Abstract
We examined the effects of 1,25 dihydroxyvitamin D3 (1,25(OH)2D3) on the distribution and mobility of the vitamin D receptor (VDR) in the enterocyte-like Caco-2 cell. Confocal microscopy showed that a green fluorescent protein-vitamin D receptor (GFP-VDR) fusion protein is predominantly nuclear (58%) and it does not associate with the apical or basolateral membrane of proliferating or polarized, differentiated cells. In contrast to the previously studied cell types, neither endogenous VDR nor GFP-VDR levels accumulate in the nucleus following 1,25(OH)2D3 treatment (100 nM, 30 min). However, in nuclear photobleaching experiments nuclear GFP-VDR import was significantly increased by 1,25(OH)2D3 during both an early (0–5 min) and later (30–35 min) period (20% per 5 min). Compared to the natural ligand, nuclear import of GFP-VDR was 60% lower in cells treated with the 1,25(OH)2D3 analog, 1-alpha-fluoro-16-ene- 20-epi-23-ene-26,27-bishomo-25-hydroxyvitamin D3 (Ro-26-9228, 5 min, 100 nM). Downstream events like ligand-induced association of VDR with chromatin at 1 h and the accumulation of CYP24 mRNA were significantly lower in Ro- 26-9228 treated cells compared to 1,25(OH)2D3 (60 and 95% lower, respectively). Collectively our data are consistent with a role for ligand-induced nuclear VDR import in receptor activation. In addition, ligand-dependent VDR nuclear import appears to be balanced by export, thus accounting for the lack of nuclear VDR accumulation even when VDR import is significantly elevated.
Keywords: intestine; nuclear trafficking; 1,25 dihydroxyvitamin D3
Intestinal calcium absorption is a critical step in the maintenance of calcium homeostasis [Fleet, 2006]. It occurs by both paracellular and transcellular pathways [Bronner, 2003; Hoenderop et al., 2005]. The transcellular calcium absorption pathway is an active process that is regulated by the active form of vitamin D, 1,25 dihydroxyvitamin D3 (1,25(OH)2D3). 1,25 (OH)2D3 increases efficiency of transcellular Calcium absorption by increasing the abundance of critical proteins: calbindin D9k (CaBPD9k), the transient receptor vanelloid family member 6 (TRPV6), and the plasma membrane calcium ATPase 1b (PMCA1b) [Fleet et al., 2002; Song et al., 2003b; Fleet, 2006].
The actions of 1,25(OH)2D3 are mediated by the vitamin D receptor (VDR), a transcription factor that belongs to nuclear hormone receptor superfamily [Haussler et al., 1998]. The deletion of VDR in the enterocyte results in a 70% reduction in intestinal calcium absorption efficiency that is coincident with a significant reduction in CaBPD9k, TRPV6, and PMCA1b gene expression [Van Cromphaut et al., 2001; Song et al., 2003a].Previous research shows that binding of 1,25(OH)2D3 to VDR in the cytoplasm of cells stimulates heterodimerization of VDR with RXR and the redistribution of the VDR-RXR- hormone complex to the nucleus [Barsony et al., 1990; Michigami et al., 1999; Racz and Barsony, 1999; Sunn et al., 2001]. The nuclear import of the VDR-RXR-hormone complex is active [Racz and Barsony, 1999;Miyauchi et al., 2005], enhanced by 1,25(OH)2D3 treatment [Michigami et al., 1999; Racz and Barsony, 1999; Sunn et al., 2001], dependent upon the presence of intact nuclear localization sequences (NLSs) in both VDR and RXR [Prufer et al., 2000], and requires various importins [Miyauchi et al., 2005; Yasmin et al., 2005]. Most of the studies examining the mechanism of VDR nuclear import have been conducted in COS-7 kidney cells (characterized by low expression of endogenous VDR) and some characteristics of VDR import have been confirmed in cells from bone, skin, or kidney. In contrast the impact of 1,25(OH)2D3 treatment on VDR distribution has not been examined in absorptive enterocytes, a primary target cell of 1,25(OH)2D3 action.
In this article we have examined the nucleocytoplasmic trafficking of VDR receptor in proliferating and differentiated Caco-2 cells. Caco-2 cells are an intestinal cell line that spontaneously differentiates and recapitulates many of the features of the absorptive epithelial cell of the small intestine, including vitamin D regulated intestinal calcium absorption [Giuliano and Wood, 1991; Fleet et al., 2002]. We find that VDR nuclear import occurs under basal conditions and that import is accelerated by 1,25(OH)2D3. This process is not influenced by the state of cellular differentiation. Our data also suggest that 1,25(OH)2D3-induced nuclear import is balanced by nuclear export in Caco-2 cells. In addition, our data are consistent with an essential role for nuclear import of VDR in the genomic responses of enterocytes to 1,25(OH)2D3.
EXPERIMENTAL PROCEDURES
Supplies
Unless otherwise noted, all chemicals were obtained from Sigma (St. Louis, MO), cell culture reagent were obtained from Cambrex (Rockland, ME), and cell culture plasticware from Corning-Costar (Cambridge, MA). 1,25 (OH)2D3 was purchased from Biomol International (Plymouth Meeting, PA). The 1,25 (OH)2D3 analog, 1α-fluoro-16-ene-20-epi-23-ene-26,27-bishomo-25-hydroxyvitamin D3 (Ro-26-9228) was kindly provided by Dr. Milan Uskokovic (Roche Bioscience) and its biological actions were previously characterized by Ismail et al. [2004]. 1,25(OH)2D3 and Ro-26-9228 were dissolved in ethanol and kept in lightprotected vials at −80°C. The green fluorescent protein-vitamin D receptor (GFP-VDR) construct used was previously described by Prufer et al. [2000].
Cell Culture
The parental Caco-2 line and the BBe clone of Caco-2 cells were purchased from American Type Cell Culture (HTB-37 and CRL-2102, respectively; ATCC, Rockville, MD). Parental Caco-2 cells were studied between passages 25 and 50, whereas BBe cells were studied between passages 52 and 77. The cells were maintained as described elsewhere [Fleet et al., 2002]. In the experiments reported in this article, we used Caco-2 cells at three different stages of cell differentiation: proliferating, 50% confluent (2-day cells), 2-day post-confluent (6-day cells), and differentiated Caco-2 cells (15-day cells). The parental Caco-2 cell line was used in experiments 1 and 5, while BBe clone was used in experiments 2, 3, and 4 described below. Our previous research demonstrates that both of these Caco-2 lines have all the components necessary for vitamin D regulated transcellular calcium transport [Fleet et al., 2002]. In addition, our initial experiments showed that GFP-VDR distribution and characteristics of VDR trafficking are identical in parental Caco-2 and BBe Caco-2. We chose to use the BBe clone of Caco-2 cells for our imaging analysis reported here because this clone is less morphologically heterogeneous than parental Caco-2 cell line [Peterson and Mooseker, 1992].
The rat osteosarcoma cell line, ROS 17/2.8 (A1G clone), was obtained from Dr. Hector DeLuca (University of Wisconsin, Madison) [Arbour et al., 1998]. ROS 17/2.8 (A1G) cells are stably transfected with a 25-hydroxyvitamin D3 24-hydroxylase (CYP24)-Luc reporter gene but are otherwise like the parent ROS 17/2.8 line. Cells were maintained in 1:1 mixture of Ham’s Nutrient Mixture F12 (Ham’s F12) and high-glucose Dulbecco’s Modified Eagle’s Medium (DMEM) supplemented with 100 U/L of penicillin, 100 µg/L of streptomycin, 1 mM sodium pyruvate, 100 µM non-essential amino acids, 50 µg/L gentamycin, 2 mM L-glutamine, 10 mM N-2-hydroxyethylpiperazine-N-2-ethanesulfonic acid (HEPES), and 10% fetal bovine serum (FBS). ROS 17/2.8 (A1G) cells were passaged by trypsinization every 3–4 days when the cells were 80% confluent. All experiments were performed in subconfluent ROS 17/2.8 (A1G) cells between passages 3 and 28.
