Abstract
Objective
Fever of unknown origin (FUO) in children presents a diagnostic challenge. Differential diagnosis includes Systemic Onset Juvenile Idiopathic Arthritis (SJIA), an auto-inflammatory syndrome associated with activation of phagocytic cells which at presentation is difficult to differentiate from severe systemic infections. In this study, we investigated whether serum concentrations of the phagocytic pro-inflammatory protein S100A12 may help in the decision between antibiotic or immunosuppressive therapy in patients with FUO.
Methods
Serum samples were obtained from 45 healthy controls and 240 patients: 60 had SJIA, 17 Familial Mediterranean Fever (FMF), 18 Neonatal-Onset Multisystem Inflammatory Disease (NOMID), 17 Muckle Wells Syndrome (MWS), 40 Acute Lymphoblastic Leukemia (ALL), 5 Acute Myeloblastic Leukemia (AML), and 83 systemic infections. All samples were collected at presentation before initiation of anytreatment, and were analyzed for S100A12 by ELISA.
Results
In SJIA patients the mean S100A12 serum level was 7,190 ng/ml (95% confidence interval ±2,690), in FMF 6,720 ng/ml (±4,960), in NOMID 720 ng/ml (±450), in MWS 150 ng/ml (±60), in infections 470 ng/ml (±160), in ALL 130 ng/ml (±80), and in AML 45 (±60) compared to 50 ng/ml (±10) in healthy controls. Sensitivity and specificity of S100A12 to distinguish SJIA from infections were 66% and 94% respectively.
Conclusions
S100A12, a marker of granulocyte activation, is highly overexpressed in SJIA and FMF, which may point to so far unknown common inflammatory mechanisms in these diseases. The measurement of S100A12 serum levels may provide a valuable diagnostic tool in the evaluation of FUO.
Fever of unknown origin (FUO) frequently presents a diagnostic challenge in the pediatric population despite of recent advances in diagnostic tools and techniques.(1, 2) FUO can be the primary manifestation of a broad spectrum of diseases, but the main causes in children are infections. Substantial progress has been achieved in the diagnosis of infectious and other causes of fever, due to new developments in nuclear medicine techniques, instrumental procedures and genetic testing for diagnosing rare hereditary auto-inflammatory conditions associated with fever. Nevertheless, there is no diagnostic checklist for children, and up to 200 conditions causing fever have to be ruled out, often leading to prolonged periods of hospitalization and treatment attempts which include various antibiotic regimens.(3, 4)
An important differential diagnosis as a cause of FUO in children is Systemic onset Juvenile Idiopathic Arthritis (SJIA, Still’s disease, OMIM 604302), an aggressive auto-inflammatory disease that resembles sepsis.(5-7) Although the pathogenesis of SJIA remains poorly understood, overwhelming activation of the innate immune system due to an imbalance between pro-inflammatory cytokines and immune deactivators without evidence of involvement of the adaptive immune responses are seen in these patients.(8), (9) Unfortunately, characteristic signs of arthritis often do not develop before the later course of this disease and therefore at initial presentation the non-specific inflammatory pattern in SJIA patients cannot be differentiated from systemic infections by clinical or laboratory parameters, and suitable biomarkers are missing. In many cases an exploratory antibiotic treatment is initiated before a definitive diagnosis is made. This clinical uncertainty impedes early initiation of an appropriate anti-inflammatory therapy.(6, 7, 10)
In previous studies we found high concentrations of S100A12 in serum from SJIA patients.(11) S100A12 is a calcium-binding protein expressed and secreted by activated phagocytes. Recently it has been assigned to the Damage Associated Molecular Pattern molecules (DAMPs), which represent endogenous ligands of pattern recognition receptors.(12) S100A12 has pro-inflammatory properties in vitro at concentrations found in SJIA serum in vivo.(11, 13) It is mainly expressed in granulocytes and binds to the Receptor for Advanced Glycation End-products (RAGE).(14) Activation of this receptor induces pro-inflammatory responses in leukocytes and endothelial cells via nuclear factor (NF)-κB.(15, 16)
S100A12 is a useful marker protein for monitoring disease activity in several inflammatory diseases.(17) In the present study we assess the diagnostic value of S100A12 serum levels in differentiating between SJIA in the initial disease phase versus acute systemic infections and childhood leukemic malignancies as the most relevant differential diagnoses. Additionally we included sera from patients with other hereditary IL-1-driven diseases namely Familial Mediterranean Fever (FMF, OMIM 249100), Neonatal-onset Multisystem Inflammatory Disease (NOMID, OMIM 607115), and Muckle Wells Syndrome (MWS, OMIM 191900). All of these disorders typically present as FUO. To the best of our knowledge, this is the largest trial on a biomarker in FUO to date.
Patients and Methods
Healthy controls
Normal S100A12 levels were determined in 45 healthy controls who gave informed consent (see table 1). These individuals without signs of inflammation underwent a routine evaluation at the University Children’s Hospital Muenster or volunteered in our laboratory. There were no significant differences between patients and controls with regard to age or gender distribution.
Table 1.
