Resolution of primary severe acute respiratory syndrome-associated coronavirus infection requires stat1. J. Virol. 2004;78:11416–11421. doi: 10.1128/JVI.78.20.11416-11421.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baas T, Roberts A, Teal TH, et al. Genomic analysis reveals age-dependent innate immune responses to severe acute respiratory syndrome coronavirus. J. Virol. 2008;82:9465–9476. doi: 10.1128/JVI.00489-08. [DOI] [PMC free article] [PubMed] [Google Scholar]