Mouse prostate epithelial cells from VDR knockout mice (MPEC VDR KO cells) were obtained from Dr. Scott Cramer (Wake Forest University Medical School) and maintained as described elsewhere [Barclay and Cramer, 2005].
Experimental Design
Experiment 1. Analysis of changes in endogenous VDR protein abundance and distribution
Two experiments were conducted. First, 15-day cultures of parental Caco- 2 cells and proliferating ROS 17/2.8 cells were treated with 100 nM 1,25(OH)2D3 for 1 h and whole cell extracts were prepared as described previously [Ismail et al., 2004]. Next, 15-day cultures of Caco-2 cells treated for 2 h with 10 nM 1,25(OH)2D3 or vehicle (0.01% ethanol) afterwards whole cell and nuclear extracts were prepared using the Active Motif Nuclear Extract Kit (Active Motif, Carlsbad, CA). The total protein concentration in the samples was determined using BioRad protein assay (Hercules, CA). The samples containing 20 µg of total protein were analyzed for VDR and general transcription factor II B(TFIIB) protein levels as described previously [Fleet et al., 2002]. The VDR protein level was normalized to TFIIB protein content in each sample to correct for protein loading. Normalized VDR protein levels were expressed relative to vehicle-treated samples. Three independent experiments were performed; each experiment used three replicates per treatment.
Experiment 2. Baseline distribution of GFP-VDR in Caco-2 and ROS 17/2.8 (A1G) cells
Proliferating (2 day) Caco-2 cells, differentiated (15 day) Caco-2 cells, and proliferating ROS 17/2.8 (A1G) cells were transiently transfected with GFP-VDR expression vector as described below. For each cell type, initial confocal images were captured in the focal plane of the cell where the circumference of the nucleus was the greatest. Additionally, we collected sequential optical sections of the cells and reconstructed 3-D images in order to examine the possibility that VDR may accumulate at specific cellular regions, for example, basolateral or brush bordermembrane of Caco-2 cells. At least 11 3-D reconstructions were prepared for ROS17/2.8 (A1G) and proliferating and differentiating Caco-2 cells.
Experiment 3. The effect of 1,25(OH)2D3 on steady state distribution of GFPVDR in Caco-2 and ROS 17/2.8 (A1G) cells
Proliferating Caco-2 and ROS17/2.8 (A1G) cells were transiently transfected with GFP-VDR. For each cell, we collected series of two images: a baseline distribution of GFP-VDR and the cellular distribution of GFP-VDR 30 min after treatment with either 100 nM 1,25(OH)2D3 or control solution (0.01% ethanol). For each image in the series we calculated the intensity of fluorescent signal associated within the nuclear and cytoplasmic compartment. Six cells were examined for each cell type and each treatment. Procedures for the analysis of each image are described in detail below in ‘Image collection and data analysis’.
Experiment 4. The effect of 1,25(OH)2D3 on GFP-VDR movement into the nucleus
Time course of GFP-VDR import in Caco-2 cells following 1,25(OH)2D3 treatment
To study the kinetics of GFP-VDR movement into the nucleus of BBe, we collected a series of nine images of vehicle (0.01% ethanol) or 1,25(OH)2D3 (100 nM) treated cells. After collecting a baseline image, an 8 µm2 area within nucleus was photobleached using 100% laser power for 10 s and an image showing a bleached nucleus was recorded. Immediately after photobleaching the treatment solution containing either 100 nM 1,25(OH)2D3 or vehicle was added to the well and a series of post-bleach images was recorded at 2.5, 5, 10, 15, 20, 25, and 30 min using the same laser intensity as for the post-photobleaching image. Six cells were examined for each cell type and each treatment. Procedures for the analysis of each image are described in detail below in ‘Image collection and data analysis’.
Early and late fluxes of GFP-VDR into the nucleus
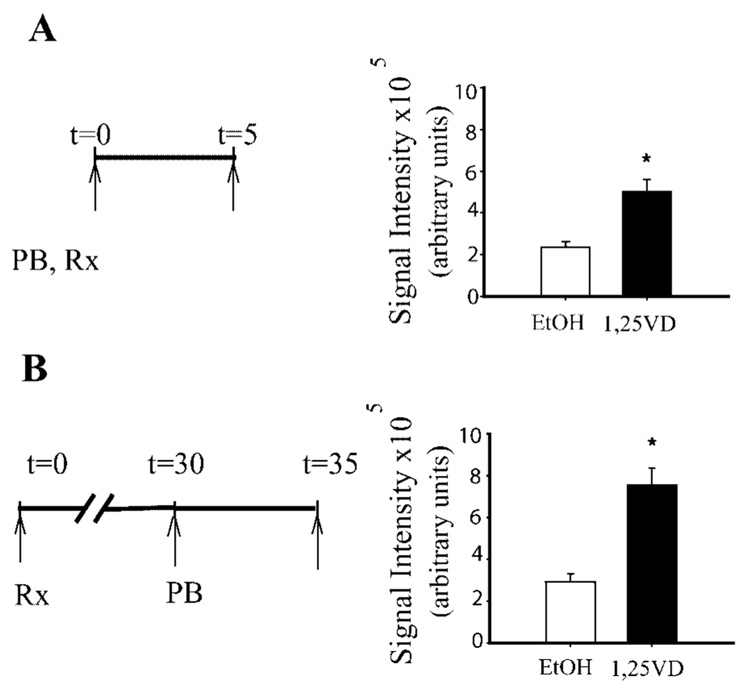
To determine whether the early flux of GFP-VDR after 1,25(OH)2D3 treatment continues throughout the hormone treatment period, we compared the effect of 1,25(OH)2D3 on nuclear import under two different protocols. The first was identical to that described above (photobleaching at t=0, treatment with 1,25(OH)2D3, examination of import at t=5 min). For the second protocol 1,25(OH)2D3 treatment was initiated at t=0 min and photobleaching of the cell nucleus was conducted 30 min afterwards. The post-bleach image was collected after 5 min recovery time. Six cells were examined for each cell type and each treatment. Procedures for the analysis of each image are described in detail below in ‘Image collection and data analysis’.
Control experiments for photobleaching
To evaluate potential confounding influences of the photobleaching protocol used in this experiment weconducted two control experiments. In the first experiment we used a propidium iodide (PI) (Molecular Probes, Inc.) exclusion test to ensure that our photobleaching conditions do not compromise the viability of the cells. In this test, Caco-2 cells were incubated with 2 µg/ml PI for 5min at 37°C. Following the incubation period, the cells were photobleached as described above. Images were collected over a 30 min period to determine whether the photobleaching technique induced cell death and PI staining of the nuclei. As expected, photobleaching did not increase cell permeability to PI. The second control experiment was conducted to ensure that any increase in fluorescence signal observed during our experiments is not due to de novo synthesis of proteins. To examine this, we completely photobleached the target cell and monitored the changes in fluorescent signal intensity of this cell within next 30 min. As expected no fluorescence signal was observed after 30 min demonstrating that the photobleached fluorochrome is destroyed and that the changes in fluorescent signal intensity we see are not due to de novo protein synthesis of GFP-VDR.