Characteristics of patients with SJIA, FMF, systemic infections, NOMID, MWS, ALL, AML and healthy controls.
| SJIA | FMF | infections | NOMID | MWS | ALL | AML | Healthy controls | |
|---|---|---|---|---|---|---|---|---|
| number | 60 | 17 | 83 | 18 | 17 | 40 | 5 | 45 |
| Age (range) | 9.1 (1.8-18.1) | 11.7 (3.8-18.6) | 8.1 (1.2-33.2) | 11.0 (4.1-32.0) | 34.6 (5.0-73.2) | 6.2 (0.9-14.9) | 11.0 (0.7-16.9) | 16 (1.2-34.3) |
| sex m/f | 32/28 | 11/6 | 43/42 | 10/8 | 8/8 | 18/21 | 3/2 | 26/24 |
| leukocytes/μl (±95%CI) | 16,120 (2,220) | n.d. | 13,300 (1,200) | 17,200 (3,600) | n.d. | 38,290 (36,400) | 33,120 (71,800) | 6,700 (1,100) |
| ESR [mm/h] (±95%CI) | 76 (23) | 38 (21) | 40 (18) | 60 (16) | 24 (9) | 75 (33) | n.d. | 11 (8) |
| CRP [mg/l] (±95%CI) | 84 (19) | 40 (29) | 111 (11) | 68 (19) | 18 (9) | 28 (17) | 17 (50) | <5 |
| S100A12 [ng/ml] (±95%CI) | 7,190 (2690) | 6,720 (4960) | 470 (160) | 720 (450) | 150 (60) | 130 (80) | 45 (60) | 50 (10) |
n.d. not determined
Patients
The study was designed as a prospective trial in which data collection was planned before the measurements of diagnostic accuracy were performed. We included patients with SJIA, FMF, NOMID, MWS, Acute Lymphoblastic Leukemia (ALL), Acute Myeloblastic Leukemia(AML), and patients with systemic infections between July 1998 and February 2007 from the University Childrens’ Hospital Muenster, the Wilhelmina Children’s Hospital, Utrecht, the National Institute of Arthritis and Musculoskeletal and Skin Diseases, Bethesda, the Charité Children’s Hospital, Berlin, and the University Children’s Hospital Tuebingen. The cohort of NOMID patients from Bethesda was the only patient group analyzed in a retrospective way, patient samples existed before the decision to analyze S100A12 was made. Recruitment of the patients required verification of the underlying disease and pro-inflammatory active disease as defined below. The study was approved by the institutional ethics committee at each center and informed consent was obtained from patients or their legal guardians.
SJIA patients fulfilled the criteria of the International League of Associations for Rheumatology with symptoms of quotidian fever, arthritis, rash, hepatomegaly, splenomegaly or serositis.(18) Clinical disease activity was determined on the basis of the core set criteria for JIA.(19, 20)
Inclusion criteria for patients with clinical and laboratory signs of severe systemic infections were CRP > 50 mg/l, and fever > 38.5°C.
Laboratory parameters
White blood cell count (cells/μl), absolute neutrophil count (cells/μl), ESR (mm/h) and CRP (mg/l) were determined as serum markers of inflammation.
Determination of concentrations of S100A12 by sandwich ELISA
Serum samples were centrifuged within 2 hours after acquisition and were frozen at -80°C until measurement. Concentrations of S100A12 were determined by a double sandwich enzyme linked immunosorbent assay (ELISA) system established in our laboratory as described.(21) Antibodies and protein standards of recombinant S100A12 (0.25 to 250 ng/ml) were generated as reported previously.(22) All samples were diluted into the linear range of the assay. The inter- and intraassay coefficients of variation were 12.1 percent and 4.8 percent, respectively.(23) The readers of the laboratory assay were blinded for the diagnosis. For comparison with earlier studies internal control sera were included in all ELISA studies.
Statistical analysis
Analysis of variance (ANOVA) was employed to analyze differences between subgroups of patients or controls. Confirmed differences were tested for statistical significance by subsequent selective post-hoc test of Dunnett and Tamhane. Rank differences were analyzed using the Mann-Whitney U test. Receiver-operating curves (ROC) were plotted to determine the accuracy of inflammatory marker measurements as diagnostic test, and for the calculation of different cut-off values with different sensitivities and specificities. SPSS version 13.0 for windows was used for statistical analyses. Data are expressed as mean ± 95% confidence interval (CI) except where stated otherwise. Box plots in figures show median, mean (bold line), 25th and 75th percentile. Error bars indicate 10th and 90th percentile. There were no missing test results, and no indeterminate or outliers were excluded.
Results
Patients
In total 240 patients were included. Patients took occasional antipyretic drugs, other concomitant medications are listed where applicable. Patient characteristics are presented in table 1.
In total, 60 SJIA patients were enrolled. Patients were diagnosed by experienced pediatric rheumatologists (MF, NW, JR) and classified according to the ILAR criteria. Three patients were between the age of 16 and 18, and in this respect did not meet the ILAR criteria but rather represented adult onset Still’s disease. Serum samples were obtained at initial presentation during episodes of fever and high disease activity, before initiation of specific therapy. Patients were enrolled in the centers of Muenster and Utrecht only and were followed until confirmation of diagnosis and initiation of appropriate anti-inflammatory treatment.
Among the 17 FMF patients included, 5 patients had mutations in the MEFV gene in M694V/M694V, 2 in M680I/M680I, 1 in S242R/M694V, 6 in M680I/M694V, 1 in M694V/R761H and in 2 patients no mutations were found. Five patients without colchicine treatment had active disease and at least one of the following clinical manifestations at the time of presentation:serositis, arthritis, fever or rash. Twelve patients on colchicine had minor disease flares with elevated acute phase reactants or symptoms related to FMF like abdominal pain, arthralgia or rash.
We also included 17 MWS patients from 7 families, 13 with heterozygous E311K and 3 with heterozygous V198M mutations in the NLRP3 gene. At time of sample acquisition patients presented with at least two of the following clinical manifestations: sensorineural hearing loss, abdominal pain, headaches, conjunctivitis, serositis, arthritis, fever, rash or clinical signs of inflammation including high inflammation markers as C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR). At that time patients did not receive anti-inflammatory treatment.
Out of 18 patients with NOMID, 12 had proven mutations in exon 3 of the CIAS-1 gene.(24) Patients presented with active disease showing at least two of the following clinical manifestations: urticarial rash, CNS involvement (e.g., papilledema, pleocytosis in the cerebrospinal fluid, and sensorineural hearing loss), or epiphyseal or patellar overgrowth on radiography. At the time of sampling all patients had inflammatory active disease despite of anti-inflammatory treatment, but none of the patients was treated with recombinant IL-1 receptor antagonist (IL-1Ra).
Out of 83 patients with severe systemic infections, 64 patients had documented bacterial infections (pneumonia 38, urinary tract infections 8, gastrointestinal tract infections 3, osteomyelitis 2, soft tissue infections 2, sepsis 6, peritonitis 3, appendicitis 2). In 19 patients the infection was classified to be of viral origin (respiratory tract infections 14, gastrointestinal tract infections 4, Epstein-Barr virus (EBV) infection 1). All serum samples were obtained prior to the start of antibiotic treatment.
Forty-five patients with hematologic malignancies were included, 40 patients had ALL and 5 patients had AML. Serum samples were taken at initial manifestation before initiation of therapy.
Classical inflammatory markers do not differentiate between SJIA and systemic infections
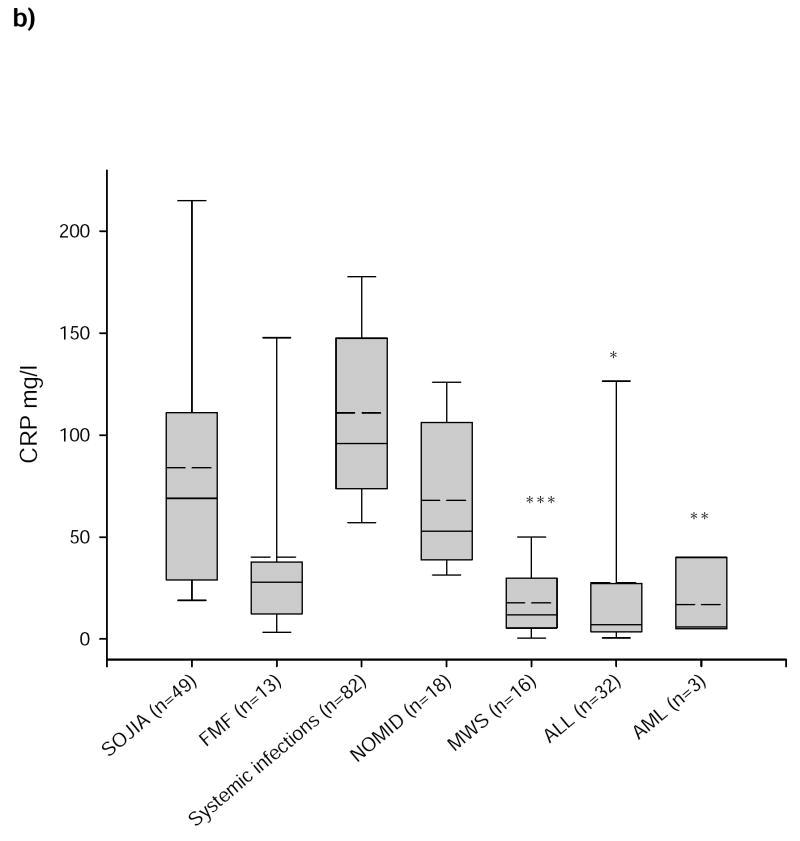
Serum CRP levels in patients with SJIA (84 ± 19 mg/l) were not significantly different from patients with severe infections (111 ± 11 mg/l; p=0.297), NOMID (68 ± 19 mg/l; p=0.99) and FMF (40 ± 29 mg/l; p=0.253), only from patients with AML (17 ± 50 mg/l; p<0.01), ALL (28 ± 17 mg/l; p=0.049), and MWS (18 ± 9 mg/l; p<0.001) (figure 1b). FMF patients had significantly lower CRP levels than patients with systemic infections (p=0.004), but did not differ significantly from patients with NOMID (p=0.892), MWS (p=0.948) or ALL (p=1.0). Determination of the diagnostic accuracy for CRP in the ROC-analysis revealed an area under the curve of 0.313 ± 0.103 confirming that CRP values were no reliable markers for the diagnosis of SJIA (figure 2).