Experiment 5. Ability of VDR ligand to stimulate nuclear import of GFP-VDR correlates with its transcriptional efficacy
The goal of our final experiment was to compare the effects of 1,25(OH)2D3 and its analog, Ro-26-9228 on three functional parameters of VDR action: nuclear import, association of VDR with chromatin, and gene expression in 2-day post-confluent parental Caco-2 cells. In the first part of this experiment, we determined the effect of two VDR ligands on GFP-VDR nuclear import using our established protocol. Briefly, photobleaching of the cell nucleus was followed by immediate addition of treatment solution containing 100 nM 1,25(OH)2D3, 100 nM Ro-26-9228 or vehicle. The post-bleach image was recorded 5 min after photobleaching. For each treatment group at least six image series were quantified as described in ‘Image collection and data analysis Section’. In the second part of this experiment, we examined the impact of 1,25(OH)2D3 treatment on the association of VDR with chromatin. Caco-2 cells (2-day post-confluent) were treated for 1 h with 100nM 1,25(OH)2D3, Ro-26-9228, or vehicle. Chromatin fractions were isolated and VDR association with chromatin was assessed as described previously [Ismail et al., 2004]. In the final experiment in this section we examined the transcriptional response of the CYP24 gene to 100 nM1,25(OH)2D3 and 100nM Ro-26-9228. Two days after reaching confluency Caco-2 cells were treated with 5% FBS containing DMEM with 100 nM 1,25(OH)2D3, 100 nM Ro-26-9228, or vehicle (0.01% ethanol). To better evaluate the effect of two ligands on induction of CYP24 gene expression we used two complementary experimental designs. In the first a 5 min-pulse with treatment solutions was followed by 7 h 55 min incubation with 5% FBS media to allow accumulation of the message (n=3 per treatment). In the second design, cells were continually treated with vitamin D compounds for 8 h prior to harvest (n=3 per treatment). Cells were harvested into 1 ml of Tri-Reagent and RNA was isolated following the manufacturer’s instructions (Molecular Research Center, Cincinnati, OH). The real-time-polymerase chain reaction (PCR) was conducted as described elsewhere [Song et al., 2003b] using the primers: CYP24 forward, CTCATGCTAAATACCCAGGTG, CY P24 reverse, TCGCTGGCAAAACGCGATGGG, GAPDH forward 5'-TCACCATCTTC CAGGAGCG-3', GAPDH reverse 5'-CTGCTTC ACCACCTTCTTGA-3' and common for both primer sets annealing temperature of 54°C. The GAPDH mRNA levels were used as an internal control because expression of this gene does not change in response to 1,25(OH)2D3 treatment. Transfection of cells with GFP-VDR. We used two protocols depending on whether we wanted to image proliferating or post-confluent cultures. In proliferating cells, 1 million cells in suspension were transiently transfected immediately following trypsinization using 6 µg of GFP-VDR vector and a 1:2.6:2.6 ratio of DNA/Lipofectamine/Plus Reagent. The cells were then seeded at a density of approximately 30,000 cells per well in an 8-chambered glass coverslip (Nalge Nunc Intl., Rochester, NY). For post-confluent non-proliferating Caco-2 cells an alternate protocol was needed to overcome the low transfection efficiency in non-proliferating cells. For this study, cells were seeded at the density of 21,333 cell per well of an 8-chamber glass coverslip and each well was transfected with 4 µg of GFP-VDR at either 2 or 9 days post-confluence using 1:1:1 ratio of DNA/Lipofectamine/Plus Reagent. With both protocols, cells were used for experiments 2 days after transfection.
Cell culture treatments
In order to avoid the effect of estrogenic agonists present in phenol red (PR) and serum on VDR distribution in living cells [Barsony et al., 1990], the cells transfected with GFP-VDR were routinely switched from their usual medium (2-day Caco-2: DMEM + 20% FBS; post-confluent Caco-2: DMEM + 10% FBS; ROS 17/2.8 (A1G) cells: Ham’s F12/DMEM + 10% FBS) to PR-free medium plus 1% insulin–transferrin–selenium (ITS, Invitrogen, Carlsbad, CA) at least 24 h prior imaging session. The treatment solutions containing 100nM 1,25(OH)2D3, 100 nM Ro-26-9228 or vehicle (ethanol at final concentration of 0.01%) were prepared in this medium and kept at 37°C water bath during imaging sessions.
Image Analysis
Criteria of choosing cells for imaging
Prior to each imaging session we evaluated cell morphology to ensure that cells were viable and healthy. Only cells that had no obvious disruption in their normal morphology were used for imaging. In addition, we avoided using cells with a low fluorescence signal as it would prevent us to use them in our protocols designed to monitoring import of GFP-VDR into the nucleus (where significant signal is lost during initial photobleaching and additional photobleaching occurs during the collection of the subsequent image collection). We also excluded cells of extremely high intensity of the signal since excessive expression of GFP-VDR construct may not reflect physiologic conditions.
Image Collection and Data Analysis
All images in this study were collected using a BioRad MRC 1024 system and the Lasersharp software package (BioRad Laboratories, Richmond, CA). BioRadMRC1024 is equipped with a water-cooled, coherent Innova Enterprise Model 622 Argon Ion UV/VIS laser, a Krypton–Argon Model 5470K laser, a Nikon Diaphot 300 inverted microscope (Nikon Corp., Tokyo, Japan), a Plan APO 60X 1.4 DIC oil objective and a heated stage. All images were collected with the heated stage set at 37°C. To detect GFP-VDR fluorescence, the 488 nm line of a krypton–argon laser was used for excitation of the sample with a bandpass 522/35 emission filter. In PI exclusion test we used the 488 nm krypton–argon laser line with a 605/32 emissions filter.
The creation of 3-D reconstructions from confocal images was accomplished using the MetaMorph software program (Molecular Devices Corporation, Sunnyvale, CA). The quantification of captured images was conducted using Adobe Photoshop (Version 6.0, Adobe System Inc., San Jose, CA).
Measurements of total, nuclear, and cytosolic GFP-VDR content were made for each cell using the histogram function to calculate the total number of pixels and the mean signal intensity per pixel. Intensity of fluorescent signal associated with each cellular compartment was calculated by multiplying the number of pixels by the mean signal intensity per pixel. Fluorescent signal intensity was used to quantitate data from all of our experiments. In experiments 2 and 3 we directly used values of fluorescent signal intensity to determine sub-cellular distribution of GFP-VDR within the nucleus and cytoplasm. In experiment 3, these values were used to calculate the percentage change in fluorescent signal intensity associated with nucleus of cells from baseline (image 1) due to treatment (image 2). In experiments 4 and 5, we normalized the total fluorescent signal intensity of each cell to a relative value of 5×106 and nuclear fluorescent signal intensity after photobleaching was assigned a value equal to 0. The absolute change in nuclear fluorescent signal intensity over time was calculated for each experimental condition. In addition, we determined the efficiency of photobleaching for each image series. For data quantification we used only those image series for which efficiency of photobleaching was greater than 50%.
Analysis of GFP-VDR Functionality in MPEC VDR KO Cells Using Reporter Gene Assay
MPEC VDR KO cells were transiently cotransfected with 5 µg of rat −298 to +74 bp 24-hydroxylase promoter luciferase gene construct [Kerry et al., 1996], 50 ng of pRL-CMV (Renilla expressing vector for assessing transfection efficiency, Promega, Madison, WI) and 5 µg of a VDR expression vector (GFP-VDR or pCR3-VDR) or pCR3.1-CAT (negative control) using the Lipofectamine Plus procedure (1:4:10 DNA/Lipofectamine/Plus reagent, Invitrogen). Eighteen hours after transfection MPEC VDR KOcells were treated with 100nM1,25(OH)2D3 or vehicle for 8 h. The cells were harvested and luciferase activity assay was measured as per the procedures in the Promega Dual Luciferase Assay (Promega).
Statistical Analysis
Data are reported as the means ± the standard error of the mean. The treatment effects in each experiment were compared by one-way ANOVA using the SYSTAT statistical software package (SYSTAT 7.0, Chicago, IL). Pairwise comparisons were conducted when appropriate using Fisher’s Protected LSD. Differences between means were considered significant at P<0.05.