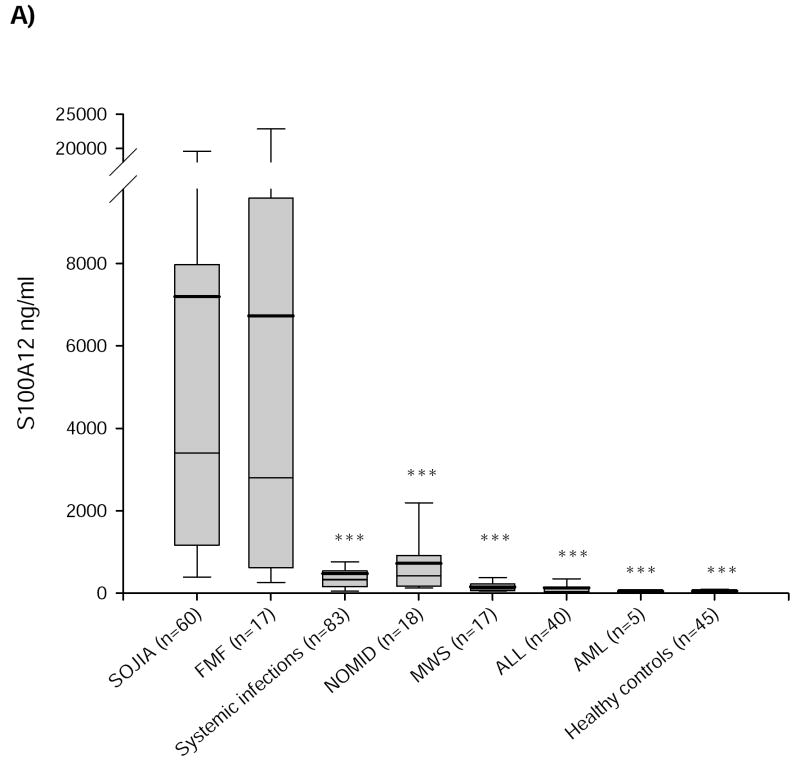
Figure 1. S100A12 and CRP in SJIA, FMF, systemic infections, NOMID, MWS, ALL, and AML.
Serum concentrations of a) S100A12 and b) CRP in patients with SJIA, FMF, systemic infections, NOMID, MWS, ALL, AML, and in a group of healthy controls. Box plots show the median (horizontal line), mean (bold horizontal line), and the 25th and 75th percentiles. Bars show the 10th and 90th percentiles. There was no significant difference for S100A12 between active SJIA and FMF but between SJIA and the other disorders and healthy controls. CRP levels between SJIA and systemic infections did not differ significantly; healthy controls had CRP levels lower than 5mg/l. (*p<0.05, **p<0.01, ***p<0.001 versus SJIA).
Figure 2. ROC-Analysis of S100A12 and CRP.
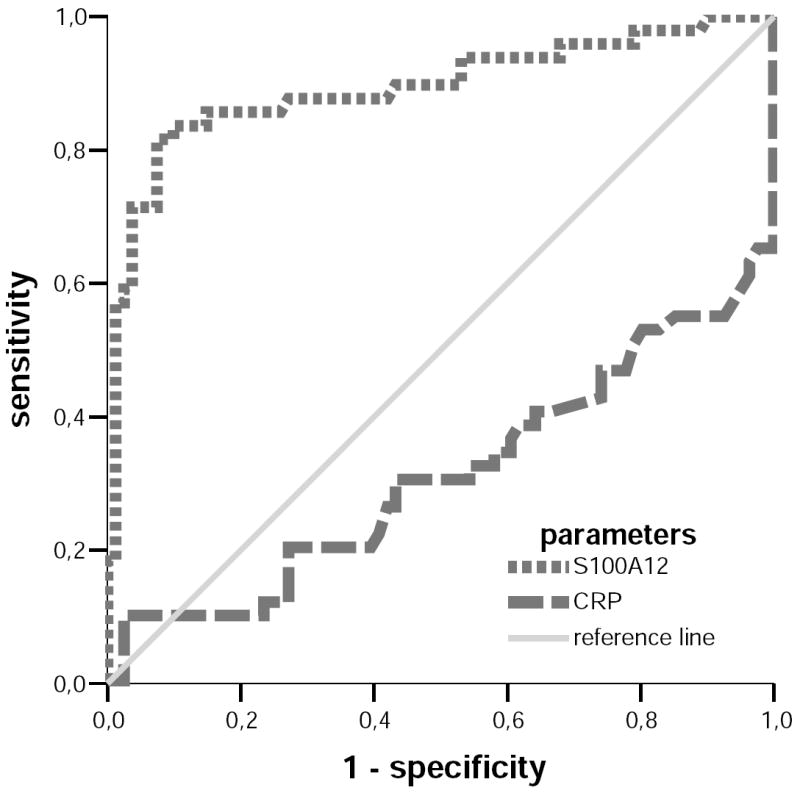
Receiver Operating Curve (ROC) analysis of S100A12 and CRP displayed as sensitivity (y-axis) against 1-specificity (x-axis) for the differentiation of SJIA and systemic infections. The area under the curve for S100A12 was 0.881 ± 0.078, and for CRP 0.313 ± 0.103.
ESR measurements were significantly elevated in patients with SJIA (p=0.002) and NOMID (p=0.002) when compared to MWS but did not significantly differ between each other, nor when compared to infections, FMF or ALL.
S100A12 levels in SJIA and FMF patients significantly differ compared to patients with severe infections, other auto-inflammatory syndromes, and hematologic leukemias
S100A12 serum levels in patients with active SJIA (7,190 ± 2,690 ng/ml) were around 145-fold higher than in healthy controls (50 ± 10 ng/ml; p<0.001), they were also significantly higher compared to patients with systemic infections (470 ± 160 ng/ml; p<0.001), NOMID (720 ± 450 ng/ml; p<0.001), MWS (150 ± 60 ng/ml; p<0.001), ALL (130 ± 80 ng/ml; p<0.001), andAML (45 ± 60; p<0.001) (figure 1a). FMF serum concentrations of S100A12 were similar to SJIA levels (6,720 ± 4,960 ng/ml) and around 135-fold higher than in healthy controls (p<0.001). There was no statistical difference between SJIA and FMF patients concerning S100A12 serum levels (p=1.0). Comparing FMF to systemic infections, NOMID, MWS, ALL, and AML differences were significant in group-to-group analysis by Mann-Whitney U test but not in selective post-hoc test of Dunnett and Tamhane probably due to the low FMF patient number.
S100A12 serum concentrations differentiate very well between SJIA and other causes of FUO beside FMF as confirmed by ROC-analyses. The areas under the curve for S100A12 were 0.881 ± 0.078 in the differentiation between SJIA and systemic infections (figure 2) and 0.866 ± 0.084 between SJIA and NOMID. At a cutoff concentration of 1,400ng/ml, S100A12 revealed a sensitivity of 66% and a specificity of 94% to distinguish SJIA from systemic infections. The corresponding positive likelihood ratio (LR) was 11.0. To distinguish between SJIA and NOMID at 1,400ng/ml S100A12 sensitivity was 66% and specificity was 89% with a respective positive LR of 6.0. To distinguish SJIA at a cut-off level of 1,000ng/ml from MWS and ALL sensitivity was in each case 78% and specificity was 100% respectively (table 2).
Table 2.
Differentiation of SJIA versus bacterial infections and NOMID with S100A12 at various cut-offs.
| Parameter | Area under the curve | Cut-off | Sensitivity | Specificity | Positive Likelihood ratio |
|---|---|---|---|---|---|
| SJIA vs infections | 0.881 ± 0.078 | 800 ng/ml | 85% | 89% | 7.7 |
| 1400 ng/ml | 66% | 94% | 11.0 | ||
| 2750 ng/ml | 55% | 97% | 18.3 | ||
| SJIA vs NOMID | 0.866 ± 0.084 | 800 ng/ml | 84% | 72% | 3.0 |
| 1400 ng/ml | 66% | 89% | 6.0 | ||
| 2750 ng/ml | 54% | 94% | 9.0 | ||
| SJIA vs MWS | 0.972 ± 0.031 | 1000 ng/ml | 78% | 100% | n.a. |
| SJIA vs ALL | 0.981 ± 0.024 | 1000 ng/ml | 78% | 100% | n.a. |
| SJIA vs AML | 1.000 ± 0.0 | 150 ng/ml | 97% | 100% | n.a. |
| SJIA vs healthy controls | 0.994 ± 0.012 | 150 ng/ml | 98% | 100% | n.a. |
vs=versus; n.a.=not applicable, LR+ is going to infinity in these cases
Discussion
The main differential diagnoses of FUO are (in this order): infections without focus, auto-inflammatory/rheumatic diseases, and malignancies. For SJIA, as a prototypic auto-inflammatory disease, there is no laboratory test available to ascertain the diagnosis and specific clinical signs, i.e. arthritis, often develop later in the course of the disease. Typically patients present with a marked elevation of acute phase reactants and a clinical course resembling sepsis, and a time consuming diagnostic workup often prevents the early initiation of appropriate, anti-inflammatory therapy. Since infectious causes of FUO by far outnumber the cases of SJIA, a surrogate marker for the latter would be very helpful in identifying the patients with SJIA.
The primary goal of our study was to investigate the potential role of S100A12 in the differential diagnosis of SJIA versus acute severe systemic infections and childhood leukemias. With the measurement of serum levels of S100A12, a diagnostic tool with high sensitivity and specificity for the early diagnosis of SJIA can now be added to the existing laboratory arsenal. The positive likelihood ratios between 6.0 and 11.0 or higher to discriminate between SJIA and other causes of FUO and a clear increased posttest probability make determination of serum levels of S100A12 a useful diagnostic tool (table 2).(25)
Interestingly, S100A12 serum concentrations in FMF and SJIA patients are comparable, therefore differentiation of both diseases via S100A12 measurement is not possible. However, FMF can easily be distinguished by other means such as family history and genetic testing, and in both conditions the general therapeutic decision against antibiotic and in favor of anti-inflammatory treatment would be appropriate for patients with both conditions. In contrast to SJIA, fever and symptoms in FMF patients occur typically episodically, even if some of the symptoms can be present in both disease entities, such as abdominal pain, serositis, lymphadenopathy, dermal rash or arthritis. Diagnosis is made on the basis of the clinical presentation, ethnic background and in consideration of diagnostic criteria sets, e.g. Tel Hashomer criteria.(26) In addition, molecular analysis of mutations in the MEFV gene helps to identify patients suggestive for FMF.(27) Interestingly, serum levels of S100A12 in the IL-1 driven syndromes NOMID and MWS are significantly lower than in SJIA and FMF. For diagnostic procedures this may help to rule out two rare causes of FUO when suspecting SJIA but even more interestingly this fact points to a common pathogenic mechanism for SJIA and FMF not present in other auto-inflammatory disorders.