RESULTS
Analysis of Changes in Endogenous VDR Protein Abundance in Response to 1,25(OH)2D3
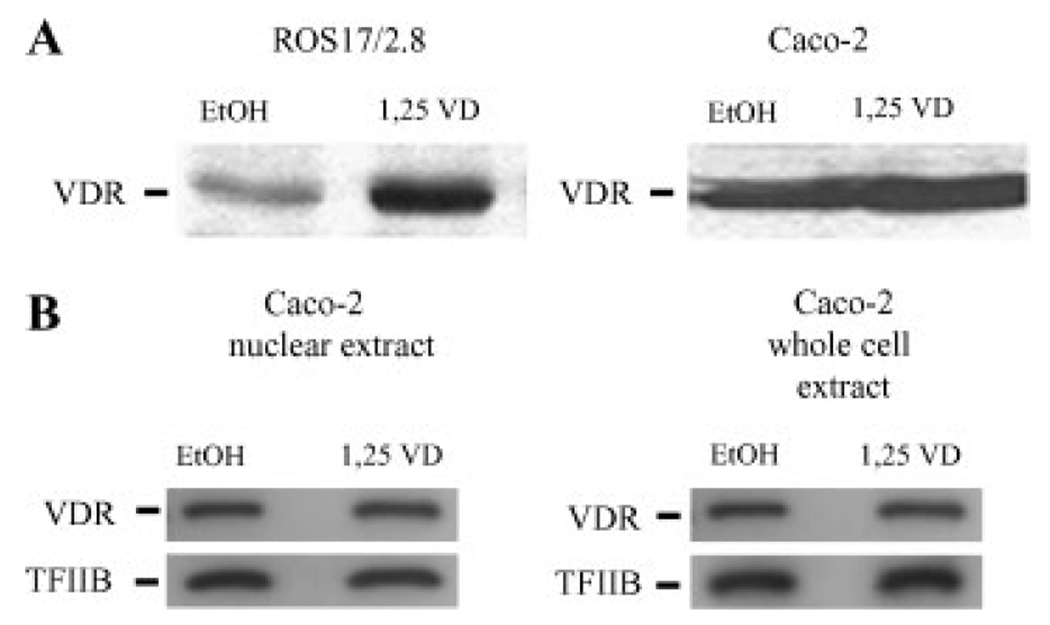
Using standard immunoblotting techniques VDR protein levels were elevated threefold by 1,25(OH)2D3 treatment in ROS cells following 1 h incubation with 100 nM 1,25(OH)2D3 (Fig. 1A). In contrast we did not observe an increase in endogenous VDR protein abundance in parental Caco-2 cells. In addition, Figure 1B shows that ligand does not significantly alter the proportion of endogenous VDR in the nuclear extracts (1.2±0.2 after vitamin D treatment vs. 1.0±0.16 arbitrary units for control, P=0.4299, n=9) or in the whole cell extracts (P=0.9768, n=9) from Caco-2 cells, even though we have previously shown that this dose can activate CYP24 and TRPV6 mRNA accumulation in Caco-2 cells [Fleet et al., 2002].
Fig. 1.
The effect of 1,25(OH)2D3 on endogenous VDR in Caco-2 and ROS 17/2.8 cells. (A) ROS 17/2.8 and parental Caco-2 cells were treated with either 100 nM 1,25(OH)2D3 (1,25 VD) or vehicle (EtOH) for 1 h and VDR protein levels were determined in whole cell extracts by Western blot analysis. (B) Caco-2 cells were treated with either 10 nM 1,25(OH)2D3 (1,25 VD) or vehicle (EtOH) for 2 h. Whole cell and nuclear extracts were examined for VDR protein levels by Western blot analysis. The figures are representative blots of individual samples from experiments with n=3 replicates; experiments were conducted three times.
The Baseline and 1,25(OH)2D3-Induced Effects on the Distribution of GFP-VDR in BBe and ROS17/2.8 (A1G) Cells
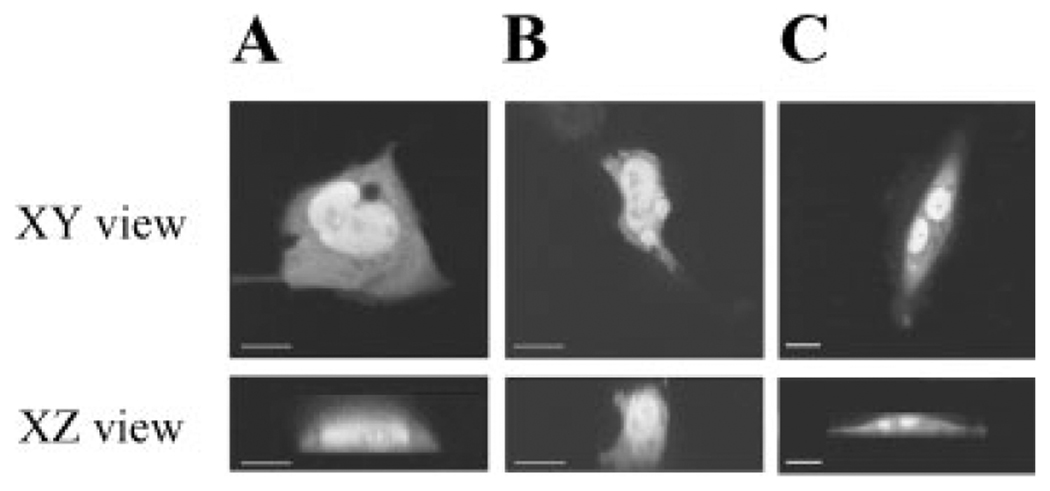
Using a transcriptionally functional GFP-VDR construct (i.e., it restored vitamin D-inducible CYP24 promoter activity in VDR null cells, data not shown) we examined basal and 1,25(OH)2D3-induced effects on the subcellular distribution of GFP-VDR in proliferating BBe and ROS17/2.8 (A1G) cells. Figure 2 shows that without hormone, GFP-VDR was evenly distributed within the cytoplasm and nucleus of BBe and ROS 17/2.8 (A1G) cells. Consistent with previous reports (Racz and Barsony, 1999) the nucleoli were devoid of GFP-VDR signal. No GFP-VDR signal was associated with either the apical or basolateroal membranes.
Fig. 2.
The distribution of GFP-VDR in proliferating BBe cells (A), differentiated BBe cells (B), and ROS 17/2.8 (AIG) cells (C). Confocal images of GFP-VDR distribution in a single optical section through the center of the cells in two axes: the XY plane and the XZ plane (reconstructed from multiple images in the XY plane).
Quantitative analysis revealed significant differences in partitioning of GFP-VDR between cytoplasm and nucleus between BBe andROS17/2.8 (A1G) cells. In untreated Caco-2 cells, the GFP-VDR is equally distributed in the nuclear and cytoplasmic compartments (Table I). In contrast, the majority of GFP-VDR resides in the cytoplasm of ROS17/2.8 (A1G) cells and only a small portion is associated with the nucleus (28.48±1.71%). Proliferating and differentiated Caco-2 cells had similar amount of nuclear GFP-VDR indicating that the process of differentiation to the absorptive phenotype did not affect the subcellular distribution of GFP-VDR.
TABLE I.
The distribution of GFR-VDR in BBe and ROS 17/2.8 (A1G) cells
| Cell type | % Nuclear | % Cytosolic |
|---|---|---|
| ROS 17/2.8 | 28.48±1.71 | 71.51±1.71 |
| BBe (2, days-culture) | 53.93±2.22 | 46.06±2.22 |
| BBe (15, days-culture) | 51.43±2.52 | 48.57±2.52 |
Confocal images from transiently transfected cells were quantified and analyzed as described in the Materials and Methods section. The data represent mean ± SEM of the fluorescent signal intensity associated with nucleus and cyptolasm (n = 2 or more per cell type).
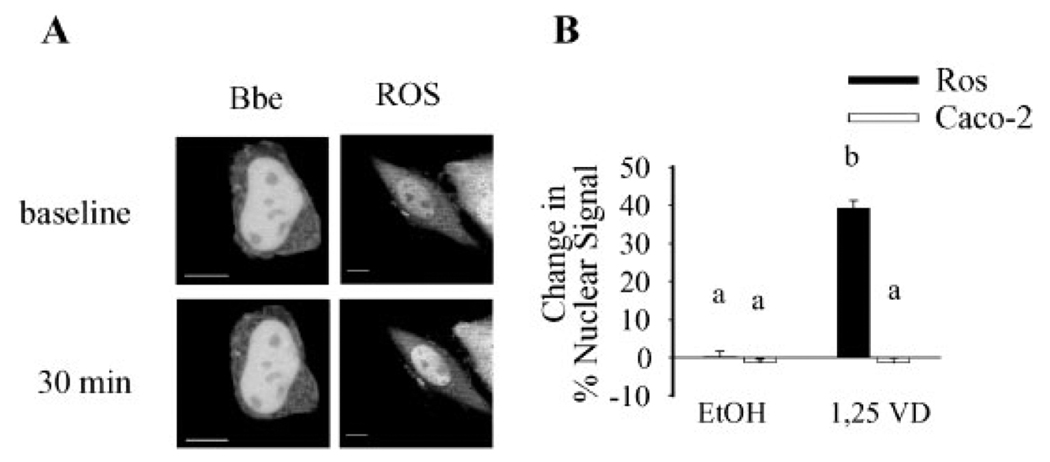
In ROS 17/2.8 (A1G) cells treatment of cells with 100 nM 1,25(OH)2D3 for 30 min increased the percentage of nuclear GFP-VDR from 28 to 37%. Treatment with 100 nM 1,25(OH)2D3 did not increase nuclear accumulation nor did it reduce the cytoplasmic signal of GFP-VDR in BBe cells (Fig. 3).