Published data revealed IL-1 as a key cytokine in SJIA. Nevertheless reports on the usefulness of IL-1Ra treatment are contradictory; while Pascual et al. found 9 of 9 patients responsive to IL1-Ra, Lequerre et al. report response in less than half of the patients.(28-31) Peripheral blood mononuclear cells (PBMC) release high amounts ofIL-1 when incubated with serum of SJIA patients, thus suggesting that SJIA serum contains factors which are responsible for the activation of leukocytes.(28) Interestingly, at concentrations we found in serum from patients with active SJIA, S100A12 can induce expression of pro-inflammatory cytokines along with other pro-inflammatory effects, but the exact role ofS100A12 in SJIA is still unclear.(13, 32) The massive overexpression of this phagocytic protein in FMF and SJIA patients points to pathogenic mechanisms closely linked to the innate immune system and specifically to the release of IL-1 and other cytokines during inflammatory responses. The MEFV -geneproduct pyrin, expressed in myeloid/monocytic cells, can bind to the NALP-3 inflammasome that induces autocatalysis of caspase-1 and may exert inhibitory functions. Secretory pathways, bypassing the classical Golgi-route, are responsible for secretion of S100-proteins and IL-1. Aberrations in these alternative pathways could represent the link between the massive elevation of S100A12 serum concentrations and the IL-1 driven pathogenic mechanisms in SJIA and FMF.(30)
Patients with active SJIA prior to the initiation of anti-inflammatory therapies present with serum concentrations of S100A12 which significantly differ from levels in other inflammatory disorders such as non-systemic forms of JIA, rheumatoid arthritis, intestinal bowel disease, giant cell arteritis and Kawasaki disease (table 3).(11, 21, 33-36) These published results can be compared with results in this study because the ELISAs were all performed in one laboratory and the same internal control sera have been used in the different studies allowing for interassay comparisons. We expand our observationsto childhood leukemias where serum concentrations of S100A12 are significantly lower than in SJIA and FMF. Previous attempts to establish biomarkers for SJIA concentrated on ferritin, which is elevated and of diagnostic value in adults-onset Still’s disease but not in SJIA.(37) Very recently gene expression profiles from PBMC of SJIA patients revealed specific upregulation of gene transcripts, differentiating active SJIA from inactive patients and other inflammatory conditions. S100A12 was among the genes significantly upregulated.(38) Allantaz et al. identified, using the same technique, 12 SJIA specific transcripts distinguishing SJIA patients from other febrile conditions including infections.(39)
Table 3.
S100A12 levels in a variety of inflammatory disorders.
| S100A12 (ng/ml) | n | Reference | |
|---|---|---|---|
| SJIA | 7190 ± 1340* | 60 | present study |
| FMF | 6720 ± 2340* | 17 | present study |
| Systemic Infections | 470 ± 80*† | 83 | present study |
| NOMID | 720 ± 210*† | 18 | present study |
| MWS | 150 ± 30*† | 17 | present study |
| ALL | 130 ± 30*† | 40 | present study |
| AML | 45 ± 20*† | 5 | present study |
| Healthy Controls | 50 ± 5*† | 45 | present study |
| JIA | 410 ± 90*† | 91 | Arthritis Rheum 2004(11) |
| Crohn’s Disease | 470 ± 125*† | 40 | Gut 2003(21) |
| Ulcerative Colitis | 400 ± 120*† | 34 | Gut 2003(21) |
| Kawasaki Vasculitis | 463 ± 125*† | 31 | Lancet 2003(33) |
| Giant Cell Arteritis | 100 ± 15*† | 42 | J Pathol 2004(35) |
| Rheumatoid Arthritis | 480 ± 75*† | 54 | Ann Rheum Dis 2007(36) |
mean ± standard error of the mean;
p<0.001 versus active SJIA
In contrast to the above mentioned gene expression studies we tried to differentiate SJIA patients by simple means of a serum biomarker. Advantages of our method in comparison to the array technology are better availability, lower costs and convenience of sampling. Analyzing cohorts of patients with very rare disease is associated with some limitations regarding interpretation of data due to the relative low number of individual patients. Although the differences in serum concentrations of S100A12 are very impressive one has to keep in mind that the age distribution between the different cohorts is not homogenously and especially in the MWS cohort we included a considerable amount of adults. However, there are no differences of S100A12 concentrations over age.(33) A second bias in MWS patients may be caused by the fact that 17 included patients descended from only 7 families, and as a consequence only two different mutations could be studied. The analysis of typical versus atypical SJIA, differences between different FMF phenotypes or between different MWS mutations are due to statistical limitations beyond the scope of our study and should be clarified in future investigations.
In conclusion, S100A12 may be a valuable laboratory biomarker expanding our arsenal of diagnostic tools for detecting SJIA which is more sensitive and specific than other available indicators of inflammation. Levels of S100A12 help to confirm the diagnosis of SJIAand allow early differentiation from patients with severe systemic infections and a number of inflammatory and malignant disorders.
Recent research suggests a key role of abnormalities in the innate immune system in the pathogenesis of SJIA and other auto-inflammatory diseases, and the upregulation of markers identifying phagocyte activation such as S100A12 are in line with these findings. The differential upregulation of phagocytic S100A12 in SJIA, FMF and to a lesser extent in the NLRP3 associated periodic syndromes (CAPS) points to a key role of neutrophil and monocyte activation in the pathogenesis of at least the first two of these disorders. Further understanding of the pathogenic mechanisms underlying the auto-inflammatory diseases may allow for more rationale therapies in the future.
Acknowledgments
The authors thank Melanie Saers and Dorothee Lagemann for excellent technical assistance.
Funding The study was supported by grants of the Interdisciplinary Centre for Clinical Research, University of Muenster, Germany (Project Foe2/005/06), and of the German Research Foundation (DFG Project FO 354/2-2). The funding sources had no involvement in the study design; in the collection, analysis, and interpretation of the data; in the writing of the report; and in the decision to submit the paper for publication.
Funding Sources: Interdisciplinary Centre for Clinical Research, University of Muenster, Germany (Project Foe2/005/06), and German Research Foundation (DFGProject FO 354/2-2)
Footnotes
Conflict of interest statement Johannes Roth has a pending patent application for S100A12 ELISA (US 20030175713). All other authors declare that they have no financial or personal conflict of interest related to this study.
References
- 1.Petersdorf RG, Beeson PB. Fever of unexplained origin: report on 100 cases. Medicine (Baltimore) 1961;40:1–30. doi: 10.1097/00005792-196102000-00001. [DOI] [PubMed] [Google Scholar]
- 2.Durack DT, Street AC. Fever of unknown origin--reexamined and redefined. Curr Clin Top Infect Dis. 1991;11:35–51. [PubMed] [Google Scholar]
- 3.Arnow PM, Flaherty JP. Fever of unknown origin. Lancet. 1997;350(9077):575–80. doi: 10.1016/S0140-6736(97)07061-X. [DOI] [PubMed] [Google Scholar]
- 4.Gaeta GB, Fusco FM, Nardiello S. Fever of unknown origin: a systematic review of the literature for 1995-2004. Nucl Med Commun. 2006;27(3):205–11. doi: 10.1097/00006231-200603000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Woo P, Wedderburn LR. Juvenile chronic arthritis. Lancet. 1998;351(9107):969–73. [PubMed] [Google Scholar]
- 6.Schneider R, Laxer RM. Systemic onset juvenile rheumatoid arthritis. Baillieres Clin Rheumatol. 1998;12(2):245–71. doi: 10.1016/s0950-3579(98)80018-6. [DOI] [PubMed] [Google Scholar]
- 7.Woo P. Systemic juvenile idiopathic arthritis: diagnosis, management, and outcome. Nat Clin Pract Rheumatol. 2006;2(1):28–34. doi: 10.1038/ncprheum0084. [DOI] [PubMed] [Google Scholar]
- 8.Jarvis JN. Pathogenesis and mechanisms of inflammation in the childhood rheumatic diseases. Curr Opin Rheumatol. 1998;10(5):459–67. doi: 10.1097/00002281-199809000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Niki Y, Yamada H, Seki S, Kikuchi T, Takaishi H, Toyama Y, et al. Macrophage- and neutrophil-dominant arthritis in human IL-1 alpha transgenic mice. J Clin Invest. 2001;107(9):1127–35. doi: 10.1172/JCI11530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Muller K, Herner EB, Stagg A, Bendtzen K, Woo P. Inflammatory cytokines and cytokine antagonists in whole blood cultures of patients with systemic juvenile chronic arthritis. Br J Rheumatol. 1998;37(5):562–9. doi: 10.1093/rheumatology/37.5.562. [DOI] [PubMed] [Google Scholar]
- 11.Foell D, Wittkowski H, Hammerschmidt I, Wulffraat NM, Schmeling H, Frosch M, et al. Monitoring neutrophil activation in juvenile rheumatoid arthritis by S100A12 serum concentrations. Arthritis Rheum. 2004;50(4):1286–1295. doi: 10.1002/art.20125. [DOI] [PubMed] [Google Scholar]
- 12.Foell D, Wittkowski H, Roth J. Mechanisms of Disease: a ‘DAMP’ view of inflammatory arthritis. Nat Clin Pract Rheumatol. 2007;3(7):382–90. doi: 10.1038/ncprheum0531. [DOI] [PubMed] [Google Scholar]
- 13.Wittkowski H, Sturrock A, van Zoelen MA, Viemann D, van der Poll T, Hoidal JR, et al. Neutrophil-derived S100A12 in acute lung injury and respiratory distress syndrome. Crit Care Med. 2007;35(5):1369–75. doi: 10.1097/01.CCM.0000262386.32287.29. [DOI] [PubMed] [Google Scholar]
- 14.Xie J, Burz DS, He W, Bronstein IB, Lednev I, Shekhtman A. Hexameric calgranulin C (S100A12) binds to the receptor for advanced glycated end products (RAGE) using symmetric hydrophobic target-binding patches. J Biol Chem. 2007;282(6):4218–31. doi: 10.1074/jbc.M608888200. [DOI] [PubMed] [Google Scholar]
- 15.Hofmann MA, Drury S, Fu C, Qu W, Taguchi A, Lu Y, et al. RAGE mediates a novel proinflammatory axis: a central cell surface receptor for S100/calgranulin polypeptides. Cell. 1999;97(7):889–901. doi: 10.1016/s0092-8674(00)80801-6. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt AM, Yan SD, Yan SF, Stern DM. The multiligand receptor RAGE as a progression factor amplifying immune and inflammatory responses. J Clin Invest. 2001;108(7):949–55. doi: 10.1172/JCI14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Foell D, Roth J. Proinflammatory S100 proteins in arthritis and autoimmune disease. Arthritis Rheum. 2004;50(12):3762–71. doi: 10.1002/art.20631. [DOI] [PubMed] [Google Scholar]
- 18.Petty RE, Southwood TR, Manners P, Baum J, Glass DN, Goldenberg J, et al. International League of Associations for Rheumatology classification of juvenile idiopathic arthritis: second revision, Edmonton, 2001. J Rheumatol. 2004;31(2):390–2. [PubMed] [Google Scholar]
- 19.Ruperto N, Ravelli A, Falcini F, Lepore L, De Sanctis R, Zulian F, et al. Performance of the preliminary definition of improvement in juvenile chronic arthritis patients treated with methotrexate. Italian Pediatric Rheumatology Study Group. Ann Rheum Dis. 1998;57(1):38–41. doi: 10.1136/ard.57.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giannini EH, Ruperto N, Ravelli A, Lovell DJ, Felson DT, Martini A. Preliminary definition of improvement in juvenile arthritis. Arthritis Rheum. 1997;40(7):1202–9. doi: 10.1002/1529-0131(199707)40:7<1202::AID-ART3>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 21.Foell D, Kucharzik T, Kraft M, Vogl T, Sorg C, Domschke W, et al. Neutrophil derived human S100A12 (EN-RAGE) is strongly expressed during chronic active inflammatory bowel disease. Gut. 2003;52(6):847–53. doi: 10.1136/gut.52.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vogl T, Propper C, Hartmann M, Strey A, Strupat K, van den Bos C, et al. S100A12 is expressed exclusively by granulocytes and acts independently from MRP8 and MRP14. J Biol Chem. 1999;274(36):25291–6. doi: 10.1074/jbc.274.36.25291. [DOI] [PubMed] [Google Scholar]