Fig. 3.
Calcitriol causes significant changes in distribution of GFP-VDR in ROS 17/2.8 but not in BBe cells. The images of proliferating BBe cells or ROS17/2.8 (A1G) cells were taken before (baseline) and 30 min after incubation with 100 nM 1,25(OH)2D3. (A) Representative confocal images. (B) Image analysis of n=6 cells per cell type and treatment. The change in % signal intensity was calculated as described in Materials and Methods Section. Data represent mean±SE. Values with different superscripts are significantly different from one another (P<0.05, n=6 cells per treatment).
The Effect of 1,25(OH)2D3 on Nuclear Import of GFP-VDR
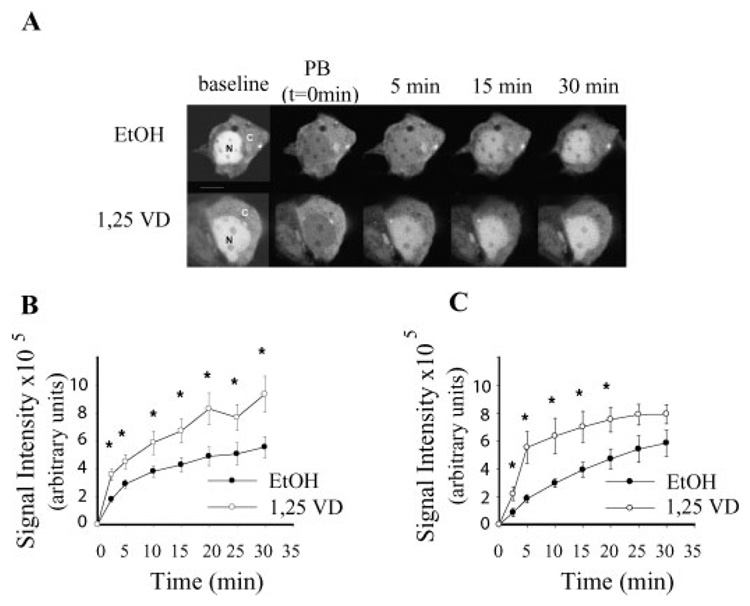
By photobleaching the GFP signal in the nucleus we were able to minimize the impact of nuclear export in our analysis and directly visualize nuclear import of GFP-VDR in BBe cells. As shown in Figure 4, ligand-independent nuclear accumulation of GFP-VDR occurred in both proliferating and differentiated BBe cells. Accumulation of GFP-VDR was significantly accelerated by 1,25(OH)2D3 treatment; the increase was significant within 2.5 min of treatment and this difference was maintained throughout the 30 min study period. At 5 min, nuclear content of the vehicle-treated cells had increased by 13% while the 1,25(OH)2D3 treatment increased nuclear GFP-VDR levels by 30%. 1,25(OH)2D3-mediated accumulation of GFP-VDR in the nucleus became saturated after 25–30 min, a point where the distribution of GFP-VDR between the nucleus and cytoplasm had reached pre-photobleaching levels. This suggests either that GFP-VDR import slowed over time or that equilibrium was reached between nuclear import and export during the 30 min study period.
Fig. 4.
Calcitriol stimulates rapid translocation of GFP-VDR to the nucleus of BBe cells. A: Representative time series for a single differentiated BBe cell response to 100 nM 1,25(OH)2D3 (1,25 VD) or vehicle (EtOH), (B) summary of the response to 1,25(OH)2D3 in proliferating BBe cells, (C) summary of the response to 1,25(OH)2D3 in differentiated BBe cells. Nuclear photobleaching of GFP-VDR transfected cells was conducted at t=0, a baseline image was collected and immediately afterwards cells were treated with either 100 nM 1,25(OH)2D3 (1,25 VD) or vehicle (EtOH). Images of cells were taken at 2.5, 5, and every 5 min until 30 min. Points represent the mean±SEM of n=6 cells per treatment. * Values for a time point are significantly different between vehicle and vitamin D treated cells, P<0.05.
Early and Late Fluxes of GFP-VDR Into Nucleus
To distinguish between the alternative hypotheses explaining the saturation of nuclear GFP-VDR accumulation following treatment with 1,25(OH)2D3 (i.e., slowing over time vs. balancing of import and export) we examined movement of GFP-VDR into the nucleus of BBe cells during the early and late phase of the response to hormone. Consistent with our initial observation, accumulation of GFP-VDR in 1,25(OH)2D3-stimulated cells was twice as high as control cells within 5 min of treatment (Fig. 5A). Similarly, we found that GFP-VDR accumulation in 1,25(OH)2D3-treated cells was 158.7% higher than in vehicle-treated cells when flux was examined 30 min after treatment (Fig. 5B). These data demonstrate that 1,25(OH)2D3-induced nuclear import of GFP-VDR inBBe cells is continuous throughout the 30 min period we examined.
Fig. 5.
Nuclear import of GFP-VDR occurs throughout a 30 min period of treatment with 1,25(OH)2D3 in BBe cells. Nuclear accumulation of GFP-VDR was examined at an early (5 min, A) and later (30 min, B) period after treatment of 6-day cultures of BBe cells with 100 nM 1,25(OH)2D3 (1,25 VD) or vehicle (EtOH). (A) Nuclear import at 5 min after treatment. The graphic on the left represents the photobleaching (PB), treatment (Rx) and image collection times (arrows). (B) Nuclear import at 30 min after addition of 1,25(OH)2D3 or vehicle. In the late assessment period protocol, cells were treated with 100 nM 1,25(OH)2D3 or vehicle at t=0. Thirty minutes later nuclei were photobleached and an image was collected.Asecond image was collected 5 min later. The confocal images were analyzed as described in Materials and Methods Section. Data represent mean±SEM (n=6 per treatment). Values with an asterisk are significantly different from one another (P<0.05).
Ability of VDR Ligand to Stimulate Nuclear Import of GFP-VDR Correlates With its Transcriptional Efficacy
We have previously shown that the analog Ro-26-9228 had reduced transcriptional potency in Caco-2 cells as compared to the effect of the analog in the hFOB osteoblast cell line [Ismail et al., 2004]. This was partially explained by reduced ability of analog-bound VDR to interact with essential protein partners, for example, RXR and glucocorticoid receptor interacting protein 1 (GRIP1). Others have observed that ligand-dependent nuclear import of VDR is dependent upon interactions between VDR and importin α [Yasmin et al., 2005]. With this in mind, we compared the ability of 1,25(OH)2D3 and Ro-26-9228 to induce nuclear GFP-VDR accumulation and subsequent downstream events: association of VDR with chromatin, and CYP24 mRNA accumulation in post-proliferating parental Caco-2 cells.
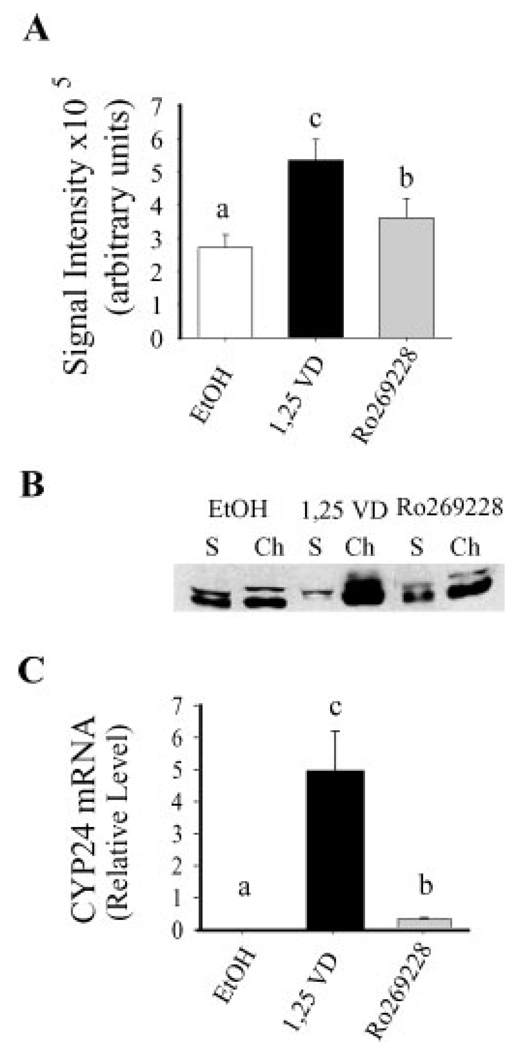
As Figure 6A shows, both 1,25(OH)2D3 and Ro-26-9228 increased GFP-VDR nuclear import within 5 min of treatment. However, while the natural ligand increased nuclear GFP-VDR by 99% compared to vehicle, the effect of the analog was significantly blunted (40% increase). The ligand-induced association of endogenous VDR with chromatin was also 60% lower in analog-treated compared to 1,25(OH)2D3-treated cells: threefold increase for Ro-26-9228 versus eightfold increase for 1,25(OH)2D3 (Fig. 6B). Finally, CYP24 mRNA accumulation was significantly lower for the analog-treated cells in both the pulse and continuous treatment protocols. For the pulse treatment protocol, the accumulation of CYP24 mRNA following analog treatment was only 6% of that observed with natural ligand (Fig. 6C). Interestingly, continuous treatment minimized the discrepancy in CYP24 mRNA accumulation between the natural ligand and the analog (analog response=58% of the natural ligand, data not shown) demonstrating partial compensation for the blunted analog response. Altogether, these data show that the analog Ro-26-9228 had reduced ability to stimulate GFP-VDR nuclear import and that this was reflected in a suppression of downstream events, for example, DNA binding and gene expression.
Fig. 6.
The ability of VDR ligands to stimulate VDR nuclear import in parental Caco-2 cells is associated with their transcriptional efficacy. (A) The effect of 5 min treatment with 100 nM 1,25(OH)2D3, 100 nM Ro269228, or vehicle (EtOH) on GFP-VDR nuclear import. Bars represent mean±SEM(n=6). (B) The effect of 1 h treatment with 100 nM 1,25(OH)2D3, Ro269228, or vehicle (EtOH) on the association of endogenous VDR with chromatin. The figure is representative data from a single sample per treatment group. S, soluble; Ch, chromatin. (C) The effect of a pulse treatment (5 min treatment, 7 h 55 min vehicle) with either 100nM1,25(OH)2D3, Ro269228, or vehicle (EtOH) on CYP24 mRNA levels as determined by RT-PCR analysis. CYP24 mRNA levels are normalized to GAPDH mRNA levels within the samples. Bars represent mean±SEM (n=3 per treatment); the experiment was repeated three times with similar results. Values with different superscripts are significantly different from one another (P<0.05).
DISCUSSION
In this study we examined the distribution of VDR in the enterocyte-like cell line Caco-2 and we found several interesting features of VDR distribution and mobility unique to this critical vitamin D target cell.
First, our quantitative analysis showed that approximately equal levels of GFP-VDR reside in the cytoplasm and nucleus of Caco-2 cells in the absence of 1,25(OH)2D3. In contrast, we found that the baseline GFP-VDR distribution in ROS17/2.8 (A1G) cells was predominantly cytoplasmic (>70%), a finding that confirms what others have reported for VDR distribution in ROS 17/2.8 cells [Racz and Barsony, 1999], COS-1 (Sunn et al., 2001), and COS-7 [Racz and Barsony, 1999] kidney cells, and microwave-fixed fibroblasts [Barsony et al., 1990]. Next, we observed that the signal produced by our GFP-VDR construct was diffusely distributed within the nucleus. This is similar to what Sunn et al. [2001] previously reported for an enhanced GFP-labeled version of the traditional VDR-A isoform in COS-1 cells. Thus, although others have reported that the traditional VDR can be found in caveolin-rich membrane domains in chick intestinal cells and in ROS 17/2.8 cells [Huhtakangas et al., 2004], we did not observe any accumulation of GFP-VDR near either the apical or basolateral membrane in our 3-D reconstructions. Our observation is consistent with what Barsony et al. [1997] reported using a BODIPY-labeled 1,25(OH)2D3 in human fibro-blasts. This does not support a role for a membrane-associated VDR as the mediator responsible for the activation of various kinases and signal transduction pathways by 1,25(OH)2D3 that have been observed in a variety of cell types [Fleet, 2004], including Caco-2 cells [Wali et al., 1992; Tien et al., 1993; Bettoun et al., 2003]. However, our inability to see membrane-associated GFP-VDR could reflect several factors including low sensitivity of the method to detect the 1–3% of VDR that is proposed to be associated with the membrane or an inability of the GFP-VDR to associate with the membrane.
Another interesting finding from our experiments is that neither endogenous VDR nor GFP-VDR accumulate in the cell or in the nucleus following 1,25(OH)2D3 treatment. This is in contrast to previous reports that consistently show a significant accumulation of VDR resulting from 1,25(OH)2D3-induced protein stabilization in other cell types [Mahonen and Maenpaa, 1994; Masuyama and MacDonald, 1998; Jaaskelainen et al., 2000]. Our data in Caco-2 cells are consistent with a report by Wiese et al. [1992] who found that 1,25(OH)2D3 treatment had modest (twofold), delayed (>4 h), and transient (normal VDR levels by 24 h) effect on VDR protein levels in the rat intestinal crypt-like cell line IEC-6. Neither this study, nor our data explain why 1,25(OH)2D3 stabilization of VDR in the enterocyte is less dramatic than in other cell types. However, even though we did not observe accumulation of total or nuclear VDR levels in Caco-2 cells after 1,25(OH)2D3 treatment, we did find that the distribution of the receptor within the nucleus shifts towards a greater association with the chromatin fraction following 1 h of treatment. Thus, the lack of nuclear VDR accumulation masks the activation of VDR as a transcription factor.
In contrast, using photobleaching to reduce GFP-VDR signal in the nucleus allowed us to document 1,25(OH)2D3-dependent nuclear import of GFP-VDR in Caco-2 cells. This increase was substantial and continuous in the presence of hormone. The difference between ligand-dependent and ligand-independent import accounted for a 20% increase in nuclear level of GFP-VDR per 5 min period; this was true whether we examined vitamin D-induced GFP-VDR movement immediately after or 30 min after hormone treatment. The mechanism mediating the nuclear import of GFP-VDR in Caco-2 cells is unclear. Ligand-independent import has been shown to be dependent upon importin 4 (Miyauchi et al., 2005) and also requires nuclear localization signals within its heterodimeric partner RXR [Prufer and Barsony, 2002]. In contrast, ligand-induced VDR movement occurs independent of the RXR nuclear localization signal [Prufer and Barsony, 2002] and a recent report by Yasmin et al. [2005] suggests that 1,25(OH)2D3-induced VDR-RXR nuclear import is due to recruitment of importin α to the VDR.