- 23.Kaiser T, Langhorst J, Wittkowski H, Becker K, Friedrich AW, Rueffer A, et al. Fecal S100A12 as non-invasive marker distinguishing inflammatory bowel disease from irritable bowel syndrome. Gut. 2007;56(12):1706–13. doi: 10.1136/gut.2006.113431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldbach-Mansky R, Dailey NJ, Canna SW, Gelabert A, Jones J, Rubin BI, et al. Neonatal-onset multisystem inflammatory disease responsive to interleukin-1beta inhibition. N Engl J Med. 2006;355(6):581–92. doi: 10.1056/NEJMoa055137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Guidelines for immunologic laboratory testing in the rheumatic diseases: an introduction. Arthritis Rheum. 2002;47(4):429–33. doi: 10.1002/art.10381. [DOI] [PubMed] [Google Scholar]
- 26.Federici L, Rittore-Domingo C, Kone-Paut I, Jorgensen C, Rodiere M, Le Quellec A, et al. A decision tree for genetic diagnosis of hereditary periodic fever in unselected patients. Ann Rheum Dis. 2006;65(11):1427–32. doi: 10.1136/ard.2006.054304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drenth JP, van der Meer JW. Hereditary periodic fever. N Engl J Med. 2001;345(24):1748–57. doi: 10.1056/NEJMra010200. [DOI] [PubMed] [Google Scholar]
- 28.Pascual V, Allantaz F, Arce E, Punaro M, Banchereau J. Role of interleukin-1 (IL-1) in the pathogenesis of systemic onset juvenile idiopathic arthritis and clinical response to IL-1 blockade. J Exp Med. 2005;201(9):1479–86. doi: 10.1084/jem.20050473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fitzgerald AA, Leclercq SA, Yan A, Homik JE, Dinarello CA. Rapid responses to anakinra in patients with refractory adult-onset Still’s disease. Arthritis Rheum. 2005;52(6):1794–803. doi: 10.1002/art.21061. [DOI] [PubMed] [Google Scholar]
- 30.Dinarello CA. Blocking IL-1 in systemic inflammation. J Exp Med. 2005;201(9):1355–9. doi: 10.1084/jem.20050640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lequerre T, Quartier P, Rosellini D, Alaoui F, De Bandt M, Mejjad O, et al. Interleukin-1 receptor antagonist (anakinra) treatment in patients with systemic-onset juvenile idiopathic arthritis or adult onset Still disease: preliminary experience in France. Ann Rheum Dis. 2008;67(3):302–8. doi: 10.1136/ard.2007.076034. [DOI] [PubMed] [Google Scholar]
- 32.Yang Z, Tao T, Raftery MJ, Youssef P, Di Girolamo N, Geczy CL. Proinflammatory properties of the human S100 protein S100A12. J Leukoc Biol. 2001;69(6):986–94. [PubMed] [Google Scholar]
- 33.Foell D, Ichida F, Vogl T, Yu X, Chen R, Miyawaki T, et al. S100A12 (ENRAGE) in monitoring Kawasaki disease. Lancet. 2003;361(9365):1270–1272. doi: 10.1016/S0140-6736(03)12986-8. [DOI] [PubMed] [Google Scholar]
- 34.Foell D, Kane D, Bresnihan B, Vogl T, Nacken W, Sorg C, et al. Expression of the pro-inflammatory protein S100A12 (EN-RAGE) in rheumatoid and psoriatic arthritis. Rheumatology (Oxford) 2003;42(11):1383–9. doi: 10.1093/rheumatology/keg385. [DOI] [PubMed] [Google Scholar]
- 35.Foell D, Hernandez-Rodriguez J, Sanchez M, Vogl T, Cid MC, Roth J. Early recruitment of phagocytes contributes to the vascular inflammation of giant cell arteritis. J Pathol. 2004;204(3):311–6. doi: 10.1002/path.1660. [DOI] [PubMed] [Google Scholar]
- 36.Wittkowski H, Foell D, af Klint E, De Rycke L, De Keyser F, Frosch M, et al. Effects of intra-articular corticosteroids and anti-TNF therapy on neutrophil activation in rheumatoid arthritis. Ann Rheum Dis. 2007;66(8):1020–5. doi: 10.1136/ard.2006.061507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sobieska M, Fassbender K, Aeschlimann A, Bourgeois P, Mackiewicz S, Muller W. Still’s disease in children and adults: a distinct pattern of acute- phase proteins. Clin Rheumatol. 1998;17(3):258–60. doi: 10.1007/BF01451062. [DOI] [PubMed] [Google Scholar]
- 38.Ogilvie EM, Khan A, Hubank M, Kellam P, Woo P. Specific gene expression profiles in systemic juvenile idiopathic arthritis. Arthritis Rheum. 2007;56(6):1954–65. doi: 10.1002/art.22644. [DOI] [PubMed] [Google Scholar]
- 39.Allantaz F, Chaussabel D, Stichweh D, Bennett L, Allman W, Mejias A, et al. Blood leukocyte microarrays to diagnose systemic onset juvenile idiopathic arthritis and follow the response to IL-1 blockade. J Exp Med. 2007;204(9):2131–44. doi: 10.1084/jem.20070070. [DOI] [PMC free article] [PubMed] [Google Scholar]