Our data from photobleaching experiments demonstrates that vitamin D-induced nuclear import should be sufficient to cause nuclear accumulation of GFP-VDR; the import rate we documented was sufficient to transfer at least 36% of the cytoplasmic signal into the nucleus in the 30 min period we studied. While we did not directly evaluate nuclear GFP-VDR export, we believe that the lack of GFP-VDR accumulation in the presence of ligand in non-photobleached cells, even in the face of significant nuclear import, is consistent with the hypothesis that VDR import and export are balanced in enterocytes. Cell-specific differences in nuclear accumulation of VDR following vitamin D treatment may therefore be due to differences in the efficiency of vitamin D-induced GFP-VDR export. Unfortunately the mechanism mediating ligand-induced nuclear export of steroid hormone receptor superfamily members, especially VDR, is not clear [Pemberton and Paschal, 2006]. For example, while chromosomal region maintenance 1 protein (CRM-1) receptor-mediated export is important for nuclear export of the unliganded VDR, ligand-enhanced export of VDR is CRM-1 independent [Prufer and Barsony, 2002]. Alternately, 1,25(OH)2D3-induced nuclear export may be mediated by binding to calreticulin, a protein previously identified as important for the export of multiple members in nuclear hormone receptor superfamily including VDR [Black et al., 2001]. However, a recent study by Walther et al. [2003] suggests that the previously identified role for calreticulin in the nuclear export of the glucocorticoid receptor is an artifact of the heterokaryon method used to identify calreticulin as a critical nuclear export protein. Other mechanisms have also been proposed to mediate nuclear export of steroid hormone receptor family members. For example, Saporita et al. [2003] identified amino acid sequences in helixes 5–8 of the ligand binding domain that were involved in the nuclear export of the androgen receptor, the mineralcorticoid receptor, and estrogen receptor α. Unfortunately, the residues proposed to be critical for nuclear export in those receptors are not well conserved in the VDR (data not shown). Future studies will be necessary to identify the critical proteins mediating export of VDR and other nuclear receptor members.
In our final study we examined the relationship between 1,25(OH)2D3 or Ro-26-9228-induced VDR import and downstream events following in Caco-2 cells, that is, association of VDR with chromatin, mRNA accumulation. Previous studies showed that Ro-26-9228 works as a tissue selective 1,25(OH)2D3 analog characterized by a tenfold lower ability to induce gene transcription in intestinal epithelial cells compared to osteoblasts [Ismail et al., 2004]. This was due in part to reduced ability of analog-bound VDR to form essential protein–protein interactions with its heterodimeric partner RXR as well as the coactivator GRIP1. Here, we add to this story by showing that the earliest step of the vitamin D-signaling process, measured by nuclear import of GFP-VDR, was less efficient in the presence of analog compared to the natural ligand. Reduced nuclear import was associated with a reduction in the next step in the process of transcription: association of VDR with chromatin. This was subsequently reflected as reduced ligand-induced CYP24 mRNA accumulation. While the role of reduced analog-induced binding of the coactivator GRIP1 to VDR is certainly contributing to the reduced transcription of the CYP24 gene, our data are consistent with the observations of Racz and Barsony [1999], who found that VDR nuclear translocation is a critical component of the receptor activation process and vitamin D-mediated gene transcription. In addition, this observation is consistent with the hypothesis that conformational changes in VDR induced by vitamin D analogs not only affect its interactions with RXR and coactivators [Ismail et al., 2004] but also with proteins critical for nuclear VDR import, that is, importins.
ACKNOWLEDGMENTS
The authors would like to thank Dr. J. Paul Robinson and the staff of the Purdue Cytometry Laboratory for their assistance with the con-focal microscopy. The Purdue Cytometry lab is supported in part by the Purdue Cancer Center (CA 23168). This work was funded by NIH grants DK54111 (to J.C.F.) and DK50583 (to S.P.).
Abbreviations used
- CaBPD9k
calbindin D9k
- CRM-1
chromosomal region maintenance 1 protein
- CYP24
25-hydroxyvitamin D3 24-hydroxylase
- DMEM
Dulbecco’s Modified Eagle’s Medium
- FBS
fetal bovine serum
- GFPVDR
chimera of green fluorescent protein with VDR
- GRIP1
glucocorticoid receptor interacting protein
- Ham’s F12
Ham’s nutrientmixture F12
- ITS
insulin–transferrin–selenium
- NLS
nuclear localization sequence
- PCR
polymerase chain reaction
- PI
propidium iodide
- PR
phenol red
- pRL-CMV
expression vector for Renilla luciferase
- PMCA1b
plasma membrane calcium ATPase 1b
- 1,25(OH)2D3
1,25 dihydroxyvitamin D3
- Ro-26-9228
1-alpha-fluoro-16-ene-20-epi-23-ene-26,27-bishomo-25-hydroxyvitamin D3
- TFIIB
general transcription factor II B
- TRPV6
transient receptor vanelloid familymember 6
- VDR
vitamin D receptor
REFERENCES
- Arbour NC, Ross TK, Zierold C, Prahl JM, DeLuca HF. A highly sensitive method for large-scale measurements of 1,25-dihydroxyvitamin D. Anal Biochem. 1998;255:148–154. doi: 10.1006/abio.1997.2439. [DOI] [PubMed] [Google Scholar]
- Barclay WW, Cramer SD. Culture of mouse prostatic epithelial cells from genetically engineered mice. Prostate. 2005;63:291–298. doi: 10.1002/pros.20193. [DOI] [PubMed] [Google Scholar]
- Barsony J, Pike JW, DeLuca HF, Marx SJ. Immunocytology with microwave-fixed fibroblasts shows 1 alpha,25-dihydroxyvitamin D3-dependent rapid and estrogen-dependent slow reorganization of vitamin D receptors. J Cell Biol. 1990;111:2385–2395. doi: 10.1083/jcb.111.6.2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsony J, Renyi I, McKoy W. Subcellular distribution of normal and mutant vitamin D receptors in living cells. J Biol Chem. 1997;272:5774–5782. doi: 10.1074/jbc.272.9.5774. [DOI] [PubMed] [Google Scholar]
- Bettoun DJ, Burris TP, Houck KA, Buck DW, Stayrook KR, Khalifa B, Lu J, Chin WW, Nagpal S. Retinoid X receptor is a non-silent major contributor to vitamin d receptor-mediated transcriptional activation. Mol Endocrinol. 2003;17:2320–2328. doi: 10.1210/me.2003-0148. [DOI] [PubMed] [Google Scholar]
- Black BE, Holaska JM, Rastinejad F, Paschal BM. DNA binding domains in diverse nuclear receptor function as nuclear export signals. Curr Biol. 2001;11:1749–1758. doi: 10.1016/s0960-9822(01)00537-1. [DOI] [PubMed] [Google Scholar]
- Bronner F. Mechanisms of intestinal calcium absorption. J Cell Biochem. 2003;88:387–393. doi: 10.1002/jcb.10330. [DOI] [PubMed] [Google Scholar]
- Fleet JC. Rapid, membrane-initiated actions of 1,25 dihydroxyvitamin d: What are they and what do they mean? J Nutr. 2004;134:3215–3218. doi: 10.1093/jn/134.12.3215. [DOI] [PubMed] [Google Scholar]
- Fleet JC. Molecular regulation of calcium metabolism. In: Weaver CM, Heaney RP, editors. Calcium in human health. Totowa, NJ: Humana Press; 2006. pp. 163–190. [Google Scholar]
- Fleet JC, Eksir F, Hance KW, Wood RJ. Vitamin Dinducible calcium transport and gene expression in three Caco-2 cell lines. Am J Physiol-Gastrointest Liver Physiol. 2002;283:G618–G625. doi: 10.1152/ajpgi.00269.2001. [DOI] [PubMed] [Google Scholar]
- Giuliano AR, Wood RJ. Vitamin D-regulated calcium transport in Caco-2 cells: Unique in vitro model. Am J Physiol. 1991;260:G207–G212. doi: 10.1152/ajpgi.1991.260.2.G207. [DOI] [PubMed] [Google Scholar]
- Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, Dominguez CE, Jurutka PW. The nuclear vitamin D receptor: Biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13:325–349. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- Hoenderop JG, Nilius B, Bindels RJ. Calcium absorption across epithelia. Physiol Rev. 2005;85:373–422. doi: 10.1152/physrev.00003.2004. [DOI] [PubMed] [Google Scholar]
- Huhtakangas JA, Olivera CJ, Bishop JE, Zanello LP, Norman AW. The vitamin D receptor is present in caveolae-enriched plasma membranes and binds 1{alpha},25(OH)2-vitamin D3 in vivo and in vitro. Mol Endocrinol. 2004;18:2660–2671. doi: 10.1210/me.2004-0116. [DOI] [PubMed] [Google Scholar]
- Ismail A, Nguyen CV, Ahene A, Fleet JC, Uskokovic MR, Peleg S. Effect of cellular environment on the selective activation of the vitamin D receptor by 1{alpha},25-dihydroxyvitamin D3 and its Analog 1{alpha}-fluoro-16-ene-20-epi-23-ene-26,27-bishomo-25- hydroxyvitamin D3 (Ro-26-9228) Mol Endocrinol. 2004;18:874–887. doi: 10.1210/me.2003-0310. [DOI] [PubMed] [Google Scholar]
- Jaaskelainen T, Ryhanen S, Mahonen A, DeLuca H, Maenpaa P. Mechanism of action of superactive vitamin D analogs through regulated receptor degradation. J Cell Biochem. 2000;76:548–558. doi: 10.1002/(sici)1097-4644(20000315)76:4<548::aid-jcb3>3.3.co;2-s. [DOI] [PubMed] [Google Scholar]
- Kerry DM, Dwivedi PP, Hahn CN, Morris HA, Omdahl JL, May BK. Transcriptional synergism between vitamin D-responsive elements in the rat 25-hydroxyvitamin D3 24-hydroxylase (CYP24) promoter. J Biol Chem. 1996;271:29715–29721. doi: 10.1074/jbc.271.47.29715. [DOI] [PubMed] [Google Scholar]
- Mahonen A, Maenpaa PH. Steroid hormone modulation of vitamin D receptor levels in human MG-63 osteosarcoma cells. Biochem Biophys Res Comm. 1994;205:1179–1186. doi: 10.1006/bbrc.1994.2790. [DOI] [PubMed] [Google Scholar]
- Masuyama H, MacDonald PN. Proteasome-mediated degradation of the vitamin D receptor (VDR) and a putative role for SUG1 interaction with the AF-2 domain of VDR. J Cell Biochem. 1998;71:429–440. [PubMed] [Google Scholar]
- Michigami T, Suga A, Yamazaki M, Shimizu C, Cai G, Okada S, Ozono K. Identification of amino acid sequence in the hinge region of human vitamin D receptor that transfer a cytosolic protein to the nucleus. J Biol Chem. 1999;274:33531–33538. doi: 10.1074/jbc.274.47.33531. [DOI] [PubMed] [Google Scholar]
- Miyauchi Y, Michigami T, Sakaguchi N, Sekimoto T, Yoneda Y, Pike JW, Yamagata M, Ozono K. Importin 4 is responsible for ligand-independent nuclear translocation of vitamin D receptor. J Biol Chem. 2005;280:40901–40908. doi: 10.1074/jbc.M509347200. [DOI] [PubMed] [Google Scholar]
- Pemberton LF, Paschal BM. Scientists share nuclear secrets at jekyll island. Traffic. 2006;7:751–760. doi: 10.1111/j.1600-0854.2006.00417.x. [DOI] [PubMed] [Google Scholar]
- Peterson MD, Mooseker MS. Characterization of the enterocyte-like brush border cytoskeleton of the C2BBe clones of the human intestinal cell line, Caco-2. J Cell Sci. 1992;102:581–600. doi: 10.1242/jcs.102.3.581. [DOI] [PubMed] [Google Scholar]
- Prufer K, Barsony J. Retinoid X receptor dominates the nuclear import and export of the unliganded vitamin D receptor. Mol Endocrinol. 2002;16:1738–1751. doi: 10.1210/me.2001-0345. [DOI] [PubMed] [Google Scholar]
- Prufer K, Racz A, Lin GC, Barsony J. Dimerization with retinoid X receptors promotes nuclear localization and subnuclear targeting of vitamin D receptors. J Biol Chem. 2000;275:41114–41123. doi: 10.1074/jbc.M003791200. [DOI] [PubMed] [Google Scholar]
- Racz A, Barsony J. Hormone-dependent translocation of vitamin D receptors is linked to transactivation. J Biol Chem. 1999;274:19352–19360. doi: 10.1074/jbc.274.27.19352. [DOI] [PubMed] [Google Scholar]
- Saporita AJ, Zhang Q, Navai N, Dincer Z, Hahn J, Cai X, Wang Z. Identification and characterization of a ligand-regulated nuclear export signal in androgen receptor. J Biol Chem. 2003;278:41998–42005. doi: 10.1074/jbc.M302460200. [DOI] [PubMed] [Google Scholar]
- Song Y, Kato S, Fleet JC. Vitamin D receptor (VDR) knockout mice reveal VDR-independent regulation of intestinal calcium absorption and ECaC2 and calbindin D9k mRNA. J Nutr. 2003a;133:374–380. doi: 10.1093/jn/133.2.374. [DOI] [PubMed] [Google Scholar]
- Song Y, Peng X, Porta A, Takanaga H, Peng JB, Hediger MA, Fleet JC, Christakos S. Calcium transporter 1 and epithelial calcium channel messenger ribonucleic acid are differentially regulated by 1,25 dihydroxyvitamin D3 in the intestine and kidney of mice. Endocrinology. 2003b;144:3885–3894. doi: 10.1210/en.2003-0314. [DOI] [PubMed] [Google Scholar]
- Sunn KL, Cock TA, Crofts LA, Eisman JA, Gardiner EM. Novel N-terminal variant of human VDR. Mol Endocrinol. 2001;15:1599–1609. doi: 10.1210/mend.15.9.0693. [DOI] [PubMed] [Google Scholar]
- Tien XY, Brasitus TA, Qasawa BM, Norman AW, Sitrin MD. Effect of 1,25 (OH)2 D3 and its analogues on membrane phosphoinositide turnover and [Ca21]i in Caco-2 cells. Am J Physiol. 1993;265:G143–G148. doi: 10.1152/ajpgi.1993.265.1.G143. [DOI] [PubMed] [Google Scholar]
- Van Cromphaut SJ, Dewerchin M, Hoenderop JG, Stockmans I, Van Herck E, Kato S, Bindels RJ, Collen D, Carmeliet P, Bouillon R, Carmeliet G. Duodenal calcium absorption in vitamin D receptor-knockout mice: Functional and molecular aspects. Proc Natl Acad Sci USA. 2001;98:13324–13329. doi: 10.1073/pnas.231474698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wali RK, Baum CL, Bolt MJ, Brasitus TA, Sitrin MD. 1,25-dihydroxyvitamin D3 inhibits Na( )-H exchange by stimulating membrane phosphoinositide turnover and increasing cytosolic calcium in CaCo-2 cells. Endocrinology. 1992;131:1125–1133. doi: 10.1210/endo.131.3.1324151. [DOI] [PubMed] [Google Scholar]
- Walther RF, Lamprecht C, Ridsdale A, Groulx I, Lee S, Lefebvre YA, Hache RJ. Nuclear export of the glucocorticoid receptor is accelerated by cell fusiondependent release of calreticulin. J Biol Chem. 2003;278:37858–37864. doi: 10.1074/jbc.M306356200. [DOI] [PubMed] [Google Scholar]
- Wiese RJ, Uhland-Smith A, Ross TK, Prahl JM, DeLuca HF. Up-regulation of the vitamin D receptor in response to 1,25-dihydroxyvitamin D3 results from ligand-induced stabilization. J Biol Chem. 1992;267:20082–20086. [PubMed] [Google Scholar]
- Yasmin R, Williams RM, Xu M, Noy N. Nuclear import of the retinoid X receptor, the vitamin D receptor, and their mutual heterodimer. J Biol Chem. 2005;280:40152–40160. doi: 10.1074/jbc.M507708200. [DOI] [PubMed] [Google Scholar]